Abstract
Objective
Narcolepsy type 1 widely affects the architecture of sleep with frequent fast transition to REM sleep at both nighttime and daytime sleep onset. The occurrence of repeated sleep onset REM periods over the Multiple Sleep Latency Test offers a unique opportunity to identify EEG patterns predictive of successful dream recall after short periods composed of only REM or NREM sleep. It also permits to disentangle state‐ from trait‐like differences in dream recall, by using a within‐subjects design.
Methods
A consecutive series of 115 first‐diagnosed drug‐free adult narcolepsy‐type 1 patients underwent Multiple Sleep Latency Tests and were asked after each nap opportunity if they had or had not a dream experience. Scalp EEG power and a specific index of cortical activation (delta/beta power ratio), obtained from naps of 43 patients with both presence and absence of dream recall in the same sleep stage, were compared separately for REM and NREM sleep.
Results
Successful dream recall was associated with an increased EEG desynchronization in both REM and NREM over partially overlapping cortical areas. Compared to unsuccessful recall, it showed (1) lower delta power over centro‐parietal areas during both stages, (2) higher beta power in the same cortical areas during NREM, and (3) lower values in the delta/beta ratio during NREM in most scalp locations.
Interpretation
A more activated electrophysiological milieu in both REM and NREM sleep promotes dream recall, strengthening the notion that the parietal areas are crucial not only in generating dream experience, as shown in brain‐damaged patients, but also in the memory processing leading to recall.
Introduction
Investigation on the neurophysiological correlates of dreaming in healthy subjects documented potential influences of ultradian (NREM/REM sleep stages), circadian (time‐of‐day/sleep cycles) and sleep‐dependent (amount of previous sleep/sleep debt) factors.1 However, both the rigid sequence of NREM/REM stages within sleep cycles and the interactions between ultradian and circadian factors make it difficult to definitely distinguish the influence of specific factors on the recall and characteristics of dream experience.1, 2 This difficulty is made apparent by the mixed results obtained in studies using protocols with serial awakenings (for example, the Sleep Interruption Technique3) to overcome the rigid sequence of NREM‐REM stages within the sleep cycle. Indeed, it has been reported that successful dream recall may be associated with reduced frontal alpha power,4 reduced delta power,5 increased occipital alpha beta power,4 and increased frontal theta power6, 7 for REM sleep, and to reduced delta activity,4, 5, 8 increased occipital gamma power,5 and decreased alpha activity6 for NREM sleep.
The variety of potential neurophysiological markers of dream recall in healthy subjects raises the issue of the effects of the desynchronization of sleep stages within cycles induced by the multiple awakenings and re‐onsets. These effects may be partly overcome by investigating dream recall in populations of individuals whose architecture of sleep is per se altered, such as patients with hypersomnolence of central origin. Among these patients, those with narcolepsy type 1 (a disease likely caused by an autoimmune attack to the hypothalamic neurons producing hypocretin and altering the wakefulness/sleep boundaries: NT1) are of interest, as often shifting rapidly into a sleep stage called sleep onset REM sleep (SOREM) regardless of time of day.9 Indeed, the repeated occurrence of periods of SOREM at the five trials of the Multiple Sleep Latency Test (MSLT) is still the most reliable neurophysiological marker discriminating NT1 from other central disorders of hypersomnolence.10
The peculiar within‐cycle sequence of sleep stages in NT1 patients at sleep onset (given the occurrence of SOREM sleep in about 70% of MSLT naps11) affords a unique opportunity to investigate the relationships between sleep neurophysiology and dreaming without two major confounding factors. Indeed, the initial sleep stage (REM or NREM) usually persists during MSLT naps (given their limited duration, 15 min from sleep onset); thus, SOREM sleep is not influenced by previous NREM sleep, differently from naps obtained through serial awakenings. Moreover, the fixed length of the interval between trials (2 h, corresponding to the duration of a trial plus a potential 90‐min sleep cycle) maintains the sleep pressure constant over MSLT trials (from 9 a.m. to 5 p.m.), at variance with serial awakenings, where the sleep debt is not homogeneous because of the desynchronization of sleep stages within cycle.
Here, we report the findings of a MSLT study on narcoleptic patients in which the predictive power of electroencephalographic (EEG) oscillations for dream recall was assessed by comparing within‐subjects couples of naps with only either REM or NREM sleep followed by successful and unsuccessful dream recall, providing an EEG substratum of the peculiar dream experience in this clinical population, as reported by previous qualitative researches12, 13, 14, 15
Methods
Patients
Two‐hundred twenty‐eight patients (aged 18–60 years) with a suspected central disorder of hypersomnolence were preliminarily considered for the study at the Outpatients Clinic for Narcolepsy of the Department of Biomedical and Neuromotor Sciences (DIBINEM) of the University of Bologna (years 2016–17). During a 3‐days hospitalization they underwent a careful and extensive clinical evaluation made by a physician expert in sleep medicine with systematic assessment of sleep symptoms and habits (including the Italian version of the Epworth Sleepiness Scale16 – ESS). These examinations were performed in drug‐free condition (all patients being drug‐naive or after drug discontinuation for at least 3 weeks). Diagnostic workout included a 48‐hours continuous polysomnographic (PSG) recording10 followed by the MSLT with five nap opportunities17 in the third day. Patients with sleep‐disordered breathing (apnea‐hypopnea index, AHI, >5) were excluded from this study. The diagnosis of NT1 was confirmed by low/absent CSF hypocretin‐1.9 According to the aims of the study, among the 115 patients with final diagnosis of NT1 only the EEG data of those (n = 43, 25 males and 18 females; mean age: 35 ± 14.2 years) showing combinations of one or more naps with (REC) and without dream recall (NREC) in MSLT trials composed exclusively of only REM or NREM sleep during the MSLT routine were considered (Fig. 1).
Figure 1.
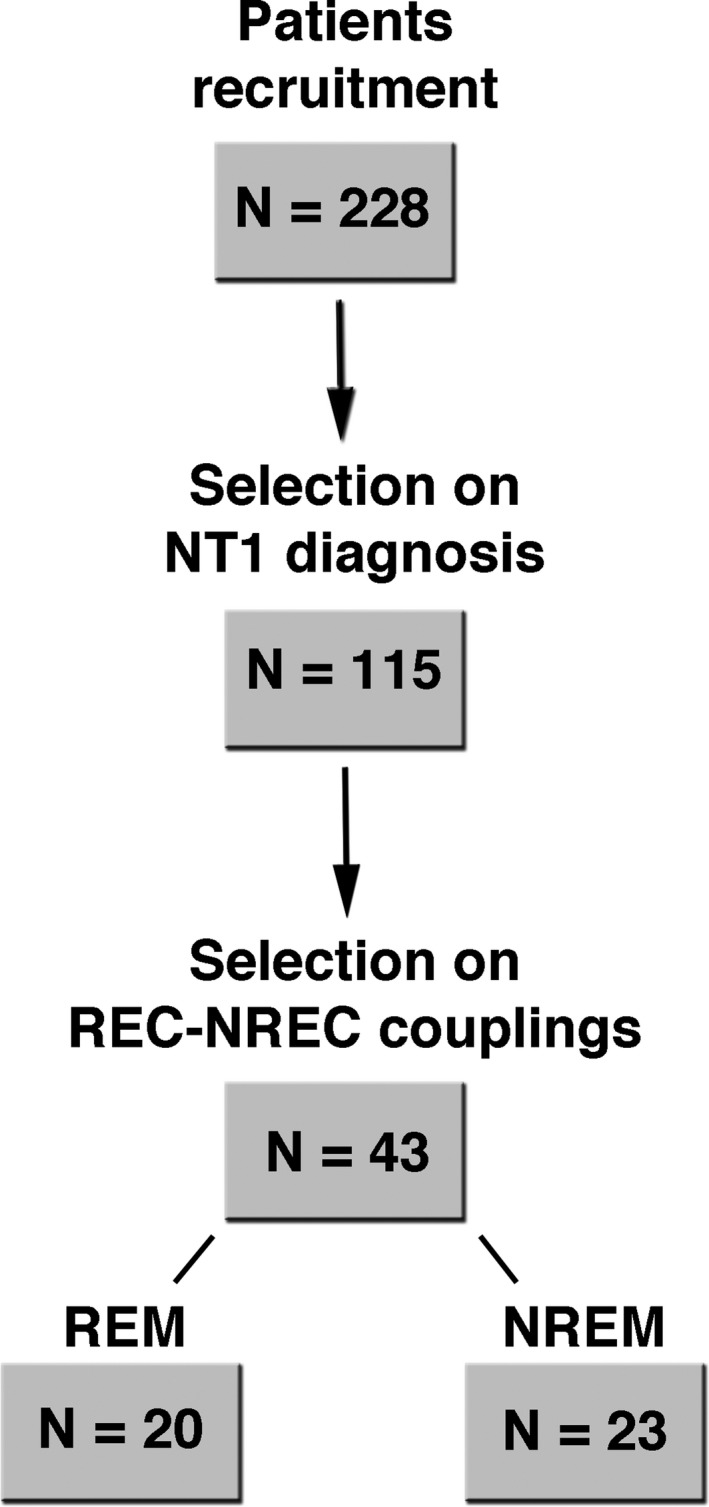
Patients’ sample selection procedure. Number of patients passing each step of the selection flow leading to the final data sample involved in the REM and NREM sleep analyses.
The study protocol was approved by the Local Ethical Committee (#17009), and all subjects signed a written informed consent.
Polysomnographic recording
The PSG recording included the acquisition of the EEG from 19 cortical derivations (C3, C4, Cz, F3, F4, F7, F8, Fp1, Fp2, Fz, O1, O2, P3, P4, Pz, T3, T4, T5, T6) and from bilateral mastoids (A1 and A2) of the international system 10–20 (notch filter: 50 Hz), the electro‐oculogram (EOG), submental and bilateral anterior tibialis electromyogram (EMG), and electrocardiogram (ECG). The EEG signals were referenced off‐line to the averaged mastoids and digitally high‐pass filtered with a time constant of 0.3 sec and low‐pass filtered at 30 Hz.
Dream recall
Dream recall was collected at the end of each nap. According to the standard MSLT procedure17 (five trials with a 2‐h interval, the first one starting at 9:00 a.m.), during each trial the subjects were allowed to fall asleep within 20 min from the lights switch‐off. After 15 min from the sleep onset they were awakened, if not spontaneously awake, and asked to report if they had or had not a dream experience before awakening.
Data analysis
Quantitative analysis of sleep EEG
Sleep recordings were visually scored according to the AASM criteria.18 The length of epochs was established at 20 sec, as a multiple of 4‐sec (i.e., the periodogram in our Fast Fourier Transform – FFT – routine). Naps including both REM and NREM (N2, N3) sleep were excluded from the subsequent analyses, while the presence of N1 during sleep onset or following intrasleep arousals was not an exclusion criterion. When more than one combination of naps was available for a patient, the temporal proximity between the naps to be compared was favored in coupling decision (e.g., in the case of REC after REM‐nap at 13:00 trial and NREC after REM‐naps at 15:00 and 17:00 trials, REC REM‐nap was coupled with NREC REM‐nap at 15:00). The final pool of data considered for the statistical analyses involved 20 within‐subject REC‐NREC combinations for REM sleep and 23 for NREM sleep. A preliminary analysis was performed on the quantitative EEG measures of a selected data sample, including only REC‐NREC combinations obtained within the same circadian phase and the same sleep stage: Morning naps (AM), occurred at 9:00 and 11:00 h, and Afternoon naps (PM), occurred at 13:00, 15:00 and 17:00 h. This control was made in order to ascertain possible effects of the circadian phase on the EEG pattern predicting dream recall.
EEG power of the REC‐NREC combinations that passed the selection procedure was calculated by a customized FFT routine on 4 sec artifact‐free epochs within the 0.50–29.75 Hz frequency range (0.25 Hz bin resolution), for the whole duration of the nap. The values of EEG power from adjacent frequency bins for each scalp derivation (expressed as percentage of the total power spectrum within the whole topography) were summed together in order to obtain the canonical frequency bands: delta (0.50–4.75 Hz), theta (5.00–7.75 Hz), alpha (8.00–11.75 Hz), sigma (12.00–15.75 Hz), and beta (16.00–24.75 Hz).
Statistical analysis
Sleep macrostructural variables were matched for REC‐NREC condition by paired t‐test, separately for couples of naps with only REM or NREM sleep.
Due to the scarcity of NREM sleep REC‐NREC combinations complying with the circadian phase criterion, the control analysis was performed only for the REM‐sleep combinations (n = 16, 6 of which in the morning). Therefore, two‐way mixed‐design ANOVAs, Recall (NREC vs. REC) × Circadian Phase (AM vs. PM), were performed on EEG power for each scalp derivation and frequency band. Since no significant interactions between dream recall and circadian phase were observed for any scalp location or frequency band, the whole pool of data passing the selection procedure described above underwent the following principal analyses.
Two‐way repeated‐measures ANOVAs, Recall (NREC vs. REC) × Frequency Band (Delta vs. Theta vs. Alpha vs. Sigma vs. Beta), were carried out for each scalp derivation, separately for REM and NREM combinations of REC‐NREC conditions. The False Discovery Rate (FDR) correction proposed by Benjamini and Yekutieli19, 20 was applied to adjust the α‐value for multiple comparisons, again separately on P‐values obtained from ANOVAs on REM and NREM sleep. The α‐value of main effects and interactions after the FDR procedure was adjusted to a critic P = 0.00244 for the REM sleep comparisons and to a critic P = 0.0002 for the NREM sleep comparisons. According to the specific aim of the study, planned comparisons (paired t‐tests) between REC vs. NREC conditions were carried out for possible significant effects of interaction, with α‐value at P ≤ 0.05.
Results
Sleep macrostructural variables
No differences were found in the sleep architecture between REC and NREC conditions for either REM or NREM coupled naps (Table 1).
Table 1.
Sleep architecture of naps associated with the REC and the NREC conditions included in the final REM and NREM sleep data samples.
| REM | NREM | |||||||
|---|---|---|---|---|---|---|---|---|
| REC | NREC | t 19 | P | REC | NREC | t 22 | P | |
| TBT (min) | 18.9 (SD 3.6) | 20.9 (SD 4.7) | −0.41 | 0.69 | 22.8 (SD 5.8) | 22.4 (SD 5.4) | 0.32 | 0.75 |
| TST (min) | 14.7 (SD 1.6) | 14.8 (SD 1.6) | −0.16 | 0.88 | 15.8 (SD 2.3) | 15.8 (SD 3.1) | 0.10 | 0. 92 |
| REM/NREM sleep duration (min) | 11.6 (SD 2.6) | 10.5 (SD 2.2) | 1.34 | 0.20 | 12.2 (SD 4.1) | 13.0 (SD 3.4) | −1.08 | 0.29 |
| WASO (min) | 0.7 (SD 1.0) | 0.5 (SD 0.8) | 0.67 | 0.51 | 0.7 (SD 0.01) | 0.5 (SD 1.2) | 1.32 | 0.20 |
| AROUSALS (#) | 6.4 (SD 3.4) | 5.7 (SD 3.4) | 0.72 | 0.48 | 5.0 (SD 3.5) | 4.0 (SD 3.6) | 1.44 | 0.16 |
| AROUSAL DURATION (sec) | 11.3 (SD 8.8) | 14.5 (SD 10.5) | −1.30 | 0.21 | 17.7 (SD 17.8) | 13.3 (SD 15.8) | 1.32 | 0.20 |
| MBM (#) | 0.3 (SD 0.7) | 0.3 (SD 0.8) | 0 | 1 | 0 | 0 | ‐ | ‐ |
TBT, total bed time; TST, total sleep time; WASO, wake after sleep onset; MBM, Major body movements.
EEG pattern of dream recall from REM sleep
Figure 2 shows the mean topographic distribution of relative EEG power in the five frequency bands for REM sleep of the REC and NREC conditions. The results from the repeated‐measures ANOVAs Recall × Frequency Band are reported in Table 2. Besides the main effects for the Frequency Band factor, encompassing the whole EEG topography, ANOVAs reveal no significant main effects for the Recall factor for any scalp location, but significant Frequency Band × Recall interactions at specific centro‐parietal areas (C3: F 4,76 = 4.66, P = 0.002; C4: F 4,76 = 6.98, P = 0.00008; Cz: F 4,76 = 6.09, P = 0.0027; P4: F 4,76 = 4.54, P = 0.0024). The statistical map for the coefficients of Recall × Frequency Band interactions is shown in Figure 3A.
Figure 2.

Topographic distribution of REM sleep EEG powers in the no‐recall (NREC) and the recall (REC) conditions. The EEG activity during REM sleep for the canonical frequency bands (delta: 0.50–4.75 Hz; theta: 5.00–7.75 Hz; alpha: 8.00–11.75 Hz; sigma: 12.00–15.75 Hz; beta: 16.00–24.75 Hz) is reported, expressed as percentage of the total EEG power within the whole topography. The maps are scaled between the minimal and maximal power values for each frequency band considering both the NREC (1st row) and REC (2nd row) conditions.
Table 2.
Results of the two‐ways repeated‐measures ANOVAs Recall (NREC vs. REC) × Frequency Band (Delta vs. Theta vs. Alpha vs. Sigma vs. Beta) on spectral powers of REM and NREM sleep.
| REM | NREM | |||||
|---|---|---|---|---|---|---|
| Recall(R) | Frequency Band (FB) | R × FB | Recall(R) | Frequency Band (FB) | R × FB | |
| F 1,19(P) | F 4,76(P) | F 4,76(P) | F 1,22(P) | F 4,88(P) | F 4,88(P) | |
| C3 | 2.44 (n.s.) | 511.17 (P < 10−15) | 4.66 (P = 0.00203) | 4.21 (n.s.) | 539.33 (P < 10−15) | 1.18 (n.s.) |
| C4 | 3.30 (n.s.) | 356.58 (P < 10−15) | 6.98 (P = 0.00008) | 0.04 (n.s.) | 173.98 (P < 10−15) | 1.86 (n.s.) |
| Cz | 5.02 (n.s.) | 421.24 (P < 10−15) | 6.09 (P = 0.00027) | 0.28 (n.s.) | 326.29 (P < 10−15) | 0.70 (n.s.) |
| F3 | 0.10 (n.s.) | 321.56 (P < 10−15) | 0.92 (n.s.) | 0.03 (n.s.) | 332.10 (P < 10−15) | 0.49 (n.s.) |
| F4 | 0.30 (n.s.) | 561.87 (P < 10−15) | 1.22 (n.s.) | 1.21 (n.s.) | 327.56 (P < 10−15) | 2.63 (n.s.) |
| F7 | 0.05 (n.s.) | 244.68 (P < 10−15) | 0.46 (n.s.) | 8.14 (n.s.) | 402.49 (P < 10−15) | 1.83 (n.s.) |
| F8 | 4.82 (n.s.) | 291.42 (P < 10−15) | 2.21 (n.s.) | 0.16 (n.s.) | 273.53 (P < 10−15) | 0.29 (n.s.) |
| Fp1 | 0.95 (n.s.) | 241.08 (P < 10−15) | 0.39 (n.s.) | 0.12 (n.s.) | 211.02 (P < 10−15) | 0.24 (n.s.) |
| Fp2 | 1.91 (n.s.) | 421.86 (P < 10−15) | 3.07 (n.s.) | 0.41 (n.s.) | 282.78 (P < 10−15) | 1.65 (n.s.) |
| Fz | 0.69 (n.s.) | 569.51 (P < 10−15) | 0.69 (n.s.) | 1.79 (n.s.) | 476.14 (P < 10−15) | 2.62 (n.s.) |
| O1 | 1.64 (n.s.) | 115.91 (P < 10−15) | 0.31 (n.s.) | 0.51 (n.s.) | 96.01 (P < 10−15) | 0.03 (n.s.) |
| O2 | 0.62 (n.s.) | 111.75 (P < 10−15) | 0.10 (n.s.) | 0.78 (n.s.) | 53.36 (P < 10−15) | 3.41 (n.s.) |
| P3 | 0.75 (n.s.) | 281.85 (P < 10−15) | 2.36 (n.s.) | 0.33 (n.s.) | 472.44 (P < 10−15) | 0.45 (n.s.) |
| P4 | 3.78 (n.s.) | 231.15 (P < 10−15) | 4.54 (P = 0.0024) | 2.56 (n.s.) | 628.18 (P < 10−15) | 12.09 (P = 1.6 × 10−08) |
| Pz | 0.07 (n.s.) | 299.66 (P < 10−15) | 0.80 (n.s.) | 0.86 (n.s.) | 531.55 (P < 10−15) | 6.33 (P = 0.00016) |
| T3 | 1.06 (n.s.) | 264.52 (P < 10−15) | 0.13 (n.s.) | 0.21 (n.s.) | 462.87 (P < 10−15) | 0.73 (n.s.) |
| T4 | 0.89 (n.s.) | 151.61 (P < 10−15) | 0.63 (n.s.) | 0.14 (n.s.) | 259.17 (P < 10−15) | 0.90 (n.s.) |
| T5 | 2.31 (n.s.) | 133. 94 (P < 10−15) | 0.84 (n.s.) | 0.07 (n.s.) | 387.80 (P < 10−15) | 0.30 (n.s.) |
| T6 | 1.01 (n.s.) | 86.16 (P < 10−15) | 0.95 (n.s.) | 0.80 (n.s.) | 229.29 (P < 10−15) | 0.13 (n.s.) |
The α‐value after the FDR correction for multiple comparisons was adjusted to a critic P = 0.00244 and P = 0.0002 for REM and NREM sleep respectively.
Figure 3.
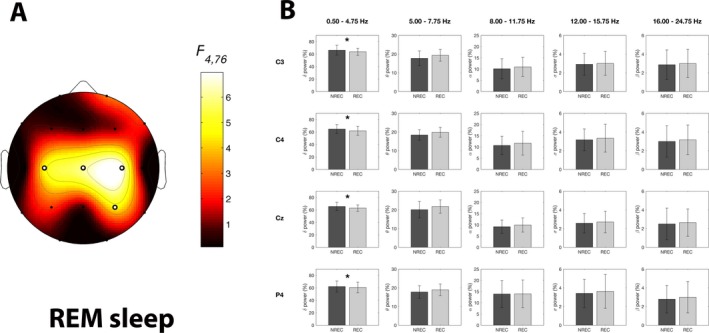
Results of the statistical comparisons on EEG power of REM sleep. Statistical map reporting the F‐values associated to the interaction effects from the two‐way repeated‐measures ANOVAs Recall (NREC vs. REC) ×Frequency Band (Delta vs. Theta vs. Alpha vs. Sigma vs. Beta) on EEG power of REM sleep for each scalp location. Significant interaction effects after the FDR‐correction for multiple comparisons are indicated by white dots (F ≥ 4.54; P ≤ 0.0024). (B) Mean EEG spectral power for the five frequency bands associated to the REM sleep in the no‐recall (NREC, dark gray) and the recall (REC, light gray) conditions, expressed as percentage of the total power spectrum. Data are reported for the electrodes showing a significant interaction effect in the ANOVAs Recall×Frequency Band. Error bars represent the standard errors. Asterisks indicate statistically significant differences between the REC and NREC conditions as revealed by planned comparisons (paired t‐tests with P ≤ 0.05).
Planned comparisons between REC and NREC conditions (Fig. 3B) show that the effect is explained by significantly lower EEG power in the delta band associated to REC relative to NREC condition (C3: t 19 = −2.15, P = 0.044; C4: t 19 = −2.78, P = 0.012; Cz: t 19 = −2.53, P = 0.020; P4: t 19 = −2.38, P = 0.028). Although the REC‐NREC differences in the beta frequency band did not reach statistical significance, the beta activity always was higher in REC condition.
EEG pattern of dream recall from NREM sleep
Figure 4 shows the mean topographic distributions of relative EEG power in the five frequency bands for NREM sleep associated with REC and NREC conditions. The ANOVA results depicted a picture quite similar to the above‐reported REM‐sleep findings (Table 2): a significant and robust main effect for the factor Frequency Band at all scalp locations, no significant effect for the factor Recall, and significant interactions Recall × Frequency Band (Fig. 5A) at two parietal scalp locations (P4: F 4,88 = 12.09, P = 1.6 × 10−8; Pz: F 4,88 = 6.33, P = 0.00016).
Figure 4.
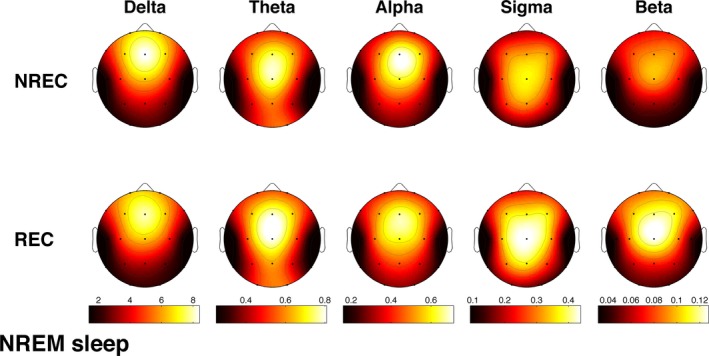
Topographic distribution of NREM sleep EEG powers in the no‐recall (NREC) and the recall (REC) conditions. The EEG activity for the canonical frequency bands (delta: 0.50–4.75 Hz; theta: 5.00–7.75 Hz; alpha: 8.00–11.75 Hz; sigma: 12.00–15.75 Hz; beta: 16.00–24.75 Hz) is reported, expressed as percentage of the total EEG power within the whole topography. The maps are scaled between the minimal and maximal power values for each frequency band considering both the NREC (1st row) and REC (2nd row) conditions.
Figure 5.
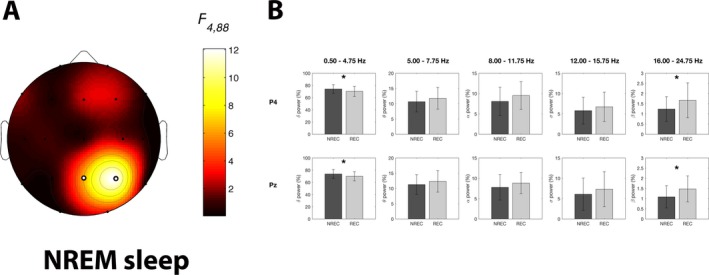
Results of the statistical comparisons on EEG power of NREM sleep. (A) Statistical maps reporting the F‐values associated to the interaction effects from the two‐way repeated‐measures ANOVAs Recall (NREC vs. REC) × Frequency Band (Delta vs. Theta vs. Alpha vs. Sigma vs. Beta) on EEG power of NREM sleep for each scalp location. Significant interaction effects after the FDR‐correction for multiple comparisons are indicated by white dots (F ≥ 6.33; P ≤ 0.0002). (B) Mean EEG power for the five frequency bands during NREM sleep in the no‐recall (NREC, dark gray) and the recall (REC, light gray) conditions, expressed as percentage of the total EEG power. Data are reported for the electrodes showing a significant interaction effect in the ANOVAs Recall × Frequency Band. Error bars represent the standard errors. Asterisks indicate statistically significant differences between the REC and NREC conditions by planned comparisons (paired t‐tests with P ≤ 0.05).
The planned comparisons between REC and NREC conditions for the significant interactions (Fig. 5B) show that the presence of dream recall at awakening from NREM sleep is significantly associated to lower delta EEG power (P4: t 22 = −3.47, P = 0.002; Pz: t 22 = −2.35, P = 0.028), together with significantly higher beta power during sleep (P4: t 22 = 2.63, P = 0.015; Pz: t 22 = 2.63, P = 0.015).
Activation index as a predictor of dream recall
The association of lower delta and higher beta power during NREM (and to a lesser extent also during REM sleep) with successful recall suggested us to evaluate the delta/beta ratio as an integrated EEG index of activation. This index had proved to be reliable to evaluate the arousal level.21, 22
The topographic distribution of the activation index values in REC and NREC conditions for NREM and REM sleep is shown in Figure 6. Comparisons of the activation index in REC and NREC conditions were carried out by paired t‐tests for each scalp location, separately for NREM and REM sleep. Results showed that dream recall after NREM‐sleep naps is associated with a significantly higher level of cortical activation (i.e., lower values of the activation index) compared with NREC condition in 17 of 19 derivations [adjusted α‐value after the FDR correction19, 20: P = 0.0324; C3:t 22 = 2.69, P = 0. 013; C4:t 22 = 2.61, P = 0.016; Cz:t 22 = 2.90, P = 0.008; F3:t 22 = 2.43, P = 0.024; F4:t 22 = 2.28, P = 0.032; F8:t 22 = 2.34, P = 0.029; Fp1:t 22 = 2.39, P = 0.026; Fp2:t 22 = 2.46, P = 0.022; Fz:t 22 = 2.46, P = 0.022; O1:t 22 = 2.47, P = 0.022; O2:t 22 = 2.76, P = 0.011; P3:t 22 = 2.44, P = 0.023; P4:t 22 = 2.95, P = 0.007; Pz:t 22 = 2.83, P = 0.010; T3:t 22 = 2.33, P = 0.029; T5:t 22 = 2.30, P = 0.031; T6:t 22 = 2.34, P = 0.029]. No significant REC versus NREC difference in the activation index during REM sleep was found for any cortical derivation, although the values were almost always lower in REC compared with NREC condition (see Fig. 6).
Figure 6.
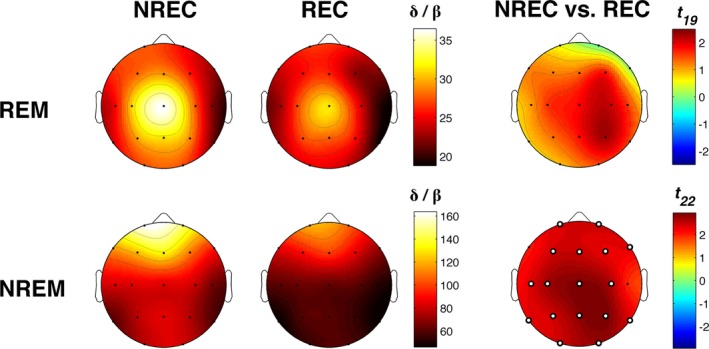
Activation index. Topographic distribution of the activation index, defined as the delta/beta ratio, for REM (1st row) and NREM (2nd row) sleep in the no‐recall (NREC, 1st column) and the recall (REC, 2nd column) conditions. The maps are scaled between the minimal and maximal considering the two experimental conditions, separately for REM and NREM sleep. Statistical maps of the comparisons between no‐recall and recall conditions by paired t‐test are also plotted (3rd column) for REM and NREM sleep. The statistical maps are scaled symmetrically according to the absolute maximal t‐value across the statistical comparisons, separately for REM and NREM sleep. Significant differences after the FDR‐correction for multiple comparisons are found only for NREM sleep and are indicated by white dots (t ≥ 2.28; P ≤ 0.0324).
Discussion
The frequent immediate wake‐REM sleep transition which characterizes NT1 patients allowed us to design a within‐subject study capable to identify EEG correlates of dream recall after MSLT naps containing solely REM or NREM sleep. This design was adequate to disentangle the state‐like differences from the trait‐like ones in SO(REM) sleep23and also to rule out the possible influence of previous NREM sleep stage on EEG pattern associated to dream bypassing the rigid sequence of NREM‐REM sleep stages typical of healthy subjects.
The whole pattern of results clearly indicated that successful dream recall is associated with an increased cortical EEG desynchronization in REM as well as NREM sleep, and involves partially overlapping cortical areas in the two sleep stages. In particular, REC condition is related to (1) a lower delta power over the centro‐parietal regions during REM sleep and over the right parietal area during NREM sleep, (2) a parallel increase in beta power in the same cortical areas during NREM sleep, and (3) lower values in the delta/beta ratio, considered as activation index, during NREM sleep in most scalp locations. This index had proved to be effective to measure the arousal level in patients with primary insomnia, whose improvement from misperception of sleep onset latency was associated with a low delta/beta ratio in sleep‐onset period.21, 22 It seems also worth stressing that REC condition was associated with lower values of delta/beta ratio compared with NREC condition in most scalp locations during NREM sleep (significantly) as well as during REM sleep (albeit without the statistical significance). The whole picture suggests a strong relationship between fast‐frequency EEG activity and effectiveness of memory encoding of dream experience as prerequisite for its successful recall after awakening and prompts to consider in future studies also the relationship of dream recall with frequencies faster than beta. This relationship appears plausible because (1) the power in the higher gamma range is associated with dreaming both during REM and NREM sleep5; (2) changes in gamma coherence are linked to lucid dreams during REM sleep in NT1 patients,24 and (3) transcranial direct current stimulation (tDCS) in the gamma band during REM sleep can induce lucid dreaming in healthy subjects.25
Taken together, our findings allow to draw some theoretical inferences.
First, the EEG correlates of dream recall observed in NT1 patients depend on state‐like factors not involving the alpha and sigma bands, in contrast with findings of studies using a between‐subjects design,6, 26 whose results should be ascribed more plausibly to interindividual differences.27 Moreover, the coherence between our findings in a clinical population and the EEG correlates of dreaming reported by previous within‐subject reports on healthy samples (i.e., decrease in low‐frequency EEG activity in relation to dream recall upon awakening from REM5 and NREM sleep5, 8) further supports this view.
Second, the localization of the greatest differences between REC and NREC conditions over the parietal and centro‐parietal cortical areas in both REM and NREM naps is consistent with the available evidence of a pivotal role of the parietal areas in the production of dream experience during sleep and its subsequent recall. Indeed, studies on both acute brain‐damaged patients28, 29 and healthy subjects5, 30 have consistently shown that the temporo‐parietal junction (TPJ) is essential for dreaming. Specifically, the regional cerebral blood flow (rCBF), as measured by PET, is greater in the TPJ during REM sleep, N3 and wakefulness in healthy subjects with higher frequency of dream recall.30 Moreover, a topographic EEG pattern associated with dream recall that partially overlaps our results [the localization in the posterior areas of high spectral power for rapid frequencies (>20 Hz) and low power in slow frequencies (<4 Hz) related to dream experience] has been observed in healthy subjects.5 Notably, the parietal lobe is also crucial in waking for spatial representation and TPJ is linked to the cortical network responsible for various cognitive processes such as mental imagery and visual memory.31
Third, the lack of significant differences in the comparisons of the PSG measures of sleep fragmentation18 (i.e., WASO, number and duration of arousals and number of major body movements reported in Table 1) when REC versus NREC conditions were compared in both REM and NREM sleep and, overall, the short duration of arousals partly contradict the prediction of the so‐called Arousal‐Retrieval model of dream recall.32, 33 Instead, our findings are consistent with an explanation of the success of dream recall in terms of (higher) cortical activation during stable sleep stages34 rather than of (number/duration of) arousals. This interpretation is also compatible with observations on individuals with specific sleep disorders. In fact, patients with sleep apnea report a decreased rate of dream recall during the treatment with CPAP, when sleep is deeper and less fragmented.35 Insomnia sufferers show a high frequency of dream recall, positively correlated with sleep fragmentation,36, 37 and patients with nightmares exhibit frequent sleep instability.38 Also, the absolute amount of A1 index (i.e. delta bursts during arousal events39) is reduced in nightmare sufferers,38 as shown by the analysis of Cycling Alternating Pattern (CAP).39 In line with this evidence, it has been found a strong decrease in dream recall in sleep‐deprived healthy subjects at the end of a recovery night, namely, a condition characterized by an increased amount of N3.40
However, a possible limitation to generalization of the present findings to other populations is the lack of a control group, given the technical impossibility of choosing an appropriate control group because of the peculiar architecture of sleep of NT1 patients. We believe, however, that a future study on patients with primary hypersomnia could definitely clarify if the association between dream recall and higher cortical activation in NREM sleep is a general trait of hypersomnias of central origin and not peculiar of NT1.
Finally, our results on the EEG correlates of dream recall allow to extend the theoretical implications of the “Activation model” of dream experience.34 This model relies on the assumption that the features of dream experience depend on the periodic and distributed activation during sleep of the cortical structures that support perceptual, cognitive, and motor processes during wakefulness.2, 34 The association between a highly activated electrophysiological milieu and successful dream recall in REM and, overall, NREM sleep in NT1 patients undoubtedly strengthens the general view that REM and NREM sleep share a similar machinery for dream recall.41 Moreover, the association of the level of activation of the parietal cortical areas with dream recall even when the contribution of a preceding sleep period is ruled out confirms that the differences between dream experiences developed during REM and NREM sleep are due to quantitative stage‐related variations in the neurophysiological activity also of these areas.
Author Contributions
Substantial contributions to the conception and design of the work: LDG, CC, GP; Acquisition, analysis of data: ADA, SS, CS, FP, SV; Interpretation of data: LDG, CC, GP, MF, FP; Drafting the work and revising it critically for important intellectual content: ADA, SS, LDG, CC, GP, MF, FP; Final approval of the version to be published: ADA, SS, CS, FP, SV, MF, CC, GP, LDG; Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: ADA, SS, CS, FP, SV, MF, CC, GP, LDG.
Conflict of Interest
The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all subjects after the nature and possible consequences of the studies were explained. The study protocol was approved by the Local Ethical Committee (#17009).
Acknowledgment
This work was partially supported by a BIAL Foundation Grant (#32/16) to S.S.
Funding InformationThis work was partially supported by a BIAL Foundation Grant (#32/16) to S.S.
Funding Statement
This work was funded by BIAL Foundation grant #32/16.
References
- 1. Nielsen TA. Chronobiological features of dream production. Sleep Med Rev 2004;8:403–424. [DOI] [PubMed] [Google Scholar]
- 2. Cipolli C, Ferrara M, De Gennaro L, Plazzi G. Beyond the neuropsychology of dreaming: insights into the neural basis of dreaming with new techniques of sleep recording and analysis. Sleep Med Rev 2017;35:8–20. [DOI] [PubMed] [Google Scholar]
- 3. Miyasita A, Fukuda K, Inugami M. Effects of sleep interruption on REM‐NREM cycle in nocturnal human sleep. Electroencephalogr Clin Neurophysiol 1989;73:107–116. [DOI] [PubMed] [Google Scholar]
- 4. Chellappa SL, Frey S, Knoblauch V, Cajochen C. Cortical activation patterns herald successful dream recall after NREM and REM sleep. Biol Psychol 2011;87:251–256. [DOI] [PubMed] [Google Scholar]
- 5. Siclari F, Baird B, Perogamvros L, et al. The neural correlates of dreaming. Nat Neurosci 2017;20:872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marzano C, Ferrara M, Mauro F, et al. Recalling and forgetting dreams: theta and alpha oscillations during sleep predict subsequent dream recall. J Neurosci 2011;31:6674–6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scarpelli S, Marzano C, D'Atri A, et al. State‐ or trait‐like individual differences in dream recall: preliminary findings from a within‐subjects study of multiple nap REM sleep awakenings. Front Psychol. 2015;6:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scarpelli S, D'Atri A, Mangiaruga A, et al. Predicting dream recall: EEG activation during NREM sleep or shared mechanisms with wakefulness? Brain Topogr 2017;30:629–638. [DOI] [PubMed] [Google Scholar]
- 9. American Academy of Sleep Medicine (AASM) . International classification of sleep disorders (ICSD‐3). 3rd ed. Darien, IL: American Academy of Sleep Medicine, 2014. [Google Scholar]
- 10. Pizza F, Moghadam KK, Vandi S, et al. Daytime continuous polysomnography predicts MSLT results in hypersomnias of central origin. J Sleep Res 2013;22:32–40. [DOI] [PubMed] [Google Scholar]
- 11. Drakatos P, Suri A, Higgins SE, et al. Sleep stage sequence analysis of sleep onset REM periods in the hypersomnias. J Neurol Neurosurg Psychiatry 2013;84:223–227. [DOI] [PubMed] [Google Scholar]
- 12. Vogel GW. Mentation reported from naps of narcoleptics. Adv Sleep Res 1976;3:161–168. [Google Scholar]
- 13. Fosse R. REM mentation in narcoleptics and normals: an empirical test of two neurocognitive theories. Conscious Cogn 2000;9:488–509. [DOI] [PubMed] [Google Scholar]
- 14. Cipolli C, Bellucci C, Mattarozzi K, et al. Story‐like organization of REM‐dreams in patients with narcolepsy–cataplexy. Brain Res Bull 2008;77(4):206–213. [DOI] [PubMed] [Google Scholar]
- 15. Mazzetti M, Bellucci C, Mattarozzi K, et al. REM‐dreams recall in patients with narcolepsy‐cataplexy. Brain Res Bull 2010;81:133–140. [DOI] [PubMed] [Google Scholar]
- 16. Vignatelli L, Plazzi G, Barbato A, et al. Italian version of the Epworth sleepiness scale: external validity. Neurol Sci 2003;23:295–300. [DOI] [PubMed] [Google Scholar]
- 17. Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep 2005;28:113–121. [DOI] [PubMed] [Google Scholar]
- 18. Iber C, Ancoli‐Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associates events: rules. Westchester, IL: Terminology and Technical Specifications, 2007. [Google Scholar]
- 19. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995;57:289–300. [Google Scholar]
- 20. Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat 2001;29:1165–1188. [Google Scholar]
- 21. Krystal AD. Non‐REM sleep EEG spectral analysis in insomnia. Psychiatr Ann 2008;38:615–620. [Google Scholar]
- 22. Maes J, Verbraecken J, Willemen M, et al. Sleep misperception, EEG characteristics and autonomic nervous system activity in primary insomnia: a retrospective study on polysomnographic data. Int J Psychophysiol 2014;91:163–171. [DOI] [PubMed] [Google Scholar]
- 23. Scarpelli S, D'Atri A, Gorgoni M, et al. EEG oscillations during sleep and dream recall: state‐ or trait‐like individual differences? Front Psychol 2015;6:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dodet P, Chavez M, Leu‐Semenescu S, et al. Lucid dreaming in narcolepsy. Sleep 2015;38:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Voss U, Holzmann R, Hobson A, et al. Induction of self awareness in dreams through frontal low current stimulation of gamma activity. Nat Neurosci 2014;17:810–812. [DOI] [PubMed] [Google Scholar]
- 26. Takeuchi T, Ogilvie RD, Murphy TI, Ferrelli AV. EEG activities during elicited sleep onset REM and NREM periods reflect different mechanisms of dream generation. Clin Neurophysiol 2003;114:210–220. [DOI] [PubMed] [Google Scholar]
- 27. De Gennaro L, Ferrara M, Vecchio F, et al. An electroencephalographic fingerprint of human sleep. NeuroImage 2005;26:114–122. [DOI] [PubMed] [Google Scholar]
- 28. Solms M. Dreaming and REM sleep are controlled by different brain mechanisms. Behav Brain Sci 2000;23:843–850. [DOI] [PubMed] [Google Scholar]
- 29. Solms, M . Neurobiology and the neurological basis of dreaming In: Montagna P., Chokroverty S., eds. Handbook of clinical neurology, 98 (3rd series) Sleep Disorders – Part 1. pp. 519–544. New York: Elsevier; 2011. [DOI] [PubMed] [Google Scholar]
- 30. Eichenlaub J‐B, Nicolas A, Daltrozzo J, et al. Resting brain activity varies with dream recall frequency between subjects. Neuropsychopharmacology 2014;39:1594–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Slotnick SD, Thompson WL, Kosslyn SM. Visual memory and visual mental imagery recruit common control and sensory regions of the brain. Cogn Neurosci 2012;3:14–20. [DOI] [PubMed] [Google Scholar]
- 32. Koulack D, Goodenough DR. Dream recall and dream recall failure: an arousal‐retrieval model. Psychol Bull 1976;83:975–984. [Google Scholar]
- 33. Vallat R, Lajnef T, Eichenlaub J‐B, et al. Increased evoked potentials to arousing auditory stimuli during sleep: implication for the understanding of dream recall. Front Hum Neurosci 2017;11:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Antrobus J. Dreaming: Cognitive processes during cortical activation and high afferent thresholds. Psychol Rev 1991;98:96–121. [DOI] [PubMed] [Google Scholar]
- 35. Carrasco E, Santamaria J, Iranzo A, et al. Changes in dreaming induced by CPAP in severe obstructive sleep apnea syndrome patients. J Sleep Res 2006;15:430–436. [DOI] [PubMed] [Google Scholar]
- 36. Charney DS, Soldatos CR, Bixler EO, Kales A. Factors contributing to dream recall in insomniac subjects. Sleep Res 1977;6:126. [Google Scholar]
- 37. Schredl M, Schäfer G, Weber B, Heuser I. Dreaming and insomnia: dream recall and dream content of patients with insomnia. J Sleep Res 1998;7:191–198. [DOI] [PubMed] [Google Scholar]
- 38. Simor P, Bódizs R, Horváth K, Ferri R. Disturbed dreaming and the instability of sleep: altered nonrapid eye movement sleep microstructure in individuals with frequent nightmares as revealed by the cyclic alternating pattern. Sleep 2013;36:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parrino L, Ferri R, Bruni O, Terzano MG. Cyclic alternating pattern (CAP): the marker of sleep instability. Sleep Med Rev 2012;16:27–45. [DOI] [PubMed] [Google Scholar]
- 40. De Gennaro L, Marzano C, Moroni F, et al. Recovery sleep after sleep deprivation almost completely abolishes dream recall. Behav Brain Res 2010;206:293–298. [DOI] [PubMed] [Google Scholar]
- 41. Fagioli I. Mental activity during sleep. Sleep Med Rev 2002;6:307–320. [DOI] [PubMed] [Google Scholar]


