Streptococcus pneumoniae causes various diseases worldwide. Current pneumococcal vaccines protect against a limited number of more than 90 pneumococcal serotypes, accentuating the urgent need to develop novel prophylactic strategies. S. pneumoniae and the commensal Streptococcus mitis share immunogenic characteristics that make S. mitis an attractive vaccine candidate against S. pneumoniae. In this study, we evaluated the potential of S. mitis and its mutant expressing pneumococcal capsule type 4 (S. mitis TIGR4cps) to induce protection against S. pneumoniae lung infection in mice. Our findings show that intranasal vaccination with S. mitis protects against S. pneumoniae strains D39 (serotype 2) and TIGR4 (serotype 4) in a serotype-independent fashion, which is associated with enhanced antibody and T cell responses. Furthermore, S. mitis TIGR4cps conferred additional protection against S. pneumoniae TIGR4, but not against D39. The findings highlight the potential of S. mitis to generate protection that combines both serotype-independent and serotype-specific responses.
KEYWORDS: commensals, infection, pneumonia, Streptococcus mitis, Streptococcus pneumoniae, vaccines
ABSTRACT
Streptococcus pneumoniae is a bacterial pathogen that causes various diseases of public health concern worldwide. Current pneumococcal vaccines target the capsular polysaccharide surrounding the cells. However, only up to 13 of more than 90 pneumococcal capsular serotypes are represented in the current conjugate vaccines. In this study, we used two experimental approaches to evaluate the potential of Streptococcus mitis, a commensal that exhibits immune cross-reactivity with S. pneumoniae, to confer protective immunity to S. pneumoniae lung infection in mice. First, we assessed the immune response and protective effect of wild-type S. mitis against lung infection by S. pneumoniae strains D39 (serotype 2) and TIGR4 (serotype 4). Second, we examined the ability of an S. mitis mutant expressing the S. pneumoniae type 4 capsule (S. mitis TIGR4cps) to elicit focused protection against S. pneumoniae TIGR4. Our results showed that intranasal immunization of mice with S. mitis produced significantly higher levels of serum IgG and IgA antibodies reactive to both S. mitis and S. pneumoniae, as well as enhanced production of interleukin 17A (IL-17A), but not gamma interferon (IFN-γ) and IL-4, compared with control mice. The immunization resulted in a reduced bacterial load in respiratory tissues following lung infection with S. pneumoniae TIGR4 or D39 compared with control mice. With S. mitis TIGR4cps, protection upon challenge with S. pneumoniae TIGR4 was superior. Thus, these findings show the potential of S. mitis to elicit natural serotype-independent protection against two pneumococcal serotypes and to provide the benefits of the well-recognized protective effect of capsule-targeting vaccines.
IMPORTANCE Streptococcus pneumoniae causes various diseases worldwide. Current pneumococcal vaccines protect against a limited number of more than 90 pneumococcal serotypes, accentuating the urgent need to develop novel prophylactic strategies. S. pneumoniae and the commensal Streptococcus mitis share immunogenic characteristics that make S. mitis an attractive vaccine candidate against S. pneumoniae. In this study, we evaluated the potential of S. mitis and its mutant expressing pneumococcal capsule type 4 (S. mitis TIGR4cps) to induce protection against S. pneumoniae lung infection in mice. Our findings show that intranasal vaccination with S. mitis protects against S. pneumoniae strains D39 (serotype 2) and TIGR4 (serotype 4) in a serotype-independent fashion, which is associated with enhanced antibody and T cell responses. Furthermore, S. mitis TIGR4cps conferred additional protection against S. pneumoniae TIGR4, but not against D39. The findings highlight the potential of S. mitis to generate protection that combines both serotype-independent and serotype-specific responses.
INTRODUCTION
Streptococcus pneumoniae is a significant bacterial pathogen that colonizes the human upper respiratory tract (1). About 14 million children suffer from serious pneumococcal diseases, including pneumonia and sepsis, and about 2 to 3 million of them, particularly children below 5 years of age, succumb to death each year worldwide (1, 2). Because this organism poses a great threat to human health, the World Health Organization (WHO) has recently included S. pneumoniae in the antibiotic-resistant priority list of 12 primary pathogens (3). Increasing emergence of pneumococcal resistance to multiple classes of antibiotics, such as penicillin and erythromycin, creates important therapeutic limitations, underscoring the need to focus on effective preventive strategies (4). Current pneumococcal vaccines, pneumococcal conjugate vaccine (PCV) and pneumococcal polysaccharide vaccine (PPSV), fail to provide optimal protection against S. pneumoniae, mainly because (i) vaccine formulations contain only up to 13 to 23 of the 97 known serotypes; (ii) PPSV has low efficacy against pneumococcal pneumonia, and despite covering more serotypes than PCV, is not immunogenic for children; and (iii) PCV has been associated with nonvaccine serotype replacement (5). Current efforts to develop optimal vaccines are focused on increasing the number of serotypes covered by vaccines, adding conserved pneumococcal proteins, but not much success has so far been achieved (6–8). Approaches to develop a live vaccine based on attenuated S. pneumoniae strains are also under investigation, as they have the potential to elicit responses that more closely resemble natural acquired immunity to S. pneumoniae (9, 10). Therefore, novel prophylactic strategies, which are focused either on protective immunity to serotypes prevalent in a specific region/country or on broad protection across all serotypes, are urgently needed to confer optimal protection against S. pneumoniae.
Streptococcus mitis is a commensal bacterium that inhabits the human oral cavity and upper respiratory tract (11, 12) and seems to colonize infants as early as their first days of life (13), in proportions that show high interindividual variability (14). Phylogenetic analysis shows that S. pneumoniae and S. mitis share a significant proportion of genes (about 80%) (15). Despite the close relatedness between the two bacterial species, S. mitis rarely causes diseases, securing a peaceful coexistence with its human host by having a reduced genome that lacks many pneumococcal virulence genes (13, 16). We have recently shown that human memory Th17 cells that are specific to S. mitis cross-react with S. pneumoniae (17) and that rabbit antibodies (Abs) raised against S. mitis recognize multiple S. pneumoniae proteins, and vice versa (18). To date, however, no studies have assessed the ability of S. mitis to protect against pneumococcal infections or characterized possible immune responses triggered by S. mitis introduced via external mucosal surfaces.
In this study, we used two experimental approaches to evaluate the potential of S. mitis, a commensal that exhibits immune cross-reactivity with S. pneumoniae, to confer protective immunity to S. pneumoniae lung infection in mice. First, we assessed whether wild-type S. mitis was effective in conferring protection against S. pneumoniae strains D39 (serotype 2) and TIGR4 (serotype 4). Secondly, we examined the ability of an S. mitis mutant expressing the S. pneumoniae type 4 capsule (S. mitis TIGR4cps) to elicit specific protection against S. pneumoniae TIGR4. Our results indicate a protective efficacy of S. mitis and the S. mitis TIGR4cps against S. pneumoniae, which could have implications in developing strategies to prevent infections with S. pneumoniae that are independent of its serotype but that can also be combined with the well-recognized protective responses targeting the capsular polysaccharides.
RESULTS
Enhanced IgG and IgA antibody responses following intranasal immunization of mice with S. mitis.
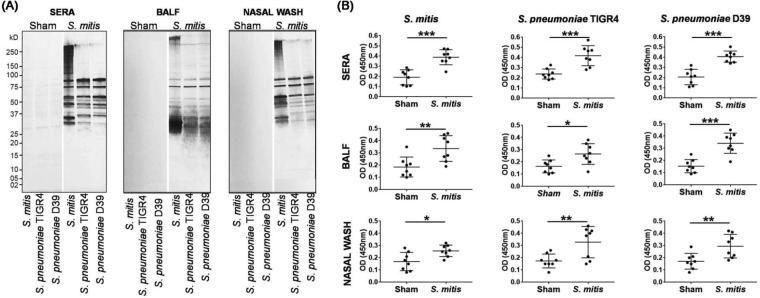
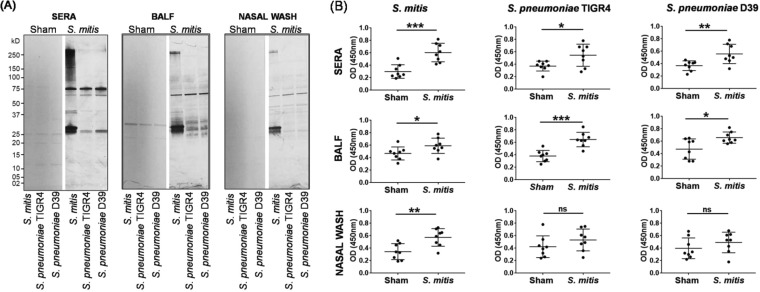
Studies involving humans and mice suggest a crucial role for IgG antibodies in protective immunity to S. pneumoniae (19, 20). Our recent findings also showed that subcutaneous immunization of rabbits with S. mitis induces IgG antibodies that react with both S. mitis and S. pneumoniae (18). Likewise, secretory IgA antibodies counter microbial pathogens, including S. pneumoniae, at the infection site (21, 22). In newborn children, salivary IgA antibodies show reactivity to several antigens of S. mitis, but whether these antibodies are generated in response to S. mitis remains unclear (23). In order to assess how S. mitis influences antibody responses, we subjected mice to intranasal immunization with S. mitis and examined IgG and IgA antibodies in the nasal wash, bronchoalveolar lavage fluid (BALF), and sera. Western blotting demonstrated that sera from the S. mitis-immunized mice cross-reacted with S. pneumoniae (TIGR4 and D39) proteins, resulting in several positive bands (Fig. 1A). This cross-reactivity was also evident in the nasal wash and BALF samples (Fig. 1A). Most visible bands were between 25 and 100 kDa (Fig. 1A). In contrast to the immunized mice, the sera from control mice inoculated with phosphate-buffered saline (PBS) reacted weakly with streptococcal proteins, reflecting very faint to almost no bands (Fig. 1A). To examine the antibody responses to pneumococci, we used a whole-cell enzyme-linked immunosorbent assay (ELISA) and measured the antibody levels in the nasal wash, BALF, and sera of the mice vaccinated with S. mitis. The immunized mice displayed significantly higher levels of IgG antibodies binding to S. mitis and S. pneumoniae (TIGR4 and D39) compared with IgG levels in control mice (Fig. 1B). Similar to IgG antibodies, the immunized mice exhibited binding of IgA antibodies to S. pneumoniae TIGR4 and D39 (Fig. 2A). Increased levels of IgA antibodies reactive to S. mitis and S. pneumoniae were seen in immunized mice (Fig. 2B). To quantify the IgG and IgA levels, we performed serial dilutions of the sera and measured the antibody titers. IgG and IgA titers from the S. mitis-immunized mice were much higher than those from mice inoculated with PBS (see Fig. S1 in the supplemental material). We were, however, unable to calculate the antibody titers in the BALF and nasal wash due to the low levels in these samples. Taken together, these findings reveal that following mucosal immunization with S. mitis, mice generate enhanced local and systemic IgG and IgA antibody responses that are specific for S. mitis but also cross-react with S. pneumoniae serotypes.
FIG 1.
IgG responses after intranasal immunization of mice with S. mitis. Mice were immunized with S. mitis, and nasal wash, BALF, and serum samples were collected to analyze IgG responses. (A) Reactivity of the sera from the immunized mice with S. mitis and S. pneumoniae serotypes by SDS-PAGE in gradient gels and by immunoblotting. Each lane was loaded with 50 µg of protein from the indicated bacterial species. (B) Levels of streptococcus-reactive IgG in antisera by whole-cell ELISA. The sera were diluted 1:1,000 and nasal wash and BALF 1:10. The data are shown as means ± standard deviations (SD) and pooled from the results of two independent experiments with 4 mice in each group. Each symbol represents data from an individual mouse, and the horizontal bars are the mean values for the groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired Student's t test.)
FIG 2.
IgA responses to intranasal immunization of mice with S. mitis. Mice were immunized with S. mitis or PBS, and nasal wash, BALF, and serum samples were collected to analyze IgA responses. (A) Reactivity of the sera from the immunized mice with S. mitis and S. pneumoniae serotypes by SDS-PAGE in gradient gels and by immunoblotting. Each lane was loaded with 50 µg of protein from the indicated bacterial species. (B) Levels of streptococcus-reactive IgA antibodies in sera by whole-cell ELISA. The sera were diluted 1:1,000 and nasal wash and BALF 1:10. The data are shown as means ± SD and were pooled from the results of two independent experiments with 4 mice in each group. Each symbol represents data from an individual mouse, and the horizontal bars are mean values for the groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant (unpaired Student's t test).
Increased IL-17A/Th17 immunity in local tissues after immunization with S. mitis intranasally.
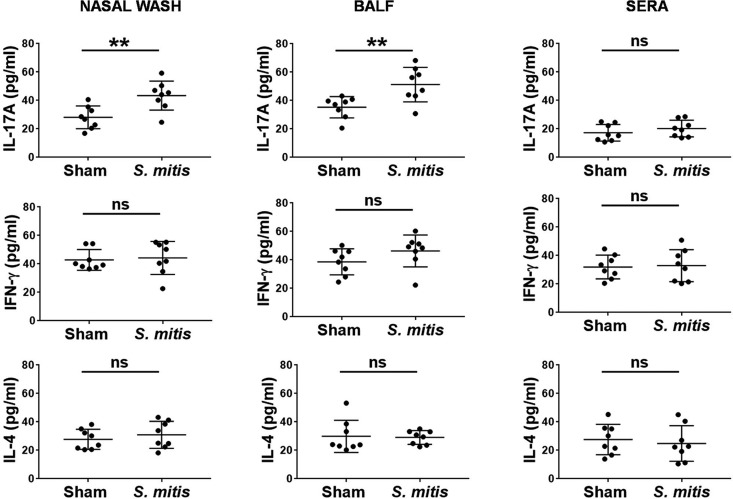
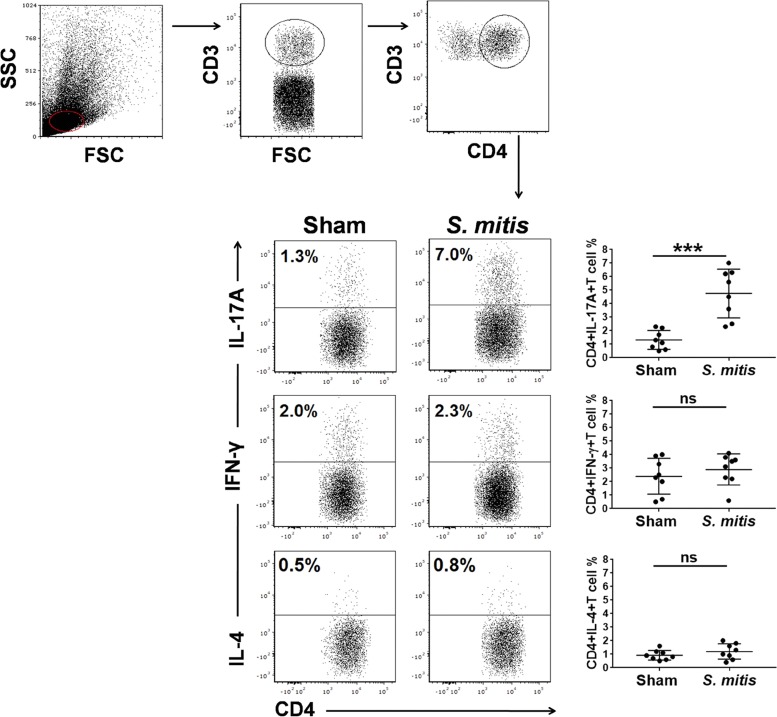
CD4+ T cell responses, in particular interleukin 17A (IL-17A)/Th17, are crucial for immunity to S. pneumoniae lung infections, as demonstrated by both murine and human studies (24, 25). How commensal bacteria influence the polarization and differentiation of T helper cells is largely unknown. Our recent study showed that S. mitis stimulates human memory CD4+ T cells to produce multiple cytokines, such as IL-17A and gamma interferon (IFN-γ), but whether this is true in vivo is unexplored (17). In order to examine the impact of S. mitis on T cell responses, we first assessed the local and systemic production of important T cell cytokines following immunization with S. mitis. Cytokine analysis of tissue samples from immunized mice showed enhanced production of IL-17A, but not IFN-γ and IL-4, in the nasal wash and BALF compared with the mice receiving PBS (Fig. 3). However, none of the cytokines (IL-17A, IFN-γ, or IL-4) was significantly elevated in sera (Fig. 3). To gain a better understanding of how S. mitis modulates the cytokine profile of T helper cells, we performed flow-cytometric intracellular-cytokine staining of CD4+ T cells in the lungs and spleens of mice after vaccination with S. mitis. We found that S. mitis induced lung CD4+ T cells to produce large amounts of IL-17A, but not IFN-γ and IL-4, that were significantly larger than those produced by control mice (Fig. 4). This increased Th17 response was not observed in splenic CD4+ T cells (Fig. 5). Cumulatively, these data reveal that nasal vaccination with S. mitis confers mucosal IL-17A/Th17 immunity to S. pneumoniae in the lungs, but not in the spleen, suggesting an S. mitis-induced Th17 bias at the local site of immunization.
FIG 3.
Cytokine responses in mice immunized intranasally with S. mitis. Samples were collected from the nasal wash, BALF, and sera from the immunized mice, and the levels of cytokines were measured using ELISA. The data are shown as means ± SD and were pooled from the results of two independent experiments with 4 mice in each group. Each symbol represents data from an individual mouse, and the horizontal bars are mean values for the groups. **, P < 0.01; ns, not significant (unpaired Student's t test).
FIG 4.
Production of cytokines by lung CD4+ T cells after S. mitis immunization. IL-17A, IFN-γ, and IL-4 production in CD4+ T cells isolated from the lungs was analyzed by flow-cytometric intracellular-cytokine staining. (Top) Cells were gated on CD3+ CD4+ T cells. (Bottom) Representative flow cytometric dot plot images (left) and a summary of the percentages of cytokine-producing CD4+ T cells (right). The data are shown as means ± SD and were pooled from the results of two independent experiments with 4 mice in each group. Each symbol represents data from an individual mouse, and the horizontal bars are mean values for the groups. ***, P < 0.001; ns, not significant (unpaired Student's t test).
FIG 5.
Production of cytokines by splenic CD4+ T cells after S. mitis immunization. IL-17A, IFN-γ, and IL-4 production in CD4+ T cells isolated from the spleen was analyzed by flow-cytometric intracellular-cytokine staining. Cells were gated on CD3+ CD4+ T cells as described in the legend to Fig. 4. Representative flow-cytometric images (left) and a summary of the percentages of cytokine-producing CD4+ T cells (right) are shown. The data are shown as means ± SD and were pooled from the results of two independent experiments with 4 mice in each group. Each symbol represents data from an individual mouse, and the horizontal bars are mean values for the groups; ns, not significant (unpaired Student's t test).
S. mitis triggers protection against S. pneumoniae serotypes 2 and 4.
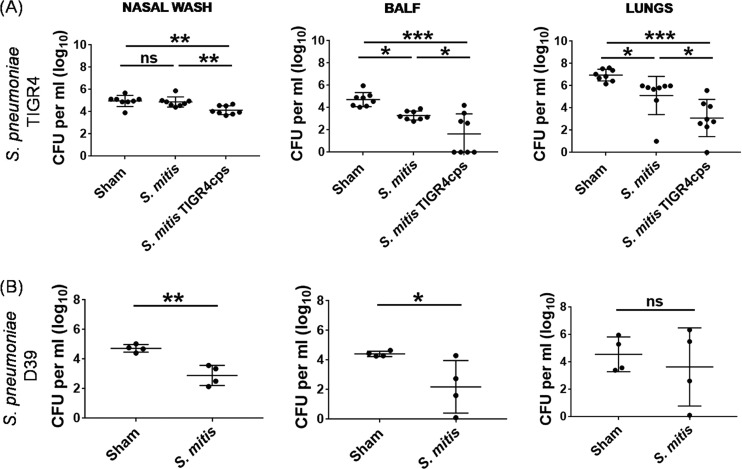
To evaluate the ability of S. mitis to confer protection against S. pneumoniae, we immunized mice with S. mitis (50 × 106 CFU in 20 µl PBS/mouse) thrice (days 0, 14, and 21) intranasally, followed by infection challenge with S. pneumoniae TIGR4 or D39 (8 × 106 CFU in 50 µl PBS/mouse) at day 7 after the last immunization, and killed them 24 h after the challenge. Mice that received PBS alone (50 µl PBS/mouse) with the same challenge were used as controls. The pneumococcal CFU count following the challenge of the immunized mice was used as an indicator of protective immunity, as described in previous studies (10, 20, 26). We found that the loads of S. pneumoniae TIGR4 in the BALF and lungs, but not the nasal wash, of the S. mitis-immunized mice were significantly lower than in the sham-treated mice (Fig. 6A). Of note, S. mitis failed to exert a significant effect on nasal colonization by S. pneumoniae EF3030 (see Fig. S2 in the supplemental material). To further assess whether S. mitis-induced protective immunity is biased toward a single pneumococcal serotype (S. pneumoniae TIGR4), we challenged the immunized mice with S. pneumoniae D39 and analyzed the bacterial burden after they were killed. The immunized mice displayed reduced S. pneumoniae D39 burden in the BALF and nasal wash compared with control mice (Fig. 6B). Pneumococcal counts in the lungs of immunized mice were lower but did not reach statistical significance (Fig. 6B). Together, these findings demonstrate that S. mitis induces defense against lung infection by two different S. pneumoniae serotypes and reduces nasal colonization by S. pneumoniae D39, but not TIGR4 and EF3030.
FIG 6.
Testing protective immunity to S. pneumoniae elicited by S. mitis or its mutant. CD1 mice were intranasally inoculated with S. mitis or S. mitis expressing the S. pneumoniae TIGR4 capsule (S. mitis TIGR4cps) or with PBS (sham) at days 0, 14, and 21 and then subjected to intranasal challenge with S. pneumoniae strain TIGR4 or D39 a week after the last immunization. The mice were sacrificed 24 h following the challenge infection, and the nasal wash, BALF, and lungs were collected for analysis of bacterial CFU. (A) Pneumococcal load in S. mitis- or S. mitis TIGR4cps-immunized mice following TIGR4 challenge. (B) Bacterial burden in mice immunized with S. mitis and subjected to D39 infection. The data are shown as means ± SD and were pooled from the results of two independent experiments with 4 mice in each group for S. pneumoniae TIGR4 challenge, whereas one experiment with 4 mice was performed for S. pneumoniae D39 challenge. Each symbol represents data from an individual mouse, and the horizontal bars are mean values for the groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001. One-way ANOVA and the Tukey test were used to compare multiple groups and an unpaired Student t test for comparing two groups.
S. mitis TIGR4cps induces enhanced protection against S. pneumoniae TIGR4 that is serotype dependent.
Our previous study showed that the mutant S. mitis TIGR4cps, which acquires the serotype 4 capsule of S. pneumoniae TIGR4 following induction of competence for natural transformation, exhibited resistance to macrophage phagocytosis and early clearance in a mouse model of lung infection (27). To move a step ahead, we sought to investigate if S. mitis TIGR4cps, being immunogenic and resistant to clearance, can generate protective immunity to S. pneumoniae. Following immunization with S. mitis TIGR4cps and subsequent challenge with S. pneumoniae TIGR4, mice displayed a significant reduction in the bacterial loads in the BALF and lungs compared with mice immunized with S. mitis (Fig. 6A). In contrast, upon challenge with S. pneumoniae D39, no significant difference was observed between the pneumococcal loads from the S. mitis- and S. mitis TIGR4cps-immunized mice (see Fig. S3 in the supplemental material). Thus, S. mitis TIGR4cps confers protection against S. pneumoniae that is specific to its pneumococcal serotype.
DISCUSSION
In the present study, our findings shed light on a novel prophylactic strategy to exploit the potential of S. mitis and its mutant expressing the pneumococcal capsule to give rise to protection against S. pneumoniae in two ways: serotype independent and serotype dependent. Intranasal immunization with S. mitis generates serotype-independent protection against lung infection with S. pneumoniae serotypes 2 and 4, whereas S. mitis TIGR4cps elicits enhanced serotype-specific protection against S. pneumoniae TIGR4. Thus, these findings provide important and novel information on S. mitis-based prophylactic approaches against clinically significant pneumococcal serotypes.
One of the most important findings of our present study encompasses the protective effect of intranasal immunization of mice with the oral commensal S. mitis against S. pneumoniae TIGR4 and D39, underscoring the capacity of S. mitis to induce protective immunity that is independent of pneumococcal serotypes 2 and 4. These findings may have implications for providing an attractive rationale for designing a live S. mitis-based vaccine that confers protection against a broad range of S. pneumoniae serotypes. Exploiting attenuated live bacteria as a vaccine has several advantages, such as mimicry of a natural infection, induction of mucosal immunity, and the possibility for oral/nasal administration (28). In spite of these advantages, attenuated live bacteria can lead to potential health hazards. For example, live attenuated bacteria can revert to virulent organisms and cause disease (28). In addition, these vaccines are not prescribed for pregnant women to rule out the risk of transplacental infection (28). Therefore, safety has been a major concern for the use of live vaccines in humans. Despite sharing many similarities with S. pneumoniae, S. mitis is a common colonizer of the oral cavity and nasopharynx that seldom causes disease, underlining its commensal relationship with humans. Previous studies have evaluated the prophylactic/therapeutic potential of oral streptococcal commensals, such as Streptococcus salivarius and S. mitis, against recurrent otitis media (middle ear inflammation) in children, which is primarily caused by S. pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, with no adverse effects (29–31). It has also been shown that natural colonization of the nasopharynx by S. pneumoniae strains BHN 418 (serotype 6B) in humans and EF3030 (serotype 19F) in mice is important for generating antibody- and T cell-mediated immunity against S. pneumoniae (10, 19). In our study, however, S. mitis failed to colonize the murine nasopharynx, as demonstrated by the lack of recovery of CFU from mice at day 1 or 28 after the first bacterial inoculation (unpublished observation), which may be attributed to the resistance offered by the respiratory microbiota. Moreover, germ-free mice and piglets, but not those with intact microbiota, are effectively colonized by S. mitis (32, 33). Our findings also throw light on the effect of S. mitis immunization on nasopharyngeal colonization of mice by S. pneumoniae TIGR4, D39, and EF3030. Although S. mitis reduced colonization by S. pneumoniae D39, it did not have a significant impact on S. pneumoniae TIGR4 and EF3030 colonization, suggesting strain-specific variations in protection against nasal colonization. Overall, live S. mitis may be a suitable pneumococcal vaccine for intranasal vaccination to confer mucosal immunity to pneumococci with broad serotype coverage, low cost, and safety.
Although our results demonstrate protective immunity induced by intranasal immunization with S. mitis, a question remains as to whether S. mitis, which inhabits the human oral and nasopharyngeal cavities, protects humans from pneumococcal infections. It is our hypothesis that S. mitis induces protection, which is anchored in previous findings that human IgG pools, representing pooled antibody responses acquired through natural exposure across a population, bind with S. mitis (34) and that the antibodies raised against S. mitis cross-react with S. pneumoniae (18). Furthermore, infants vary in their colonization status with commensal streptococci acquired when they are born, and such individual differences are also observed later in life (14, 35). Also, children resistant to tonsillitis are reported to have higher numbers of commensal streptococci (36). It is important to note that having tolerogenic features does not exclude the induction of effector immunity by commensals (37, 38). Regarding protection in the absence of carriage, our results are in accordance with a previous report that intranasal challenge of healthy individuals with live S. pneumoniae without subsequent carriage induced increased antigen-specific IgG antibody responses in the lungs, suggesting that immunity can be induced in the absence of carriage (39).
We have previously identified certain pneumococcal proteins, such as choline-binding protein D (CbpD), as targets of rabbit IgG antibodies raised against S. mitis (18). Our Western blotting data from the present study also show the binding of IgG and IgA antibodies from sera raised against S. mitis to multiple proteins of S. pneumoniae serotypes 2 and 4. Hence, various protein antigens conserved between S. mitis and S. pneumoniae might be responsible for invoking protective humoral immunity to different serotypes, as seen in this study. S. mitis also possesses capsules that can potentially generate anticapsular antibodies cross-reactive to pneumococcal polysaccharide antigens (40). Nevertheless, S. mitis immunization results in IgG and IgA antibodies that cross-react with different pneumococcal serotypes. It is notable that several pathogenic bacteria, like S. pneumoniae, H. influenzae, and N. meningitidis, secrete IgA1 proteases that destroy the structure and function of human IgA1 antibodies, which may induce susceptibility to invasive infections (41, 42). However, murine IgA is not susceptible to the IgA1 proteases of these human pathogens, which should be taken into account when extrapolating our findings to humans. On the other hand, IL-17/Th17 responses have been shown to promote immunity to S. pneumoniae lung infection (10). Our previous study demonstrated that human memory Th17 cells that are stimulated with antigen-presenting cells pulsed with S. mitis elicit cross-reactivity to S. pneumoniae (17). In accordance with these findings, our data show that nasal vaccination of mice with live S. mitis generates IL-17A responses in the nasal wash and BALF. Of note, cytokine levels in the sera, nasal wash, and BALF were quite low, which may be due to the cytokine measurement at day 7 after the last immunization. Moreover, Th17 immunity was significantly increased in the lungs, but not the spleen, suggesting local protective immunity induced by S. mitis. Emerging evidence also points out the importance of local mucosal immunity in providing defense against respiratory infections (43, 44). Taken together, our data shed light on the impact of mucosal immunization of mice with S. mitis on local T cell-mediated immunity against pneumococci. Although vaccination with S. mitis induces both antibody and Th17 responses against S. mitis and S. pneumoniae D39 and TIGR4, it has yet to be determined if the humoral and/or Th17 immunity is actually protective. We hypothesize that both antibody and Th17 immunities are important for protection against pneumococcal lung infection. This is in line with a previous study where intranasal inoculation of mice with a mild S. pneumoniae EF3030 strain gave rise to protective immunity against pneumococcal lung infection that was directly dependent on antibody and Th17 responses (10). Our future studies will investigate the roles of humoral and T cell immunity in protective immunity using mouse models that are deficient in specific immune components.
Our data also show that S. mitis TIGR4cps elicits better protective immunity against S. pneumoniae TIGR4 than the S. mitis type strain. Also, S. mitis TIGR4cps not only expressed the S. pneumoniae TIGR4 capsule, but also retained its serotype 4 capsule-specific immunogenicity. This reflects the potential of S. mitis as an efficient vector for expressing the pneumococcal antigens. Although we have previously shown that having the TIGR4 capsule may provide the S. mitis TIGR4cps mutant an advantage in surviving clearance from the lungs, such an advantage was not observed in a mouse model of septicemia (27). In any case, the capsule locus is found in at least half of the sequenced S. mitis strains, despite the fact that they are seldom invasive (27). Accumulating evidence further shows that several S. mitis clinical isolates express capsules that are similar or nearly identical to those from S. pneumoniae serotypes (45, 46). As more genome sequences of S. mitis, which is a large and diverse group, become available, these numbers are likely to increase. Overall, developing S. mitis mutants expressing the pneumococcal capsular antigens and identifying naturally occurring S. mitis strains with capsules of S. pneumoniae serotypes could be used for serotype-dependent prophylactic strategies, as seen in our current vaccine formulations, which can confer focused protective immunity to serotypes prevalent in specific areas. In general, live attenuated vaccines induce responses that are seldom matched by those using selected antigens. Thus, in contrast to serotype-specific vaccines, live nasal vaccines would provide multiple benefits, e.g., a high level of antigen exposure, easy accessibility and little discomfort to patients, and induction of mucosal and systemic immunity (9, 47). However, the possibility of developing virulence and creating a balance between attenuation and antigenicity pose challenges to the success of such vaccines (47). Being a natural attenuated version of S. pneumoniae that does not need to be constructed or selected in the laboratory, S. mitis, in combination with adjuvants, may elicit effective protection against a broad range of S. pneumoniae serotypes, including serotype 4. Furthermore, although pneumococcal capsular polysaccharides are characterized as T cell-independent (TI) antigens, T cells can influence the humoral immunity to these antigens (48). On the other hand, pneumococcal protein antigens, like surface proteins, function as T cell-dependent (TD) antigens (49). Since S. mitis TIGR4cps expresses the pneumococcal TIGR4 capsule, as well as surface proteins like PsaA and CbpD, which are similar to and cross-reactive with their pneumococcal counterparts (18), it might combine the features of both TI and TD antigens, mounting effective humoral responses to S. pneumoniae.
In conclusion, our findings demonstrate that intranasal vaccination of mice with live S. mitis induces humoral (IgG and IgA) and cell-mediated (IL-17A/Th17) responses, followed by protection against lung infection with two S. pneumoniae serotypes, 2 and 4. Moreover, protection was significantly increased using an S. mitis strain that was constructed to express one of the serotypes. It is worth mentioning that the protection shown in this study was based on partial reduction in pneumococcal loads in the lungs and that the survival of mice after challenge was not evaluated. Overall, our findings provide significant insights into how commensal bacteria can be exploited for defense against pathogens in general and against S. pneumoniae serotypes in particular, paving the way to the design of effective prophylactic and/or therapeutic strategies.
MATERIALS AND METHODS
Bacterial strains and media.
The S. mitis type strain, CCUG31611, which is equivalent to NCTC12261, and its mutant, S. mitis TIGR4caps, were used throughout this study (27). S. pneumoniae strains included D39 (serotype 2), TIGR4 (serotype 4), and EF3030 (serotype 19F). The bacterial strains were suspended in Trypticase soy broth (Becton Dickinson, Franklin Lakes, NJ, USA) and 15% glycerol and stored in a −80°C refrigerator. Bacterial stock cultures were diluted and grown at 37°C to an optical density at 600 nm (OD600) of 0.5 in a 5% CO2 incubator. The bacterial cells were harvested by centrifugation at 5,000 × g for 10 min at 4°C and washed in endotoxin-free Dulbecco’s PBS (Sigma-Aldrich, St. Louis, MO, USA). For cell lysis, bacteria were treated using a Precellys lysing kit and homogenizer (Precellys 24; Bertin Instruments) according to the manufacturer’s instructions (18). In brief, the bacterial cells were mixed with high-quality glass beads (0.5 mm) in the Precellys lysing tube, which was subjected to homogenization (Precellys 24 homogenizer; program 2). The bacterial cell lysate was centrifuged at 1,000 × g for 5 min at 4°C, and the supernatant was collected and stored at −80°C for further use. The total protein in the cell lysate was quantified using a bicinchoninic acid (BCA) protein assay kit (ThermoFisher Scientific).
Animals.
The CD1 mice used in this study were specific-pathogen-free (SPF) females 6 to 8 weeks of age bought from Charles River and were quarantined and housed in a Minimal Disease Unit at the animal facility at Oslo University Hospital, Rikshospitalet, Oslo, Norway. The mice were kept in cages that were environmentally enriched with impellers and paper for nest building and were given standard feed and water ad libitum.
Ethics statement.
All animal experiments were approved by the Norwegian Food Safety Authority, Oslo, Norway (project license numbers FOTS-8481 and -10515) and performed in accordance with the guidelines of the Norwegian Animal Welfare Act (10 June 2009, no. 97), the Norwegian Regulation on Animal Experimentation (REG 2015-06-18-761), and European Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. The mice were allowed a 1-week acclimatization period before experiments were started.
Immunization and challenge of mice.
To perform immunization, mice (4 or 5 per group) were anesthetized with isoflurane (4%), followed by intranasal administration of 5 × 107 CFU of S. mitis or S. mitis TIGR4cps in 20 µl of PBS or 20 µl of PBS only (sham) for each mouse at days 0, 14, and 21. The mice were euthanized at day 7 after the last immunization to collect the nasal wash, BALF, lungs, spleen, and blood. To obtain antisera, the freshly isolated blood was kept at 4°C for 1 h and then centrifuged at 1,000 × g for 5 min. The supernatant antisera were collected and preserved at −80°C in a refrigerator for further analysis. The nasal wash, BALF, and lungs were collected from the euthanized mice as described previously (10). To recover the BALF and nasal wash, a small cut in the trachea was made with scissors, and 1 ml of sterile cold PBS was inoculated with a syringe (19-gauge needle) and recovered for plating, as well as antibody measurements. For challenge experiments with pneumococcal lung infection, S. mitis- or S. mitis TIGR4caps-immunized mice were anesthetized with isoflurane (4%) at day 7 after the last immunization, followed by intranasal instillation with 8 × 106 CFU of S. pneumoniae TIGR4 or D39 bacteria suspended in 50 µl of PBS, as described previously (50, 51). For the nasal colonization model, the S. mitis-immunized mice were intranasally inoculated with 10 × 106 CFU of S. pneumoniae EF3030 bacteria suspended in 10 µl of PBS (10). All the mice were euthanized 24 h after bacterial inoculation, and nasal wash, BALF, and lungs were collected and stored on ice for immediate dilution and plating. For euthanasia, mice were anesthetized with isoflurane (4%) and then inoculated with an intraperitoneal injection of pentobarbital (0.5 ml per mouse). All samples were plated onto blood agar plates containing gentamicin (5 µg · ml−1) for differentiation from other species and CFU calculation (10).
Measurement of antibody responses.
To measure antibody responses, we employed Western blotting and whole-cell ELISA. For Western blotting, SDS-PAGE-separated proteins were transferred from gradient gels (4 to 20%) to nitrocellulose membranes with a constant voltage of 100 V for 2 h using the Bio-Rad Midi-Protean Western blotting module (Bio-Rad, CA, USA) according to the manufacturer’s instructions. The membranes were blocked with blocking solution (Tris-buffered saline [TBS] [pH 7.4] containing 0.1% Tween 20 [Sigma-Aldrich] and 5% bovine serum albumin [BSA]) for 1 h at room temperature, washed three times with TBS-Tween, and incubated overnight with sera, BALF, or nasal wash at 4°C. The sera were diluted 1:1,000 and the BALF and nasal wash 1:10. Following incubation, the membranes were washed and finally incubated with allophycocyanin (AP)-conjugated anti-mouse IgG or IgA secondary antibodies and later developed with the substrate (4-chloro-1-naphthol). Of note, the exposure times were similar for the membranes treated with samples from immunized and sham-immunized mice. To determine antibody levels in mouse samples, a whole-cell ELISA was used as described previously (18). In brief, each well of a 96-well plate (Maxi-Sorb; Nunc, Thermo Scientific) was coated overnight with 100 µl of bacterial suspension (OD600 = 0.5), which was washed and then fixed with 10% formalin. The plate was washed and blocked with a blocking buffer (PBS, 0.05% Tween, and 1% BSA) and incubated for 1 h at 37°C. The sera, BALF, and nasal wash were diluted, added to wells in duplicate, and incubated for 2 h at room temperature before addition of the anti-IgG-horseradish peroxidase (HRP) or anti-IgA-HRP secondary antibody (1:10,000), followed by incubation for 2 h at room temperature. The plates were washed, and 100 µl of TMB substrate (ThermoFisher Scientific, Rockford, IL, USA) was added to each well. The plates were incubated in the dark at room temperature for 15 min, after which stop solution (ThermoFisher Scientific, Rockford, IL, USA) was added to each well to terminate the reaction. The plate absorbance was measured by reading the plates at 450 nm using a Multi-Mode reader (BioTek Cytation 3; ThermoFisher Scientific). We performed 2-fold serial dilutions of sera ranging from 1:50 to 1:3,200. Titration curves were drawn for each measurement, and cutoffs were applied to convert serum activities into titers. For estimation of titers, cutoffs were placed at an OD450 of 0.4, because those values were within the linear parts of the titration curves.
Isolation of cells from lungs and spleen.
The lungs and spleen were removed from the euthanized mice and processed into single‐cell suspensions, as described previously (43). In brief, the lungs were digested in 10 mg/ml collagenase XI (Sigma-Aldrich, Israel) in RPMI 1640 (Sigma-Aldrich, United Kingdom) for 1 hour at 37°C. The lung cell suspension was treated with lysis buffer (eBioscience, San Diego, CA, USA) to lyse contaminating red blood cells (RBCs). The lung cells were washed with washing buffer (PBS, 0.5% BSA, and 5 mM EDTA). The spleen was mashed on a 70-µm cell strainer (ThermoFisher Scientific, Rockford, IL, USA) with the plunger of a 3-ml syringe and washed with the washing buffer. The cell suspension was treated with RBC lysis buffer and washed twice. A hemocytometer and trypan blue were used to count viable cells.
Cytokine measurement by ELISA.
Sera, nasal wash, and BALF were collected from the mice inoculated with S. mitis or PBS and frozen at −80°C for further cytokine analysis with commercial ELISA kits. The concentrations of IFN-γ and IL-17A were measured with Ready-Set-Go ELISA kits (eBioscience, San Diego, CA, USA), whereas the concentration of IL-4 was measured with an ELISA kit from Invitrogen (Vienna, Austria), in accordance with the instructions of the respective manufacturers. The cytokine detection limits of the ELISA kits for IL-17, IL-4, and IFN-γ were 4, 3.9, and 15 pg/ml, respectively.
Flow-cytometric intracellular-cytokine staining.
To perform intracellular-cytokine staining in T cells, lung and splenic cells were incubated at 5 × 106 cells per ml of complete RPMI medium containing 10% heat‐inactivated fetal bovine serum (FBS), 25 μg/ml gentamicin, l‐glutamine, and sodium bicarbonate (Sigma-Aldrich, United Kingdom) in 48‐well plates at 37°C and stimulated with a cell stimulation cocktail according to the instructions of the manufacturer (eBioscience, San Diego, CA, USA). In brief, lung or spleen cells (5 million cells/ml of complete medium) were incubated with a cell stimulation cocktail (2 µl in 1 ml of medium) for 18 h. Following incubation, the cells were washed (Dulbecco's PBS containing 0.5% BSA and 1 mM EDTA) and incubated with FcR-blocking Abs (anti‐16/32; eBioscience) for 15 min at 4°C. The cell surface markers were first stained with anti‐CD3‐phycoerythrin (PE)‐Cy7 and anti‐CD4‐PE or respective isotype control antibodies (eBioscience, San Diego, CA, USA). The cells were fixed with IC fixation buffer (Invitrogen, CA, USA) and permeabilized with permeabilization buffer (eBioscience, San Diego, CA, USA) according to the manufacturer's instructions, which was followed by intracellular staining with anti‐IL‐17A–allophycocyanin (APC), anti‐IFN‐γ–APC, anti-IL-4–APC, or isotype control antibodies (eBioscience, San Diego, CA, USA). Fluorescence minus one (FMO) controls were also used. Finally, the cells were washed, resuspended in Dulbecco's PBS containing 0.5% BSA and 1 mM EDTA, and analyzed by flow cytometry. Sample data were collected using a BD LSR II flow cytometer (BD Biosciences, San Diego, CA, USA), and the data were analyzed using FCS Express software (De Novo Software, Los Angeles, CA, USA).
Statistics.
One-way analysis of variance (ANOVA) and the Tukey test were used to compare multiple groups, while an unpaired Student's t test was used for comparing two groups (GraphPad Prism software, version 7; Graph Pad, San Diego, CA, USA). A P value of less than 0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Norwegian Research Council (grant number 241011).
We thank the animal facility staff, especially Henrik Rasmussen and Victor Garcia, for their cooperation in conducting mouse experiments at the Oslo University Hospital, Rikshospitalet, Oslo, Norway.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02235-18.
REFERENCES
- 1.O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, Hib and Pneumococcal Global Burden of Disease Study Team 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 2.Weiser JN, Ferreira DM, Paton JC. 2018. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol 16:355–367. doi: 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. 2017. WHO publishes list of bacteria for which new antibiotics are urgently needed. http://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. World Health Organization, Geneva, Switzerland.
- 4.Cherazard R, Epstein M, Doan TL, Salim T, Bharti S, Smith MA. 2017. Antimicrobial resistant Streptococcus pneumoniae: prevalence, mechanisms, and clinical implications. Am J Ther 24:e361–e369. doi: 10.1097/MJT.0000000000000551. [DOI] [PubMed] [Google Scholar]
- 5.Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH. 2015. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev 28:871–899. doi: 10.1128/CMR.00024-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels CC, Rogers PD, Shelton CM. 2016. A review of pneumococcal vaccines: current polysaccharide vaccine recommendations and future protein antigens. J Pediatr Pharmacol Ther 21:27–35. doi: 10.5863/1551-6776-21.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pichichero ME, Khan MN, Xu Q. 2016. Next generation protein based Streptococcus pneumoniae vaccines. Hum Vaccin Immunother 12:194–205. doi: 10.1080/21645515.2015.1052198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarahomjoo S. 2014. Recent approaches in vaccine development against Streptococcus pneumoniae. J Mol Microbiol Biotechnol 24:215–227. doi: 10.1159/000365052. [DOI] [PubMed] [Google Scholar]
- 9.Roche AM, King SJ, Weiser JN. 2007. Live attenuated Streptococcus pneumoniae strains induce serotype-independent mucosal and systemic protection in mice. Infect Immun 75:2469–2475. doi: 10.1128/IAI.01972-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson R, Cohen JM, Jose RJ, de Vogel C, Baxendale H, Brown JS. 2015. Protection against Streptococcus pneumoniae lung infection after nasopharyngeal colonization requires both humoral and cellular immune responses. Mucosal Immunol 8:627–639. doi: 10.1038/mi.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frandsen EV, Pedrazzoli V, Kilian M. 1991. Ecology of viridans streptococci in the oral cavity and pharynx. Oral Microbiol Immunol 6:129–133. [DOI] [PubMed] [Google Scholar]
- 12.Bek-Thomsen M, Tettelin H, Hance I, Nelson KE, Kilian M. 2008. Population diversity and dynamics of Streptococcus mitis, Streptococcus oralis, and Streptococcus infantis in the upper respiratory tracts of adults, determined by a nonculture strategy. Infect Immun 76:1889–1896. doi: 10.1128/IAI.01511-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilian M, Riley DR, Jensen A, Bruggemann H, Tettelin H. 2014. Parallel evolution of Streptococcus pneumoniae and Streptococcus mitis to pathogenic and mutualistic lifestyles. mBio 5:e01490-14. doi: 10.1128/mBio.01490-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright MS, McCorrison J, Gomez AM, Beck E, Harkins D, Shankar J, Mounaud S, Segubre-Mercado E, Mojica AMR, Bacay B, Nzenze SA, Kimaro SZM, Adrian P, Klugman KP, Lucero MG, Nelson KE, Madhi S, Sutton GG, Nierman WC, Losada L. 2017. Strain level Streptococcus colonization patterns during the first year of life. Front Microbiol 8:1661. doi: 10.3389/fmicb.2017.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilian M, Poulsen K, Blomqvist T, Håvarstein LS, Bek-Thomsen M, Tettelin H, Sørensen UBS. 2008. Evolution of Streptococcus pneumoniae and its close commensal relatives. PLoS One 3:e2683. doi: 10.1371/journal.pone.0002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denapaite D, Bruckner R, Nuhn M, Reichmann P, Henrich B, Maurer P, Schahle Y, Selbmann P, Zimmermann W, Wambutt R, Hakenbeck R. 2010. The genome of Streptococcus mitis B6—what is a commensal? PLoS One 5:e9426. doi: 10.1371/journal.pone.0009426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engen SA, Rukke HV, Becattini S, Jarrossay D, Blix IJ, Petersen FC, Sallusto F, Schenck K. 2014. The oral commensal Streptococcus mitis shows a mixed memory Th cell signature that is similar to and cross-reactive with Streptococcus pneumoniae. PLoS One 9:e104306. doi: 10.1371/journal.pone.0104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shekhar S, Khan R, Ferreira DM, Mitsi E, German E, Rorvik GH, Berild D, Schenck K, Kwon K, Petersen F. 2018. Antibodies reactive to commensal Streptococcus mitis show cross-reactivity with virulent Streptococcus pneumoniae serotypes. Front Immunol 9:747. doi: 10.3389/fimmu.2018.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira DM, Neill DR, Bangert M, Gritzfeld JF, Green N, Wright AKA, Pennington SH, Bricio-Moreno L, Moreno AT, Miyaji EN, Wright AD, Collins AM, Goldblatt D, Kadioglu A, Gordon SB. 2013. Controlled human infection and rechallenge with Streptococcus pneumoniae reveals the protective efficacy of carriage in healthy adults. Am J Respir Crit Care Med 187:855–864. doi: 10.1164/rccm.201212-2277OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen JM, Khandavilli S, Camberlein E, Hyams C, Baxendale HE, Brown JS. 2011. Protective contributions against invasive Streptococcus pneumoniae pneumonia of antibody and Th17-cell responses to nasopharyngeal colonisation. PLoS One 6:e25558. doi: 10.1371/journal.pone.0025558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janoff EN, Fasching C, Orenstein JM, Rubins JB, Opstad NL, Dalmasso AP. 1999. Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J Clin Invest 104:1139–1147. doi: 10.1172/JCI6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantis NJ, Rol N, Corthesy B. 2011. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol 4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nogueira RD, Sesso MLT, Borges MCL, Mattos-Graner RO, Smith DJ, Ferriani VPL. 2012. Salivary IgA antibody responses to Streptococcus mitis and Streptococcus mutans in preterm and fullterm newborn children. Arch Oral Biol 57:647–653. doi: 10.1016/j.archoralbio.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Clarke TB, Weiser JN. 2009. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest 119:1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright AK, Bangert M, Gritzfeld JF, Ferreira DM, Jambo KC, Wright AD, Collins AM, Gordon SB. 2013. Experimental human pneumococcal carriage augments IL-17A-dependent T-cell defence of the lung. PLoS Pathog 9:e1003274. doi: 10.1371/journal.ppat.1003274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen JM, Chimalapati S, de Vogel C, van Belkum A, Baxendale HE, Brown JS. 2012. Contributions of capsule, lipoproteins and duration of colonisation towards the protective immunity of prior Streptococcus pneumoniae nasopharyngeal colonisation. Vaccine 30:4453–4459. doi: 10.1016/j.vaccine.2012.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rukke HV, Kalluru RS, Repnik U, Gerlini A, Jose RJ, Periselneris J, Marshall H, Griffiths G, Oggioni MR, Brown JS, Petersen FC. 2014. Protective role of the capsule and impact of serotype 4 switching on Streptococcus mitis. Infect Immun 82:3790–3801. doi: 10.1128/IAI.01840-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Detmer A, Glenting J. 2006. Live bacterial vaccines—a review and identification of potential hazards. Microb Cell Fact 5:23. doi: 10.1186/1475-2859-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Mantia I, Varricchio A, Ciprandi G. 2017. Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a nasal spray for preventing recurrent acute otitis media in children: a real-life clinical experience. Int J Gen Med 10:171–175. doi: 10.2147/IJGM.S137614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchisio P, Santagati M, Scillato M, Baggi E, Fattizzo M, Rosazza C, Stefani S, Esposito S, Principi N. 2015. Streptococcus salivarius 24SMB administered by nasal spray for the prevention of acute otitis media in otitis-prone children. Eur J Clin Microbiol Infect Dis 34:2377–2383. doi: 10.1007/s10096-015-2491-x. [DOI] [PubMed] [Google Scholar]
- 31.Tano K, Hakansson EG, Holm SE, Hellstrom S. 2002. A nasal spray with alpha-haemolytic streptococci as long term prophylaxis against recurrent otitis media. Int J Pediatr Otorhinolaryngol 62:17–23. doi: 10.1016/S0165-5876(01)00581-X. [DOI] [PubMed] [Google Scholar]
- 32.Daifalla N, Cayabyab MJ, Xie E, Kim HB, Tzipori S, Stashenko P, Duncan M, Campos-Neto A. 2015. Commensal Streptococcus mitis is a unique vector for oral mucosal vaccination. Microbes Infect 17:237–242. doi: 10.1016/j.micinf.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie E, Kotha A, Biaco T, Sedani N, Zou J, Stashenko P, Duncan MJ, Campos-Neto A, Cayabyab MJ. 2015. Oral delivery of a novel recombinant Streptococcus mitis vector elicits robust vaccine antigen-specific oral mucosal and systemic antibody responses and T cell tolerance. PLoS One 10:e0143422. doi: 10.1371/journal.pone.0143422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson R, Cohen JM, Reglinski M, Jose RJ, Chan WY, Marshall H, de Vogel C, Gordon S, Goldblatt D, Petersen FC, Baxendale H, Brown JS. 2017. Naturally acquired human immunity to pneumococcus is dependent on antibody to protein antigens. PLoS Pathog 13:e1006137. doi: 10.1371/journal.ppat.1006137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kononen E, Jousimies-Somer H, Bryk A, Kilp T, Kilian M. 2002. Establishment of streptococci in the upper respiratory tract: longitudinal changes in the mouth and nasopharynx up to 2 years of age. J Med Microbiol 51:723–730. doi: 10.1099/0022-1317-51-9-723. [DOI] [PubMed] [Google Scholar]
- 36.Grahn E, Holm SE. 1983. Bacterial interference in the throat flora during a streptococcal tonsillitis outbreak in an apartment house area. Zentralbl Bakteriol Mikrobiol Hyg A 256:72–79. [PubMed] [Google Scholar]
- 37.Ivanov II, Littman DR. 2010. Segmented filamentous bacteria take the stage. Mucosal Immunol 3:209–212. doi: 10.1038/mi.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnupf P, Gaboriau-Routhiau V, Sansonetti PJ, Cerf-Bensussan N. 2017. Segmented filamentous bacteria, Th17 inducers and helpers in a hostile world. Curr Opin Microbiol 35:100–109. doi: 10.1016/j.mib.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Wright AK, Ferreira DM, Gritzfeld JF, Wright AD, Armitage K, Jambo KC, Bate E, El Batrawy S, Collins A, Gordon SB. 2012. Human nasal challenge with Streptococcus pneumoniae is immunising in the absence of carriage. PLoS Pathog 8:e1002622. doi: 10.1371/journal.ppat.1002622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skov Sorensen UB, Yao K, Yang Y, Tettelin H, Kilian M. 2016. Capsular polysaccharide expression in commensal Streptococcus species: genetic and antigenic similarities to Streptococcus pneumoniae. mBio 7:e01844-16. doi: 10.1128/mBio.01844-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mistry D, Stockley RA. 2006. IgA1 protease. Int J Biochem Cell Biol 38:1244–1248. doi: 10.1016/j.biocel.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kilian M, Reinholdt J, Lomholt H, Poulsen K, Frandsen EV. 1996. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS 104:321–338. [DOI] [PubMed] [Google Scholar]
- 43.Shekhar S, Peng Y, Gao X, Joyee AG, Wang S, Bai H, Zhao L, Yang J, Yang X. 2015. NK cells modulate the lung dendritic cell-mediated Th1/Th17 immunity during intracellular bacterial infection. Eur J Immunol 45:2810–2820. doi: 10.1002/eji.201445390. [DOI] [PubMed] [Google Scholar]
- 44.Iwasaki A, Foxman EF, Molony RD. 2017. Early local immune defences in the respiratory tract. Nat Rev Immunol 17:7–20. doi: 10.1038/nri.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carvalho MDG, Pimenta FC, Moura I, Roundtree A, Gertz RE, Li Z, Jagero G, Bigogo G, Junghae M, Conklin L, Feikin DR, Breiman RF, Whitney CG, Beall BW. 2013. Non-pneumococcal mitis-group streptococci confound detection of pneumococcal capsular serotype-specific loci in upper respiratory tract. PeerJ 1:e97. doi: 10.7717/peerj.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kjaer TR, Hansen AG, Sorensen UB, Holm AT, Sorensen GL, Jensenius JC, Thiel S. 2013. M-ficolin binds selectively to the capsular polysaccharides of Streptococcus pneumoniae serotypes 19B and 19C and of a Streptococcus mitis strain. Infect Immun 81:452–459. doi: 10.1128/IAI.01148-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosch JW. 2014. Promises and pitfalls of live attenuated pneumococcal vaccines. Hum Vaccin Immunother 10:3000–3003. doi: 10.4161/21645515.2014.970496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mond JJ, Lees A, Snapper CM. 1995. T cell-independent antigens type 2. Annu Rev Immunol 13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 49.Briles DE, King JD, Gray MA, McDaniel LS, Swiatlo E, Benton KA. 1996. PspA, a protection-eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine 14:858–867. [DOI] [PubMed] [Google Scholar]
- 50.Brown JS, Hussell T, Gilliland SM, Holden DW, Paton JC, Ehrenstein MR, Walport MJ, Botto M. 2002. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc Natl Acad Sci U S A 99:16969–16974. doi: 10.1073/pnas.012669199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chimalapati S, Cohen J, Camberlein E, Durmort C, Baxendale H, de Vogel C, van Belkum A, Brown JS. 2011. Infection with conditionally virulent Streptococcus pneumoniae Deltapab strains induces antibody to conserved protein antigens but does not protect against systemic infection with heterologous strains. Infect Immun 79:4965–4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.