Abstract
Meprin metalloendopeptidases, comprising α and β isoforms, are widely expressed in mammalian cells and organs including kidney, intestines, lungs, skin, and bladder, and in a variety of immune cells and cancer cells. Meprins proteolytically process many inflammatory mediators, including cytokines, chemokines, and other bioactive proteins and peptides that control the function of immune cells. The knowledge of meprin-mediated processing of inflammatory mediators and other target substrates provides a pathophysiologic link for the involvement of meprins in the pathogenesis of many inflammatory disorders. Meprins are now known to play important roles in inflammatory diseases including acute kidney injury, sepsis, urinary tract infections, bladder inflammation, and inflammatory bowel disease. The proteolysis of epithelial and endothelial barriers including cell junctional proteins by meprins promotes leukocyte influx into areas of tissue damage to result in inflammation. Meprins degrade extracellular matrix proteins; this ability of meprins is implicated in the cell migration of leukocytes and the invasion of tumor cells that express meprins. Proteolytic processing and maturation of procollagens provides evidence that meprins are involved in collagen maturation and deposition in the fibrotic processes involved in the formation of keloids and hypertrophic scars and lung fibrosis. This review highlights recent progress in understanding the role of meprins in inflammatory disorders in both human and mouse models.
Keywords: IL-1β, IL-6, IL-18, CCL2, IL-6R, meprin A, meprin α, meprin β, inflammation, metalloproteinase
Introduction
Meprins are multidomain oligomeric metalloproteinases of the ‘astacin’ family that are expressed in two isoforms, meprin α and meprin β. Initially identified in apical brush-border membranes of the kidneys and intestines, meprin isoforms are widely found in many mammalian tissues and cells, including the kidney, intestines, lungs, leukocytes, skin, and bladder, as well as in a variety of cancer cells [1–3]. Meprin α and meprin β are related proteases but are encoded by two different genes. Meprin α is encoded by the MEP1A locus on chromosome 6p in humans and Mep1a locus on chromosome 17 in mice, whereas meprin β is encoded by the MEP1B locus in human and Mep1b locus in mice on chromosome 18 of both species [4]. Structurally, both meprin α, and β have multiple domains that may participate in protein-protein interactions [1] (Figure 1).
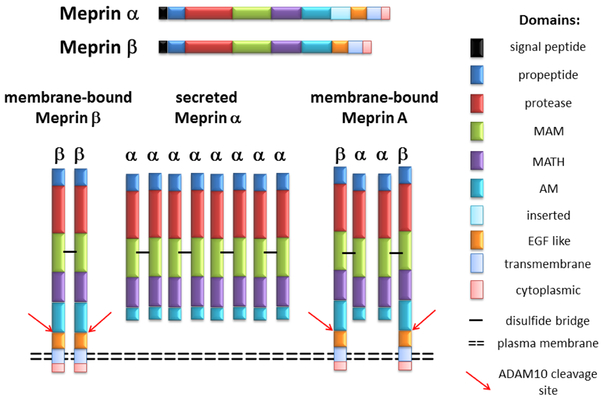
Figure 1:
Structural domains and oligomeric forms of meprin α and β. Structural domains of meprins include an N-terminal signal peptide, propeptide, protease domain (HExxHxxGxxH), MAM (meprin A5 protein receptor protein tyrosine phosphatase μ), TRAF (tumor necrosis factor receptor-associated factor), AM (after MATH), I (inserted domain), EGF (epidermal growth factor -like), TM (transmembrane spanning), and cytoplasmic domain. Meprin β is a membranebound protease. Meprin subunits composed of α2β2 heterotetramer form Meprin A which is bound to membranes through meprin β. The meprin subunits are bound together by disulfide bridges (horizontal black bars). Meprin α oligomerizes to become a higher molecular mass soluble from of protease and is secreted as a homooligomer.
The structural domains of meprin subunits are comprised of a signal peptide, propeptide, protease catalytic domain containing the zinc-binding active site motif HExxHxxGxxH/N, MAM (meprin A5 protein tyrosine phosphatase μ) domain, MATH [meprin-and-TRAF (tumor necrosis factor receptor-associated factor) homology domain] domain, an AM (after MATH) domain, EGF (epidermal growth factor)-like domain, transmembrane domain, and a cytosolic domain. The function of each structural domain has been described in previous reviews [1–3]. Briefly, the propeptide domain contains the signal peptide that must be cleaved for the proteolytic activity. Different proteases including plasmin, pancreatic trypsin, and tissue kallikrein related peptidase (KLK) 5 are knwn to activate meprin subunits. The zinc-binding active site motif HExxHxxGxxH/N forms a deep cleft for the substrate binding. Both the MAM and TRAF domains participate in the oligomerization of meprin subunits. The MAM domain participates in protein-protein interactions and the cysteine residues of the MAM domain are involved in the formation of disulfide bonds that contribute to the production of oligomeric structures in meprins. The TRAF domain is involved in protein-protein interactions and plays a role in a disulfide bond formation between the meprin subunits. The specific function of the AM domain is unknown but may facilitate in the catalytic function. The C-terminus transmembrane and cytoplasmic domain of the β subunit facilitate membrane insertion as well as in intracellular transport. Meprin α when expressed contains one more domain, called the intervening domain (I-domain) than meprin β. This additional I-domain present between the TRAF and EGF-like domains is proteolytically cleaved by furin during translocation across the membranes of the secretory pathway, resulting in the loss of the membrane anchor and leading to the release of soluble meprin α in the extracellular space [5–7]. The soluble form of meprin α further oligomerizes to become a higher molecular mass protease [7, 8]. Due to the absence of the I-domain, meprin β is membrane-bound and expressed as a type-1 protein anchored to the plasma membrane (Figure 1).
The heterodimer of meprin α and β subunits is called meprin A. Meprin A (meprin αβ form) is bound to the membranes through the β subunit. Meprin α and meprin β are expressed independently or co-expressed depending on the particular tissue or cell type. In murine kidneys, upon co-expression of murine meprin α and β subunits, meprin α can either associate with membrane-bound meprin β to form meprin A (meprin αβ) or self-associate to form homooligomer of meprin α subunits, which is secreted [7, 8]. The ectodomain of meprin β can be shed in soluble form by ADAM10 [9, 10] suggesting that ADAM10 plays a regulatory role in the proteolytic action of membrane-bound meprin A and meprin β. Meprin A (meprin αβ) has been purified and characterized from murine kidneys as described [2, 11]. Though membrane-associated meprin has been purified from human kidney [12], it is not known if the purified meprin is heteromeric meprin A or meprin β. In the large intestine, meprin α, but not meprin β is expressed, whereas both meprin α and β are expressed in the small intestine [13–15]. Meprin α and β are expressed in separate layers of the human epidermis [16]. Leukocytes of the lamina propria of the human inflamed bowel [17] and mouse mesenteric lymph nodes [18] express both meprin α and β.
Meprins target a wide variety of substrates including basement membrane proteins, cytokines, adherens junction proteins, growth factors, protein kinases, bioactive peptides, and cell-surface proteins (Table 1). Meprin α and meprin β also show some differences in cleavage specificities of the various substrates [2, 19]. Meprin-mediated proteolysis of these substrates provides a link between meprin and pathophysiology of the target substrates and associated diseases. This review describes how meprins participate in pathogenesis of many inflammatory diseases (described below).
Table 1.
Substrates of meprins
| Substrate identified in vitro | Meprin Isoform and Species | Reference (s) |
|---|---|---|
| CCL2/MCP1 | meprin β (human), meprin α (rat), meprin A (rat) | [20] |
| FGF-19 | meprin β (human) | [21] |
| Pro-IL-1β | meprin α (human), meprin β (human), meprin A (rat) | [22, 23] |
| IL-6 | meprin α and β (human), meprin β (rat), meprin α (mouse) | [24] |
| IL-6R | meprin α and β (human) | [25] |
| Pro-IL-18 | meprin β (mouse) | [26] |
| Actin | meprin β (rat), meprin α (mouse) | [27] |
| Pro-ADAM10 | meprin β (human) | [9] |
| Angiotensin-I and -II | meprin A (mouse), meprin A (human) | [28–30] |
| CD99 | meprin β (mouse) | [31] |
| E-Cadherin | meprin α and β (human) | [32] |
| Pro-Collagen-III | meprin α and β (human) | [33] |
| Collagen-IV | meprin A (rat), meprin α and β (human) | [11, 34] |
| Fibronectin | meprin A (rat), meprin α and β (human) | [11, 34] |
| Insulin | meprin A (mouse), meprin A (human) | [28–30] |
| Laminin-1 and −5 | rec human meprin α | [11, 35] |
| Nidogen-I | meprin A (rat), meprin α and β (human) | [34, 36] |
| Protein Kinase A | meprin A (rat) | [37] |
| Thymosin-β4 | meprin A (rat) | [38] |
| Villin | meprin β (rat), meprin α (mouse) | [27] |
| Substrate identified in vivo | ||
| Pro-IL-1β | meprin A (mouse) | [23, 39] |
| IL-6 | meprin α and β (mouse) | [24, 40] |
| IL-6R | meprin β (human) | [25] |
| Pro-IL-18 | meprin β (mouse) | [26] |
| Amyloid precursor protein | meprin β (mouse) | [41] |
| CD99 | meprin β (mouse) | [42] |
| Pro-Collagen-I | meprin α and β (mouse) | [43, 44] |
| Mucin-2 | meprin β (mouse) | [45] |
| Nidogen-I | meprin A (rat), meprin β (mouse) | [36, 46] |
| Thymosin-β4 | meprin α (mouse) | [38] |
Other in vitro substrates have been previously reported [2].
Role of meprins in inflammation
Inflammation is a biological response triggered upon exposure of tissues and organs to harmful stimuli including microbial infections, tissue injury, or toxic cellular components. In response to inflammation stimulus, the inflammatory cells recruited at the inflammatory sites release specialized substances including proinflammatory cytokines and acute-phase proteins to mediate the process and prevent further tissue damage. In inflammatory processes, cytokines and chemokines function alone or together to help regulate immune-cell recruitment and migration [47]. Meprins are involved in these inflammatory processes and therefore, play important roles in the pathogenesis of many inflammatory disorders (described below). The role of meprins in inflammation and modulation of immune cells was revealed from the evidence that (i) meprins process cytokines, chemokines, and other bioactive proteins and peptides; (ii) meprins are involved in inflammatory diseases including acute kidney injury (AKI), sepsis, urinary tract infections (UTIs), bladder inflammation, and idiopathic pulmonary arterial hypertension (PAH), and are associated with susceptibility to inflammatory bowel disease (IBD); and (iii) meprins affect monocyte and leukocyte migration and infiltration at sites of inflammation due to meprin-mediated loss of integrity of epithelial barriers and cleavage of tight junction proteins.
(i). Meprins process cytokines and chemokines:
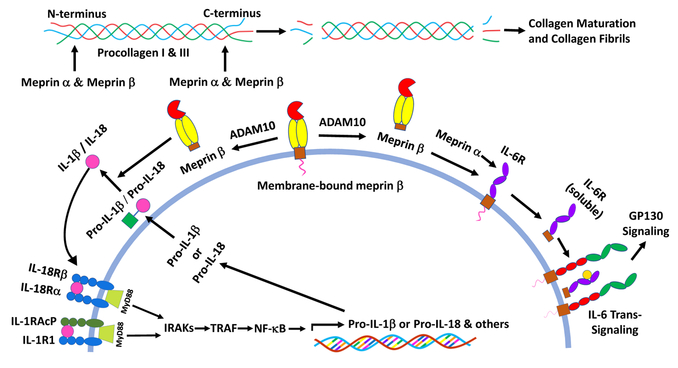
Cytokines and chemokines are endogenous inflammatory mediators that upon activation regulate migration of leukocytes to sites of injury to mount an immune response [47]. Meprins proteolytically process many cytokines, and chemokines and influence their bioavailability [1, 2]. Our studies have demonstrated that recombinant meprin α, recombinant meprin β, or heteromeric meprin A purified from kidney cortex can produce biologically functional proinflammatory cytokine IL-1β from its inactive proform [22, 23].
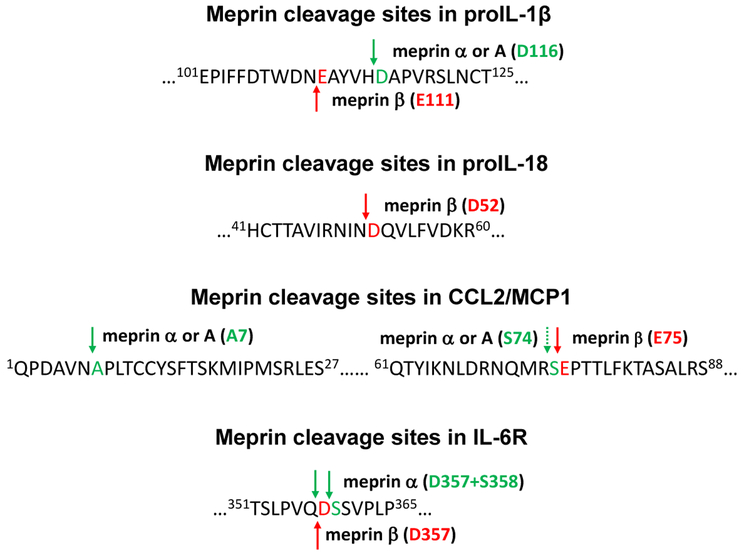
Meprin A and meprin α cleave pro-IL-1 β at the His115-Asp116 bond, which is one amino acid N-terminal to the caspase-1 cleavage site and five amino acids C-terminal to the meprin β site at Asn110-Glu111 [23] (Figure 2). This is consistent with the specificity of peptide bond cleavage sites for meprins as meprin β has a preference to cleave peptide bonds N-terminal to the negatively charged amino acids [21]. The biological activity of the pro-IL-1 β cleaved product that meprin A produces, as determined by the proliferative response of helper T-cells, was 2-fold higher compared to those of the IL-1β products that meprin β or caspase-1 produced [23]. In a mouse model of sepsis induced by cecal ligation puncture, the meprin inhibitor actinonin significantly reduced levels of serum IL-1 β and the initial peritubular capillary dysfunction, as well as the subsequent tubular injury [39]. Actinonin is a naturally occurring hydroxamate found in actinomycetes and has proven to be a most effective inhibitor of meprin α (Ki = 20 nM) and meprin β (Ki =1.7 μM) [34]. In response to lipopolysaccharide (LPS) challenge serum levels of IL-1 β were reduced in meprin α-knockout (KO) mice and these mice were protected from LPS-induced renal injury compared to wild-type (WT) mice [48]. These studies suggest that prolonged elevation of cytokine levels in WT mice upon LPS treatment contributes to the more extensive kidney damage compared with meprin α-KO mice. Meprin A may therefore play a critical role in the production of active IL-1β during inflammation and tissue injury in addition to the role in degradation of extracellular matrix (ECM) proteins.
Figure 2:
Sites of cleavage by meprins in pro-IL-1β, pro-IL-18, CCL2/MCP-1, and IL-6R. Cleavage sites of meprin A, meprin α, and meprin β, in the pro-IL-1β sequence were analyzed as described [22, 23]. Meprin A and meprin α cleave pro-IL-1β at the His115-Asp116 peptide bond and meprin β cleaves at As n110-Glu111 peptide bond. Cleavage sites of heteromeric meprin A and meprin β in the pro-IL-18 at the Asn51-Asp52 peptide bond were determined as described [26]. Cleavage sites of meprin α, heteromeric meprin A, and meprin β for CCL2/MCP-1 were analyzed as described [20]. Meprin α and meprin A cleaves the N-terminal domain at the Asn6-Ala7 bond and meprin β cleaves the C-terminal region between Ser74 and Glu75 of mouse CCL2/MCP-1. Meprin α and β cleave and membranebound IR-6R at the Gln357-Asp358 peptide bond as described [25].
Another pro-inflammatory cytokine, IL-18, similar in structure to IL-1β that plays a role in host defense and inflammatory processes [49, 50], is also a target of meprins. Meprin β and heteromeric meprin A, but not meprin α, cleave pro-IL-18 at the Asn51-Asp52 peptide bond [26], similar to the cleavage specificity of meprin β for IL-1β (Figure 2). The cleaved 17-kDa product was biologically active as determined by activation of NF-kB in EL-4 cells. The exact mechanism of release of proforms of cytokines is not completely understood. ProIL-18 has been shown to be actively released from the live epithelial and leukocytic cells and passively from dead cells [26]. Cytokines in their proforms can be released from the cell in the absence of proteolytically functional caspase-1 [51, 52]. Pro-IL18 is exposed to extracellular proteases and activated. For example, PR-3, a membrane-bound protease of the azurophil granules of polymorphonuclear neutrophils activates proIL-18 at the plasma membrane via a caspase-1-independent pathway [53]. IL-18 is known to contribute to the pathogenesis of inflammatory bowel diseases (IBD) in animals [54] as well as in humans [55]. Meprin β-KO mice subjected to experimental IBD by dextran sulfate sodium (DSS) exhibited reduced serum levels of IL-18 compare to WT mice indicating an in vivo interaction of meprin β with the cytokine [26]. However, IL-18 levels were not decreased, but rather increased in meprin α-KO mice with IBD, further suggesting that meprin α is not involved in the pro-IL-18 processing. Increased levels of IL-18 in meprin α-KO mice are due to the processing by meprin β present in meprin α-KO mice. Thus, meprin β-mediated activation of IL-18 from pro-IL-18 may affect the inflammatory response in IBD.
IL-6, another cytokine, is secreted in its active form that does not require proteolytic activation. Secreted active IL-6 is cleaved and inactivated by meprins [24]. Therefore, cleavage of IL-6 by meprins may control IL-6 activity in vivo. IL-6 has been implicated in inflammatory diseases including IBD [56] and increased levels of IL-6 were reported in meprin α-KO and meprin β-KO mice subjected to IBD [40], suggesting interaction between meprins and IL-6. Indeed, both meprin α and β were able to cleave IL-6 into a smaller product when incubated for a short time, but extensive degradation occurred upon prolonged incubation [24]. Removal of 3–5 amino acids from the C-terminus by limited meprin proteolysis inactivated IL-6, as demonstrated by a B9 cell proliferation assay. This is consistent with the previous o bservation that carboxy-terminal amino acids of human IL-6 are essential for its biological activity [57]. IL-6 is a pleiotropic cytokine that mediates its signaling via interaction with membrane-bound IL-6 receptor (IL-6R) and soluble IL-6 receptor (sIL-6R) [58]. The complex of IL-6 and membrane bound IL-6R associates with gp130, subsequently transducing classic intracellular signaling, which is protective and regenerative; whereas signaling via IL-6 and sIL-6R is called IL-6 trans-signaling, which is pro-inflammatory [59]. Upon binding with gp130, the signaling by IL-6 and membrane-bound IL-6R is restricted to cells that express IL-6 R, including hepatocytes, some leukocytes, and epithelial cells, whereas IL-6-sIL-6R can bind to gp130 and trans-signaling can occur in cells that do not express IL-6R [60].
Until recently, ADAM10 and ADAM17 were thought to be the enzymes responsible for ectodomain shedding of IL-6 R. Initially, the soluble IL-6 R produced upon cleavage at Gln357-Asp358 was identified as the cleavage site of ADAM10/17 [61]. However, hydrophobic rather than acidic amino acids are the predicted preferences for the cleavage site for ADAMs, and recombinant ADAM17 has been shown to cleave IL-6R between Pro355 and Val356 [62]. A recent study has confirmed that ADAM10/17 cleave the IL-6R between Pro-355 and Val-356 [63]. Importantly, this cleavage site was not only identified in vitro, but also in sIL-6R isolated from human serum. The cleavage specificity for Gln357-Asp358 , on the other hand, is consistent with meprin cleavage rather than ADAM specificity and indeed a recent study showed that meprin α and β cleave and shed membrane-bound IL-6R at the previously identified Gln357-Asp358 site to generate biologically active soluble IL-6R [25] (Figure 3). The biological activity of the soluble IL-6R shed by meprins was demonstrated by a Ba/F3-gp130 cell proliferation assay. Further, high expression of meprins was found associated with low receptor levels on the surface of granulocytes in human blood samples. This striking negative correlation between the expression of meprin β and IL-6R was attributed to the in vivo cleavage of the IL-6R by meprin β [25]. The processing of IL-6 R, IL-6 and other cytokines is summarized (Figure 3). The active cytokines then can bind their receptors to transduce NF-kB and MAPKs signaling.
Figure 3:
Processing of cytokines and procollagens by meprins. Membrane-bound meprin β can be shed by ADAM 10. Following activation by trypsin-like serine proteases, shed meprin β can process pro-IL- 1β and pro-IL-18 to their active forms. Meprin α can also process pro-IL-1β to its active form. The active cytokines then bind thei r receptors to transduce NF-kB and MAPKs signaling. Membrane bound meprin β may be activated by matriptase-2 (not shown). Meprin α and β can also cleaves ectodomain of IL-6R to produce soluble IL-6R that promotes IL-6 trans-signaling. Meprin α and β can process N- and C-termini of procollagen I and III resulting in the collagen maturation and formation of collagen fibrils.
Meprin α, meprin β, and meprin A can also process the chemokine CCL2/MCP-1 that results in significant reduction in the chemotactic activity as measured by chemotactic migration assay using THP-1 cells [20]. CCL2/MCP-1 belongs to the CC-type chemokine family and is known to play a role in the recruitment and trafficking of mononuclear immune cells to inflammation sites in the pathogenesis of chronic inflammatory diseases including asthma, atherosclerosis, rheumatoid arthritis, multiple sclerosis, and renal inflammatory diseases [64, 65]. Meprin α and meprin A, but not meprin β, cleaves the N-terminal domain of mouse CCL2/MCP-1 at the Asn6 and Ala7 bond, resulting in a reduction in its chemotactic activity. Meprin β does not cleave the N-terminus of mouse CCL2/MCP-1, but rather cleaves the C-terminal region between Ser74 and Glu75. Meprin α also cleaves the N-terminus of human CCL2/MCP-1 which lacks the murine C-terminal region, resulting in significant loss of CCL2/MCP-1 biological activity, whereas cleavage by meprin β does not affect the biological activity of human CCL2/MCP-1. Deletion of amino acid residues at the N-terminus of CCL2/MCP-1 by site-directed mutagenesis also renders CCL2/MCP-1 inactive for chemotaxis [66]. These studies suggest the importance of CCL2/MCP-1 N-terminus in chemotactic activity since CCL2/MCP-1 devoid of the N-terminus no longer possesses chemotactic activity. It is not yet known whether meprins process CCL2/MCP-1 and related chemokines in vivo in models of inflammatory diseases including acute kidney injury, cancer, and chronic inflammation of the gastrointestinal tract.
Thus, meprins may modulate the immune environment by processing and activating pro-inflammatory cytokines and chemokines that induce the migration of leukocytes to sites of injury or infection.
(ii). Meprins are involved in inflammatory diseases:
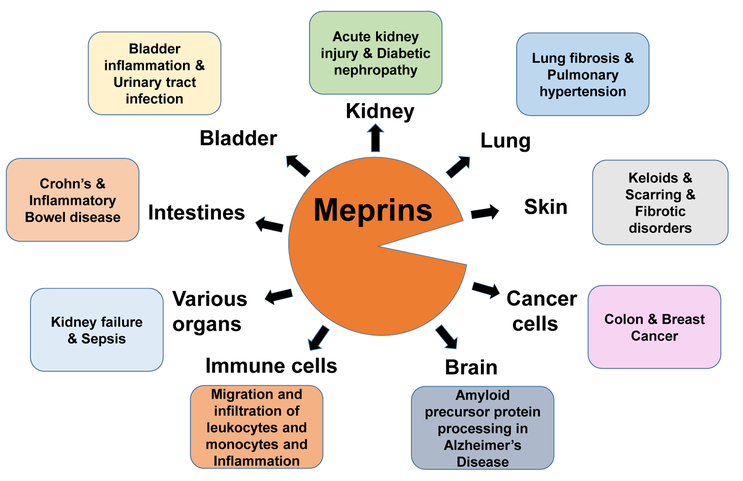
Meprins are involved in inflammatory diseases including AKI, sepsis, PAH, UTIs, bladder inflammation, and IBD (Figure 4).
Figure 4:
Schematic diagram depicting the involvement of meprins in organs and cells. Meprins are expressed in various organs and cells and regulate pathophysiologic outcome as shown.
Acute kidney injury (AKI):
The role of meprins in AKI has been studied in models of ischemia-reperfusion (IR), cisplatin, and sepsis [2]. Meprin A, a heteromeric form composed of meprin α and β subunits, is exclusively localized to the luminal surface of the proximal tubules in normal kidneys. In experimental models of AKI, meprin A is redistributed from apical membranes of polarized proximal tubular epithelial cells and is noticeable basolaterally to the underlying basement membrane of the proximal tubules [36, 67–69].
Due to its enormous proteolytic potential in cytokine processing and degradation of ECM and other bioactive proteins, altered distribution of meprin A is considered detrimental by promoting cellular damage and proinflammatory response in renal injury [2, 67]. Meprin β-KO mice subjected to IR injury exhibited reduced infiltration of inflammatory cells and improved renal function compared to wild-type mice [67].
The idea that meprin A contributes to renal injury was also supported by the finding that meprin-mediated cleavage of cytoskeleton components actin and villin associated with IR injury was absent in meprin α- and meprin β-KO mice [27]. In IR- and cisplatin-induced AKI, actinonin, a potent inhibitor of meprins, afforded protection as reflected in reduced loss in renal function accompanied by significant reduction in leukocyte infiltration and apoptosis [69, 70]. In a sepsis model of AKI induced by cecal ligation and puncture (CLP), actinonin markedly diminished production of IL-1β as well as improved renal function and histology [39]. Meprin α-KO mice subjected to sepsis induced by LPS displayed marked reduction in proinflammatory cytokines IL-1 β and TNF-α, and lower blood urea nitrogen levels compared with WT and meprin β-KO mice [48]. Also, meprin α-KO mice in this model exhibited significantly less bladder edema, leukocyte infiltration, and bladder permeability than WT mice [48]. These studies indicate that meprin A contributes to the renal and urogenital pathogenesis in LPS model of sepsis.
Idiopathic pulmonary arterial hypertension:
In human and animal models of PAH, pulmonary vascular lesions are associated with increased accumulation of perivascular immune cells and intravascular infiltration of these cells [71]. Mice genetically modified to overexpress Fos-related protein Fra-2 are used as a model of idiopathic pulmonary fibrosis since Fra-2 has profibrogenic activity and overexpression of Fra-2 causes fibrosis preferentially in pulmonary tissues [72]. In Fra-2 over-expressing mice, meprin β was the most upregulated gene in the lungs [71]. Since meprin β is known to enhance inflammatory cell infiltration, it may promote invasion of fibroblasts to alveolar space and extracellular matrix (ECM) deposition [71].
Inflammatory bowel disease (IBD):
The MEP1A gene encoding meprin α, has been identified as a susceptibility gene for IBD in patients with ulcerative colitis [40, 73]. Functional data on the MEP1A gene association with ulcerative colitis (UC) display four single-nucleotide polymorphisms in the coding region (P = 0.0012−0.04), and one in the 3’-untranslated region (P = 2 × 10−7) [40, 73]. Meprin α is secreted into the intestinal lumen or is retained by meprin β at the brush-border membranes [74]. Decreased levels of this protease correlated with the severity of inflammation in patients with inflammatory bowel disease. Further, meprin α-KO mice were more susceptible to DSS-induced experimental colitis and displayed more severe colon damage and inflammation than WT mice [40, 73]. However, DSS-treated meprin β-KO mice were less susceptible to IBD probably because of reduced meprin-mediated activation of IL-18 [26]. In patients with Crohn’s disease (CD), mRNA levels of meprin β in ileal mucosa are reduced [75]. In CD, pathogenic adherent-invasive Escherichia coli (AIEC) adhere and severely infect intestinal epithelial cells (IEC). Since meprins inhibit the abilities of AIEC to adhere to and invade intestinal epithelial cells, reduced levels of protective meprins, as observed in CD patients may contribute to increased AIEC colonization. Along similar lines, in small intestines, ADAM10-mediated soluble meprin β shed from membranes, cleaves mucin 2 (MUC2) and modulates the immune environment. MUC2 is the main structural component of the intestinal mucus layer and its cleavage by meprins promotes mucus detachment to prevent bacterial outgrowth [45, 76]. Taken together, these studies provide evidence of that a defect in the MEP1A gene results in vulnerability to IBD, particularly ulcerative colitis and meprins are important in preventing bacterial growth in intestinal mucus.
(iii). Meprins impact monocyte and leukocyte migration/infiltration.
Accumulating evidence show that meprins are involved in leukocyte migration and infiltration. The impact of 0meprins on the homeostasis of leukocytes was demonstrated in meprin-KO mice. For example, meprin α/β-KO mice had reduced monocytes (R-MC) and natural killer (NK) cells in blood, but increased prevalence in bone marrow which suggested that meprins are involved in migration of these cells from bone marrow and in homing to the periphery [77]. Mononuclear cells from human and mouse peripheral blood express both meprin α and β mRNAs. Leukocytes prepared from meprin β-KO mice displayed reduced ability to migrate through extracellular matrix compared to leukocytes isolated from WT mice [18]. In a model of intestinal inflammation, meprin β-expressing leukocytes infiltrate into the lamina propria [15, 26].
Meprin β-KO mice display reduced leukocyte infiltration following renal IR [67] and LPS injury [48]. This proinflammatory activity of meprin β was validated recently using an acute inflammation model (air pouch/carrageenan) that showed significantly fewer infiltrated immune cells in meprin β-KO animals [31]. Monocytes from meprin-KO mice exhibited reduced migration through an MDCK monolayer compared to monocytes from WT mice [78].
Proteolysis of epithelial and endothelial barriers, including breakdown of epithelial and endothelial cell junctional proteins, can promote leukocyte influx into areas of tissue damage and result in tissue inflammation. Meprins cleave tight junction proteins E-cadherin and occludin in MDCK monolayers that disrupts epithelial cell barrier function [69, 70]. Meprin-mediated proteolysis of endothelial cell barriers also influences infiltration of monocytes in injured tissue. For example, meprin β cleaves cell adhesion molecule CD99 [9] that regulates transendothelial cell migration (TEM) of immune cells [79]. Cleavage of CD99 by meprin β promotes TEM by reducing cell adhesion [42]. Thus, meprins promote leukocyte migration by disrupting epithelial and endothelial cell barriers by cleaving tight junction proteins and matrix proteins.
Meprin-mediated degradation and processing of extracellular matrix (ECM) proteins and its pathophysiological role
Meprins are capable of cleaving and processing many substrates including basement membrane proteins, cytokines, cell adhesion proteins, hormones, bioactive peptides, and cell-surface proteins [1, 80]. It was initially demonstrated that heteromeric meprin A purified from rat kidneys was capable of degrading ECM proteins including collagen IV, laminin, nidogen, fibronectin, and gelatin in vitro [11, 36]. Further studies showed that recombinant human meprin α and β are able to degrade collagen IV, fibronectin and nidogen-1 [34] while human meprin α can degrade laminin-1 and laminin-5 in vitro [35]. Only a few of the meprin-mediated degradation of ECM proteins have been demonstrated under in vivo conditions. In experimental models of acute kidney injury (AKI), meprin was able to cleave nidogen which functions as a connecting element between the collagen IV and laminin networks and integrates other basement membrane components into the extracellular matrix [81–83]. A nidogen fragment was detected in the urine from AKI mice and urinary excretion of cleaved nidogen was significantly suppressed by the meprin inhibitor actinonin, and this suppression was confirmed in meprin β-KO mice [46]. These observations are consistent with the observations that meprin A gains access to the basement membrane in AKI as it undergoes redistribution from the apical side toward the basolateral side of the proximal tubule. Thus, meprins that are normally restricted to the brush border membranes of tubules may be detrimental in renal injury due to altered localization. Cell migration of leukocytes of mesenteric lymph nodes [18] and invasion of tumor cells that express meprin have been attributed to the matrix-degrading activity of meprins. Breast cancer MDA-MB-435 cells were less invasive when treated with the meprin inhibitor actinonin [84]. In metastatic colon cancer cells expression of meprin α and its matrix degrading activity was responsible for migration of tumor cells and the progression of colon cancer [17].
Meprins also participate in proteolytic processing and maturation of procollagens by cleaving and removing both N- and C-propeptides (Figure 2). Previous studies have shown that disintegrin and metalloproteinases with thrombospondin motifs (ADAMTS)-2, −3, and −14 are involved in the cleavage of N-propeptides and bone morphogenetic protein-1 (BMP-1), and related tolloid-like proteinases are involved in the removal of C-propeptides of procollagen I [85, 86]. The proteolytic processing is necessary for self-assembly of collagen molecules to form normal collagen fibrils. Meprins can efficiently cleave both the N- and C-termini of procollagen propeptides, in contrast to previous reports that different enzymes were responsible for processing of the C-terminal and N-terminal propeptides. Initially, a proteomic based approach called TAILS identified procollagen type I as a substrate of meprin α and β. Following treatment of recombinant procollagen type I with meprin α and meprin β, the resulting mature collagen molecules spontaneously assembled to form collagen fibrils [44]. Meprin α and β also cleaved C- and N-propeptides of procollagen III in vitro [33]. Thus, both meprins are the only known proteases that cleave the procollagen proteins at both the N- and C-termini [44]. Compared to WT mice, meprin α-KO and meprin β-KO mice exhibited relatively decreased collagen deposition in the skin and reduced tensile strength, both characteristic of impaired connective tissue [44].
In a fibrotic skin disease called keloids, and in pulmonary hypertension characterized by the fibrotic condition of the lung, expression of meprin β was increased [33, 87] providing further in vivo evidence that meprins are involved in the collagen maturation and deposition in fibrotic processes. In keloids and hypertrophic scars, deposition of collagen fibers, primarily of collagen I and collagen III, have been reported [88]. Meprins are expressed in the lung epithelial and inflammatory cells in human and mouse lungs and meprin β is upregulated in a mouse model of idiopathic pulmonary fibrosis [87]. Meprin β-KO, but not meprin α-KO mice, subjected to bleomycin-induced lung fibrosis accumulated less collagen than bleomycin treated WT mice [43]. Taken together, these studies indicate that meprins play important roles in the fibrosis of lungs and skin.
Conclusions
This review summarizes recent advances in the role of meprins in inflammation. Recently, many substrates have been identifies that are targets of meprins that provides link to the pathophysiology. Cytokines and chemokines known to regulate inflammatory responses and control immune-cell recruitment in inflammatory processes are target substrates of meprins. Meprins generate biologically active IL-1β and IL-18 from their inactive proforms and the meprin-mediated processing of these cytokines has been demonstrated in in vivo models of sepsis and IBD. It is not yet known whether meprins process these cytokines in various other inflammatory disorders. One such pro-inflammatory role of meprin in vivo has been recently shown for processing of IL-6R and inducing IL-6 trans-signaling. Meprins may also regulate inflammation by inactivating pro-inflammatory cytokines and chemokines. For example, both meprin α and β cleave and inactivate IL-6. Therefore, the inflammatory effect of meprin will depend on the net effect of the activation of IL-1 β and IL-18 and inactivation of IL-6. More studies are further needed to determine whether other cytokines are targets of meprins. Meprin A and meprin α cleave the N-terminal domain of mouse CCL2/MCP-1, resulting in significant reduction in CCL2’s chemotactic activity. Further studies are required to determine whether other related chemokines are targets and activated by meprins. Meprins have been implicated in inflammatory diseases including AKI, sepsis, UTIs, bladder inflammation, and IBD. The evidence for some of these studies is limited to animal models so more studies are required to translate the information into applications for patient care. Much of the information on the role of meprins has been obtained using meprin-KO mice. There is always a possibility that loss of a protease is compensated to some extent by other proteases directly or by modifying an important activation cascade. For example, studies from our lab and that of others have shown that meprin β and meprin A are involved in shedding ADAM 10, which itself is involved in shedding many important molecules, including ectodomain shedding of a number of transmembrane proteins (e.g., Notch, EGFR ligands, cytokines or other signaling proteins involved in many functions). Thus, assigning a phenotype to a specific substrate in meprin-KO remains a challenge since these enzymes, including ADAM10, have a wide range of substrates. These issues may be partly addressed if specific and potent inhibitors are designed and developed to examine the effect of inhibition of each of the substrates. Meprins are new players for the processing and maturation of procollagens involved in the fibrillation process. Further studies are required to determine the role of meprin in models of fibrosis including that of chronic kidney disease. Nevertheless, current knowledge on the role of meprins in inflammation and fibrosis has potential for application in the development of meprin inhibitors and modulators for therapeutic use.
Highlights.
Meprin metalloproteinases comprised of α and β subunits are widely expressed in mammalian cells.
Meprins proteolytically process many inflammatory cytokines and chemokines.
Defect in expression of meprins is associated with inflammatory disorders.
Meprins are involved in matrix degradation and procollagen I and III maturation.
Acknowledgements
This work was supported by NIH grant R01DK081690 and VA Merit Award BX000444 to GPK and VA Merit Award BX000828 to RSH. We thank Bowen Jeffery for generously providing help in drawing figures for these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].] Sterchi EE, Stocker W, Bond JS, Meprins, membrane-bound and secreted astacin metalloproteinases, Mol Aspects Med 29(5) (2008) 309–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kaushal GP, Haun RS, Herzog C, Shah SV, Meprin A metalloproteinase and its role in acute kidney injury, Am J Physiol Renal Physiol 304(9) (2013) F1150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Prox J, Arnold P, Becker-Pauly C, Meprin alpha and meprin beta: Procollagen proteinases in health and disease, Matrix Biol 44-46 (2015) 7–13. [DOI] [PubMed] [Google Scholar]
- [4].Bond JS, Beynon RJ, The astacin family of metalloendopeptidases, Protein Sci 4(7) (1995)1247–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tang J, Bond JS, Maturation of secreted meprin alpha during biosynthesis: role of the furin site and identification of the COOH-terminal amino acids of the mouse kidney metalloprotease subunit, Arch Biochem Biophys 349(1) (1998) 192–200. [DOI] [PubMed] [Google Scholar]
- [6].Becker C, Kruse MN, Slotty KA, Kohler D, Harris JR, Rosmann S, Sterchi EE, Stocker W, Differences in the activation mechanism between the alpha and beta subunits of human meprin, Biol Chem 384(5) (2003) 825–31. [DOI] [PubMed] [Google Scholar]
- [7].Bertenshaw GP, Norcum MT, Bond JS, Structure of homo- and hetero-oligomeric meprin metalloproteases. Dimers, tetramers, and high molecular mass multimers, J Biol Chem 278(4) (2003) 2522–32. [DOI] [PubMed] [Google Scholar]
- [8].Ishmael FT, Norcum MT, Benkovic SJ, Bond JS, Multimeric structure of the secreted meprin A metalloproteinase and characterization of the functional protomer, J Biol Chem 276(25) (2001) 23207–11. [DOI] [PubMed] [Google Scholar]
- [9].Jefferson T, Auf dem Keller U, Bellac C, Metz VV, Broder C, Hedrich J, Ohler A, Maier W, Magdolen V, Sterchi E, Bond JS, Jayakumar A, Traupe H, Chalaris A, Rose-John S, Pietrzik CU, Postina R, Overall CM, Becker-Pauly C, The substrate degradome of meprin metalloproteases reveals an unexpected proteolytic link between meprin beta and ADAM10, Cellular and molecular life sciences : CMLS 70(2) (2013) 309–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Herzog C, Haun RS, Ludwig A, Shah SV, Kaushal GP, ADAM10 is the major sheddase responsible for the release of membrane-associated meprin A, J Biol Chem 289(19) (2014) 13308–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kaushal GP, Walker PD, Shah SV, An old enzyme with a new function: purification and characterization of a distinct matrix-degrading metalloproteinase in rat kidney cortex and its identification as meprin, J Cell Biol 126(5) (1994) 1319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yamaguchi T, Fukase M, Sugimoto T, Kido H, Chihara K, Purification of meprin from human kidney and its role in parathyroid hormone degradation, Biol Chem Hoppe Seyler 375(12) (1994) 821–4. [PubMed] [Google Scholar]
- [13].Bankus JM, Bond JS, Expression and distribution of meprin protease subunits in mouse intestine, Arch Biochem Biophys 331(1) (1996) 87–94. [DOI] [PubMed] [Google Scholar]
- [14].Bond JS, Matters GL, Banerjee S, Dusheck RE, Meprin metalloprotease expression and regulation in kidney, intestine, urinary tract infections and cancer, FEBS Lett 579(15) (2005) 3317–22. [DOI] [PubMed] [Google Scholar]
- [15].Lottaz D, Hahn D, Muller S, Muller C, Sterchi EE, Secretion of human meprin from intestinal epithelial cells depends on differential expression of the alpha and beta subunits, Eur J Biochem 259(1–2) (1999) 496–504. [DOI] [PubMed] [Google Scholar]
- [16].Becker-Pauly C, Howel M, Walker T, Vlad A, Aufenvenne K, Oji V, Lottaz D, Sterchi EE, Debela M, Magdolen V, Traupe H, Stocker W, The alpha and beta subunits of the metalloprotease meprin are expressed in separate layers of human epidermis, revealing different functions in keratinocyte proliferation and differentiation, J Invest Dermatol 127(5) (2007) 1115–25. [DOI] [PubMed] [Google Scholar]
- [17].Lottaz D, Maurer CA, Hahn D, Buchler MW, Sterchi EE, Nonpolarized secretion of human meprin alpha in colorectal cancer generates an increased proteolytic potential in the stroma, Cancer Res 59(5) (1999) 1127–33. [PubMed] [Google Scholar]
- [18].Crisman JM, Zhang B, Norman LP, Bond JS, Deletion of the mouse meprin beta metalloprotease gene diminishes the ability of leukocytes to disseminate through extracellular matrix, J Immunol 172(7) (2004) 4510–9. [DOI] [PubMed] [Google Scholar]
- [19].Bertenshaw GP, Turk BE, Hubbard SJ, Matters GL, Bylander JE, Crisman JM, Cantley LC, Bond JS, Marked differences between metalloproteases meprin A and B in substrate and peptide bond specificity, J Biol Chem 276(16) (2001) 13248–55. [DOI] [PubMed] [Google Scholar]
- [20].Herzog C, Haun RS, Shah SV, Kaushal GP, Proteolytic processing and inactivation of CCL2/MCP-1 by meprins, Biochemistry and biophysics reports 8 (2016) 146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Becker-Pauly C, Barre O, Schilling O, Auf dem Keller U, Ohler A, Broder C, Schutte A, Kappelhoff R, Stocker W, Overall CM, Proteomic analyses reveal an acidic prime side specificity for the astacin metalloprotease family reflected by physiological substrates, Molecular & cellular proteomics : MCP 10(9) (2011) M111 009233.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Herzog C, Kaushal GP, Haun RS, Generation of biologically active interleukin-1beta by meprin B, Cytokine 31(5) (2005) 394–403. [DOI] [PubMed] [Google Scholar]
- [23].Herzog C, Haun RS, Kaushal V, Mayeux PR, Shah SV, Kaushal GP, Meprin A and meprin alpha generate biologically functional IL-1beta from pro-IL-1beta, Biochem Biophys Res Commun 379(4) (2009) 904–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Keiffer TR, Bond JS, Meprin metalloproteases inactivate interleukin 6, J Biol Chem 289(11) (2014) 7580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Arnold P, Boll I, Rothaug M, Schumacher N, Schmidt F, Wichert R, Schneppenheim J, Lokau J, Pickhinke U, Koudelka T, Tholey A, Rabe B, Scheller J, Lucius R, Garbers C, Rose-John S, Becker-Pauly C, Meprin Metalloproteases Generate Biologically Active Soluble Interleukin-6 Receptor to Induce Trans-Signaling, Scientific reports 7 (2017) 44053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Banerjee S, Bond JS, Prointerleukin-18 is activated by meprin beta in vitro and in vivo in intestinal inflammation, J Biol Chem 283(46) (2008) 31371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ongeri EM, Anyanwu O, Reeves WB, Bond JS, Villin and actin in the mouse kidney brush-border membrane bind to and are degraded by meprins, an interaction that contributes to injury in ischemia-reperfusion, Am J Physiol Renal Physiol 301(4) (2011) F871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Butler PE, McKay MJ, Bond JS, Characterization of meprin, a membrane-bound metalloendopeptidase from mouse kidney, Biochem J 241(1) (1987) 229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sterchi EE, Naim HY, Lentze MJ, Biosynthesis of N-benzoyl-L-tyrosyl-p-aminobenzoic acid hydrolase: disulfide-linked dimers are formed at the site of synthesis in the rough endoplasmic reticulum, Arch Biochem Biophys 265(1) (1988) 119–27. [DOI] [PubMed] [Google Scholar]
- [30].Sterchi EE, Naim HY, Lentze MJ, Hauri HP, Fransen JA, N-benzoyl-L-tyrosyl-p-aminobenzoic acid hydrolase: a metalloendopeptidase of the human intestinal microvillus membrane which degrades biologically active peptides, Arch Biochem Biophys 265(1) (1988) 105–18. [DOI] [PubMed] [Google Scholar]
- [31].Bedau T, Schumacher N, Peters F, Prox J, Arnold P, Koudelka T, Helm O, Schmidt F, Rabe B, Jentzsch M, Rosenstiel P, Sebens S, Tholey A, Rose-John S, Becker-Pauly C, Cancer-associated mutations in the canonical cleavage site do not influence CD99 shedding by the metalloprotease meprin beta but alter cell migration in vitro, Oncotarget 8(33) (2017) 54873–54888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huguenin M, Muller EJ, Trachsel-Rosmann S, Oneda B, Ambort D, Sterchi EE, Lottaz D, The metalloprotease meprinbeta processes E-cadherin and weakens intercellular adhesion, PLoS One 3(5) (2008) e2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kronenberg D, Bruns BC, Moali C, Vadon-Le Goff S, Sterchi EE, Traupe H, Bohm M, Hulmes DJ, Stocker W, Becker-Pauly C, Processing of Procollagen III by Meprins: New Players in Extracellular Matrix Assembly?, J Invest Dermatol (2010). [DOI] [PubMed] [Google Scholar]
- [34].Kruse MN, Becker C, Lottaz D, Kohler D, Yiallouros I, Krell HW, Sterchi EE, Stocker W, Human meprin alpha and beta homo-oligomers: cleavage of basement membrane proteins and sensitivity to metalloprotease inhibitors, Biochem J 378(Pt 2) (2004) 383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Köhler D, Kruse M, Stocker W, Sterchi EE, Heterologously overexpressed, affinity-purified human meprin alpha is functionally active and cleaves components of the basement membrane in vitro, FEBS Lett 465(1) (2000) 2–7. [DOI] [PubMed] [Google Scholar]
- [36].Walker PD, Kaushal GP, Shah SV, Meprin A, the major matrix degrading enzyme in renal tubules, produces a novel nidogen fragment in vitro and in vivo, Kidney Int 53(6) (1998) 1673–80. [DOI] [PubMed] [Google Scholar]
- [37].Chestukhin A, Muradov K, Litovchick L, Shaltiel S, The cleavage of protein kinase A by the kinase-splitting membranal proteinase is reproduced by meprin beta, J Biol Chem 271(47) (1996) 30272–80. [DOI] [PubMed] [Google Scholar]
- [38].Kumar N, Nakagawa P, Janic B, Romero CA, Worou ME, Monu SR, Peterson EL, Shaw J, Valeriote F, Ongeri EM, Niyitegeka JM, Rhaleb NE, Carretero OA, The anti-inflammatory peptide Ac-SDKP is released from thymosin-beta4 by renal meprin-alpha and prolyl oligopeptidase, Am J Physiol Renal Physiol 310(10) (2016) F1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang Z, Herzog C, Kaushal GP, Gokden N, Mayeux PR, Actinonin, a meprin A inhibitor, protects the renal microcirculation during sepsis, Shock 35(2) (2011) 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Banerjee S, Jin G, Bradley SG, Matters GL, Gailey RD, Crisman JM, Bond JS, Balance of meprin A and B in mice affects the progression of experimental inflammatory bowel disease, American journal of physiology. Gastrointestinal and liver physiology 300(2) (2011) G273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schonherr C, Bien J, Isbert S, Wichert R, Prox J, Altmeppen H, Kumar S, Walter J, Lichtenthaler SF, Weggen S, Glatzel M, Becker-Pauly C, Pietrzik CU, Generation of aggregation prone N-terminally truncated amyloid beta peptides by meprin beta depends on the sequence specificity at the cleavage site, Molecular neurodegeneration 11 (2016) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bedau T, Peters F, Prox J, Arnold P, Schmidt F, Finkernagel M, Kollmann S, Wichert R, Otte A, Ohler A, Stirnberg M, Lucius R, Koudelka T, Tholey A, Biasin V, Pietrzik CU, Kwapiszewska G, Becker-Pauly C, Ectodomain shedding of CD99 within highly conserved regions is mediated by the metalloprotease meprin beta and promotes transendothelial cell migration, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 31(3) (2017) 1226–1237. [DOI] [PubMed] [Google Scholar]
- [43].Biasin V, Wygrecka M, Marsh LM, Becker-Pauly C, Brcic L, Ghanim B, Klepetko W, Olschewski A, Kwapiszewska G, Meprin beta contributes to collagen deposition in lung fibrosis, Scientific reports 7 (2017) 39969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Broder C, Arnold P, Vadon-Le Goff S, Konerding MA, Bahr K, Muller S, Overall CM, Bond JS, Koudelka T, Tholey A, Hulmes DJ, Moali C, Becker-Pauly C, Metal loproteases meprin alpha and meprin beta are C- and N-procollagen proteinases important for collagen assembly and tensile strength, Proc Natl Acad Sci U S A 110(35) (2013) 14219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schutte A, Ermund A, Becker-Pauly C, Johansson ME, Rodriguez-Pineiro AM, Backhed F, Muller S, Lottaz D, Bond JS, Hansson GC, Microbial-induced meprin beta cleavage in MUC2 mucin and a functional CFTR channel are required to release anchored small intestinal mucus, Proc Natl Acad Sci U S A 111(34) (2014) 12396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Herzog C, Marisiddaiah R, Haun RS, Kaushal GP, Basement membrane protein nidogen-1 is a target of meprin beta in cisplatin nephrotoxicity, Toxicology letters 236(2) (2015) 110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Turner MD, Nedjai B, Hurst T, Pennington DJ, Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease, Biochim Biophys Acta 1843(11) (2014) 2563–2582. [DOI] [PubMed] [Google Scholar]
- [[48].] Yura RE, Bradley SG, Ramesh G, Reeves WB, Bond JS, Meprin A metalloproteases enhance renal damage and bladder inflammation after LPS challenge, Am J Physiol Renal Physiol 296(1) (2009) F135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dinarello CA, Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process, The American journal of clinical nutrition 83(2) (2006) 447S–455S. [DOI] [PubMed] [Google Scholar]
- [50].Dinarello CA, Overview of the IL-1 family in innate inflammation and acquired immunity, Immunological reviews 281(1) (2018) 8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mehta VB, Hart J, Wewers MD, ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage, J Biol Chem 276(6) (2001) 3820–6. [DOI] [PubMed] [Google Scholar]
- [52].Andrei C, Margiocco P, Poggi A, Lotti LV, Torrisi MR, Rubartelli A, Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: Implications for inflammatory processes, Proc Natl Acad Sci U S A 101(26) (2004) 9745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sugawara S, Uehara A, Nochi T, Yamaguchi T, Ueda H, Sugiyama A, Hanzawa K, Kumagai K, Okamura H, Takada H, Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells, J Immunol 167(11) (2001) 6568–75. [DOI] [PubMed] [Google Scholar]
- [54].Sivakumar PV, Westrich GM, Kanaly S, Garka K, Born TL, Derry JM, Viney JL, Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage, Gut 50(6) (2002) 812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kanai T, Kamada N, Hisamatsu T, Clinical strategies for the blockade of IL-18 in inflammatory bowel diseases, Current drug targets 14(12) (2013) 1392–9. [DOI] [PubMed] [Google Scholar]
- [56].Atreya R, Neurath MF, Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer, Clinical reviews in allergy & immunology 28(3) (2005) 187–96. [DOI] [PubMed] [Google Scholar]
- [57].Kruttgen A, Rose-John S, Dufhues G, Bender S, Lutticken C, Freyer P, Heinrich PC, The three carboxy-terminal amino acids of human interleukin-6 are essential for its biological activity, FEBS Lett 273(1–2) (1990) 95–8. [DOI] [PubMed] [Google Scholar]
- [58].Schaper F, Rose-John S, Interleukin-6: Biology, signaling and strategies of blockade, Cytokine Growth Factor Rev 26(5) (2015) 475–87. [DOI] [PubMed] [Google Scholar]
- [59].Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S, The pro- and anti-inflammatory properties of the cytokine interleukin-6, Biochim Biophys Acta 1813(5) (2011) 878–88. [DOI] [PubMed] [Google Scholar]
- [60].Rose-John S, The Soluble Interleukin 6 Receptor: Advanced Therapeutic Options in Inflammation, Clinical pharmacology and therapeutics 102(4) (2017) 591–598. [DOI] [PubMed] [Google Scholar]
- [61].Mullberg J, Oberthur W, Lottspeich F, Mehl E, Dittrich E, Graeve L, Heinrich PC, Rose-John S, The soluble human IL-6 receptor. Mutational characterization of the proteolytic cleavage site, J Immunol 152(10) (1994) 4958–68. [PubMed] [Google Scholar]
- [62].Goth CK, Halim A, Khetarpal SA, Rader DJ, Clausen H, Schjoldager KT, A systematic study of modulation of ADAM-mediated ectodomain shedding by site-specific O-glycosylation, Proc Natl Acad Sci U S A 112(47) (2015) 14623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Riethmueller S, Somasundaram P, Ehlers JC, Hung CW, Flynn CM, Lokau J, Agthe M, Dusterhoft S, Zhu Y, Grotzinger J, Lorenzen I, Koudelka T, Yamamoto K, Pickhinke U, Wichert R, Becker-Pauly C, Radisch M, Albrecht A, Hessefort M, Stahnke D, Unverzagt C, Rose-John S, Tholey A, Garbers C, Proteolytic Origin of the Soluble Human IL-6R In Vivo and a Decisive Role of N-Glycosylation, PLoS biology 15(1) (2017) e2000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Deshmane SL, Kremlev S, Amini S, Sawaya BE, Monocyte chemoattractant protein-1 (mCp-1): an overview, J Interferon Cytokine Res 29(6) (2009) 313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Moser B, Loetscher P, Lymphocyte traffic control by chemokines, Nature immunology 2(2) (2001) 123–8. [DOI] [PubMed] [Google Scholar]
- [[66].] Zhang YJ, Rutledge BJ, Rollins BJ, Structure/activity analysis of human monocyte chemoattractant protein-1 (MCP-1) by mutagenesis. Identification of a mutated protein that inhibits MCP-1-mediated monocyte chemotaxis, J Biol Chem 269(22) (1994) 15918–24. [PubMed] [Google Scholar]
- [67].Bylander J, Li Q, Ramesh G, Zhang B, Reeves WB, Bond JS, Targeted disruption of the meprin metalloproteinase beta gene protects against renal ischemia-reperfusion injury in mice, Am J Physiol Renal Physiol 294(3) (2008) F480–90. [DOI] [PubMed] [Google Scholar]
- [68].Oneda B, Lods N, Lottaz D, Becker-Pauly C, Stocker W, Pippin J, Huguenin M, Ambort D, Marti HP, Sterchi EE, Metalloprotease meprin beta in rat kidney: glomerular localization and differential expression in glomerulonephritis, PLoS One 3(5) (2008) e2278.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Carmago S, Shah SV, Walker PD, Meprin, a brush-border enzyme, plays an important role in hypoxic/ischemic acute renal tubular injury in rats, Kidney Int 61(3) (2002) 959–66. [DOI] [PubMed] [Google Scholar]
- [70].Herzog C, Seth R, Shah SV, Kaushal GP, Role of meprin A in renal tubular epithelial cell injury, Kidney Int 71(10) (2007) 1009–18. [DOI] [PubMed] [Google Scholar]
- [71].Rabinovitch M, Guignabert C, Humbert M, Nicolls MR, Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension, Circulation research 115(1) (2014) 165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Maurer B, Reich N, Juengel A, Kriegsmann J, Gay RE, Schett G, Michel BA, Gay S, Distler JH, Distler O, Fra-2 transgenic mice as a novel model of pulmonary hypertension associated with systemic sclerosis, Annals of the rheumatic diseases 71(8) (2012) 1382–7. [DOI] [PubMed] [Google Scholar]
- [73].Banerjee S, Oneda B, Yap LM, Jewell DP, Matters GL, Fitzpatrick LR, Seibold F, Sterchi EE, Ahmad T, Lottaz D, Bond JS, MEP1A allele for meprin A metalloprotease is a susceptibility gene for inflammatory bowel disease, Mucosal Immunol 2(3) (2009) 220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Eldering JA, Grunberg J, Hahn D, Croes HJ, Fransen JA, Sterchi EE, Polarised expression of human intestinal N-benzoyl-L-tyrosyl-p-aminobenzoic acid hydrolase (human meprin) alpha and beta subunits in Madin-Darby canine kidney cells, Eur J Biochem 247(3) (1997) 920–32. [DOI] [PubMed] [Google Scholar]
- [75].Vazeille E, Bringer MA, Gardarin A, Chambon C, Becker-Pauly C, Pender SL, Jakob C, Muller S, Lottaz D, Darfeuille-Michaud A, Role of meprins to protect ileal mucosa of Crohn’s disease patients from colonization by adherent-invasive E. coli, PLoS One 6(6) (2011) e21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wichert R, Ermund A, Schmidt S, Schweinlin M, Ksiazek M, Arnold P, Knittler K, Wilkens F, Potempa B, Rabe B, Stirnberg M, Lucius R, Bartsch JW, Nikolaus S, Falk-Paulsen M, Rosenstiel P, Metzger M, Rose-John S, Potempa J, Hansson GC, Dempsey PJ, Becker-Pauly C, Mucus Detachment by Host Metalloprotease Meprin beta Requires Shedding of Its Inactive Pro-form, which Is Abrogated by the Pathogenic Protease RgpB, Cell reports 21(8) (2017) 2090–2103. [DOI] [PubMed] [Google Scholar]
- [77].Sun Q, Jin HJ, Bond JS, Disruption of the meprin alpha and beta genes in mice alters homeostasis of monocytes and natural killer cells, Exp Hematol 37(3) (2009) 346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bao J, Yura RE, Matters GL, Bradley SG, Shi P, Tian F, Bond JS, Meprin A impairs epithelial barrier function, enhances monocyte migration, and cleaves the tight junction protein occludin, Am J Physiol Renal Physiol 305(5) (2013) F714–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Muller WA, Localized signals that regulate transendothelial migration, Current opinion in immunology 38 (2016) 24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [[80].] Broder C, Becker-Pauly C, The metalloproteases meprin alpha and meprin beta: unique enzymes in inflammation, neurodegeneration, cancer and fibrosis, Biochem J 450(2) (2013) 253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Mayer U, Zimmermann K, Mann K, Reinhardt D, Timpl R, Nischt R, Binding properties and protease stability of recombinant human nidogen, Eur J Biochem 227(3) (1995) 681–6. [DOI] [PubMed] [Google Scholar]
- [82].Aumailley M, Battaglia C, Mayer U, Reinhardt D, Nischt R, Timpl R, Fox JW, Nidogen mediates the formation of ternary complexes of basement membrane components, Kidney Int 43(1) (1993) 7–12. [DOI] [PubMed] [Google Scholar]
- [83].Ries A, Gohring W, Fox JW, Timpl R, Sasaki T, Recombinant domains of mouse nidogen-1 and their binding to basement membrane proteins and monoclonal antibodies, Eur J Biochem 268(19) (2001) 5119–28. [DOI] [PubMed] [Google Scholar]
- [84].Matters GL, Manni A, Bond JS, Inhibitors of polyamine biosynthesis decrease the expression of the metalloproteases meprin alpha and MMP-7 in hormone-independent human breast cancer cells, Clin Exp Metastasis 22(4) (2005) 331–9. [DOI] [PubMed] [Google Scholar]
- [85].Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS, Bone morphogenetic protein-1: the type I procollagen C-proteinase, Science 271(5247) (1996) 360–2. [DOI] [PubMed] [Google Scholar]
- [86].Bella J, Hulmes DJ, Fibrillar Collagens, Sub-cellular biochemistry 82 (2017) 457–490. [DOI] [PubMed] [Google Scholar]
- [87].Biasin V, Marsh LM, Egemnazarov B, Wilhelm J, Ghanim B, Klepetko W, Wygrecka M, Olschewski H, Eferl R, Olschewski A, Kwapiszewska G, Meprin beta, a novel mediator of vascular remodelling underlying pulmonary hypertension, The Journal of pathology 233(1) (2014) 7–17. [DOI] [PubMed] [Google Scholar]
- [88].Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG, Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies, Molecular medicine 17(1–2) (2011) 113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]