Abstract
Background
We aimed to screen a specific secretory protein that could serve as blood diagnostic marker for cholangiocarcinoma (CCA).
Methods
Starting with the analysis of gene expression profiles in tumor tissues and matched normal tissues from cases with CCA and hepatocellular carcinoma (HCC), we identified peptidase inhibitor 15 (PI15) was a potential diagnostic marker for CCA. We demonstrated PI15 expression levels in CCA, HCC, and normal liver tissues. Furthermore, quantitative enzyme-linked immunosorbent assay (ELISA) assessed plasma PI15 levels in CCA (n = 61), HCC (n = 72), benign liver disease (n = 28), chronic hepatitis B (CHB) patients (n = 45), and healthy individuals (n = 45). The diagnostic value of PI15 was estimated by the area under the receiver operating characteristic (ROC) curve (AUC).
Findings
The positive rate of PI15 expression was 70% in CCA and only 9.1% in HCC; PI15 was not detected in normal liver tissue. High levels of plasma PI15 were evident in CCA patients, whereas only low levels were observed in cases involving HCC, benign liver disease, CHB patients, and healthy individuals. Plasma PI15 levels in CCA patients were obviously reduced (p = .0014) after surgery. The AUC of plasma PI15 for discriminating between CCA and HCC was 0.735. Furthermore, with a specificity of 94.44%, the combination of CA19–9 (>98.5 U/ml) and PI15 (>13 ng/ml) yielded a sensitivity of 80.39% for CCA and HCC.
Interpretation
PI15 exhibits promise as a novel marker for predicting the diagnosis and follow-up of CCA patients.
Fund
Natural Science Research Foundation of Anhui Province and Natural Science Foundation of China
Keywords: Cholangiocarcinoma, PI15, Biomarker, Blood diagnosis
Research in context.
Evidence before this study
The incidence and mortality of cholangiocarcinoma, a primary hepatic malignancy, are rising in the world. The absence of accurate diagnostic marker restricts early detection and treatment choices. Elevated levels of serum carbohydrate antigen 19–9, a widely used biomarker for cholangiocarcinoma, are also known to occur with other forms of tumors and benign liver disease, which restricts the sensitivity and specificity of diagnosis. We found the expression of peptidase inhibitor 15 (PI15), a secretory trypsin inhibitor, was significantly upregulated in cholangiocarcinoma by analyzing the microarray and TCGA database. Previous study indicated that PI15 could not be detected in a range of healthy human tissues (heart, brain, placenta, lung, liver, skeletal muscle, kidney, and pancreas), suggesting it may be a potential diagnostic marker for cholangiocarcinoma with high specificity.
Added value of this study
We demonstrated the PI15 was highly expressed in cholangiocarcinoma tumor tissues, and could not be detected in normal liver tissues. We detected high levels of plasma PI15 in cholangiocarcinoma patients, but low levels in patients with hepatocellular carcinoma, benign liver disease, chronic hepatitis B patients, and healthy individuals. Moreover, plasma PI15 levels in cholangiocarcinoma patients were obviously reduced after surgery. Altogether, PI15 holds potential diagnostic and follow-up value for patients with cholangiocarcinoma.
Implications of all the available evidence
This study suggests plasma PI15 holds significant value for predicting diagnosis for cholangiocarcinoma patients, and the combination of PI15 and carbohydrate antigen 19–9 improves diagnostic performance for cholangiocarcinoma.
Alt-text: Unlabelled Box
1. Introduction
Cholangiocarcinoma (CCA) is the second most common liver cancer (10%–15%) and is associated with high levels of invasiveness and a poor prognosis [1]. In recent years, the incidence of CCA has been increasing worldwide [2,3]. Unfortunately, CCA is frequently diagnosed at an advanced stage, which restricts the treatment options to only radical surgery or liver transplantation [4,5]. Serum carbohydrate antigen 19–9 (CA19–9) is the most widely used biomarker for CCA [6]. However, 10% of the general population are negative for Lewis-antigen, meaning that CA19–9 levels are undetectable in the serum; furthermore, elevated levels of serum CA19–9 are also known to occur with other forms of tumors and benign liver disease [7,8]. Therefore, a sensitive and specific biomarker is urgently required to facilitate the detection of CCA.
Secretory proteins may serve as diagnostic markers for a variety of tumors [[9], [10], [11], [12], [13]]. Peptidase inhibitor 15 (PI15), a secretory trypsin inhibitor, was originally identified and purified from the serum-free conditioned medium of human glioblastoma T98G cells as a novel 25-kDa trypsin-binding protein [14]. PI15 belongs to the cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins (CAP) superfamily. CAP superfamily proteins are frequently secreted with an extracellular endocrine or paracrine function [15]. Northern blotting analysis previously indicated that PI15 could not be detected in a range of healthy human tissues (heart, brain, placenta, lung, liver, skeletal muscle, kidney, and pancreas), and could only be found in glioblastoma and neuroblastoma cell lines among multiple cancer cell lines (5 glioblastoma lines, 7 neuroblastoma lines, 8 gastric carcinoma lines, 6 squamous cell carcinoma lines, 5 hepatocellular carcinoma lines, 2 bladder carcinoma lines, and 1 fibrosarcoma line) [16].
In the present study, we demonstrated that PI15, a secretory trypsin inhibitor, was highly expressed in CCA tumor tissue compared to matched normal tissue. In addition, plasma PI15 levels in CCA patients were higher than that in patients with hepatocellular carcinoma (HCC), benign liver disease, chronic hepatitis B (CHB), and healthy individuals, thus indicating its potential diagnostic value for CCA. Receiver operating characteristic (ROC) curve analysis suggested that PI15 had a high diagnostic value for CCA, especially for iCCA patients, an important subtype of CCA. Furthermore, the combination of plasma PI15 and serum CA19–9 improved diagnostic performance. Additionally, plasma PI15 level was significantly reduced after surgery in CCA patients, which further illustrated that the origin of the elevated plasma PI15 concentration was the CCA tumor. Collectively, these results suggest that PI15 is a potential diagnostic and follow-up biomarker for CCA patients.
2. Materials and methods
2.1. Patient samples
Fresh samples of CCA patients (n = 67), HCC patients (n = 83), benign liver disease patients (n = 33; 13 as hepatic hemangioma; 20 as intrahepatic stones), CHB patients (n = 45), and healthy individuals (n = 45) were collected from the First Affiliated Hospital of Anhui Medical University. The pre- and postoperative plasma and serum of CCA, HCC, and benign liver disease patients were included. In addition, fresh normal liver tissues, tumor tissues, and matched normal tissues were collected. Healthy controls were matched to the CCA patients by age. CCA and HCC patients were diagnosed as primary cases by histological and clinical examination. Plasma and tissue samples were stored at −80 °C until they were used. HCC and CCA patients did not receive radiotherapy, chemotherapy or targeted therapy prior to surgery. The clinical characteristics of CCA, HCC, benign liver disease, CHB, and healthy individuals are shown in Supplementary Tables S1–4, respectively.
This research was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (Quick-PJ 2018-07-22), and all patients provided signed informed consent for the use of their samples for biomedical research.
2.2. Gene expression profile assay
The gene expression profiles of tumor tissues and matched normal tissues were analyzed using a whole human genome oligo microarray (G4112F; Agilent). Agilent's Feature-Extraction software (version 9.1.3; Agilent Technologies) was used for microarray image analysis. The gene expression values were log2-transformed, and the following analysis was performed using online SAS statistical software (http://sas.ebioservice.com/). Cluster 3.0 (Complete Linkage Clustering) was used to accomplish hierarchical clustering. Heat maps and green-red scale schemes were constructed using MultiExperiment Viewer (MEV). The microarray data were deposited into the National Center for Biotechnology Information Gene Expression Omnibus (GEO) repository under accession number GSE117361.
2.3. Analysis of The Cancer Genome Atlas (TCGA) datasets
RNA-seq data from multiple tumors was obtained from The Cancer Genome Atlas (TCGA, http://cancergenome.nih.gov/), including data for cholangiocarcinoma (CHOL/CCA, 36 cancer and 9 normal), liver hepatocellular carcinoma (LIHC/HCC, 374 cancer and 50 normal), pancreatic adenocarcinoma (PAAD, 178 cancer and 4 normal), stomach adenocarcinoma (STAD, 375 cancer and 32 normal), colon adenocarcinoma (COAD, 480 cancer and 41 normal), rectum adenocarcinoma (READ, 167 cancer and 10 normal), lung squamous cell carcinoma (LUSC, 502 cancer and 49 normal), lung adenocarcinoma (LUAD, 535 cancer and 59 normal), kidney renal papillary cell carcinoma (KIRP, 289 cancer and 32 normal), kidney renal clear cell carcinoma (KIRC, 539 cancer and 72 normal), kidney chromophobe (KICH, 65 cancer and 24 normal), breast invasive carcinoma (BRCA, 312 cancer and 36 normal), and prostate adenocarcinoma (PRAD, 499 cancer and 52 normal). The analysis of differentially expressed genes (DEGs) between tumor tissue and normal tissue was conducted using the Edger package in R [17]. The criteria for defining DEGs was as follows: false discovery rate (FDR) < 0.05 and |log2(FC)| > 1, where FC represents the fold change.
2.4. Quantitative polymerase chain reaction
Total RNA was isolated from tissue specimens using TRIzol (Invitrogen), and reverse transcribed into cDNA with Moloney Murine Leukemia Virus (M-MLV; Invitrogen). Quantitative polymerase chain reaction (qPCR) analysis was then performed on a Roche LightCycler 96 using SYBR premix Ex Tap II (Takara). The data analysis involved the ΔΔCt method. All primers were synthesized by Sangon (Shanghai, China). The tumor markers were AFP, CEA, CA125, PSA, and GH, and the corresponding genes were AFP, CEACAM5, MUC16, KLK3, and GH1, respectively. We designed two PI15 primers for PCR, which were designated PI15–1 and PI15–2, respectively. Supplementary Table S5 shows detailed information relating to the PCR primers used for PI15, AFP, CEACAM5 [18], MUC16, KLK3 [19], and GH1 [20].
2.5. Enzyme-linked immunosorbent assay
Plasma PI15 level was measured using a Human-PI15 ELISA kit (QY-E01315; China) in accordance with the manufacturer's instructions. We first prepared the reagents, samples, and standards. We then incubated each prepared sample and standard with HRP-Conjugate Reagent for 60 min at 37 °C. Each plate was then washed five times, chromogen solution A and B were added, and the mixture was incubated for 5 min at room temperature away from light. Finally, the stop solution was added and the optical density (OD) at 450 nm was measured within 15 min. A standard curve linear regression equation was then estimated based on standard concentrations and the corresponding OD values. The OD value for each sample was then added into the regression equation to calculate the sample's concentration. Each sample was analyzed in duplicate.
2.6. Electrochemiluminescence
The concentration of serum carbohydrate antigen 19–9 (CA19–9) was measured using Electrochemical luminescence kit (Roche; Switzerland) in accordance with the manufacturer's instructions. The Roche Cobas e601 was used to analyze the detection data.
2.7. Statistical analysis
Data were summarized and represented as mean ± standard error of the mean (SEM). Statistical analysis was conducted using SPSS (version 22.0) and GraphPad Prism (version 6.0) software programs. The student's t-test and paired t-test were used to analyze the statistical significance between independent groups and paired data, respectively. ROC curve analysis was used to evaluate the diagnostic value of the different markers. The area under the ROC curve (AUC) was used to assess the accuracy of each marker. Univariate and multivariate logistic regression models were used to consider the diagnostic value of PI15 alone and PI15 combined with CA19–9 [21]. P < .05 was considered statistically significant.
3. Results
3.1. Identification of a potential marker for CCA
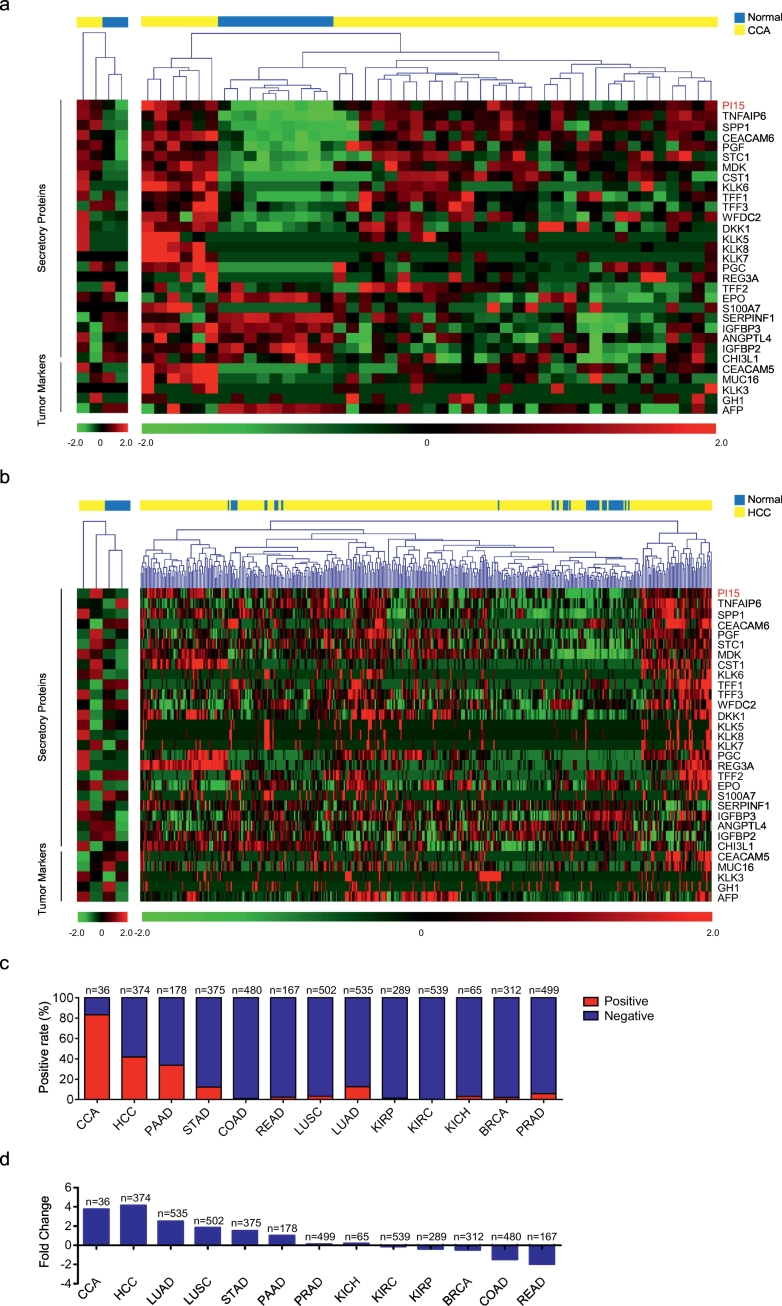
In order to identify a potential diagnostic marker for CCA, we analyzed the gene expression profiles of CCA and HCC tissue samples by gene expression profile assays. The detection of secretory protein biomarkers in plasma is a non-invasive diagnostic method, which is imperative in the clinic due to the need for duplicate tests and low costs. Thus, we screened a range of secretory proteins and found that PI15, a secretory trypsin inhibitor, was overexpressed in CCA tumor tissues compared to matched normal tissues (Fig. 1a, Left). To further demonstrate our finding, we analyzed CCA (CHOL, n = 36) mRNA expression data accessed by RNA-seq from TCGA using Edger analysis to identify differentially expressed genes (DEGs). We found that PI15 expression was elevated by 3.8-fold in CCA tumor tissues relative to normal tissues, which was consistent with our initial finding.
Fig. 1.
Discovery of a candidate marker for CCA.
(a) Gene expression profile assay of CCA tumor tissues and matched normal tissues for screening candidate diagnostic markers. Heat map shown differentially expressed secretory proteins and tumor markers in CCA. The left heat map was based on the gene expression profile of CCA tumor tissues and matched normal tissues. The right heat map was based on CCA (CHOL, n = 36) RNA-seq data from the TCGA database. (b) Gene expression profile assay of HCC tumor tissues and matched normal tissues. Heat map shown selected secretory proteins and tumor markers in HCC. The left heat map was based on microarray data of HCC tumor tissues and matched normal tissues. The right heat map was based on HCC (LIHC, n = 374) RNA-seq data from the TCGA database. Each column depicts an individual sample. Blue squares represent normal tissues, yellow squares represent tumor tissues. RNA-seq data were normalized with MultiExperiment Viewer. (c) The positive rate of PI15 expression in various tumors. (d) The fold change of PI15 expression in various tumors. CCA (CHOL), cholangiocarcinoma (n = 36); HCC (LIHC), hepatocellular carcinoma (n = 374); PAAD, pancreatic adenocarcinoma (n = 178); STAD, stomach adenocarcinoma (n = 375); COAD, colon adenocarcinoma (n = 480); READ, rectum adenocarcinoma (n = 167); LUSC, lung squamous cell carcinoma (n = 502); LUAD, lung adenocarcinoma (n = 535); KIRP, kidney renal papillary cell carcinoma (n = 289); KIRC, kidney renal clear cell carcinoma (n = 539); KICH, kidney chromophobe (n = 65); BRCA, breast invasive carcinoma (n = 312); and PRAD, prostate adenocarcinoma (n = 499). Tumor samples are from the TCGA database. Red columns indicate positive expression of PI15, blue columns indicate negative expression of PI15.
To further illustrate whether PI15 possessed potential as a diagnostic marker for CCA, we analyzed the sensitivity and specificity of the expression of PI15 and other tumor markers in CCA. We selected tumor markers used in the clinic, consisting of AFP, CEA, CA125, GH, and PSA [[22], [23], [24], [25], [26]]. The corresponding genes were AFP, CEACAM5, MUC16, GH1, and KLK3, respectively. We characterized the expression of PI15, other secretory proteins, and tumor markers in CCA (Fig. 1a). We found that the sensitivity and specificity of PI15 expression was superior to those of other tumor markers. Furthermore, we performed the same analysis in HCC samples; we analyzed the expression of PI15, other secretory proteins, and 5 tumor markers in HCC samples (Fig. 1b), and found that PI15 was upregulated in a fraction of HCC. Thus, PI15 might be used as a novel diagnostic marker for CCA, with better sensitivity and specificity than other markers.
After the analysis of PI15 expression in cases with CCA and HCC, we focused on the positive rate and fold change of PI15 expression in various human tumors (Fig. 1c and d). The highest expression of PI15 in normal tissue was regarded as the upper limit of normal expression. When PI15 expression exceeded this upper value in tumor tissue, we defined such cases as positive-expression. The positive rate and fold change analysis were performed in various human tumors, accessed by RNA-seq deposited in TCGA. Consequently, we observed that the positive rate of PI15 expression was the highest (83.3%) in CCA (CHOL, n = 36) among various tumors (Fig. 1c), and that the fold change of PI15 was 3.8 in CCA (CHOL, n = 36) and 4.1 in HCC (LIHC, n = 374), which were higher than that in other tumors (Fig. 1d). Therefore, PI15 expression had higher specificity in CCA, indicating utility as a diagnostic marker.
3.2. Increased expression of the secretory protein PI15 in CCA patients
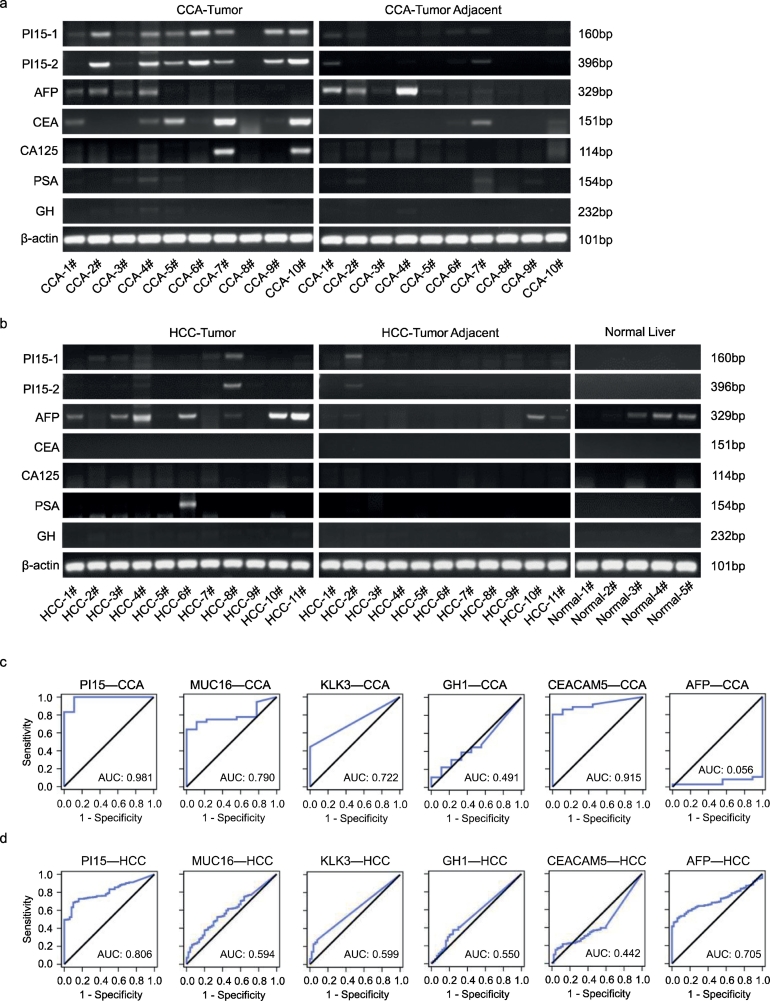
To further investigate the sensitivity and specificity of PI15 expression in CCA, we measured the expression of PI15 and tumor markers (AFP, CEA, CA125, PSA, and GH) in 10 pairs of CCA tumor tissues and matched tumor adjacent normal tissues, 11 pairs of HCC tumor tissues and matched tumor adjacent normal tissues, and 5 normal liver tissues. As shown in Fig. 2a and b, the positive rate of PI15 expression was higher in CCA cases (70%, 7/10) than in HCC cases (9.1%, 1/11), and PI15 could not be detected in tumor adjacent normal tissues and normal liver tissues. Additionally, the positive rates of tumor markers expression in CCA were lower than that of PI15, including for AFP (40%, 4/10), CEA (50%, 5/10), and CA125 (20%, 2/10), GH and PSA could not be detected in CCA. Therefore, the sensitivity and specificity of PI15 expression was higher than those of other tumor markers in CCA. Moreover, we evaluated the relative expression levels of PI15 and tumor markers by qPCR in CCA and HCC, and found that only PI15 expression was higher in CCA tumor tissues compared with normal tissues (p < .05, paired t-test), but this was not the case in HCC, and no significant differences of other tumor markers expression were observed in CCA and HCC (Supplementary Fig. 1a and b).
Fig. 2.
Expression of secretory protein PI15 was significantly upregulated in CCA.
(a) The expression of PI15 and tumor markers (AFP, CEA, CA125, PSA, and GH) were determined by reverse transcription PCR (RT-PCR) in CCA tumor tissues (n = 10) and matched normal tissues (n = 10). Each band represents a different patient sample. (b) The expression of PI15 and tumor markers (AFP, CEA, CA125, PSA, and GH) were determined by RT-PCR in normal liver tissues (n = 5), HCC tumor tissues (n = 11), and matched normal tissues (n = 11). Each band represents a different patient sample. (c-d) ROC curve analysis of the expression of PI15 and tumor markers (assessed by RNA-seq) for discriminating tumor tissue from normal tissue in CCA (CHOL, n = 36) and HCC (LIHC, n = 374). The genes that encode the tumor markers (AFP, CEA, CA125, PSA, and GH) were AFP, CEACAM5, MUC16, KLK3, and GH1, respectively. PI15–1 and PI15–2 were the two primers used to amplify PI15. CCA/CHOL, cholangiocarcinoma; HCC/LIHC, hepatocellular carcinoma; Tumor adjacent, matched normal tissue; Normal liver, normal liver tissue.
Next, we focused on the positive rate of PI15 and tumor marker expression in CCA (CHOL, n = 36) and HCC (LIHC, n = 374) (Supplementary Fig. 1c), and observed that the positive rates of PI15 and tumor markers in CCA (CHOL, n = 36) were 83.3% (PI15), 69.4% (MUC16), 69.4% (CEACAM5), 44.4% (KLK3), 11.1% (GH1), and 2.8% (AFP). In HCC (LIHC, n = 374), the positive rates were 41.7% (PI15), 36.4% (AFP), 5.4% (MUC16), 6.7% (KLK3), 1.3% (GH1), and 0.5% (CEACAM5). Thus, PI15 expression in CCA showed a higher positive rate than other tumor markers, and the positive rate of PI15 expression was much higher in CCA than in HCC.
ROC curve analysis was then conducted to determine the diagnostic value of PI15 and tumor markers at the mRNA level in CCA (CHOL, n = 36) and HCC (LIHC, n = 374). We found that PI15 showed an AUC of 0.981 (95% confidence interval [CI], 0.943 to 1.000) for discriminating CCA tumor tissue from normal tissue, which was superior to the diagnostic performance of other tumor markers (Fig. 2c). In addition, PI15 exhibited an AUC of 0.806 (95% CI, 0.760 to 0.853) for discriminating HCC tumor tissue from normal tissue, which was higher than AFP with an AUC of 0.705 (95% CI, 0.653 to 0.758) (Fig. 2d). Thus, PI15 expression was significantly upregulated in CCA, and the diagnostic sensitivity and specificity of PI15 at the mRNA level were superior to those for other tumor markers in CCA.
3.3. PI15 as a potential diagnostic blood marker for CCA patients
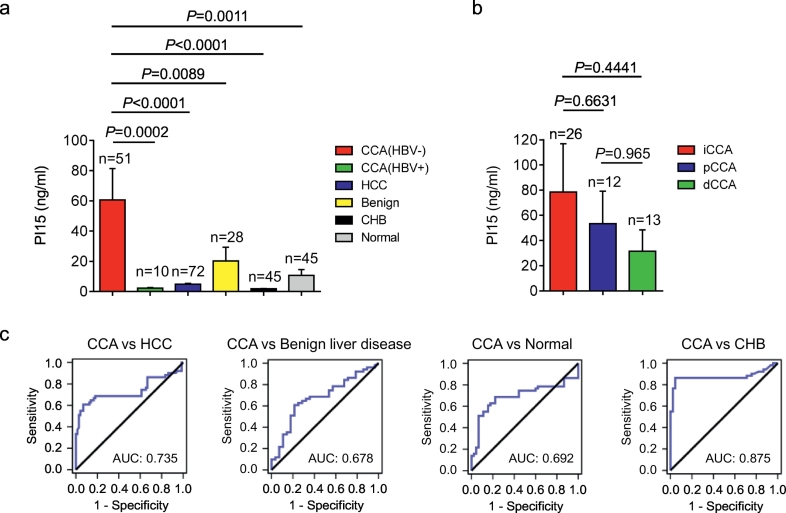
To determine the potential diagnostic value of PI15 in CCA, we examined the plasma PI15 level in CCA patients (n = 61), HCC patients (n = 72), benign liver disease patients (n = 28), CHB patients (n = 45), and healthy individuals (n = 45) using a quantitative ELISA assay. Consequently, we found that the plasma PI15 concentration was upregulated in CCA patients (Fig. 3a). Specifically, the plasma PI15 concentration was significantly increased in HBV negative CCA patients (60.64 ± 20.78 ng/ml) but not in HBV positive CCA patients (2.34 ± 0.39 ng/ml) (Fig. 3a). In addition, the plasma PI15 mean concentration was only 4.91 ± 0.50 ng/ml in HCC patients, which was significantly lower than in CCA patients (p < .0001, unpaired t-test), and the plasma PI15 mean concentration was 20.26 ± 9.13 ng/ml in benign liver disease patients, 10.81 ± 3.84 ng/ml in healthy individuals, and 1.83 ± 0.24 ng/ml in CHB patients (Fig. 3a).
Fig. 3.
PI15 as a potential diagnostic marker for CCA.
(a) Plasma PI15 levels were measured by quantitative ELISA in CCA (HBV-) patients (n = 51), CCA (HBV+) patients (n = 10), HCC patients (n = 72), benign liver disease patients (n = 28), CHB patients (n = 45), and healthy individuals (n = 45). (b) Plasma PI15 levels in iCCA (n = 26), pCCA (n = 12), and dCCA (n = 13) patients. (c) ROC curves for PI15 levels in plasma samples from patients with CCA (HBV-) patients (n = 51) versus HCC patients (n = 72), benign liver disease patients (n = 28), CHB patients (n = 45), and healthy individuals (n = 45). HBV-, HBV negative; HBV+, HBV positive; Benign, benign liver disease; CHB, chronic hepatitis B; Normal, healthy individuals; iCCA, intrahepatic cholangiocarcinoma; pCCA, perihilar cholangiocarcinoma; dCCA, distal cholangiocarcinoma. Unpaired t-test; Data are presented as mean ± SEM.
We further compared the plasma PI15 level in different CCA subtypes categorized by anatomical location as intrahepatic cholangiocarcinoma (iCCA), perihilar cholangiocarcinoma (pCCA), and distal cholangiocarcinoma (dCCA). The mean plasma PI15 concentration was 78.5 ng/ml, 53.48 ng/ml, and 31.51 ng/ml in the iCCA (n = 26), pCCA (n = 12), and dCCA (n = 13) patients, respectively (Fig. 3b). Thus, the plasma PI15 level in iCCA patients was higher than in pCCA (p = .6631, unpaired t-test) patients and dCCA patients (p = .4441, unpaired t-test) (Fig. 3b).
ROC curve analysis was performed to further illustrate the diagnostic value of plasma PI15 for CCA patients. PI15 exhibited an AUC of 0.735 (95% CI, 0.632 to 0.838) for CCA samples compared to HCC controls (Fig. 3c; Table 1). Additionally, the AUC of PI15 was 0.678, 0.692, and 0.875 for discriminating CCA patients from benign liver disease patients, healthy individuals, and CHB patients, respectively (Fig. 3c; Table 1). In conclusion, plasma PI15 was able to discriminate effectively between CCA cases and other controls, suggesting great potential as a diagnostic marker.
Table 1.
AUC calculations of ROC analysis for patients with CCA and iCCA versus HCC, benign liver disease, CHB, and healthy individuals.
| PI15 |
CA19–9 |
PI15 + CA19–9 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | AUC | 95% CI | AUC | 95% CI | AUC | 95% CI | ||||
| CCA versus Controls | ||||||||||
| CCA versus HCC | 51/72 | 0.735 | 0.632 | 0.838 | 0.875 | 0.805 | 0.946 | 0.908 | 0.846 | 0.97 |
| CCA versus Benign | 51/28 | 0.678 | 0.555 | 0.8 | 0.737 | 0.624 | 0.85 | 0.75 | 0.642 | 0.858 |
| CCA versus CHB | 51/45 | 0.875 | 0.793 | 0.957 | 0.888 | 0.813 | 0.964 | 0.962 | 0.915 | 1.000 |
| CCA versus Normal | 51/45 | 0.692 | 0.58 | 0.804 | 0.881 | 0.803 | 0.96 | 0.878 | 0.799 | 0.958 |
| iCCA versus Controls | ||||||||||
| iCCA versus HCC | 26/72 | 0.75 | 0.614 | 0.886 | 0.849 | 0.735 | 0.963 | 0.921 | 0.841 | 1.000 |
| iCCA versus Benign | 26/28 | 0.699 | 0.558 | 0.84 | 0.712 | 0.573 | 0.85 | 0.761 | 0.634 | 0.888 |
| iCCA versus CHB | 26/45 | 0.899 | 0.8 | 0.998 | 0.855 | 0.729 | 0.98 | 0.963 | 0.897 | 1.000 |
| iCCA versus Normal | 26/45 | 0.709 | 0.564 | 0.855 | 0.854 | 0.728 | 0.98 | 0.85 | 0.724 | 0.977 |
3.4. 3.4 Use of the PI15/CA19–9 marker panel improved diagnostic performance for CCA
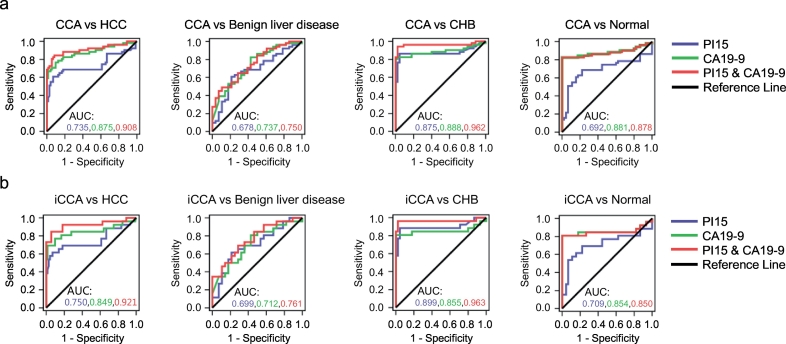
To investigate whether a combination of plasma PI15 and serum CA19–9 could constitute a combined diagnostic panel with higher discriminatory ability than each alone, we performed logistic regression to evaluate the diagnostic capacity of the combination of PI15 and CA19–9. A combination of PI15 and CA19–9 for CCA cases versus HCC controls yielded an AUC of 0.908 (95% CI, 0.846 to 0.97), outperforming either of the markers alone (Fig. 4a; Table 1). In addition, the AUC of the two-marker panel discriminating CCA from benign liver disease was 0.750 (95% CI, 0.642 to 0.858), which was higher than 0.678 (PI15 alone) and 0.737 (CA19–9 alone) (Fig. 4a; Table 1). The PI15/CA19–9 panel was able to discriminate CCA cases versus CHB patients with an AUC of 0.962 (Fig. 4a; Table 1). Moreover, the PI15/CA19–9 panel for CCA versus healthy individuals exhibited an AUC of 0.878, which was close to 0.881, the AUC of CA19–9 alone (Fig. 4a; Table 1). Thus, the PI15/CA19–9 panel was able to distinguish CCA from CHB patients and helped to distinguish CCA from HCC and benign liver disease compared to CA19–9 alone.
Fig. 4.
The combination of PI15 and CA19–9 improves diagnostic performance for CCA.
(a) ROC curves for PI15, CA19–9, and PI15 + CA19–9 levels in patients with CCA (HBV-) patients (n = 51) versus HCC patients (n = 72), benign liver disease patients (n = 28), CHB patients (n = 45), and healthy individuals (n = 45). (b) ROC curves for PI15, CA19–9, and PI15 + CA19–9 levels in patients with iCCA (HBV-) patients (n = 26) versus HCC patients (n = 72), benign liver disease patients (n = 28), CHB patients (n = 45), and healthy individuals (n = 45). HBV-, HBV negative; CHB, chronic hepatitis B; Normal, healthy individuals; iCCA, intrahepatic cholangiocarcinoma.
In the clinic, iCCA is usually diagnosed as a hepatic mass, frequently similar to the imaging performance of HCC with cirrhosis; thus, the differential diagnosis of HCC and iCCA can be difficult. In our present study, the plasma PI15 level in iCCA patients was highest among the different CCA subtypes tested, suggesting better diagnostic value for iCCA. Therefore, we further investigated the diagnostic performance of PI15 alone and the PI15/CA19–9 panel for iCCA. PI15 exhibited an AUC of 0.750 (95% CI, 0.614 to 0.886) for iCCA samples compared to HCC controls (Fig. 4b; Table 1). In the same sample set, CA19–9 had a comparable AUC of 0.849 for iCCA samples compared to HCC controls (Fig. 4b; Table 1). Furthermore, the PI15/CA19–9 panel displayed an AUC of 0.921 (95% CI, 0.841 to 1.000), indicating the superiority of the two-marker panel (Fig. 4b; Table 1). When considering iCCA samples versus benign liver disease, the AUC including CA19–9 increased from 0.712 (alone) to 0.761 (with PI15) (Fig. 4b; Table 1). The PI15/CA19–9 panel yielded the AUC of 0.963 for discriminating iCCA samples versus CHB controls (Fig. 4b; Table 1). For healthy individuals, the combination of PI15 and CA19–9 was not able to increase the ability to discriminate between iCCA samples and healthy individuals (Fig. 4b; Table 1). In conclusion, our results indicated that the PI15/CA19–9 marker panel exhibited better performance in diagnosing iCCA patients.
3.5. Establishing a cutoff concentration for plasma PI15 in iCCA patients
In order to determine a plasma PI15 concentration which could act as a diagnostic cut off value with which to distinguish between iCCA and HCC. We firstly analyzed the concentration distribution of plasma PI15 in HCC patients to obtain cut-off values corresponding to the false-positive rates (FPRs) of 0%, 3%, and 5%. Subsequently, these cut off values were further analyzed and evaluated for their sensitivity and specificity in diagnosing iCCA patients. As seen in Table 2, plasma PI15 could detect approximately 57.7% of iCCA patients (sensitivity) with 94.4% specificity when the cut off value was set to 13 ng/ml. Furthermore, when we used CA19–9 > 98.5 U/ml, and a cut off value of 13 ng/ml for plasma PI15, the two-marker panel yielded 84.62% sensitivity and 94.44% specificity (Table 2).
Table 2.
PI15 concentration cut off values for iCCA and CCA based on percentiles of distribution in HCC plasma controls.
| Marker | Cutoff | iCCA versus HCC |
CCA versus HCC |
||
|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | ||
| CA19-9 (>98.5) | 69.20 | 98.60 | 66.70 | 98.60 | |
| PI15 (ng/ml) | |||||
| 95% | 11 | 57.70 | 93.10 | 54.90 | 93.10 |
| 97% | 13 | 57.70 | 94.40 | 54.90 | 95.80 |
| 100% | 25.2 | 38.50 | 98.60 | 33.30 | 98.60 |
| CA19-9 (>98.5) and PI15 (ng/ml) | |||||
| 95% | 11 | 84.62 | 93.06 | 80.39 | 93.06 |
| 97% | 13 | 84.62 | 94.44 | 80.39 | 94.44 |
| 100% | 25.2 | 80.77 | 98.61 | 74.51 | 98.61 |
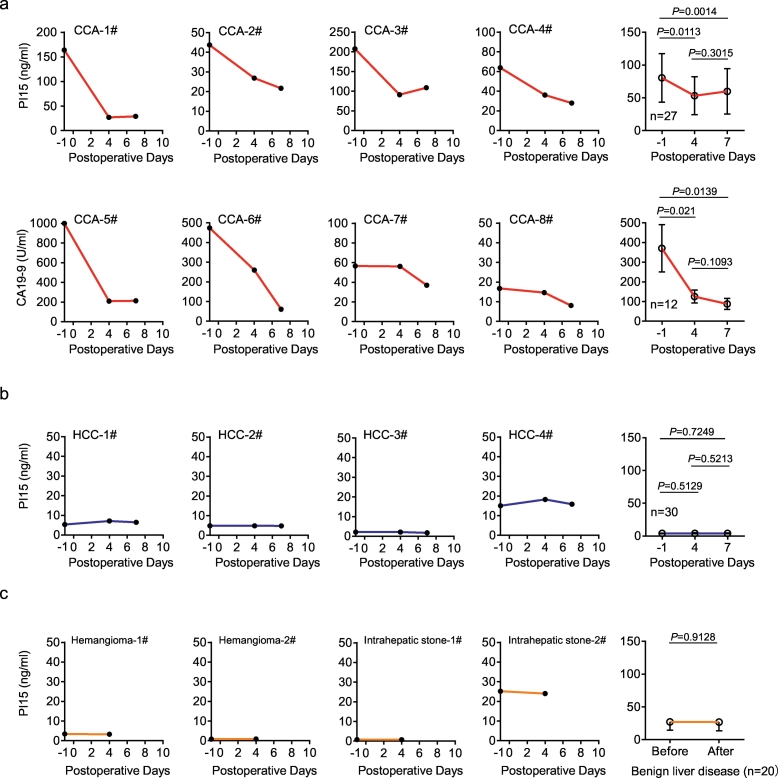
3.6. Evaluation of the postoperative recovery of CCA patients using plasma PI15 level
Due to our results suggested that plasma PI15 could be used as a diagnostic marker for CCA patients, we further investigated whether plasma PI15 level could evaluate the postoperative recovery of CCA patients. We measured the pre- and postoperative plasma PI15 levels in CCA patients (n = 27), HCC patients (n = 30), and benign liver disease patients (n = 20); Meanwhile, we also detected the pre- and postoperative serum CA19–9 levels in CCA patients (n = 12), all of patients underwent curative hepatectomy. We observed that PI15 and CA19–9 were significantly reduced after surgery in CCA patients (Fig. 5a). The pre-operative mean plasma PI15 concentration in CCA patients reached 80.42 ng/ml. However, on the 4th and 7th days after surgery, the plasma PI15 mean concentration decreased to 53.17 ng/ml and 59.75 ng/ml, respectively (Fig. 5a). In the pre-operative plasma of HCC patients, the mean PI15 concentration was only 3.98 ng/ml, and the mean concentration was 4.12 ng/ml and 4.05 ng/ml on the postoperative 4th and 7th days, respectively (Fig. 5b). For benign liver disease patients, the mean pre- and postoperative mean plasma PI15 concentrations were 27.02 ng/ml and 26.79 ng/ml, respectively (Fig. 5c). Thus, plasma PI15 level was obviously reduced after surgery in CCA patients (Fig. 5a), whereas there was no significant change in HCC and benign liver disease patients (Fig. 5b and c). The dynamic change of plasma PI15 level in CCA patients confirmed that the elevated plasma PI15 concentration originated from the CCA tumor, demonstrating outstanding potential diagnostic and follow-up value for CCA patients. Consequently, the detection of plasma PI15 was able to evaluate the outcomes of surgical treatment, and could be used as potential follow-up marker for CCA patients after surgery.
Fig. 5.
Determination of plasma PI15 level for the prediction of postoperative recovery in CCA patients.
(a) The levels of plasma PI15 and serum CA19–9 in preoperative (day “-1”) and postoperative (days “4” and “7”) CCA patients (n = 27) were measured by quantitative ELISA and electrochemiluminescence respectively. The PI15 and CA19–9 concentration of representative CCA cases were shown. (b) Plasma PI15 levels in preoperative (day “-1”) and postoperative (days “4” and “7”) HCC patients (n = 30) were measured by quantitative ELISA. The PI15 concentration of representative HCC cases were shown. (c) Plasma PI15 levels in preoperative (day “-1”) and postoperative (days “4” and “7”) benign liver disease patients (n = 20) were measured by quantitative ELISA. The PI15 concentration of representative benign liver disease cases were shown. The abscissa of the coordinate axis is the number of days after surgery (postoperative days), and “-1” refers to the preoperative day. Before, before surgery; After, after surgery. Paired t-test; Data are presented as mean ± SEM.
4. Discussion
In this study, our present results indicated that PI15 was highly expressed in CCA tumor tissues, and could not be detected in normal liver tissues. PI15 was also increased in the plasma of CCA patients, demonstrating that PI15 represents a potential diagnostic marker for CCA. The differentially methylated CpG of PI15 was previously found to be a potential novel prognostic marker capable of distinguishing prostate cancer patients with metastatic-lethal tumors from nonrecurrent tumors [27]. The PI15 gene had also been identified as a candidate oncogene in colorectal cancer [28]. However, no previous study has investigated the expression of PI15 in CCA. Human PI15 is situated on chromosome 8q21.11, and is adjacent to Cysteine Rich Secretory Protein LCCL Domain Containing 1 (CRISPLD1), another mammalian CAP superfamily gene. Most of the CAP superfamily proteins are structurally conserved, which leads to members with a CAP domain exhibiting similar fundamental functions. CAP superfamily proteins are frequently secreted with an extracellular endocrine or paracrine function. N-terminal sequencing of PI15 derived from the serum-free conditioned medium of glioblastoma cells indicated that the predicted secretory signal peptide was active [15], suggesting that the secretory protein PI15 has the potential to be a marker for detection in the peripheral blood.
In our present study, plasma PI15 levels in CCA patients were significantly higher compared to patients with HCC, benign liver disease, CHB patients, or healthy individuals, indicating that PI15 could be regarded as a diagnostic marker for CCA patients. Importantly, the combination of PI15 and CA19–9 exhibited superior diagnostic performance.
Moreover, the PI15 concentration in postoperative plasma was significantly decreased compared with that in the preoperative plasma in CCA patients, thus confirming that the elevated plasma PI15 concentration was related to CCA tumor tissue, which may help us to judge whether tumor tissue has been completely removed. However, there was no significant change in the plasma PI15 levels of benign liver disease patients, ruling out surgery, health care interventions, and other factors as contributors to the change in plasma PI15 in CCA patients. Meanwhile, PI15 and CA19–9 showed same decreasing trendency after surgery in CCA patients, which further illustrated the potential diagnostic value of PI15. Thus, we propose that the plasma PI15 is able to evaluate the outcomes of surgical treatment. Further work needs to be performed to investigate how PI15 is released into the plasma of CCA patients.
Further research still needs to be performed in the future. Long-term follow-up data collection and multi-center study are necessary to further validate the diagnostic and follow-up value of PI15 for CCA. Moreover, we will determine plasma PI15 levels at different stages of CCA to further investigate the early diagnostic value, thereby potentially enhancing its value for clinical application. Additionally, the combination of plasma PI15 and serum CA19–9 improved the diagnostic performance for CCA. However, for serum CA19–9 positive patients with liver diseases including cholangitis and duct obstruction [29], or serum CA19–9 negative CCA patients, the diagnostic performance of plasma PI15 needs to be further explored.
In conclusion, PI15 has the potential to act as a novel blood diagnostic marker for CCA, and could also be used as an indicator to evaluate postoperative recovery in CCA patients. The combination of plasma PI15 and serum CA19–9 improves the diagnostic performance for CCA.
The following are the supplementary data related to this article.
Evaluation of PI15 and other tumor markers expression in CCA and HCC. (a-b) The relative expression level of PI15 and other tumor markers accessed by real-time qPCR in CCA and HCC; (c) The positive rate of PI15 and other tumor markers expression in CCA (CHOL, n = 36) and HCC (LIHC, n = 374). The genes that encode the tumor markers (AFP, CEA, CA125, PSA, and GH) were AFP, CEACAM5, MUC16, KLK3, and GH1, respectively. Red columns indicate positive expression of PI15, blue columns indicate negative expression of PI15. CCA/CHOL, cholangiocarcinoma; HCC/LIHC, hepatocellular carcinoma; Normal, normal tissue; Paired t-test; ns, nonsignificant; *, p < .05.
Supplementary Table 1-5
Funding sources
This work was supported by the Natural Science Research Foundation of Anhui Province (1508085MH173, KJ2015A137), and this work was also supported by the Natural Science Foundation of China (81602491).
Declaration of interests
The authors declare no potential conflict of interest.
Authors' contributions
Conception and design: Yong Jiang, Xiaohu Zheng, Yeben Qian, Haiming Wei.
Acquisition of data: Yong Jiang, Xiaohu Zheng, Defeng Jiao, Peng Chen.
Data analysis and interpretation: Yong Jiang, Xiaohu Zheng, Defeng Jiao, Yechuan Xu.
Manuscript writing: Yong Jiang, Xiaohu Zheng, Yeben Qian, Haiming Wei.
Final approval of manuscript: All authors.
Acknowledgements
We thank Mr. Wang Dongyao, Mr. Chen He and Ms. Ren Chunxia for their assistance in collecting the specimens involved in this study.
Contributor Information
Haiming Wei, Email: ustcwhm@ustc.edu.cn.
Yeben Qian, Email: qianyeben@hotmail.com.
References
- 1.Sia D., Villanueva A., Friedman S.L., Llovet J.M. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152(4):745–761. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bridgewater J., Galle P.R., Khan S.A. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Moeini A., Sia D., Bardeesy N., Mazzaferro V., Llovet J.M. Molecular pathogenesis and targeted therapies for intrahepatic cholangiocarcinoma. Cancer Res. 2016;22(2):291–300. doi: 10.1158/1078-0432.CCR-14-3296. Clinical cancer Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizvi S., Khan S.A., Hallemeier C.L., Kelley R.K., Gores G.J. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charatcharoenwitthaya P., Enders F.B., Halling K.C., Lindor K.D. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48(4):1106–1117. doi: 10.1002/hep.22441. [DOI] [PubMed] [Google Scholar]
- 6.Rizvi S., Gores G.J. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan S.A., Davidson B.R., Goldin R.D. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657–1669. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 8.Lempinen M., Isoniemi H., Makisalo H. Enhanced detection of cholangiocarcinoma with serum trypsinogen-2 in patients with severe bile duct strictures. J Hepatol. 2007;47(5):677–683. doi: 10.1016/j.jhep.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Bonney G.K., Craven R.A., Prasad R., Melcher A.F., Selby P.J., Banks R.E. Circulating markers of biliary malignancy: opportunities in proteomics? Lancet Oncol. 2008;9(2):149–158. doi: 10.1016/S1470-2045(08)70027-5. [DOI] [PubMed] [Google Scholar]
- 10.Egland K.A., Vincent J.J., Strausberg R., Lee B., Pastan I. Discovery of the breast cancer gene BASE using a molecular approach to enrich for genes encoding membrane and secreted proteins. Proc Natl Acad Sci U S A. 2003;100(3):1099–1104. doi: 10.1073/pnas.0337425100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikuta Y., Nakatsura T., Kageshita T. Highly sensitive detection of melanoma at an early stage based on the increased serum secreted protein acidic and rich in cysteine and glypican-3 levels. Clin Cancer Res. 2005;11(22):8079–8088. doi: 10.1158/1078-0432.CCR-05-1074. [DOI] [PubMed] [Google Scholar]
- 12.Welsh J.B., Sapinoso L.M., Kern S.G. Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci U S A. 2003;100(6):3410–3415. doi: 10.1073/pnas.0530278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawrenson K., Grun B., Lee N. NPPB is a novel candidate biomarker expressed by cancer-associated fibroblasts in epithelial ovarian cancer. Int J Cancer. 2015;136(6):1390–1401. doi: 10.1002/ijc.29092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koshikawa N., Nakamura T., Tsuchiya N. Purification and identification of a novel and four known serine proteinase inhibitors secreted by human glioblastoma cells. J Biochem. 1996;119(2):334–339. doi: 10.1093/oxfordjournals.jbchem.a021244. [DOI] [PubMed] [Google Scholar]
- 15.Gibbs G.M., Roelants K., O'Bryan M.K. The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins--roles in reproduction, cancer, and immune defense. Endocr Rev. 2008;29(7):865–897. doi: 10.1210/er.2008-0032. [DOI] [PubMed] [Google Scholar]
- 16.Yamakawa T., Miyata S., Ogawa N. cDNA cloning of a novel trypsin inhibitor with similarity to pathogenesis-related proteins, and its frequent expression in human brain cancer cells. Biochim Biophys Acta. 1998;1395(2):202–208. doi: 10.1016/s0167-4781(97)00149-8. [DOI] [PubMed] [Google Scholar]
- 17.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng M., Chen Y., Yu X., Tian Z., Wei H. Diagnostic utility of LunX mRNA in peripheral blood and pleural fluid in patients with primary non-small cell lung cancer. BMC Cancer. 2008;8:156. doi: 10.1186/1471-2407-8-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuessel S., Sickert D., Meye A. Multiple tumor marker analyses (PSA, hK2, PSCA, trp-p8) in primary prostate cancers using quantitative RT-PCR. Int J Oncol. 2003;23(1):221–228. [PubMed] [Google Scholar]
- 20.Kong X., Wu W., Yuan Y. Human growth hormone and human prolactin function as autocrine/paracrine promoters of progression of hepatocellular carcinoma. Oncotarget. 2016;7(20):29465–29479. doi: 10.18632/oncotarget.8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J., Bamlet W.R., Oberg A.L. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers. Sci Transl Med. 2017;9(398) doi: 10.1126/scitranslmed.aah5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Serag H.B., Kanwal F., Davila J.A., Kramer J., Richardson P. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology. 2014;146(5):1249–1255. doi: 10.1053/j.gastro.2014.01.045. [e1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lilja H., Ulmert D., Vickers A.J. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8(4):268–278. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 24.Li Y., Cao H., Jiao Z. Carcinoembryonic antigen interacts with TGF-{beta} receptor and inhibits TGF-{beta} signaling in colorectal cancers. Cancer Res. 2010;70(20):8159–8168. doi: 10.1158/0008-5472.CAN-10-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jelovac D., Armstrong D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;61(3):183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahlbusch R., Keller B., Ganslandt O., Kreutzer J., Nimsky C. Transsphenoidal surgery in acromegaly investigated by intraoperative high-field magnetic resonance imaging. Eur J Endocrinol. 2005;153(2):239–248. doi: 10.1530/eje.1.01970. [DOI] [PubMed] [Google Scholar]
- 27.Zhao S., Geybels M.S., Leonardson A. Epigenome-Wide Tumor DNA Methylation Profiling Identifies Novel Prognostic Biomarkers of Metastatic-Lethal Progression in Men Diagnosed with Clinically Localized Prostate Cancer. Clin Cancer Res. 2017;23(1):311–319. doi: 10.1158/1078-0432.CCR-16-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuupanen S., Hanninen U.A., Kondelin J. Identification of 33 candidate oncogenes by screening for base-specific mutations. Br J Cancer. 2014;111(8):1657–1662. doi: 10.1038/bjc.2014.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razumilava N., Gores G.J. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11(1):13–21. doi: 10.1016/j.cgh.2012.09.009. [e1; quiz e3–4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Evaluation of PI15 and other tumor markers expression in CCA and HCC. (a-b) The relative expression level of PI15 and other tumor markers accessed by real-time qPCR in CCA and HCC; (c) The positive rate of PI15 and other tumor markers expression in CCA (CHOL, n = 36) and HCC (LIHC, n = 374). The genes that encode the tumor markers (AFP, CEA, CA125, PSA, and GH) were AFP, CEACAM5, MUC16, KLK3, and GH1, respectively. Red columns indicate positive expression of PI15, blue columns indicate negative expression of PI15. CCA/CHOL, cholangiocarcinoma; HCC/LIHC, hepatocellular carcinoma; Normal, normal tissue; Paired t-test; ns, nonsignificant; *, p < .05.
Supplementary Table 1-5