Abstract
Introduction
It is unknown if treatment with rt‐PA in mild acute ischemic stroke (MIS) is associated with improvement in long term cognition.
Methods
Forty‐five patients with suspected acute mild stroke or transient ischemic attacks with NIHSS ≤6 were enrolled in a prospective cohort. Cognitive testing was performed within 24 h of symptom onset. Follow‐up assessment was performed at Day 90 on 25 patients. Prestroke baseline cognition was based on age, years of education (YrE), history of cognitive impairment, and the Fazekas score.
Results
Eighty‐five percent patients with suspected MIS or TIA showed cognitive abnormalities within 24 h of onset. There was no significant difference in age, sex, Fazekas score, or YrE between rt‐PA versus No‐rt‐PA groups (N = 8 vs. 17).Two sample t‐test for change in performance in the WMS‐III sub‐tests (follow‐up – baseline) ± SD, indicated a difference between rt‐PA 0.74 ± 0.77 and no‐rt‐PA groups ‐0.02 ± 0.83 (P = 0.044). Logistic regression for predicting normal status using the mental control subtest, at follow‐up showed an OR 8.96, CI 0.98–82.12 (P = 0.05) favoring the rt‐PA group. Improvement in Mental Control at 90 days occurred in patients with low white matter disease compared to high white matter disease, 0.60 ± 0.46 (P = 0.048). A statistical trend was observed and suggested an improvement on SDMT and Trail Making tests, 1.43 ± 0.8 (P = 0.077).
Conclusion
Suspected MIS and TIA patients have cognitive impairment within 24 h of onset. rt‐PA administration might be associated with improvement on some cognitive tests at 90 days.
Introduction
Of nearly 800,000 strokes occurring in the United States annually,1 over half are considered mild. When patients present less than 3 h from onset with acute ischemic stroke, mild or improving symptoms are the most common reasons physicians withhold tissue‐plasminogen‐activator (rt‐PA).2, 3, 4, 5, 6 It is believed mild symptoms will resolve, or are benign, and do not justify the risk of bleeding associated with thrombolysis.7, 8 Traditionally, treatment decisions are based on the National Institute of stroke scale (NIHSS); occasionally exceptions are made if the neurologist identifies a debilitating deficit, such as vision loss or severe aphasia. However, outcomes after acute mild ischemic stroke (MIS) are unfavorable in a substantial proportion of patients. Studies reveal approximately 30% of patients presenting less than 3 hours from onset with MIS who don not receive rt‐PA are either dead or dependent at discharge.9, 10
Furthermore, a significant proportion of MIS patients have poor outcomes at 90 days and beyond.11, 12 Long‐term studies of MIS show that impaired concentration, memory, and overall reduced quality of life are quite common despite lack of motor impairments or global cognitive screening abnormalities.13, 14, 15, 16 Patients with transient ischemic attack (TIA) are more likely to have impaired cognition at follow‐up,17 and poststroke setting, there is an association with acutely impaired cognition and chronically decreased quality of life.18, 19, 20 Other studies show early executive function impairment after stroke is a risk factor for long term cognitive impairment, and neglect; perceptual deficits predict poor functional outcomes.21
Similarly, deficits in processing speed and memory are also prevalent.22, 23 Executive function abnormalities can be subtle and easy to miss on rapid neurological examination of suspected AIS. Assessment of cognition beyond basic language and consciousness is not routinely performed in the emergency department (ED) or stroke unit setting, therefore, significant cognitive dysfunction (CD) may not be easy to be detect on patients in the acute phase, when symptoms or deficits are mild or have rapidly resolved.
In order to justify performing cognitive screening acutely, assessment must be feasible and, as clinicians, we need to offer an intervention based on the screening results. However, at this time no rapid cognitive test has been identified for use pre‐tPA.
Further, to our knowledge no study has examined the association between rt‐PA treatment and cognitive outcomes in severe strokes, where outcomes are measured by a modified Rankin Scale (mRS), nor in MIS and TIA, where cognitive deficits may be the only sequelae of the event. Thus, we conducted a pilot study of acute MIS patients to determine the effects of rt‐PA on cognitive outcomes; and cognitive testing was performed within 24‐h of symptom onset and at 90 days. This study was designed as an exploratory pilot study; rt‐PA was administered as standard of care but was not an inclusion criterion. The study investigators were not blinded to whether rt‐PA was given to patients.
Our study had two goals: (1) to determine if screening in the acute phase of MIS or TIA is feasible and sensitive for identifying cognitive deficits while examining the incidence of cognitive impairment in this population, and (2) to assess for preliminary evidence suggesting that rt‐PA administration is associated with improvement in cognitive outcomes. We sought to determine the need to incorporate cognitive testing in patients with MIS and TIA, in the acute to chronic phase and if large studies are needed to establish if rt‐PA reduces cognitive impairment after MIS.
Materials and Methods
Patient selection
We recruited patients admitted to our comprehensive stroke center. The inclusion criteria were: (1) clinical diagnosis of mild or rapidly improving AIS (NIHSS < 6 at presentation) or TIA and (2) age ≥ 18 years. TIA was defined as stroke‐like symptoms that resolved within 24 h after alternate etiologies were ruled out with diagnostic and clinical examinations. All TIA cases were eligible, regardless of ABCD2 score if all other criteria were met. Patients were excluded if cognitive assessment was not completed >24 h after symptom onset; impaired level of consciousness for example drowsiness, stupor, or coma (≥1 point on NIHSS item 1a), prior CNS illness/injury (e.g. brain tumor, inflammatory or infectious neurological diseases, traumatic brain injury with loss of consciousness, neurodegenerative disorder), known history of any degree of cognitive impairment; unable to complete paper and pen cognitive assessments (e.g. blindness, non‐English speaking, etc.). The patients with prior stroke were included if the patient or legal representative confirmed no cognitive or behavioral sequela.
Medical care
Basic demographic, clinical, and years of education data were collected. Acute stroke guidelines, including r‐tPA, were followed. All patients underwent complete neurologic history and examination prior to any cognitive testing as per standard of care. The investigators determined the protocol would not influence or alter medical management. Thus there was no delay in care to perform cognitive testing; cognitive testing would have necessitated a 30–50 min delay in treatment.
Cognitive covariates
Potential baseline covariates that might impact cognitive function were: age, years of education (YrE), history, and extent of white matter lesions (WML). The WML on MRI were defined by Fazekas score24 from 0–3 points based on the extent of WML seen on T2 FLAIR MRI in subcortical and periventricular domains ‐ with a maximum of six areas.
Cognitive assessment
The complete battery was repeated at each study visit time‐point in a predetermined standardized sequence. Assessments included tests of: (1) learning and short/long term verbal memory [California Verbal Learning Test (CVLT‐II), (2) visuospatial function and figural memory [Rey‐Osterrieth Complex Figure test (ROCF): copy, immediate‐recall, and delayed‐recall subtests], (3) working memory (automaticity) and processing speed [Mental Control]: sub‐tests of the Wechsler Memory Scale (WMS‐III), the Symbol Digit Modality Test (SDMT), and the Trail‐Making Tests A & B], (4) immediate and working memory [Digit Span WMS‐III)]. (5) Fluency [FAS test], and (6) a brief global cognitive function screening [Montreal Cognitive Assessment (MoCA), original English version]. These domains were the primary focus. Executive and perceptual impairments are known as the best predictors of the lasting dysfunction that impact quality of life after stroke.17, 18, 19, 25, 26
Administration and scoring
Cognitive tests were carefully selected to ensure the battery could be: (1) completed in ≤60 min, (2) scored using easily obtained normative data distributed across a broad range of ages and levels of education, and (3) easily administered by personnel with minimal neuropsychiatric testing experience. Tests were administered under the supervision of a neuropsychologist to ensure accuracy of results and consistency of interpretation. Abnormal performance on any of the cognitive tests was defined as z‐score of ≤ −1.5. The oral SDMT format was offered to all participants with dominate hand or arm impairment, as indicated by NIHSS, but each declined and completed the written format.
Statistics/analysis
Descriptive statistics are presented as percentages and proportions with distribution of variables, means ± standard deviation or medians with range reported. Two sample t‐test and logistical regression analysis were performed correcting for age and Fazekas score.
Functional baseline
To establish functional baseline of patients prior to acute event, we relied on a series of questions similar to an instrument to evaluate dementia in the community, the Informant Questionnaire on Cognitive Decline (IQCODE)27, 28 In addition, we examined factors that would contribute to limited cognitive function ‐ age, years of education, and extent of white matter lesions.24, 29 Age, years of education, and extent of white matter lesions were included in our linear regression analysis to determine the effect of these cognitive covariates on cognitive outcome testing.
Results: demographic and clinical data
From February 2012 to March 2013 all patients with acute MIS or TIA and met inclusion criteria were screened for enrollment. The patients were excluded if unable to be assessed <24 h from onset (35%) or had prior neurologic or psychiatric illness (30%). Forty‐Five patients were enrolled and consented. Six were withdrawn after enrollment due to diagnosis of a stroke mimic: three with migraine; one with syncope, Bell's palsy, and CNS neoplasm. Thus, 39 patients were included in analyses of cognitive function at baseline and 25 patients completed cognitive assessment at 90 days. There was no difference between participants in terms to t‐PA use (P = 0.4), age (P = 0.14), education (P = 0.06), and NIHSS (P = 0.94(See Table 1). At baseline, 16 (41%) patients had no deficits on NIHSS at the time of cognitive testing, whereas the remaining 23 (59%) had persistent deficits and diagnosed with MIS. NIHSS was 0 in 18 (46%) patients and between 1 and 5 in 21 (54%) patients, of which only one scored points for mild aphasia (This patient was later excluded when it was determined he was experiencing receptive aphasia, per the study exclusion criteria. The patient's data were not aggregated into the study analysis) and none had points for neglect. The frequency of NIHSS components is shown in Table 2.
Table 1.
Basic demographic and clinical information
| Demographic & Clinical information for screening | Summary Measure |
|---|---|
| Age – median (range) in years | 64 (26–86) |
| Male gender – n (%) | 23 (59) |
| Education – median (range) in years | 13 (8–20) |
| NIHSS at enrollment –median (range) in points | 1 (0–5) |
| Time from onset of symptoms to testing median (range) in hrs. | 18.3 (1.1–28.5a) |
2 patients were later determined to have earlier onset time than first reported resulting in a protocol deviation: one tested at 24.5 h and another at 28.5 h after symptom onset.
Table 2.
NIHSS Component score frequency at baseline
| NIHSS Component | Number of patients with deficit: n (%) |
|---|---|
| Level of Consciousness | 0 (0%) |
| Questions | 0 (0%) |
| Commands | 0 (0%) |
| Best Gaze | 1 (3%) |
| Visual | 2 (5%) |
| Facial Palsy | 9 (23%) |
| Motor Arm | 11 (28%) |
| Motor Leg | 5 (13%) |
| Limb Ataxia | 3 (8%) |
| Sensory | 10 (26%) |
| Best Language | 1 (3%) |
| Dysarthria | 8 (21%) |
| Inattention/Extinction | 0 (0%) |
Cognitive testing experience
Cognitive testing was performed within 24‐h of reported symptom onset and did not interfere with or delay standard care. Stroke onset time was adjusted on two patients after testing completion. Because the main purpose of the 24‐h window was to evaluate the study's feasibility, the patients were retained in the study. All patients completed the cognitive assessment battery. Testing time for the individual components varied slightly by component and by patient, but most required less than 5 min, except the MoCA, which averages 10 min to administer.22 Thirty‐three (85%) patients completed the full battery in <60 min. All testing was completed within 70 min except for one patient with mild aphasia whom testing took 80 minutes due to comprehension difficulties.
Cognitive testing results within 24 h of stroke
No cognitive testing data were missing in the 39 patients. Thirty‐three (85%) had detected abnormalities: 10 (26%) on 1–2 assessments while 23 (59%) on ≥3 assessments (see Table 3). The most frequently abnormal tests were the MoCA (64%), ROCF (54%), and Trail Making Tests (54%). Among patients with a NIHSS of 0, 78% had an abnormal score on at least one cognitive test and 30% had ≥3 abnormal tests. Patients with a NIHSS of 1–5 had higher incidences of cognitive abnormal tests than those with a NIHSS of 0.
Table 3.
Baseline characteristics and cognitive results (z‐scores) by TPA
| TPA (n = 8) | Control (n = 17) | P value | |
|---|---|---|---|
| Age, mean ± SD | 64.9 ± 12.5 | 63.8 ± 13.7 | 0.86 |
| Fazekas‐score | 1.00 | ||
| 0/1 | 4 (50%) | 7 (41%) | |
| 2/3 | 2 (25%) | 6 (35%) | |
| 4/5 | 1 (12.5%) | 1 (6%) | |
| No MRI | 1 (12.5%) | 3 (18%) | |
| Year of education | 0.87 | ||
| 8–12 | 2 (25%) | 5 (29%) | |
| 13–15 | 2 (25%) | 6 (35%) | |
| ≥16 | 4 (50%) | 6 (35%) | |
| Cognitive Test (Z‐score) | |||
| SDMT | −1.44 ± 1.50 | −1.65 ± 1.07 | 0.69 |
| Digit Span | 0.98 ± 1.16 | 0.18 ± 1.50 | 0.20 |
| CVLT2‐Tr4 | −1.02 ± 1.41 | −0.75 ± 1.43 | 0.66 |
| CVLT2‐SD | −0.90 ± 1.33 | −1.0 ± 1.31 | 0.88 |
| CVLT2‐LD | −0.63 ± 1.09 | −0.82 ± 1.20 | 0.70 |
| MOCA | −2.41 ± 2.61 | −2.77 ± 3.25 | 0.79 |
| ROCF‐Copy | −2.4 ± 2.45 | −1.97 ± 2.43 | 0.68 |
| ROCF‐IR | −1.2 ± 0.88 | −0.90 ± 1.19 | 0.50 |
| ROCF‐DR | −1.13 ± 1.04 | −1.15 ± 0.91 | 0.96 |
| Mental Control | −0.41 ± 1.25 | −0.35 ± 1.25 | 0.90 |
| Trails A | −3.05 ± 3.73 | −0.95 ± 1.23 | 0.16 |
| Trails B | −0.62 ± 2.06 | −1.09 ± 1.88 | 0.61 |
| FAS | −1.16 ± 0.64 | −1.22 ± 1.45 | 0.88 |
TPA pilot cohort
For the second aim, 25 patients successfully completed the cognitive battery at 90 days. There was no difference between participants who completed the study at Day 90 and those who did not, related to tPA use (P = 0.4), age (P = 0.14), education (P = 0.06), and NIHSS (P = 0.94). The effects of rt‐PA on cognitive outcomes was analyzed using the same screening 90 day tool.. Eight (32%) of the 25 patients received rt‐PA alongside standard of care medical management. The overall Fazekas scores were weighted toward the low end in both groups indicating low pre‐existing white matter disease, consistent with the exclusion criteria. There were no significant differences between groups for cognitive confounders, which would predispose to poor cognitive function and limited recovery potential; specifically age, years of education, Fazekas score, and baseline cognitive impairments (see Table 3).
TPA pilot outcomes
Analysis of rt‐PA versus no rt‐PA, Z‐scores indicated differences in scores for each of the nine tests in the cognitive battery. The only significant finding was for the Mental Control sub‐test of the WMS III (a test of attention and automaticity) (rt‐PA 0.74 ± 0.77, control −0.02 ± 0.83, P: 0.0395) (Table 4). A linear regression for predicting normal cognitive status at follow‐up was performed, with baseline cognitive testing and rt‐PA as covariates. We found a potential statistical trend suggesting an association between rt‐PA and improvement in Mental Control (OR 8.96, 95% CI 0.98–82.12, P: 0.053) (Table 5).
Table 4.
Z‐scores improvement (follow‐up – baseline) by TPA
| Cognitive test | TPA Yes (N = 8) | TPA No (N = 17) | P value |
|---|---|---|---|
| SDMT | 0.56 ± 1.18 | 0.94 ± 1.32 | 0.50 |
| Digit Span | 0.15 ± 0.88 | ‐0.03 ± 1.69 | 0.78 |
| CVLT2‐Tr4 | −0.13 ± 0.64 | ‐0.26 ± 1.09 | 0.36 |
| CVLT2‐SD | 0.25 ± 1.10 | 0.18 ± 1.86 | 0.92 |
| CVLT2‐LD | 1.00 ± 0.93 | 0.74 ± 1.11 | 0.57 |
| MOCA | 0.93 ± 2.20 | 1.24 ± 2.51 | 0.77 |
| ROCF‐Copy | −1.78 ± 1.89 | −1.5 ± 0.71 | 0.75 |
| ROCF‐IR | −0.94 ± 0.70 | −0.85 ± 0.84 | 0.80 |
| ROCF‐DR | 0.02 ± 0.74 | 0.02 ± 0.74 | 0.75 |
| Mental Control | 0.74 ± 0.77 | −0.02 ± 0.83 | 0.04 |
| Trails A | 2.42 ± 3.75 | 0.57 ± 1.07 | 0.21 |
| Trails B | 0.55 ± 1.50 | 0.67 ± 1.82 | 0.89 |
| FAS | 0.86 ± 0.67 | 0.76 ± 0.81 | 0.75 |
Bolded value highlights statistical significance.
Table 5.
Logistic regression model for predicting normal status at follow‐up with baseline status and TPA as covariates
| Baseline normal | TPA Yes | |||||
|---|---|---|---|---|---|---|
| Test | OR | 95% CI | P | OR | 95% CI | P |
| SDMT | 10.84 | 0.62–188.9 | 0.102 | 0.21 | 0.02–2.13 | 0.185 |
| Digit Span | 12.34 | 1.44–105.5 | 0.022 | 7.11 | 0.51–98.6 | 0.144 |
| CVLT4Lon | 7.45 | 0.74–75.6 | 0.089 | 1.59 | 0.21–12.07 | 0.653 |
| MOCA | 7.10 | 1.05–48.13 | 0.045 | 1.29 | 0.20–8.39 | 0.789 |
| ROCFDR | 11.00 | 0.45–270.1 | 0.142 | 0.80 | 0.05–13.53 | 0.879 |
| Mental Control | 11.15 | 1.26–98.79 | 0.030 | 8.96 | 0.98–82.12 | 0.053 |
| Trails A | 6.75 | 0.54–83.70 | 0.137 | 0.64 | 0.09–4.28 | 0.641 |
| Trails B | 28.46 | 2.34–345.9 | 0.009 | 0.22 | 0.01–3.48 | 0.281 |
| FAS | 6.95 | 0.77–∞ | 0.147 | 2.30 | 0.23–24.10 | 0.668 |
Patient stratification by extent of WMD on Fazekas score 0–1 versus 2–5, revealed significant improvement in outcomes associated with rt‐PA in patients with fewer cognitive covariates (Table 6). Improvement in Mental Control on WMS III in the low disease‐burden group (Fazekas 0–1) (N = 4) was −0.51 ± 0.61 as compared to the group with more extensive WMD burden (Fazekas 2–5) (N = 3) 0.60 ± 0.46 (P = 0.048). Another statistical trend suggested improvement on the SDMT and Trail Making tests, in patients with low WMD burden (N = 4) 0.40 ± 0.37 when compared to high disease‐burden (N = 3) 1.43 ± 0.8 (P = 0.0696).
Table 6.
Z‐score improvement (follow‐up – baseline) by Faz‐score in patients having rt‐PA
| Faz score 0/1 | Faz‐score 2–5 | ||
|---|---|---|---|
| Test | (N = 4) | (N = 3) | P value |
| SDMT | 0.25 ± 1.55 | 1.17 ± 0.58 | 0.3840 |
| Digit Span | 0.20 ± 0.76 | 0.37 ± 1.19 | 0.8284 |
| CVLT4Lon | 0.88 ± 1.18 | 1.50 ± 0 | 0.3677 |
| MOCA | 0.18 ± 1.43 | 2.23 ± 3.11 | 0.2866 |
| ROCFDR | −0.51 ± 0.61 | 0.60 ± 0.46 | 0.0484 |
| Mental Control | 0.40 ± 0.37 | 1.43 ± 0.81 | 0.0696 |
| Trails A | 0.22 ± 0.34 | 5.83 ± 4.58 | 0.1670 |
| Trails B | 0.15 ± 0.27 | 1.37 ± 3.02 | 0.6694 |
| FAS | 0.95 ± 0.75 | 0.83 ± 0.81 | 0.8518 |
Mean ± SD are shown in table.
Two‐sample t‐test was used to compare z‐score improvement.
Discussion
The aims of our study were dual: (1) determine feasibility of cognitive testing and prevalence of cognitive impairment in the acute phase of MIS and TIA and (2) Acquire preliminary data on the association between rt‐PA administration in acute MIS and improvement on follow‐up cognitive testing. Potentially, more than ten cognitive functions can be impaired by stroke.30 Our protocol was designed as an exploratory study, due to the paucity of information on cognitive impairment within 72 h after stroke. Multiple aspects of cognition were assessed to determine which, if any, were significantly impaired and if assessment could be performed in the hyper‐acute to acute phase. In designing our screening battery, the nine tests selected addressed a broad range of cognitive deficits, focusing primarily on executive function, memory, and visual/spatial function. These cognitive domains have previously been found most associated with impaired quality of life after MIS,18, 21, 22, 23 are unlikely to be detected by NIHSS, and are important aspects of vascular cognitive impairment.31, 32, 33
The battery included many components recommended by the NINDS‐CNS Vascular Cognitive Impairment Harmonization Standards23; They are based on a population with more severe vascular events. Thus, this shorter battery was more focused on identifying deficits in the domains most affected in mild strokes and TIA. Also, it was easily administered by staff trained by our neuropsychologist without prior test administration experience, and, in all cases testing was completed within 60 min.
A surprisingly high prevalence of cognitive abnormalities was noted among patients with MIS or TIA. More than one‐half had significant cognitive impairment using our relatively high threshold of ≥3 abnormal tests. Cognitive impairment after MIS in one domain is not unexpected; however, the intent of this study was to assess significant cognitive impairment. Therefore, more stringent criteria were selected. Significant impairment was defined as >3 impaired domains and could include multi‐domain amnesiac (i.e. memory impairment) or multi‐domain non‐amnesiac, if at least three domains were impaired. Jokinen et al. indicated this should include at least 50% of all stroke survivors.34 Choosing a lower threshold, such as abnormalities in ≥1 domain, or a MoCA of ≤26, for example, would have resulted in markedly higher prevalence of significant impairment (64% vs. 85%, respectively). Deficits in executive, visuospatial, and memory dysfunctions were detected in the majority of cases, findings consistent with previous studies, and supportive of our focus on screening tests of these domains.18, 21, 22, 23, 25 In no case did NIHSS deficits suggest cognitive impairment, and in several with NIHSS 0, bedside cognitive evaluation detected significant abnormalities, with 30% of impaired patients having ≥3 abnormal tests.
To avoid confounders such as aphasia, our exclusion criteria ensured that any participant unable to perform testing would be excluded. Also, any participant who was unable to perform graphomotor tasks have been excluded. However, no participant had severe dominate hand impairment and could complete tasks with their non‐dominate hand, as the written tasks were not timed‐tests. One study participant gave an indication (Figure 1) that minimal injury can influence overall neuro‐processing in multiple cognitive domains. Processing speed is more closely associated with global tract integrity than even memory or general intelligence24 and therefore IPS may also be more sensitive to any injury than other cognitive functions.35
Figure 1.
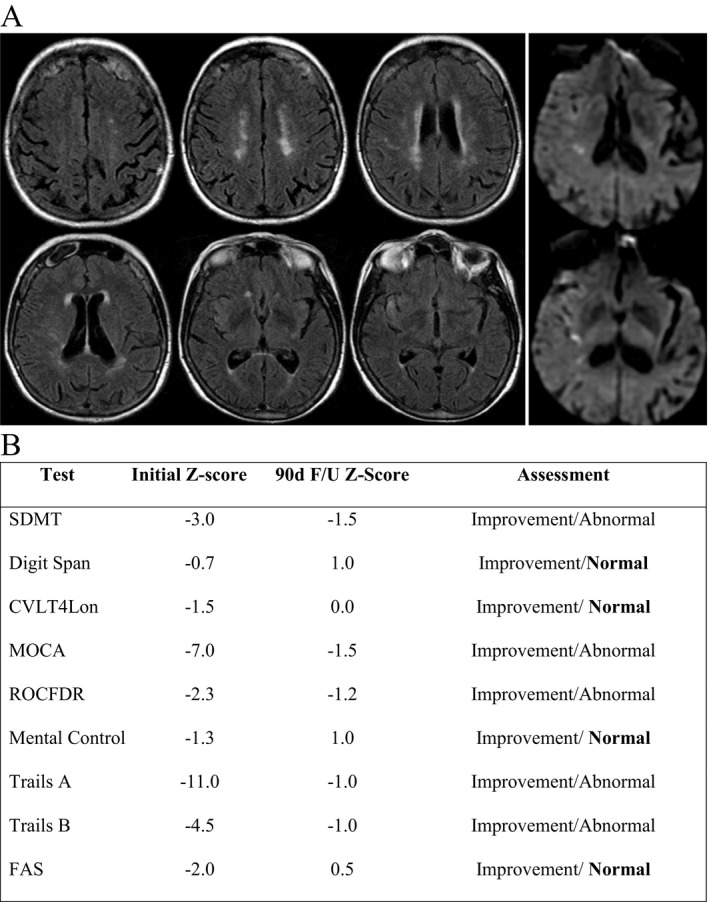
Demonstrative case showing cognitive improvement after TPA in patient with mild‐moderate baseline cognitive deficit. Eighty‐two years old R‐handed woman presents with sudden onset left sided hemiparesis and facial droop – received TPA with complete resolution of symptoms. MRI (Fig. 1A) showed few foci of restricted diffusion in right insula, temporal lobe, and corona‐radiata. Fazekas score of 4 (2 periventricular + 2 white matter). Patient determined to have moderate‐low cognitive baseline based on impact of covariates age, imaging, and YrE (16). Initial cognitive screening showed deficits on nine of nine tests. Three month follow‐up demonstrated improvement in all nine tests as well as normalization in four of nine tests (Fig. 1B).
Of 45 patients initially enrolled, 25 with MIS successfully completed re‐evaluation at 90 days. These 25 formed the cohort for examining the effect of rt‐PA on cognitive outcomes in MIS. By assessing patients without deficits in language on NIHSS, we could more accurately assess deficits that address other areas of processing, memory, and abstraction otherwise confounded by aphasia. These areas are not tested by NIHSS, but can result in significant functional morbidities.33 Moreover, persistent deficits in cognition are often overlooked in studies evaluating stroke outcomes, as the mRS does not account for cognitive deficits.19, 31, 32
Although our sample size was small, we found evidence suggesting rt‐PA was associated with significant improvement in processing speed and automaticity as indexed by the mental control component of the WMS III. This was valid both when examining the z‐score of overall outcome, as well as post linear‐regression, accounting for factors that contribute to poor cognitive baseline. Additionally, there was a statistical trend suggesting improvement in the SDMT and Digit Span subtest of the WMS III in patients receiving rt‐PA. Both measurements are designed to assess processing speed. One could explain this potential finding as these tests reflects global cognitive function, and be most affected by poor physiologic neuro‐infrastructure on the cellular level.
To describe the underlying mechanism of cognitive deficits in TIA/MIS is beyond the scope of this study. Considering that TIA/MIS can result from multiple etiologies, the specific pathophysiology of each mechanism of injury would need to be addressed individually. It is possible that for certain etiologies, such as pure hypoperfusion, rt‐PA would provide no benefit. Nonetheless, based on our findings, these deficits are not the result of an injury to a specific eloquent anatomical region. It is more likely a multifactorial process that involves the disruption of complex neuronal networks on a cellular level, beyond the sensitivity of our diagnostic tools. This is supported by our observation that patients with more severe WMD showed better recovery at 90 days, as these patients are more likely to be sensitive to even minor insults.
The major limitation to our pilot study is small sample size, particularly in the cohort examining potential effects of rt‐PA in MIS. As an exploratory study, a‐priori sample size was not calculated; thus, outcome predictions cannot be made. Additionally, the study clearly demonstrated feasibility of early cognitive assessment in patients with MIS and TIA, and high prevalence of detectable cognitive impairment within 24 h of symptom onset. Given our strict exclusion criteria, we believe that our screening battery is effective in selecting for cognitive deficits in the acute phase. Nonetheless, we acknowledge that a brief screening is not a substitute for a comprehensive neuro‐psychological evaluation that could more accurately define areas impaired. In most situations, a complete evaluation would be impractical in the acute phase, given the extensive time and resources such assessments require. Thus, a single rapid screening tool could function to both determine if deficits are present acutely, as well as enable monitoring impairment over time.36 While too small a study to reach definitive interpretations, this pilot cohort's results support that larger studies may add value to our body of knowledge and ultimately improve patient outcomes.
Summary
Cognitive deficits are common and overlooked sequelae of MIS and TIA. Screening for deficits in the acute phase is feasible and could focus on areas of processing speed, executive functioning, and functional memory. Based on our data, further studies are needed to assess for treatment effects of rt‐PA on cognitive deficits in this population. Detecting a treatment effect would make cognitive screening an important element of AIS evaluation. Lastly as suggested by other studies, persistent cognitive deficits continue for months after the acute event; thus, screening for deficits might help design better medical regimens to improve functional recovery.
Conflict of Interest
The authors listed in the title of this manuscript certify that they have no affiliations with or involvement in any organization, financial interest, or non‐financial interest in the subject matter or materials discussed in this manuscript.
Funding Information
National Institutes of Health (2 T32 NS007412).
Funding Statement
This work was funded by National Institutes of Health grant 2 T32 NS007412.
References
- 1. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dhamoon MS, Moon YP, Paik MC, et al. Long‐term functional recovery after first ischemic stroke: the Northern Manhattan Study. Stroke 2009;40:2805–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reeves M, Khoury J, Alwell K, et al. Distribution of National Institutes of Health stroke scale in the Cincinnati/Northern Kentucky Stroke Study. Stroke 2013;44:3211–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hills NK, Johnston SC. Why are eligible thrombolysis candidates left untreated? Am J Prev Med 2006;31(6 Suppl 2):S210–S216. [DOI] [PubMed] [Google Scholar]
- 5. Kleindorfer D, Kissela B, Schneider A, et al. Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: a population‐based study. Stroke 2004;35:e27–e29. [DOI] [PubMed] [Google Scholar]
- 6. Barber PA, Zhang J, Demchuk AM, et al. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility Neurology 2001;56:1015–1020. [DOI] [PubMed] [Google Scholar]
- 7. Smith EE, Abdullah AR, Petkovska I, et al. Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke 2005;36:2497–2499. [DOI] [PubMed] [Google Scholar]
- 8. Smith EE, Fonarow GC, Reeves MJ, et al. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue‐type plasminogen activator: findings from Get With The Guidelines‐Stroke. Stroke 2011;42:3110–3115. [DOI] [PubMed] [Google Scholar]
- 9. Guerrero WR, Savitz SI. Mild acute ischaemic stroke–the case for thrombolytic therapy. Nat Rev Neurol 2013;9:653–656. [DOI] [PubMed] [Google Scholar]
- 10. DeGraba TJ, Hallenbeck JM, Pettigrew KD, et al. Progression in acute stroke: value of the initial NIH stroke scale score on patient stratification in future trials. Stroke 1999;30:1208–1212. [DOI] [PubMed] [Google Scholar]
- 11. Khatri P, Conaway MR, Johnston KC. Ninety‐day outcome rates of a prospective cohort of consecutive patients with mild ischemic stroke. Stroke 2012;43:560–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duncan PW, Samsa GP, Weinberger M, et al. Health status of individuals with mild stroke. Stroke 1997;28:740–745. [DOI] [PubMed] [Google Scholar]
- 13. Edwards DF, Hahn M, Baum C, Dromerick AW. The impact of mild stroke on meaningful activity and life satisfaction. J Stroke Cerebrovasc Dis 2006;15:151–157. [DOI] [PubMed] [Google Scholar]
- 14. Carlsson GE, Moller A, Blomstrand C. Consequences of mild stroke in persons < 75 years ‐ A 1‐year follow‐up. Cerebrovasc Dis 2003;16:383–388. [DOI] [PubMed] [Google Scholar]
- 15. Mahon S, Parmar P, Barker‐Collo S, et al. Determinants, prevalence, and trajectory of long‐term post‐stroke cognitive impairment: results from a 4‐year follow‐up of the ARCOS‐IV study. Neuroepidemiology 2017;49:129–134. [DOI] [PubMed] [Google Scholar]
- 16. Kapoor A, Lanctot KL, Bayley M, et al. “Good Outcome” isn't good enough: cognitive impairment, depressive symptoms, and social restrictions in physically recovered stroke patients. Stroke 2017;48:1688–1690. [DOI] [PubMed] [Google Scholar]
- 17. van Rooij F, Schaapsmeerders P, Maaijwee N, et al. Persistent cognitive impairment after transient ischemic attack. Stroke 2014;45:2270–2274. [DOI] [PubMed] [Google Scholar]
- 18. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sivakumar L, Kate M, Jeerakathil T, et al. Serial montreal cognitive assessments demonstrate reversible cognitive impairment in patients with acute transient ischemic attack and minor stroke. Stroke 2014;45:1709–1715. [DOI] [PubMed] [Google Scholar]
- 20. Nys GM, van Zandvoort MJ, van der Worp HB, et al. Early cognitive impairment predicts long‐term depressive symptoms and quality of life after stroke. J Neurol Sci 2006;247:149–156. [DOI] [PubMed] [Google Scholar]
- 21. Rahbar MH, Gonzales NR, Ardjomand‐Hessabi M, et al. The University of Texas Houston Stroke Registry (UTHSR): implementation of enhanced data quality assurance procedures improves data quality. BMC Neurol 2013;13:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nasreddine Z. Montreal Cognitive Assessment (MoCA) Administration and Scoring Instructions. 2010. mocatest.org2010 [cited 2013]; Available from: http://www.mocatest.org/pdf_files/MoCA-Instructions-English_2010.pdf
- 23. Hachinski V, Iadecola C, Petersen RC, et al. National institute of neurological disorders and stroke‐canadian stroke network vascular cognitive impairment harmonization standards. Stroke 2006;37:2220–2241. [DOI] [PubMed] [Google Scholar]
- 24. Penke L, Maniega SM, Bastin ME, et al. Brain white matter tract integrity as a neural foundation for general intelligence. Mol Psychiatry 2012;17:1026–1030. [DOI] [PubMed] [Google Scholar]
- 25. Nys GMS, Zandvoort MJEV, Kort PLMD, Worp HBVD. The prognostic value of domain‐specific cognitive abilities in acute first‐ever stroke. Neurology 2005;64:821–827. [DOI] [PubMed] [Google Scholar]
- 26. Burton L, Tyson SF. Screening for cognitive impairment after stroke: a systematic review of psychometric properties and clinical utility. J Rehabil Med 2015;47:193–203. [DOI] [PubMed] [Google Scholar]
- 27. McGovern A, Pendlebury ST, Mishra NK, et al. Test accuracy of informant‐based cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke 2016;47:329–335. [DOI] [PubMed] [Google Scholar]
- 28. Blackburn DJ, Bafadhel L, Randall M, Harkness KA. Cognitive screening in the acute stroke setting. Age Ageing 2013;42:113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Snaphaan L, de Leeuw FE. Poststroke memory function in nondemented patients: a systematic review on frequency and neuroimaging correlates. Stroke 2007;38:198–203. [DOI] [PubMed] [Google Scholar]
- 30. Cumming TB, Marshall RS, Lazar RM. Stroke, cognitive deficits, and rehabilitation: still an incomplete picture. Int J Stroke 2013;8:38–45. [DOI] [PubMed] [Google Scholar]
- 31. Lees R, Corbet S, Johnston C, et al. Test accuracy of short screening tests for diagnosis of delirium or cognitive impairment in an acute stroke unit setting. Stroke 2013;44:3078–3083. [DOI] [PubMed] [Google Scholar]
- 32. Lees R, Selvarajah J, Fenton C, et al. Test accuracy of cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke 2014;45:3008–3018. [DOI] [PubMed] [Google Scholar]
- 33. Kauranen T, Laari S, Turunen K, et al. The cognitive burden of stroke emerges even with an intact NIH Stroke Scale Score: a cohort study. J Neurol Neurosurg Psychiatry 2014;85:295–299. [DOI] [PubMed] [Google Scholar]
- 34. Jokinen H, Melkas S, Ylikoski R, et al. Post‐stroke cognitive impairment is common even after successful clinical recovery. Eur J Neurol 2015;22:1288–1294. [DOI] [PubMed] [Google Scholar]
- 35. Su CY, Wuang YP, Lin YH, Su JH. The role of processing speed in post‐stroke cognitive dysfunction. Arch Clin Neuropsychol 2015;30:148–160. [DOI] [PubMed] [Google Scholar]
- 36. Campbell N, Rice D, Friedman L, et al. Screening and facilitating further assessment for cognitive impairment after stroke: application of a shortened Montreal Cognitive Assessment (miniMoCA). Disabil Rehabil 2016;38:601–604. [DOI] [PubMed] [Google Scholar]


