There is a need for novel, mercury-free UV lamp technology to replace germicidal lamps containing harmful mercury, which are routinely utilized for UV pasteurization of apple juice. In addition, consideration of the changes in response to antimicrobial treatments that may occur when pathogens are adapted to the acid in an apple juice matrix is critical to the practical application of this technology. Based on this, an investigation using 222-nm KrCl excilamp technology, an attractive alternative to mercury lamps, was conducted. Our study demonstrated increased resistance to 222-nm KrCl excilamp treatment as pathogens adapted to acids, and this was due to changes in reactivity to ROS with changes in the fatty acid composition of the cell membrane. Despite increased resistance, the 222-nm KrCl excilamp achieved pathogen reductions of 5 log or more at laboratory scale without affecting apple juice quality. These results provide valuable baseline data for application of 222-nm KrCl excilamps in the apple juice industry.
KEYWORDS: 222-nm KrCl excilamp, Escherichia coli O157:H7, ROS, Salmonella Typhimurium, acid adaptation, apple juice, cell membrane fatty acid, ultraviolet irradiation
ABSTRACT
In this study, we examined the change in resistance of Salmonella enterica serovar Typhimurium and Escherichia coli O157:H7 to 222-nm krypton-chlorine (KrCl) excilamp treatment as influenced by acid adaptation and identified a mechanism of resistance change. In addition, we measured changes in apple juice quality indicators, such as color, total phenols, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity, during treatment. Non-acid-adapted and acid-adapted pathogens were induced by growing the cells in tryptic soy broth without dextrose (TSB w/o D) at pH 7.3 and in TSB w/o D at pH 5.0 (adjusted with HCl), respectively. For the KrCl excilamp treatment, acid-adapted pathogens exhibited significantly (P < 0.05) higher D5d values, which indicate dosages required to achieve a 5-log reduction, than those for non-acid-adapted pathogens in both commercially clarified apple juice and phosphate-buffered saline (PBS), and the pathogens in the juice showed significantly (P < 0.05) higher D5d values than those for pathogens in PBS because of the UV-absorbing characteristics of apple juice. Through mechanism identification, it was found that the generation of lipid peroxidation in the cell membrane, inducing cell membrane destruction, was significantly (P < 0.05) lower in acid-adapted cells than in non-acid-adapted cells for the same amount of reactive oxygen species (ROS) generated at the same dose because the ratio of unsaturated to saturated fatty acids (USFA/SFA) in the cell membrane was significantly (P < 0.05) decreased as a result of acid adaptation. Treated apple juice showed no significant (P > 0.05) difference in quality indicators compared to those of untreated controls during treatment at 1,773 mJ/cm2.
IMPORTANCE There is a need for novel, mercury-free UV lamp technology to replace germicidal lamps containing harmful mercury, which are routinely utilized for UV pasteurization of apple juice. In addition, consideration of the changes in response to antimicrobial treatments that may occur when pathogens are adapted to the acid in an apple juice matrix is critical to the practical application of this technology. Based on this, an investigation using 222-nm KrCl excilamp technology, an attractive alternative to mercury lamps, was conducted. Our study demonstrated increased resistance to 222-nm KrCl excilamp treatment as pathogens adapted to acids, and this was due to changes in reactivity to ROS with changes in the fatty acid composition of the cell membrane. Despite increased resistance, the 222-nm KrCl excilamp achieved pathogen reductions of 5 log or more at laboratory scale without affecting apple juice quality. These results provide valuable baseline data for application of 222-nm KrCl excilamps in the apple juice industry.
INTRODUCTION
Fruit juices are widely popular and are consumed because of their characteristics of freshness, flavor, and health benefits, due to high vitamin, polyphenol, and antioxidant content (1–3). Apple juice is one of the most popular fruit juices, and its consumption has risen over the past 30 years because of its taste, flavor, and health benefits (4, 5). Increasing consumption of fruit juice has a positive impact on the economy, but it has a negative effect when problems occur, such as foodborne disease outbreaks or spoilage (6). To date, outbreaks associated with unpasteurized commercial apple juice consumption continue to occur, mainly due to Escherichia coli O157:H7 and Salmonella spp. (7). In fact, a large outbreak (70 cases) of E. coli O157:H7 infection occurred in 1996, during which 25 people were hospitalized, 14 experienced hemolytic-uremic syndrome, and 1 died (8, 9). In response to the increasing outbreaks associated with fruit juice, the U.S. Food and Drug Administration (USFDA) introduced regulations for all fruit juice processes that require manufacturers to perform processing steps that can achieve a minimum 5-log reduction of the pertinent pathogenic bacteria in the finished juice (10, 11).
The USFDA considers thermal processing to be an effective and proven way to ensure the safety of fruit juices from pathogenic microorganisms as well as prolonging the shelf life of refrigerated juices (11). Therefore, currently, thermal pasteurization is an industrial intervention that has been predominantly applied to ensure the microbial safety of juice (12–14). However, the adverse effects of high temperatures on the organoleptic and nutritional properties of juices and increased consumer demand for minimal processing and fresh-tasting products encourage researchers to investigate alternative, nonthermal food processing techniques (14–16). Among them, UV technology is considered one of the most promising alternatives to the conventional thermal pasteurization process due to its numerous advantages, such as no disinfectant residuals, an inactivation effect on a wide range of pathogens, ease of fit into existing processes, and low energy consumption (17, 18). In addition, the USFDA has approved UVC radiation to be applied to fruit juice processing to control human pathogens and other microorganisms (19). In the UV disinfection system, mercury vapor-filled lamps producing germicidal UV radiation are typically used, which are either low-pressure (LP) lamps, emitting a monochromatic light at 254.7 nm, or medium-pressure (MP) lamps, emitting polychromatic light across the electromagnetic spectrum (20, 21). To date, many studies have demonstrated that conventional mercury UV lamps, which replace thermal treatment, can effectively reduce pathogenic microorganisms in apple juice (22–25). However, the use of such UV radiation sources poses a potential risk of mercury release and resulting human health and environmental hazards due to their fragile quartz construction (21, 26). In addition, as the United Nations Environment Program (UNEP) has signed to the Minamata Convention on Mercury, which aims to gradually phase out the usage of mercury present in numerous products and processes by 2020, the application of a novel UV system to replace mercury UV lamps is essential (27, 28).
In that regard, UV-LEDs or dielectric barrier discharge (DBD)-driven excilamps (excimer or exciplex lamps) are a promising technology that may substitute for mercury lamps in the future. In particular, excilamps are considered an attractive UV source due to characteristics such as their wavelength selectivity, fast warm-up, long lifetime, and high radiant intensity, as well as the absence of mercury (29–31). Excilamps can emit nearly monochromatic radiation, with wavelengths ranging from 172 to 345 nm, depending on the type of rare gas and halogen used (31, 32). Recent studies, including ours, to evaluate the germicidal effects of various forms of excilamps (Xe2, KrCl, and XeBr excilamps with maximum emissions at 172, 222, and 282 nm, respectively) and conventional mercury lamps emitting 254-nm radiation revealed that the KrCl excilamp, emitting at a 222-nm wavelength, resulted in the highest log reductions of pathogens among other excilamps as well as mercury lamps emitting at a 254-nm wavelength at the same incident dose, which means that the 222-nm KrCl excilamp has a greater inactivation effect than those of other excilamps or the mercury vapor lamp (29, 32–34). In particular, the Yin et al. study (35) comparing the inactivation effect at the same energy level that is imposed entirely on cells, excluding the matrix effect of the medium, by using the actual dose imparted to apple juice, which was calculated by taking into account the absorption coefficient of the apple juice sample to be treated, reported that the 222-nm KrCl excilamp reduced E. coli O157:H7 in apple juice by almost 1 log more than the 282-nm XeBr excilamp or 254-nm mercury lamp. Meanwhile, according to Buonanno et al. (36), unlike the 254-nm wavelength of the conventional mercury lamp, the 222-nm wavelength of the KrCl excilamp did not show cytotoxicity on mammalian skin and rarely produced premutagenic DNA lesions associated with UV in a three-dimensional (3D) human skin model. This is due to the 222-nm wavelength being unable to penetrate either all of the tissues with a stratum corneum or the mammalian cell cytoplasm (37, 38). Thus, 222-nm KrCl excilamp technology can likely be utilized for juice processing without causing harmful effects to the operator by exposure to UV light. For these reasons, the 222-nm KrCl excilamp has great potential as an alternative to mercury lamps in the apple juice industry.
Bacteria can be exposed to various stresses in all areas of the farm-to-table food chain, especially in fruit juice processing, where low pH is a major source of stress (39). Under such low-pH conditions, bacteria are induced to increase resistance to acidic environments, which has been termed the acid adaptation response, increasing their survival and consequently causing fruit juice to become contaminated more easily (40). Indeed, several studies have demonstrated that E. coli and Salmonella enterica serovar Typhimurium that are adapted to acidic conditions can survive longer in acidic environments (41–44). In addition, it has been reported that the acid adaptation response also leads to increased resistance of E. coli O157:H7 and S. Typhimurium in fruit juices to inactivation by other stresses in food processing, such as heat treatments (45–48), ozone (49), and ultrasound treatment (50). Another crucial concern is that acid-adapted pathogens may have lower infectious doses, leading to increased pathogenicity, because it is inferred that the greater the resistance to the acidity of the gastrointestinal environment, the more likely they are to overcome the human gastric barrier after ingestion (51, 52). Therefore, the acid adaptation response must be considered a real possibility which can have a great influence on the development of processing protocols by the fruit juice industry. Although a recent study reported that the 222-nm KrCl excilamp achieved a 2.81-log reduction of E. coli O157:H7 in apple juice following treatment at 75 mJ/cm2 (35) and it yielded a D value of 28.48 mJ/cm2 in another study (32) using a batch-type UV treatment system, to the best of our knowledge, no published research has been carried out to investigate the bactericidal effect of this intervention on acid-adapted pathogens.
Consequently, in our study, the changes in responses of E. coli O157:H7 and S. Typhimurium, the main organisms associated with apple juice outbreaks, to 222-nm KrCl excilamp treatment (Fig. 1) after acid adaptation were investigated, and the mechanism was elucidated. Also, apple juice qualities, such as color, total phenols, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity, were observed under conditions in which pathogenic bacteria with higher resistance decreased 5 log or more in order to propose a practical pathogen inactivation protocol for apple juice without overestimating the sterilization effect of 222-nm KrCl excilamps.
FIG 1.
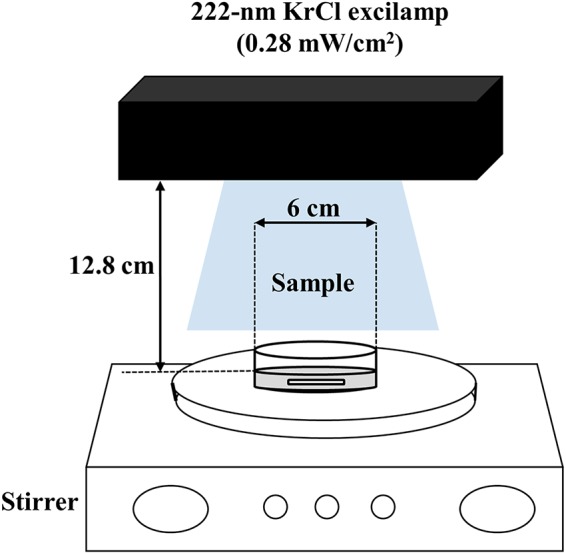
Schematic diagram of the batch-type 222-nm KrCl excilamp treatment system used in this study.
RESULTS
Comparative resistance of acid-adapted and non-acid-adapted cells of S. Typhimurium and E. coli O157:H7 to 222-nm KrCl excilamp treatment.
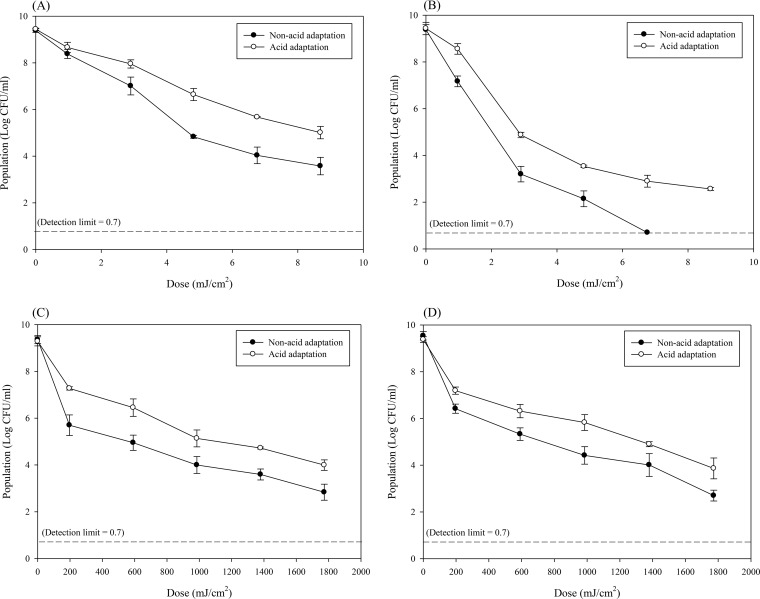
Figure 2 shows surviving populations of non-acid-adapted and acid-adapted S. Typhimurium and E. coli O157:H7 suspended in phosphate-buffered saline (PBS) (Fig. 2A and B) or apple juice (Fig. 2C and D) during treatment with the 222-nm KrCl excilamp. Incidentally, in order to ascertain whether the apple juice environment can affect pathogen reduction, non-acid-adapted or acid-adapted cells of S. Typhimurium and E. coli O157:H7 were inoculated into apple juice and maintained at room temperature (22°C ± 1°C) for 105.7 min (1,773 mJ/cm2), the maximum treatment time for this experiment, without subjecting them to 222-nm KrCl excilamp treatment, following which survival populations were enumerated. Results showed that a holding time of 105.7 min did not significantly (P > 0.05) change the survival population of the pathogens used in this study (data not shown). Thus, for this study, the reduction of pathogens that occurred during the UV treatment can be attributed solely to UV. In both PBS and apple juice, surviving populations of non-acid-adapted and acid-adapted S. Typhimurium and E. coli O157:H7 significantly (P < 0.05) decreased as the treatment dosage increased. To quantitatively compare the inactivation effects of the 222-nm KrCl excilamp based on the suspending matrix (PBS or apple juice) or cell conditions (non-acid adaptation or acid adaptation), survival curves were fitted to the suitable model equation to derive the D5d value, which is the dosage required to achieve a 5-log reduction. The parameters of the models and the D5d values derived from them are shown in Table 1. For both S. Typhimurium and E. coli O157:H7, when cells were adapted to acid, D5d values were significantly (P < 0.05) increased compared to those for cells that were not acid adapted. This trend was the same when cells were treated in PBS or in apple juice. Specifically, non-acid-adapted cells of S. Typhimurium and E. coli O157:H7 treated in PBS yielded D5d values of 6.32 and 2.42 mJ/cm2, respectively, while cells of S. Typhimurium and E. coli adapted to acid and treated in PBS were 9.83 and 3.07 mJ/cm2, respectively. In the case of pathogens treated in apple juice, cells of S. Typhimurium and E. coli O157:H7 that were not adapted to acid showed D5d values of 927.71 and 1,023.72 mJ/cm2, respectively, while acid-adapted cells of S. Typhimurium and E. coli had values of 1,887.09 and 1,807.29 mJ/cm2, respectively. Also, this result revealed that regardless of cell condition (non-acid adaptation or acid adaptation), when cells were treated in apple juice, the D5d value was significantly (P < 0.05) higher than that for cells treated in PBS.
FIG 2.
Surviving populations of non-acid-adapted or acid-adapted Salmonella Typhimurium suspended in PBS (A) or apple juice (C) or Escherichia coli O157:H7 suspended in PBS (B) or apple juice (D) and treated with a 222-nm KrCl excilamp. Each point and set of error bars represent the mean value for three replicates and the standard deviation, respectively.
TABLE 1.
Parameters of the Weibull or Weibull-with-tail modela for inactivation of Salmonella Typhimurium and Escherichia coli O157:H7 treated with a 222-nm KrCl excilamp
| Pathogen | Treatment condition | Cell condition | Parameterb
|
D5d (mJ/cm2)c | ||||
|---|---|---|---|---|---|---|---|---|
| δ | p | Log10(Nres) | RMSE | R2 | ||||
| S. Typhimurium | PBS | Non-acid adaptation | 0.69 ± 0.20 A | 0.72 ± 0.08 C | 0.34 | 0.97 | 6.32 ± 0.54 A | |
| Acid adaptation | 1.58 ± 0.26 B | 0.89 ± 0.11 C | 0.17 | 0.99 | 9.83 ± 0.72 B | |||
| Apple juice | Non-acid adaptation | 9.59 ± 9.49 ABC | 0.33 ± 0.08 A | 0.27 | 0.98 | 927.74 ± 184.27 C | ||
| Acid adaptation | 156.27 ± 40.97 D | 0.64 ± 0.06 B | 0.31 | 0.97 | 1,887.09 D | |||
| E. coli O157:H7 | PBS | Non-acid adaptation | 0.15 ± 0.03 A | 0.58 ± 0.03 A | 0.42 | 0.98 | 2.42 ± 0.15 A | |
| Acid adaptation | 1.07 ± 0.26 B | 1.55 ± 0.30 B | 3.00 ± 0.10 | 0.29 | 0.99 | 3.07 ± 0.11 B | ||
| Apple juice | Non-acid adaptation | 34.11 ± 37.45 C | 0.44 ± 0.12 A | 0.35 | 0.97 | 1,023.72 ± 271.854 C | ||
| Acid adaptation | 95.94 ± 61.46 C | 0.54 ± 0.13 A | 0.31 | 0.97 | 1,807.29 ± 103.58 D | |||
The Weibull-with-tail model was applied only to the inactivation data for acid-adapted E. coli O157:H7 in PBS.
The values are means ± standard deviations for three replications. Mean values with different uppercase letters within the same column for each pathogen are significantly different (P < 0.05). δ, time to first decimal reduction; p, shape parameter; log10(Nres), residual population density; RMSE, root mean square error.
Dosage necessary to achieve a 5-log reduction.
When injured-cell recovery methods were compared to those for samples directly plated on selective media, there were no significant (P > 0.05) differences between either plating method at all treatment doses (197 to 1,773 mJ/cm2) (Table 2). That is, 222-nm KrCl excilamp treatment did not produce injured cells of either acid-adapted or non-acid-adapted S. Typhimurium and E. coli O157:H7.
TABLE 2.
Reduction levels of non-acid-adapted and acid-adapted Salmonella Typhimurium and Escherichia coli O157:H7 in apple juice treated with a 222-nm KrCl excilamp at doses of 197 to 1,773 mJ/cm2
| Organism and treatment dose (mJ/cm2) | Log reductiona [log10(N0/N)] by treatment type and selection medium |
|||
|---|---|---|---|---|
| Non-acid adaptation | Acid adaptation | |||
| Salmonella Typhimurium | XLD | OV-XLD | XLD | OV-XLD |
| 0 | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa |
| 197 | 3.65 ± 0.39 Bb | 3.65 ± 0.29 Bb | 2.01 ± 0.19 Ba | 2.12 ± 0.03 Ba |
| 591 | 4.40 ± 0.29 Cb | 4.31 ± 0.39 Cb | 2.83 ± 0.20 Ca | 2.92 ± 0.33 Ca |
| 985 | 5.36 ± 0.19 Db | 5.17 ± 0.52 Db | 4.14 ± 0.20 Da | 4.23 ± 0.17 Da |
| 1,379 | 5.76 ± 0.09 Db | 5.40 ± 0.26 Db | 4.56 ± 0.17 Ea | 4.45 ± 0.06 Da |
| 1,773 | 6.65 ± 0.17 Eb | 6.34 ± 0.14 Eb | 5.29 ± 0.05 Fa | 5.33 ± 0.43 Ea |
| E. coli O157:H7 | SMAC | SPRAB | SMAC | SPRAB |
| 0 | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa |
| 197 | 3.11 ± 0.29 Bb | 3.00 ± 0.02 Bb | 2.20 ± 0.11 Ba | 2.28 ± 0.10 Ba |
| 591 | 4.21 ± 0.34 Cb | 4.19 ± 0.32 Cb | 3.06 ± 0.24 Ca | 3.22 ± 0.21 Ca |
| 985 | 5.12 ± 0.35 Db | 4.84 ± 0.36 Db | 3.55 ± 0.36 Ca | 3.64 ± 0.19 Da |
| 1,379 | 5.53 ± 0.31 Db | 5.43 ± 0.26 Eb | 4.48 ± 0.22 Da | 4.60 ± 0.17 Ea |
| 1,773 | 6.84 ± 0.10 Eb | 6.69 ± 0.10 Fb | 5.51 ± 0.33 Ea | 5.55 ± 0.17 Fa |
The values are means ± standard deviations for three replications. Mean values with the same uppercase letter within the same column are not significantly different (P > 0.05). Mean values with the same lowercase letter within the same row are not significantly different (P > 0.05). XLD, xylose lysine desoxycholate agar; OV-XLD, overlay of XLD agar on TSA; SMAC, sorbitol MacConkey agar; SPRAB, phenol red agar base with 1% sorbitol; N0, initial population (initial populations of S. Typhimurium and E. coli O157:H7 were approximately 9.0 and 9.5 log CFU/ml, respectively); N, population after treatment.
Incidence of cell membrane damage and intracellular reactive oxygen species (ROS) generation during 222-nm KrCl excilamp treatment.
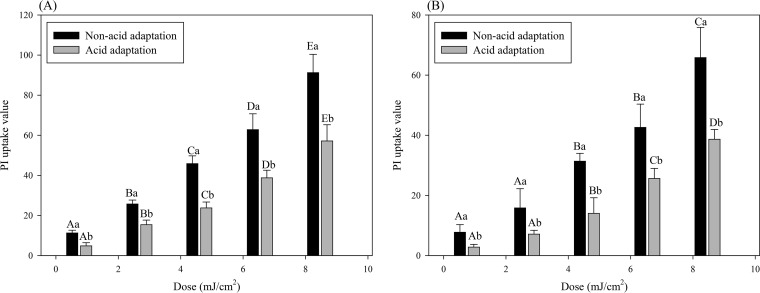
Propidium iodide (PI) is a fluorescent dye that cannot pass through a structurally intact cell membrane because of its size and charge, but when pores form in the cell membrane, it can enter the cell and bind with nucleic acids (DNA or RNA) and emit fluorescence when exposed to an excitation wavelength of 493 nm (53, 54). Thus, the degree of pore formation in the cell membrane can be assessed by the increased fluorescence signal generated from PI binding with nucleic acids within the cell (55, 56). As shown in Fig. 3, PI uptake values significantly (P < 0.05) increased with increasing treatment doses for both pathogens. Moreover, for all treatments, PI uptake values for acid-adapted cells were significantly (P < 0.05) lower than those for non-acid-adapted cells. Therefore, treatment with the 222-nm KrCl excilamp caused membrane damage in the form of pore formation, and less membrane damage occurred to acid-adapted cells with the same UV treatment.
FIG 3.
Destruction of cell membrane of non-acid-adapted or acid-adapted Salmonella Typhimurium (A) or Escherichia coli O157:H7 (B) as determined by the PI uptake assay. Data represent averages for three independent experiments, and error bars indicate standard deviations. The data were normalized by subtracting fluorescence values obtained for untreated cells and against the OD600, as follows: (fluorescence value after treatment − fluorescence value for untreated control)/OD600. Different uppercase letters for cells grown in the same matrix indicate significant differences (P < 0.05). Different lowercase letters for the same treatment dose indicate significant differences (P < 0.05).
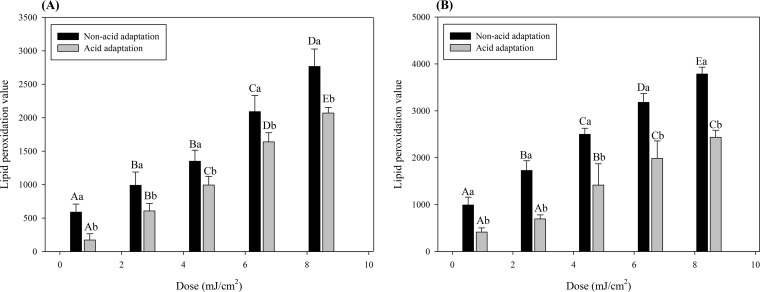
To measure the degree of lipid peroxidation of the cell membrane, we used the fluorescent dye diphenyl-1-pyrenylphosphine (DPPP) in this study. Nonfluorescent DPPP reacts with the lipid hydroperoxide in the cell membrane and is converted to fluorescent diphenyl-1-pyrenylphosphine oxide (DPPP=O) (57). Therefore, the generation of fluorescence resulting from formation of DPPP=O represents the degree of lipid peroxidation of the cell membrane. Figure 4 shows that lipid peroxide values for both non-acid-adapted and acid-adapted cells increased significantly (P < 0.05) as the treatment dose increased (0.97 to 8.69 mJ/cm2) for both pathogens. However, on comparing non-acid-adapted and acid-adapted cells, the lipid peroxidation value for non-acid-adapted cells was significantly (P < 0.05) higher for all treatments at the same treatment dose for both pathogens. Therefore, when S. Typhimurium and E. coli O157:H7 were adapted to acid, they exhibited a lower level of lipid peroxidation in the cell membrane in response to 222-nm KrCl excilamp treatment.
FIG 4.
Lipid peroxidation of cell membrane of non-acid-adapted or acid-adapted Salmonella Typhimurium (A) or Escherichia coli O157:H7 (B) as determined by the DPPP assay. Data represent averages for three independent experiments, and error bars indicate standard deviations. The data were normalized by subtracting fluorescence values obtained for untreated cells and against the OD600, as follows: (fluorescence value after treatment − fluorescence value for untreated control)/OD600. Different uppercase letters for cells grown in the same matrix indicate significant differences (P < 0.05). Different lowercase letters for the same treatment dose indicate significant differences (P < 0.05).
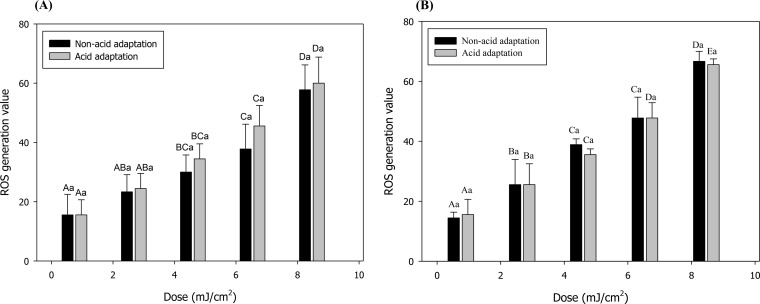
Intracellular ROS were measured using 5-(and -6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate (CM-H2DCFDA; Invitrogen), which is a cellular assay probe. This probe can readily penetrate the cell membrane and be hydrolyzed into the dichlorofluorescein (DCFH) carboxylate anion in the cell and then converted to 2',7'-dichlorofluorescein (DCF), which is highly fluorescent, upon oxidation by ROS (58–60). Therefore, the degree of fluorescence of DCF reflects the degree of generation of intracellular ROS. As shown in Fig. 5, ROS generation values significantly (P < 0.05) increased with increasing treatment doses for both non-acid-adapted and acid-adapted cells of both pathogens, while there were no significant differences (P > 0.05) in ROS generation values between non-acid-adapted and acid-adapted cells at each treatment dose for both pathogens. That is, 222-nm KrCl excilamp treatment generated the same amount of ROS whether or not S. Typhimurium and E. coli O157:H7 were adapted to acid.
FIG 5.
Intracellular ROS generation values for non-acid-adapted or acid-adapted Salmonella Typhimurium (A) or Escherichia coli O157:H7 (B), obtained by the ROS detection assay using CM-H2DCFDA. Data represent averages for three independent experiments, and error bars indicate standard deviations. The data were normalized by subtracting fluorescence values obtained for untreated cells and against the OD600, as follows: (fluorescence value after treatment − fluorescence value for untreated control)/OD600. Different uppercase letters for cells grown in the same matrix indicate significant differences (P < 0.05). Different lowercase letters for the same treatment dose indicate significant differences (P < 0.05).
Changes in fatty acid composition of the cell membrane.
Changes in the membrane fatty acid composition, including the contents of individual major fatty acids, total minor fatty acid (MFA) content, and the ratio of unsaturated to saturated fatty acids (USFA/SFA), are shown in Table 3 for S. Typhimurium and E. coli O157:H7 relative to acid adaptation. Overall, for both pathogens, saturated fatty acid content increased and unsaturated fatty acid content decreased as a result of acid adaptation. Specifically, in the case of S. Typhimurium, the content of C16:0 increased significantly (P < 0.05), from 53.51 to 66.78, while the content of C16:1 and C18:1 decreased significantly (P < 0.05), from 13.30 to 4.74 and from 6.64 to not detected (ND), respectively. Also, in the case of E. coli O157:H7, C14:0 and C15:0 contents increased significantly (P < 0.05), from 12.26 to 20.46 and from 5.18 to 9.16, respectively, while the contents of C16:1 and C18:1 decreased significantly (P < 0.05), from 10.70 to 0.77 and from 1.78 to ND, respectively. Furthermore, we derived the value of USFA divided by SFA because the calculated USFA/SFA ratio can be used as an indirect indicator of membrane fluidity (61). Accordingly, significantly (P < 0.05) decreased USFA/SFA ratio values were observed following acid adaptation in both S. Typhimurium and E. coli O157:H7. In other words, S. Typhimurium and E. coli O157:H7 showed reduced fluidity of the cell membrane under acidic conditions.
TABLE 3.
Membrane fatty acid compositions of non-acid-adapted and acid-adapted Salmonella Typhimurium and Escherichia coli O157:H7
| Fatty acid | Content (%)a |
|||
|---|---|---|---|---|
|
S. Typhimurium |
E. coli O157:H7 |
|||
| Non-acid adaptation | Acid adaptation | Non-acid adaptation | Acid adaptation | |
| C12:0 | 10.23 ± 3.80 A | 9.20 ± 1.53 A | 12.26 ± 2.19 A | 20.46 ± 2.39 B |
| C14:0 | 13.85 ± 1.06 A | 12.80 ± 1.28 A | 14.26 ± 1.73 A | 16.01 ± 1.52 A |
| C15:0 | 2.13 ± 2.18 A | 4.20 ± 0.73 A | 5.18 ± 1.82 A | 9.16 ± 0.95 B |
| C16:0 | 53.51 ± 6.68 A | 66.78 ± 3.64 B | 52.58 ± 3.25 A | 52.44 ± 2.84 B |
| C16:1 | 13.30 ± 1.38 A | 4.74 ± 4.13 B | 10.70 ± 2.63 A | 0.77 ± 1.33 B |
| C18:1 | 6.64 ± 6.51 | ND | 1.78 ± 1.61 | ND |
| MFA | 0.15 ± 0.27 A | 2.28 ± 4.74 A | 3.24 ± 4.08 A | 1.15 ± 0.25 A |
| USFA/SFA | 0.25 ± 0.09 A | 0.05 ± 0.04 B | 0.15 ± 0.05 A | 0.01 ± 0.01 B |
The values are means ± standard deviations for three replications. MFA, total minor fatty acids; USFA, total unsaturated fatty acids; SFA, total saturated fatty acids; ND, not detected (absent or below the detection limit). Mean values with the same uppercase letter within the same row for each pathogen are not significantly different (P > 0.05).
Observation of apple juice quality during 222-nm KrCl excilamp treatment.
Color (L*, a*, and b*, indicating color lightness, redness, and yellowness, respectively), total phenols, and DPPH free radical scavenging activity values were chosen as quality indicators because these values are commonly investigated for comparisons of quality changes in studies involving apple juice disinfection (62–66), and accordingly, these values were measured after 222-nm KrCl excilamp treatment. During treatment at 197 to 1,773 mJ/cm2, there were no significantly (P > 0.05) different values compared to those for untreated control samples for all three values (Table 4). This means that 222-nm KrCl excilamps can reduce the number of cells of S. Typhimurium and E. coli O157:H7 that are not acid adapted, as well as those of S. Typhimurium and E. coli O157:H7 that are adapted to acid, more than 5 log without inducing quality deterioration of apple juice.
TABLE 4.
Changes in color, DPPH free radical scavenging activity, and total phenols in apple juice during 222-nm KrCl excilamp treatment
| Treatment dose (mJ/cm2) | Quality valuea
|
||||
|---|---|---|---|---|---|
| Color |
DPPH free radical scavenging activity (%) | Total phenols (mg GAE/liter) | |||
| L* | a* | b* | |||
| 0 | 24.52 ± 0.63 | 0.41 ± 0.05 | 4.76 ± 0.10 | 84.12 ± 0.52 | 42.39 ± 0.63 |
| 197 | 24.44 ± 0.61 | 0.43 ± 0.01 | 4.75 ± 0.08 | 84.44 ± 0.16 | 42.20 ± 0.36 |
| 591 | 24.69 ± 0.85 | 0.42 ± 0.03 | 4.78 ± 0.10 | 84.48 ± 0.44 | 42.58 ± 0.51 |
| 985 | 24.35 ± 0.67 | 0.43 ± 0.06 | 4.79 ± 0.23 | 84.76 ± 0.50 | 42.44 ± 0.60 |
| 1,379 | 24.43 ± 0.43 | 0.42 ± 0.06 | 4.82 ± 0.13 | 84.26 ± 0.60 | 42.29 ± 0.55 |
| 1,773 | 24.00 ± 0.92 | 0.43 ± 0.08 | 4.86 ± 0.09 | 84.58 ± 0.18 | 42.63 ± 0.36 |
Data are means ± standard deviations for three replications. All quality values showed no significant (P > 0.05) difference from untreated controls during treatment at 197 to 1,773 mJ/cm2.
DISCUSSION
Our results showed that in both PBS and apple juice, both acid-adapted S. Typhimurium and E. coli O157 exhibited increased resistance to the 222-nm KrCl excilamp compared to that of cells not adapted to acid (Fig. 2 and Table 1). Acid-exposed cells exhibit cross protection against many other environmental challenges, such as heat, oxidative stress, high osmolarity, and DNA damage, which may be related to the activation of various global regulators due to changes in internal and external pH (67). This means that stress from the acidic environment led to increased resistance of S. Typhimurium and E. coli O157:H7 to 222-nm KrCl excilamp treatment by inducing the acid adaptation response. Indeed, this increase in pathogen resistance due to acid adaptation is consistent with other studies showing increased resistance when acid-adapted cells are subjected to fruit juice processing interventions, such as heating, ultrasound, and ozone treatment (45–50). Therefore, it is important to consider the acid adaptation response, because there is a possibility of overestimating the bactericidal effect of 222-nm KrCl excilamp treatment on pathogenic bacteria in apple juice if these bacteria are adapted to acid and exhibit increased resistance.
Regardless of cell condition, more UV energy was needed to inactivate pathogens in apple juice than those in PBS at the sample matrix level. This tendency is consistent with the results of Gabriel and Nakano (12), who showed that pathogens in apple juice exhibited greater D values on exposure to UV radiation treatment than those for pathogens in PBS. Ngadi et al. (68) reported that the pH of the suspending food matrix did not affect UV inactivation of E. coli O157:H7, as pathogens in apple juice and liquid egg white (pH 3.5 and 9.1, respectively) showed similar inactivation rates for UV radiation treatment. Therefore, it can be considered that PBS and apple juice exhibit different D5d values that are not due to the pH of the medium. Instead, this phenomenon can be explained by the transmission characteristics of UV radiation in the suspending medium. Because of the presence of organic solutes, colored compounds, or suspended matter, fresh juice products allow only relatively little UV light transmission, and this low UV light transmission in fresh juice affects the effectiveness of the UV pasteurization process (69). In fact, it is widely reported that the inactivation effect of UV light decreases as the absorbance or amount of suspended particles in the suspension medium increases (70, 71). Specifically, Murakami et al. (71) showed that the turbidity and absorption coefficient of apple juice reduce the UV inactivation capacity as their levels increase. They interpreted this to mean that as the turbidity of apple juice increased, the UV light shielding effect of the particles increased, which would have a negative impact on the UV inactivation effect. Also, they explained the effect of the absorption coefficient of apple juice on the UV inactivation effect by using Beer-Lambert-Bouger’s law (72), by which the amount of light transmitted through the solution decreases as the absorption coefficient of the solution increases. Since the apple juice used in this study had a turbidity of 0 nephelometric turbidity units (NTU) and an absorption coefficient of 20.8 cm−1, the results of this study indicate that pathogens in apple juice show larger D5d values than those for pathogens in PBS, which can be explained by the absorption coefficient of apple juice. That is, unlike the case in PBS, in which 222-nm UV light is transmitted completely (data not shown), in apple juice, with an absorption coefficient of 20.8 cm−1 for the 222-nm wavelength, 222-nm UV light penetrates as its light intensity decreases in proportion to the absorption coefficient value of apple juice according to Beer-Lambert-Bouger’s law, so the actual UV dose imparted in apple juice is lower than the UV dose imparted in PBS, which ultimately results in a lower UV inactivation effect on the pathogens in apple juice than on those in PBS. Expanding on this, it can be expected that juice samples with different turbidities or absorption coefficients for the same UV treatment can produce different inactivation results. Moreover, the decreased capability of UV radiation to penetrate a food medium with high turbidity or absorption coefficient characteristics can create limitations in the practical application of UV light by the juice industry. In response, various strategies have been proposed to optimize the efficacy of UV radiation. In one of them, new UV reactors have been devised to guarantee turbulent flow patterns to ensure that all portions of the liquid sample are exposed to UV (69, 73, 74). Another strategy is to incorporate UV light and other novel processing techniques or milder conventional conservation methods, called hurdle technology, which can achieve acceptable inactivation of pathogens (75). In particular, a study by Yin et al. (35) reported that the absorption coefficient increased from 16.15 to 30.25 cm−1 as the wavelength decreased from 282 to 222 nm, indicating that the shorter the UV wavelength, the smaller the penetration depth of UV photons becomes. Therefore, for practical application of 222-nm KrCl excilamps by the apple juice industry, it is necessary to consider the transmittance of the UV light and to establish an appropriate strategy for optimizing treatment.
Since elucidation of the mechanism of increased resistance can be provided as important baseline data for future related research or industrial applications, we sought to determine the mechanism by which resistance of pathogens to 222-nm KrCl excilamp inactivation increases as they become adapted to acid. Our previous studies that identified the inactivation mechanism of the 222-nm KrCl excilamp suggested that it mainly induces lipid peroxidation of the cell membrane, resulting in physical damage with increased permeability and eventually leading to cell death (34, 76). Thus, we hypothesized that the cause of differential resistance to 222-nm KrCl excilamp treatment between non-acid-adapted and acid-adapted cells is related to the occurrence of cell membrane damage. From the results shown in Fig. 3, it can be seen that when pathogens are adapted to acid, less physical membrane damage, such as pore formation, occurs than that in pathogens not adapted to acid when exposed to the same UV treatment dose. This physical damage in the cell membrane induces events such as increased permeability of the cell membrane, changes in the membrane potential and the internal pH, and a decrease in the concentration of the cellular ATP, which can make it difficult for cells to maintain homeostasis, eventually leading to cell death (56, 77, 78). Thus, the results for cell membrane damage can be linked to results which show that more acid-adapted cells tend to survive against 222-nm KrCl excilamp treatment than is the case for non-acid-adapted cells. In other words, for 222-nm KrCl excilamp treatment, acid-adapted cells show greater resistance because less membrane damage occurs than that for cells that are not acid adapted. For a specific mechanism analysis, it is necessary to identify the cause of membrane damage by investigating what form of changes are caused by 222-nm KrCl excilamp treatment and result in generation of physical damage, such as pore formation. In particular, this cause of cell membrane damage must exhibit a difference in the degree of occurrence between acid-adapted and non-acid-adapted cells. As mentioned above, it is known that 222-nm KrCl excilamps induce lipid peroxidation, resulting in cell membrane damage. Thus, we measured the extent of lipid peroxidation of the cell membrane during 222-nm KrCl excilamp treatment through DPPP assay. As shown in Fig. 4, it was confirmed that 222-nm KrCl excilamp treatment induced lipid peroxidation of the cell membrane for both pathogens, and at the same 222-nm KrCl excilamp treatment dose, the generation of lipid peroxidation of the cell membrane was lower in acid-adapted than in non-acid-adapted cells. If lipid peroxidation occurs in the cell membrane, it will cause damages, such as loss of fluidity, decreasing cell membrane potential, and increasing permeability, leading to cell death (79). Therefore, 222-nm KrCl excilamp treatment induces lipid peroxidation in the cell membrane, which results in physical damages leading to cell death, such as pore formation, and when cells become acid adapted, less lipid peroxidation occurs in the cell membrane than that in cells that are not acid adapted, resulting in increased resistance to 222-nm KrCl excilamp treatment.
In order to fully analyze the mechanism, there was still the question of how different amounts of lipid peroxidation occurred with the same treatment dose. In general, it is known that when intracellular constituents, such as chromophoric amino acids, absorb UV light, excited state species or radicals are generated as a result of photoionization through the photosensitization process (80–82). Based on this principle, it is widely reported that UV-induced ROS cause oxidative damage to cells (82–85). In particular, based on several studies, it is well demonstrated that ROS are one of the major causes that induce lipid peroxidation in cell membranes, resulting in cell death (86–89). Indeed, our previous study (34) found that the 222-nm KrCl excilamp produced lipid peroxidation in the cell membrane as a result of ROS generation. Therefore, we assumed that different amounts of ROS were produced with the same treatment dose, resulting in a difference in the occurrence of lipid peroxidation, and thus we measured the intracellular ROS of cells that were adapted as well as not adapted to acid and subjected to treatment. However, unlike our hypothesis, during 222-nm KrCl excilamp treatment, the same amounts of ROS were generated in both non-acid-adapted and acid-adapted cells at the same dose for both pathogens. In other words, different levels of lipid peroxidation of cell membranes occur between non-acid-adapted and acid-adapted cells for the same amount of intracellular ROS. Additionally, it is known that an acid stress environment causes various physiological changes to bacteria, including a decrease in cytoplasmic pH (90), an increase in activity and production of the F1Fo-ATPase that excretes protons out of the cell (91), and an alteration in the fatty acid composition of the cell membrane (92). Based on these facts, along with the results of this study showing that levels of lipid peroxidation in fatty acids of the cell membrane between non-acid-adapted and acid-adapted cells were different, we hypothesized that the different levels of lipid peroxidation might be related to a change in fatty acid composition of the cell membrane among several physiological changes induced by the acid adaptation response, and thus we examined the change of the fatty acid composition of the cell membrane after acid adaptation. We found that the content of saturated fatty acids increased and that of unsaturated fatty acids decreased, and thus the value of the USFA/SFA ratio, which can indicate fluidity of the cell membrane, decreased remarkably, when cells were adapted to acid (Table 2). Other studies investigating the fatty acid composition of bacteria adapted to acidic conditions have also reported a decrease in cell membrane fluidity (increased USFA and decreased SFA) as a result of acid adaptation (92–95). Therefore, our results are consistent with the results of several other studies. A study by Jordan et al. (96) demonstrated that resistance of E. coli O157:H7 to acids increased due to reduced proton penetration through cell membranes in acidic environments when cells were adapted to acid. Thus, if bacteria become adapted to acid, they improve their ability to maintain intracellular pH, thereby increasing their survival under acidic conditions. Linking this to our results, the increase in USFA and the decrease in SFA in the cell membrane as a result of acid adaptation are interpreted to mean that pathogens reduce their membrane fluidity by altering the fatty acid composition as a way of decreasing their permeability to protons in order to maintain their internal pH, consequently increasing their resistance to acids under acidic conditions. Meanwhile, it is well known that the presence of the double bond in fatty acids weakens the C-H bonds adjacent to the double bond to facilitate the removal of the hydrogen ion, so the unsaturated fatty acid is particularly sensitive to the induction of peroxidation and thus the unsaturated fatty acid in the cell membrane is extremely vulnerable to damage caused by ROS, in contrast to saturated fatty acids (79, 97). Based on this principle, our findings can be interpreted as follows: fluidity of the cell membrane is reduced by decreasing USFA and increasing SFA levels, which increases resistance to acidic conditions as a result of acid adaptation, and thus damage to the cell membrane caused by ROS is reduced, resulting in an increase in the resistance of pathogens to 222-nm KrCl excilamp treatment.
In conclusion, S. Typhimurium and E. coli O157:H7, which are the predominant pathogens implicated in apple juice-related outbreaks, were resistant to 222-nm KrCl excilamp treatment when adapted to acid. Therefore, acid adaptation of pathogens must be considered in order to avoid overestimation of the bactericidal effect in application of 222-nm KrCl excilamps in the apple juice pasteurization process. Conventional 254-nm mercury lamp treatment has been reported to effectively reduce the microbial load in fruit juice while minimizing quality changes (23, 98–100). In the present study, the 222-nm KrCl excilamp reduced levels of acid-adapted pathogens more than 5 log without adversely affecting apple juice quality indicators, such as color, total phenols, and DPPH free radical scavenging activity. This result supports the possibility that the 222-nm KrCl excilamp is a promising technology for apple juice disinfection processing by replacing existing mercury UV lamps. However, this study shows only the laboratory-level disinfection effect of 222-nm KrCl excilamps and the limited quality analysis results. For practical application of 222-nm KrCl excilamps, further studies on commercial-scale application, changes in nutritional or organoleptic properties, sensory evaluation by use of panels, consumer acceptance, and cytotoxicity after treatment are necessary. Nevertheless, since this study, which confirmed the disinfection effect of the 222-nm KrCl excilamp in apple juice and identified the increased resistance of pathogens to the 222-nm KrCl excilamp as a result of acid adaptation, provides valuable baseline data for practical industry application, it can be expected to be utilized in further studies to help facilitate 222-nm KrCl excilamp treatment by the juice industry in the near future.
MATERIALS AND METHODS
Bacterial strains.
Three strains each of E. coli O157:H7 (ATCC 35150, ATCC 43889, and ATCC 43890) and S. Typhimurium (ATCC 19585, ATCC 43971, and DT104), obtained from the bacterial culture collection of Seoul National University (Seoul, South Korea), were used in this study. Stock cultures were prepared by incubating strains in 5 ml of tryptic soy broth (TSB; Difco, BD) at 37°C for 24 h, mixing 0.7 ml with 0.3 ml of sterile 50% glycerol, and then storing cultures at −80°C. To prepare working cultures, bacterial strains were streaked onto tryptic soy agar (TSA; Difco, BD), incubated at 37°C for 24 h, and then stored at 4°C and used within 1 week.
Preparation of non-acid-adapted and acid-adapted bacterial cultures and inoculums.
All strains of E. coli O157:H7 and S. Typhimurium were separately cultured in 5 ml of TSB overnight at 37°C, and then 200-μl aliquots of overnight cultures were transferred to 20 ml of TSB without dextrose (TSB w/o D; pH 7.3) (Difco, BD) and TSB w/o D at pH 5.0 (adjusted with 6 N HCl) to develop the non-acid adaptation and acid adaptation responses, respectively (39, 68, 101), and incubated for 18 h at 37°C. The incubation time of 18 h was the time required for the cells to grow to early stationary phase in TSB w/o D, pH 7.3 or 5.0, which was determined from growth curves plotting the mean optical density at 600 nm (OD600) versus the incubation time of each strain (data not shown). This incubation time was applied because it is commonly known that bacteria in stationary phase exhibit increased UV resistance (102, 103). Furthermore, in this study, TSB w/o D instead of the usual TSB formulation (containing 0.25% dextrose) was used to prevent the acid adaptation response of the pathogens from being induced in non-acid-adapted cells by acids produced as a result of dextrose fermentation (101, 104). After incubation, all three strains of each pathogen were combined, and the cell pellet was harvested by centrifugation at 4,000 × g for 20 min at 4°C and washed twice with phosphate-buffered saline (PBS; 0.1 M). To prepare the inoculum, the final pelleted cells were resuspended in PBS at a concentration of approximately 1010 to 1011 CFU/ml.
UV treatment.
In this study, a DBD excilamp (29 × 9 × 8 cm; Unilam, Ulsan, South Korea) with 20 W of output power and filled with a KrCl gas mixture was used as a UV radiation source; detailed specifications of this apparatus were described in our previous study (34). A batch-type processing system was used in this study and was constructed as follows: a 222-nm KrCl excilamp was placed vertically at a 12.8-cm distance from the center of the sample surface to allow the sample to vertically face the UV radiation (Fig. 1). In this experimental setup, a light intensity of 0.29 mW/cm2 at the center of the sample surface (incidence intensity) was measured using a UV fiber optic spectrometer (AvaSpec-ULS2048; Avantes, Eerbeek, Netherlands). However, since the UV light of the excilamp used in this study is emitted without being collimated, the intensity decreases as the distance from the center of the sample surface contained in the petri dish increases, resulting in no uniform intensity throughout the sample surface. Therefore, the petri factor, defined as the ratio of the average incident irradiance over the petri dish area to the irradiance at the center of the petri dish, was used to correct the intensity at the petri dish center to more accurately reflect the incident fluence average over the surface (105). The petri factor was calculated by scanning the probe over every 5 mm of the surface area of the sample, and the value was 0.97. Therefore, the incident intensity (0.29 mW/cm2) measured at the center of the sample surface was corrected to 0.28 mW/cm2 by multiplying by the petri factor of 0.97, and this value was used in this study. Commercially clarified apple juice (Woongjin Foods Co., Seoul, South Korea) purchased from a local grocery market and PBS were used as samples. Samples were kept in a refrigerator at 4°C until use. Before inoculation, samples were taken out of the refrigerator for more than 1 h to equilibrate to room temperature (22°C ± 1°C). Five milliliters of each sample was dispensed into a petri dish (60 × 15 mm2), inoculated with 0.1 ml of inoculum, and then gently mixed by hand for approximately 5 s to allow for an even distribution. At this time, the sample depth was 2 mm. Immediately after inoculation and mixing, apple juice and PBS samples were treated with a 222-nm KrCl excilamp at dosages of 0.97 to 8.69 mJ/cm2 and 197 to 1,773 mJ/cm2, respectively, while being mixed with a magnetic stirrer (1.5 cm × 0.1 cm; TM-17R; Jeio Tech, South Korea) at 300 rpm to allow for even irradiation at room temperature (22°C ± 1°C) in a dark chamber. UV doses were calculated by multiplying the incident intensity of UV light by the irradiation times. The value of the UV dose in our study implies the amount of energy delivered to the sample surface, with the change in energy due to the sample matrix not taken into consideration, because it is expressed as the energy value for the intensity of the UV light reaching the sample surface. In order to make the results of this study comparable to those of other relevant studies, the characteristics of apple juice, such as pH, sugar concentration (degrees Brix [°Bx]), turbidity (NTU), and UV absorption coefficient (α), were investigated. The pH, sugar concentration, and turbidity of apple juice samples were measured with a pH meter (Mettler Toledo, Switzerland), a digital refractometer (Atago Co., Ltd., Japan), and a turbidity meter (Lutron Electronic Enterprise Co., Ltd., Taiwan), respectively, and their values were pH 2.95, 12.6 °Bx, and 0 NTU, respectively. To obtain the absorption coefficient (α) of apple juice samples at 222 nm, absorbance values for various dilutions (1:10, 1:25, 1:50, 1:100, 1:250, and 1:500 [vol/vol]) of apple juice sample were measured at 222 nm by use of a quartz cuvette with a 1-cm path length in a spectrophotometer (Spectramax M2e; Molecular Devices, Sunnyvale, CA, USA), and then the absorption coefficient was estimated from the slope of the absorbance value-versus-sample concentration plot; the absorption coefficient of the apple juice sample was 20.8 cm−1.
For investigation of destruction and lipid peroxidation of the cell membrane and ROS generation, non-acid-adapted or acid-adapted E. coli O157:H7 and S. Typhimurium were adjusted to an OD600 of approximately 0.3 in PBS and then treated with a 222-nm KrCl excilamp at 8.69 mJ/cm2.
Bacterial enumeration.
For microbial enumeration after treatment, 1-ml aliquots of treated samples were 10-fold serially diluted in 9 ml of sterile 0.2% buffered peptone water, and 0.1-ml aliquots of samples or diluents were spread plated onto each selective medium. If small populations of surviving cells were anticipated, 1-ml aliquots of the undiluted original samples were equally divided between four plates of each medium and spread plated. Xylose lysine desoxycholate agar (XLD; Difco) and sorbitol MacConkey agar (SMAC; Difco) were used as selective media for the enumeration of S. Typhimurium and E. coli O157:H7, respectively. All plates were incubated at 37°C for 24 h, and typical colonies were enumerated and calculated as log10 CFU per milliliter (detection limit, 0.70 log CFU/ml).
Enumeration of sublethally injured cells.
To identify the occurrences of sublethally injured cells of S. Typhimurium in apple juice after treatment, the overlay (OV) method was used (106). A nonselective medium, tryptic soy agar (TSA; Difco), was used to resuscitate injured cells. Aliquots of appropriate dilutions or undiluted original samples were duplicate spread plated onto TSA, and the plates were incubated at 37°C for 2 h to facilitate injured-cell recovery. Following incubation, the plates were overlaid with 7 ml of the selective medium XLD. After solidification, the plates were incubated for an additional 22 h at 37°C, and then typical black colonies were counted.
In the case of enumeration of injured E. coli O157:H7 cells, phenol red agar base with 1% sorbitol (SPRAB; Difco) was used (29). After incubation at 37°C for 24 h, typical white colonies characteristic of E. coli O157:H7 were enumerated, and simultaneously, serological confirmation (RIM E. coli O157:H7 latex agglutination test; Remel, Lenexa, KS, USA) was performed on randomly selected presumptive E. coli O157:H7 colonies because SPRAB is not generally used as a selective medium for enumerating E. coli O157:H7.
Modeling of survival curves and calculation of D5d.
In order to quantitatively compare our inactivation results, the D5d value, which indicates the UV dosage necessary to achieve a 5-log reduction by 222-nm KrCl excilamp treatment, was calculated using predictive modeling equations. To identify the appropriate model to apply to our results among the various models, the goodness of fit of each model for each data set was evaluated using R2 and root mean square error (RMSE) values, which meant that the closer the value was to 1 and the lower the value, respectively, the better the model fit the data. When survival curves were fitted with several model equations by use of GInaFiT (107), it was shown that the Weibull model and the Weibull-with-tail model were well fitted to all inactivation data except those for acid-adapted E. coli O157:H7 in PBS and to the inactivation data only for acid-adapted E. coli O157:H7 in PBS, respectively, because these models represented high (≥0.97) R2 and low (<0.50) RMSE values. Thus, these two models were applied in this study. The related model equations are as follows:
The Weibull model equation (108) is described as follows:
where N (CFU/ml) is the surviving population of microorganisms, N0 is the initial population, d (mJ/cm2) is the treatment dosage, δ (mJ/cm2) is the dosage for the first decimal reduction, and p is the parameter related to the shape of the line, such as a concave upward curve when p < 1 and a concave downward survival curve when p > 1.
The Weibull-with-tail equation (109) is a modification of the Weibull model and is described as follows:
where N (CFU/ml) is the surviving population of microorganisms, N0 is the initial population, d (mJ/cm2) is the treatment dosage, δ (mJ/cm2) is the dosage for the first decimal reduction, Nres is the residual population density, and p is the parameter describing the shape of the line. There is no point of inflection when 0 < p < 1, but the line has an inflection point when p > 1.
After the model parameters for each model (Weibull or Weibull with tail) were obtained by fitting survival curves to model equations by use of GInaFiT, D5d was derived using Microsoft Excel 2010: describing the appropriate equations for independent variables followed by calculating the dose value for a particular log reduction was performed using the “goal seek” function.
Measurement of incidence of cell membrane damage and generation of intracellular ROS.
Treated samples were incubated with PI (Sigma-Aldrich), DPPP (Sigma-Aldrich), or CM-H2DCFDA (Molecular Probes, Thermo Fisher Scientific) at a final concentration of 2.9, 50, or 5 μM for 10, 20, or 15 min at 37°C to assess pore formation in the cell membrane, incidence of lipid peroxidation of the cell membrane, or generation of intracellular ROS, respectively. After incubation, cells were collected by centrifugation at 10,000 × g for 10 min and washed twice with PBS, and fluorescence was measured with a spectrofluorophotometer (Spectramax M2e; Molecular Devices, Sunnyvale, CA, USA) at excitation/emission wavelengths of 493/630 nm for PI uptake, 351/380 nm for DPPP assay, and 495/520 nm for CM-H2DCFDA assay. For quantitative comparison of the values, the obtained fluorescence signal was divided by the OD600. Because this study was intended to quantitatively compare only the extents of damage to the cell membrane and generation of ROS, quantitative comparisons were performed using average values at the population level rather than comparing values at the individual cell level by using a tool such as flow cytometry.
Analysis of fatty acid composition of the cell membrane.
Cells grown under nonacidic or acidic conditions were collected by centrifugation at 4,000 × g for 20 min at 4°C, and the obtained pellets were subjected to fatty acid extraction following protocols described in MIDI technical note 101 (110). The fatty acid methyl esters (FAMEs), extracted with mixtures of hexane and methyl tert-butyl ether, were analyzed with an Agilent gas chromatograph (model 7890 A; Agilent Technologies, Santa Clara, CA, USA) equipped with a split-capillary injector and a flame ionization detector (111). A DB-23 column (60 mm × 0.25-mm internal diameter × 0.25 μm; Agilent Technologies) was used to separate fatty acids. The injection and detector temperatures were set at 250 and 280°C, respectively. The initial column oven was maintained at 50°C for 1 min, and then the temperature was increased to 130°C at a rate of 15°C/min, 170°C at a rate of 8°C/min, and 215°C at a rate of 2°C/min and then held for 10 min. The carrier gases were hydrogen, air, and helium at flow rates of 35, 350, and 35 ml/min, respectively. The fatty acid compositions were analyzed using a Supelco 37 component FAME mix (Supelco, Inc., PA, USA). The relative contents of fatty acids were expressed as percentages by peak area normalization.
Quality measurement.
To analyze changes of apple juice quality after treatment, color, total phenols, and DPPH free radical scavenging activity of apple juice were investigated. Apple juice was treated with the 222-nm KrCl excilamp at doses of 197 to 1,773 mJ/cm2, and untreated apple juice was used as a control. Color values for apple juice were measured with a Minolta colorimeter (CR400; Minolta Co., Osaka, Japan) and expressed as L*, a*, and b* values, indicating color lightness, redness, and yellowness, respectively (112).
Total phenols were measured by the Folin-Ciocalteu assay (113, 114). Briefly, 1 ml of apple juice sample diluted 10-fold in distilled water was incubated with 5 ml of 0.2 N Folin-Ciocalteu reagent (Sigma-Aldrich, St. Louis, MO) for 3 min at room temperature (22°C ± 1°C). This mixture was then mixed with 4 ml of 7.5% sodium carbonate (Samchun Pure Chemicals Co., Ltd., Pyeongtaek, South Korea) solution. After maintenance at room temperature (22°C ± 1°C) for 2 h in the dark with shaking, the absorbance at 765 nm was measured against that of a blank sample by use of a spectrophotometer. The blank sample was prepared using distilled water instead of apple juice. Gallic acid (Sigma-Aldrich) was used as a standard solution to construct the calibration curve, and the results were expressed as milligrams of gallic acid equivalents (GAE) per liter.
DPPH free radical scavenging activity was measured according to the method described by Abid et al. (62), with some modification. Two milliliters of 0.2 mM DPPH solution dissolved in methanol was added to 2 ml of apple juice sample. This mixture was incubated for 30 min at room temperature (22°C ± 1°C) in the dark. After the reaction, the absorbance at 517 nm was measured using a spectrophotometer (Spectramax M2e; Molecular Devices, Sunnyvale, CA). A control was prepared with methanol instead of apple juice, and the absorbance of the control was measured with the same procedure. DPPH free radical scavenging activity was calculated as follows: DPPH free radical scavenging activity (%) = (1 − absorbance of sample/absorbance of control) × 100.
Statistical analysis.
All experiments were duplicate plated and replicated three times. All data were analyzed by the analysis of variance (ANOVA) procedure of SAS (version 9.4; SAS Institute Inc., Cary, NC, USA) and the least significant difference (LSD) t test to determine whether there were significant differences in mean values for log reduction, fluorescence signal normalized to OD600, fatty acid composition, color, DPPH free radical scavenging activity, and total phenols at a probability level (P) of <0.05.
ACKNOWLEDGMENTS
This research was part of a project entitled “Development and Commercialization of Marine Products Applicable Rapid Detection Method for Hazardous Microorganisms (Bacteria & Viruses) and Construction Safety Management System by Application New Technology,” funded by the Ministry of Oceans and Fisheries, South Korea. This work was also supported by a National Research Foundation of Korea (NRF) grant funded by the South Korean government (grant NRF-2018R1A2B2008825).
REFERENCES
- 1.Raybaudi-Massilia RM, Mosqueda-Melgar J, Martín-Belloso O. 2009. Antimicrobial activity of malic acid against Listeria monocytogenes, Salmonella Enteritidis and Escherichia coli O157:H7 in apple, pear and melon juices. Food Control 20:105–112. doi: 10.1016/j.foodcont.2008.02.009. [DOI] [Google Scholar]
- 2.Ait-Ouazzou A, Espina L, García-Gonzalo D, Pagán R. 2013. Synergistic combination of physical treatments and carvacrol for Escherichia coli O157:H7 inactivation in apple, mango, orange, and tomato juices. Food Control 32:159–167. doi: 10.1016/j.foodcont.2012.11.036. [DOI] [Google Scholar]
- 3.Chueca B, Ramírez N, Arvizu-Medrano SM, García-Gonzalo D, Pagán R. 2016. Inactivation of spoiling microorganisms in apple juice by a combination of essential oils’ constituents and physical treatments. Food Sci Technol Int 22:389–398. doi: 10.1177/1082013215606832. [DOI] [PubMed] [Google Scholar]
- 4.USDA. 2012. Apple statistics table 18, apple juice and cider: supply and utilization in the United States, 1980/81 to date. http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1825.
- 5.AIJN. 2012. Liquid fruit market report 2012, p 6–7. European Fruit Juice Association; Brussels, Belgium: http://www.aijn.eu/pages/main/file.handler?f=AIJNMarketReport2012.pdf. [Google Scholar]
- 6.Lima Tribst AA, de Souza Sant’Ana A, de Massaguer PR. 2009. Microbiological quality and safety of fruit juices—past, present and future perspectives. Crit Rev Microbiol 35:310–339. doi: 10.3109/10408410903241428. [DOI] [PubMed] [Google Scholar]
- 7.Danyluk MD, Goodrich-Schneider RM, Schneider KR, Harris LJ, Worobo RW. 2012. Outbreaks of foodborne disease associated with fruit and vegetable juices, 1922–2010. EDIS publication FSHN12-04. http://ucfoodsafety.ucdavis.edu/files/223883.pdf.
- 8.Cody SH, Glynn MK, Farrar JA, Cairns KL, Griffin PM, Kobayashi J, Fyfe M, Hoffman R, King AS, Lewis JH, Swaminathan B, Bryant RG, Vugia DJ. 1999. An outbreak of Escherichia coli O157:H7 infection from unpasteurized commercial apple juice. Ann Intern Med 130:202–209. [DOI] [PubMed] [Google Scholar]
- 9.CDC. 1996. Outbreak of Escherichia coli O157:H7 infections associated with drinking unpasteurized commercial apple juice—British Columbia, California, Colorado, and Washington, October 1996. MMWR Morb Mortal Wkly Rep 45:975. [PubMed] [Google Scholar]
- 10.Basaran N, Quintero-Ramos A, Moake M, Churey J, Worobo R. 2004. Influence of apple cultivars on inactivation of different strains of Escherichia coli O157:H7 in apple cider by UV irradiation. Appl Environ Microbiol 70:6061–6065. doi: 10.1128/AEM.70.10.6061-6065.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.USFDA. 2001. Hazard analysis and critical control point (HACCP); procedures for the safe and sanitary processing and importing of juice. Fed Regist 66:6137–6202. [Google Scholar]
- 12.Gabriel AA, Nakano H. 2009. Inactivation of Salmonella, E. coli and Listeria monocytogenes in phosphate-buffered saline and apple juice by ultraviolet and heat treatments. Food Control 20:443–446. doi: 10.1016/j.foodcont.2008.08.008. [DOI] [Google Scholar]
- 13.Gouma M, Álvarez I, Condón S, Gayán E. 2015. Modelling microbial inactivation kinetics of combined UV-H treatments in apple juice. Innov Food Sci Emerg Technol 27:111–120. doi: 10.1016/j.ifset.2014.11.004. [DOI] [Google Scholar]
- 14.Gouma M, Gayán E, Raso J, Condón S, Álvarez I. 2015. Influence of dimethyl dicarbonate on the resistance of Escherichia coli to a combined UV-heat treatment in apple juice. Front Microbiol 6:501. doi: 10.3389/fmicb.2015.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tahiri I, Makhlouf J, Paquin P, Fliss I. 2006. Inactivation of food spoilage bacteria and Escherichia coli O157:H7 in phosphate buffer and orange juice using dynamic high pressure. Food Res Int 39:98–105. doi: 10.1016/j.foodres.2005.06.005. [DOI] [Google Scholar]
- 16.Aguilar-Rosas S, Ballinas-Casarrubias M, Nevarez-Moorillon G, Martin-Belloso O, Ortega-Rivas E. 2007. Thermal and pulsed electric fields pasteurization of apple juice: effects on physicochemical properties and flavour compounds. J Food Eng 83:41–46. doi: 10.1016/j.jfoodeng.2006.12.011. [DOI] [Google Scholar]
- 17.Guerrero-Beltran JA, Barbosa-Canovas GV. 2004. Advantages and limitations on processing foods by UV light. Food Sci Technol Int 10:137–147. doi: 10.1177/1082013204044359. [DOI] [Google Scholar]
- 18.Chen J, Loeb S, Kim J-H. 2017. LED revolution: fundamentals and prospects for UV disinfection applications. Environ Sci Water Res Technol 3:188–202. doi: 10.1039/C6EW00241B. [DOI] [Google Scholar]
- 19.USFDA. 2001. Irradiation in the production, processing and handling of food Code of federal regulations. 21 CFR, title 21, vol 3. Part 179.39. USFDA, Silver Spring, MD. [Google Scholar]
- 20.Beck SE, Ryu H, Boczek LA, Cashdollar JL, Jeanis KM, Rosenblum JS, Lawal OR, Linden KG. 2017. Evaluating UV-C LED disinfection performance and investigating potential dual-wavelength synergy. Water Res 109:207–216. doi: 10.1016/j.watres.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chevremont A-C, Boudenne J-L, Coulomb B, Farnet A-M. 2013. Impact of watering with UV-LED-treated wastewater on microbial and physico-chemical parameters of soil. Water Res 47:1971–1982. doi: 10.1016/j.watres.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Char CD, Mitilinaki E, Guerrero SN, Alzamora SM. 2010. Use of high-intensity ultrasound and UV-C light to inactivate some microorganisms in fruit juices. Food Bioprocess Technol 3:797–803. doi: 10.1007/s11947-009-0307-7. [DOI] [Google Scholar]
- 23.Caminiti IM, Palgan I, Muñoz A, Noci F, Whyte P, Morgan DJ, Cronin DA, Lyng JG. 2012. The effect of ultraviolet light on microbial inactivation and quality attributes of apple juice. Food Bioprocess Technol 5:680–686. doi: 10.1007/s11947-010-0365-x. [DOI] [Google Scholar]
- 24.Gachovska T, Kumar S, Thippareddi H, Subbiah J, Williams F. 2008. Ultraviolet and pulsed electric field treatments have additive effect on inactivation of E. coli in apple juice. J Food Science 73:M412–M417. doi: 10.1111/j.1750-3841.2008.00956.x. [DOI] [PubMed] [Google Scholar]
- 25.Keyser M, Műller IA, Cilliers FP, Nel W, Gouws PA. 2008. Ultraviolet radiation as a non-thermal treatment for the inactivation of microorganisms in fruit juice. Innov Food Sci Emerg Technol 9:348–354. doi: 10.1016/j.ifset.2007.09.002. [DOI] [Google Scholar]
- 26.Close J, Ip J, Lam K. 2006. Water recycling with PV-powered UV-LED disinfection. Renew Energy 31:1657–1664. doi: 10.1016/j.renene.2005.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenny RM, Jasper MN, Simmons OD, Shatalov M, Ducoste JJ. 2015. Heuristic optimization of a continuous flow point-of-use UV-LED disinfection reactor using computational fluid dynamics. Water Res 83:310–318. doi: 10.1016/j.watres.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 28.UNEP. 2013. Minamata Convention on Mercury. http://www.mercuryconvention.org/Convention/Text. Accessed 17 April 2018.
- 29.Ha J-W, Kang D-H. 2018. Effect of intermittent 222 nm krypton-chlorine excilamp irradiation on microbial inactivation in water. Food Control 90:146–151. doi: 10.1016/j.foodcont.2018.02.025. [DOI] [Google Scholar]
- 30.Sosnin EA. 2007. Excimer lamps and based on them a new family of ultraviolet radiation sources. Light Eng 15:49–57. [Google Scholar]
- 31.Matafonova G, Batoev V. 2012. Recent progress on application of UV excilamps for degradation of organic pollutants and microbial inactivation. Chemosphere 89:637–647. doi: 10.1016/j.chemosphere.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Orlowska M, Koutchma T, Kostrzynska M, Tang J. 2015. Surrogate organisms for pathogenic O157:H7 and non-O157 Escherichia coli strains for apple juice treatments by UV-C light at three monochromatic wavelengths. Food Control 47:647. doi: 10.1016/j.foodcont.2014.08.004. [DOI] [Google Scholar]
- 33.Wang D, Oppenländer T, El‐Din MG, Bolton JR. 2010. Comparison of the disinfection effects of vacuum‐UV (VUV) and UV light on Bacillus subtilis spores in aqueous suspensions at 172, 222 and 254 nm. Photochem Photobiol 86:176–181. doi: 10.1111/j.1751-1097.2009.00640.x. [DOI] [PubMed] [Google Scholar]
- 34.Kang J-W, Kim S-S, Kang D-H. 2018. Inactivation dynamics of 222 nm krypton716 chlorine excilamp irradiation on Gram-positive and Gram-negative foodborne pathogenic bacteria. Food Res Int 109:325–333. doi: 10.1016/j.foodres.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 35.Yin F, Zhu Y, Koutchma T, Gong J. 2015. Inactivation and potential reactivation of pathogenic Escherichia coli O157:H7 in apple juice following ultraviolet light exposure at three monochromatic wavelengths. Food Microbiol 46:329–335. doi: 10.1016/j.fm.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Buonanno M, Ponnaiya B, Welch D, Stanislauskas M, Randers-Pehrson G, Smilenov L, Lowy FD, Owens DM, Brenner DJ. 2017. Germicidal efficacy and mammalian skin safety of 222-nm UV light. Radiat Res 187:493–501. doi: 10.1667/RR0010CC.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buonanno M, Stanislauskas M, Ponnaiya B, Bigelow AW, Randers-Pehrson G, Xu Y, Shuryak I, Smilenov L, Owens DM, Brenner DJ. 2016. 207-nm UV light—a promising tool for safe low-cost reduction of surgical site infections. II. In-vivo safety studies. PLoS One 11:e0138418. doi: 10.1371/journal.pone.0138418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buonanno M, Randers-Pehrson G, Bigelow AW, Trivedi S, Lowy FD, Spotnitz HM, Hammer SM, Brenner DJ. 2013. 207-nm UV light—a promising tool for safe low-cost reduction of surgical site infections. I. In vitro studies. PLoS One 8:e76968. doi: 10.1371/journal.pone.0076968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patil S, Bourke P, Kelly B, Frías JM, Cullen PJ. 2009. The effects of acid adaptation on Escherichia coli inactivation using power ultrasound. Innov Food Sci Emerg Technol 10:486–490. doi: 10.1016/j.ifset.2009.06.005. [DOI] [Google Scholar]
- 40.Cheng H-Y, Yu R-C, Chou C-C. 2003. Increased acid tolerance of Escherichia coli O157:H7 as affected by acid adaptation time and conditions of acid challenge. Food Res Int 36:49–56. doi: 10.1016/S0963-9969(02)00107-2. [DOI] [Google Scholar]
- 41.Foster JW. 1991. Salmonella acid shock proteins are required for the adaptive acid tolerance response. J Bacteriol 173:6896–6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garren DM, Harrison MA, Russell SM. 1997. Retention of acid tolerance and acid shock responses of Escherichia coli O157:H7 and non-O157:H7 isolates. J Food Prot 60:1478–1482. doi: 10.4315/0362-028X-60.12.1478. [DOI] [PubMed] [Google Scholar]
- 43.Goodson M, Rowbury R. 1989. Habituation to normally lethal acidity by prior growth of Escherichia coli at a sub‐lethal acid pH value. Lett Appl Microbiol 8:77–79. doi: 10.1111/j.1472-765X.1989.tb00227.x. [DOI] [Google Scholar]
- 44.Lin J, Smith MP, Chapin KC, Baik HS, Bennett GN, Foster JW. 1996. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl Environ Microbiol 62:3094–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Usaga J, Worobo RW, Padilla-Zakour OI. 2014. Effect of acid adaptation and acid shock on thermal tolerance and survival of Escherichia coli O157:H7 and O111 in apple juice. J Food Prot 77:1656–1663. doi: 10.4315/0362-028X.JFP-14-126. [DOI] [PubMed] [Google Scholar]
- 46.Sharma M, Adler B, Harrison M, Beuchat L. 2005. Thermal tolerance of acid‐adapted and unadapted Salmonella, Escherichia coli O157:H7, and Listeria monocytogenes in cantaloupe juice and watermelon juice. Lett Appl Microbiol 41:448–453. doi: 10.1111/j.1472-765X.2005.01797.x. [DOI] [PubMed] [Google Scholar]
- 47.Mazzotta AS. 2001. Thermal inactivation of stationary-phase and acid-adapted Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes in fruit juices. J Food Prot 64:315–320. [DOI] [PubMed] [Google Scholar]
- 48.Ryu J-H, Beuchat LR. 1998. Influence of acid tolerance responses on survival, growth, and thermal cross-protection of Escherichia coli O157:H7 in acidified media and fruit juices. Int J Food Microbiol 45:185–193. [DOI] [PubMed] [Google Scholar]
- 49.Patil S, Valdramidis V, Cullen P, Frias J, Bourke P. 2010. Ozone inactivation of acid stressed Listeria monocytogenes and Listeria innocua in orange juice using a bubble column. Food Control 21:1723–1730. doi: 10.1016/j.foodcont.2010.04.031. [DOI] [Google Scholar]
- 50.Gabriel AA. 2012. Microbial inactivation in cloudy apple juice by multi-frequency Dynashock power ultrasound. Ultrason Sonochem 19:346–351. doi: 10.1016/j.ultsonch.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Gorden J, Small P. 1993. Acid resistance in enteric bacteria. Infect Immun 61:364–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferreira A, Sue D, O’Byrne CP, Boor KJ. 2003. Role of Listeria monocytogenes σB in survival of lethal acidic conditions and in the acquired acid tolerance response. Appl Environ Microbiol 69:2692–2698. doi: 10.1128/AEM.69.5.2692-2698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breeuwer P, Abee T. 2000. Assessment of viability of microorganisms employing fluorescence techniques. Int J Food Microbiol 55:193–200. [DOI] [PubMed] [Google Scholar]
- 54.Hewitt CJ, Nebe-Von-Caron G. 2004. The application of multi-parameter flow cytometry to monitor individual microbial cell physiological state. Adv Biochem Eng Biotechnol 89:197–223. doi: 10.1007/b93997. [DOI] [PubMed] [Google Scholar]
- 55.Ha J-W, Kang D-H. 2013. Simultaneous near-infrared radiant heating and ultraviolet radiation for inactivating Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in powdered red pepper (Capsicum annum L.). Appl Environ Microbiol 79:6568–6575. doi: 10.1128/AEM.02249-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park I-K, Kang D-H. 2013. Effect of electropermeabilization by ohmic heating for inactivation of E. coli O157:H7, Salmonella enterica serovar Typhimurium and Listeria monocytogenes in buffered peptone water and apple juice. Appl Environ Microbiol 79:7122–7129. doi: 10.1128/AEM.01818-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okimoto Y, Watanabe A, Niki E, Yamashita T, Noguchi N. 2000. A novel fluorescent probe diphenyl‐1‐pyrenylphosphine to follow lipid peroxidation in cell membranes. FEBS Lett 474:137–140. doi: 10.1016/S0014-5793(00)01587-8. [DOI] [PubMed] [Google Scholar]
- 58.Wojtala A, Bonora M, Malinska D, Pinton P, Duszynski J, Wieckowski MR. 2014. Methods to monitor ROS production by fluorescence microscopy and fluorometry. Methods Enzymol 542:243–262. doi: 10.1016/B978-0-12-416618-9.00013-3. [DOI] [PubMed] [Google Scholar]
- 59.Negre-Salvayre A, Augé N, Duval C, Robbesyn F, Thiers J-C, Nazzal D, Benoist H, Salvayre R. 2002. Detection of intracellular reactive oxygen species in cultured cells using fluorescent probes. Methods Enzymol 352:62–71. [DOI] [PubMed] [Google Scholar]
- 60.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, Ischiropoulos H. 2012. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y, Khoo WJ, Zheng Q, Chung H-J, Yuk H-G. 2014. Growth temperature alters Salmonella Enteritidis heat/acid resistance, membrane lipid composition and stress/virulence related gene expression. Int J Food Microbiol 172:102–109. doi: 10.1016/j.ijfoodmicro.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 62.Abid M, Jabbar S, Wu T, Hashim MM, Hu B, Lei S, Zhang X, Zeng X. 2013. Effect of ultrasound on different quality parameters of apple juice. Ultrason Sonochem 20:1182–1187. doi: 10.1016/j.ultsonch.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Islam MS, Patras A, Pokharel B, Wu Y, Vergne MJ, Shade L, Xiao H, Sasges M. 2016. UV-C irradiation as an alternative disinfection technique: study of its effect on polyphenols and antioxidant activity of apple juice. Innov Food Sci Emerg Technol 34:344–351. doi: 10.1016/j.ifset.2016.02.009. [DOI] [Google Scholar]
- 64.Zhao L, Wang Y, Qiu D, Liao X. 2014. Effect of ultrafiltration combined with high-pressure processing on safety and quality features of fresh apple juice. Food Bioprocess Technol 7:3246–3258. doi: 10.1007/s11947-014-1307-9. [DOI] [Google Scholar]
- 65.Liao X, Li J, Muhammad AI, Suo Y, Chen S, Ye X, Liu D, Ding T. 2018. Application of a dielectric barrier discharge atmospheric cold plasma (Dbd‐Acp) for Escherichia coli inactivation in apple juice. J Food Sci 83:401–408. doi: 10.1111/1750-3841.14045. [DOI] [PubMed] [Google Scholar]
- 66.Song WJ, Sung HJ, Kang DH. 2015. Inactivation of Escherichia coli O157:H7 and Salmonella Typhimurium in apple juices with different soluble solids content by combining ozone treatment with mild heat. J Appl Microbiol 118:112–122. doi: 10.1111/jam.12671. [DOI] [PubMed] [Google Scholar]
- 67.Audia JP, Webb CC, Foster JW. 2001. Breaking through the acid barrier: an orchestrated response to proton stress by enteric bacteria. Int J Med Microbiol 291:97–106. doi: 10.1078/1438-4221-00106. [DOI] [PubMed] [Google Scholar]
- 68.Ngadi M, Smith JP, Cayouette B. 2003. Kinetics of ultraviolet light inactivation of Escherichia coli O157:H7 in liquid foods. J Sci Food Agric 83:1551–1555. doi: 10.1002/jsfa.1577. [DOI] [Google Scholar]
- 69.Koutchma T, Parisi B, Patazca E. 2007. Validation of UV coiled tube reactor for fresh juices. J Environ Eng Sci 6:319–328. doi: 10.1139/s06-058. [DOI] [Google Scholar]
- 70.Oteiza JM, Peltzer M, Gannuzzi L, Zaritzky N. 2005. Antimicrobial efficacy of UV radiation on Escherichia coli O157:H7 (EDL 933) in fruit juices of different absorptivities. J Food Prot 68:49–58. [DOI] [PubMed] [Google Scholar]
- 71.Murakami EG, Jackson L, Madsen K, Schickedanz B. 2006. Factors affecting the ultraviolet inactivation of Escherichia coli K12 in apple juice and a model system. J Food Process Eng 29:53–71. doi: 10.1111/j.1745-4530.2006.00049.x. [DOI] [Google Scholar]
- 72.Willard HH, Merritt LL Jr, Dean JA, Settle FA Jr. 1988. Instrumental methods of analysis, 7th ed Wadsworth Publishing Co, Belmont, CA. [Google Scholar]
- 73.Franz CM, Specht I, Cho G-S, Graef V, Stahl MR. 2009. UV-C-inactivation of microorganisms in naturally cloudy apple juice using novel inactivation equipment based on Dean vortex technology. Food Control 20:1103–1107. doi: 10.1016/j.foodcont.2009.02.010. [DOI] [Google Scholar]
- 74.Geveke DJ. 2008. UV inactivation of E. coli in liquid egg white. Food Bioprocess Technol 1:201–206. doi: 10.1007/s11947-008-0070-1. [DOI] [Google Scholar]
- 75.Leistner L. 1992. Food preservation by combined methods. Food Res Int 25:151–158. doi: 10.1016/0963-9969(92)90158-2. [DOI] [Google Scholar]
- 76.Ha J-W, Lee J-I, Kang D-H. 2017. Application of a 222-nm krypton-chlorine excilamp to control foodborne pathogens on sliced cheese surfaces and characterization of the bactericidal mechanisms. Int J Food Microbiol 243:96–102. doi: 10.1016/j.ijfoodmicro.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 77.Pagán R, Mackey B. 2000. Relationship between membrane damage and cell death in pressure-treated Escherichia coli cells: differences between exponential- and stationary-phase cells and variation among strains. Appl Environ Microbiol 66:2829–2834. doi: 10.1128/AEM.66.7.2829-2834.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sánchez E, García S, Heredia N. 2010. Extracts of edible and medicinal plants damage membranes of Vibrio cholerae. Appl Environ Microbiol 76:6888–6894. doi: 10.1128/AEM.03052-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gutteridge J. 1995. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem 41:1819–1828. [PubMed] [Google Scholar]
- 80.Bensasson RV, Land EJ, Truscott TG. 2013. Flash photolysis and pulse radiolysis: contributions to the chemistry of biology and medicine. Pergamon Press, Oxford, UK. [Google Scholar]
- 81.Kramer B, Muranyi P. 2014. Effect of pulsed light on structural and physiological properties of Listeria innocua and Escherichia coli. J Appl Microbiol 116:596–611. doi: 10.1111/jam.12394. [DOI] [PubMed] [Google Scholar]
- 82.Pattison DI, Davies MJ. 2006. Actions of ultraviolet light on cellular structures, p 131–157. In Cancer: cell structures, carcinogens and genomic instability. Birkhäuser Basel Publisher, Boston, MA. [DOI] [PubMed] [Google Scholar]
- 83.Matsuda N, Horikawa M, Wang L-H, Yoshida M, Okaichi K, Okumura Y, Watanabe M. 2000. Differential activation of ERK 1/2 and JNK in normal human fibroblast-like cells in response to UVC radiation under different oxygen tensions. Photochem Photobiol 72:334–339. [DOI] [PubMed] [Google Scholar]
- 84.Zeeshan M, Prasad S. 2009. Differential response of growth, photosynthesis, antioxidant enzymes and lipid peroxidation to UV-B radiation in three cyanobacteria. S Afr J Bot 75:466–474. doi: 10.1016/j.sajb.2009.03.003. [DOI] [Google Scholar]
- 85.Zhang X, Rosenstein BS, Wang Y, Lebwohl M, Wei H. 1997. Identification of possible reactive oxygen species involved in ultraviolet radiation-induced oxidative DNA damage. Free Radic Biol Med 23:980–985. [DOI] [PubMed] [Google Scholar]
- 86.Joshi SG, Cooper M, Yost A, Paff M, Ercan UK, Fridman G, Friedman G, Fridman A, Brooks AD. 2011. Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob Agents Chemother 55:1053–1062. doi: 10.1128/AAC.01002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lovrić J, Cho SJ, Winnik FM, Maysinger D. 2005. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem Biol 12:1227–1234. doi: 10.1016/j.chembiol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 88.von Moos N, Slaveykova VI. 2014. Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae—state of the art and knowledge gaps. Nanotoxicology 8:605–630. doi: 10.3109/17435390.2013.809810. [DOI] [PubMed] [Google Scholar]
- 89.Wickens AP. 2001. Ageing and the free radical theory. Respir Physiol 128:379–391. [DOI] [PubMed] [Google Scholar]
- 90.Sánchez B, Champomier-Vergès M-C, del Carmen Collado M, Anglade P, Baraige F, Sanz Y, Clara G, Margolles A, Zagorec M. 2007. Low-pH adaptation and the acid tolerance response of Bifidobacterium longum biotype longum. Appl Environ Microbiol 73:6450–6459. doi: 10.1128/AEM.00886-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuhnert WL, Zheng G, Faustoferri RC, Quivey RG. 2004. The F-ATPase operon promoter of Streptococcus mutans is transcriptionally regulated in response to external pH. J Bacteriol 186:8524–8528. doi: 10.1128/JB.186.24.8524-8528.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fozo EM, Quivey RG Jr. 2004. Shifts in the membrane fatty acid profile of Streptococcus mutans enhance survival in acidic environments. Appl Environ Microbiol 70:929–936. doi: 10.1128/AEM.70.2.929-936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Álvarez-Ordóñez A, Fernández A, López M, Arenas R, Bernardo A. 2008. Modifications in membrane fatty acid composition of Salmonella Typhimurium in response to growth conditions and their effect on heat resistance. Int J Food Microbiol 123:212–219. doi: 10.1016/j.ijfoodmicro.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 94.Chang YY, Cronan JE. 1999. Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol Microbiol 33:249–259. [DOI] [PubMed] [Google Scholar]
- 95.Di Pasqua R, Hoskins N, Betts G, Mauriello G. 2006. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J Agric Food Chem 54:2745–2749. doi: 10.1021/jf052722l. [DOI] [PubMed] [Google Scholar]
- 96.Jordan KN, Oxford L, O’Byrne CP. 1999. Survival of low-pH stress by Escherichia coli O157:H7: correlation between alterations in the cell envelope and increased acid tolerance. Appl Environ Microbiol 65:3048–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bielski B, Arudi RL, Sutherland MW. 1983. A study of the reactivity of HO2/O2− with unsaturated fatty acids. J Biol Chem 258:4759–4761. [PubMed] [Google Scholar]
- 98.Pala ÇU, Toklucu AK. 2013. Effects of UV-C light processing on some quality characteristics of grape juices. Food Bioprocess Technol 6:719–725. doi: 10.1007/s11947-012-0808-7. [DOI] [Google Scholar]
- 99.Pala ÇU, Toklucu AK. 2011. Effect of UV-C light on anthocyanin content and other quality parameters of pomegranate juice. J Food Compost Anal 24:790–795. doi: 10.1016/j.jfca.2011.01.003. [DOI] [Google Scholar]
- 100.Pala ÇU, Toklucu AK. 2013. Microbial, physicochemical and sensory properties of UV-C processed orange juice and its microbial stability during refrigerated storage. LWT Food Sci Technol 50:426–431. doi: 10.1016/j.lwt.2012.09.001. [DOI] [Google Scholar]
- 101.Singh R, Jiang X. 2012. Thermal inactivation of acid-adapted Escherichia coli O157:H7 in dairy compost. Foodborne Pathog Dis 9:741–748. doi: 10.1089/fpd.2011.1110. [DOI] [PubMed] [Google Scholar]
- 102.Child M, Strike P, Pickup R, Edwards C. 2002. Salmonella Typhimurium displays cyclical patterns of sensitivity to UV-C killing during prolonged incubation in the stationary phase of growth. FEMS Microbiol Lett 213:81–85. doi: 10.1111/j.1574-6968.2002.tb11289.x. [DOI] [PubMed] [Google Scholar]
- 103.Bucheli‐Witschel M, Bassin C, Egli T. 2010. UV‐C inactivation in Escherichia coli is affected by growth conditions preceding irradiation, in particular by the specific growth rate. J Appl Microbiol 109:1733–1744. doi: 10.1111/j.1365-2672.2010.04802.x. [DOI] [PubMed] [Google Scholar]
- 104.Buchanan RL, Edelson SG. 1996. Culturing enterohemorrhagic Escherichia coli in the presence and absence of glucose as a simple means of evaluating the acid tolerance of stationary-phase cells. Appl Environ Microbiol 62:4009–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bolton JR, Linden KG. 2003. Standardization of methods for fluence (UV dose) determination in bench-scale UV experiments. J Environ Eng 129:209–215. doi: 10.1061/(ASCE)0733-9372(2003)129:3(209). [DOI] [Google Scholar]
- 106.Lee S-Y, Kang D-H. 2001. Suitability of overlay method for recovery of heat-injured Listeria monocytogenes and Salmonella Typhimurium. Food Sci Biotechnol 10:323–326. [Google Scholar]
- 107.Geeraerd A, Valdramidis V, Van Impe J. 2005. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int J Food Microbiol 102:95–105. doi: 10.1016/j.ijfoodmicro.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 108.van Boekel MA. 2002. On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. Int J Food Microbiol 74:139–159. doi: 10.1016/S0168-1605(01)00742-5. [DOI] [PubMed] [Google Scholar]
- 109.Albert I, Mafart P. 2005. A modified Weibull model for bacterial inactivation. Int J Food Microbiol 100:197–211. doi: 10.1016/j.ijfoodmicro.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 110.Sasser M. 1990. MIDI technical note 101. Identification of bacteria by gas chromatography of cellular fatty acids. MIDI, Newark, DE. [Google Scholar]
- 111.Garcés R, Mancha M. 1993. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal Biochem 211:139–143. doi: 10.1006/abio.1993.1244. [DOI] [PubMed] [Google Scholar]
- 112.Chen Z, Zhu C, Zhang Y, Niu D, Du J. 2010. Effects of aqueous chlorine dioxide treatment on enzymatic browning and shelf-life of fresh-cut asparagus lettuce (Lactuca sativa L.). Postharvest Biol Technol 58:232–238. doi: 10.1016/j.postharvbio.2010.06.004. [DOI] [Google Scholar]
- 113.Song WJ, Shin JY, Ryu S, Kang DH. 2015. Inactivation of Escherichia coli O157:H7, Salmonella Typhimurium and Listeria monocytogenes in apple juice at different pH levels by gaseous ozone treatment. J Appl Microbiol 119:465–474. doi: 10.1111/jam.12861. [DOI] [PubMed] [Google Scholar]
- 114.Singleton VL, Rossi JA Jr. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158. [Google Scholar]