Abstract
We utilized ultrasensitive single molecule technology to measure plasma alpha‐synuclein in 221 subjects (51 controls, 170 PD). Plasma alpha‐synuclein levels were significantly higher in PD than controls (15506.3 vs. 13057.0 pg/mL, P = 0.037), adjusting for age and gender. In PD, alpha‐synuclein levels did not vary by H&Y stage or UPDRS motor scores but were significantly higher in PD patients with poorer cognition (MMSE ≤ 25) than controls (P = 0.016, Bonferroni corrected P = 0.047). Alpha‐synuclein levels quantified using ultrasensitive single molecule technology discriminate PD from controls and correlate with cognitive severity. These preliminary findings require independent validation to determine the utility of this assay.
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized by degeneration of nigrostriatal dopaminergic neurons and Lewy body deposits containing misfolded alpha‐synuclein.1 Discriminating between healthy subjects and PD using blood‐based biomarkers, particularly those measuring alpha‐synuclein, has been limited by very low concentrations of circulating “pathological” rather than ubiquitous alpha‐synuclein in blood that are difficult to quantify using existing methods.2 Both lower and higher plasma total alpha‐synuclein levels have been reported in PD compared to healthy controls measured by Western blotting and ELISA.3, 4, 5 To address this gap, we utilized a novel ultrasensitive single molecule array technology to measure plasma alpha‐synuclein levels in 170 PD patients and 51 healthy controls. We hypothesized that PD patients would show higher plasma alpha‐synuclein levels than healthy controls; and that alpha‐synuclein levels will correlate with motor, functional, and cognitive severity.
Methods
Clinical recruitment
Subjects were recruited from the movement disorder clinics at the National Neuroscience Institute, Singapore, between November 2014 and February 2018. All PD patients fulfilled National Institute of Neurological Disorders and Stroke (NINDS) criteria for the diagnosis of PD. Functional status was determined using the Hoehn and Yahr (H&Y) rating scale6 and motor severity was ascertained using the Movement Disorders Society Unified Parkinson's Disease Rating Scale part III (MDS‐UPDRS).7 Patients were classified into motor subtypes of tremor dominant (TD) or postural instability and gait disorders (PIGD) based on MDS‐UPDRS part II and III components.8 Global cognition was measured using the Mini‐Mental State Examination (MMSE).9 Healthy controls were recruited from the community and were free of significant neurological, psychiatric or systemic disease. Ethics approval was obtained from the institutional review committee, and all participants provided informed written consent.
Plasma alpha‐synuclein level measurement
EDTA blood was collected from each subject by venipuncture and centrifuged at 1500 g for 15 min within 1 h after blood collection. Plasma was aliquoted and stored at −80°C until further analysis. Visibly hemolyzed plasma samples were excluded. Thawed plasma samples were centrifuged at 10,000 g for 5 min to remove particulate matter, and diluted 40 × before assay. Total alpha‐synuclein levels were measured using ultrasensitive single molecule array (Simoa) Human Alpha‐synuclein assay and Simoa HD‐1 Analyzer (Quanterix, MA), according to the manufacturer's protocol.10 This assay was developed with two monoclonal antibodies specific to alpha synuclein protein. For more details see Data S1 and SIMOA human alpha‐synuclein assay data sheet (Data S2 file).
Statistical analysis
Differences in the demographic data between PD and controls were assessed by two‐tailed unpaired t‐test for continuous variables, and Chi‐square test for categorical variables. Correlations between clinical data and alpha‐synuclein levels were calculated using Spearman's rank order correlation. Plasma alpha‐synuclein levels were log‐transformed to achieve a normal distribution for subsequent analysis. For group‐wise comparisons of plasma alpha‐synuclein, we used univariate general linear models controlling for possible confounders (e.g., age, sex, disease duration) and least significant difference (LSD) post hoc test. Where necessary, multiple comparisons were corrected for using the Bonferroni method. The diagnostic accuracy of plasma alpha‐synuclein was assessed with the receiving operating characteristic (ROC) curve analysis. P ≤ 0.05 was considered statistically significant. All statistics were done using SPSS version 22 (IBM).
Results
A total of 221 subjects were included (51 controls and 170 PD). Their demographic and clinical details are listed in Table 1. There was no significant association between alpha‐synuclein and age (rho = 0.007, P = 0.920), gender (P = 0.869) or disease duration (rho = 0.076, P = 0.265). We did not detect significant association between age and synuclein levels in the PD (rho = −0.005, P = 0.950) or the HC group (rho = −0.116, P = 0.418). Plasma alpha‐synuclein levels were significantly higher in PD patients than controls (15506.3 ± 8480.8 vs. 13057.0 ± 7770.9 pg/mL, P = 0.037; Fig. 1A), adjusting for age and gender. Patients were stratified by H&Y stages 1–4, and UPDRS Motor Part III score (median cut off in PD = 23). In the PD group, there was no significant difference in alpha‐synuclein levels across H&Y stages (P = 0.900; Fig. 1B), but there was a trend toward lower alpha‐synuclein levels at later stages of disease (H&Y 3‐4). PD patients with motor scores ≥24 (n = 84) had higher alpha‐synuclein levels than controls (P = 0.045, corrected P = 0.134), while patients with motor scores ≤23 (n = 86) had levels similar to controls (P = 0.085, corrected P = 0.265) after adjusting for age, gender, and disease duration (Fig. 1C). 60% of our PD patients had the PIGD phenotype and 32% had the TD phenotype; the remaining patients (8%) had indeterminate subtypes and were excluded from the analysis. We found that alpha‐synuclein levels were higher in PIGD than TD (15890.6 ± 9203.6 vs. 14474.9 ± 7341.5 pg/mL), but the difference did not reach significance after adjusting for confounders.
Table 1.
Demographics of all subjects
| Controls | PD | P value | |
|---|---|---|---|
| Total subjects, n | 51 | 170 | |
| Age, years | 63.3 ± 7.4 | 66.6 ± 9.5 | 0.026 |
| Gender, % males | 43 | 58 | 0.057 |
| Age at onset, years | – | 61.5 ± 10.0 | |
| Disease duration, years | – | 5.0 ± 5.0 | |
| Hoehn & Yahr (%) | – | ||
|
Stage 1.0 Stage 1.5 Stage 2.0 Stage 2.5 Stage 3.0 Stage 4.0 |
6.5 7.7 64.5 9.5 5.3 6.5 |
||
| UPDRS II: ADLs | – | 9.0 ± 6.5 | |
| UPDRS III: Motor | – | 24.8 ± 12.5 | |
| MMSE score | 28.8 ± 1.3 | 25.4 ± 3.7 | <0.001 |
Data are expressed as mean ± standard deviation or percentages (%).
PD, Parkinson's disease; UPDRS, Unified Parkinson's disease rating scale; ADL, activities of daily living; MMSE, mini‐mental status examination.
Figure 1.
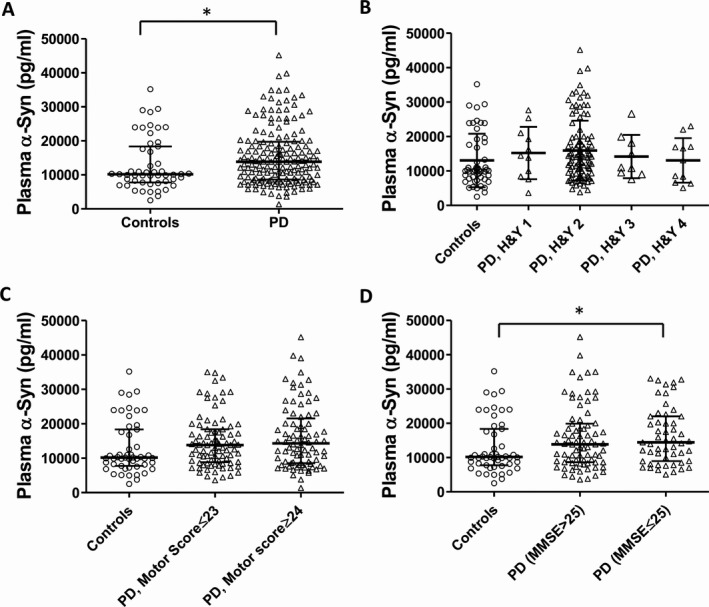
Plasma alpha‐synuclein levels in (A) PD versus healthy controls, (B) Hoehn‐Yahr stage, (C) UPDRS Motor scores, and (D) MMSE scores. Values are medians ± interquartile range. A value of P ≤0.05 was considered significant after correction for covariates and multiple comparisons.
The mean ± SD levels of plasma alpha‐synuclein in controls, PD patients with better cognitive scores (MMSE > 25), and patients with worse cognitive scores (MMSE ≤ 25) were 13057.0 ± 7770.9, 15742.1 ± 9212.9, and 16290.2 ± 8253.8 pg/mL, respectively (P = 0.046, adjusted for age, gender, and disease duration; Fig. 1D). Post‐hoc analysis revealed higher alpha‐synuclein levels in PD with MMSE ≤ 25 than in controls (P = 0.016, Bonferroni corrected P = 0.047), controlled for age, gender, and disease duration. Receiver operating characteristic (ROC) analysis revealed that plasma alpha‐synuclein levels differentiated PD patients from healthy controls (AUC = 0.599, 95% CI = 0.509–0.690), and PD patients with MMSE ≤ 25 from healthy controls (AUC = 0.630, 95% CI = 0.521–0.739).
Discussion
Clear genetic links between alpha‐synuclein and PD risk and the identification of aggregated alpha‐synuclein as the main constituent of Lewy body pathology has highlighted alpha‐synuclein as the major therapeutic target in PD.11 We utilized novel ultrasensitive single molecule technology and found significantly higher plasma alpha‐synuclein levels in PD patients than healthy controls, correlating with poorer cognition but not with motor/disability scores or motor subtype.
Both lower and higher plasma total alpha‐synuclein levels have been reported in PD compared to controls,3, 4, 5 while other studies reported increased alpha‐synuclein in PD‐derived plasma exosomes.12 Mechanistically, accumulation of alpha‐synuclein in the periphery is consistent with the “Braak staging” hypothesis, where alpha‐synuclein pathology has been shown to start in the peripheral autonomic nervous system before spreading to the central nervous system.13 While our results showing higher alpha‐synuclein levels in PD are similar to those published by Lin et al. measuring plasma alpha‐synuclein using an immunomagnetic reduction‐based immunoassay,14 the mean levels reported here are much higher, suggesting higher sensitivity of the ultrasensitive method used in our study. While both platforms are based on ultrasensitive immunoreactivity between specific antibodies and analytes, it remains difficult to conclude which platform confers greater sensitivity due to differing methods used for protein detection.
In our study, we found that plasma alpha‐synuclein levels did not vary significantly by H&Y stage, nor by UPDRS Part III motor scores, consistent with previous reports suggesting a lack of association of alpha‐synuclein levels with motor severity in PD.14 Of note, while plasma synuclein levels were higher in milder stages of PD, there was a trend toward lower levels in later disease stages (H&Y 3‐4). While interpretation of this finding is limited by the small number of patients in the later stages of disease (H&Y 3‐4, n = 20), this phenomenon has also been reported by other groups.15 Additionally, the carboxy‐terminal (C‐terminal) truncated form of alpha‐synuclein which is more prone to aggregation,16 is enriched in pathological aggregates of alpha‐synuclein in lewy bodies of sporadic PD brains.17 As the current assay uses monoclonal antibodies targeting the C‐terminal of alpha‐synuclein, we hypothesize that lower levels of alpha‐synuclein seen in later disease stages could be attributed to more truncated aggregates of alpha‐synuclein lacking their C‐terminals, which escape detection.
In our cohort, PD patients with lower cognitive scores (MMSE ≤ 25) had significantly higher plasma alpha‐synuclein levels than controls. These results add to recent reports of peripheral alpha‐synuclein level as a potential biomarker of cognitive impairment in PD,14 and are not unexpected given that multiplications in the synuclein gene (SNCA) are associated with cognitive impairment in monogenic PD.18 These results were not corrected for education status, with another limitation being the use of the MMSE for cognitive assessment, rather than the Montreal Cognitive Assessment (MoCA) tool which is more sensitive toward frontal‐executive deficits and lacks the ceiling effect of the MMSE.
The strength of our study remains the use of a novel ultrasensitive assay for detecting plasma alpha‐synuclein levels in a good sample size of PD patients and controls. Given that alpha‐synuclein is known to increase in red blood cells and lymphocytes of patients with PD,19 we took extra caution to remove all potentially hemolysed samples, but our results do not account for hemoglobin levels and its potential effects on alpha synuclein measures. Alpha‐synuclein levels were not adjusted for concomitant PD treatment or levodopa therapy, but an earlier study suggested that PD treatment does not alter plasma synuclein levels.5
In summary, we used novel ultrasensitive single molecule technology to show that plasma alpha‐synuclein levels are higher in PD versus controls, and may associate with cognition in PD. To the best of our knowledge, this will be the first study reporting the utility of Single Molecule Array (Simoa) technology in quantifying alpha‐synuclein levels in PD and controls. The growing number of studies utilizing this technology, particularly with neurofilament light chain (NFL) in other neurodegenerative diseases makes this study important for expanding the use of ultrasensitive technology in our search for PD biomarkers. Furthermore, a multi‐site evaluation of Simoa undertaken by scientists from the AAPS Emerging Technologies Focus Group demonstrated improved sensitivity compared to other commercially available kits with excellent site‐to‐site reproducibility,20 offering excellent sensitivity important for low‐abundance analytes like alpha‐synuclein. These preliminary results will require validation in independent cohorts to determine the utility of this assay.
Author Contribution
YJT, ASLN, EKT, and LCST involved in drafting or revising the manuscript for intellectual content. YJT, ASLN, EKT, and LCST involved in study design. ZYX, KYT, KMP, WLA, EKT, and LCST involved in patient recruitment. ZHL, SYEN, NSYC, FS, EYLN, and YJT involved in acquisition of data. YJT, and ASLN involved in analysis and interpretation of the data. All authors approved the final version of the manuscript.
Conflict of Interest
The authors declare no financial disclosures or competing interests.
Supporting information
Data S1. Detailed description on total alpha‐synuclein measurement using SIMOA technology as well as inter‐ and intra‐assay variations and limit of detection.
Data S2. SIMOA human alpha‐synuclein assay data sheet.
Acknowledgments
The authors thank our patients and their families for their valuable contribution to the study. This study was funded by Singapore's National Medical Research Council (ASLN by the Clinician‐Scientist New Investigator Grant (CIRG/1416/2015); EKT and LCST by the Singapore Translational Research (STaR) Investigator award (NMRC/STaR/014/2013), and Parkinson's disease Translational and Clinical Research flagship grant (TCR12dec010)).
Funding Information
This study was funded by Singapore's National Medical Research Council (ASLN by the Clinician‐Scientist New Investigator Grant (CIRG/1416/2015); EKT and LCST by the Singapore Translational Research (STaR) Investigator award (NMRC/STaR/014/2013), and Parkinson's disease Translational and Clinical Research flagship grant (TCR12dec010)).
Funding Statement
This work was funded by Singapore's National Medical Research Council grant ; Clinician‐Scientist New Investigator Grant grant CIRG/1416/2015; Singapore Translational Research grant NMRC/STaR/014/2013; Parkinson's disease Translational and Clinical Research grant TCR12dec010.
Contributor Information
Adeline S. L. Ng, Email: adeline.ng.s.l@singhealth.com.sg
Louis C. S. Tan, Email: louis.tan.c.s@singhealth.com.sg
References
- 1. Kalia LV, Lang AE. Parkinson's disease. Lancet 2015;386:896–912. [DOI] [PubMed] [Google Scholar]
- 2. Chahine LM, Stern MB, Chen‐Plotkin A. Blood‐based biomarkers for Parkinson's disease. Parkinsonism Relat Disord 2014;20(Suppl 1):S99–S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Q‐X, Mok SS, Laughton KM, et al. Plasma alpha‐synuclein is decreased in subjects with Parkinson's disease. Exp Neurol 2007;204:583–588. [DOI] [PubMed] [Google Scholar]
- 4. Gorostidi A, Bergareche A, Ruiz‐Martínez J, et al. Αlpha‐synuclein levels in blood plasma from LRRK2 mutation carriers. PLoS ONE 2012;7:e52312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duran R, Barrero FJ, Morales B, et al. Plasma alpha‐synuclein in patients with Parkinson's disease with and without treatment. Mov Disord Off J Mov Disord Soc 2010;25:489–493. [DOI] [PubMed] [Google Scholar]
- 6. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 7. Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease . The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord Off J Mov Disord Soc 2003;18:738–750. [DOI] [PubMed] [Google Scholar]
- 8. Stebbins GT, Goetz CG, Burn DJ, et al. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: comparison with the unified Parkinson's disease rating scale. Mov Disord Off J Mov Disord Soc 2013;28:668–670. [DOI] [PubMed] [Google Scholar]
- 9. Folstein MF, Robins LN, Helzer JE. The mini‐mental state examination. Arch Gen Psychiatry 1983;40:812. [DOI] [PubMed] [Google Scholar]
- 10. Wilson DH, Rissin DM, Kan CW, et al. The simoa HD‐1 analyzer: a novel fully automated digital immunoassay analyzer with single‐molecule sensitivity and multiplexing. J Lab Autom 2016;21:533–547. [DOI] [PubMed] [Google Scholar]
- 11. Brundin P, Dave KD, Kordower JH. Therapeutic approaches to target alpha‐synuclein pathology. Exp Neurol 2017;298(Pt B):225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi M, Liu C, Cook TJ, et al. Plasma exosomal α‐synuclein is likely CNS‐derived and increased in Parkinson's disease. Acta Neuropathol 2014;128:639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braak H, Ghebremedhin E, Rüb U, et al. Stages in the development of Parkinson's disease‐related pathology. Cell Tissue Res 2004;318:121–134. [DOI] [PubMed] [Google Scholar]
- 14. Lin C‐H, Yang S‐Y, Horng H‐E, et al. Plasma α‐synuclein predicts cognitive decline in Parkinson's disease. J Neurol Neurosurg Psychiatry 2017;88:818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malec‐Litwinowicz M, Plewka A, Plewka D, et al. The relation between plasma α‐synuclein level and clinical symptoms or signs of Parkinson's disease. Neurol Neurochir Pol 2018;52:243–251. [DOI] [PubMed] [Google Scholar]
- 16. Li W, West N, Colla E, et al. Aggregation promoting C‐terminal truncation of α‐synuclein is a normal cellular process and is enhanced by the familial Parkinson's disease‐linked mutations. Proc Natl Acad Sci USA 2005;102:2162–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baba M, Nakajo S, Tu PH, et al. Aggregation of alpha‐synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 18. Mata IF, Leverenz JB, Weintraub D, et al. APOE, MAPT, and SNCA genes and cognitive performance in Parkinson disease. JAMA Neurol 2014;71:1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang X, Yu S, Li F, Feng T. Detection of α‐synuclein oligomers in red blood cells as a potential biomarker of Parkinson's disease. Neurosci Lett 2015;599:115–119. [DOI] [PubMed] [Google Scholar]
- 20. Chunyk AG, Joyce A, Fischer SK, et al. A multi‐site in‐depth evaluation of the quanterix simoa from a user's perspective. AAPS J 2017;20:10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Detailed description on total alpha‐synuclein measurement using SIMOA technology as well as inter‐ and intra‐assay variations and limit of detection.
Data S2. SIMOA human alpha‐synuclein assay data sheet.


