Abstract
Objective
We present an exploratory study for identification of sex differences in imaging biomarkers that could further refine selection of patients for acute reperfusion therapy and trials based on sex and imaging targets.
Methods
The Lesion Evolution in Stroke and Ischemia On Neuroimaging (LESION) study included consecutive acute stroke patients who underwent MRI within 24 h of time from last known well and prior to therapy. Those demonstrating a potential therapeutic target on imaging were identified by presence of: (1) arterial occlusion on angiography, (2) focal ischemic region on perfusion maps, or (3) a mismatch of perfusion versus diffusion imaging lesion size. The prevalence of imaging targets within clinically relevant time intervals was calculated for each patient and examined. The relationship of time from stroke onset to probability of detection of imaging targets was evaluated.
Results
Of 7007 patients screened, of which 86.7% were scanned with MRI, 1092 patients (477/615 men/women) were included in LESION. The probability of imaging target detection was significantly different between men and women, with women more likely to present with all assessed imaging targets, odds ratios between 1.36 and 1.59, P < 0.02, adjusted for NIHSS, age, and time from last known well to MRI scan. This trend held for the entire 24‐h studied.
Interpretation
Women present more often with treatable ischemic stroke than men. The greater probability of potentially viable and/or treatable imaging targets in women at all time points suggests that tissue injury is slower to evolve in women.
Introduction
Although the overall decline in stroke incidence and stroke mortality are encouraging, these gains are driven by the decreasing incidence in men but not women.1 As of 2014, stroke is the fifth leading cause of death in the United States2 but the fourth one in women.3 While the incidence and prevalence of stroke is higher in men than in women,4 the overall lifetime risk of stroke is higher for women.5 Women have worse outcome from stroke:6, 7, 8 the 1‐month case fatality for women is 24.7% compared with 19.7% for men.4 Women also have greater disability from stroke.6 Improvements in stroke incidence and mortality have not been realized for women;2 the reasons for these disparities are unclear.
Cerebrovascular factors known to predict stroke outcomes and functional independence are measurable by advanced imaging methods. The ischemic cerebrovascular bed typically exhibits two zones of injury: the ischemic core and the ischemic penumbra. The ischemic core zone has decreased blood flow (10–25% of normal) and rapid depletion of energy stores with possible necrosis. The ischemic penumbra includes the rim of tissue in which the infarction is evolving between the ischemic core and normally perfused tissue. This ischemic but still viable penumbral tissue may remain viable for a few hours due to blood supply from the collateral arteries.9, 10, 11 The overall goal of this study was to identify if there are differences by sex in the imaging‐based biomarkers described above in acute ischemic stroke patients. We hypothesized that imaging‐based evidence of core and penumbral tissue could explain clinical observations of sex differences in ischemic stroke. This identification of differences in imaging targets represents a step toward further refining the imaging selection of patients for acute reperfusion therapy and trials as well as stratification of trials based on patient sex.
Methods
Patient selection
The Lesion Evolution in Stroke and Ischemia On Neuroimaging (LESION) Study includes data from consecutive patients with suspected stroke and baseline MRI scan screened by the NIH Stroke team at Suburban Hospital in Bethesda, Maryland between August 1999 and October 2009 and at MedStar Washington Hospital Center in Washington, DC between September 2004 and October 2009 with the purpose to characterize the population of potentially treatable stroke patients. (Fig. 1) The LESION population had a baseline admit National Institutes of Health Stroke Scale (NIHSS) score ≥4, an evaluable baseline MRI scan done within 24 h since time last known well (TLKW) and obtained before any acute intervention. Treatment‐based analysis is not presented herein. Patients with unknown TLKW to scan initiation (onset time) were excluded. For patients with multiple admissions during the study period, only the first qualifying admission was included. Ethics approval was obtained from the local institutional review boards to analyze images and clinical data fields in our clinical database for the purposes of this research.
Figure 1.

LESION study inclusion. Consecutive patients were screened for acute ischemic stroke with the eligibility criteria of a baseline MRI performed within 24 h from last known well, an NIHSS greater than or equal to 4, no treatment prior to the MRI scan, and first eligible admission.
MRI protocol
MR imaging was performed using either 1.5 T (Twinspeed, General Electric) or 3.0 T (Achieva, Philips) clinical scanners, depending on hospital site. Typical sequences have been described.12, 13 The diffusion‐weighted imaging (DWI) was a spin‐echo planar series. The perfusion‐weighted imaging (PWI) was a dynamic gradient‐echo series with single‐dose gadolinium contrast injection of 0.1 mmol/kg of gadolinium (gadolinium‐DTPA; Magnevist; Bayer Schering Pharma) through a power injector using 25–40 phase measurements. The mean transit time (MTT) maps were calculated as the first moment of the time concentration curves divided by the zeroth moment with no arterial input correction or deconvolution. Only MTT maps were used for the perfusion assessments. The DWI, FLAIR, and PWI series were acquired co‐localized over the entire brain with a superior to inferior coverage of 14 cm. The magnetic resonance angiography (MRA) was an intracranial 3D time‐of‐flight (TOF) centered in the region of the Circle of Willis. The detailed imaging protocol parameters are found in Table S1.
Image analysis
The images used for this analysis were assessed by a team of trained readers consisting of vascular neurologists and experienced imaging scientists. Readers identified the acute ischemic lesion on DWI, the corresponding perfusion abnormality on PWI, and the affected vessel on MRA and classified any DWI/PWI mismatch as obvious mismatch (PWI > DWI), no mismatch (PWI~DWI) or reverse mismatch (DWI > PWI). Before interpreting the LESION images, readers were trained on the mismatch classification with a series of training images and had to achieve >80% agreement with the “gold standard” for each assessment. The “gold standard” was a consensus read by the entire team when identifying the imaging targets: presence of the acute ischemic DWI lesion, presence of the corresponding PWI lesion (perfusion abnormality), mismatch classification, and the corresponding affected vessel on MRA. Qualitative analysis of acute stroke imaging as described is comparable to quantitative evaluation.12
For LESION, each image series was assigned randomly and analyzed by a single trained reader, who was blinded to any clinical data. For each assigned image, the reader determined whether the scan was evaluable and completed the baseline imaging case report form including the assessments described above. From these data, we identified if each patient had a potential imaging target at baseline: (1) a focal region of delayed perfusion on MTT maps (PERFUSION), (2) an obvious PWI‐DWI mismatch (MISMATCH), or (3) an arterial occlusion on MRA (ARTERY). If an arterial occlusion was identified, it was further characterized as MCA occlusion (M1 or M2 branches) or as an endovascular therapy target (ENDOVASCULAR) for ischemia in M1 or internal carotid artery.
Statistical analysis
Patient characteristics (demographics, medical history, presentation, chronic biomarkers, and acute therapeutic targets on imaging) were tested for difference by sex. Differences were assessed by measuring absolute risk differences, odds ratios (ORs), and Chi‐squared tests or Wilcoxon ranked sum tests, as appropriate.
The prevalence of imaging targets within clinically relevant time intervals was calculated for each patient. Univariate regression tests were used to estimate the magnitude and variations of associations among sex, age, TLKW, and admit NIHSS. Relationships between time and therapeutic target were also stratified by arterial region, when appropriate, to look for effect measure modification.
Therapeutic targets were modeled separately using logistic regression across the three steps: Model 1: univariate model of sex and target, Model 2: an a priori multivariate model (sex, age, NIHSS, and onset), and Model 3: a stepwise inclusion process of all potential covariates. A full model was first tested including onset time, age, sex, race, admit NIHSS, Body Mass Index (BMI), MCA target, and chronic lesion biomarkers on imaging.
The a priori multivariate Model 2, which included age, NIHSS, and onset time, identified the presence of any arterial target (ARTERY) and a middle cerebral artery (MCA) occlusion as the imaging biomarkers that differed most between men and women.
The stepwise model (Model 3) of each target tested the potential covariates identified for possible influence on the measures of association between imaging targets and sex. Inclusion of each potential covariate was assessed for influence on the relationship between sex and imaging target using a 10% threshold for change in the OR. All confounders identified, if any, were then included in the model to calculate the adjusted OR for sex.
To characterize those patients presenting outside of the therapeutic time windows for thrombolytic therapy (4.5 h) and endovascular therapy (24 h), we examined the probability of detection of therapeutic targets on imaging as they related to onset for both men and women. Additional eligibility criteria were considered and adjustments were performed for age and admit NIHSS.
Results
Patient demographics
For this analysis, 1092 patients met the inclusion criteria (Fig. 1); 477 were men and 615 were women. Anterior and posterior ischemic strokes were included. Patient characteristics including demographics, medical history, presentation, and acute therapeutic targets on imaging by sex are presented (Table 1). Women were older at the time of symptom commencement (P < 0.001) and more often had atrial fibrillation (P = 0.002), an infection within 1 week of stroke (P = 0.041), more severe stroke as indicated by a higher admit NIHSS (nine for men and 10 for women, P = 0.016), lower partial thromboplastin time (PTT) (P = 0.028), and a higher platelet count (P < 0.001). Risk factors more prevalent in men were coronary artery disease (32.1%/22.3%, men/women), history of alcohol use (22.9%/12/7%, men/women), current/former smoker (17.1%/11.2%, men/women), and higher BMI (26.8/26/0, men/women, P = 0.025).
Table 1.
LESION study results for sex differences of risk factors, presentation characteristics, and pathological targets on acute imaging
| Men n = 477 (% or IQR) | Women n = 615 (% or IQR) | Odds ratio (confidence intervals) | P‐value | |
|---|---|---|---|---|
| Risk factors | ||||
| Age (median, IQR) | 70 (60–79) | 78 (67–85) | <0.001* | |
| Caucasian (%) | 265 (56.3) | 362 (59.5) | 0.279 | |
| African American/Black (%) | 184 (39.1) | 224 (36.8) | 0.89 (0.693–1.146) | 0.455 |
| Other (%) | 22 (4.7) | 22 (3.6) | 0.73 (0.397–1.351) | 0.386 |
| Hispanic ethnicity (%) | 12 (2.5) | 30 (4.9) | 1.98 (1.002–3.919) | 0.045* |
| Hypertension (%) | 365 (78.2) | 493 (81.4) | 1.22 (0.903–1.646) | 0.195 |
| Diabetes mellitus (%) | 155 (33.2) | 177 (29.2) | 0.83 (0.640–1.078) | 0.162 |
| Coronary artery disease (%) | 150 (32.1) | 135 (22.3) | 0.61 (0.460–0.797) | <0.001* |
| Atrial fibrillation (%) | 118 (25.3) | 205 (33.8) | 1.51 (1.155–1.979) | 0.002* |
| Stroke/TIA, previous | 126 (27.0) | 177 (29.2) | 1.12 (0.853–1.462) | 0.422 |
| Cancer | 82 (17.6) | 93 (15.4) | 0.85 (0.615–1.178) | 0.331 |
| Infection within 1 week | 68 (14.6) | 117 (19.3) | 1.40 (1.012–1.948) | 0.041* |
| Alcohol, history of any | 109 (22.9) | 78 (12.7) | 0.49 (0.355–0.677) | <0.001* |
| Alcohol, heavy use history | 9 (1.9) | 3 (0.5) | 0.26 (0.068–0.950) | 0.028* |
| Smoker, current | 46 (9.9) | 44 (7.3) | 0.72 (0.465–1.105) | 0.129 |
| Smoker, current/former | 80 (17.1) | 68 (11.2) | 0.61 (0.431–0.868) | 0.005* |
| Presentation | ||||
| NIHSS at admission (n = 1092) | 9 (5–16) | 10 (6–18) | 0.016* | |
| Alcohol, at presentation | 10 (6.7) | 7 (4.1) | 0.59 (0.219–1.605) | 0.298 |
| Illicit drugs (included only sympathomimetic) | 21 (4.5) | 7 (1.2) | 0.25 (0.104–0.592) | 0.001* |
| Body mass index (BMI) | 26.8 (24.5–30.1) | 26.0 (22.3–29.8) | 0.025* | |
| Blood glucose (n = 871) | 121 (104–152) | 123.5 (103–160) | 0.579 | |
| Prothrombin time (PT) (n = 736) | 12.4 (10.8–14.1) | 12.0 (10.8–13.9) | 0.183 | |
| Partial thromboplastin time (PTT) (n = 679) | 30.2 (27.0–34.4) | 29.3 (26.3–33.1) | 0.028* | |
| Platelets (n = 777) | 226 (182–276) | 246 (201–293) | <0.001* | |
| International normalized ratio (INR) | 1.10 (1.00–1.16) | 1.03 (0.96–1.12) | 0.103 | |
| Mean arterial pressure (MAP) | 107.3 (95–121) | 106.7 (92–121) | 0.523 | |
| Therapeutic targets on imaging | ||||
| Any arterial target (MRA; n = 1011) | 210 (47.5) | 336 (59.1) | 1.59 (1.238–2.050) | <0.001* |
| Middle cerebral artery (MCA, M1/M2) occlusion (n = 1011) | 129 (29.2) | 212 (37.3) | 1.44 (1.103–1.883) | 0.007* |
| Endovascular target (ICA/M1, n = 1011) | 92 (20.8) | 157 (27.6) | 1.45 (1.079–1.948) | 0.013* |
| Perfusion weighted imaging (PWI) target (n = 882) | 285 (72.2) | 385 (79.1) | 1.46 (1.076–1.988) | 0.017* |
| PWI‐diffusion weighted imaging (DWI) mismatch (n = 881) | 177 (44.8) | 256 (52.7) | 1.37 (1.049–1.791) | 0.020* |
Significant differences for a variety of variables indicated by an *.
Imaging results
Of the 1092 patients that met inclusion criteria, 1011 had an evaluable MRA, 882 had an evaluable PWI series, 1088 had an evaluable DWI series, and 881 had an evaluable mismatch (patients with both DWI and PWI imaging without severe artifacts). Lesions in the PWI series were found in 670/882 (76.0%) patients while lesions in the DWI series were found in 1022/1088 (93.9%) patients. An obvious PWI‐DWI mismatch (MISMATCH) demonstrated by 433/881 (49.1%) patients. An occlusion on MRA was observed in 546/1011 (54.0%) patients with 339/1011 (33.7%) being in the MCA (either M1 or M2).
Imaging target presence
Women were more likely to present with all five imaging targets including: the presence of any arterial target (ARTERY), focal region of delayed perfusion on MTT maps (PERFUSION), an obvious PWI‐DWI mismatch (MISMATCH), an occlusion in the M1 or M2 (MCA), or occlusion in M1 or internal carotid artery (ENDOVASCULAR), (Table 1 and Fig. 2). Table 2 presents the results of these imaging targets modeled separately using logistic regression across the three steps previously outlined in the Methods. Model 1: univariate model of sex and target, Model 2: an a priori multivariate model (sex, age, NIHSS, and onset time), and Model 3: a stepwise inclusion process of all potential covariates for possible adjustment. The a priori multivariate Model 2, which included age, NIHSS, and onset time, identified the presence of any arterial target and a middle cerebral artery occlusion as the imaging biomarkers that differed most between men and women. The stepwise Model 3 of each target tested the covariates identified for influence on the measures of association between imaging targets and sex. Inclusion of each potential covariate was determined by influence on the relationship between sex and imaging target using a 10% threshold change in the OR. All covariates identified, if any, were then included in the model and the adjusted OR for sex was reported (right column, Table 2). No confounders were identified for ARTERY, ENDOVASCULAR, and MISMATCH. The platelet count was identified as a confounder for the MCA imaging target resulting in a reduced effect size of 1.30 (95% CI: 0.92–1.82). Platelets are important mediators of atherothrombosis and play a major role in ischemic stroke, with known platelet activation in the ischemic zone of MCA occlusion patients14 and demonstrated increase in serum platelets in all ischemic stroke patients.15 The PTT and BMI were identified for the PERFUSION imaging target resulting in an effect size of 1.87 (95% CI: 1.01–3.45).
Figure 2.
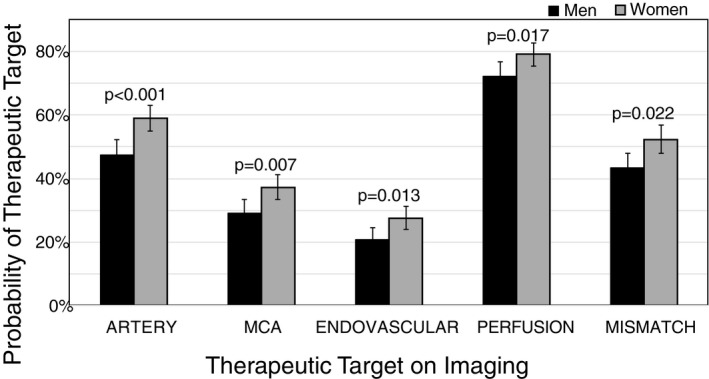
Probability of detecting therapeutic targets on imaging for men (black) and women (gray). ARTERY: Any arterial target, MCA: Occlusion in M1 or M2, ENDOVASCULAR: Occlusion in M1 or ICA, PERFUSION: lesion detected on perfusion, MISMATCH: perfusion‐diffusion mismatch, error bars indicate 95% confidence intervals.
Table 2.
LESION results of imaging targets were modeled separately using logistic regression across 3 steps: univariate model of sex and target, an a priori multivariate model including sex, age, NIHSS, and onset time (Model 2), then a stepwise inclusion process of all potential confounders for possible adjustment
| Model 1: univariate differences | Model 2 (sex, age, NIHSS, onset time) | Confounders identified by 10% change in effect from significant P‐values in Table 1 | Model 3: Stepwise adjusted for additional confounders | ||||
|---|---|---|---|---|---|---|---|
| Effect size of sex difference (OR) | P‐value | Effect size of sex difference (OR) | P‐value | Effect size of sex difference (OR) | P‐value | ||
| Any arterial target | 1.59 | <0.001 | 1.60 | 0.001 | None | ||
| MCA occlusion (M1/M2) | 1.44 | 0.007 | 1.51 | 0.005 | Platelets | 1.30 | 0.132 |
| Endovascular target (ICA/M1) | 1.45 | 0.013 | 1.32 | 0.088 | None | ||
| Perfusion target | 1.46 | 0.017 | 1.42 | 0.040 | PTT, BMI | 1.87 | 0.045 |
| Perfusion‐diffusion mismatch | 1.36 | 0.022 | 1.36 | 0.034 | None | ||
All imaging targets were predicted by women with platelets, PTT and BMI being confounding factors for MCA and perfusion targets, respectively. No mediation or interactions were found.
Therapeutic target timing
Figure 3 plots the probability of detecting imaging targets as a function of TLKW and sex. Women (gray) consistently had a higher probability when compared to men (black) for all therapeutic targets examined and this trend held for the extent of the 24‐h time to MRI start time evaluated. The models in Figure 3 were adjusted for age and admit NIHSS. The increased probability of PERFUSION and MISMATCH demonstrates that tissue injury is slower to evolve in women for the duration of the 24‐h period studied.
Figure 3.
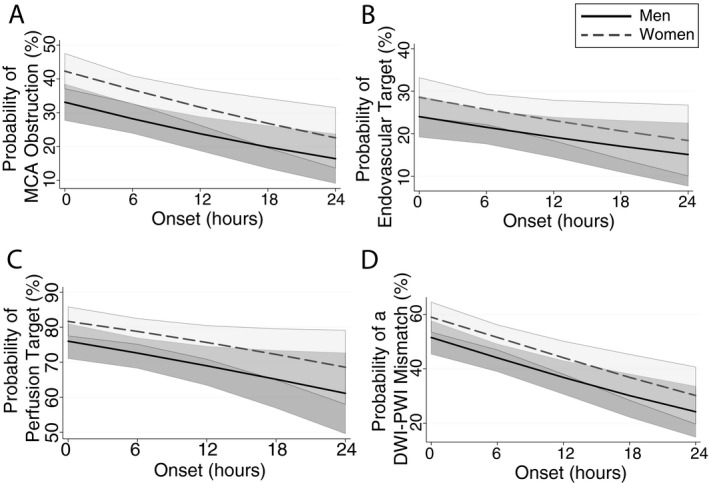
Probability of detecting a therapeutic target based on time from last known well to imaging time (onset); data fit shown with 95% confidence intervals. Women (gray‐dashed) had consistently higher probability of detecting a target than men (black‐solid), including (A) M1 or M2 occlusion, (B) endovascular target (M1 or ICA), (C) hypoperfusion lesion, and (D) perfusion‐diffusion mismatch.
Imaging target presence in clinically relevant time intervals
Importantly, imaging targets were found in a substantial proportion of patients beyond the proven time windows for thrombolytic (4.5 h) or previous endovascular therapy (6 h). Table 3 presents the number and probability (ORs) of patients presenting outside of the therapeutic time windows for thrombolytic therapy (4.5 h) and previous endovascular therapy (6 h). The probability of detection of therapeutic targets on imaging as they related to TLKW (onset time) for both men and women with adjustment for age and admit NIHSS are shown. Although not significant, a higher percentage of women presented with imaging targets of ischemia (72.2% men, 79.1% women), occlusion on MRA (47.5% men, 59.1% women), a tPA‐eligible (presenting within 4.5 h of symptom commencement) occlusion in the MCA (29.2% men, 37.3% women), an endovascular therapy eligible occlusion in the intracranial ICA or M1 branch of the MCA (20.8% men, 27.6% women), and MISMATCH (44.8% men, 52.7% women) outside of the 4.5‐h time window with ORs ranging from 1.11 to 1.50. Similarly, 88.2% of women and 75.0% of men meeting endovascular therapy criteria of admit NIHSS ≥6 presented with PERFUSION outside of the 6‐h time window.
Table 3.
Examination of sex differences in imaging target in the context of former treatment time windows and NIHSS criteria
| Selection scenario | Within window | OR P‐values | Beyond window | OR P‐values | n | ||||
|---|---|---|---|---|---|---|---|---|---|
| Men (% targets) | Women (% targets) | Sex OR (W/M) | Men (% targets) | Women (% targets) | Sex OR (W/M) | ||||
| iv TPA (0–4.5 h window) | |||||||||
| Perfusion target | 150/195 (76.9%) | 223/267 (83.5%) | 1.52 | 0.08 | 135/200 (67.5%) | 162/220 (73.6%) | 1.34 | 0.168 | 882 |
| Any arterial target | 115/228 (50.4%) | 195/310 (62.9%) | 1.67 | 0.00 | 95/214 (44.4%) | 141/259 (54.4%) | 1.50 | 0.03 | 1,011 |
| TPA OCCLUSION | 76/228 (33.3%) | 132/310 (42.6%) | 1.48 | 0.03 | 135/200 (67.5%) | 162/220 (73.6%) | 1.34 | 0.168 | 1,011 |
| EVT OCCLUSION | 51/228 (22.4%) | 103/310 (33.2%) | 1.73 | 0.01 | 41/214 (19.2%) | 54/259 (20.8%) | 1.11 | 0.648 | 1,011 |
| Perfusion‐diffusion mismatch | 101/195 (51.8%) | 160/267 (59.9%) | 1.39 | 0.08 | 76/200 (38.0%) | 96/219 (43.8%) | 1.27 | 0.226 | 881 |
| Guideline EVT (0–6 h window); limited to ICA and M1 occlusions & NIHSS ≥6 | |||||||||
| Perfusion target | 42/42 (100%) | 86/92 (93.5%) | 0.00 | 0.09 | 21/28 (75.0%) | 30/34 (88.2%) | 2.50 | 0.178 | 196 |
| Perfusion‐diffusion mismatch | 36/42 (85.7%) | 73/92 (79.4%) | 0.64 | 0.38 | 19/28 (67.9%) | 23/34 (67.7%) | 0.99 | 0.986 | 196 |
| Extended window EVT (6–24 h window); limited to ICA and M1 occlusions & NIHSS ≥10 | |||||||||
| Perfusion‐diffusion mismatch | 32/38 (84.2%) | 59/72 (81.9%) | 0.85 | 0.77 | 16/20 (80.0%) | 18/26 (69.2%) | 0.56 | 0.415 | 156 |
Discussion
Cerebrovascular factors known to predict stroke outcomes and functional independence are measurable by advanced clinical imaging methods and data presented herein suggest that differences in these imaging biomarkers may explain observed sex disparities. We have used neuroimaging to identify potential pathological therapeutic targets reflective of clinical observations of differences between sexes. The presented results indicate increased occurrence of large vessel occlusions in women, supporting recent consecutive cohort studies,18, 19, 20 though the literature is inconsistent.21, 22 This imaging‐based evidence of sex disparities is particularly apparent in the presence of a therapeutic target as a function of time, with women maintaining imaging targets longer than men. These results, though not yet applicable on an individual level, provide rationale for further study of imaging presentation and treatment window for women stroke patients.
Potentially confounding effects were found for biological variables for the imaging markers of MCA occlusion and PWI target (Table 2). Platelets are important mediators of atherothrombosis and play a major role in ischemic stroke, with known platelet activation in the ischemic zone of MCA occlusion patients14 and demonstrated increase in serum platelets in all ischemic stroke patients.15 The confounders for perfusion deficit were PTT and BMI, both being higher in men. The PTT is essentially how long it takes for blood to clot so an association with ischemia is understandable. In fact, studies have demonstrated that PTT is an independent risk factor for ischemic stroke, stroke severity, and neurological worsening after acute stroke.16 Additionally, lower PTT values have been seen in women17 corroborating a relatively higher procoagulant imbalance consequent to increased levels of coagulation factors compared to men. Higher BMI was also found in men and to be a confounder in the Model 3 for PWI target. Data on the overall association of obesity and stroke as well as stroke subtypes are limited and inconclusive. It is hypothesized that an increase in prothrombic factors observed among overweight and obese individuals may contribute to their increased risk for ischemic events. Inclusion of these variables in the model may cause overestimation of the effect of sex and further prospective studies are necessary to elucidate the significant confounding variables for sex‐based differences in imaging target presentation.
Women have more severe and worse clinical deficits at presentation from ischemic stroke than men, although evidence is contradictory once accounting for age and stroke subtype.23 Sex differences in response to stroke reperfusion therapies, thrombolysis and thrombectomy, have been suggested, but the literature is inconsistent and interactions with pretreatment severity limit generalizations.24, 25, 26, 27, 28, 29 Although severity of deficits and disability after stroke is often worse for women, women are more likely to survive.30 There are many potential confounding factors and it is clear that more research is necessary to shed light on this complex relationship. Surprisingly little is known about sex differences in cerebrovascular and hemodynamic factors as potential determinants of sex differences in severity and outcome.
Given differences in cerebrovascular physiology and stroke mechanism between women and men,23, 31 sex‐related differences in response to r‐tPA are plausible and have been demonstrated through an analysis of five clinical trials of acute stroke,24 although updated meta‐analysis was contradictory,25 as are other studies reporting sex‐related differences in response to IV rt‐PA.24, 27, 28, 29, 32, 33, 34, 35, 36, 37 The few studies of sex‐related differences in response to endovascular therapy are also conflicting.38, 39, 40, 41 HERMES found no interaction between sex and treatment. Of note, although the incidence of first stroke in the US is higher in women; in the HERMES database, only 47% of the subjects were women.42 DAWN enrolled a higher proportion of women to men, and showed a higher OR of good outcome in women (albeit, not significantly different).43 No studies to date have definitively determined whether there are sex‐related differences in treatment response for the newer thrombectomy trials. Prior studies have neglected sex differences in stroke severity and treatment outcome in respect to therapeutic window while our study suggests that patient sex influences stroke biology at presentation and should be considered, particularly with regards to acute stroke therapy.
The limitations of this study are primarily due to the qualitative nature of imaging target definitions, though any limitations due to qualitative definition of targets will be present in both sexes. This precludes analysis of the size of patients’ specific mismatch volume and how it relates to sex and other risk factors. Since the upper and lower limits for recanalization are currently unknown for both the volume of ischemic core, mismatch thresholds are not a good indication of treatment potential.44, 45 Furthermore, the use of qualitative perfusion evaluation may lead artefactual delays resulting in overestimation of ischemic volumes hence the MTT was chosen since it is relatively insensitive to time issues. In order to extend the applicability of this data, quantitative analyses applying commonly used mismatch criteria are underway.
The LESION cohort was created to model that of a clinical trial while being a representative sample of all strokes presenting to the hospitals. Those with minor stroke (NIHSS ≤3) were excluded. This could lead to a bias in conclusions though studies have found that women presenting with an NIHSS ≤3 have worse outcomes compared to men,46 though infarct volume does not correlate with NIHSS at these low values (NIHSS 0–5).47 Finally, known factors that can impact cerebrovascular hemodynamics and structure, such as hormone exposure and parity, were not collected as part of this study. Finally, a proportion of arterial occlusions may be underestimated due to MRA's ability to detect branch occlusions. Therefor we are unable to comment on sex differences in branch occlusions as detected by MRA.
Conclusion
Imaging biomarkers that are known to be prognostic for stroke outcome revealed higher prevalence in women for all imaging targets and all times. Consideration of risk factors affecting stroke severity and outcome measures allowed further examination of sex differences in stroke presentation and evolution. The increased prevalence of imaging targets in women persisted for the full 24‐h period included in the study indicating the presence of potentially viable and/or treatable tissue exists well beyond the traditional therapeutic windows for thrombolytic and endovascular therapies, particularly for women.
Author Contribution
ML, LD, JM, AH, LL, and SW designed the study with input from the other authors and were responsible for the conduct of the study. AD, ML, BK, SS, AM, LD, GG, JM, AH, LL, and SW collected study data and gave input on analysis. AD, BK, ML, and SW analyzed the data, and all authors were involved in data interpretation.
Conflicts of Interest
Dula reports has nothing to disclose; Luby has nothing to disclose; King has nothing to disclose; Sheth has nothing to disclose; Magadan has nothing to disclose; Davis has nothing to disclose; Gealogo has nothing to disclose; Merino has nothing to disclose; Hsia has nothing to disclose; Merino has nothing to disclose; and Latour has nothing to disclose. Warach reports personal fees from Merck Sharp & Dohme Corporation, personal fees from Genentech, grants from Boehringer Ingelheim, grants from Valtari Bio, personal fees from AbbVie, outside the submitted work; In addition, Warach has a patent Biomarkers for acute ischemic stroke; US Patent number: 9200322 issued.
Supporting information
Table S1. MRI imaging protocol for baseline scan.
Acknowledgment
The LESION database was created and funded by the Basic Neuroscience Program of the Intramural Research Program of the National Institute of Neurological Disorders and Stroke. This analysis and publication were also made possible by funding made available by the Texas Legislature to the Lone Star Stroke Clinical Trial Network. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Government of the United States or the State of Texas.
Funding Information
The LESION database was created and funded by the Basic Neuroscience Program of the Intramural Research Program of the National Institute of Neurological Disorders and Stroke. This analysis and publication were also made possible by funding made available by the Texas Legislature to the Lone Star Stroke Clinical Trial Network.
Funding Statement
This work was funded by Basic Neuroscience Program of the Intramural Research Program of the National Institute of Neurological Disorders and Stroke grant ; Lone Star Stroke Clinical Trial Network grant .
References
- 1. Madsen TE, Khoury JC, Alwell KA, et al. Stroke incidence in the greater cincinnati/northern kentucky stroke study is decreasing significantly in men but not women. 2016.
- 2. Kochanek KD, Murphy SL, Xu J, Tejada‐Vera B. Deaths: final data for 2014. Natl Vital Stat Rep 2016;65:1–122. [PubMed] [Google Scholar]
- 3. National Center for Health Statistics. Health, United States , 2017: Age‐adjusted death rates for selected causes of death, by sex, race, and Hispanic origin: United States, selected years 1950‐2013 Hyattsville, Maryland. 2018.
- 4. Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke 2009;40:1082–1090. [DOI] [PubMed] [Google Scholar]
- 5. Carandang R, Seshadri S, Beiser A, et al. Trends in incidence, lifetime risk, severity, and 30‐day mortality of stroke over the past 50 years. JAMA 2006;296:2939–2946. [DOI] [PubMed] [Google Scholar]
- 6. Roquer J, Campello AR, Gomis M. Sex differences in first‐ever acute stroke. Stroke 2003;34:1581–1585. [DOI] [PubMed] [Google Scholar]
- 7. Olivotto I, Maron MS, Adabag AS, et al. Gender‐related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J Am Coll Cardiol 2005;46:480–487. [DOI] [PubMed] [Google Scholar]
- 8. Andersen MN, Andersen KK, Kammersgaard LP, Olsen TS. Sex differences in stroke survival: 10‐year follow‐up of the Copenhagen stroke study cohort. J Stroke Cerebrovasc Dis 2005;14:215–220. [DOI] [PubMed] [Google Scholar]
- 9. Saver JL. Time is brain–quantified. Stroke 2006;37:263–266. [DOI] [PubMed] [Google Scholar]
- 10. Saver JL, Levine SR. Alteplase for ischaemic stroke–much sooner is much better. Lancet 2010;375:1667–1668. [DOI] [PubMed] [Google Scholar]
- 11. Liebeskind DS. Collateral circulation. Stroke 2003;34:2279–2284. [DOI] [PubMed] [Google Scholar]
- 12. Luby M, Ku KD, Latour LL, et al. Visual perfusion‐diffusion mismatch is equivalent to quantitative mismatch. Stroke 2011;42:1010–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shah S, Luby M, Poole K, et al. Screening with MRI for accurate and rapid stroke treatment: SMART. Neurology 2015;84:2438–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. del Zoppo GJ, Schmid‐Schonbein GW, Mori E, et al. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke; a journal of cerebral circulation. 1991;22:1276–1283. [DOI] [PubMed] [Google Scholar]
- 15. Tamer D, Fevzi Y, Deniz AE, et al. The value of serum mean platelet volume in ischaemic stroke patient. J Pak Med Assoc 2013;63:1509–1510. [PubMed] [Google Scholar]
- 16. Lin CH, Kuo YW, Kuo CY, et al. Shortened activated partial thromboplastin time is associated with acute ischemic stroke, stroke severity, and neurological worsening. J Stroke Cerebrovasc Dis 2015;24:2270–2276. [DOI] [PubMed] [Google Scholar]
- 17. Fourel V, Gabastou JM, Desroys du Roure F, et al. Influence of age, sex and ABO blood group on activated partial thromboplastin time. Haemostasis 1993;23:321–326. [DOI] [PubMed] [Google Scholar]
- 18. Carvalho A, Cunha A, Gregorio T, et al. Is the efficacy of endovascular treatment for acute ischemic stroke sex‐related. Interv Neurol 2018;7:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vanacker P, Heldner MR, Amiguet M, et al. Prediction of large vessel occlusions in acute stroke: national institute of health stroke scale is hard to beat. Crit Care Med 2016;44:e336–e343. [DOI] [PubMed] [Google Scholar]
- 20. Piccini JP, Simon DN, Steinberg BA, et al. Differences in clinical and functional outcomes of atrial fibrillation in women and men: two‐year results from the ORBIT‐AF registry. JAMA Cardiol 2016;1:282–291. [DOI] [PubMed] [Google Scholar]
- 21. Beumer D, Mulder MJHL, Saiedie G, et al. Occurrence of intracranial large vessel occlusion in consecutive, non‐referred patients with acute ischemic stroke. Neurovasc Imaging 2016;2:11. [Google Scholar]
- 22. Smith WS, Lev MH, English JD, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke 2009;40:3834–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reeves MJ, Bushnell CD, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 2008;7:915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kent DM, Price LL, Ringleb P, et al. Sex‐based differences in response to recombinant tissue plasminogen activator in acute ischemic stroke: a pooled analysis of randomized clinical trials. Stroke 2005;36:62–65. [DOI] [PubMed] [Google Scholar]
- 25. Emberson J, Lees KR, Lyden P, et al. Thrombolysis in acute stroke–authors’ reply. Lancet 2015;385:1396. [DOI] [PubMed] [Google Scholar]
- 26. Saposnik G, Di Legge S, Webster F, Hachinski V. Predictors of major neurologic improvement after thrombolysis in acute stroke. Neurology 2005;65:1169–1174. [DOI] [PubMed] [Google Scholar]
- 27. Elkind MS, Prabhakaran S, Pittman J, et al. Sex as a predictor of outcomes in patients treated with thrombolysis for acute stroke. Neurology 2007;68:842–848. [DOI] [PubMed] [Google Scholar]
- 28. Lorenzano S, Ahmed N, Falcou A, et al. Does sex influence the response to intravenous thrombolysis in ischemic stroke?: answers from safe implementation of treatments in Stroke‐International Stroke Thrombolysis Register. Stroke 2013;44:3401–3406. [DOI] [PubMed] [Google Scholar]
- 29. Savitz SI, Schlaug G, Caplan L, Selim M. Arterial occlusive lesions recanalize more frequently in women than in men after intravenous tissue plasminogen activator administration for acute stroke. Stroke 2005;36:1447–1451. [DOI] [PubMed] [Google Scholar]
- 30. Dehlendorff C, Andersen KK, Olsen TS. Sex disparities in stroke: women have more severe strokes but better survival than men. J Am Heart Assoc 2015;4:e001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arboix A, Cartanya A, Lowak M, et al. Gender differences and woman‐specific trends in acute stroke: results from a hospital‐based registry (1986‐2009). Clin Neurol Neurosurg 2014;127:19–24. [DOI] [PubMed] [Google Scholar]
- 32. Hametner C, Kellert L, Ringleb PA. Impact of sex in stroke thrombolysis: a coarsened exact matching study. BMC Neurol 2015;15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Forster A, Gass A, Kern R, et al. Gender differences in acute ischemic stroke: etiology, stroke patterns and response to thrombolysis. Stroke 2009;40:2428–2432. [DOI] [PubMed] [Google Scholar]
- 34. Kent DM, Buchan AM, Hill MD. The gender effect in stroke thrombolysis: of CASES, controls, and treatment‐effect modification. Neurology 2008;71:1080–1083. [DOI] [PubMed] [Google Scholar]
- 35. Martinez‐Sanchez P, Fuentes B, Fernandez‐Dominguez J, et al. Young women have poorer outcomes than men after stroke. Cerebrovasc Dis 2011;31:455–463. [DOI] [PubMed] [Google Scholar]
- 36. Al‐hussain F, Hussain MS, Molina C, et al. Does the sex of acute stroke patients influence the effectiveness of rt‐PA? BMC Neurol 2014;14:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mandava P, Kent TA. A method to determine stroke trial success using multidimensional pooled control functions. Stroke 2009;40:1803–1810. [DOI] [PubMed] [Google Scholar]
- 38. Hill MD, Kent DM, Hinchey J, et al. Sex‐based differences in the effect of intra‐arterial treatment of stroke: analysis of the PROACT‐2 study. Stroke 2006;37:2322–2325. [DOI] [PubMed] [Google Scholar]
- 39. Shah SH, Liebeskind DS, Saver JL, et al. Influence of gender on outcomes after intra‐arterial thrombolysis for acute ischemic stroke. Neurology 2006;66:1745–1746. [DOI] [PubMed] [Google Scholar]
- 40. Arnold M, Kappeler L, Nedeltchev K, et al. Recanalization and outcome after intra‐arterial thrombolysis in middle cerebral artery and internal carotid artery occlusion: does sex matter? Stroke 2007;38:1281–1285. [DOI] [PubMed] [Google Scholar]
- 41. Hametner C, MacIsaac RL, Kellert L, et al. Sex and stroke in thrombolyzed patients and controls. Stroke 2017;48:367–374. [DOI] [PubMed] [Google Scholar]
- 42. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 43. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 h after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 44. Bandera E, Botteri M, Minelli C, et al. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke 2006;37:1334–1339. [DOI] [PubMed] [Google Scholar]
- 45. Luby M, Hong J, Merino JG, et al. Stroke mismatch volume with the use of ABC/2 is equivalent to planimetric stroke mismatch volume. AJNR Am J Neuroradiol 2013;34:1901–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sato S, Uehara T, Ohara T, et al. Factors associated with unfavorable outcome in minor ischemic stroke. Neurology 2014;83:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yaghi S, Herber C, Boehme AK, et al. The association between diffusion MRI‐defined infarct volume and NIHSS score in patients with minor acute stroke. J Neuroimaging 2017;27:388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. MRI imaging protocol for baseline scan.


