We identified two novel α-l-arabinofuranosidases, BlArafC and BlArafB, from B. longum subsp. longum JCM 1217, both of which are predicted to be extracellular membrane-bound enzymes. The former specifically acts on α1,2/3-l-arabinofuranosyl linkages, while the latter acts on the α1,5-l-arabinofuranosyl linkage. These enzymes cooperatively degrade arabinan and are required for the efficient growth of bifidobacteria in arabinan-containing medium. The genes encoding these enzymes are located side by side in a gene cluster involved in metabolic pathways for plant-derived polysaccharides, which may confer adaptability in adult intestines.
KEYWORDS: dietary fiber, gut microbiota, hemicellulose, intestinal microbiota, prebiotics, probiotics
ABSTRACT
Arabinose-containing poly- or oligosaccharides are suitable carbohydrate sources for Bifidobacterium longum subsp. longum. However, their degradation pathways are poorly understood. In this study, we cloned and characterized the previously uncharacterized glycoside hydrolase family 43 (GH43) enzymes B. longum subsp. longum ArafC (BlArafC; encoded by BLLJ_1852) and B. longum subsp. longum ArafB (BlArafB; encoded by BLLJ_1853) from B. longum subsp. longum JCM 1217. Both enzymes exhibited α-l-arabinofuranosidase activity toward p-nitrophenyl-α-l-arabinofuranoside but no activity toward p-nitrophenyl-β-d-xylopyranoside. The specificities of the two enzymes for l-arabinofuranosyl linkages were different. BlArafC catalyzed the hydrolysis of α1,2- and α1,3-l-arabinofuranosyl linkages found on the side chains of both arabinan and arabinoxylan. It released l-arabinose 100 times faster from arabinan than from arabinoxylan but did not act on arabinogalactan. On the other hand, BlArafB catalyzed the hydrolysis of the α1,5-l-arabinofuranosyl linkage found on the arabinan backbone. It released l-arabinose from arabinan but not from arabinoxylan or arabinogalactan. Coincubation of BlArafC and BlArafB revealed that these two enzymes are able to degrade arabinan in a synergistic manner. Both enzyme activities were suppressed with EDTA treatment, suggesting that they require divalent metal ions. The GH43 domains of BlArafC and BlArafB are classified into GH43 subfamilies 27 and 22, respectively, but show very low similarity (less than 15% identity) with other biochemically characterized members in the corresponding subfamilies. The B. longum subsp. longum strain lacking the GH43 gene cluster that includes BLLJ_1850 to BLLJ_1853 did not grow in arabinan medium, suggesting that BlArafC and BlArafB are important for assimilation of arabinan.
IMPORTANCE We identified two novel α-l-arabinofuranosidases, BlArafC and BlArafB, from B. longum subsp. longum JCM 1217, both of which are predicted to be extracellular membrane-bound enzymes. The former specifically acts on α1,2/3-l-arabinofuranosyl linkages, while the latter acts on the α1,5-l-arabinofuranosyl linkage. These enzymes cooperatively degrade arabinan and are required for the efficient growth of bifidobacteria in arabinan-containing medium. The genes encoding these enzymes are located side by side in a gene cluster involved in metabolic pathways for plant-derived polysaccharides, which may confer adaptability in adult intestines.
INTRODUCTION
Bifidobacteria are common inhabitants of the large intestine of mammals, including humans. In humans, bifidobacteria are among the dominant members of the intestinal microbiota and are believed to exert various health-promoting effects (1, 2). The species compositions of bifidobacterial populations vary with age. Bifidobacterium bifidum, B. breve, and B. longum subsp. infantis are frequently found in infant intestines, while B. adolescentis, B. catenulatum, and B. pseudocatenulatum are mainly found in adult intestines. B. longum subsp. longum is found in the intestines of both infants and adults (3). B. longum subsp. longum is also added to food products as a probiotic because of its adaptive ability and beneficial health effects to the host (1).
In the large intestine, where bifidobacteria reside, monosaccharides are limited. Therefore, bifidobacteria possess various glycosidases and sugar transporters to assimilate indigestible polysaccharides, oligosaccharides, and complex carbohydrates (4). It is also known that bifidobacterial glycosidases play important roles in glycan cross-feeding within the gut microbiota (5–7). Whole-genome sequencing revealed that approximately 8.5% of the identified open reading frames (ORFs) of B. longum NCC 2705 are related to glycan degradation and metabolism (8). In the past decade, several groups, including our own, reported that bifidobacteria in infant intestines—B. bifidum, B. longum subsp. infantis, and B. longum subsp. longum—utilize oligosaccharides from breast milk (9–18) and glycoprotein glycans secreted from the gastrointestinal tract (19–22). We also reported that B. longum subsp. longum possesses a unique metabolic pathway to assimilate glycans from plant cell wall glycoproteins called extensins (23–25). Previous research suggests that oligosaccharides derived from plant matrix polysaccharides, such as arabinan, arabinoxylan, and arabinogalactan, are potential carbohydrate sources for adult-type bifidobacteria (26, 27). Arabinan is composed of α1,5-linked poly-l-arabinofuranose as a backbone with branched side chains of α1,2- or α1,3-linked l-arabinofuranose (28). However, how bifidobacteria utilize arabinan is poorly understood. Therefore, we investigated the mechanism of arabinan utilization in B. longum subsp. longum.
In the Carbohydrate-Active Enzymes (CAZy) database (http://www.cazy.org), there are 59 putative and experimentally characterized glycosidases belonging to 23 glycoside hydrolase (GH) families from B. longum subsp. longum JCM 1217. Of these GH families, we focused on GH family 43 (GH43), which contains hemicellulose-degrading glycosidases, including α-l-arabinofuranosidase, β-xylosidase, arabinanase, and xylanase. Nine ORFs with 11 GH43 domains occur in the genome of B. longum subsp. longum JCM 1217. Notably, five previously uncharacterized GH43 ORFs, BLLJ_1850 to BLLJ_1854, form a gene cluster. In this study, we cloned and characterized two GH43 enzymes, B. longum subsp. longum ArafC (BlArafC; encoded by BLLJ_1852), belonging to GH43 subfamily 27 (GH43_27), and B. longum subsp. longum ArafB (BlArafB; encoded by BLLJ_1853), belonging to GH43 subfamily 22 (GH43_22), and found that these glycosidases cooperatively degrade arabinan by acting on different glycosidic bonds.
RESULTS
Bifidobacterium longum subsp. longum JCM 1217 utilizes arabinan as a carbon source.
Arabino-oligosaccharides are considered potential prebiotics for bifidobacteria (26, 27). In terms of arabinan, it has been reported that the utilization ability of B. longum subsp. longum differs depending on the strain (29, 30). First, we tested whether B. longum subsp. longum JCM 1217 grows in a medium with arabinan as the sole carbon source (Fig. 1). This strain grew well in d-glucose-containing medium (optical density at 600 nm [OD600] = 1.81 after 48 h of incubation) and moderately in an l-arabinose-containing one (OD600 = 1.38 after 48 h). In arabinan-containing medium, it reached a level (OD600 = 1.31 after 48 h) similar to that in l-arabinose-containing medium, although the growth rate was slightly lower. Growth was suppressed in sugar-restricted basal medium (OD600 = 0.65 after 48 h). These results suggest that B. longum subsp. longum JCM 1217 degrades arabinan into l-arabinose and utilizes that as a carbon source.
FIG 1.
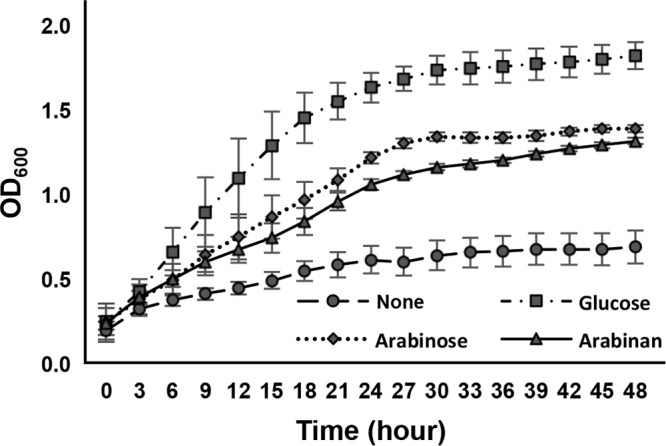
Growth of B. longum subsp. longum JCM 1217 in media containing different carbon sources. The strain was cultured at 37°C in sugar-restricted basal medium supplemented with 2.0% different carbon sources under anaerobic conditions. The OD600 was measured every 3 h for 48 h. Error bars indicate SD (n = 6).
Expression of BLLJ_1852 and BLLJ_1853 was enhanced in the presence of arabinan.
To identify the glycosidases responsible for the degradation of arabinan, we focused on the gene cluster consisting of BLLJ_1850 to BLLJ_1854, all of which encode GH43 domain-containing proteins. Of these, we have recently reported that BLLJ_1854 encodes BlArafA α-l-arabinofuranosidase, preferentially acting on arabinogalactan (31). Therefore, we investigated the expression of BLLJ_1850 to BLLJ_1853 in the presence of arabinan using semiquantitative reverse transcription-PCR (RT-PCR). The mRNA levels of BLLJ_1852 and BLLJ_1853 were estimated to be 4.0- and 5.4-fold higher in arabinan medium, respectively, than in d-glucose medium (see Fig. S1 in the supplemental material). However, the expression of BLLJ_1850 was not significantly enhanced by arabinan (less than 1.2-fold), and the mRNA of BLLJ_1851 could not be detected under these RT-PCR conditions. This result suggests that BLLJ_1852 and BLLJ_1853 are involved in the utilization of arabinan in this strain.
Molecular cloning of BLLJ_1852 and BLLJ_1853.
The ORF of BLLJ_1852 consists of 3,741 bp and encodes a polypeptide with 1,246 amino acid (aa) residues, which is predicted to contain a signal peptide (aa 1 to 30), a LamG domain (aa 95 to 253), a bacterial Ig-like domain (aa 669 to 725), a GH43 domain (aa 738 to 1,087), and a transmembrane region (aa 1,217 to 1,238) (Fig. 2A). The ORF of BLLJ_1853 consists of 3,294 bp and encodes a 1,097-aa polypeptide, which contains a signal peptide (aa 1 to 35), LamG domain (aa 81 to 258), GH43 domain (aa 491 to 782), bacterial Ig-like domain (aa 823 to 877), and transmembrane region (aa 1,069 to 1,090) (Fig. 2A). Therefore, both enzymes are predicted to be extracellular membrane-bound enzymes. LamG and bacterial Ig-like domains are frequently found in bacterial glycosidases, but their functions are unknown.
FIG 2.
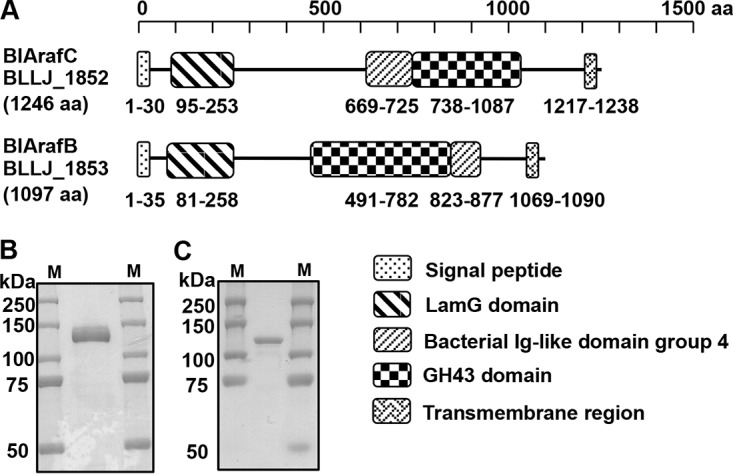
Characterization of BlArafC (BLLJ_1852) and BlArafB (BLLJ_1853). (A) Domain structure of BlArafC and BlArafB. (B) SDS-PAGE of purified BlArafC. (C) SDS-PAGE of purified BlArafB. Lanes M, molecular size markers.
We amplified BLLJ_1852 and BLLJ_1853 without the regions encoding the N-terminal signal peptide and C-terminal transmembrane region using high-fidelity PCR on the genomic DNA from B. longum subsp. longum JCM 1217 as a template. The PCR product was cloned into a plasmid vector, and the recombinant protein was expressed in Escherichia coli and affinity purified. Purified proteins migrated as a single protein band at about 130 kDa for the product of BLLJ_1852 and at about 115 kDa for that of BLLJ_1853 on SDS-PAGE (Fig. 2B and C, respectively), which is consistent with the predicted molecular masses.
Characterization of recombinant enzymes.
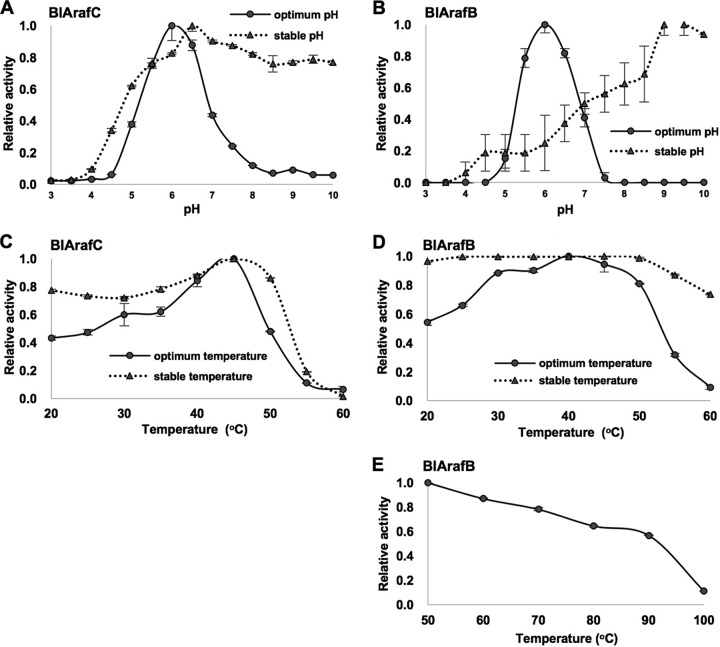
The recombinant proteins expressed from BLLJ_1852 and BLLJ_1853 hydrolyzed p-nitrophenyl-α-l-arabinofuranoside (pNP-α-Araf), indicating that both enzymes are exo-α-l-arabinofuranosidases, and thus, they were named BlArafC and BlArafB, respectively. The biochemical properties of recombinant BlArafC and BlArafB were investigated using pNP-α-Araf as a substrate. The optimum pH for both glycosidases was 6.0. However, the stable pH ranges in which enzymes retained more than 80% activity were quite different: pH 5.0 to 10.0 for BlArafC and pH 9.0 to 10.0 for BlArafB (Fig. 3A and B, respectively). The optimum temperatures for BlArafC and BlArafB were 45°C and 40°C, respectively. BlArafC was stable below 50°C for 1 h (Fig. 3C). In contrast, BlArafB was shown to be still active at 60°C for 1 h (Fig. 3D). Therefore, we tested the stability of BlArafB at higher temperatures and found that greater than 50% activity was maintained below 90°C, although it gradually declined (Fig. 3E). The kinetic parameters of recombinant BlArafC for pNP-α-Araf were determined: the Km, kcat, and kcat/Km values were 9.8 mM, 19.2 s−1, and 1.9 s−1 mM−1, respectively. These parameters of BlArafB could not be determined due to the low activity for pNP-α-Araf. We tested the activities of these enzymes for other pNP-glycosides, such as pNP-α-l-arabinopyranoside, pNP-β-xylopyranoside, and pNP-β-galactopyranoside, but no hydrolysis was observed (Fig. 4A), indicating that they are exo-α-l-arabinofuranosidases with a strict glycone specificity.
FIG 3.
General properties of recombinant BlArafC and BlArafB. pNP-α-Araf was incubated with recombinant enzymes at 37°C. (A) Effect of pH on BlArafC. (B) Effect of pH on BlArafB. (C) Effect of temperature on BlArafC. (D) Effect of temperature on BlArafB. (E) Thermal stability of BlArafB at high temperatures. Error bars indicate SD (n = 3). For stability tests, enzymes were preincubated in each pH buffer for 16 h at 37°C or in sodium acetate buffer (pH 6.0) at each temperature for 1 h.
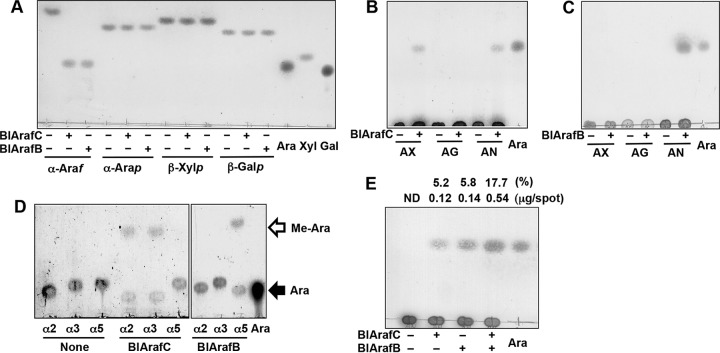
FIG 4.
Substrate specificities of BlArafC and BlArafB. The substrates were incubated with recombinant BlArafC or BlArafB at 37°C overnight and analyzed by TLC. (A) Action on synthetic substrates. α-Araf, pNP-α-l-arabinofuranoside; α-Arap, pNP-α-l-arabinopyranoside; β-Xylp, pNP-β-xylopyranoside; β-Galp, pNP-β-galactopyranoside; Ara, standard l-arabinose; Xyl, standard d-xylose; Gal, standard d-galactose. (B) Action of BlArafC on natural substrates. AX, arabinoxylan; AG, arabinogalactan; AN, arabinan; Ara, standard l-arabinose. (C) Action of BlArafB on natural substrates. (D) Action of BlArafC and BlArafB on synthetic disaccharide substrates. α2, Arafα1,2Arafα1-OMe; α3, Arafα1,3Arafα1-OMe; α5, Arafα1,5Arafα1-OMe; Ara, standard l-arabinose. (E) Synergy effect of coincubation of BlArafC and BlArafB on arabinan degradation. Arabinan was incubated with either BlArafC or BlArafB, or both. The amount of released l-arabinose was determined and the hydrolysis (in percent) of arabinan is shown above the panel. Ara, standard l-arabinose. ND, not detected. 1-Butanol–acetic acid–water (2:1:1, by volume) was used as a developing solvent in the assays whose results are shown in panels A to C and E; chloroform-methanol-acetic acid (6:1:1, by volume) was used in the assay whose results are shown in panel D.
Different effects of divalent metal ions on the activities of BlArafC and BlArafB.
Some GH43 glycosidases have been reported to have a calcium ion at their active site (32, 33). The activities of the recombinant BlArafC and BlArafB for pNP-α-Araf were measured in the presence of various divalent metal ions. BlArafC activity increased approximately 1.5-fold in the presence of all divalent metal cations tested (Zn2+, Mn2+, Cu2+, Ca2+, Co2+, Mg2+, and Ni2+). In contrast, BlArafB activity was not enhanced by these cations and was severely inhibited by Cu2+ (Fig. 5A). Both glycosidases were inhibited by EDTA, suggesting a requirement for divalent metal ions (Fig. 5A). BlArafC was more sensitive to EDTA than BlArafB (Fig. 5B). Next, we tested whether these EDTA-treated enzymes could be restored by adding cations. BlArafC and BlArafB were treated with 100 μM EDTA at 4°C for 16 h, and then the activities were measured in the presence of 1.0 mM various cations (Zn2+, Mn2+, Ca2+, Co2+, Mg2+, and Ni2+). The activity of BlArafB was fully restored by the addition of all these cations except Zn2+, whereas the activity of BlArafC was enhanced 1.7 to 2.5 times compared with that of EDTA-untreated enzyme (Fig. 5C). Notably, Zn2+, Mn2+, Co2+, Mg2+, and Ni2+ more strongly activated BlArafC than Ca2+, suggesting that divalent cations in the native enzyme can be replaced with other suitable cations.
FIG 5.
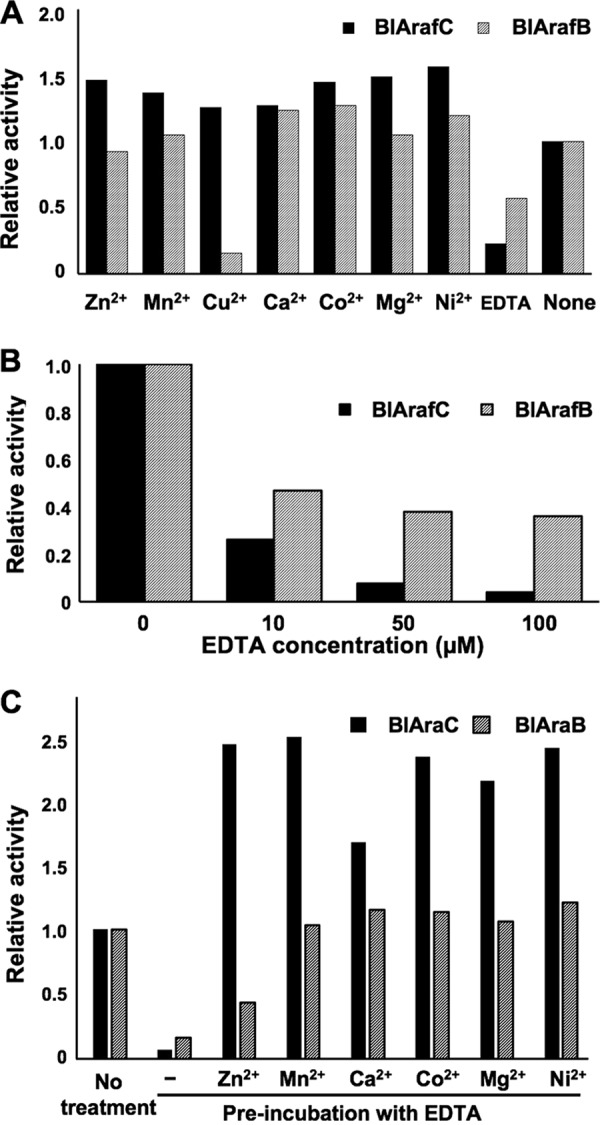
Effects of divalent metal ions on the activities of BlArafC and BlArafB. (A) Effects of metal ions on the activities of recombinant enzymes. The divalent metal ions were added at 5.0 mM. (B) Effects of various concentrations of EDTA on recombinant enzymes. (C) Restoration of enzyme activity by addition of divalent metal ions after treatment with EDTA. pNP-α-Araf was used as a substrate in all experiments. Error bars indicate SD (n = 3).
Substrate specificities of BlArafC and BlArafB.
We tested the specificities of BlArafC and BlArafB for natural substrates. BlArafC released l-arabinose from both arabinan and arabinoxylan, while BlArafB released l-arabinose from arabinan only (Fig. 4B and C, respectively). There are α1,2- and α1,3-arabinofuranosyl side chains on the α1,5-poly-l-arabinofuranosyl backbone of arabinan. The same α1,2- and/or α1,3-arabinofuranosyl side chains are on the xylan backbone of arabinoxylan. Thus, to address the linkage specificities, we used synthetic arabinobioses: Arafα1,2Arafα1-methoxy (OMe), Arafα1,3Arafα1-OMe, and Arafα1,5Arafα1-OMe. BlArafC acted on the α1,2 and α1,3 linkages, whereas BlArafB acted only on the α1,5 linkage (Fig. 4D). These results indicate that BlArafC hydrolyzes the side chains of arabinan and arabinoxylan and that BlArafB hydrolyzes the backbone of arabinan. Since BlArafC and BlArafB have different linkage specificities, we evaluated the effect of coincubation of BlArafC and BlArafB for arabinan degradation (Fig. 4E). When arabinan was incubated with either BlArafC or BlArafB, 5.2% and 5.8% of the arabinan was degraded into l-arabinose, respectively. Interestingly, when arabinan was incubated with both BlArafC and BlArafB, 17.7% of the arabinan was degraded. From this result, we concluded that synergy occurred when BlArafC and BlArafB were coincubated with arabinan.
We quantified the release rate of l-arabinose from five substrates (Table 1). Both enzymes showed the highest activity toward arabinan. BlArafC also acted on arabinoxylan, but the rate was only 0.9% of that for arabinan. The synthetic glycosides pNP-α-Araf and 4-methylumbelliferyl-α-Araf (4MU-α-Araf) were very slowly hydrolyzed by both enzymes. The specific activities of BlArafC toward pNP-α-Araf and 4MU-α-Araf were only 1.4% and 0.9% of the activity for arabinan, respectively, and those of BlArafB were 0.2% and 5.2% of the activity for arabinan, respectively. These results indicate that both enzymes have strict aglycone and linkage specificities.
TABLE 1.
Relative specific activities of BlArafC and BlArafB for various substrates
| Enzyme | Relative sp acta
|
||||
|---|---|---|---|---|---|
| pNP | 4MU | AN | AX | AG | |
| BlArafC | 1.00 | 0.62 | 70.23 | 0.62 | ND |
| BlArafB | 0.009 | 0.23 | 4.42 | ND | ND |
The reaction mixtures were incubated at 37°C for 30 min. The released l-arabinose was determined by the enzymatic measurement method, and the relative values are shown. pNP, pNP-α-l-arabinofuranoside; 4MU, 4-methylumbelliferyl-α-l-arabinofuranoside; AN, sugar beet arabinan; AX, wheat arabinoxylan; AG, larch wood arabinogalactan; ND, not detected.
Comparison of GH43 cluster in various strains of B. longum subsp. longum.
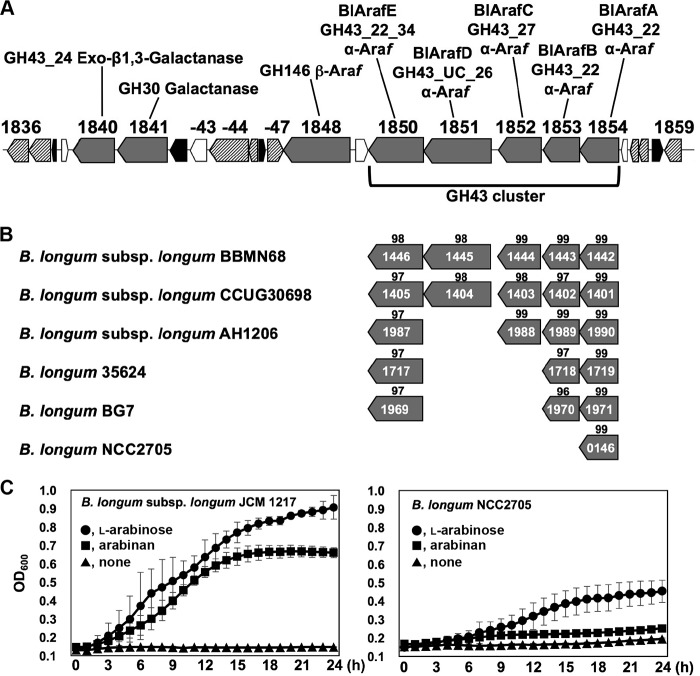
Five GH43 genes (BLLJ_1850 to BLLJ_1854) form a gene cluster in the genome of B. longum subsp. longum JCM 1217 (Fig. 6A). We compared the genomes of several strains of B. longum subsp. longum and found that this GH43 cluster is not completely conserved in all strains (Fig. 6B). Among these, B. longum NCC2705 lacks BLLJ_1850 to BLLJ_1853 homologs. To confirm the importance of BlArafC and BlArafB for assimilation of arabinan, we cultured two strains in arabinan medium (Fig. 6C). As expected, B. longum NCC2705 did not grow in arabinan medium (OD600 = 0.25 after 24 h of incubation) or in sugar-restricted basal medium (OD600 = 0.19 after 24 h) but grew well in the presence of l-arabinose (OD600 = 0.45 after 24 h). B. longum subsp. longum JCM 1217 grew well in both arabinan and l-arabinose media (OD600 = 0.66 and OD600 = 0.90, respectively, after 24 h). Although the genomic background is different between these two strains, it is highly possible that this gene cluster including BLLJ_1852 and BLLJ_1853 is essential for the assimilation of arabinan.
FIG 6.
(A) Map of the hemicellulose-degrading gene cluster in B. longum subsp. longum JCM 1217. Gray boxes, glycosidases; striped boxes, sugar transporters; black boxes, transcriptional regulators; white boxes, hypothetical proteins. BlArafE (BLLJ_1850) and BlArafD (BLLJ_1851) have two GH43 domains, and the N-terminal GH43 domain of BlArafD belongs to an unclassified (UC) subfamily. (B) Conservation of the GH43 cluster in various B. longum strains. The scores indicated above the boxes indicate the percent identity of the amino acid sequences with those of B. longum subsp. longum JCM 1217. (C) Growth of B. longum subsp. longum JCM 1217 and B. longum NCC2705 in sugar-restricted basal medium supplemented with 1.0% arabinan or l-arabinose. The strains were cultured at 37°C under anaerobic conditions. Error bars indicate SD (n = 3).
DISCUSSION
GH43 is a large family composed of more than 10,000 sequences and currently classified into 37 subfamilies (34). Nine enzymes with 11 GH43 domains from B. longum subsp. longum JCM 1217 are listed in the CAZy database. They are highly diversified, with 10 domains belonging to eight different subfamilies and 1 unclassified domain. Four GH43 enzymes, including BlArafC (GH43_27) and BlArafB (GH43_22), have been characterized. BLLJ_0213 encodes HypAA α-l-arabinofuranosidase (GH43_29), which specifically degrades α1,3-linked Araf in Arafα1,3Arafβ1-2Arafβ1-2Arafβ1-hydroxyproline of plant glycoproteins (24). BLLJ_1840 encodes an exo-β1,3-galactanase (GH43_24) that acts on the backbone of type II arabinogalactan (35). In this study, we found that BlArafC (GH43_27) and BlArafB (GH43_22), which are exo-α-l-arabinofuranosidases, cooperatively degrade arabinan. BlArafC removes α1,2- and α1,3-linked Araf side chains in arabinan, and BlArafB degrades α1,5-linked l-arabinose of the arabinan backbone from the nonreducing terminus. BlArafC also acted very slowly on arabinoxylan, which contains Arafα1,2/3Xylpβ1-R, but not on arabinogalactan, which contains Arafα1,3Galpβ1-R. Since Araf, Xylp, and Galp have equatorial hydroxyl groups at the C-2 and C-3 positions but only Galp has an axial hydroxyl group at the C-4 position, BlArafC may have a strict recognition mechanism for equatorial hydroxyl groups at the C-4 position in aglycone.
Our preliminary results suggest that the gene products of BLLJ_1850 (GH43_22_34), BLLJ_1851 (GH43_unclassified subfamily_26), and BLLJ_1854 (GH43_22; BlArafA) are also α-l-arabinofuranosidases. The products of BLLJ_1850 and BLLJ_1851 are tentatively named BlArafE and BlArafD, respectively. BlArafE rapidly acts on arabinoxylan but weakly on arabinogalactan, and BlArafD rapidly acts on arabinan and weakly on arabinoxylan. The details will be reported elsewhere (M. Komeno, Y. Yoshihara, W. Nabeshima, and H. Ashida, unpublished data). BlArafA (BLLJ_1854) rapidly acts on arabinogalactan and α1,3-Araf-Gal3 [Galβ1,6(Arafα1,3)Galβ1,6Gal] but weakly on α1,2/3/5-arabinobioses (31). Except for BLLJ_0213, these GH43 enzymes form a gene cluster, and there is almost no functional redundancy (Fig. 6A).
GH43_27 is a small subfamily whose members are restricted to bacterial enzymes. So far, only the Abf43B α-l-arabinofuranosidase from Paenibacillus sp. strain E18 (GenBank accession number AFC38437) (36) and the GbtXyl43B β-d-xylosidase from Geobacillus thermoleovorans IT-08 (GenBank accession number ABD48561) (37) have been experimentally characterized. The former hydrolyzed pNP-α-l-Araf but neither arabinan nor arabinoxylan, and the latter hydrolyzed pNP-β-d-Xylp and xylan. Since BlArafC is an arabinan-degrading exo-1,2-1,3-α-l-arabinofuranosidase, the substrate specificity of BlArafC is quite different from the substrate specificities of Abf43B and GbtXyl43B. The amino acid sequence of the GH43_27 domain (aa 738 to 1,087) of BlArafC had only 10 and 9% identity with the corresponding domains of Abf43B and GbtXyl43B, respectively. Adult-type bifidobacteria have putative homologs with higher identities of 45 to 50%: BAD_0149 from B. adolescentis ATCC 15703, BBCT_0647 from B. catenulatum, BBPC_0158 from B. pseudocatenulatum, and BBSC_0258 from B. scardovii. However, within the closely related strains of B. longum whose genomic data are available, the BlArafC homolog was lost in several strains, such as B. longum 35264, B. longum BG7, and B. longum NCC2705 (Fig. 6B) as well as in B. longum subsp. infantis.
In comparison to GH43_27, the subfamily GH43_22 is relatively larger and more widely distributed in eukaryotes (several fungi) and archaea, in addition to bacteria. In this subfamily, BAD_1527 from B. adolescentis has been predicted to be an endo-β-1,4-xylanase, although it has not been experimentally confirmed (38). In our strain, there are three enzymes belonging to this subfamily, BlArafE (GH43_22_34; BLLJ_1850), BlArafB (BLLJ_1853), and BlArafA (BLLJ_1854), which exhibit distinct substrate preferences for arabinoxylan, arabinan, and arabinogalactan, respectively, as described above. Within B. longum strains, the homologs of BLLJ_1853 and BLLJ_1850 are conserved, except in B. longum NCC2705, while BLLJ_1854 homologs are conserved in all strains (Fig. 6B). BlArafB (BLLJ_1853) is an arabinan-degrading exo-1,5-α-l-arabinofuranosidase. The enzymes with similar substrate specificity are classified in the subfamily GH43_26: Araf43A (GenBank accession number BAC68753) from Streptomyces avermitilis MA-4680 (39, 40) and Abf43K (GenBank accession number ACE82749) and Abf43L (GenBank accession number ACE84379) from Cellvibrio japonicus Ueda107 (41). However, the amino acid sequence of BlArafB shares very low identities with the amino acid sequences of Araf43A (13%), Abf43K (11%), and Abf43L (15%), suggesting that the evolutionary origins may be different. The exo-1,5-α-l-arabinofuranosidase that belongs to the other families of GH51 was also reported: AbfA (GenBank accession number CAA99595) from Bacillus subtilis strain 168 (42).
It is known that B. longum subsp. longum is widely distributed in the intestines of infants and adults. A recent study on the comparative genome analyses of human isolates revealed that this GH43 gene cluster (BLLJ_1850 to BLLJ_1854 homologs) is enriched in strains from elderly subjects (3). The essentiality of this gene cluster (BLLJ_1850 to BLLJ_1853 homologs) in arabinan utilization has also been shown in several human isolates, as revealed by in vitro culture tests and pan-genome analysis (29, 30). These findings suggest that this gene cluster is generally important for B. longum subsp. longum colonization of the intestines of adults who consume vegetables and grains. Such strains possessing this gene cluster may also play an important role in cross-feeding of arabinan within bifidobacterial populations and also the intestinal ecosystem.
MATERIALS AND METHODS
Bacterial strains and culture.
B. longum subsp. longum JCM 1217 was obtained from the Japan Collection of Microorganisms (RIKEN Bioresource Center, Japan). B. longum strain NCC2705 was provided by Nestec Ltd., Switzerland. The strains were cultured in GAM broth (Nissui Pharmaceutical, Japan) or sugar-restricted basal medium (1.0% Bacto peptone, 0.5% Bacto yeast extract, 0.5% sodium acetate trihydrate, 0.2% diammonium hydrogen citrate, 0.08% l-cysteine hydrochloride monohydrate, 0.02% magnesium sulfate heptahydrate, 1.36% l-ascorbic acid, 0.44% sodium carbonate anhydrous) at 37°C under anaerobic conditions using an AnaeroPack Anaero anaerobic chamber (Mitsubishi Chemical, Japan). Escherichia coli DH5α and E. coli BL21(λDE3) ΔlacZ (a gift from T. Katayama, Kyoto University, Kyoto, Japan) were cultured in Luria-Bertani (LB) broth (BD, USA) at 37°C under aerobic conditions.
Chemicals and substrates.
p-Nitrophenyl (pNP)-glycosides were purchased from Sigma-Aldrich, MO, USA; sugar beet arabinan and wheat arabinoxylan were purchased from Megazyme, Ireland; larch wood arabinogalactan was purchased from Tokyo Chemical Industry, Japan; and l-arabinose was purchased from Wako Chemicals, Japan. Synthetic 1-O-methylated arabinobioses (Arafα1,2Arafα1-OMe, Arafα1,3Arafα1-OMe, and Arafα1,5Arafα1-OMe) were gifts from S. Kaneko, University of the Ryukyus, Japan (43). Other chemicals were obtained from Nacalai Tesque Inc., Japan.
Growth assay of B. longum subsp. longum.
The strains were inoculated in sugar-restricted basal medium supplemented with 1.0% l-arabinose and cultured at 37°C under anaerobic conditions until the optical density at 600 nm (OD600) reached 1.0. A 10% volume seed culture was added to basal medium containing 2.0% arabinan, 2.0% l-arabinose, or 2.0% d-glucose. The wells of a 96-well plate were filled with these mixtures and sealed with Microseal B plate-sealing film (Bio-Rad, USA). The plate was maintained at 37°C, and the OD600 was monitored every 3 h using a Powerscan HT plate reader (Dainippon Sumitomo Pharma, Japan).
Semiquantitative RT-PCR.
B. longum subsp. longum JCM 1217 was cultured at 37°C under anaerobic conditions in sugar-restricted basal medium with either 2.0% arabinan or 2.0% d-glucose. RNA extraction was carried out using the TRIzol reagent (Thermo Fisher Scientific, MA, USA), and purification was carried out using a PureLink RNA minikit (Thermo Fisher Scientific). Reverse transcription was performed using PrimeScript reverse transcriptase (TaKaRa Bio Inc., Japan). Primers for RT-PCR were designed and were as follows: 5′-CCCAAGCTTGATACCACCGATTCATCGGCCGC and 5′-GTGCCGCTCGAGGGAGATGACGGCACCCGGCTTCTTG for BLLJ_1850, 5′-CGCGGATCCGGACGACGCGACGCCTGCGGTG and 5′-CCCAAGCTTAGAGGACGGCAGACCGGAGTCTGC for BLLJ_1851, 5′-GGAATTCCATATGGATACCGTTCCGACCAATAATCTCATC and 5′-CCCAAGCTTAGACAGACCGAGCTTGTTGCCCG for BLLJ_1852, and 5′-CGGAATTCGGAGAGCGCATCGCCAATCGATG and 5′-GTGCCGCTCGAGGGTGTTGGACAGAGCGCTGCCCGG for BLLJ_1853. RT-PCR was performed using TaKaRa Ex Taq HS polymerase (TaKaRa Bio Inc.). The PCR products were separated by electrophoresis on a 0.8% agarose gel. The gel was stained using SYBR Gold nucleic acid gel stain (Thermo Fisher Scientific). The densities of the bands were quantified using the ImageJ program.
Cloning, expression, and purification of enzymes.
The DNA fragment for base pairs 106 to 3645 of BLLJ_1852 (GenBank accession number BAJ67516) without the coding sequences of the N-terminal signal peptide and the C-terminal transmembrane region was amplified by high-fidelity PCR using KOD plus (version 2) DNA polymerase (Toyobo, Japan) and genomic DNA from B. longum subsp. longum JCM 1217 as a template. The same pair of primers for RT-PCR with the NdeI and HindIII sites was used. The amplified fragment was ligated into the NdeI and HindIII sites of the pET23b(+) vector (Novagen, USA). Similarly, the DNA fragment of base pairs 91 to 3203 of BLLJ_1853 (GenBank accession number BAJ67517) was amplified using the same the pair of primers used for the RT-PCR containing the EcoRI and XhoI sites. The product was ligated into the corresponding sites of pET23b(+). The nucleotide sequences were confirmed by sequencing. The E. coli BL21(λDE3) ΔlacZ strain was transformed with these plasmids and cultured in LB liquid medium containing 100 μg/ml ampicillin at 37°C under aerobic conditions until the OD600 reached 0.35. Then the flask was put on ice in order to lower the temperature. Next, to induce expression, 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the culture, which was further incubated at 25°C for 15 h. The cells were harvested by centrifugation and lysed with the BugBuster protein extraction reagent (Novagen). The cell-free protein extract was applied to a His GraviTrap column (GE Healthcare, USA), and the adsorbed proteins were eluted by a stepwise imidazole concentration gradient in 20 mM sodium acetate buffer at pH 6.5 containing 500 mM NaCl. The active fraction was dialyzed with a size 36 dialysis membrane (Wako Chemicals) against 0.5 mM sodium acetate buffer at pH 6.5 overnight at 4°C. The protein concentration was measured using a Bio-Rad protein assay dye reagent concentrate (Bio-Rad).
Glycosidase assay.
α-l-Arabinofuranosidase activity was measured based on the release of p-nitrophenyl (pNP) from pNP-α-Araf. The reaction mixture containing 1.0 mM pNP-α-Araf, an appropriate amount of purified enzyme, and 1.0 mM sodium acetate buffer at pH 5.5 for a total volume of 50 μl was incubated at 37°C. After the incubation, 1.5 volumes of 1.0 M Na2CO3 were added as a stop solution, and the released pNP was measured at 405 nm. For optimum pH tests, sodium acetate (pH 3.0 to 6.0), sodium phosphate (pH 6.5 to 7.5), and Tris-HCl (pH 8.0 to 10.0) buffers were used. The 50-μl reaction mixture containing 1.0 mM each pH buffer, 1.0 mM pNP-α-Araf, and 5 μg enzyme was incubated at 37°C for 30 min (BlArafC) or overnight (BlArafB). For the stable pH assay, the enzyme was pretreated in 10 mM buffer at 4°C for 24 h, and then the remaining activity was measured in sodium acetate buffer at pH 5.5. For the metal ion test, 5 mM chloride salts (ZnCl2, MnCl2, CuCl2, CaCl2, CoCl2, MgCl2, and NiCl2) or EDTA 2Na was added to the reaction mixtures. For the assays for natural substrates, such as arabinan, arabinoxylan, and arabinogalactan, the reaction mixture containing 0.5% substrate, 1.0 mM sodium acetate buffer at pH 5.5, and the amount of enzyme indicated above was incubated at 37°C for the appropriate period. The reaction mixture was analyzed by thin-layer chromatography (TLC) or applied to enzymatic measurements of the released l-arabinose using an l-arabinose/d-galactose assay kit (Megazyme).
TLC.
TLC analysis was carried out using silica gel 60 plates (Merck, Germany) with 1-butanol–acetic acid–water (2:1:1 by volume) or chloroform-methanol-acetic acid (6:1:1 by volume) as the developing solvent. Sugars were visualized by spraying acetone-aniline-diphenyl amine-phosphoric acid (100:1:1:10 by volume) and heating to 150°C for 15 min.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Kaneko (University of the Ryukyus, Japan) for the kind gifts of Arafα1,2Arafα1-OMe, Arafα1,3Arafα1-OMe, and Arafα1,5Arafα1-OMe and T. Katayama (Kyoto University, Japan) for providing E. coli BL21(λDE3) ΔlacZ. We also thank M. N. Ojima (Kyoto University, Japan) for critical reading of the manuscript.
This work was supported by JSPS KAKENHI grant numbers 15K07448 and 18K05494 (to H.A.).
We declare that there is no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02582-18.
REFERENCES
- 1.O’Callaghan A, Sinderen D. 2016. Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol 7:925. doi: 10.3389/fmicb.2016.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitsuoka T. 2014. Establishment of intestinal bacteriology. Biosci Microbiota Food Health 33:99–116. doi: 10.12938/bmfh.33.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odamaki T, Bottacini F, Kato K, Mitsuyama E, Yoshida K, Horigome A, Xiao J-ZZ, van Sinderen D. 2018. Genomic diversity and distribution of Bifidobacterium longum subsp. longum across the human lifespan. Sci Rep 8:85. doi: 10.1038/s41598-017-18391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garrido D, Barile D, Mills DA. 2012. A molecular basis for bifidobacterial enrichment in the infant gastrointestinal tract. Adv Nutr 3:415S–421S. doi: 10.3945/an.111.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogowski A, Briggs JA, Mortimer JC, Tryfona T, Terrapon N, Lowe EC, Baslé A, Morland C, Day AM, Zheng H, Rogers TE, Thompson P, Hawkins AR, Yadav MP, Henrissat B, Martens EC, Dupree P, Gilbert HJ, Bolam DN. 2015. Glycan complexity dictates microbial resource allocation in the large intestine. Nat Commun 6:7481. doi: 10.1038/ncomms8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turroni F, Milani C, Duranti S, Mahony J, van Sinderen D, Ventura M. 2018. Glycan utilization and cross-feeding activities by bifidobacteria. Trends Microbiol 26:339–350. doi: 10.1016/j.tim.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Gotoh A, Katoh T, Sakanaka M, Ling Y, Yamada C, Asakuma S, Urashima T, Tomabechi Y, Katayama-Ikegami A, Kurihara S, Yamamoto K, Harata G, He F, Hirose J, Kitaoka M, Okuda S, Katayama T. 2018. Sharing of human milk oligosaccharides degradants within bifidobacterial communities in faecal cultures supplemented with Bifidobacterium bifidum. Sci Rep 8:13958. doi: 10.1038/s41598-018-32080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schell MA, Karmirantzou M, Snel B, Vilanova D, Berger B, Pessi G, Zwahlen M-CC, Desiere F, Bork P, Delley M, Pridmore RD, Arigoni F. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc Natl Acad Sci U S A 99:14422–14427. doi: 10.1073/pnas.212527599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitaoka M, Tian J, Nishimoto M. 2005. Novel putative galactose operon involving lacto-N-biose phosphorylase in Bifidobacterium longum. Appl Environ Microbiol 71:3158–3162. doi: 10.1128/AEM.71.6.3158-3162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada J, Ando T, Kiyohara M, Ashida H, Kitaoka M, Yamaguchi M, Kumagai H, Katayama T, Yamamoto K. 2008. Bifidobacterium bifidum lacto-N-biosidase, a critical enzyme for the degradation of human milk oligosaccharides with a type 1 structure. Appl Environ Microbiol 74:3996–4004. doi: 10.1128/AEM.00149-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, Lapidus A, Rokhsar DS, Lebrilla CB, German JB, Price NP, Richardson PM, Mills DA. 2008. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci U S A 105:18964–18969. doi: 10.1073/pnas.0809584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashida H, Miyake A, Kiyohara M, Wada J, Yoshida E, Kumagai H, Katayama T, Yamamoto K. 2009. Two distinct α-l-fucosidases from Bifidobacterium bifidum are essential for the utilization of fucosylated milk oligosaccharides and glycoconjugates. Glycobiology 19:1010–1017. doi: 10.1093/glycob/cwp082. [DOI] [PubMed] [Google Scholar]
- 13.Miwa M, Horimoto T, Kiyohara M, Katayama T, Kitaoka M, Ashida H, Yamamoto K. 2010. Cooperation of β-galactosidase and β-N-acetylhexosaminidase from bifidobacteria in assimilation of human milk oligosaccharides with type 2 structure. Glycobiology 20:1402–1409. doi: 10.1093/glycob/cwq101. [DOI] [PubMed] [Google Scholar]
- 14.Kiyohara M, Tanigawa K, Chaiwangsri T, Katayama T, Ashida H, Yamamoto K. 2011. An exo-α-sialidase from bifidobacteria involved in the degradation of sialyloligosaccharides in human milk and intestinal glycoconjugates. Glycobiology 21:437–447. doi: 10.1093/glycob/cwq175. [DOI] [PubMed] [Google Scholar]
- 15.Bunesova V, Lacroix C, Schwab C. 2016. Fucosyllactose and l-fucose utilization of infant Bifidobacterium longum and Bifidobacterium kashiwanohense. BMC Microbiol 16:248. doi: 10.1186/s12866-016-0867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James K, Motherway MO, Bottacini F, van Sinderen D. 2016. Bifidobacterium breve UCC2003 metabolises the human milk oligosaccharides lacto-N-tetraose and lacto-N-neo-tetraose through overlapping, yet distinct pathways. Sci Rep 6:38560. doi: 10.1038/srep38560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thongaram T, Hoeflinger JL, Chow J, Miller MJ. 2017. Human milk oligosaccharide consumption by probiotic and human-associated bifidobacteria and lactobacilli. J Dairy Sci 100:7825–7833. doi: 10.3168/jds.2017-12753. [DOI] [PubMed] [Google Scholar]
- 18.Yamada C, Gotoh A, Sakanaka M, Hattie M, Stubbs K, Katayama-Ikegami A, Hirose J, Kurihara S, Arakawa T, Kitaoka M, Okuda S, Katayama T, Fushinobu S. 2017. Molecular insight into evolution of symbiosis between breast-fed infants and a member of the human gut microbiome Bifidobacterium longum. Cell Chem Biol 24:515–524.e5. doi: 10.1016/j.chembiol.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Fujita K, Oura F, Nagamine N, Katayama T, Hiratake J, Sakata K, Kumagai H, Yamamoto K. 2005. Identification and molecular cloning of a novel glycoside hydrolase family of core 1 type O-glycan-specific endo-α-N-acetylgalactosaminidase from Bifidobacterium longum. J Biol Chem 45:37415–37422. doi: 10.1074/jbc.M506874200. [DOI] [PubMed] [Google Scholar]
- 20.Kiyohara M, Nakatomi T, Kurihara S, Fushinobu S, Suzuki H, Tanaka T, Shoda S, Kitaoka M, Katayama T, Yamamoto K, Ashida H. 2012. α-N-Acetylgalactosaminidase from infant-associated bifidobacteria belonging to a novel glycoside hydrolase family 129 is implicated in an alternative mucin degradation pathway. J Biol Chem 287:693–700. doi: 10.1074/jbc.M111.277384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakinaka T, Kiyohara M, Kurihara S, Hirata A, Chaiwangsri T, Ohnuma T, Fukamizo T, Katayama T, Ashida H, Yamamoto K. 2013. Bifidobacterial α-galactosidase with unique carbohydrate-binding module specifically acts on blood group B antigen. Glycobiology 23:232–240. doi: 10.1093/glycob/cws142. [DOI] [PubMed] [Google Scholar]
- 22.Shimada Y, Watanabe Y, Wakinaka T, Funeno Y, Kubota M, Chaiwangsri T, Kurihara S, Yamamoto K, Katayama T, Ashida H. 2015. N-Acetylglucosaminidase from Bifidobacterium bifidum specifically hydrolyzes α-linked N-acetylglucosamine at nonreducing terminus of O-glycan on gastric mucin. Appl Microbiol Biotechnol 99:3941–3948. doi: 10.1007/s00253-014-6201-x. [DOI] [PubMed] [Google Scholar]
- 23.Fujita K, Sakamoto S, Ono Y, Wakao M, Suda Y, Kitahara K, Suganuma T. 2010. Molecular cloning and characterization of a β-l-arabinobiosidase in Bifidobacterium longum that belongs to a novel glycoside hydrolase family. J Biol Chem 7:5143–5150. doi: 10.1074/jbc.M110.190512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita K, Kitahara K, Suganuma T. 2012. Functional analysis of degradative enzymes for hydroxyproline-linked β-l-arabinofuranosides in Bifidobacterium longum. Trends Glycosci Glycotech 24:215–224. doi: 10.4052/tigg.24.215. [DOI] [Google Scholar]
- 25.Fujita K, Takashi Y, Obuchi E, Kitahara K, Suganuma T. 2014. Characterization of a novel β-l-arabinofuranosidase in Bifidobacterium longum: functional elucidation of a DUF1680 family member. J Biol Chem 8:5240–5249. doi: 10.1074/jbc.M113.528711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Laere K, Hartemink R, Bosveld M, Schols H, Voragen A. 2000. Fermentation of plant cell wall derived polysaccharides and their corresponding oligosaccharides by intestinal bacteria. J Agric Food Chem 48:1644–1652. doi: 10.1021/jf990519i. [DOI] [PubMed] [Google Scholar]
- 27.Holck J, Lorentzen A, Vigsnæs LK, Licht TR, Mikkelsen JD, Meyer AS. 2011. Feruloylated and nonferuloylated arabino-oligosaccharides from sugar beet pectin selectively stimulate the growth of Bifidobacterium spp. in human fecal in vitro fermentations. J Agric Food Chem 59:6511–6519. doi: 10.1021/jf200996h. [DOI] [PubMed] [Google Scholar]
- 28.Westphal Y, Kühnel S, de Waard P, Hinz SWA, Schols HA, Voragen AGJ, Gruppen H. 2010. Branched arabino-oligosaccharides isolated from sugar beet arabinan. Carbohydr Res 345:1180–1189. doi: 10.1016/j.carres.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 29.O'Callaghan A, Bottacini F, O'Connell Motherway M, van Sinderen D. 2015. Pangenome analysis of Bifidobacterium longum and site-directed mutagenesis through by-pass of restriction-modification systems. BMC Genomics 16:832. doi: 10.1186/s12864-015-1968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arboleya S, Bottacini F, O'Connell-Motherway M, Ryan CA, Ross RP, van Sinderen D, Stanton C. 2018. Gene-trait matching across the Bifidobacterium longum pan-genome reveals considerable diversity in carbohydrate catabolism among human infant strains. BMC Genomics 19:33. doi: 10.1186/s12864-017-4388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita K, Sakamoto A, Kaneko S, Kotake T, Tsumuraya Y, Kitahara K. 18 December 2018. Degradative enzymes for type II arabinogalactan side chains in Bifidobacterium longum subsp. longum. Appl Microbiol Biotechnol doi: 10.1007/s00253-018-9566-4. [DOI] [PubMed] [Google Scholar]
- 32.De Sanctis D, Inácio JM, Lindley PF, de Sá-Nogueira I, Bento I. 2010. New evidence for the role of calcium in the glycosidase reaction of GH43 arabinanases. FEBS J 277:4562–4574. doi: 10.1111/j.1742-4658.2010.07870.x. [DOI] [PubMed] [Google Scholar]
- 33.Jordan DB, Braker JD, Wagschal K, Lee CC, Chan VJ, Dubrovska I, Anderson S, Wawrzak Z. 2015. X-ray crystal structure of divalent metal-activated β-xylosidase, RS223BX. Appl Biochem Biotechnol 117:637–648. doi: 10.1007/s12010-015-1767-z. [DOI] [PubMed] [Google Scholar]
- 34.Mewis K, Lenfant N, Lombard V, Henrissat B. 2016. Dividing the large glycoside hydrolase family 43 into subfamilies: a motivation for detailed enzyme characterization. Appl Environ Microbiol 82:1686–1692. doi: 10.1128/AEM.03453-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita K, Sakaguchi T, Sakamoto A, Shimokawa M, Kitahara K. 2014. Bifidobacterium longum subsp. longum exo-β-1,3-galactanase is an enzyme for the degradation of type II arabinogalactan. Appl Environ Microbiol 80:4577–4584. doi: 10.1128/AEM.00802-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi P, Chen X, Meng K, Huang H, Bai Y, Luo H, Yang P, Yao B. 2013. Distinct actions by Paenibacillus sp. E18 α-l-arabinofuranosidases and xylanase on xylan degradation. Appl Environ Microbiol 79:1990–1995. doi: 10.1128/AEM.03276-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ratnadewi AAI, Fanani M, Kurniasih SD, Sakka M, Wasito EB, Sakka K, Nurachman Z, Puspaningsih NNT. 2013. β-d-Xylosidase from Geobacillus thermoleovorans IT-08: biochemical characterization and bioinformatics of the enzyme. Appl Biochem Biotechnol 170:1950–1964. doi: 10.1007/s12010-013-0329-5. [DOI] [PubMed] [Google Scholar]
- 38.Amaretti A, Bernardi T, Leonardi A, Raimondi S, Zanoni S, Rossi M. 2013. Fermentation of xylo-oligosaccharides by Bifidobacterium adolescentis DSMZ 18350: kinetics, metabolism, and β-xylosidase activities. Appl Microbiol Biotechnol 97:3109–3117. doi: 10.1007/s00253-012-4509-y. [DOI] [PubMed] [Google Scholar]
- 39.Ichinose H, Yoshida M, Fujimoto Z, Kaneko S. 2008. Characterization of a modular enzyme of exo-1,5-α-l-arabinofuranosidase and arabinan binding module from Streptomyces avermitilis NBRC14893. Appl Microbiol Biotechnol 80:399–408. doi: 10.1007/s00253-008-1551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujimoto Z, Ichinose H, Maehara T, Honda M, Kitaoka M, Kaneko S. 2010. Crystal structure of an exo-1,5-α-l-arabinofuranosidase from Streptomyces avermitilis provides insights into the mechanism of substrate discrimination between exo- and endo-type enzymes in glycoside hydrolase family 43. J Biol Chem 44:34134–34143. doi: 10.1074/jbc.M110.164251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cartmell A, McKee L, Peña MJ, Larsbrink J, Brumer H, Kaneko S, Ichinose H, Lewis RJ, Viksø-Nielsen A, Gilbert HJ, Marles-Wright J. 2011. The structure and function of an arabinan-specific α-1,2-arabinofuranosidase identified from screening the activities of bacterial GH43 glycoside hydrolases. J Biol Chem 17:15483–15495. doi: 10.1074/jbc.M110.215962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inácio JM, Correia IL, de Sá-Nogueira I. 2008. Two distinct arabinofuranosidases contribute to arabino-oligosaccharide degradation in Bacillus subtilis. Microbiology 154:2719–2729. doi: 10.1099/mic.0.2008/018978-0. [DOI] [PubMed] [Google Scholar]
- 43.Kawabata Y, Kaneko S, Kusakabe I, Gama Y. 1995. Synthesis of regioisomeric methyl α-l-arabinofuranobiosides. Carbohydr Res 267:39–47. doi: 10.1016/0008-6215(94)00290-V. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.