Abstract
Following noise overexposure and tinnitus-induction, fusiform cells of the dorsal cochlear nucleus (DCN) show increased spontaneous firing rates (SFR), increased spontaneous synchrony and altered stimulus-timing dependent plasticity (StDP), which correlate with behavioral measures of tinnitus. Sodium salicylate, the active ingredient in aspirin, which is commonly used to induce tinnitus, increases SFR and activates NMDA receptors in the ascending auditory pathway. NMDA receptor activation is required for StDP in many brain regions, including the DCN. Blocking NMDA receptors can alter StDP timing rules and decrease synchrony in DCN fusiform cells. Thus, systemic activation of NMDA receptors with sodium salicylate should elicit pathological changes to StDP, thereby increasing SFR and synchrony and induce tinnitus. Herein, we examined the action of salicylate in tinnitus generation in guinea pigs in vivo by measuring tinnitus using two behavioral measures and recording single unit responses from DCN fusiform cells pre- and post-salicylate administration in the same animals. First, we show that animals administered salicylate show evidence of tinnitus using both behavioral paradigms, cross-validating the tests. Second, fusiform cells in animals with tinnitus showed increased SFR, synchrony and altered StDP timing rules, like animals with noise-induced tinnitus. These findings suggest that alterations to fusiform-cell plasticity is an essential component of tinnitus, regardless of induction technique.
Keywords: tinnitus, stimulus-timing-dependent plasticity, dorsal cochlear nucleus, fusiform cell, salicylate, spontaneous firing rate
Introduction
The mammalian dorsal cochlear nucleus (DCN) is a layered, cerebellar-like structure that receives input from both the cochlea and other sensory systems (Oertel and Young, 2004; Zhou and Shore, 2004). Fusiform cells, the principle output neurons of the DCN, receive input from the cochlea via auditory nerve fiber (ANF) synapses on their basal dendrites (Pfeiffer, 1966). In addition, fusiform cells receive somatosensory input via granule-cell axons, parallel-fibers (Mugnaini et al., 1980), which synapse on their apical dendrites (Ryugo et al., 2003; Haenggeli et al., 2005). This dendritic bipolarity allows fusiform cells to integrate somatosensory and auditory information for the processing of sound location and suppression of self-generated signals (Sutherland et al., 1998b; Sutherland et al., 1998a; May, 2000; Singla et al., 2017).
In vitro, fusiform cells exhibit spike-timing dependent plasticity (STDP) (Tzounopoulos et al., 2004): when EPSPs in parallel-fiber synapses are followed by post-synaptic spikes in fusiform cells then long-term potentiation (LTP) occurs, while stimulating the basal dendrites first results in long-term depression (LTD). In vivo fusiform cells exhibit stimulus-timing-dependent plasticity (StDP) (Koehler and Shore, 2013a; Wu et al., 2015), the macroscopic equivalent of STDP in which LTP or LTD occurs depending on the order of auditory and somatosensory stimulation (Koehler and Shore, 2013a). In vivo, the apical dendrites of fusiform cells are activated through deep brain stimulation of somatosensory nuclei (Dehmel et al., 2012b; Koehler and Shore, 2013a), or transdermal activation of the face overlying the trigeminal ganglion or the neck overlying the C2 ganglion (Wu et al., 2015; Marks et al., 2018), while the basal dendritic synapses are activated with sound (Liberman, 1993). Thus, the combination of sound and somatosensory stimulation elicits StDP in fusiform cells (Koehler and Shore, 2013a; Wu et al., 2015).
Altered StDP has been demonstrated in animals with tinnitus, which show StDP timing-rule inversions, in which bimodal auditory-somatosensory stimuli that normally result in LTP now result in LTD, and those that would normally result in LTD, now result in LTP (Koehler and Shore, 2013b). StDP timing rules from animals with tinnitus also show enhancement, i.e. the timing rules show more bimodal intervals eliciting LTP than LTD compared to exposed animals without evidence of tinnitus or non-exposed control animals with balanced LTP and LTD (Koehler and Shore, 2013b; Marks et al., 2018). Enhanced LTP biases the fusiform-cell firing rates toward excitation, contributing to increased SFR, increased bursting and increased pairwise synchrony, the physiological hallmarks of tinnitus (Wu et al., 2016; Marks et al., 2018).
While noise-induced tinnitus is the most common form of tinnitus in humans (Shore et al., 2016), tinnitus can also be temporarily induced in humans through the acute administrations of high doses of aspirin (Sheppard et al., 2014). In many different species and behavioral models, administration of the active ingredient in aspirin, sodium salicylate, leads to tinnitus (Jastreboff et al., 1988; Bauer et al., 1999; Guitton et al., 2003; Ruttiger et al., 2003; Yang et al., 2007; Turner and Parrish, 2008). However, the mechanisms through which salicylate induced tinnitus occurs are not well understood and appear to be multifactorial. In the present study, we hypothesized that guinea pigs with salicylate-induced tinnitus would show inversions of fusiform-cell StDP timing rules as well as increases in fusiform-cell SFR and synchrony like those previously demonstrated with noise overexposure (Koehler and Shore, 2013b; Wu et al., 2016; Marks et al., 2018). Consistent with our hypotheses, animals with behavioral evidence of tinnitus assessed with GPIAS and operant conditioning, following salicylate administration, demonstrated tinnitus-frequency-specific StDP timing-rule enhancements. In addition, we also observed increased SFR and enhanced pairwise unit synchrony between fusiform cells. These findings highlight similarities in mechanisms of action in noise-induced and salicylate-induced tinnitus and suggest maladaptive timing-dependent plasticity is a necessary ingredient for tinnitus induction.
Methods
Ethical Treatment of Animals
All animal procedures were performed in accordance with protocols established by the National Institutes of Health (Publication 80–23) and approved by the University Committee on Use and Care of Animals at the University of Michigan. Seven juvenile female, pigmented guinea pigs were obtained from the University of Michigan colony at 2–3 weeks of age.
Experimental Design
Guinea pigs were tested for tinnitus using two behavioral paradigms (Fig 1A): gap-prepulse inhibition of the acoustic startle reflex (GPIAS) (Fig 1B), and a custom developed operant conditioning technique (Fig 1C, D). Baseline GPIAS results were collected for six experiment days, following which animals were administered salicylate for an additional six experiment days. After GPIAS testing, animals underwent the operant conditioning procedure. Learning rates were assessed until animals correctly demonstrated 65% success rates (2–6 experiment days) or were removed from further study if they failed to learn. Baseline crossing rates were measured for six experiment days. Saline then salicylate testing periods were also measured for six experiment days. Following behavioral assessments, DCN electrophysiology was assessed (Fig 1E). DCN was surgically accessed (Fig 1F), fusiform cells identified, and their activity recorded with multichannel electrodes (Fig 1G, H). STDP learning rules, SFR, synchrony and ABRs were measured pre- and post-salicylate administration. At the end of the experiment, animals were killed by intraperitoneal injection of sodium pentobarbital (SomnaSol, 1mL) and decapitation.
Figure 1. Experimental Design and Timeline.
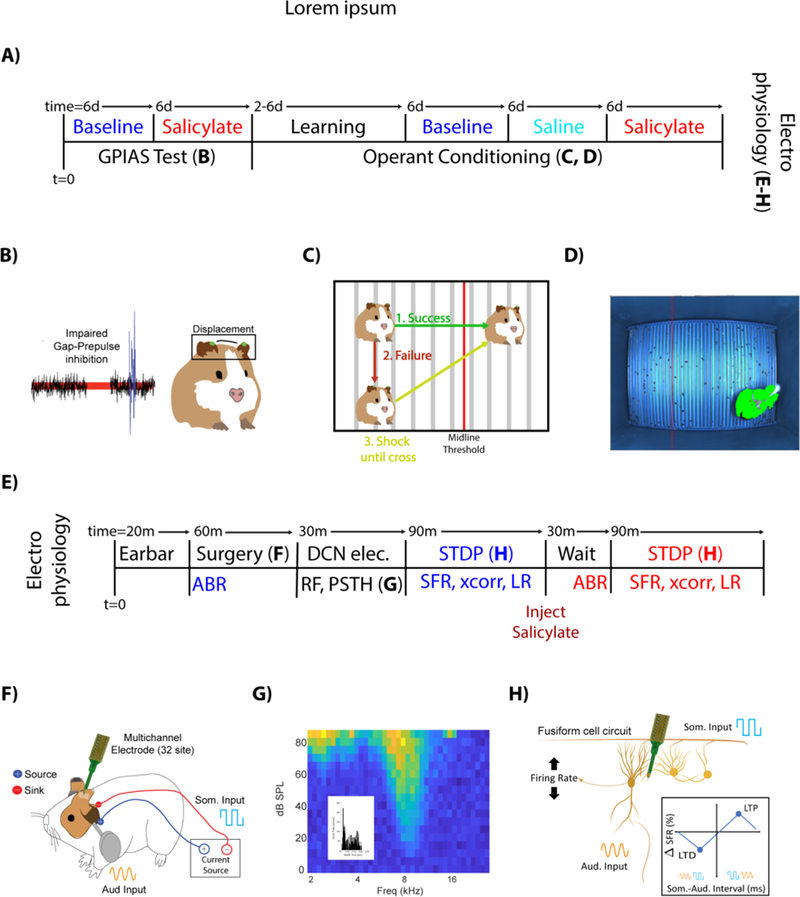
A) Animals were tested for tinnitus baseline using the adapted GPIAS paradigm (see METHODS) 6 days (Mon/Thurs, or Tue/Fri) and with salicylate for 6 days. Following GPIAS, animals were trained to move when a sound was presented (2–6 days; Mon/Wed/Fri). Animals that successfully learned crossing received 6 days of baseline testing, followed by administration of saline and then salicylate, each for 6 days. After operant conditioning, DCN electrophysiology was performed. B) Tinnitus impairs gap-prepulse inhibition when spectrally like a background carrier band. Guinea pig pinna tips were painted green, tracked using high speed cameras and the pinna-startle displacement computed. C) If guinea pigs crossed the midline when a sound was introduced, no shock was given (Green). If they failed to cross the midline during a sound (Red), the guinea pig received a footshock until it crossed the midline (Yellow). D) Sample frame, with the adaptive midline (vertical red line) and guinea pig location (green, with red star on centroid). E) ABRs were recorded (20 min) followed by single unit recordings to identify DCN fusiform cells (30 min) and record spontaneous firing rates (SFR) and STDP learning rules (LR) (~90 min). Salicylate was then injected (i.p.), After 30 min, ABRs and single unit recordings were repeated. F) Schematic of multichannel recording electrode placements DCN and Ag/AgCl stimulating electrodes over C2 DRG region for STDP evaluation. G) Fusiform cells were identified by their receptive fields and temporal response patterns (inset), and stereotaxic location with the DCN (See methods for coordinates). H) Somatosensory (Blue square waves) and auditory (yellow sine waves) stimulation was applied to assess StDP and quantified by learning rules (boxed inset).
Gap-prepulse inhibition of the acoustic startle (GPIAS).
Animals startle in the presence of a rapid-onset sound (the startle pulse), while the presentation of a stimulus (detectable above a background noise) before the startle pulse will reduce the resultant startle amplitude. Similarly, a gap placed in the background noise before the startle pulse will decrease the startle amplitude. Tinnitus that is spectrally similar to the background noise is thought to impair detection of the gap (Fig 1B) (Turner et al., 2006).
The guinea pig’s pinna-reflex displacement was measured in response to the startle pulse (Berger et al., 2013). Pinna tips were marked with non-toxic, water-soluble green paint, manually applied by trained investigators. Green pixels were identified using a custom-written k-nearest neighbors classifier algorithm (Mathworks MATLAB) (Friedman, 1977; Altman, 1992). Frames where green points constituted less than 0.01% of pixels were excluded, as this indicated the animal’s ears were not located in the frame. Pinna locations were identified by clustering green pixels and computing the centroids of a two-dimensional Gaussian mixture model (McLachlan and Chang, 2004). The Euclidean distance between (Xear (t), Year (t)) points was computed over the trial duration. Startle amplitudes were computed by fitting the Euclidean distance to a Gaussian-windowed sine-wave cycle and computed as the resultant amplitude parameter.
To assess tinnitus, gaps in constant background noise (65 dB SPL; 50 ms with 5 ms rise/fall times) were presented 100 ms before a broadband noise startle pulse (90 dB SPL; 20 ms with 2 ms rise/fall times). At a given background frequency band (center frequencies of 9, 13, 17 kHz with 2 kHz bandwidths, or Gaussian broadband noise), a randomized series of 10 pre-pulse (either a gap of silence, or a pre-pulse of noise at 75 dB SPL) and 10 no-prepulse sounds were delivered. All testing was performed in sound-proof booths (Acoustic Systems, Inc), with greater than 100 dB acoustic isolation between testing chambers. Trials were randomly presented every 20 to 30 seconds, with prepulse and no-prepulse trials combined into a single per-frequency testing session, and randomly interleaved. Each per-frequency testing session lasted approximately 10 minutes due to random variation of intertrial intervals. Eight testing sessions (one gap and one PPN testing session for each frequency band) were performed each testing day, for an average testing time of approximately 80 minutes. Animals were not kept in their restraints for more than two hours. Testing occurred twice per week, with at least two non-testing days in between each testing day (Mondays and Thursdays or Tuesdays and Fridays) to prevent habituation. Per-background frequency testing session results were pooled over three weeks. Startle amplitudes greater than two standard deviations above the mean were identified and excluded. In each frequency band, a normalized startle ratio (R) was computed as the mean with pre-pulse conditions were normalized by the mean without pre-pulse values. Tinnitus was assessed by measuring the amplitude of the startle reflex at baseline (blue) and after salicylate treatment (red). An animal was defined as having tinnitus if, at a given frequency, the mean of the post-exposure distribution was significantly greater than the mean of the pre-exposure distribution (Mann-Whitney U-test if distributions are non-normal as assessed through the Kolmogorov-Smirnoff test, otherwise two-sample t-test; alpha = 0.05). The changes in gap R values from pre- to post-exposure were quantified by the standardized tinnitus index [(x – µ)/σ] (Kalappa et al., 2014), where x is the post-exposure gap R value, µ and σ are the mean and standard deviation of pre-exposure gap R value. A larger positive index indicates more impaired gap detection (“more tinnitus”).
Operant Conditioning Paradigm
We modified operant conditioning tests previously developed for rats (Ruttiger et al., 2003; Yang et al., 2011) for use in guinea pigs. Guinea pigs are notoriously difficult to train through positive reinforcement. To increase learning rates, fear conditioning was exclusively used. Further, light-dark preference testing was not used as guinea pigs are generally non-responsive to classical operant conditioning paradigms (Anderson and Wedenberg, 1965; Crifo and Antonelli, 1972).
Seven guinea pigs were recruited for training. All operant behavioral testing was conducted in a double-walled sound proof booth (Acoustic Systems, Inc). These animals were trained to cross a custom-built operant chamber in response to sounds (Fig 1C). There were no distinguishing features on either side of the chamber (Fig 1D). The midpoint of the chamber was computed digitally, and dynamically switched to from 35% away from the left side of the box to 65% away from the left on crossing, ensuring that an animal had to complete cross the box to advance the protocol. Animals were tracked using custom-written MATLAB software and high-speed cameras (Point Grey, Inc). A single tracking camera was placed over the midline and two speakers (Pyle Wave PLX32 4 ohm speakers; Parasound Zamp Zone Amplifier) were fixed into the chamber ceiling two feet above the animal. The speakers were positioned ½ way in between the midline and the nearest side of the chamber. The system transfer function was measured using a ¼” microphone (B&K 4136 and Stanford Research Systems SR760 spectrum analyzer) and flatted in FFT space from 4 kHz to 30 kHz with custom written software. The sound field at the bottom of the chamber varied by 2 dB but was symmetric across the midline.
For each trial, the animal was required to remain still for a randomly-determined holding period (uniformly distributed from 5–45 seconds) followed immediately by a sound (2 kHz noise band with center frequencies at 8, 10, 12, 14, 16 kHz, or carriers as tones for 12 unique sounds; intensity range: 40–90 dBSPL in 10 dB steps for 6 unique intensities). Each sound-intensity pair was presented once for 72 trials per testing session, with ordering randomized per testing session. The animal had 30 seconds to cross from one side of the box to the other before electrical shocks were presented if the animal failed to cross in time (I = 1.25 mA; Med Associates ENV-414S with custom built Arduino controller; applied to front and hind paws by custom built electrode grid). Shocks were applied uniformly across the entire grid, for at most one minute after a failed trial to prevent harm to the animal. However, if the animal crossed before the end of sound presentation, the trial was considered a success and the next trial was immediately started. The non-learning animals demonstrating “freezing” behavior, where no electrical stimulus could elicit crossing. These animals were removed after two weeks of testing. Four out of the seven guinea pigs successfully learned the operant conditioning.
After each animal achieved a success crossing rate of 65%, probe trials were introduced. Ten punishment-free silence probe trials were randomly interspersed with regular trials, with a duration of 2 minutes each. The average number of crossings per silence period was normalized by the average number of successful crossings to control for differences in animal learning and locomotion, as previous studies have shown this measure is independent of motor impairments, auditory masking and hearing loss (Ruttiger et al., 2003; Tan et al., 2007). An animal was defined as tinnitus-positive if it’s probe trial crossing rate during silence trials post-induction was greater than the baseline rate (Chi-square test of proportions; alpha = 0.05).
Tinnitus Induction
To induce tinnitus, animals received a daily dose of sodium salicylate dissolved in saline (intraperitoneal 300 mg/kg; concentration: 250 mg/mL, solution provided and used as-is by Racehorse Meds) (Norena et al., 2010), which reliably and rapidly induces tinnitus (Jastreboff et al., 1988). An equivalent volume of 0.9% saline was administered as a control. Behavioral and physiological assessments of tinnitus commenced within thirty minutes of injection and were completed within three hours, corresponding to the peak effect of salicylate (Norena et al., 2010). This duration provided adequate time to complete all behavioral tests as well as electrophysiology: GPIAS testing sessions lasted no more than two hours, operant conditioning sessions 1 hour, and electrophysiology recordings 2 hours.
Auditory Brainstem Responses
All electrophysiology testing occurred in a double-walled, sound proof booth (Acoustic Systems, Inc). Animals were anesthetized and auditory brainstem responses (ABRs) were measured pre-baseline and 30 minutes post-salicylate administration (Fig 1A, E) (tone pip, 1024 repetitions, 5ms duration, 0.5 ms rise/fall time, cos^2 gating; 8, 12, 16, 20, 24 kHz; TDT RX8 DAC, HB7 amplifier, and PA-5 attenuator). Sounds were presented close-field (DT770 Speaker) and were coupled to the ear drum through custom-built hollow ear bars. Calibration was performed using TDT SigCalRP and a ¼” microphone (B&K 4136 and Stanford Research Systems SR760 spectrum analyzer; RX8 and PA5). The system transfer function was flattened in FFT space from 200 Hz-32kHz. Stainless steel needle electrodes were placed into the skin overlying the bullae and at vertex. Evoked potentials were digitized and filtered (TDT RA4LI headstage; PZ2–64 pre-amp; filtered between 300 Hz-3kHz with a 60 Hz notch). Sound intensities were presented starting at 90 dB SPL and decreased in 10 dB steps to 0 dB SPL. Thresholds were identified by a trained experimenter as documented previously (Dehmel et al., 2012a). Threshold was defined as the one-step greater than lowest sound pressure level that did not elicit ABRs with at least three identifiable peaks and troughs.
Surgery
After ketamine/xylazine (40:10 mg/kg) anesthesia, animals were held in a Kopf stereotaxic frame with hollow ear bars. Fur overlying the head and neck were removed (while ensuring that whiskers were not affected) by clippers. Skin was cleaned with an alcohol wipe. Body temperature was kept constant (38 degree C) throughout the experiment by a custom-built heating pad with closed-loop controller. The state of the animal was checked, and supplemental anesthesia (0.15 mg of same ketamine/xylazine dose) was administered every 30 minutes by the experimenter. Tissue overlying the occipital ridge was removed without impacting the ear muscles, and a craniotomy and duratomy performed to expose the cerebellum. Surgical manipulations were performed consistently across all animals, and are similar to previous experiments performed in this lab (Dehmel et al., 2012b; Koehler and Shore, 2013b; Basura et al., 2015; Stefanescu et al., 2015; Wu et al., 2016; Marks et al., 2018).
Single Unit Electrophysiology
Multichannel recording electrodes (Neuronexus; 32 channels with 16 channels per 2 shanks; custom headstage) were used to record in vivo neural responses (Fig 1F). Voltages from each electrode site were digitized (PZ2–64 pre-amp) and bandpass filtered (300 Hz-3kHz, with a 60 Hz and harmonic comb-filter). Spikes were identified when voltage amplitude crossed 2 standard deviations above the mean voltage arising from spontaneous activity. The fusiform cell layer was consistently found when the electrode was placed 25 degrees off the vertical, 3–4 mm lateral to the midline and 3–4 mm posterior to earbar zero, and from 5–6 mm ventral to the surface of the cerebellum. Units were identified using 65 dB SPL broadband search stimuli. Unit thresholds were stable throughout the experiment. Fusiform cells were identified by their build-up and pause-build-up peri-stimulus time histograms (PSTH) and locations within the DCN (Stabler et al., 1996) (Fig 1G). Once a set of fusiform cells were identified, the electrode was not moved until the end of the experiment after the salicylate experiments were complete. Unit consistency was maintained by clustering all waveform PCA coefficients throughout the experiment. Neural spike data was imported into MATLAB and analyzed offline. Spike waveforms were projected into principle component space and clustered by the first three coefficients by a trained user. Timestamps were grouped by cluster into isolated units, and spiketrains constructed in MATLAB.
StDP Induction
StDP was elicited by applying non-invasive transdermal electrical stimulation (Rhythymlink Ag/AgCl electrodes; custom-built linear isolated current source; biphasic square wave; 100 us/phase, 1 kHz, 3 pulses) briefly before or after tone bursts (50 ms duration, 2 ms rise and fall times, Cos2-ramps) 40 dB above unit threshold (SL) at a neurons best frequency (Wu et al., 2015; Marks et al., 2018) (Fig 1F, H). Electrodes were applied to the skin after ABRs but before surgery. Current level was determined as mA less than the level that elicited muscle contractions. Source electrodes were placed on the skin over the C2 dorsal ganglion, while sink electrodes were placed lateral to the spinal column. Timing rules were measured as the percent change in firing rate from pre- to post-pairing (Wu et al., 2015; Marks et al., 2018). Each bimodal interval recording session lasted 15 minutes, with six recordings per experimental condition for a total of 90 minutes trial time. Two recording sessions were performed in the same experiment, without moving the electrode in-between sessions (Baseline, Salicylate).
Synchrony Analysis
Spontaneous activity (at least 150 seconds) was recorded prior to starting each STDP recording session. SFR was computed as the average spike rate during this trial. Cross-unit spatial synchrony was computed using cross-correlograms (Voigt and Young, 1990; Norena and Eggermont, 2003; Wu et al., 2016; Marks et al., 2018). Spikes co-occurring within 150 us were removed. Cross-correlation coefficients (p(τ)) were computed as a function of time lag for each pairwise combination of spike trains (Eq. 1).
| (1–2) |
𝑅AB (𝜏) s the unbiased cross-correlation of spike trains A and B; NA and NB indicate spike counts in the respective spike trains. E is the mean probability of coincident firing for Poisson-distributed data (Eq. 2), defined by the multiplication of NA and NB over the number of bins (n). Bin size was constant at 0.3 ms (Voigt and Young, 1990). A unit-pair was considered synchronous when the peak p value was greater than ±4 standard deviations from the mean p(τ). In the present study, negative cross-correlations were removed from further analysis.
Data Analysis
Data normality was assessed using the Kolmogorov-Smirnoff test. Linear correlations were computed using Pearson’s linear correlation. Distribution differences were assessed for significance with ANOVAs or Kruskal-Wallis tests where appropriate (alpha = 0.05). Chi-square test of proportions was used to assess tinnitus status for the operant paradigm (alpha = 0.05).
Results
GPIAS and operant conditioning diagnose salicylate-induced tinnitus in guinea pigs
To first assess animals for tinnitus, we utilized a modified variant of the GPIAS paradigm, tracking an animal’s pinna-tip, or Preyer’s, reflex instead of a whole body amplitude startle (Berger et al., 2013) (Fig 1B). Fig. 2A shows an example animal positive for tinnitus at 12–14 kHz and 16–18 kHz, but not at 8–10 kHz or BBN. For each animal, tinnitus strength was quantified through the tinnitus index (TI). All tested animals (n=4) demonstrated evidence of tinnitus in at least one frequency band, but no animals showed evidence of broadband noise tinnitus (Fig 2B). The animals demonstrated a high-frequency tinnitus, with the peak of the average tinnitus spectrum occurring at 12 kHz and consistent with other studies utilizing GPIAS to assess salicylate-induced tinnitus (Yang et al., 2007; Ralli et al., 2010). The mean TI was significantly greater within tinnitus frequency bands than outside tinnitus frequency bands (p=7.142e-4; two-sample t-test) (Fig 2C). Animals demonstrated tinnitus-positive TIs with a similar range and variance (current study: min=0.31, max=1.21, st.dev.=0.32) compared to animals tested in Marks et al. (2018) (min=0.33, max=2.01, st.dev.=0.46). Further, the current TI distributions and the TI distributions from Marks et al. (2018) were not significantly different (two-way ANOVA, P=0.476).
Figure 2. GPIAS identifies guinea pigs with tinnitus after salicylate.
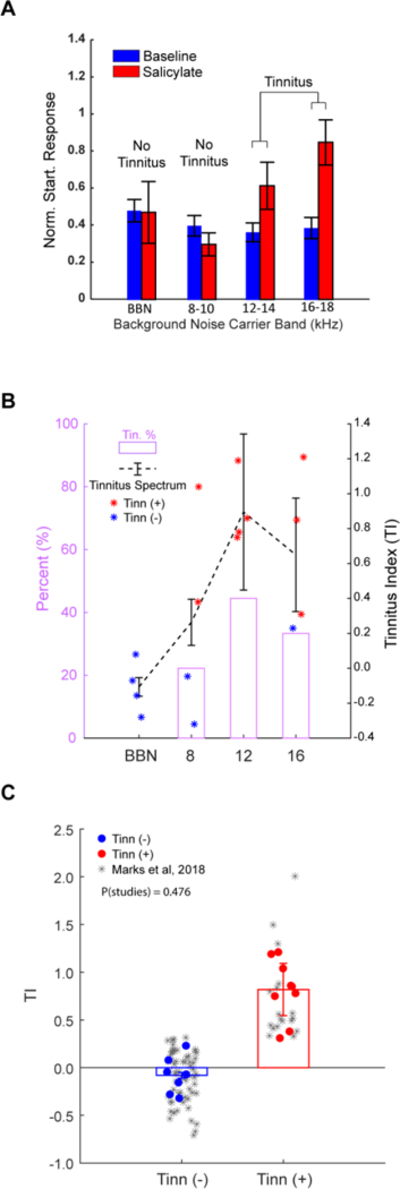
A) An example animal shows significantly increased normalized startle response ratios in the 12–14 and 16–18 kHz noise bands after receiving salicylate (red) compared to baseline (blue), but not in the BBN and 8–10 kHz bands. B) The left axis shows the percentage of animals within having tinnitus within the band (pink), while the right axis shows the average tinnitus spectrum (dashed black lines; data are mean+/−SEM). C) Pooled Tis from all tested animals for within tinnitus frequency bands (red) and outside tinnitus frequency bands (blue) are not significantly different compared to TI distributions from Marks et al. (2018). Data shown are mean+/−SEM. Significance assessed using two-way ANOVA. Alpha = 0.05.
To validate the presence of tinnitus, we modified operant conditioning procedures previously developed for use in rats. Guinea pigs were trained to cross from one side of the operant box to the other in the presence of sound, while remaining on the original side when no sound was present (Fig 1C, D). Four animals successfully demonstrated crossing rates greater than 65% within six experiment days (Fig 3A). Next, we presented silence trials interspersed with sound trials, and measured the animal’s baseline crossing rate (Fig 3B). To control for differences in baseline locomotion for each animal, crossing rates in silence were normalized by the animal’s crossing rate during sound, as previous studies have shown that normalization corrects for differences in mobility and learning rate (Ruttiger et al., 2003). Finally, we administered salicylate to the animals, and measured the crossing rate again. Intraperitoneal administration of salicylate (300 mg/kg) significantly increased the normalized crossing rate when compared to baseline or equivolume saline time points (Fig 3B) (p=5.03e-4, n=4 animals, Chi-Square test of proportions).
Figure 3. Operant conditioning identifies tinnitus in guinea pigs after salicylate, but not saline injections.
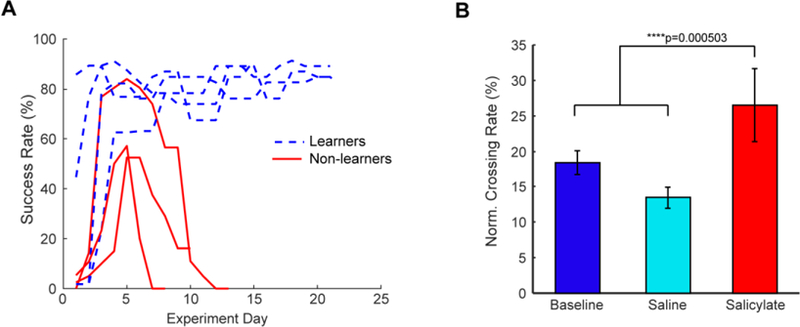
A) Guinea pigs that consistently showed high learning rates moved onto tinnitus testing (dashed blue lines), while guinea pigs that failed to do so were removed from further study (solid red lines). B) When administered saline during silence trials, animals did not show significant changes from baseline in their normalized crossing rate (light blue). However, after salicylate administration, animals showed significantly increased crossing rates (red) (Chi-square test of proportions, p=5.03e-4, n=4).
Animals with salicylate-induced tinnitus have increased SFR, synchrony and altered StDP timing rules in DCN fusiform cells.
Fusiform cells show increased SFR, increased synchrony and altered StDP timing rules following noise-overexposure induced tinnitus (Koehler and Shore, 2013b; Wu et al., 2016; Marks et al., 2018). In the present study, we wanted to explore the possibility that the mechanisms by which salicylate induced tinnitus are like those of noise-overexposure-induced tinnitus. We measured the electrophysiological activity in animals that had been positively screened using GPIAS and operant conditioning tests (Figs 1–3). Prior to surgery, transdermal electrodes were placed ipsilateral to the neck region overlying the C2 dorsal root ganglion (Fig. 1F). Multichannel single-unit electrodes were stereotaxically implanted into the DCN of anesthetized guinea pigs (Fig. 1F–H). Fusiform cells were identified by their characteristic build-up and pause build-up PSTH, receptive fields and coordinates within the DCN (Stabler et al., 1996) (Fig 1G). Once stable fusiform cell responses were identified, electrodes were not moved throughout the remainder of the experiment. Units were found with BFs ranging from 2kHz to 24kHz, with a preponderance of units located between 6kHz and 18kHz (Fig 4A). Fusiform cells with BFs in a GPIAS carrier band showing evidence of tinnitus constituted 53.15% of recorded units, while fusiform cells with BFs outside tinnitus bands constituted 46.85% of units (Fig. 4A). Auditory brainstem responses (ABRs) indicate threshold shifts of approximately 10 dB after salicylate administration at frequencies at and above 12 kHz (Fig 4B), consistent with previous studies (Stolzberg et al., 2012).
Figure 4. Salicylate induces increased ABR thresholds, increased SFR and synchrony in DCN fusiform cells.
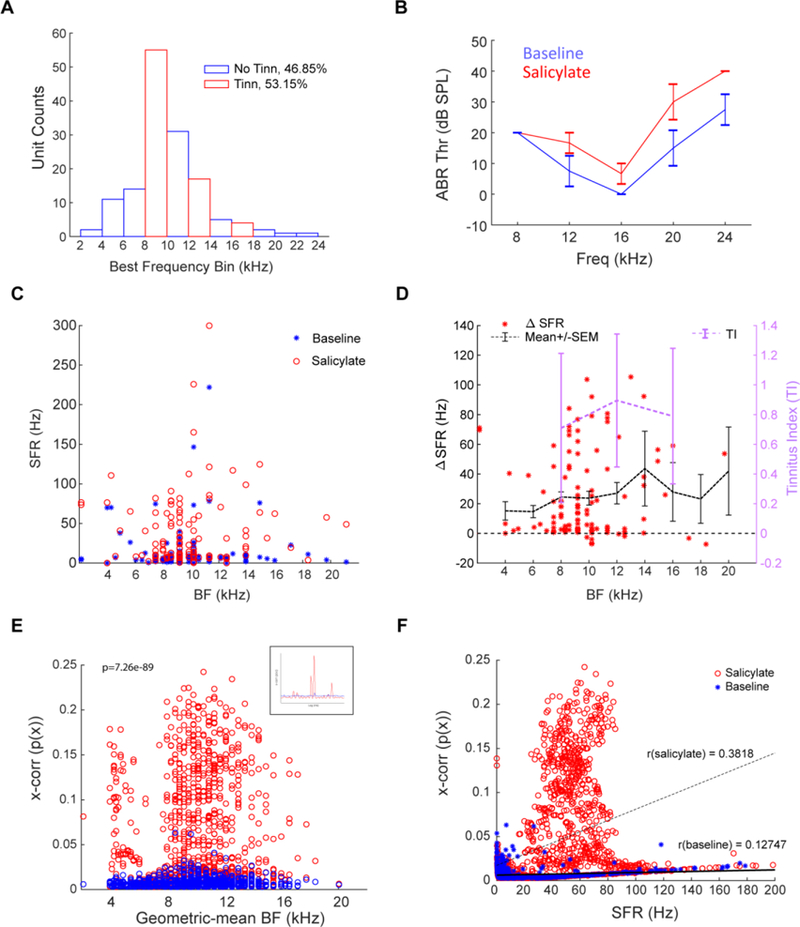
A) Fraction of units by best frequency, with no-tinnitus frequencies (blue bars) and tinnitus frequencies (red bars) indicated. 46.85% of units were within a no-tinnitus band, while 53.15% of units were in a tinnitus band. B) ABRs were measured before surgery (blue) and post-salicylate administration after 30 minutes had past (red). C) Fusiform cells in all test animals showed significant increases in SFR after salicylate administration (red circles) compared to baseline (blue stars). D) Change in SFR for each unit from baseline to salicylate (red stars; mean+/− SEM indicated by dashed black line). Most increases occur in frequencies where GPIAS-measured tinnitus was confirmed (pink line). E) Cross-unit synchrony between pairs of spiketrains was computed at baseline (blue) and post-salicylate (red; see inset for sample cross-correlations) and is significantly increased over range of geometric mean BFs of sampled pairwise spiketrains (ANOVA2; p=7.26e-89, n=4404). F) Synchrony and SFR increase their correlation (r=0.3818) post-salicylate administration compared to baseline (r=0.127; Pearson’s linear correlation). Data shown are mean+/− SEM; alpha = 0.05.
Fusiform cell spontaneous activity was recorded before (blue) and after (red) intraperitoneal administration of salicylate (Fig. 4C). After salicylate administration, fusiform-cell SFR was increased significantly across animals when compared to baseline (ANOVA, p=1.79e-11, n=199; Fig. 4C). Increases in SFR were most pronounced in the 8–16 kHz regions, corresponding to frequencies with GPIAS-based evidence of tinnitus (purple tinnitus spectrum in Fig. 4D). Furthermore, cross-unit synchrony was significantly increased over the geometric mean BF between sampled units (two-way ANOVA, p=7.26e-89, n=4404) (Fig. 4E). Importantly, salicylate administration significantly increased the correlation between SFR and synchrony (r-baseline=0.13; r-salicylate=0.38; Pearson’s linear correlation), consistent with previous studies (Wu et al., 2016; Marks et al., 2018) (Fig. 4F).
To induce StDP in the fusiform cells (Fig. 1H), transdermal Ag/AgCl electrodes were placed on the skin overlying the C2 ganglion (Wu et al., 2015; Marks et al., 2018). These electrodes were not moved during the experiment. StDP timing rules were assessed using bimodal stimulation with variable auditory (orange sinewave in Fig. 1H) -somatosensory (blue pulse in Fig. 1H) stimulus intervals. Timing rules were assessed as previously described (Wu et al., 2015; Marks et al., 2018). At baseline, the guinea pigs exhibited StDP timing rules consistent with those obtained from non-tinnitus animals in previous studies (Blue line in Fig. 5A) (Wu et al., 2015; Marks et al., 2018). Post-salicylate administration, StDP timing rules were significantly enhanced and inverted compared to baseline (ANOVA, p=0.0036, n=199) (Fig. 5A). Interestingly, partitioning timing rules into groups based on whether the unit BF was in a tinnitus-band or not revealed a divergence in learning rule enhancement or suppression. Timing rules in a GPIAS-measured tinnitus band exhibited predominantly LTP, while those outside the tinnitus band exhibited LTD (Fig. 5B).
Figure 5. Salicylate induces frequency-specific enhancements of STDP timing rules in DCN fusiform cells.
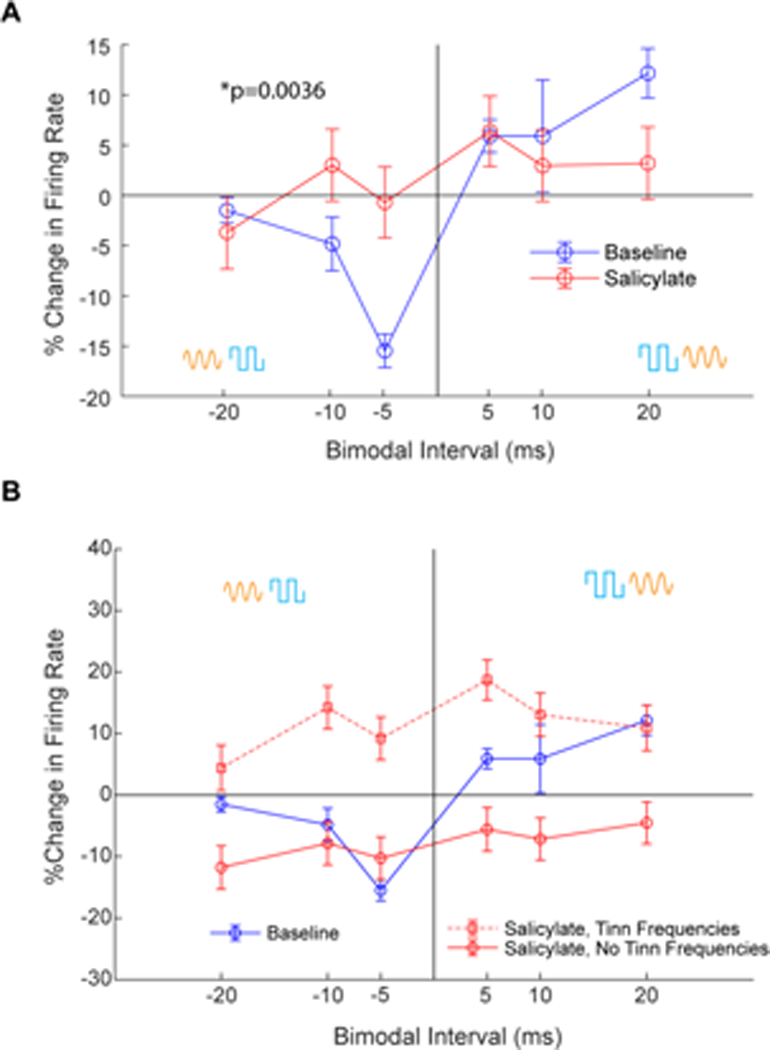
A) Mean StDP timing rules show inversion of the timing rules and LTP at more pairing protocol intervals after salicylate administration (red) compared to baseline (blue) (ANOVA, p=0.0036, N=199). Bimodal ordering (Aud-Som vs Som-Aud) indicated by yellow and blue symbols. Data shown are mean+/−SEM pooled across frequencies. B) Timing rules from units in a GPIAS-confirmed tinnitus band (dashed red line with square markers) show LTP, while timing rules from units outside GPIAS-confirmed tinnitus bands show mostly LTD (solid red line with circle markers).
Discussion
In the present study, we demonstrate that guinea pigs administered salicylate, but not saline, show behavioral evidence of tinnitus using two behavioral tests, GPIAS and operant conditioning. Furthermore, we demonstrate that fusiform cells in these same animals show increased SFR and synchrony post-salicylate administration compared to baseline, as well as altered StDP timing rules that show tinnitus-related increases in LTP. This evidence suggests that like noise overexposure, salicylate triggers important tinnitus-related changes in fusiform cell plasticity.
Following salicylate administration, animals are reliably diagnosed with tinnitus by both GPIAS and our operant conditioning paradigm.
All mammals have a startle response, which consists of a contraction of major muscles in the presence of loud and unexpected noise (Holt and Koch, 1999). Furthermore, mammals also exhibit prepulse inhibition, wherein the startle response is reduced by presenting a weaker stimulus in the form of a background noise before the stronger stimuli (Fendt et al., 2001). Similarly, if a silent gap is inserted in the background noise before the stronger stimuli, then the animal will startle less. Therefore, any noise at the same frequency as the background noise that masks the gap will result in the animals exhibiting a full startle response. The GPIAS reflex test takes advantage of this “masking” effect to detect the presence of tinnitus (Turner et al., 2006; Yang et al., 2007) (Fig 1B). Further, GPIAS can be used to estimate the tinnitus spectrum and strength if multiple background frequency bands are presented (Kalappa et al., 2014; Wu et al., 2016; Marks et al., 2018).
However, GPIAS has been criticized as a tinnitus assessment (Fournier and Hebert, 2013; Galazyuk and Hebert, 2015). Cross-validating GPIAS with another widely accepted behavioral paradigm is important for the tinnitus research field (Fig. 1A). To this end, we performed an operant conditioning test based on fear-conditioning, with modifications for guinea pigs (Ruttiger et al., 2003; Yang et al., 2011). Our operant conditioning test pairs an unconditioned stimulus (sound) to a conditioned stimulus (footshock), a well-established paradigm. Here, once the sound goes on, the animal must cross the chamber to avoid a footshock. In our paradigm, we used the phantom perception of sound to act as the unconditioned stimulus, given that the phantom perception of sound had a frequency found within the unconditioned stimuli. Therefore, if the animal had tinnitus, it would cross from one side of the chamber to the other to avoid the supposed incoming footshock, thereby increasing the crossing rate when compared to baseline. The data gathered with this test was consistent with other behavioral tests assessing salicylate- and noise-induced tinnitus (Guitton et al., 2003; Ruttiger et al., 2003; Turner et al., 2006; Yang et al., 2007; Stolzberg et al., 2013). For example, Guitton et al. (2003) created a behavioral model to test for tinnitus, where they conditioned rats to jump on to a pole whenever they heard a sound to avoid being foot shocked. Therefore, if they heard a phantom perception of sound after salicylate treatment, they would jump onto the pole to avoid being footshocked, even in the absence of an external stimuli. Like our test, this operant conditioning relied on the phantom perception of sound to avoid a footshock. Nevertheless, contrary to the operant conditioning task employed in our study, GPIAS allowed us to gather more information on the characteristics of the tinnitus by allowing us to measure the guinea pig’s tinnitus spectrum. To generalize operant conditioning, multiple tones at several intensities should be used to assess for the frequency and intensity of the tinnitus. Further, additional analyses, such reduced successful crossing rates for specific frequency bands, could be employed. Another major limitation of the operant paradigm compared to GPIAS is that many animals will not learn the crossing behavior. The non-learning animals exhibited freezing behavior, where an animal would huddle in a corner of the box and not leave it, regardless of electrical current levels applied to the animal. Further training periods could help improve our learning rates. Additionally, providing an additional sensory cue could help reduce freezing rates. Non-learning necessarily reduces testing throughput, as well as potentially selects for animals that are physiologically different from the non-learners. Further, operant conditioning outcomes are parameter sensitive. Increasing the sound duration and pre-sound holding period could potentially increase learning rates. Indeed, in several pilot animals, we found that using 5 second sound stimuli, with 10–20 second holding periods resulted in a lower success rate compared to the present results. In any case, further optimization of the protocol is essential to increase the usability of the test.
Salicylate is an important tool for assessing tinnitus behavior as it reliably induces tinnitus in both humans and non-human species (Guitton et al., 2003; Stolzberg et al., 2012). However, elevated auditory thresholds that arise following its administration complicate tinnitus testing (Fig. 4B), as impaired hearing can result in false-positive diagnoses of tinnitus. For example, GPIAS requires detection of a gap-prepulse; hearing loss at the same frequency as the background carrier will reduce the salience of the gap, increasing normalized startle ratios and indicating tinnitus. However, not all hearing loss results in tinnitus (Roberts et al., 2008; Roberts et al., 2010), and neither cochlear synaptopathy nor ABR threshold shifts reliably distinguish tinnitus animals from no-tinnitus animals (Li et al., 2013; Li et al., 2015; Wu et al., 2016; Marks et al., 2018). Further, noise-overexposure is the most common cause of tinnitus in humans (Axelsson and Ringdahl, 1989; Shore et al., 2016), making its study more relevant for understanding the pathophysiology in humans. Thus, while the present findings are an important proof-of-concept, future studies are essential to cross-validate operant conditioning and GPIAS with noise-induced tinnitus.
Increased SFR, synchrony and altered StDP timing rules in DCN fusiform cells following salicylate administration underlie tinnitus behavior
The present results suggest that salicylate-induced tinnitus may have a similar pathophysiology as noise-overexposure-induced tinnitus (Koehler and Shore, 2013b; Marks et al., 2018). Both salicylate and noise-overexposure result in enhanced StDP timing rules, increased SFR and synchrony in tinnitus animals compared to non-tinnitus animals (Figs. 4, 5). In the present study, these changes were specific to units with BFs within the tinnitus frequencies: salicylate induced increases in LTP from fusiform cells with BFs within the tinnitus frequencies, while inducing LTD outside the tinnitus frequencies. Furthermore, fusiform cells in tinnitus animals have been demonstrated to be hyperexcitable and hypersynchronous by several other groups, utilizing a variety of tinnitus assessment techniques (Middleton et al., 2011; Dehmel et al., 2012b; Pilati et al., 2012; Li et al., 2013; Li et al., 2015). Hypersynchronous fusiform cell firing could bind increased SFR into an auditory object that the brain interprets as a phantom sound (Singer, 1999). This notion is consistent with the increased correlation between synchrony and SFR found post-salicylate administration (Fig. 4E, F), potentially increasing the salience of spontaneous neural activity. Thus, the present findings reinforce the link between tinnitus, fusiform cell hyperexcitability and pathologically enhanced LTP arising from reduced ANF output.
In contrast to the present findings, Wei et al. (2010) showed that in vitro bath application of salicylate to a fusiform cell culture reduced SFR through a reduction in membrane excitability. One factor to explain these differences is that In vitro preparations remove multisensory and other descending input into the fusiform cell circuit, and therefore may not fully reflect pathological processes that underlie salicylate-induced tinnitus. Furthermore, in vitro preparations necessarily remove afferent input to the fusiform cell. Many labs have shown that salicylate alters cochlear function through multiple, distinct mechanisms. Altered afferent input could in turn have complex effects on fusiform cell activity that is not accounted for in vitro. Alternatively, since the present findings show that salicylate induces increases in LTP from fusiform cells with BFs within the tinnitus frequencies, while inducing LTD outside the tinnitus frequencies, the Wei et al. (2010) study may have sampled from fusiform cells outside a tinnitus frequency region in DCN, and thus consistent with our present findings. Finally, Wei et al only apply salicylate briefly, whereas our recordings are delayed until 30 minutes after salicylate injection and lasted for several hours. Treatment duration can change physiological responses to salicylate. Cazals et al. (1998) demonstrated that short-term application of salicylate can reduce ANF activity, while long-term, regular dosing reduces the magnitude of this effect.
There are several ways that salicylate could induce fusiform cell hyperexcitability. Following cochlear-damage, reduced ANF output triggers upregulation of excitatory somatosensory inputs to DCN fusiform cells and cartwheel cells (Shore et al., 2008; Zeng et al., 2009). At high doses, salicylate increases hearing thresholds and reduces ANF output, with a maximal reduction occurring between 2 to 4 after administration (Fig. 4B) (Cazals et al., 1998; Muller et al., 2003). Reduced ANF output caused by salicylate could therefore trigger an upregulation of excitatory inputs to DCN fusiform cell- and cartwheel cell-apical dendrites, which could increase synchrony through enhanced parallel fiber input or by enhancing NMDA receptor activity. Alternatively, increased SFR and synchrony could result from disinhibition of the fusiform cell. In addition, salicylate is a glycine receptor antagonist (Lu et al., 2009), the application of which could result in increased SFR in fusiform cells. Reduced glycinergic inhibition has been observed in DCN fusiform cells from animals with noise-induced tinnitus (Wang et al., 2009). Salicylate could enhance fusiform cell firing through its activity on N-methyl-D-aspartate (NMDA) receptors since it acts as an NMDA receptor agonist (Guitton et al., 2003). NMDA receptors have been found on fusiform cells and have been shown to mediate StDP timing rules and firing rate changes (Stefanescu and Shore, 2015). Stefanescu and Shore (2015) demonstrated that antagonizing NMDA receptors lead to reduced synchrony between fusiform cells. Blocking NMDA receptors results in less intracellular Ca2+ and leads to an inhibition of LTP and LTD that is regulated by STDP and StDP timing rules in the DCN (Magee and Johnston, 1997; Bi and Poo, 1998; Han et al., 2000; Tao et al., 2001). Thus, it is not surprising that by activating NMDA receptors, salicylate leads to increases in synchrony, SFR, enhanced LTP and thus, tinnitus.
Highlights.
Stimulus-timing-dependent plasticity is altered in animals administered salicylate
Cross-unit synchrony and spontaneous firing rates are increased in animals with salicylate-induced tinnitus
Gap-prepulse inhibition of the acoustic startle reflex and an operant conditioning test both indicate animals administered salicylate have tinnitus
Salicylate-induced tinnitus and noise-exposure-induced tinnitus could have a similar mechanism
Acknowledgements
The authors acknowledge Shaowen Bao for assisting with the development of the operant conditioning paradigm, Chris Ellinger and Dwayne Valliencourt for engineering assistance, Calvin Wu for technical and analytical assistance for single unit data, Liqin Zhang for assisting with behavioral data collection, and Michael Roberts for comments on an earlier version of the manuscript.
Funding
National Institutes of Health R01-DC004825 (SES), T32-DC00011 (DTM), P30-DC005188 (KHRI)
Abbreviations
- DCN
Dorsal cochlear nucleus
- SFR
spontaneous firing rate
- StDP
stimulus-timing dependent plasticity
- STDP
spike-timing dependent plasticity
- NMDA
N-methyl-D-aspartate
- VGluT
vesicular glutamate transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability
Data and analysis code used for this study are available upon request to the authors.
References
- Altman NS (1992) An introduction to kernel and nearest-neighbor nonparametric regression. The American Statistician 46:10. [Google Scholar]
- Anderson H, Wedenberg E (1965) A new Method for Hearing Tests in the Guinea Pig. Acta Oto-Laryngologica 60. [Google Scholar]
- Axelsson A, Ringdahl A (1989) Tinnitus—a study of its prevalence and characteristics. British journal of audiology 23:53–62. [DOI] [PubMed] [Google Scholar]
- Basura GJ, Koehler SD, Shore SE (2015) Bimodal stimulus timing-dependent plasticity in primary auditory cortex is altered after noise exposure with and without tinnitus. J Neurophysiol 114:3064–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Rojas R, Boley J, Wyder M (1999) Behavioral model of chronic tinnitus in rats. Otolaryngol Head Neck Surg 121:457–462. [DOI] [PubMed] [Google Scholar]
- Berger JI, Coomber B, Shackleton TM, Palmer AR, Wallace MN (2013) A novel behavioural approach to detecting tinnitus in the guinea pig. J Neurosci Methods 213:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi GQ, Poo MM (1998) Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci 18:10464–10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazals Y, Horner KC, Huang ZW (1998) Alterations in average spectrum of cochleoneural activity by long-term salicylate treatment in the guinea pig: a plausible index of tinnitus. J Neurophysiol 80:2113–2120. [DOI] [PubMed] [Google Scholar]
- Crifo S, Antonelli M (1972) [Ig G,A,M in the nasal secretion in cystic fibrosis]. Folia Allergol (Roma) 19:126–128. [PubMed] [Google Scholar]
- Dehmel S, Eisinger D, Shore SE (2012a) Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs. Front Syst Neurosci 6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmel S, Pradhan S, Koehler S, Bledsoe S, Shore S (2012b) Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus--possible basis for tinnitus-related hyperactivity? J Neurosci 32:1660–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS (2001) Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology (Berl) 156:216–224. [DOI] [PubMed] [Google Scholar]
- Fournier P, Hebert S (2013) Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: does tinnitus fill in the gap? Hear Res 295:16–23. [DOI] [PubMed] [Google Scholar]
- Friedman JH, Bentely Jon L., Finkel Raphael A. (1977) An algorithm for finding best matches in logarithmic expected time. ACM Transactions on Mathematical Software 3:209–226. [Google Scholar]
- Galazyuk A, Hebert S (2015) Gap-Prepulse Inhibition of the Acoustic Startle Reflex (GPIAS) for Tinnitus Assessment: Current Status and Future Directions. Front Neurol 6:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL (2003) Salicylate induces tinnitus through activation of cochlear NMDA receptors. J Neurosci 23:3944–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenggeli CA, Pongstaporn T, Doucet JR, Ryugo DK (2005) Projections from the spinal trigeminal nucleus to the cochlear nucleus in the rat. J Comp Neurol 484:191–205. [DOI] [PubMed] [Google Scholar]
- Han VZ, Grant K, Bell CC (2000) Reversible associative depression and nonassociative potentiation at a parallel fiber synapse. Neuron 27:611–622. [DOI] [PubMed] [Google Scholar]
- Holt GR, Koch C (1999) Electrical interactions via the extracellular potential near cell bodies. J Comput Neurosci 6:169–184. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Brennan JF, Coleman JK, Sasaki CT (1988) Phantom auditory sensation in rats: an animal model for tinnitus. Behav Neurosci 102:811–822. [DOI] [PubMed] [Google Scholar]
- Kalappa BI, Brozoski TJ, Turner JG, Caspary DM (2014) Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. J Physiol 592:5065–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler SD, Shore SE (2013a) Stimulus-timing dependent multisensory plasticity in the guinea pig dorsal cochlear nucleus. PLoS One 8:e59828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler SD, Shore SE (2013b) Stimulus timing-dependent plasticity in dorsal cochlear nucleus is altered in tinnitus. J Neurosci 33:19647–19656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Choi V, Tzounopoulos T (2013) Pathogenic plasticity of Kv7.2/3 channel activity is essential for the induction of tinnitus. Proc Natl Acad Sci U S A 110:9980–9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kalappa BI, Tzounopoulos T (2015) Noise-induced plasticity of KCNQ2/3 and HCN channels underlies vulnerability and resilience to tinnitus. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC (1993) Central projections of auditory nerve fibers of differing spontaneous rate, II: Posteroventral and dorsal cochlear nuclei. J Comp Neurol 327:17–36. [DOI] [PubMed] [Google Scholar]
- Lu YG, Tang ZQ, Ye ZY, Wang HT, Huang YN, Zhou KQ, Zhang M, Xu TL, Chen L (2009) Salicylate, an aspirin metabolite, specifically inhibits the current mediated by glycine receptors containing alpha1-subunits. Br J Pharmacol 157:1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Johnston D (1997) A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science 275:209–213. [DOI] [PubMed] [Google Scholar]
- Marks KL, Martel DT, Wu C, Basura GJ, Roberts LE, Schvartz-Leyzac KC, Shore SE (2018) Auditory-somatosensory bimodal stimulation desynchronizes brain circuitry to reduce tinnitus in guinea pigs and humans. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May BJ (2000) Role of the dorsal cochlear nucleus in the sound localization behavior of cats. Hear Res 148:74–87. [DOI] [PubMed] [Google Scholar]
- McLachlan GJ, Chang SU (2004) Mixture modelling for cluster analysis. Stat Methods Med Res 13:347–361. [DOI] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T (2011) Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A 108:7601–7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini E, Warr WB, Osen KK (1980) Distribution and light microscopic features of granule cells in the cochlear nuclei of cat, rat, and mouse. J Comp Neurol 191:581–606. [DOI] [PubMed] [Google Scholar]
- Muller M, Klinke R, Arnold W, Oestreicher E (2003) Auditory nerve fibre responses to salicylate revisited. Hear Res 183:37–43. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ (2003) Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res 183:137–153. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Moffat G, Blanc JL, Pezard L, Cazals Y (2010) Neural changes in the auditory cortex of awake guinea pigs after two tinnitus inducers: salicylate and acoustic trauma. Neuroscience 166:1194–1209. [DOI] [PubMed] [Google Scholar]
- Oertel D, Young ED (2004) What’s a cerebellar circuit doing in the auditory system? Trends Neurosci 27:104–110. [DOI] [PubMed] [Google Scholar]
- Pfeiffer RR (1966) Classification of response patterns of spike discharges for units in the cochlear nucleus: tone-burst stimulation. Exp Brain Res 1:220–235. [DOI] [PubMed] [Google Scholar]
- Pilati N, Large C, Forsythe ID, Hamann M (2012) Acoustic over-exposure triggers burst firing in dorsal cochlear nucleus fusiform cells. Hear Res 283:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralli M, Lobarinas E, Fetoni AR, Stolzberg D, Paludetti G, Salvi R (2010) Comparison of salicylate- and quinine-induced tinnitus in rats: development, time course, and evaluation of audiologic correlates. Otol Neurotol 31:823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LE, Moffat G, Baumann M, Ward LM, Bosnyak DJ (2008) Residual inhibition functions overlap tinnitus spectra and the region of auditory threshold shift. J Assoc Res Otolaryngol 9:417–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA (2010) Ringing ears: the neuroscience of tinnitus. J Neurosci 30:14972–14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttiger L, Ciuffani J, Zenner HP, Knipper M (2003) A behavioral paradigm to judge acute sodium salicylate-induced sound experience in rats: a new approach for an animal model on tinnitus. Hear Res 180:39–50. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Haenggeli CA, Doucet JR (2003) Multimodal inputs to the granule cell domain of the cochlear nucleus. Exp Brain Res 153:477–485. [DOI] [PubMed] [Google Scholar]
- Sheppard A, Hayes SH, Chen GD, Ralli M, Salvi R (2014) Review of salicylate-induced hearing loss, neurotoxicity, tinnitus and neuropathophysiology. Acta Otorhinolaryngol Ital 34:79–93. [PMC free article] [PubMed] [Google Scholar]
- Shore SE, Roberts LE, Langguth B (2016) Maladaptive plasticity in tinnitus--triggers, mechanisms and treatment. Nat Rev Neurol 12:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, Koehler S, Oldakowski M, Hughes LF, Syed S (2008) Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci 27:155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W (1999) Neuronal synchrony: a versatile code for the definition of relations? Neuron 24:49–65, 111–125. [DOI] [PubMed] [Google Scholar]
- Singla S, Dempsey C, Warren R, Enikolopov AG, Sawtell NB (2017) A cerebellum-like circuit in the auditory system cancels responses to self-generated sounds. Nat Neurosci 20:943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler SE, Palmer AR, Winter IM (1996) Temporal and mean rate discharge patterns of single units in the dorsal cochlear nucleus of the anesthetized guinea pig. J Neurophysiol 76:1667–1688. [DOI] [PubMed] [Google Scholar]
- Stefanescu RA, Shore SE (2015) NMDA Receptors Mediate Stimulus-Timing-Dependent Plasticity and Neural Synchrony in the Dorsal Cochlear Nucleus. Frontiers in neural circuits 9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanescu RA, Koehler SD, Shore SE (2015) Stimulus-timing-dependent modifications of rate-level functions in animals with and without tinnitus. J Neurophysiol 113:956–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzberg D, Salvi RJ, Allman BL (2012) Salicylate toxicity model of tinnitus. Front Syst Neurosci 6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzberg D, Hayes SH, Kashanian N, Radziwon K, Salvi RJ, Allman BL (2013) A novel behavioral assay for the assessment of acute tinnitus in rats optimized for simultaneous recording of oscillatory neural activity. J Neurosci Methods 219:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland DP, Masterton RB, Glendenning KK (1998a) Role of acoustic striae in hearing: reflexive responses to elevated sound-sources. Behavioural brain research 97:1–12. [DOI] [PubMed] [Google Scholar]
- Sutherland DP, Glendenning KK, Masterton RB (1998b) Role of acoustic striae in hearing: discrimination of sound-source elevation. Hear Res 120:86–108. [DOI] [PubMed] [Google Scholar]
- Tan J, Ruttiger L, Panford-Walsh R, Singer W, Schulze H, Kilian SB, Hadjab S, Zimmermann U, Kopschall I, Rohbock K, Knipper M (2007) Tinnitus behavior and hearing function correlate with the reciprocal expression patterns of BDNF and Arg3.1/arc in auditory neurons following acoustic trauma. Neuroscience 145:715–726. [DOI] [PubMed] [Google Scholar]
- Tao HW, Zhang LI, Engert F, Poo M (2001) Emergence of input specificity of ltp during development of retinotectal connections in vivo. Neuron 31:569–580. [DOI] [PubMed] [Google Scholar]
- Turner JG, Parrish J (2008) Gap detection methods for assessing salicylate-induced tinnitus and hyperacusis in rats. American journal of audiology 17:S185–192. [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM (2006) Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci 120:188–195. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO (2004) Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci 7:719–725. [DOI] [PubMed] [Google Scholar]
- Voigt HF, Young ED (1990) Cross-correlation analysis of inhibitory interactions in dorsal cochlear nucleus. J Neurophysiol 64:1590–1610. [DOI] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM (2009) Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience 164:747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Ding D, Sun W, Xu-Friedman MA, Salvi R (2010) Effects of sodium salicylate on spontaneous and evoked spike rate in the dorsal cochlear nucleus. Hear Res 267:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Martel DT, Shore SE (2015) Transcutaneous induction of stimulus-timing-dependent plasticity in dorsal cochlear nucleus. Front Syst Neurosci [DOI] [PMC free article] [PubMed]
- Wu C, Martel DT, Shore SE (2016) Increased Synchrony and Bursting of Dorsal Cochlear Nucleus Fusiform Cells Correlate with Tinnitus. J Neurosci 36:2068–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W (2007) Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res 226:244–253. [DOI] [PubMed] [Google Scholar]
- Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S (2011) Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci U S A 108:14974–14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Nannapaneni N, Zhou J, Hughes LF, Shore S (2009) Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and nonauditory inputs to the cochlear nucleus. J Neurosci 29:4210–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Shore S (2004) Projections from the trigeminal nuclear complex to the cochlear nuclei: a retrograde and anterograde tracing study in the guinea pig. J Neurosci Res 78:901–907. [DOI] [PubMed] [Google Scholar]


