Abstract
Sex differences in mu-opioid receptor agonist-induced antinociception have been reported in nonhuman primates. The degree to which mu-opioid receptor agonist sex differences in nonhuman primates extend to other behavioral endpoints remains unknown. The present study compared the behavioral effects of three mu-opioid receptor ligands (fentanyl, buprenorphine and naltrexone) that varied in efficacy to stimulate [35S]-GTPγS binding (from highest to lowest: fentanyl, buprenorphine and naltrexone) in male and female rhesus monkeys. Male (n=3) and female (n=3) monkeys were trained to respond under a fixed-ratio 10 schedule of food presentation during daily sessions consisting of multiple components. Once rates of responding were stable, cumulative dose-effect functions were determined for intramuscular fentanyl (0.00032–0.032 mg/kg), buprenorphine (0.001–1 mg/kg) and naltrexone (0.01–0.1 mg/kg). Fentanyl dose-dependently decreased rates of responding in both sexes and the corresponding ED50 values were not significantly different. Buprenorphine dose-dependently decreased rates of responding in females, but not males. Naltrexone did not significantly alter behavior in either females or males. Overall, these results suggest the expression of sex differences in MOR pharmacology depends upon both the efficacy of the MOR ligand and the behavioral endpoint.
Keywords: fentanyl, buprenorphine, naltrexone, rhesus monkey, sex differences, mu-opioid receptors
Introduction:
Females have been disproportionately under-represented in preclinical biomedical research. In 2015, the National Institutes of Health mandated preclinical biomedical researchers to consider sex as a biological variable in both experimental design and analysis (National Institutes of Health, 2015; Miller et al, 2016). Nonhuman primates may have utility in investigating sex-dependent pharmacological effects because of a similar reproductive system (Hotchkiss et al, 1971; Shively and Clarkson, 2009) and G-protein coupled receptor densities and neuroanatomical locations to humans (for review, see Weerts et al, 2007; Banks et al, 2017). For example, low efficacy mu-opioid receptor (MOR) agonists produced greater antinociception in male monkeys compared to females (Negus and Mello, 1999). However, the degree to which sex differences in basic opioid behavioral pharmacology extend to other endpoints is unknown.
Schedule-controlled responding is an operant procedure that has demonstrated utility in elucidating the basic pharmacology of MOR ligands (Negus et al, 1993; Withey et al, 2018). For example, the high-efficacy MOR agonist fentanyl suppresses rates of responding in male nonhuman primates ( Stevenson et al, 2003; Banks et al, 2010) whereas the low-efficacy MOR agonist buprenorphine and the MOR antagonist naltrexone do not significantly alter rates of responding (Goldberg et al, 1981; Negus et al, 2002a). MOR ligand effects on schedule-controlled responding in female monkeys have not been systematically evaluated. Therefore, the present study aim was to evaluate the expression of sex differences in an assay of schedule-controlled responding for three MOR ligands fentanyl, buprenorphine, and naltrexone that vary in efficacy to stimulate [35S]-GTPγS binding from highest to lowest (Selley et al, 1997; Yuan et al, 2013).
Methods:
Subjects:
Studies were conducted in 3 adult male and 3 adult female rhesus monkeys (Macaca mulatta). 5 (2 males and 3 females) were of Indian origin and one male was of Chinese origin. All monkeys had previous experimental and drug histories including MOR ligands. Monkeys were maintained on food biscuits (Teklad Global 20% Protein Primate Diet, Envigo, Madison WI) provided after the behavioral procedure. Water was continuously available in the housing chamber which also served as the experimental chamber. A 12 h light-dark cycle was in effect (lights on from 06.00 to 18.00 h). During the behavioral procedure, monkeys could earn up to 60 1-g banana-flavored sucrose pellets (5TUQ, Test Diets, Richmond, IL). Environmental enrichment was provided after behavioral sessions and consisted of dry or fresh fruit, foraging boards, destructible bags, and videos. Monkeys were afforded opportunities to interact socially using olfactory and auditory cues and mirrors provided opportunities for visual interactions. The vivarium was licensed by the United States Department of Agriculture and accredited by AAALAC international. Both experimental and enrichment protocols were approved by the Institutional Animal Care and Use Committee.
Apparatus:
Monkeys were individually housed in well-ventilated, stainless steel chambers. Each chamber was equipped with a custom-made, stainless steel screen enclosure (Lafayette Instrument, Lafayette, USA) that was mounted on the chamber front to provide access to a 15″ touch-sensitive screen (Model 1537L; Elo TouchSystems, Menlo Park, CA). A pellet dispenser (ENV-203–1000; Med Associates, St Albans, VT) was mounted on a shelf above the chamber. All experimental events and data were collected using custom programming in ABET II Touch software (Lafayette Instrument,) in tandem with Whisker server (Cambridge University, UK).
Behavioral Procedure:
Experimental sessions were conducted 5–7 days per week and consisted of multiple components. Each component consisted of a 15-min time-out period and 5-min response period. During the response period, two square stimuli were presented horizontally with a green square on the left side and a red square on the right side. Ten consecutive touches (i.e., fixed-ratio 10 schedule of reinforcement) on the green square resulted in food pellet delivery and a 1-s presentation of a white frame around the touch screen border. Touches on the right red square had no programmed consequences. The response period terminated when the subject completed the maximum number of ratio requirements (10) or five min had elapsed. Training sessions consisted of 1–6 components. Training criteria were met when rates of responding were stable following intramuscular (IM) saline administration for 6 sequential components. Stability was defined as no significant effect of saline on rates as determined using a repeated measures one-way ANOVA.
Once monkeys reached training criteria as described above, test sessions were introduced in addition to training sessions, and test sessions were typically conducted on Tuesdays and Fridays. On test days, a test drug dose was administered IM 15 min before each response period, and each dose increased the total cumulative dose by one-fourth or one-half log units in 20-min intervals. Dose-effect functions were determined for fentanyl (males: 0.001–0.032 mg/kg; females: 0.00032–0.01 mg/kg), buprenorphine (males: 0.032–1 mg/kg; females: 0.001–0.01 mg/kg), and naltrexone (males: 0.0032–0.1 mg/kg; females: 0.0032–0.1 mg/kg). Each dose effect curve was determined once in each monkey, except for buprenorphine which was determined twice in two out of three female monkeys. Buprenorphine effects in these two females were averaged for both graphical and statistical analysis purposes.
Drugs:
Fentanyl HCl and (−)-naltrexone HCl were supplied by the National Institute on Drug Abuse Supply Program (Bethesda, MD). (±)-Buprenorphine HCl was purchased from Spectrum Chemicals (Gardena, CA). Fentanyl, naltrexone, and buprenorphine were dissolved in sterile water. All drugs were administered intramuscularly, and all doses were expressed as the salt forms listed above.
Data Analysis:
Raw response rates for each component were calculated by dividing the total number of responses by the component run time. Raw rates of responding after test drug administration were converted to a percent saline rate using the average response rate from all six components from the saline test session in that monkey. The effective dose (ED50) that produced a 50% reduction in percent saline rates of responding was determined by interpolation when only 2 data points were available (one below and one >50% effect) or by linear regression when at least 3 data points on the linear portion of the dose-effect function were available. Individual ED50 values were subsequently averaged to yield mean ED50 values and 95% confidence intervals. ED50 values were considered significantly different if the 95% confidence limits were nonoverlapping. An area under the dose-response was also calculated (GraphPad Prism, La Jolla, CA) and total area was considered significantly different if the 95% confidence limits were nonoverlapping.
Results:
Raw mean ± SEM rates of responding on saline training days were not significantly different between males (1.6 ± 0.3 responses/s) and females (1.2 ± 0.2 responses/s). Figure 1 shows fentanyl, buprenorphine, and naltrexone effects on rates of responding in male and female monkeys. Fentanyl produced dose-dependent decreases in rates of responding in both males and females. Fentanyl ED50 and total area values were not significantly different between sexes (Table 1). In contrast to fentanyl, buprenorphine produced dose-dependent decreases in responding only in females and the corresponding ED50 and total area values are reported in Table 1. Buprenorphine decreased rates of responding to a maximum of 65.9 ± 8.4 percent saline rate in males and larger buprenorphine doses were not evaluated due to solubility issues. Naltrexone did not significantly alter rates of responding in either males or females up to the largest dose tested.
Figure 1:
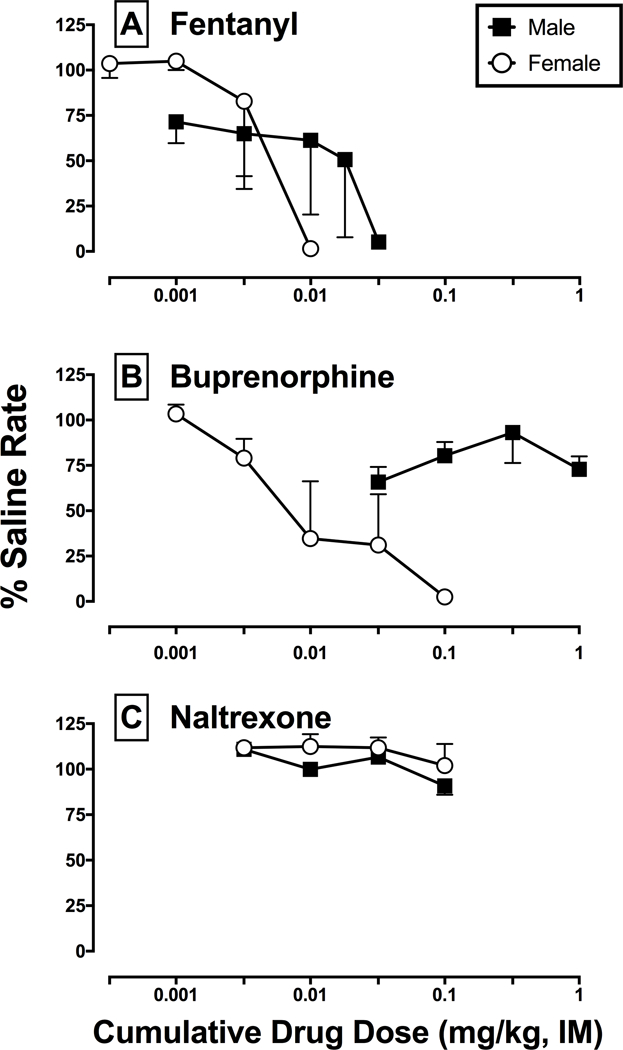
Effects of MOR ligands to decrease rates of responding in male (n=3) and female (n=3) monkeys. Horizontal axis: cumulative intramuscular drug dose in mg/kg. Vertical axis: percent saline rate. Each point represents the mean ± SEM of three male and three female monkeys.
Table 1.
Group mean ED50 values (95% confidence limits; CL) and total area (95% CL) for three MOR ligands to decrease rates of responding in male (n=3) and female (n=3) monkeys.
| Drug | Male | Female | ||
|---|---|---|---|---|
| ED50 in mg/kg (95% CL) | Total area (95% CL) |
ED50 in mg/kg (95% CL) | Total area (95% CL) | |
| Fentanyl | 0.007 (0.001, 0.04) |
1.42 (0, 2.85) |
0.004 (0.002, 0.009) |
0.564 (0.06, 1.07) |
| Buprenorphine | NC | 80.48 (58.31, 102.7) |
0.010 (0.002, 0.05) |
2.453 (0, 6.08) |
| Naltrexone | NC | 9.701 (9.04, 10.36) |
NC | 10.49 (8.94, 12.05) |
NC: Not calculable because no drug dose decreased rates of responding > 50% in any monkey
Discussion:
The present study compared the potency and effectiveness of three MOR ligands that varied in efficacy to stimulate GTPγS activity to decrease rates of responding in male and female rhesus monkeys. The main finding was the expression of sex differences in MOR ligand effectiveness to decrease rates of responding depended upon MOR ligand efficacy. Specifically, buprenorphine selectively decreased rates of responding in females, but not males. These results provide evidence that sex differences in opioid pharmacology depend upon not only the efficacy of the MOR agonist, but also the dependent measure.
The present results with the high-efficacy MOR agonist fentanyl and the MOR antagonist naltrexone in an assay of schedule-controlled responding in male monkeys were consistent with previous monkey studies (Negus et al, 1993; Banks et al, 2010; Paronis and Bergman, 2011). The present study extended these previous findings to female monkeys and suggest no sex difference with regard to potency or effectiveness of fentanyl and naltrexone to alter rates of responding. The present results were also consistent with previous fentanyl antinociception results demonstrating no sex differences in rhesus monkeys (Negus et al, 1999, 2002b). Overall, the present results and the existing literature do not suggest sex differences in the basic pharmacology of either high efficacy MOR ligands or MOR antagonists.
In contrast to fentanyl effects, there was a robust sex difference in the effectiveness of buprenorphine to disrupt schedule-controlled responding. Buprenorphine produced dose-dependent decreases in responding in female monkeys similar to effects observed with the high efficacy MOR agonist fentanyl. In male monkeys, buprenorphine failed to alter food-maintained responding similar to effects observed with the MOR antagonist naltrexone. Although sex-dependent effects of buprenorphine have not been previously reported, buprenorphine effects on schedule-controlled responding in monkeys have yielded mixed results. In two previous studies, buprenorphine failed to significantly alter rates of responding up to a cumulative dose of 3.2 mg/kg in two different cohorts, one cohort of 1 male and 3 female monkeys (Negus et al, 2002a) and another cohort of 7 male and female monkeys from which 3 monkeys participated in the acute buprenorphine studies (Paronis et al, 2011). These results would potentially suggest a lack of sex difference in buprenorphine effects on schedule-controlled responding. In another cohort of male rhesus monkeys in our laboratory, buprenorphine also did not significantly disrupt schedule-controlled responding up to a cumulative dose of 3.2 mg/kg (unpublished observations). In contrast, buprenorphine produced dose-dependent decreases in rates of food-maintained responding in male rhesus (Mello et al, 1985) and male squirrel (Withey et al, 2018) monkeys at doses of 0.1 mg/kg. Sex differences in buprenorphine-induced disruption of schedule-controlled responding observed in the present study may help clarify one potential reason for these previously reported mixed buprenorphine effects.
There are at least two possible explanations for these apparent differential buprenorphine results in female rhesus monkeys. First, differential buprenorphine effects between previous studies (Mello et al, 1985; Negus et al, 2002a; Paronis et al, 2011) and the present results could be related to differences in the schedule of reinforcement. The present study used a FR10 schedule of reinforcement, whereas Negus (2002) and Paronis (2011) used a FR30. A second-order FR4 (variable-ratio 16:S) schedule of reinforcement was used in the study of Mello (1985). These different operant schedules result in different baseline rates of responding that might alter the sensitivity of buprenorphine to produce rate suppression consistent with the principle of rate dependency (Kelleher and Morse, 1968). For example, buprenorphine doses of 0.1 and 0.3 mg/kg were sufficient to significantly decrease rates of behavior in male monkeys in the Mello (1985) study whereas a cumulative buprenorphine dose of 1 mg/kg failed to significantly alter rates of responding in male monkeys (present study). Although rate dependency might explain differences in buprenorphine effects in male monkeys, baseline rates of responding in the present study were not significantly different between male and female monkeys.
Another potential explanation for the apparent differential results between the present study and previous studies could be related to differences in experimental or drug history. The experimental history of monkeys in the study of Paronis and Bergman (2011) were not reported. Female monkeys in studied by Negus (2002a) had a cocaine self-administration history. Chronic cocaine self-administration disrupts the menstrual cycle in female monkeys and previous studies have shown female gonadal hormones to play a role in the potency of the low-efficacy agonist nalbuphine to produce antinociception (Negus et al, 1999; Mello et al, 2004). In male rhesus (Mello et al, 1984) and squirrel (Withey et al, 2018) monkeys where acute buprenorphine decreased rates of food-maintained responding, experimental/drug history appears to be an important factor. For example, the male rhesus monkeys in the study of Mello (1985) were drug naïve and the male squirrel monkeys studied by Withey (2018) were drug free for 3 months prior to experimentation. Although experimental history may explain differences in buprenorphine effects in male monkeys, experimental history alone does not explain the observed sex differences in buprenorphine effectiveness to alter rates of responding in the present study. Female monkeys in the present study had a 15-month opioid exposure history similar to the male monkeys. Other experimental factors such as where the experimental session was conducted (i.e. housing or experimental chamber) or type of food reinforcer also do not adequately explain differences in buprenorphine effects between the present study and previous studies. Overall, the present findings and the existing literature support the expression of sex differences in MOR ligand basic pharmacology. These sex differences in nonhuman primates appear to be most evident for low to moderate efficacy MOR ligands.
Acknowledgements:
This research was supported by grants from the National Institutes of Health and the National Institute on Drug Abuse [F31 DA043921 (KLS) and R01DA037287 (MLB)].
Footnotes
Conflicts of Interest: There are no conflicts of interest.
References:
- Banks ML, Folk JE, Rice KC, Negus SS (2010). Selective enhancement of fentanyl-induced antinociception by the delta agonist SNC162 but not by ketamine in rhesus monkeys: further evidence supportive of delta agonists as candidate adjuncts to mu opioid analgesics. Pharmacol Biochem Behav 97: 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Czoty PW, Negus SS (2017). Utility of Nonhuman Primates in Substance Use Disorders Research. ILAR Jl 58(2): 202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S, Morse W, Goldberg D (1981). Acute and chronic effects of naltrexone and naloxone on schedule-controlled behavior of squirrel monkeys and pigeons. J Pharmacol Exp Ther 216(3): 500–509. [PubMed] [Google Scholar]
- Hotchkiss J, Atkinson L, Knobil E (1971). Time course of serum estrogen and luteinizing hormone (LH) concentrations during the menstrual cycle of the rhesus monkey. Endocrinology 89(1): 177–183. [DOI] [PubMed] [Google Scholar]
- Kelleher R, Morse W (1968). Determinants of the specificity of behavioral effects of drugs Reviews of Physiology Biochemistry and Experimental Pharmacology , Volume 60 Springer, pp 1–56. [DOI] [PubMed] [Google Scholar]
- Mello NK, Bree MP, Lukas SE, Mendelson JH (1985). Buprenorphine effects on food-maintained responding in macaque monkeys. Pharmacol Biochem Behav 23(6): 1037–1044. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Negus SS, Kelly M (2004). Ovarian steroid hormone modulation of the acute effects of cocaine on luteinizing hormone and prolactin levels in ovariectomized rhesus monkeys. J Pharmacol Exp Ther 308(1): 156–167. [DOI] [PubMed] [Google Scholar]
- Miller LR, Marks C, Becker JB, Hurn PD, Chen W-J, Woodruff T, et al. (2016). Considering sex as a biological variable in preclinical research. FASEB J 31(1): 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health (2015). Consideration of sex as a biological variable in NIH-funded research. National Institutes of Health. [Google Scholar]
- Negus SS, Mello NK (1999). Opioid antinociception in ovariectomized monkeys: comparison with antinociception in males and effects of estradiol replacement. J Pharmacol Exp Ther 290(3): 1132–1140. [PubMed] [Google Scholar]
- Negus SS, Burke TF, Medzihradsky F, Woods J (1993). Effects of opioid agonists selective for mu, kappa and delta opioid receptors on schedule-controlled responding in rhesus monkeys: antagonism by quadazocine. J Pharmacol Exp Ther 267(2): 896–903. [PubMed] [Google Scholar]
- Negus S, Bidlack J, Mello N, Furness M, Rice K, Brandt M (2002a). Delta opioid antagonist effects of buprenorphine in rhesus monkeys. Behav Pharmacol 13(7): 557–570. [DOI] [PubMed] [Google Scholar]
- Negus SS, Zuzga DS, Mello NK (2002b). Sex differences in opioid antinociception in rhesus monkeys: antagonism of fentanyl and U50, 488 by quadazocine. J Pain 3(3): 218–226. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Bergman J (2011). Buprenorphine and opioid antagonism, tolerance, and naltrexone-precipitated withdrawal. J Pharmacol Exp Ther 336(2): 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Sim LJ, Xiao R, Liu Q, Childers SR (1997). μ-Opioid receptor-stimulated guanosine-5′-O-(γ-thio)-triphosphate binding in rat thalamus and cultured cell lines: signal transduction mechanisms underlying agonist efficacy. Mol Pharmacol 51(1): 87–96. [DOI] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB (2009). The unique value of primate models in translational research. Am J Primatol 71(9): 715–721. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Folk JE, Linsenmayer DC, Rice KC, Negus SS (2003). Opioid interactions in rhesus monkeys: effects of δ+ μ and δ+ κ agonists on schedule-controlled responding and thermal nociception. J Pharmacol Exp Ther 307(3): 1054–1064. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK (2007). The Value of Nonhuman Primates in Drug Abuse Research. Exp Clin Psychopharmacol 15(4): 309–327. [DOI] [PubMed] [Google Scholar]
- Withey SL, Paronis CA, Bergman J (2018). Concurrent Assessment of the Antinociceptive and Behaviorally Disruptive Effects of Opioids in Squirrel Monkeys. J Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Zaidi SA, Elbegdorj O, Aschenbach LC, Li G, Stevens DL, et al. (2013). Design, synthesis, and biological evaluation of 14-heteroaromatic-substituted naltrexone derivatives: pharmacological profile switch from mu opioid receptor selectivity to mu/kappa opioid receptor dual selectivity. J Med Chem 6(22): 9156–9169. [DOI] [PMC free article] [PubMed] [Google Scholar]


