Abstract
For patients who require post-operative radiotherapy after endoscopic resection of skull base tumors, proton therapy with pencil beam scanning (PBS) may allow sparing of normal tissue compared to intensity modulated photon radiation (IMRT). We compared PBS and IMRT radiation plans in the pre-operative and post-operative settings for two patients with advanced skull base tumors following endoscopic resection. The benefits of PBS over IMRT appear greater in the post-operative setting following endoscopic resection with improved sparing of critical organs at risk. The multidisciplinary approach of endoscopic resection followed by PBS represents a treatment paradigm with potential for improvements in toxicity reduction.
Keywords: Skull base, endoscopic surgery, proton therapy, head and neck cancer
INTRODUCTION:
In the appropriately selected patient, endoscopic resection of anterior skull base tumors offers the ability to perform resection of locally advanced disease in a less invasive manner than traditional open approaches. For patients who require post-operative radiation therapy after endoscopic resection, proton therapy may provide substantial sparing of surrounding normal tissue and superior dosimetry to photon techniques, though the combined approach of endoscopic surgery followed by proton therapy with pencil beam scanning (PBS) has not been formally assessed.
We performed radiation dosimetry comparisons between intensity modulated photon radiation therapy (IMRT) plans and PBS proton plans in the the pre-operative and post-operative setting following endoscopic resection using two illustrative cases.
CASE 1
An 80 year old man presented with locally advanced intestinal-type adenocarcinoma of the ethmoid sinus with extensive involvement of the anterior skull base and paranasal sinuses. After multidisciplinary assessment, he underwent endoscopic surgical resection. He was then treated with adjuvant proton radiotherapy with PBS to a dose of 6600 cGy to the tumor bed. Comparison IMRT and PBS plans were constructed with pre-operative (figure 1) and post-operative (figure 2) imaging and target volumes. For pre-operative plans, a dose of 7000 cGy was prescribed to the gross disease and 6000 cGy to the surrounding areas at risk.
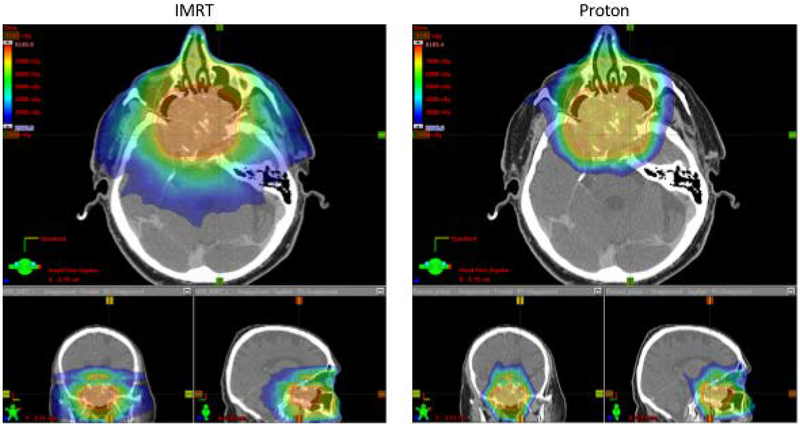
Figure 1.
Comparison of IMRT and proton pre-operative plans for case 1.
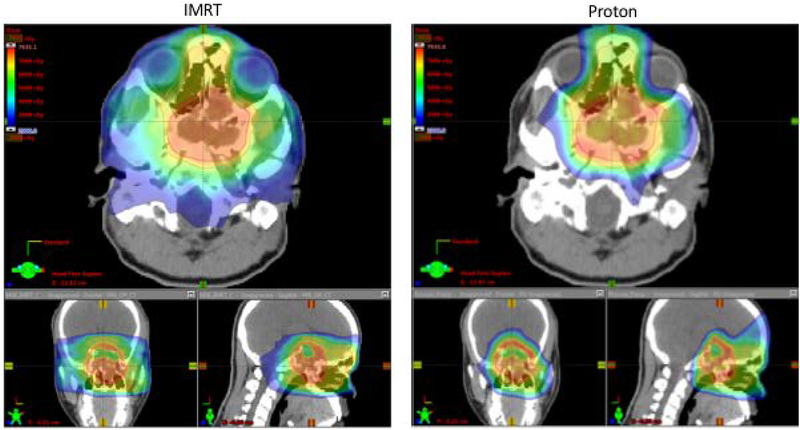
Figure 2.
Comparison of IMRT and proton post-operative plans for case 1 following endoscopic resection.
Dose-volume characteristics for the IMRT and PBS plans are shown in table 1. In the pre-operative setting, significant reductions in dose were achieved to the brain (mean dose 14.5 Gy IMRT, 8.0 PBS CGE), brainstem (max dose 60.0 Gy IMRT, 35.8 PBS CGE), temporal lobes (mean doses IMRT 28.6 Gy and 25.9 Gy, PBS 10.7 and 8.5 Gy), lacrimal glands (mean doses IMRT 41.4 Gy and 21.9 Gy, PBS 7.2 CGE and 4.7 CGE) and cochleae (mean doses IMRT 31.7 Gy and 29.3 Gy, PBS 2.7 CGE and 2.7 CGE). In the post-operative setting these dose reductions were maintained and significant dose reductions were also achieved for the optic structures (Chiasm, R nerve, L nerve dose to 0.03cc: IMRT 49.1 Gy, 51.3 Gy, 43.3 Gy; PBS 37.4 CGE, 38.0 CGE, 43.1 CGE) amounting to ~20% reduction in dose to the optic tracts overall. In the pre-operative and post-operative settings, target coverage was comparable with IMRT or PBS as assessed by V95 (volume reciving 95% of the prescription dose). As of 14 months following completion of post-operative radiation, the patient remains without evidence of recurrent disease.
Table 1.
Dose-volume statistics for case 1
| Pre-op | Post-op | |||
|---|---|---|---|---|
| IMRT | PROTON | IMRT | PROTON | |
| Organs at risk | ||||
| Brain mean dose (Gy) | 14.5 | 8 | 12.8 | 4.0 |
| Brainstem mean dose(Gy) | 9.6 | 9.6 | 24.9 | 4.1 |
| Brainstem max dose/D0.03cc(Gy) | 60.0/57.3 | 35.8/34.6 | 49.4/47.8 | 26.5/21.5 |
| Temporal lobe R max/D0.03cc(Gy) | 75.9/74.9 | 77.0/74.5 | 72.5/70.8 | 73.4/72.4 |
| Temporal lobe L max/D0.03cc(Gy) | 74.9/74.0 | 72.8/70.0 | 70.5/67.9 | 72.0/70.1 |
| Temporal lobe R mean(Gy) | 28.6 | 10.7 | 9.7 | 6.4 |
| Temporal lobe L mean(Gy) | 25.9 | 8.5 | 8.1 | 4.1 |
| Optic Chiasm max dose/D0.03cc(Gy) | 52.8/48.7 | 51.9/50.5 | 52.8/49.1 | 47.4/37.4 |
| Optic nerve R max/D0.03cc(Gy) | 53.8/48.7 | 52.4/46.2 | 54.3/51.3 | 46.4/38.0 |
| Optic nerve L max/D0.03cc(Gy) | 49.3/45.4 | 50.3/45.5 | 54.1/43.3 | 50.9/43.1 |
| Cochlea R max/D0.03cc/mean(Gy) | 38.4/35.0/31.7 | 9.6/5.0/2.7 | 36.9/31.7/30.5 | 6.9/3.6/3.3 |
| Cochlea L max/D0.03cc/mean(Gy) | 35.8/31.8/29.3 | 7.1/4.4/2.7 | 17.7/14.9/14.5 | 5.2/3.2/2.9 |
| Lacrimal gland R mean dose (Gy) | 31.4 | 7.2 | 13.1 | 1.8 |
| Lacrimal gland L mean dose (Gy) | 21.9 | 4.7 | 11.9 | 3.3 |
| Target coverage | ||||
| CTV60 V95 (%) | 97.90% | 97.70% | ||
| PTV60 V95 (%) | 97.1% | 96.5% | ||
| PTV70 V95 (%) | 93.4% | 92.3% | ||
| CTV66 V95 (%) | 98.1% | 98.0% | ||
| PTV 66 V95 (%) | 95.9% | 91.7% | ||
CASE 2
21 year old man with a locally advanced chondrosarcoma of the sphenoid sinus with extensive involvement of the skull base. He underwent endoscopic surgical resection. He was then treated with adjuvant proton radiotherapy with PBS to a dose of 7000 cGy to the high risk tumor bed and 6000 cGy to surrounding areas at risk. Comparison IMRT and PBS plans were constructed with pre-operative (figure 3) and post-operative imaging (figure 4) and target volumes. For pre-operative plans, a dose of 7000 cGy was prescribed to the gross disease and 6000 cGy to the surrounding areas at risk.
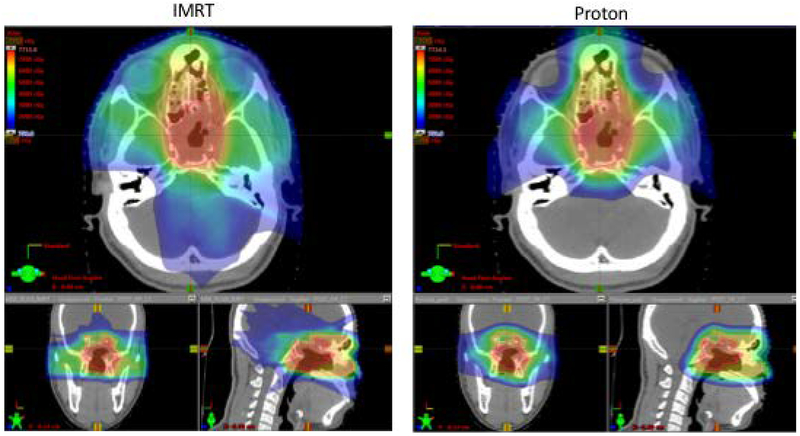
Figure 3.
Comparison of IMRT and proton pre-operative plans for case 2.
Figure 4.
Comparison of IMRT and proton post-operative plans following endoscopic resection for case 2.
Dose-volume characteristics for the IMRT and PBS plans are shown in table 2. For the pre-operative plans, lower dose was observed to the brain, brainstem and temporal lobes with PBS (mean doses: IMRT 17.1 Gy, 36.1 Gy, 34 Gy, 37.6 Gy; PBS 12.1 CGE, 13.3 CGE, 17.5 CGE, 25.6 CGE). Lower doses were delieverd to the cochleae with PBS (mean doses IMRT 34.9 Gy, 42.7 Gy; PBS 14.5 CGE, 16.1 CGE) as well as the lacrimal glands (IMRT 38.9 Gy and 38.3 Gy, PBS 8.9 CGE and 9.3 CGE). In the post-operative plans, lower mean doses to the brain, brainstem and cochleae were maintained. In this case, comparable dose was delivered to the optic chiasm and right optic nerve but dose reduction to the left optic nerve was achieved (D0.03cc IMRT 73.1 Gy, PBS 65.5 Gy) as the chaism was encompassed by tumor. As of 27 months following completion of post-operative radiation, the patient remains without evidence of recurrent disease.
Table 2.
Dose-volume statistics for case 2
| Pre-op | Post-op | |||
|---|---|---|---|---|
| IMRT | PROTON | IMRT | PROTON | |
| Organs at risk | ||||
| Brain mean dose(Gy) | 17.1 | 12.1 | 11.1 | 7.5 |
| Brainstem mean dose (Gy) | 36.1 | 13.3 | 28.4 | 17 |
| Brainstem max dose/D0.03cc(Gy) | 65.7/62.6 | 52.7/48.8 | 56.7/52.1 | 68.5/65.4 |
| Temporal lobe R max/D0.03cc(Gy) | 76.5/75.6 | 73.8/73.1 | 69.5/66.6 | 71.1/66.9 |
| Temporal lobe L max/D0.03cc(Gy) | 77.3/76.5 | 78.0/77.0 | 72.7/71.4 | 73.2/72.2 |
| Temporal lobe R mean(Gy) | 34 | 17.5 | 8.3 | 11.6 |
| Temporal lobe L mean(Gy) | 37.6 | 25.6 | 23.9 | 19.5 |
| Optic Chiasm max dose/D0.03cc(Gy) | 54.8/50.1 | 57.7/52.1 | 53.4/51.0 | 52.8/50.9 |
| Optic nerve R max/D0.03cc(Gy) | 54.6/51.9 | 55.3/52.1 | 54.7/53.0 | 54.8/53.1 |
| Optic nerve L max/D0.03cc(Gy) | 54.9/50.8 | 56.1/53.0 | 74.7/73.1 | 66.3/65.5 |
| Cochlea R max/D0.03cc/mean(Gy) | 42.9/37.6/34.9 | 21.6/18.1/14.5 | 7.4/6.0/6.2 | 5.8/4.7/4.9 |
| Cochlea L max/D0.03cc/mean(Gy) | 48.8/46.6/42.7 | 25.2/20.5/16.1 | 23.8/22.3/22.6 | 5.7/3.8/4.3 |
| Lacrimal gland R mean dose (Gy) | 38.9 | 8.9 | 25.7 | 8.1 |
| Lacrimal gland L mean dose (Gy) | 38.3 | 19.3 | 23.9 | 15.1 |
| Target coverage | ||||
| CTV60 V95 (%) | 96.20% | 95.90% | 98.2% | 98.3% |
| PTV60 V95 (%) | 96.20% | 94.5% | 97.5% | 97.4% |
| CTV70 V95 (%) | 96.5% | 95.9% | ||
| PTV70 V95 (%) | 86.4% | 83.9% | 94.6% | 94.5% |
In both cases, doses to brain, brainstem, temporal lobes, optic structures and chiasm were significantly reduced following endoscopic resection compared to in the pre-operative setting.
Each proton case was planned using five gantry fields, with unique orientations chosen to maximize PTV coverage and organ-at-risk sparing. Spot weights were optimized using Multi-Field Optimization Intensity Modulated Proton Therapy (MFO-IMPT). Beams treating to shallow depth included a 7cm range shifter placed 2 cm away from the patient surface. For the 100 MeV to 180 MeV energy layers used, spot size in air at isocenter σ ranged from 7 mm to 4 mm. Photon IMRT plans were generated using the same structure sets and prescription doses as the proton plans. IMRT plans utilized 9 coplanar beams from 120 CCW to 240 degrees at 30 degree intervals, plus two anterior oblique beams, all used 6MV photons.
DISCUSSION
Advanced tumors of the anterior skull base present a challenging clinical scenario given the proximity and involvement of multiple critical structures including cranial nerves, optic tracts, brainstem and temporal lobes. Endoscopic skull base surgery has emerged as an effective approach for resection of selected tumors while having the potential for low treatment related morbidity. Compared to traditional craniofacial resection, endoscopic surgery has been demonstrated to result in lower rates of post-operative complication, shorter mean duration of hospitalization[1] and less cosmetic deformity[2]. Although there is clearly selection bias, endoscopic surgery may be associated with improved locoregional control, disease-specific and overall survival in the management of sinonasal malignancies more broadly [3].
Following resection of skull base tumors, adjuvant radiotherapy is often indicated to minimize risk of locoregional recurrence. Proton radiotherapy carries significant advantages over conventional photon-based therapy due to the physical properties of the proton beam. Specifically, due to the deposition of dose at a narrow specified depth known as the Bragg peak, no radiation dose is delivered beyond the target, whereas photon IMRT is inherently limited by delivery of “exit dose” beyond the tumor volume. Head and neck cancers, and skull base tumors in particular, are often intimately assocatied with sensitive structures and organs such as the optic tracts, the brainstem, temporal lobes and cranial nerves. As a result, the benefits of proton therapy are magnified in the context of skull base tumors due to the ability to spare adjacent critical structures[4]. A systematic review and meta-analysis has demonstrated improved overall survival and disease-free survival with the use of particle therapy for sinonasal tumors compared to photon radiation[5]. Spot-scanning proton therapy has demonstrated particularly impressive rates of local control for typically radioresistant histologies such as chordoma and chondrosarcoma[6–8].
The two cases presented in this study demonstrate the situational advanatages of the combined multidisciplinary approach of endoscopic surgery followed by proton radiotherapy. In the first case, a patient with locally advanced intestinal-type adenocarcinoma of the ethmoid sinus with extensive involvement of the anterior skull base underwent resection with an endoscopic approach followed by adjuvant proton therapy. The lesion is abutting the brainstem and optic chiasm such that a non-operative radiation based approach with proton therapy (figure 1) results in only moderate sparing of the optics, brainstem and temporal lobes with proton therapy compared to IMRT. Following endoscopic resection, however, the radiation dose can be reduced (70 Gy to 60 Gy) and, furthermore, lower maximum doses to the brainstem, optic nerves, optic chiasm are achieved with protons compared to than in the pre-operative setting (table 1, figure 2). In this case, because the tumor is only marginally associated with the chiasm and optic nerves, proton therapy allows for significant sparing in the post-operative setting. PBS also delivers markedly lower doses to the temporal lobes which would be expected to result in lower risk of necrosis. These benefits are achieved while maintaining excellent coverage of the target volume.
The second case describes a patient with a locally advanced chondrosarcoma of the sphenoid sinus with extensive involvement of the skull base who underwent endoscopic resection followed by adjuvant proton therapy. In this case, the optic chiasm is encompassed within the target volume so sparing cannot be technically achieved with protons either in the pre-operative or post-operative settings. However, the conformality of PBS allows for carving of dose around the optics in a manner similar to what can be achieved with IMRT (figure 4). Meanwhile, as is also true in the first case, significantly less dose in the low-intermediate range is deposited to surrounding normal tissue, mostly brain. This is particulary important in a young patient such as this to minimize the risk of secondary malignancy by reducing the volume of irradiated healthy tissue.
CONCLUSION
A multidisciplinary approach involving endoscopic resection followed by post-operative proton beam therapy with PBS for anterior skull base tumors represents a treatment paradigm with potential for improvements in neurological sparing and toxicity reduction. Proton therapy with PBS offers particular benefit in terms of dose reduction to organs at risk outside of the planning target volume. Formal comparative studies and clinical trials evaluating this combined approach are needed.
Footnotes
Financial disclosures: None
Conflicts of interest: None
Prior presentation: Multidisciplinary Head and Neck Cancer Symposium, Scottsdale, AZ, February 15–17, 2018
REFERENCES
- 1.Kim BJ, Kim DW, Kim SW, et al. Endoscopic versus traditional craniofacial resection for patients with sinonasal tumors involving the anterior skull base. Clin Exp Otorhinolaryngol 2008;1(3):148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MA, Liang J, Cohen IJ, et al. Endoscopic resection of advanced anterior skull base lesions: oncologically safe? ORL J Otorhinolaryngol Relat Spec 2009;71(3):123–8. [DOI] [PubMed] [Google Scholar]
- 3.Higgins TS, Thorp B, Rawlings BA, et al. Outcome results of endoscopic vs craniofacial resection of sinonasal malignancies: a systematic review and pooled-data analysis. Int Forum Allergy Rhinol 2011;1(4):255–61. [DOI] [PubMed] [Google Scholar]
- 4.Leeman JE, Romesser PB, Zhou Y, et al. Proton therapy for head and neck cancer: expanding the therapeutic window. Lancet Oncol 2017;18(5):e254–e265. [DOI] [PubMed] [Google Scholar]
- 5.Patel SH, Wang Z, Wong WW, et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol 2014;15(9):1027–38. [DOI] [PubMed] [Google Scholar]
- 6.Ares C, Hug EB, Lomax AJ, et al. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys 2009;75(4):1111–8. [DOI] [PubMed] [Google Scholar]
- 7.Rombi B, Ares C, Hug EB, et al. Spot-scanning proton radiation therapy for pediatric chordoma and chondrosarcoma: clinical outcome of 26 patients treated at paul scherrer institute. Int J Radiat Oncol Biol Phys 2013;86(3):578–84. [DOI] [PubMed] [Google Scholar]
- 8.Rutz HP, Weber DC, Goitein G, et al. Postoperative spot-scanning proton radiation therapy for chordoma and chondrosarcoma in children and adolescents: initial experience at paul scherrer institute. Int J Radiat Oncol Biol Phys 2008;71(1):220–5. [DOI] [PubMed] [Google Scholar]