Abstract
Dysregulated Krϋppel-like factor (KLF) gene expression appears in many disease-associated pathologies. In this review, we discuss physiological functions of KLFs in the kidney with a focus on potential pharmacological modulation/therapeutic applications of these KLF proteins. KLF2 is critical to maintaining endothelial barrier integrity and preventing gap formations and in prevention of glomerular endothelial cell and podocyte damage in diabetic mice. KLF4 is renoprotective in the setting of AKI and is a critical regulator of proteinuria in mice and humans. KLF6 expression in podocytes preserves mitochondrial function and prevents podocyte apoptosis, while KLF5 expression prevents podocyte apoptosis by blockade of ERK/p38 MAPK pathways. KLF15 is a critical regulator of podocyte differentiation and is protective against podocyte injury. Loss of KLF4 and KLF15 promotes renal fibrosis, while fibrotic kidneys have increased KLF5 and KLF6 expression. For therapeutic modulation of KLFs, continued screening of small molecules will promote drug discoveries targeting KLF proteins.
Keywords: Krϋppel-like factors (KLFs), Kidney disease, Podocytes, Renal tubule cells, Fibrosis, Interstitial inflammation
1. Introduction
Krϋppel-like factors (KLFs) belong to a group of transcription factors that contain conserved zinc (Zn)-finger domains in their C-terminal regions that bind to target DNA sequences. KLFs share homology with Sp1-like transcription factors, one of the first transcription factors to be identified and classified [1]. Subsequently, other Zn-finger containing transcription factors like KLF proteins were identified. This nomenclature Krϋppel, came from drosophila which means “cripple” in German, as a critical mutation in drosophila, led to their severe body malformation. These KLF transcription factors are important constituents of the eukaryotic transcriptional machinery in cells. KLF proteins regulate the gene expression of a wide variety of genes.
2. Structure and function
The first mammalian Krϋppel, erythroid Krϋppel-like factor (EKLF/KLF1) was identified in red blood cells [2]. Since the initial discovery of KLF1, 17 more KLF proteins were identified in the human genome and each factor was designated in the chronological order of their discovery. KLF17 and KLF18 are chromosomal neighbors, and it is suggested that KLF18 resulted from a local gene duplication of KLF17 [3]. These KLF genes comprise of three phylogenetic groups. Group 1: contains KLF proteins 3, 8 and 12, which serve as transcriptional repressors through their interaction with C-terminal binding protein (CtBP). Group 2: KLF proteins are transcriptional activators including KLF1, KLF2, 4, 5, 6, and 7. However, in KLF2 and KLF4, repression domains have been identified next to their activation domains [[4], [5], [6]]. Similarly, KLF5, can also repress expression of its gene targets. However, unlike KLF2 and KLF4, KLF5-repression domain is not identified to date. While group 3: KLF proteins 9, 10, 11, 13, 14, and 16 share a conserved α-helical motif AA/VXXL that mediates their binding to Sin3A and their activities as transcriptional repressors [[7], [8], [9], [10], [11], [12]]. KLF15 and KLF17 do not cluster in among these three phylogenetic groups as their protein interaction domains have yet to be determined. The KLF proteins known to regulate kidney injury/disease include KLF2, KLF4, KLF5, KLF6 and KLF15. Thus, this review addresses our current understanding of these aforementioned KLF proteins in regulating kidney injury/kidney disease.
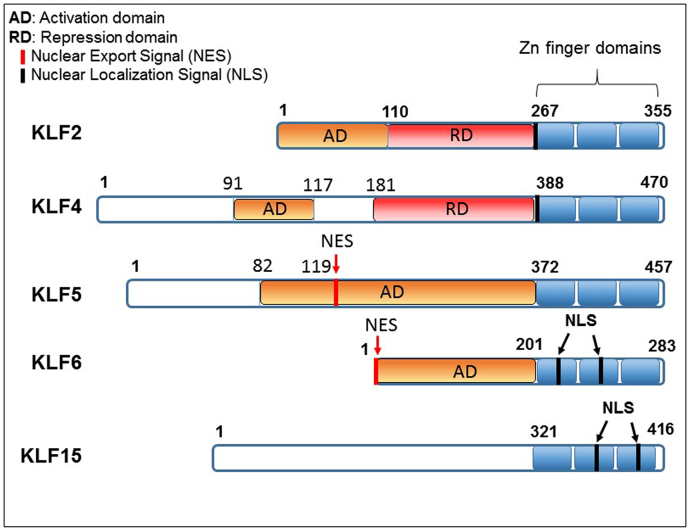
The most frequently encountered Zn-finger motif is the C2H2 type, in which a Zn atom is tetrahedrally coordinated by 2 conserved cysteine and histidine residues, such that they fold into a ββα structure. The interfinger space amino acid (aa) sequence contains a highly conserved 7-aa sequence, TGEKP(Y/F)X [13]. The regions outside the Zn-finger domain are unique. The Zn-finger motifs facilitate DNA binding and nuclear localization [14,15]. The transcriptional regulation by KLFs are facilitated by their activation and repression domains as shown in Fig. 1. Therefore, they can serve as transcriptional activators, repressors or both by interacting with GC rich concensus, including 5’-CACCC-3’ DNA sequences [16,17]. The amino-terminal regions of KLFs are divergent and modulate the specificity of protein-protein and protein-DNA interactions. Thus, specificity of KLF-mediated transcription depends on their N-terminal sequences. A number of KLFs possess a transactivation domain (TAD) within their N-terminal regions [4,6,18]. Certain KLF proteins contain functional binding domains including the C-terminal binding proteins (CtBP) and Sin3A binding sites that allow KLF proteins to function as repressor proteins [7,19]. However, KLF proteins discussed in this review (KLF2, 4, 5, 6 and 15) do not contain CtBP nor Sin3A binding sites (Fig. 1).
Fig. 1.
Schematic comparison of various domains of KLFs discussed in this review.
3. Physiological functions in kidney
The glomerulus is a filtering unit of the kidney and comprises of four cell types. The parietal epithelial cells that form the Bowman's capsule, the podoctyes that cover the outermost layer of the glomerular filtration barrier, the fenestrated endothelial cells that are in contact with the blood and the mesangial cells that are located within the capillary loops. A tight coordination and cross talk between these cell types is necessary to maintain a functional glomerular filtration barrier. During filtration, plasma first passes through the fenestra in the capillary endothelium, then through the basement membrane, and finally through filtration slits found between interdigitating podocyte foot processes. A slit diaphragm consists of proteins synthesized by the podocyte that extend across the filtration slits. Therefore, podocytes are an important component of the filtration barrier. Stress or injury to the podocytes results in foot process effacement and loss of the slit diaphragm resulting in proteinuria, an abnormal accumulation of protein in the urine [20]. Normally, a very small amount of protein is present in the urine, as the proximal tubule cells reabsorb filtered protein by endocytosis. However, when the filtration barrier is dysregulated, urinary protein concentration is elevated. Glomerulonephritis, diabetes mellitus and hypertension are some of the major disorders that can disrupt barrier function.
KLF proteins are critical regulators of physiological systems including cardiovascular, hematological, respiratory, digestive, and immune system. They are involved in disorders like cardiovascular disease, cancer, obesity and inflammatory diseases. KLF-like factors also regulate key physiological processes in the kidney, which range from maintaining glomerular filtration barrier to tubulointerstitial inflammation to progression of kidney fibrosis. Gene expression arrays from deep sequencing of microdissected nephron segments of rat renal cortex demonstrated the expression pattern of various KLF proteins in the kidney [21].
3.1. KLFs in endothelial biology
Endothelial cells (ECs) play an important role in regulating cellular processes that are critical to cell survival which include barrier function, inflammation, coagulation, vascular tone and angiogenesis. Thus, ECs function is critical in tissue development, growth and repair [22]. Endothelial dysfunction results when one of the aforementioned processes are dysregulated. This results in loss of vascular homeostasis leading to vascular pathologies such as diabetic nephropathy [[23], [24], [25]].
KLFs 2, 4, 5, 6 and 15 are expressed in ECs [[26], [27], [28], [29]]. KLF2 protein regulates endothelial barrier integrity and prevents gap formation between ECs by inducing expression of occludin, a key tight junction protein [30]. Accumulation of uremic solutes in End Stage Renal Disease (ESRD) patients increases their risk of developing cardiovascular disease (CVD) [31]. Uremic solutes decreased endothelial transcription factor, KLF2 expression. Decreased KLF2 expression, promoted endothelial dysfunction and resultant CVD [32]. In addition to causing cardiovascular injury, loss of KLF2 has been associated with glomerular endothelial injury in diabetic nephropathy and in mice with unilateral nephrectomy [33,34]. Glomerular hyperfiltration is associated with sheer stress and therefore role of KLF2 in compensatory response in unilateral nephrectomy (UNX) was examined. EC-specific KLF2 heterozygous knockout mice (KO) and their wild type littermates (WT) were subjected to UNX or sham operation. While WT-UNX mice developed compensatory hypertrophy as expected, KO-UNX mice developed high blood pressure, reduced glomerular filtration rate (GFR), a significant increase in proteinuria, and glomerulosclerosis when compared to wild type mice. Significant decrease in expression of KLF2 target gene, endothelial nitric oxide synthase (Nos3) along with other endothelial genes were detected in the glomeruli of KO-UNX compared to WT mice. Moreover, decreased renal KLF2 expression in nephrectomy patients was associated with progression to Chronic Kidney Disease (CKD) [34]. Thus, KLF2 plays a protective role in glomerular endothelial injury and progression to CKD in model of compensatory kidney hypertrophy.
KLF2 is down-regulated in glomerular ECs of patients with diabetic kidney disease and EC-specific reduction of KLF2 expression in experimental model of diabetic kidney disease promotes glomerular EC injury and promotes disease progression [35]. The aforementioned KLF2 WT and KO mice were treated with vehicle or streptozotocin. Diabetic KLF2 KO mice developed increased glomerular hypertrophy and proteinuria when compared to diabetic WT mice. Loss of KLF2 caused more injury to glomerular ECs in diabetic mice. A number of glomerular KLF2 target genes such as eNOS, ZO-1, glycocalyx, Flt1, tie2 and angiopoietin 1 were markedly decreased in diabetic KLF2 KO mice compared to diabetic WT mice. Down regulation of KLF2 protein during hyperglycemia was mediated by FOXO1-dependent transcriptional KLF2 gene silencing [36]. Thus, KLF2 via regulation of its endothelial gene targets, controls injury to the glomerular ECs during diabetes [33]. Moreover, KLF2 is a prototypical vasoprotective factor, as it is induced by stimuli that activate ECs, it is known to inhibit pro-inflammatory and pro-thrombotic gene expression, and lastly, it is known to be protective against vascular inflammatory diseases.
Endothelial KLF4 is renoprotective in the setting of acute kidney injury and in cultured ECs treated with TNF-α [37]. Conditional knockout of KLF4 from ECs (KLF4 cKO mice), promoted ischemic acute kidney injury (AKI) by modulating the expression of cell adhesion molecules and infiltration of neutrophils and lymphocytes [37]. KLF2 and KLF4 are enriched in the endothelium and have overlapping functions in ECs [26]. In addition to KLF2, laminar shear stress also induces KLF4 expression [38,39]. Moreover, both share similar downstream targets as well [26].
3.2. KLFs in podocyte biology
Glucose treatment decreased, while insulin treatment increased KLF2 expression in cultured ECs. Similarly, KLF2 expression decreased in the glomeruli of streptozotocin-induced diabetic mice and insulin treatment resulted in significant induction of KLF2 expression in diabetic mice compared to non-diabetic mice. EC specific KLF2 KO mice treated with STZ were more susceptible to glomerular EC damage. Interestingly, increased podocyte injury was also detected in these mice suggesting a cross-talk from glomerular ECs to podocytes in early diabetic nephropathy (DN) [33]. Similar to KLF2 KO-diabetic mice, KLF2 KO-UNIX mice demonstrated increased glomerular endothelial injury as well as podocyte injury suggesting an important role for KLF2 in regulation of EC and podocyte injury in early diabetes as well as in progressive kidney disease [34].
KLF4 is expressed in podocytes and is a critical regulator of proteinuria. In proteinuric animals and humans, decreased KLF4 expression contributes to proteinuria. Gene transfer by tail vein injections or podocyte-specific transgenic restoration of KLF4 in diseased glomeruli, induced recovery of podocyte epithelial marker nephrin with a concurrent decrease in albuminuria. Moreover, adriamycin-induced proteinuria was found to be significantly exacerbated in podocyte-specific KLF4 KO mice. The mechanism by which KLF4 regulated expression of nephrin gene and other epithelial and mesenchymal genes was shown to involve epigenetic modification of promoters of these genes [40].
KLF6 is also expressed in the podocytes and is critical for preservation of mitochondrial function and prevention of podocyte apoptosis [41]. KLF6 expression is decreased in renal biopsies of patients with HIV-associated nephropathy (HIVAN) and focal segmental glomerulosclerosis (FSGS). Additionally, loss of KLF6 in podocyte-specific KLF6 KO mice increased susceptibility of a resistant mouse strain to adriamycin-induced FSGS [41]. KLF6 regulated the mitochondrial function by modulating expression of its target protein mitochondrial cytochrome c oxidase assembly gene (SCO2). [41]. Over-expression of KLF5 in podocytes prevented PAN-induced cell cycle arrest and podocyte apoptosis by blocking the activation of ERK/p38 MAPK pathways [42].
Podocyte dedifferentiation is the hallmark of glomerular kidney diseases and KLF15 expression is down-regulated in diseased glomeruli. KLF15 is critical regulator of podocyte differentiation. Loss of KLF15 in podocyte-specific KLF15 KO mice, resulted in increased proteinuria, podocyte foot process effacement, and a decrease in podocyte differentiation when compared to WT mice. Thus, enhancing KLF15 expression can promote podocyte differentiation and protect against podocyte injury [43]. Adriamycin (ADR) and lipopolysaccharide (LPS) treated KLF15 (−/−) mice had increased proteinuria and podocyte foot process effacement. KLF15 expression was also decreased in glomeruli isolated from HIV transgenic mice and in kidney biopsies from patients with HIV-associated nephropathy and FSGS [43]. Loss of KLF15 increases susceptibility to podocyte injury. Podocyte specific induction of KLF15 had renoprotective effects in HIV-1 Tg mice. Podocyte-specific KLF15 induction in HIV-1 Tg mice attenuated podocyte injury, glomerulosclerosis, tubulointerstitial fibrosis, and inflammation. It also improved renal function and overall survival. Moreover, ADR-induced podocyte injury was also attenuated when KLF15 expression was induced [44]. CCR5−/− and WT mice were subjected to 5/6 nephrectomy-induced chronic podocyte injury. KLF15 expression was decreased in both WT and CCR5−/− mice after injury, with a further significant decrease in podocyte KLF15 expression in injured CCR5−/− mice compared to WT mice. Moreover, decreased KLF15 expression in patients was correlated with worse renal outcomes [45]. Thus, modulating expression of KLF proteins may serve as a therapeutic option to treat renal diseases.
3.3. KLFs in renal fibrosis and interstitial inflammation
With progressing renal fibrosis, normal tissue architecture is replaced with extracellular matrix (ECM). Progressive fibrosis leads to End Stage Renal Disease (ESRD). Renal inflammation in general is induced as a protective mechanism in response to injury and/or infection. However, prolonged or uncontrolled inflammation promotes progressive renal fibrosis. The mechanisms underlying progressive renal fibrosis are unknown and identifying these mechanisms is critical to identification of effective anti-fibrotic therapies.
In the kidney, TGF-β is mediator of renal fibrosis. TGF-β promotes cellular proliferation, differentiation and induces synthesis of ECM [46]. TGF-β promotes renal tubular epithelial cell to mesenchymal transition (EMT) a process that is critical to development of tubulointerstitial fibrosis [47,48]. Various KLF proteins also regulate renal fibrosis. Enhanced KLF4 expression blocked myofibroblast activation and inhibited fibrosis [49]. High glucose decreased KLF4 expression and increased TGF-β expression [50]. Similarly, decrease in KLF4 expression was also demonstrated in two in vivo models of unilateral ureteral obstruction [48,51], and in the renal tubular cells in an animal model of diabetic nephropathy [55], suggesting an anti-fibrotic role for KLF4 in the kidney. KLF4 modulates renal fibrosis by inhibiting inflammation. KLF4 inhibits TGF-β-induced release of pro-inflammatory cytokines MIF and MCP-1 [50].
In two in vivo models of unilateral ureteral obstruction namely UUO and 5/6 nephrectomy, KLF5 expression was increased in proliferating renal tubule cells located in the cortex and medulla. Co-localization studies with KLF5 and aquaporin 1, demonstrated KLF5 expression in the proximal renal tubules of fibrotic kidneys. While KLF5 expression was induced, KLF4 expression was suppressed. YAP stabilized KLF5 expression by preventing its degradation at the proteasome. Thus, inhibition of collagen crosslinking by lysyl oxidase inhibitor, decreased UUO-induced renal tubular dilatation and proliferation by inducing KLF4 expression and down-regulating YAP1/KLF5 pathway [51]. Moreover, KLF5 in renal collecting duct plays a critical role in the initiation and progression of tubulointerstitial inflammation [52]. KLF6 expression was induced in diabetic Ren-2 rat kidneys as well as in high glucose (HG) treated renal tubular cells. HG-induced KLF6 expression in renal tubular cells was dependent upon TGF-β and increased KLF6 expression promoted EMT [53].
Ang II treatment of mice and NRK-49F cells demonstrated decreased KLF15 expression and increased CTGF expression. Over-expression of KLF15 in the NRK-49F cells prevented Ang II-induced CTGF expression by inhibiting the co-activator P/CAF recruitment to the CTGF promoter [54]. Loss of KLF15, promoted renal fibrosis in various murine models [55]. In 5/6 nephrectomized rats a model of progressive interstitial fibrosis, KLF15 expression was decreased in the renal interstitium at 24-week time point. TGF-β-induced CTGF expression and decreased KLF15 expression in NRK-49F cells in an ERK and JNK MAPK dependent pathways. Moreover, over-expression of KLF15 in NRK-49F cells prevented TGF-β-induced CTGF expression in these cells. Thus, KLF15 is a critical anti-fibrotic factor that controls renal interstitial pathways by possibly modulating ERK and JNK MAPK pathways [56]. Krüppel-like factor 10 (KLF10), originally named TGF-β (Transforming growth factor beta) inducible early gene 1 (TIEG1), and KLF11 regulate the TGF-βsignal transduction pathway [57]. While KLF10 modulates fibrosis in dystrophic skeletal muscles [58], endometriosis-related fibrosis is regulated by KLF11 [59]. However, the role of KLF10 and KLF11 in modulating kidney fibrosis is not investigated.
Therapies to halt progression of renal fibrosis are limited. Importantly, myofibroblast differentiation and proliferation play an important role in progression of renal fibrosis. Therefore, identifying pathways that control myofibroblast differentiation and proliferation is critical to generating therapies to slow down or halt progression of renal fibrosis.
4. Pharmacological modulation of KLFs/therapeutic applications of KLFs
Various KLF proteins regulate different aspects of kidney disease as discussed in this review. Specific KLF proteins could serve as therapeutic targets and agents that increase or decrease expression and/or function of specific KLF proteins, could serve as drugs to ameliorate kidney injury and/or slow down or halt progression to fibrosis and CKD. Endothelial KLF4 is protective against ischemia/reperfusion induced AKI. Loss of KLF4 in KLF4 cKO mice exacerbated renal I/R injury. Statins protected control mice by increasing KLF4 expression but not KLF4 cKO mice, suggesting that the protective effects of statins are mediated by KLF4. Increased KLF4 expression, in the presence of statins, suppressed inflammation-induced expression of cell adhesion molecules during AKI [37]. Similarly, statins also induce expression of KLF2 in ECs and circulating immune cells [[60], [61], [62]]. Loss of KLF2 expression promotes glomerular EC injury [35]. Moreover, suberanilohydroxamic acid is a pharmacological KLF2 activator and can inhibit vascular inflammation [63]. In addition to statins and suberanilohydroxamic acid, tannic acid and resveratrol induce endothelial KLF2 expression and prevent inflammation [64,65]. Thus, modulation of KLF2 expression and/or function could lead to generation of new therapies to treat kidney fibrosis as inflammation is considered to be the main driving force of fibrosis. Resveratrol is also renoprotective [66]. However, more work needs to be done to examine role of KLF proteins in mediating resveratrol's protective effects.
Angiotensin receptor blocker (ARB) reduces proteinuria and slows down the progression to ESRD in humans. Recently, the protective effects of ARB were mediated by induction of KLF4 expression in podocytes [67]. Hypermethylation of KLF4 promoter during UUO-induced renal fibrosis, mediated by DNA methyltransferase I, resulted in KLF4 degradation. Treatment with 5-aza-2′-deoxycytidine attenuated TGF-β-induced KLF4 and E-cadherin downregulation and upregulation of α-smooth muscle actin (α-SMA) in human renal proximal tubular HK-2 cells. Moreover, 5-aza-2′-deoxycytidine could prevent renal fibrosis by inhibiting DNA methyltransferase I activity and preserving KLF4 expression [48].
Epigenetic modifications of genes play a critical role in renal fibrosis and CKD. In the future epigenetic modification drugs that induce KLF4 expression could serve as good candidate for treatment of CKD [68]. KLF4 and KLF6 expression is decreased in renal cell carcinoma [69,70], there by promoting cellular proliferation and metastasis. KLF4 expression is also decreased in colorectal cancer [71]. Sulforaphane and Iberin, both derived from broccoli, are known to inhibit colon cancer cell proliferation by induction of KLF4 expression [72]. Recently, a traditional Chinese medicine, sijunzi decoction (SJZD), is being prescribed for prevention and treatment of colorectal cancer. KLF4 was shown to be an mRNA target of SJZD for treatment of colorectal cancer [71]. As opposed to KLF4, KLF5 expression is enhanced in proliferating epithelial cells. Down-regulation of KLF5 is associated with decreased proliferation of colorectal cancer cells. Thus, down-regulation of KLF5 expression can be targeted to treat colorectal cancer. A ultrahigh-throughput screening has identified small molecule inhibitors that reduce KLF5 expression and thee small molecules have been shown to inhibit colon cancer cell proliferation by targeting KLF5 protein [73]. KLF5 proteins have opposing roles in various cancers. KLFs promote its oncogenic function by promoting cancer cell proliferation and survival in some type of cancers, while they serve a tumor suppressor function promoting cancer cell growth inhibition in some cancers [74]. Thus, small molecules targeting KLF5 as inhibitors as well as activators have been identified [75]. KLF5 inhibitors could also be targeted to reduce KLF5 expression in proliferating renal tubular cells or in fibrotic kidneys in efforts to block pro-fibrotic signaling.
Angiotensin II (Ang II) treatment of mice resulted in renal fibrosis, with concurrent decrease in renal KLF15 expression. Lorsartan treatment reversed the effects of Ang II treatment [54]. Loss of KLF15 expression and podocyte differentiation markers during kidney disease can be restored with dexamethasone treatment. The protective effects of dexamethasone were shown to be mediated by enhanced KLF15 expression. Accordingly, dexamethasone had no protective effect on podocyte differentiation in podocyte-specific KLF15 KO mice. Furthermore, glucocorticoid responsiveness in 35 patients with minimal change disease (MCD) and FSGS were correlated with KLF15 expression in the podocytes and glomeruli from human biopsies [76]. Retinoic acid can also induce KLF15 expression in podocytes [43]. Cyclosporin A (CsA), a calcineurin inhibitor, is administered clinically to patients with podocytopathy. TGF-β decreased KLF15 and ZO-1 expression and induced fibronectin expression in cultured podocytes. While, treatment of podocytes with CsA induced KLF15 and ZO-1 expression and decreased fibronectin expression in TGF-β treated podocytes. The protective effects of CsA on TGF-β treated podocytes were dependent on KLF15 expression, as the protective effects of CsA were lost in KLF15 silenced podocytes [45]. Interestingly, low protein diet is shown to induce KLF15 expression and limiting renal fibrosis [77]. The various KLFs discussed in this review could serve as therapeutic drug targets in treatment of various diseases, including kidney diseases.
5. Outstanding questions and challenges
In the current review article, we highlight the role of KLFs 2, 4, 5, 6, and 15 in kidney diseases. Recent studies on various KLFs have continued to broaden our knowledge of their diverse biological and physiological functions and of their roles in a variety of organs/tissues and in a number of cellular processes. While the KLF proteins have normal biological roles, dysregulated KLF expression/function is involved in disease processes. Therefore, although a number of initial studies have highlighted roles of KLFs in development and regeneration, KLFs play a critical role in progression of a number of diseases including kidney disease. Table 1 summarizes the contributions of various KLFs in renal physiology and disease.
Table 1.
Contributions of various KLFs in renal physiology and disease.
| Kidney | KLFs | Function | Cell culture/animal model | Comments | Refs |
|---|---|---|---|---|---|
| Glomerular Endothelial Cell (GEC) Dysfunction | KLF2 | A key regulator of endothelial function and activation | Porcine 5/6 nephrectomy model | Increased uremic advanced glycation end products (AGEs) decreased KLF2 expression in ECs and induced GEC dysfunction. | [32] |
| GEC Dysfunction | KLF2 | KLF2 regulates GEC dysfunction | WT and EC-specific KLF2 KO mice were injected with STZ or vehicle (KO-STZ/KO-Vehicle and WT-STZ/WT-Vehicle) | Loss of KLF2 caused glomerular hypertrophy, proteinuria, and GEC dysfunction. | [33] |
| Podocyte Injury | KLF2 | KLF2 regulates proteinuria | KO-STZ/KO-Vehicle and WT-STZ/WT-Vehicle | Loss of KLF2 in KO-STZ mice resulted in albuminuria and podocyte injury possibly through glomerular endothelial cell-podocyte cross-talk. | [33] |
| GEC Dysfunction | KLF2 | KLF2 regulates GEC dysfunction | WT and EC-specific KLF2 KO mice were subjected to sham or unilateral nephrectomy (UNX) | WT-UNX but not KO-UNIX mice developed compensatory kidney Hypertrophy. KO-UNIX mice had increased proteinuria and GEC injury compared to WT-UNIX mice. | [34] |
| Podocyte Injury | KLF2 | KLF2 regulates podocyte damage | KO-UNIX/KO-Sham and WT-Sham/WT-UNIX | KO-UNIX mice had increased podocyte injury compared to WT-UNIX possibly through glomerular endothelial cell-podocyte cross-talk. | [34] |
| Acute Kidney Injury | KLF4 | Endothelial KLF4 is renoprotective | Endothelial KLF4 conditional knockout (KLF4 cKO) and WT mice subjected to renal ischemia-reperfusion injury | Loss of KLF4 led to enhanced neutrophil and lymphocyte accumulation and enhanced expression of cell adhesion molecules in injured kidneys of KLF4 cKO mice. | [37] |
| Podocyte Injury | KLF4 | KLF4 is a critical regulator of proteinuria | 1. KLF4fl/flCre+ KO & KLF4fl/flCre− WT mice treated with/without adriamycin (ADR). | KLF expression is decreased in podocytes from proteinuric animal models and from patients with proteinuric glomerular disease. Loss of KLF4 causes proteinuria and podocyte damage. Restoring KLF4 (KLF4-Tg) in diseased glomeruli attenuated proteinuria. | [40] |
| 2.KLF4-Tg and Control mice. | |||||
| 3. Puromycin-induced nephropathy | |||||
| 4. db/db type 2 diabetes mice. | |||||
| Podocyte Injury | KLF5 | Promotes podocyte survival | Podocytes (MPC-5 cells) treated with puromycin aminonucleoside (PAN) | PAN promoted podocyte apoptosis by activating intrinsic apoptotic cascade. KLF5 blocked PAN-induced podocyte apoptosis by inhibiting activation of ERK/p38 MAPK pathways. | [42] |
| Podocyte Injury | KLF6 | Critical for podocyte survival and for preserving mitochondrial function. | Podocyte-specific KLF6 KO (Podocin-Cre Klf6fl/fl) WT-KLF6 controls (Podocin-Cre Klf6+/+) subjected to Adriamycin nephropathy | Loss of KLF6 increased susceptibility to ADR nephropathy and resulted in albuminuria and glomerular sclerosis. Loss of KLF6 also increased mitochondrial injury and promoted podocyte apoptosis by activation of intrinsic apoptotic pathway. KLF6 expression was decreased in HIV-1 Tg (Tg26) mice as well as in biopsies of patients with HIV-associated nephropathy (HIVAN) and in patients with focal segmental glomerulosclerosis (FSGS). | [41] |
| Podocyte Injury | KLF15 | KLF15 is a critical regulator of podocyte differentiation | WT or Podocyte-specific KLF15 KO mice treated with or without lipopolysaccharide (LPS) or ADR | Loss of KLF15 resulted in decreased podocyte differentiation and increased susceptibility to podocyte injury. ADR and LPS treated KLF15 (−/−) mice had increased proteinuria and podocyte foot process effacement. KLF15 expression was also decreased in glomeruli isolated from Tg26 mice and in kidney biopsies from patients with HIVAN and FSGS. | [43] |
| Podocyte Injury | KLF15 | KLF15 is renoprotective | WT and podocyte –specific KLF15 Tg mice were cross bred with HIV-1 Tg (Tg26) mice | Loss of KLF15 increased susceptibility to podocyte injury. Podocyte specific induction of KLF15 had renoprotective effects in Tg26 mice. Podocyte-specific KLF15 induction in Tg26 mice attenuated podocyte injury, glomerulosclerosis, tubulointerstitial fibrosis, and inflammation. It also improved renal function and overall survival; ADR-induced podocyte injury was also attenuated. | [44] |
| Podocyte Injury | KLF15 | KLF15 promotes podocyte differentiation | WT and CCR5(−/−) mice were subjected to sham or 5/6 Nephrectomy | KLF15 expression was decreased in 5/6 nephrectomized WT animals and further decreased in CCR5(−/−) mice and this correlated with increased podocyte injury. | [45] |
| Renal Fibrosis and Interstitial Inflammation | KLF4 | KLF4 blocks epithelial to mesenchymal transition (EMT) in renal fibrosis | 1. Unilateral Ureteral Obstruction (UUO) model of renal fibrosis | UUO treatment resulted in decreased KLF4 expression and increase in TGF-β expression in renal tissue. Over-expression of KLF4 suppressed TGF-β-induced progression of EMT in HK-2 cells. |
[49] |
| 2. TGF-β treated Human renal proximal tubule cells (HK−2) | |||||
| Renal Fibrosis and Interstitial Inflammation | KLF4 | KLF4 is involved in renal fibrosis by regulating renal inflammation` | 1. TGF-β-induced HK-2 cells | KLF4 reduced renal inflammation by abrogating the TGF-β1-induced production of pro-inflammatory MIF and MCP-1 in human renal tubular cells. | [50] |
| 2.STZ-induced diabetic mice | |||||
| Renal Fibrosis and Interstitial Inflammation | KLF4/KLF5 | KLF4 is an anti-fibrotic and KLF5 is pro-fibrotic protein | mouse proximal tubular epithelial cells (mPTECs) in ex vivo culture on soft-matrix or stiff-matrix | Soft-matrix decreases KLF5 and increases KLF4 and induced growth arrest. Stiff-matrix induced high levels of KLF5 and decreased KLF4 promoting mPTECs proliferation and fibrosis progression. | [51] |
| Renal Fibrosis and Interstitial Inflammation | KLF5 | KLF5 is pro-inflammatory protein | WT or KLF5 haploinsufficient mice (Klf5+/− mice) subjected to UUO | Klf5 is expressed in renal collecting duct epithelial cells. KLF5 haploinsufficiency reduced UUO-induced M1-macrophages, blocking release of pro-inflammatory cytokines that induced apoptosis of epithelial cells. KLF5 haploinsufficiency promoted fibrosis after UUO treatment. | [52] |
| Renal Fibrosis and Interstitial Inflammation | KLF6 | KLF6 regulates EMT | 1.STZ-treated control or STZ-treated mRen-2 rats | KLF6 expression was increased HG-treated HK-2 cells and STZ-treated mRen-2 renal tubular cells with concurrent increase in EMT. Blockade of KLF6 with KLF6 siRNA in HK-2 cells preserved E-cadherin expression and prevented EMT. | [53] |
| 2. HK-2 cells were treated with TGF-β and low glucose LG)/high glucose (HG) for varying times. | |||||
| Renal Fibrosis and Interstitial Inflammation | KLF15 | KLF15 is an anti-fibrotic protein | 1. Murine model of angiotensin II (Ang II)-induced renal fibrosis | Ang-II induced fibrosis in mice and decreased KLF15 expression. Ang II decreased KLF15 expression in NRK-49 Cells and induced expression of pro-fibrotic proteins and extracellular matrix proteins. Over-expression of KLF16 blocked Ang II effects in NRK-49 cells. | [54] |
| 2.Rat renal fibroblasts (NRK—49F) stimulated with or without Ang II | |||||
| Renal Fibrosis and Interstitial Inflammation | KLF15 | KLF15 attenuates renal fibrosis by inhibiting the canonical Wnt/β-catenin pathway | Foxd1-Cre Klf15fl/fl and Foxd1-Cre Klf15+/+ (control) mice were subjected to sham or UUO. These mice were also treated with vehicle or Ang II | Loss of KLF15 in Foxd1+ stromal cells induced pro-fibrotic markers after UUO and accelerated fibrosis after Ang II treatment. Aged Foxd1-Cre Klf15fl/fl mice demonstrated an increase in fibrotic markers with concurrent renal dysfunction. |
[55] |
| Renal Fibrosis and Interstitial Inflammation | KLF15 | KLF15 regulates renal tubular interstitial injury | 1. Sham control or 5/6 kidney nephrectomy in rats | KLF15 was expressed in the interstitium of control rats. KLF15 expression decreased and renal tubular interstitial injury increased after nephrectomy. TGF-β treatment reduced KLF15 expression in NRK-49F cells, while FN, CTGF, and ColIII expressions were increased. Over-expression of KLF15 blocked these effects. | [56] |
| 2. NRK-49F cells treated with or without TGF-β |
Although, a role for KLFs in kidney diseases are identified, significant gaps exist in understanding role of KLF5, KLF6, and KLF15 in glomerular endothelial cells, role of KLF10 and KLF11 in kidney fibrosis, and determining gender specific differences in various KLF protein expressions in the kidney. Moreover, there is a need to understanding the mechanisms that control KLF expression/function in renal physiology and disease. A number of KLFs have context-dependent functions. At some point, they promote a certain function (e.g. act as an oncogene) while at other times they inhibit the same function (e.g. act as a tumor suppressor) in a different cell type. These studies highlight the roles of accessory proteins, binding partners that interact with these KLFs to alter their functional outcomes. Thus, opposing actions of KLFs can occur, when changes in protein-protein interactions within the KLF complex occurs, possibly regulating KLF protein post-translational modifications, resulting in induction/repression of their gene targets.
KLFs are expressed in various cell types and targeting KLFs for therapeutic applications in specific tissue will be challenging. Therefore, given the redundancy of KLF family members and their common transcriptional targets, identifying specific KLF protein binding partners, accessory proteins and downstream gene targets in specific cell type and disease is critical. Such studies will lead to mechanistic insights into specific KLF functions in different tissues, providing novel therapeutic targets and paving the way to precision medicine approaches.
Search strategy and selection criteria
Data for this Review were identified by searches of MEDLINE, Current Contents, PubMed, and references from relevant articles using search terms “kidney fibrosis”, “Krupple-like Factor 2”, “Krupple-like Factor 4” “Krupple-like Factor 5” “Krupple-like Factor 6” “Krupple-like Factor 15”, “vascular endothelial damage”, “podocytes injury/damage”, “renal tubule injury/damage”, and “interstitial inflammation”. Abstracts and reports from meetings were included only when they related directly to previously published work. Only articles published in English between 1993 and 2018 were included.
Conflict of interest statement
The authors declare no conflict of Interest.
Author contributions
Madhavi Rane and Lu Cai conceived the idea of the review article. Madhavi Rane and Lu Cai designed the layout of the review article. The literature search was done by Madhavi Rane and Yuguang Zhao. Fig. 1 and Table 1 was created by Madhavi Rane. The manuscript was written by Madhavi Rane. Yuguang Zhao and Lu Cai reviewed and edited the manuscript.
Acknowledgements
The authors were supported by the National Institute of Health grant R01A1075212 (MJR), Division of Nephrology, the University of Louisville (MJR). The National Science Foundation of China (81670221, to YZ) and the American Diabetes Association Grant 1-18-IBS-082 (MJR and CL). Only the authors listed in the article were involved in writing of this review, and Funders did not have any role in paper design, data collection, data analysis and interpretation.
Contributor Information
Madhavi J. Rane, Email: madhavi.rane@louisville.edu.
Lu Cai, Email: L0cai001@louisville.edu.
References
- 1.Kaczynski J., Cook T., Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4(2):206. doi: 10.1186/gb-2003-4-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller I.J., Bieker J.J. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Kruppel family of nuclear proteins. Mol Cell Biol. 1993;13(5):2776–2786. doi: 10.1128/mcb.13.5.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pei J., Grishin N.V. A new family of predicted krüppel-like factor genes and pseudogenes in placental mammals. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conkright M.D., Wani M.A., Lingrel J.B. Lung Kruppel-like factor contains an autoinhibitory domain that regulates its transcriptional activation by binding WWP1, an E3 ubiquitin ligase. J Biol Chem. 2001;276(31):29299–29306. doi: 10.1074/jbc.M103670200. [DOI] [PubMed] [Google Scholar]
- 5.Geiman D.E., Ton-That H., Johnson J.M., Yang V.W. Transactivation and growth suppression by the gut-enriched Kruppel-like factor (Kruppel-like factor 4) are dependent on acidic amino acid residues and protein-protein interaction. Nucleic Acids Res. 2000;28(5):1106–1113. doi: 10.1093/nar/28.5.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wani M.A., Conkright M.D., Jeffries S., Hughes M.J., Lingrel J.B. cDNA isolation, genomic structure, regulation, and chromosomal localization of human lung Kruppel-like factor. Genomics. 1999;60(1):78–86. doi: 10.1006/geno.1999.5888. [DOI] [PubMed] [Google Scholar]
- 7.Cook T., Gebelein B., Belal M., Mesa K., Urrutia R. Three conserved transcriptional repressor domains are a defining feature of the TIEG subfamily of Sp1-like zinc finger proteins. J Biol Chem. 1999;274(41):29500–29504. doi: 10.1074/jbc.274.41.29500. [DOI] [PubMed] [Google Scholar]
- 8.Daftary G.S., Lomberk G.A., Buttar N.S., Allen T.W., Grzenda A., Zhang J. Detailed structural-functional analysis of the Kruppel-like factor 16 (KLF16) transcription factor reveals novel mechanisms for silencing Sp/KLF sites involved in metabolism and endocrinology. J Biol Chem. 2012;287(10):7010–7025. doi: 10.1074/jbc.M111.266007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaczynski J., Zhang J.S., Ellenrieder V., Conley A., Duenes T., Kester H. The Sp1-like protein BTEB3 inhibits transcription via the basic transcription element box by interacting with mSin3A and HDAC-1 co-repressors and competing with Sp1. J Biol Chem. 2001;276(39):36749–36756. doi: 10.1074/jbc.M105831200. [DOI] [PubMed] [Google Scholar]
- 10.Lomberk G., Grzenda A., Mathison A., Escande C., Zhang J.S., Calvo E. Kruppel-like factor 11 regulates the expression of metabolic genes via an evolutionarily conserved protein interaction domain functionally disrupted in maturity onset diabetes of the young. J Biol Chem. 2013;288(24):17745–17758. doi: 10.1074/jbc.M112.434670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truty M.J., Lomberk G., Fernandez-Zapico M.E., Urrutia R. Silencing of the transforming growth factor-beta (TGFbeta) receptor II by Kruppel-like factor 14 underscores the importance of a negative feedback mechanism in TGFbeta signaling. J Biol Chem. 2009;284(10):6291–6300. doi: 10.1074/jbc.M807791200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J.S., Moncrieffe M.C., Kaczynski J., Ellenrieder V., Prendergast F.G., Urrutia R. A conserved alpha-helical motif mediates the interaction of Sp1-like transcriptional repressors with the corepressor mSin3A. Mol Cell Biol. 2001;21(15):5041–5049. doi: 10.1128/MCB.21.15.5041-5049.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dang D.T., Pevsner J., Yang V.W. The biology of the mammalian Kruppel-like family of transcription factors. Int J Biochem Cell Biol. 2000;32(11−12):1103–1121. doi: 10.1016/s1357-2725(00)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McConnell B.B., Yang V.W. Mammalian Kruppel-like factors in health and diseases. Physiol Rev. 2010;90(4):1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brayer K.J., Segal D.J. Keep your fingers off my DNA: protein-protein interactions mediated by C2H2 zinc finger domains. Cell Biochem Biophys. 2008;50(3):111–131. doi: 10.1007/s12013-008-9008-5. [DOI] [PubMed] [Google Scholar]
- 16.Lomberk G., Urrutia R. The family feud: turning off Sp1 by Sp1-like KLF proteins. Biochem J. 2005;392(Pt 1):1–11. doi: 10.1042/BJ20051234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suske G., Bruford E., Philipsen S. Mammalian SP/KLF transcription factors: bring in the family. Genomics. 2005;85(5):551–556. doi: 10.1016/j.ygeno.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Lahiri S.K., Zhao J. Kruppel-like factor 8 emerges as an important regulator of cancer. Am J Transl Res. 2012;4(3):357–363. [PMC free article] [PubMed] [Google Scholar]
- 19.Turner J., Crossley M. Cloning and characterization of mCtBP2, a co-repressor that associates with basic Kruppel-like factor and other mammalian transcriptional regulators. EMBO J. 1998;17(17):5129–5140. doi: 10.1093/emboj/17.17.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grahammer F., Schell C., Huber T.B. The podocyte slit diaphragm--from a thin grey line to a complex signalling hub. Nat Rev Nephrol. 2013;9(10):587–598. doi: 10.1038/nrneph.2013.169. [DOI] [PubMed] [Google Scholar]
- 21.Lee J.W., Chou C.L., Knepper M.A. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-specific Transcriptomes. J Am Soc Nephrol. 2015;26(11):2669–2677. doi: 10.1681/ASN.2014111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monahan-Earley R., Dvorak A.M., Aird W.C. Evolutionary origins of the blood vascular system and endothelium. J Thromb Haemost. 2013;11(Suppl. 1):46–66. doi: 10.1111/jth.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng H., Harris R.C. Renal endothelial dysfunction in diabetic nephropathy. Cardiovasc Hematol Disord Drug Targets. 2014;14(1):22–33. doi: 10.2174/1871529x14666140401110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung W.K., Gao L., Siu P.M., Lai C.W. Diabetic nephropathy and endothelial dysfunction: current and future therapies, and emerging of vascular imaging for preclinical renal-kinetic study. Life Sci. 2016;166:121–130. doi: 10.1016/j.lfs.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Stehouwer C.D. Endothelial dysfunction in diabetic nephropathy: state of the art and potential significance for non-diabetic renal disease. Nephrol Dial Transplant. 2004;19(4):778–781. doi: 10.1093/ndt/gfh015. [DOI] [PubMed] [Google Scholar]
- 26.Sangwung P., Zhou G., Nayak L., Chan E.R., Kumar S., Kang D.W. KLF2 and KLF4 control endothelial identity and vascular integrity. JCI Insight. 2017;2(4) doi: 10.1172/jci.insight.91700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumekawa M., Fukuda G., Shimizu S., Konno K., Odawara M. Inhibition of monocyte chemoattractant protein-1 by Kruppel-like factor 5 small interfering RNA in the tumor necrosis factor- alpha-activated human umbilical vein endothelial cells. Biol Pharm Bull. 2008;31(8):1609–1613. doi: 10.1248/bpb.31.1609. [DOI] [PubMed] [Google Scholar]
- 28.Botella L.M., Sanchez-Elsner T., Sanz-Rodriguez F., Kojima S., Shimada J., Guerrero-Esteo M. Transcriptional activation of endoglin and transforming growth factor-beta signaling components by cooperative interaction between Sp1 and KLF6: their potential role in the response to vascular injury. Blood. 2002;100(12):4001–4010. doi: 10.1182/blood.V100.12.4001. [DOI] [PubMed] [Google Scholar]
- 29.Helbing T., Volkmar F., Goebel U., Heinke J., Diehl P., Pahl H.L. Kruppel-like factor 15 regulates BMPER in endothelial cells. Cardiovasc Res. 2010;85(3):551–559. doi: 10.1093/cvr/cvp314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Z., Natesan V., Shi H., Dong F., Kawanami D., Mahabeleshwar G.H. Kruppel-like factor 2 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol. 2010;30(10):1952–1959. doi: 10.1161/ATVBAHA.110.211474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunet P., Gondouin B., Duval-Sabatier A., Dou L., Cerini C., Dignat-George F. Does uremia cause vascular dysfunction? Kidney Blood Press Res. 2011;34(4):284–290. doi: 10.1159/000327131. [DOI] [PubMed] [Google Scholar]
- 32.Saum K., Campos B., Celdran-Bonafonte D., Nayak L., Sangwung P., Thakar C. Uremic Advanced Glycation End Products and Protein-Bound Solutes Induce Endothelial Dysfunction through suppression of Kruppel-like factor 2. J Am Heart Assoc. 2018;7(1) doi: 10.1161/JAHA.117.007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong F., Chen H., Wei C., Zhang W., Li Z., Jain M.K. Reduced Kruppel-like factor 2 expression may aggravate the endothelial injury of diabetic nephropathy. Kidney Int. 2015;87(2):382–395. doi: 10.1038/ki.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong F., Mallipattu S.K., Estrada C., Menon M., Salem F., Jain M.K. Reduced Kruppel-like factor 2 aggravates glomerular endothelial cell injury and kidney disease in mice with unilateral nephrectomy. Am J Pathol. 2016;186(8):2021. doi: 10.1016/j.ajpath.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong F., Lee K., He J.C. Role of Kruppel-like factor-2 in kidney disease. Nephrol Ther (Carlton, Vic) 2018;23(Suppl. 4):53–56. doi: 10.1111/nep.13456. [DOI] [PubMed] [Google Scholar]
- 36.Lee H.Y., Youn S.W., Cho H.J., Kwon Y.W., Lee S.W., Kim S.J. FOXO1 impairs whereas statin protects endothelial function in diabetes through reciprocal regulation of Kruppel-like factor 2. Cardiovasc Res. 2013;97(1):143–152. doi: 10.1093/cvr/cvs283. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida T., Yamashita M., Iwai M., Hayashi M. Endothelial Kruppel-like factor 4 mediates the protective effect of statins against ischemic AKI. J Am Soc Nephrol. 2016;27(5):1379–1388. doi: 10.1681/ASN.2015040460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slater S.C., Ramnath R.D., Uttridge K., Saleem M.A., Cahill P.A., Mathieson P.W. Chronic exposure to laminar shear stress induces Kruppel-like factor 2 in glomerular endothelial cells and modulates interactions with co-cultured podocytes. Int J Biochem Cell Biol. 2012;44(9):1482–1490. doi: 10.1016/j.biocel.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 39.Hamik A., Lin Z., Kumar A., Balcells M., Sinha S., Katz J. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007;282(18):13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi K., Sasamura H., Nakamura M., Azegami T., Oguchi H., Sakamaki Y. KLF4-dependent epigenetic remodeling modulates podocyte phenotypes and attenuates proteinuria. J Clin Invest. 2014;124(6):2523–2537. doi: 10.1172/JCI69557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mallipattu S.K., Horne S.J., D'Agati V., Narla G., Liu R., Frohman M.A. Krüppel-like factor 6 regulates mitochondrial function in the kidney. J Clin Invest. 2015;125(3):1347–1361. doi: 10.1172/JCI77084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y., Sui X., Hu X., Hu Z. Overexpression of KLF5 inhibits puromycininduced apoptosis of podocytes. Mol Med Rep. 2018;18(4):3843–3849. doi: 10.3892/mmr.2018.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mallipattu S.K., Liu R., Zheng F., Narla G., Ma'ayan A., Dikman S. Kruppel-like factor 15 (KLF15) is a key regulator of podocyte differentiation. J Biol Chem. 2012;287(23):19122–19135. doi: 10.1074/jbc.M112.345983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo Y., Pace J., Li Z., Ma'ayan A., Wang Z., Revelo M.P. Podocyte-specific induction of Kruppel-like factor 15 restores differentiation markers and attenuates kidney injury in proteinuric kidney disease. J Am Soc Nephrol. 2018;29(10):2529–2545. doi: 10.1681/ASN.2018030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han S.S., Yu M.Y., Yoo K.D., Lee J.P., Kim D.K., Kim Y.S. Loss of KLF15 accelerates chronic podocyte injury. Int J Mol Med. 2018;42(3):1593–1602. doi: 10.3892/ijmm.2018.3726. [DOI] [PubMed] [Google Scholar]
- 46.Meng X.M., Huang X.R., Xiao J., Chung A.C., Qin W., Chen H.Y. Disruption of Smad4 impairs TGF-beta/Smad3 and Smad7 transcriptional regulation during renal inflammation and fibrosis in vivo and in vitro. Kidney Int. 2012;81(3):266–279. doi: 10.1038/ki.2011.327. [DOI] [PubMed] [Google Scholar]
- 47.Carew R.M., Wang B., Kantharidis P. The role of EMT in renal fibrosis. Cell Tissue Res. 2012;347(1):103–116. doi: 10.1007/s00441-011-1227-1. [DOI] [PubMed] [Google Scholar]
- 48.Xiao X., Tang W., Yuan Q., Peng L., Yu P. Epigenetic repression of Kruppel-like factor 4 through Dnmt1 contributes to EMT in renal fibrosis. Int J Mol Med. 2015;35(6):1596–1602. doi: 10.3892/ijmm.2015.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ke B., Zhang A., Wu X., Fang X. The role of Kruppel-like factor 4 in renal fibrosis. Front Physiol. 2015;6:327. doi: 10.3389/fphys.2015.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mreich E., Chen X.M., Zaky A., Pollock C.A., Saad S. The role of Kruppel-like factor 4 in transforming growth factor-beta-induced inflammatory and fibrotic responses in human proximal tubule cells. Clin Exp Pharmacol Physiol. 2015;42(6):680–686. doi: 10.1111/1440-1681.12405. [DOI] [PubMed] [Google Scholar]
- 51.Chen W.C., Lin H.H., Tang M.J. Matrix-stiffness-regulated inverse expression of Kruppel-like factor 5 and Kruppel-like factor 4 in the pathogenesis of renal fibrosis. Am J Pathol. 2015;185(9):2468–2481. doi: 10.1016/j.ajpath.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 52.Fujiu K., Manabe I., Nagai R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest. 2011;121(9):3425–3441. doi: 10.1172/JCI57582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holian J., Qi W., Kelly D.J., Zhang Y., Mreich E., Pollock C.A. Role of Kruppel-like factor 6 in transforming growth factor-beta1-induced epithelial-mesenchymal transition of proximal tubule cells. Am J Physiol Renal Physiol. 2008;295(5):F1388–F1396. doi: 10.1152/ajprenal.00055.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu X., Xu D., Fu L., Wang Y., Mei C., Gao X. KLF15 works as an early anti-fibrotic transcriptional regulator in Ang II-induced renal fibrosis via down-regulation of CTGF expression. Kidney Blood Press Res. 2017;42(6):999–1012. doi: 10.1159/000485349. [DOI] [PubMed] [Google Scholar]
- 55.Gu X., Mallipattu S.K., Guo Y., Revelo M.P., Pace J., Miller T. The loss of Kruppel-like factor 15 in Foxd1(+) stromal cells exacerbates kidney fibrosis. Kidney Int. 2017;92(5):1178–1193. doi: 10.1016/j.kint.2017.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao X., Wu G., Gu X., Fu L., Mei C. Kruppel-like factor 15 modulates renal interstitial fibrosis by ERK/MAPK and JNK/MAPK pathways regulation. Kidney Blood Press Res. 2013;37(6):631–640. doi: 10.1159/000355743. [DOI] [PubMed] [Google Scholar]
- 57.Memon A., Lee W.K. KLF10 as a tumor suppressor gene and its TGF-beta signaling. Cancer. 2018;10(6) doi: 10.3390/cancers10060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DiMario J.X. KLF10 gene expression modulates Fibrosis in dystrophic skeletal muscle. Am J Pathol. 2018;188(5):1263–1275. doi: 10.1016/j.ajpath.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 59.Shenoy C.C., Khan Z., Zheng Y., Jones T.L., Khazaie K., Daftary G.S. Progressive fibrosis: a progesterone- and KLF11-mediated sexually dimorphic female response. Endocrinology. 2017;158(10):3605–3619. doi: 10.1210/en.2017-00171. [DOI] [PubMed] [Google Scholar]
- 60.Sen-Banerjee S., Mir S., Lin Z., Hamik A., Atkins G.B., Das H. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112(5):720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- 61.Parmar K.M., Nambudiri V., Dai G., Larman H.B., Gimbrone M.A., Jr., Garcia-Cardena G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem. 2005;280(29):26714–26719. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 62.Tuomisto T.T., Lumivuori H., Kansanen E., Hakkinen S.K., Turunen M.P., van Thienen J.V. Simvastatin has an anti-inflammatory effect on macrophages via upregulation of an atheroprotective transcription factor, Kruppel-like factor 2. Cardiovasc Res. 2008;78(1):175–184. doi: 10.1093/cvr/cvn007. [DOI] [PubMed] [Google Scholar]
- 63.Xu Y., Xu S., Liu P., Koroleva M., Zhang S., Si S. Suberanilohydroxamic acid as a pharmacological Kruppel-like factor 2 activator that represses vascular inflammation and atherosclerosis. J Am Heart Assoc. 2017;6(12) doi: 10.1161/JAHA.117.007134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu Y., Liu P., Xu S., Koroleva M., Zhang S., Si S. Tannic acid as a plant-derived polyphenol exerts vasoprotection via enhancing KLF2 expression in endothelial cells. Sci Rep. 2017;7(1):6686. doi: 10.1038/s41598-017-06803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gracia-Sancho J., Villarreal G., Jr., Zhang Y., Yu J.X., Liu Y., Tullius S.G. Flow cessation triggers endothelial dysfunction during organ cold storage conditions: strategies for pharmacologic intervention. Transplantation. 2010;90(2):142–149. doi: 10.1097/TP.0b013e3181e228db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kitada M., Koya D. Renal protective effects of resveratrol. Oxid Med Cell Longev. 2013;2013 doi: 10.1155/2013/568093. (568093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayashi K., Sasamura H., Nakamura M., Sakamaki Y., Azegami T., Oguchi H. Renin-angiotensin blockade resets podocyte epigenome through Kruppel-like factor 4 and attenuates proteinuria. Kidney Int. 2015;88(4):745–753. doi: 10.1038/ki.2015.178. [DOI] [PubMed] [Google Scholar]
- 68.Hayashi K., Itoh H. Transcription factors and epigenetic modulation: its therapeutic implication in chronic kidney disease. Arch Immunol Ther Exp (Warsz) 2015;63(3):193–196. doi: 10.1007/s00005-014-0326-6. [DOI] [PubMed] [Google Scholar]
- 69.Li H., Wang J., Xiao W., Xia D., Lang B., Yu G. Epigenetic alterations of Kruppel-like factor 4 and its tumor suppressor function in renal cell carcinoma. Carcinogenesis. 2013;34(10):2262–2270. doi: 10.1093/carcin/bgt189. [DOI] [PubMed] [Google Scholar]
- 70.Gao Y., Li H., Ma X., Fan Y., Ni D., Zhang Y. KLF6 suppresses metastasis of clear cell renal cell carcinoma via transcriptional repression of E2F1. Cancer Res. 2017;77(2):330–342. doi: 10.1158/0008-5472.CAN-16-0348. [DOI] [PubMed] [Google Scholar]
- 71.Jie Y., He W., Yang X., Chen W. Kruppel-like factor 4 acts as a potential therapeutic target of Sijunzi decoction for treatment of colorectal cancer. Cancer Gene Ther. 2017;24(9):361–366. doi: 10.1038/cgt.2017.25. [DOI] [PubMed] [Google Scholar]
- 72.Traka M.H., Chambers K.F., Lund E.K., Goodlad R.A., Johnson I.T., Mithen R.F. Involvement of KLF4 in sulforaphane- and iberin-mediated induction of p21(waf1/cip1) Nutr Cancer. 2009;61(1):137–145. doi: 10.1080/01635580802348641. [DOI] [PubMed] [Google Scholar]
- 73.Bialkowska A.B., Crisp M., Bannister T., He Y., Chowdhury S., Schurer S. Identification of small-molecule inhibitors of the colorectal cancer oncogene Kruppel-like factor 5 expression by ultrahigh-throughput screening. Mol Cancer Ther. 2011;10(11):2043–2051. doi: 10.1158/1535-7163.MCT-11-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diakiw S.M., D'Andrea R.J., Brown A.L. The double life of KLF5: opposing roles in regulation of gene-expression, cellular function, and transformation. IUBMB Life. 2013;65(12):999–1011. doi: 10.1002/iub.1233. [DOI] [PubMed] [Google Scholar]
- 75.Gao Y., Ding Y., Chen H., Chen H., Zhou J. Targeting Kruppel-like factor 5 (KLF5) for cancer therapy. Curr Top Med Chem. 2015;15(8):699–713. doi: 10.2174/1568026615666150302105052. [DOI] [PubMed] [Google Scholar]
- 76.Mallipattu S.K., Guo Y., Revelo M.P., Roa-Pena L., Miller T., Ling J. Kruppel-like factor 15 mediates glucocorticoid-induced restoration of podocyte differentiation markers. J Am Soc Nephrol. 2017;28(1):166–184. doi: 10.1681/ASN.2015060672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y., Mitch W.E. Proteins and renal fibrosis: low-protein diets induce Kruppel-like factor-15, limiting renal fibrosis. Kidney Int. 2011;79(9):933–934. doi: 10.1038/ki.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]