Abstract
During 2014–2016, severe marine heatwaves in the northeast Pacific triggered well-documented disturbances including mass mortalities, harmful algal blooms, and declines in subtidal kelp beds. However, less attention has been directed towards understanding how changes in sea surface temperature (SST) and alongshore currents during this period influenced the geographic distribution of coastal taxa. Here, we examine these effects in northern California, USA, with a focus on the region between Point Reyes and Point Arena. This region represents an important biogeographic transition zone that lies <150 km north of Monterey Bay, California, where numerous southern species have historically reached their northern (poleward) range limits. We report substantial changes in geographic distributions and/or abundances across a diverse suite of 67 southern species, including an unprecedented number of poleward range extensions (37) and striking increases in the recruitment of owl limpets (Lottia gigantea) and volcano barnacles (Tetraclita rubescens). These ecological responses likely arose through the combined effects of extreme SST, periods of anomalous poleward flow, and the unusually long duration of heatwave events. Prolonged marine heatwaves and enhanced poleward dispersal may play an important role in longer-term shifts in the composition of coastal communities in northern California and other biogeographic transition zones.
Introduction
Marine heatwaves are defined as periods of extreme sea surface temperature (SST) persisting for days to months1,2. Although the ecological consequences of gradual, long-term increases in mean SST have generally received greater attention3–5, a series of recent marine heatwaves has highlighted the importance of extreme events in coastal ecosystems2,6. For example, extreme SST events in the Mediterranean Sea and Australia have been associated with extensive disturbances in subtidal communities including mass mortalities, coral bleaching, loss of habitat-forming kelps, and cascading ecological effects7–10. Marine heatwaves have increased in frequency and duration over the past century11, prompting calls for increased study of their ecological effects1,2,11.
During 2014–2016, the northeast Pacific experienced an unprecedented period of anomalously warm water, which has been characterized as the largest marine heatwave on record12. Oceanographic changes began during the winter of 2013–2014 in the Gulf of Alaska with the formation of a large warm-water anomaly, also referred to as the “warm-water Blob”13. By Fall 2014, this warm water had spread south and influenced much of the California Current Large Marine Ecosystem (CCLME). Subsequently, a strong El Niño event developed in the equatorial Pacific in 2015, with warm water spreading poleward into the northeast Pacific12,14. The combination of these events in the CCLME during 2014–2016 led to persistent sea surface temperature (SST) anomalies that were often 2–4 °C above mean climatological values15.
A broad range of ecological disturbances was observed in the CCLME during 2014–2016 including disruption of pelagic food webs and mortality of seabirds and marine mammals12,16,17. There were also striking declines in the abundance of subtidal kelp in northern California with cascading negative impacts on red abalone populations, which prompted closure of the recreational abalone fishery in 201818. A toxic algal bloom of unprecedented extent and severity also occurred throughout much of the CCLME, leading to the closure of an important commercial crab fishery19. Although it has been suggested that this marine heatwave might have been the most ecologically significant in recorded history12,17, documentation of these ecological effects has been incomplete. In particular, there has been relatively little consideration of numerous shifts in the geographic distribution and abundance of coastal species in the CCLME during 2014–201619,20.
During past strong El Niño events, marine species have often been documented poleward of their typical geographic ranges in the CCLME21–23. However, prior studies of these ecological effects in the CCLME have been limited with regards to habitat, taxonomic scope, and geographic region. Most studies to date have examined responses in pelagic ecosystems and have focused primarily on fish and other vertebrates19,23–26, with some consideration of shifts in the composition of zooplankton communities27,28. The few studies that have considered the influence of El Niño events on benthic intertidal communities in the CCLME have suggested that effects on these coastal ecosystems may be negligible29,30. Geographically, there has been a strong focus on effects of warm-water events in the Southern California Bight30–32, with some studies in Oregon and Washington state26,28, but few studies in northern California.
The paucity of studies of marine heatwaves in northern California is a significant gap as many coastal species with southern biogeographic affinities reach their northern (poleward) range limits in the CCLME at Monterey Bay, California33–35. Thus, northern California is an important transition zone between the mild-temperate taxa of the Montereyan biogeographic province (ranging from Point Conception in southern California to Monterey Bay) and the cool-temperate taxa of the Mendocinian province (ranging from Monterey Bay north to Cape Flattery, Washington33,36). Such transition zones are considered ideal barometers for documenting geographic range expansions and shifts in community composition as species from lower latitudes disperse poleward in response to warmer water5,37,38. Indeed, recent decades have seen a global trend towards tropicalization, as warm-adapted species disperse further poleward and increase in abundance in well-studied transition zones such as those found along the coasts of Europe and Australia8,39,40. It is increasingly clear that such shifts in community composition often involve not only increased SST, but also changes in the oceanographic currents that transport larvae and other reproductive propagules poleward8,41–44. Documenting the effects of marine heatwaves on the distribution of taxa in biogeographic transition zones thus provides a lens through which to advance our understanding of alongshore transport, dispersal, geographic range expansions, and the influence of climate change on coastal ecosystems.
Here, we document oceanographic changes and associated shifts in the distribution of coastal biota in northern California, USA, during the marine heatwaves of 2014–2016. Our primary study region extended from Point Reyes to Point Arena (Fig. 1), although we include some records of southern taxa transported farther north, to regions ranging from northern California to British Columbia, Canada. We analyze SST and current anomalies in northern California, but also consider oceanographic conditions in central California (e.g., at Point Sur and Año Nuevo, Fig. 1) to explore potential mechanisms of poleward transport from Monterey Bay and lower latitudes.
Figure 1.
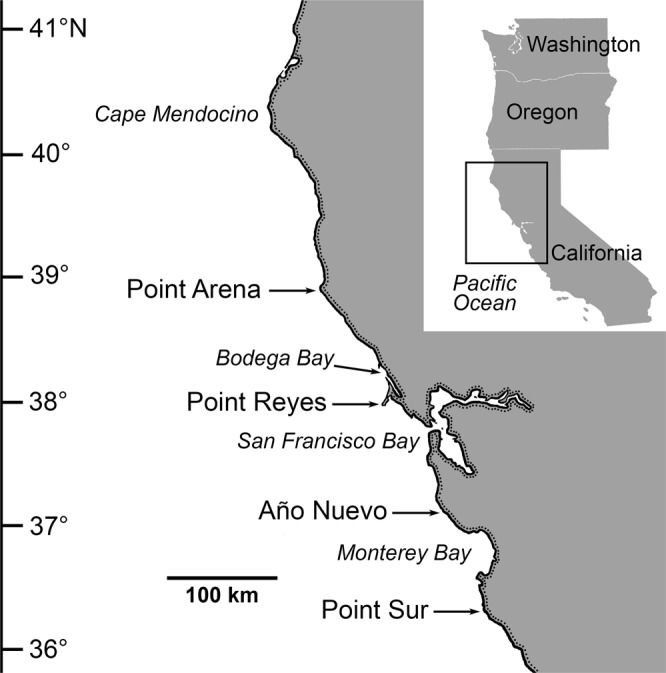
Primary study region in northern California, USA. Inset map (upper right) shows location of study region along the Pacific coast of the United States.
Results
Oceanographic conditions
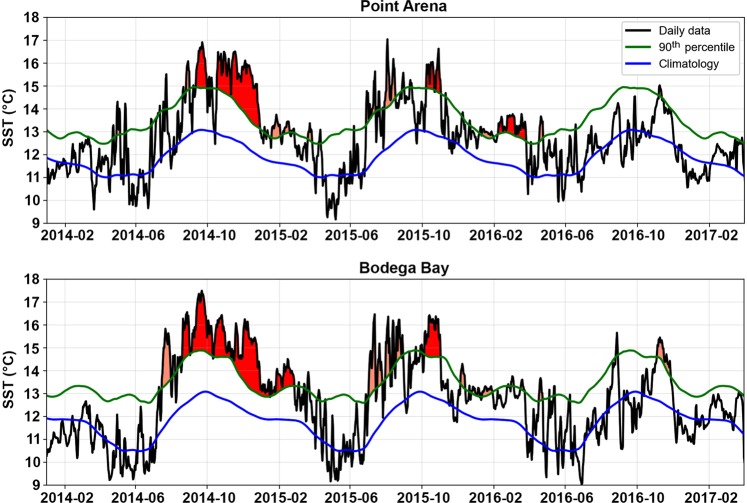
During 2014–2016, SST in our study region were frequently 2–4 °C above the climatological mean for 1981–2011 (Fig. 2). Using previously defined metrics1, our analyses of SST during this period identified 14 heatwaves (see Methods) of varying duration (range = 5–199 days; Fig. 2, Tables S2 and S3) at both Bodega Bay and Point Arena. Based on cumulative intensity, two heatwaves at Bodega Bay ranked among the 10 most intense events since 1981. The most intense lasted 199 days (August 2014 to late February 2015), had a cumulative intensity of 518.3 °C days, and a maximum intensity of 4.4 °C above the climatological mean. The duration and intensity of this heatwave surpassed those that occurred at this location even during strong El Niño events in the past (e.g., 1997–1998, Table S2). At Point Arena, four shorter heatwaves during 2014–2016 ranked among the 10 most intense events since 1981 (Table S3). The most intense occurred during October–December 2014 (74 days), and had a cumulative intensity of 231.8 °C days, and a maximum intensity of 3.9 °C.
Figure 2.
Sea surface temperature (SST) for January 2014 to March 2017 from NOAA/NDBC buoys at Point Arena (upper panel), and Bodega Bay (lower panel). Black line indicates daily SST, blue line indicates the climatology based on the 1981–2011 period, green line indicates the 90th percentile threshold that defines marine heatwaves, and shaded areas indicate events identified as heatwaves1. Red shading indicates events that rank among the 10 most intense marine heatwaves based on cumulative intensity of events during 1981–2017.
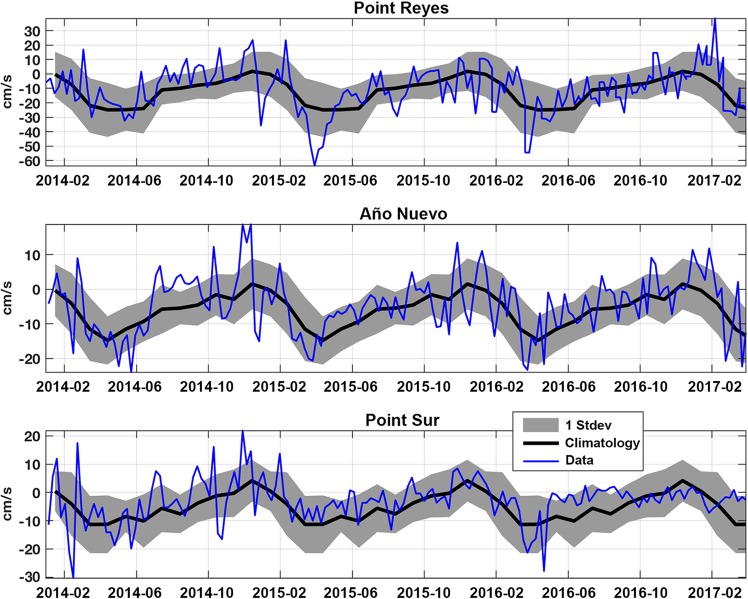
Anomalous poleward surface currents were common along the coast from Point Sur to Point Reyes during July–November 2014 (Fig. 3). Several shorter periods of anomalous northward flow were also recorded at Año Nuevo and Point Reyes in late 2015/early 2016, during the El Niño event. Although warming dissipated by mid-2016, several short periods of anomalous poleward currents were observed in late 2016 and early 2017.
Figure 3.
Temporal variation in alongshore flow velocity (North-South) at three locations: northwest of Point Reyes (upper panel), Año Nuevo (middle panel), Point Sur (lower panel). Values are weekly-averaged velocities (cm/s) derived from high-frequency radar. Positive values indicate northward flow, and negative values indicate southward flow. Data compare 2014–2017 (blue line) to mean climatology (2002–2011) and standard deviation (black line and gray shading, respectively).
During 1991–2017, oceanographic conditions in northern California varied substantially among years, as reflected by the Multivariate Ocean Climate Indicator (MOCI; Fig. S68). The period of 2014–2016 was characterized by many (non-continuous) seasons with warm conditions, comparable only to El Niño periods in magnitude (e.g., the 1982–1983 and 1997–1998 events), although more persistent than during prior El Niño events. Warm conditions were broken by quasi-normal upwelling season conditions in northern California during 2014–2016.
New records of northern geographic range boundaries
During 2014–2017, we recorded poleward range extensions into northern California and higher latitudes for 37 species with primarily southern distributions (Table 1; see also Supplementary Information). These species spanned a diversity of taxa including molluscs (n = 18), crustaceans (6), cnidarians (4), echinoderms (3), ctenophores (1), tunicates (1), fish (1), seabirds (1), marine mammals (1), and red algae (1). In contrast, during this period we observed an equatorward range expansion into our study region of only one northern species (a poorly studied sea cucumber, Pentamera pediparva). Of these 37 species, nine belonged to the pelagic community (including gastropods, siphonophores, a jellyfish, a ctenophore, a salp, and pelagic red crabs). Twenty-one were benthic animals with planktonic, feeding larvae. An additional five benthic species had life histories associated with limited dispersal including direct developers, crawling larvae (i.e., the nudibranch Phidiana hiltoni45), or macroalgal spores. The distance of the range extensions varied significantly among these three groups of taxa (Table 1; ANOVA, F2,32 = 17.28, p < 0.0001). Range extensions for pelagic species (mean distance ± SE = 690.0 ± 154.3 km) were 2.6x greater than those of benthic species with planktonic larvae (265.7 ± 33.3 km; Tukey HSD, p = 0.044), and were 9.6x greater than those of benthic species with limited dispersal potential (72.2 ± 48.1 km; Tukey HSD, p < 0.0001). Range extensions for benthic species with planktonic larvae were 3.7x greater than those of benthic species with limited dispersal (Tukey HSD, p = 0.0002).
Table 1.
Geographic range extensions (n = 37).
| Common name | Scientific name | New northern record | Former range limit | Habitat/Life history | Range extension |
|---|---|---|---|---|---|
| Red Alga (A) | Dasya binghamiae | Bodega Harbor, CA | Estero de San Antonio, CA | Benthic/LD | 7 |
| Sunburst Sea Anemone (CN) | Anthopleura sola | Bruhel Point, CA | Bodega Marine Reserve, CA | Benthic/PL | 165 |
| Siphonophore (CN) | Hippopodius hippopus | Salmon Creek Beach, CA | Point Conception, CA | Pelagic | 490 |
| Hula Skirt Siphonophore (CN) | Physophora hydrostatica | Mad River Beach, CA | Monterey Bay, CA | Pelagic | 530 |
| Purple-striped Jellyfish (CN) | Chrysaora colorata | Arcadia Beach, OR | Bodega Bay, CA | Pelagic | 875 |
| Venus’ Girdle Ctenophore (CT) | Cestum veneris | Rainy Bay, BC | Monterey Bay, CA | Pelagic | 1400 |
| Angular Unicorn Snail (M) | Acanthinucella spirata | Cape Mendocino, CA | Bodega Harbor, CA | Benthic/LD | 260 |
| Appleseed Erato Snail (M) | Hespererato vitellina | Cape Mendocino, CA | Bodega Marine Reserve, CA | Benthic/PL | 255 |
| Purple Sea Snail (M) | Janthina umbilicata | Leadbetter Point, WA | Neskowin, OR | Pelagic | 140 |
| Scaled Tube Snail (M) | Thylacodes squamigerus | Salt Point State Park, CA | Bodega Marine Reserve, CA | Benthic/PL | 36 |
| Curved Needle Pteropod (M) | Creseis virgula | Bodega Line Station 4 | off Central California | Pelagic | 150 |
| Striated Sea Butterfly (M) | Hyalocylis striata | Bodega Line Station 4 | Baja California, Mexico | Pelagic | 1400 |
| Strong’s Sidegill (M) | Berthella strongi | MacKerricher State Park, CA | Moss Beach, CA | Benthic/PL | 255 |
| Black-tipped Spiny Dorid (M) | Acanthodoris rhodoceras | Chup Point, BC | Cape Arago, OR | Benthic/PL | 620 |
| Olive’s Nudibranch (M) | Anteaeolidiella oliviae | Fort Bragg, CA | Duxbury Reef, CA | Benthic/PL | 210 |
| Rabbit Dorid Nudibranch (M) | Crimora coneja | Boiler Bay, OR | Cape Arago, OR | Benthic/PL | 170 |
| Colorful Dirona Nudibranch (M) | Dirona picta | Bamfield, BC | Cape Meares, OR | Benthic/PL | 385 |
| White-spotted Sea Goddess (M) | Doriopsilla albopunctata | Whiskey Creek, OR | Mendocino, CA | Benthic/PL | 335 |
| White-spotted Dorid (M) | Doriopsilla fulva | Netarts Bay, OR | Abalone Beach, CA | Benthic/PL | 485 |
| Janolus Nudibanch (M) | Janolus barbarensis | Bodega Harbor, CA | San Francisco Bay, CA | Benthic/PL | 87 |
| Los Angeles Okenia (M) | Okenia angelensis | Miwok Beach, CA | San Francisco Bay, CA | Benthic/PL | 94 |
| Hilton’s Nudibranch (M) | Phidiana hiltoni | Pinnacle Gulch, CA | Duxbury Reef, CA | Benthic/LD | 63 |
| Orange-spike Polycera (M) | Polycera atra | Monas Island, BC | Westport, WA | Benthic/PL | 290 |
| Spotted Triopha Nudibranch (M) | Triopha maculata | Port Hardy, BC | Bamfield, BC | Benthic/PL | 425 |
| Spiny Lobster (CR) | Panulirus interruptus | Horseshoe Cove (BMR), CA | San Francisco Bay, CA | Benthic/PL | 87 |
| Chocolate Porcelain Crab (CR) | Petrolisthes manimaculis | Point St. George, CA | Trinidad, CA | Benthic/PL | 80 |
| Xantus’ Swimming Crab (CR) | Portunus xantusii | Tomales Bay, CA | Morro Bay, CA | Benthic/PL | 390 |
| Pelagic Red Crab (CR) | Pleuroncodes planipes | Agate Beach, OR | Fort Bragg, CA | Pelagic | 595 |
| Pink-striped Barnacle (CR) | Megabalanus californicus | Humbug State Park, OR | Humboldt Bay, CA | Benthic/PL | 215 |
| Red-striped Barnacle (CR) | Paraconcavus pacificus | MacKerricher State Park, CA | San Francisco, CA | Benthic/PL | 240 |
| Glass-spined Brittle Star (E) | Ophiothrix spiculata | Patrick’s Point State Park, CA | Cordell Bank, CA | Benthic/PL | 360 |
| Scarlet Sea Cucumber (E) | Lissothuria nutriens | Fort Ross Reef, CA | Bodega Marine Reserve, CA | Benthic/LD | 25 |
| Red Sea Cucumber (E) | Pachythyone rubra | Bodega Marine Reserve, CA | Pinnacle Gulch, CA | Benthic/LD | 6 |
| Salp (T) | Thetys vagina | Calvert Island, BC | Grays Canyon, WA | Pelagic | 630 |
| Pacific Snake Eel (F) | Ophichthus triserialis | Lincoln City, OR | Klamath River, CA | Benthic/PL | 395 |
| Wedge-rumped Storm-Petrel (B) | Oceanodroma tethys | Humboldt Bay, CA | Monterey Bay, CA | N/A | 500 |
| Common Bottlenose Dolphin (MM) | Tursiops truncatus | Little River, CA | Doran Beach, CA | N/A | 130 |
New records of southern-ranging species observed north of Point Reyes, California, during 2014–2017. Taxonomic group indicated in parentheses: A = alga, B = bird, CN = cnidarian, CR = crustacean, CT = ctenophore, E = echinoderm, F = fish, M = mollusc, MM = marine mammal, T = tunicate. Columns 3–4 show the new northern range limit and the former range limit, respectively (see Supplementary Information). Column 5 classifies species based on whether adults are pelagic vs. benthic, and whether benthic species have planktonic, feeding larvae (PL), or limited dispersal (LD) associated with direct development, for example. N/A = not applicable. The final column shows the distance of the range extension (km). Coordinates for all geographic locations are listed in Table S1.
Unusual records of occurrence
In addition to records of new northern geographic range limits, we observed a large influx of 21 primarily southern species that are rare in northern California (Table 2). Historical records and museum collections suggest that many of these species had been observed in northern California only in association with past El Niño events (Supplementary Information). For example, we are aware of only three prior records of the pelagic snail Janthina janthina occurring north of Point Conception, California, and all are associated with El Niño events (Supplementary Information). During 2014–2016, some of these species were recorded at very low densities (e.g., one or a few individuals at a particular site, such as the nudibranch Hancockia californica). Others were locally abundant and were observed at many sites throughout northern California (e.g., the nudibranch Okenia rosacea45).
Table 2.
Rare occurrences (n = 21).
| Common name | Scientific name | Local site | Known range limit |
|---|---|---|---|
| Siphonophore (CN) | Diphyes dispar | Salmon Creek Beach, CA | off Brookings, OR |
| White Flatworm (PL) | Pseudoceros luteus | Bodega Harbor, CA | Bodega Harbor, CA |
| Violet Sea Snail (M) | Janthina janthina | Salmon Creek Beach, CA | Neskowin, OR |
| Spanish Shawl Nudibranch (M) | Flabellinopsis iodinea | Pinnacle Gulch, CA | Vancouver Island, BC |
| Hancock’s Nudibranch (M) | Hancockia californica | Pinnacle Gulch, CA | Trinidad, CA |
| Hopkins’ Rose Nudibranch (M) | Okenia rosacea | Bodega Head, CA | Gregory Point, OR |
| Hedgpeth’s Nudibranch (M) | Polycera hedgpethi | Bodega Harbor, CA | Bodega Harbor, CA |
| California Sea Hare (M) | Aplysia californica | Miwok Beach, CA | Yaquina Bay, OR |
| California Aglaja (M) | Navanax inermis | Bodega Harbor, CA | Bodega Harbor, CA |
| Blue Buoy Barnacle (CR) | Dosima fascicularis | Salmon Creek Beach, CA | Salisbury Sound, AK |
| Salp (T) | Thalia democratica | Salmon Creek Beach, CA | Newport, OR |
| Ocean Whitefish (F) | Caulolatilus princeps | Farallon Islands, CA | Vancouver Island, BC |
| Black Storm-Petrel (B) | Oceanodroma melania | Cordell Bank, CA | Seaside, OR |
| Black-vented Shearwater (B) | Puffinus opisthomelas | off Bodega Marine Reserve, CA | Vancouver Island, BC |
| Guadalupe Murrelet (B) | Synthliboramphus hypoleucus | Cordell Bank, CA | Washington |
| Brown Booby (B) | Sula leucogaster | Tomales Bay, CA | Alaska |
| Green Sea Turtle (R) | Chelonia mydas | Golden Gate, CA | Alaska |
| Olive Ridley Sea Turtle (R) | Lepidochelys olivacea | Salmon Creek Beach, CA | Alaska |
| Guadalupe Fur Seal (MM) | Arctocephalus townsendi | Doran Beach, CA | Alaska |
| Long-beaked Common Dolphin (MM) | Delphinus capensis | Cordell Bank, CA | British Columbia |
| Short-beaked Common Dolphin (MM) | Delphinus delphis | Cordell Bank, CA | British Columbia |
Southern-ranging species not typically found in northern California, but with rare historical records in this and other northern regions (often in association with El Niño events, see Supplementary Information). Taxonomic group indicated in parentheses: B = bird, CN = cnidarian, CR = crustacean, F = fish, M = mollusc, MM = marine mammal, PL = platyhelminth, R = reptile, T = tunicate. Columns 3 and 4 show where the species was observed locally during the study, and the known northern geographic range limit, respectively. Coordinates for all geographic locations are listed in Table S1.
Increases in the abundance and larval recruitment of southern species
An additional set of primarily southern species also responded strongly during the marine heatwaves (Table 3). These species had been present routinely at our field sites in very low abundance during 2004–2013 (see Supplementary Information), but each experienced a pronounced increase in local abundance during 2014–2017. For two of these species with planktonic larvae (the owl limpet Lottia gigantea and volcano barnacle Tetraclita rubsecens), we conducted targeted field surveys to quantify changes in recruitment and abundance through time.
Table 3.
Southern-ranging species that experienced a pronounced increase in local abundance during 2014–2016 (n = 14).
| Common name | Scientific name | Local site(s) | Known range limit |
|---|---|---|---|
| By-the-wind Sailor (CN) | Velella velella | Salmon Creek Beach, CA | Alaska |
| Sunburst Sea Anemone (CN) | Anthopleura sola | Bodega Marine Reserve, CA | Bruhel Point, CA* |
| Owl Limpet (M) | Lottia gigantea | Bodega Marine Reserve, CA | Crescent City, CA |
| Monterey Tube Snail (M) | Petaloconchus montereyensis | Bodega Marine Reserve, CA | Van Damme State Park, CA** |
| White-spotted Dorid (M) | Doriopsilla fulva | Salt Point State Park, CA | Netarts Bay, OR* |
| Bryozoan (BR) | Jellyella tuberculata | Bodega Marine Reserve, CA | Bodega Marine Reserve, CA |
| Pink-striped Barnacle (CR) | Megabalanus californicus | Bodega Marine Reserve, CA | Humbug State Park, OR* |
| Pink Volcano Barnacle (CR) | Tetraclita rubescens | Salt Point & Van Damme, CA | Burnt Hill, OR |
| Spiny Mole Crab (CR) | Blepharipoda occidentalis | Salmon Creek Beach, CA | Salmon Creek Beach, CA** |
| Mole Crab (CR) | Emerita analoga | Salmon Creek Beach, CA | Alaska |
| Chocolate Porcelain Crab (CR) | Petrolisthes manimaculis | Pinnacle Gulch, CA | Point St. George, CA* |
| Scarlet Sea Cucumber (E) | Lissothuria nutriens | Bodega Marine Reserve, CA | Fort Ross Reef, CA* |
| Pyrosome (T) | Pyrosoma atlanticum | Salmon Creek Beach, CA | Sitka, AK |
| Ocean Sunfish (F) | Mola mola | Bodega Bay & Cordell Bank, CA | Alaska |
Taxonomic group indicated in parentheses: BR = bryozoan, CN = cnidarian, CR = crustacean, E = echinoderm, F = fish, M = mollusc, T = tunicate. Column 3 shows the local site where the increase was observed. Column 4 shows the known northern geographic range limit. One asterisk (*) indicates a new northern range limit recorded during this study (Table 1), whereas two asterisks (**) indicates an unpublished northern range record that we observed prior to 2014 (see Supplementary Information). Coordinates for all geographic locations are listed in Table S1.
Owl limpets
Only larger size classes of Lottia gigantea were present at Bodega Marine Reserve in 2010 with no evidence of recent recruitment of smaller individuals (Fig. 4). During 2011–2014, we noted the gradual loss of these larger owl limpets, but never recorded the presence of small owl limpets (J.L.S. and E.S., personal observations). In June 2016, we recorded a striking increase in recruitment of owl limpets (Fig. 4). The majority of these individuals were 15–35 mm long, and based on published age-size relationships for this species46, likely settled during late 2014 in association with anomalously warm conditions and poleward currents. By May 2017, many of these owl limpets had grown into the larger size classes (30–50 mm) expected for ~2.5-year old owl limpets46. Also in May 2017, a second large cohort of small (15–35 mm) owl limpets was apparent, and these likely represented ~1.5-year old limpets that settled in late 2015 in association with the El Niño event. By May 2018, limpets from the two years of successful recruitment (late 2014, late 2015) continued to grow into larger size classes (30–55 mm). Small numbers of new recruits (15–35 mm) were also recorded, suggesting some recruitment in late 2016 after warm-water conditions had dissipated (Fig. 4). Survival of L. gigantea recruits was high; 90.6% of juvenile limpets survived between the 2016 and 2017 annual surveys, and 82.4% of juvenile limpets survived between the 2017 and 2018 annual surveys.
Figure 4.
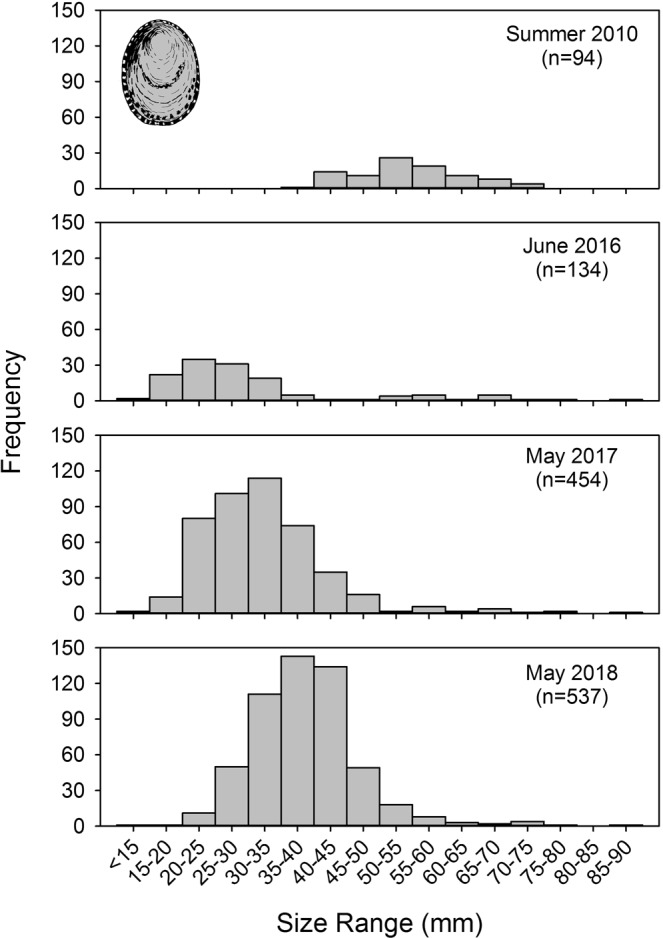
Owl limpet (Lottia gigantea) size frequency distributions at Bodega Marine Reserve, California. During 2006–2013, the population was characterized by little to no recruitment and almost all individuals were in larger size classes. Two cohorts of juvenile owl limpets (15–40 mm long) recruited during late 2014/early 2015 and late 2015/early 2016 in association with heatwave events, with subsequent growth into larger size classes in 2017 and 2018. n = sample size.
Volcano barnacles
In May 2006, the population of Tetraclita rubescens at Van Damme State Park was composed almost entirely of larger barnacles (Fig. 5). Observations at this site during 2006–2014 (at least one visit per year) indicated a similar distribution of sizes, with very low recruitment of Tetraclita during this period (E.S. and J.L.S., personal observations). In June 2015, we recorded a large recruitment pulse of small Tetraclita (basal diameter ≤11 mm). Based on published age-size relationships for this species47, these individuals recruited during Fall 2014 in association with the anomalously warm conditions and poleward currents. By June 2016, many of these barnacles had grown into larger size classes (11–20 mm), and there was evidence of a second, smaller recruitment event from Fall 2015 associated with the El Niño event. In May 2017 and May 2018, a continued shift of recruits from the warm-water events into larger size classes was apparent, but these surveys suggested minimal recruitment of new individuals (basal diameter <11 mm) during Fall 2016 and Fall 2017, respectively.
Figure 5.
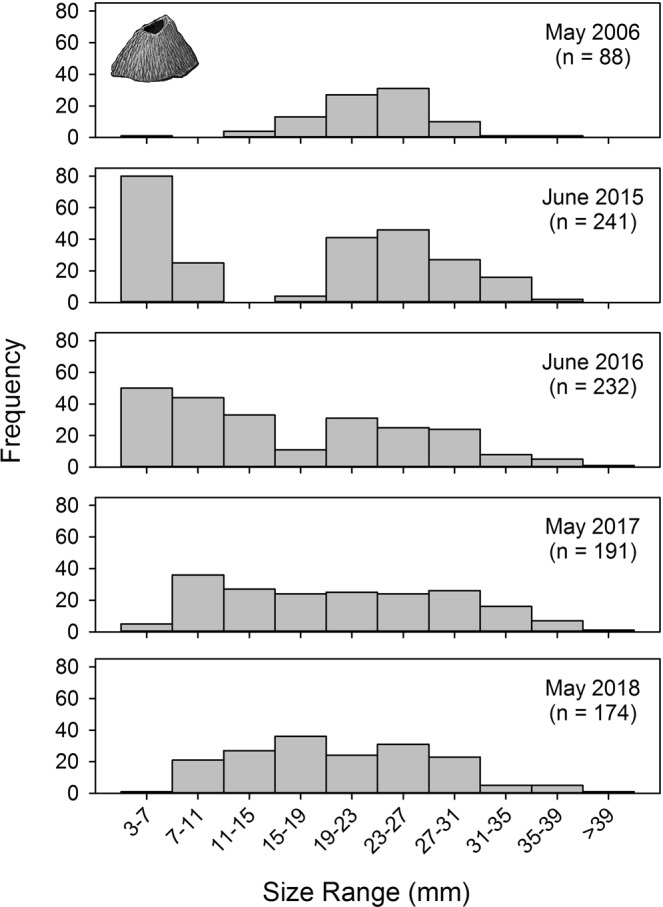
Volcano barnacle (Tetraclita rubescens) size frequency distributions at Van Damme State Park, California. During 2006–2013, the population was characterized by infrequent recruitment and almost all individuals were in larger size classes. Discrete recruitment events (barnacles < 11 mm diameter) occurred during late Summer/Fall 2014 and late Summer/Fall 2015, with minimal recruitment during late Summer/Fall or 2016 and 2017. n = sample size.
Discussion
Oceanographic changes and the distribution and abundance of southern taxa
Although shifts in geographic distribution have frequently been associated with strong El Niño events21,22,26,48 and other marine heatwaves49, the number of poleward range extensions observed during this study appears unprecedented. Conditions in northern California during 2014–2016 were characterized by SST that were often 2–4 °C above the climatological mean, and included a series of more than a dozen heatwaves (i.e., events lasting ≥5 days with SSTs warmer than the 90th percentile based on the 30-year climatology1). The most intense heatwave at Bodega Bay lasted 199 days (August 2014 to late February 2015), with a cumulative intensity (518.3 °C days) that exceeded that of other extreme heatwaves recorded in Western Australia (2011), the NW Atlantic (2012), the Mediterranean Sea (2014), and the Tasman Sea (2015–2016)1,50. Given that cooler SST can limit the development and successful recruitment of larval stages of biota from lower latitudes43,51, the unusually long duration of anomalously warm temperatures in northern California during 2014–2016 was likely a primary driver of poleward range shifts observed in our study.
In addition, anomalous poleward transport might have contributed to shifts in the distribution of southern biota during 2014–2016, a hypothesis proposed to explain geographic range expansions during some previous El Niño events21,23,52,53. Larval dispersal is influenced by a combination of advective and diffusive processes54, both of which may be important in explaining poleward transport in our study. Alongshore currents during 2014–2016 exhibited anomalous poleward flows, especially during the second half of 2014. Although such anomalies were infrequent overall, the persistent 15 cm/s poleward flow past Point Año Nuevo during November 2014 could have advected plankton over distances of ~500 km in one month. Moreover, it is possible that some plankton were transported north through a sequence of poleward flows rather than a single advection event. During late 2014, alongshore flow often did not revert to equatorward flow between poleward flow anomalies. This pulsed advection, such as the stop-start poleward flow past Point Año Nuevo during July–August 2014, can account for ~300 km displacement. Lastly, poleward transport might arise via a diffusion mechanism in which some of the plankton transported north during a brief event are not returned south as flows reverse, but are retained and transported farther north during the next brief poleward event. This process might ultimately result in a portion of the larval pool moving considerable distances north through a series of poleward flow events, such as flows past Point Reyes during July–October 2014 (potential displacement of ~400 km). This last diffusive transport scenario can be enhanced by retention features like bays (e.g., Monterey Bay, or the Gulf of Farallones) or offshore mesoscale eddies and requires that some flows be directed poleward, especially from March to August when mean flow off central and northern California is typically equatorward. Thus, even marginally anomalous flows, as observed during 2014–2016, might result in a marked increase in the probability of poleward transport of propagules.
There are other possible explanations for anomalous transport of warm-water biota into our region during 2014–2016. In particular, enhanced onshore transport might have delivered organisms to coastal waters that are typically found in warmer, offshore waters, including many of the pelagic species reported in this study. Although there was no evidence of anomalous downwelling along the California coast during 2014–201615, previous studies suggest additional mechanisms of onshore transport during El Niño years55.
It is likely that both temperature and transport played a role in the poleward shift of biota, and that the combination of these effects would be more effective than either one alone41,42,56. For example, warmer temperatures shorten planktonic larval durations57 and thus may increase the chances that southern species will be transported poleward, complete development, and settle in benthic habitats within the timespan of temporary current reversals51,58. Indeed, the combined influence of changes in temperature and currents has facilitated range expansions in other geographic regions8,9,41–43,56,59.
While we recorded range extensions and/or increased recruitment of many southern species, others species with northern range boundaries at Monterey Bay did not undergo range expansions into our study region. Individualistic responses of this kind have been observed in other studies of geographic range shifts and likely depend in part on the specific life histories, dispersal potentials, and habitat requirements of a given species39,42,60,61. For example, many of the nudibranch species in our study reproduce over a large proportion of the year, including production of larvae during fall and winter (Table S4). Similarly, the larvae of owl limpets and volcano barnacles both occur in the plankton primarily during the second half of the year (September–January, and July–November, respectively62,63). The timing of larval dispersal in these species thus overlapped with periods of strong surface poleward flow and warm SST during 2014–2016 (Figs 2 and 3). In contrast, planktonic larvae of species that reproduce during the spring likely encountered primarily equatorward flow and more typical, cooler SST during 2014–2016.
The magnitude of geographic range extensions was greatest for taxa that are members of the pelagic community as adults (e.g., jellyfish, ctenophores, pteropods, and pelagic red crabs). The prolonged occurrence of these species in the pelagic environment presumably increases the chances that some individuals will be transported poleward by advective and diffusive processes. For benthic taxa that experienced range extensions, species with planktonic, feeding larvae exhibited greater range extensions than those species with direct development and limited dispersal potential, such as brooding sea cucumbers. Although some meta-analyses have found faster range extensions in pelagic organisms and highly mobile fish than in benthic species64, others have failed to find a significant effect of reproductive mode and dispersal potential on the rate of range shifts65,66. When evaluated over the same timescale as anomalous conditions during 2014–2016, dispersal potential likely had a direct influence on the colonization of poleward regions. In contrast, when range shifts are assessed over decadal timescales65,66, initial patterns of dispersal and colonization during heatwaves may sometimes be reshaped by additional abiotic and biotic factors.
Persistence of ecological changes
Ecological changes associated with marine heatwaves can lead to population changes that range from short-lived to persistent9,67. Poleward appearances of southern biota beyond their typical range boundaries are often ephemeral, with species disappearing from the region when the oceanographic event ends, due to unsuitable conditions in the northern habitat and/or the short lifespan of some species53. This was the case, for example, for many of the primarily southern nudibranchs that occurred only ephemerally in our study region during the marine heatwaves (e.g., Flabellinopsis iodinea, Anteaeolidiella oliviae, Janolus barbarensis, and others20). In other cases, larval recruitment may establish “relict populations” that decline in abundance more slowly before eventually disappearing67. This may be the case for the Hopkins’ Rose Nudibranch (Okenia rosacea), which was still present in northern California during summer 2018, over two years after the El Niño ended, but at much lower densities than occurred in the region during 2015–2016 (see Supplementary Information). A final possible outcome of marine heatwaves is that warmer water and increased larval transport from lower latitudes may establish or sustain “marginal” or sink populations that can persist indefinitely67. Such sink populations are maintained primarily by episodic larval recruitment from southern source populations68, as appears to be the case for owl limpets and volcano barnacles in our study region63,69.
Whether or not northern populations established during marine heatwaves persist will likely be influenced by several other factors beyond future larval recruitment and connectivity with southern source populations. First, the physiological tolerances of a species will determine if new recruits can persist in poleward habitats when more typical temperatures return42,51,56,59. Second, the lifespan of a species will also influence the persistence of marginal populations. For example, volcano barnacles and owl limpets are relatively long-lived species, and individuals can live 10–15 years62 or >20 years69, respectively. Moreover, our results suggest that survival of juvenile owl limpets in northern California is relatively high. Thus, even infrequent recruitment associated with El Niño events may be sufficient to maintain marginal populations of some southern species68,69. Finally, mode of reproduction and habitat are likely important in determining whether marginal populations will contribute to future recruitment in the northern region. For example, for broadcast spawners, low population density may reduce fertilization success via Allee effects39. Open coast populations that produce planktonic larvae near the northern edge of a geographic range might contribute minimally to future recruitment in this region if prevailing coastal currents carry most larvae equatorward58. In contrast, species with direct development (e.g., brooders) might be self-sustaining once a sufficiently dense population is established in a northern location. During our study, two species with direct development (Lissothuria nutriens, Petaloconchus montereyensis) underwent striking increases in local abundance in northern populations (see Supplementary Information). The capacity for self-recruitment may allow southern species with direct development to respond rapidly to favorable, warm-water conditions.
Range expansions in a temperate transition zone
Temperate transition zones are often hotspots of diversity because these regions contain a mix of both warm-adapted and cool-adapted species37,39. The coast of California from Monterey Bay to Point Arena represents such a transition zone with high species richness that arises in part from benthic communities that include a mix of species with differing biogeographic affinities5. For example, of the 10 common intertidal barnacles in California, two are cosmopolitan species found coastwide, four are primarily northern, and four are primarily southern34. All 10 species occur in the region from Monterey Bay to Point Arena, and the boundary of this transition zone has shifted poleward since the 1970s (Fig. 6). Although El Niño events are typically viewed as ecological disturbances, not all associated effects on marine ecosystems are negative70. Indeed, episodic periods of warmer water and enhanced northward transport during warm-water events may be critical to the recruitment and population persistence of some primarily southern species in northern California. This may be particularly true for relatively long-lived species where northern populations can persist through extended periods of low recruitment. These recruitment dynamics may play an underappreciated role in maintaining the high species richness of transition zones. For example, during 2014–2016, all four southern barnacles in our study region increased in abundance and/or experienced geographic range extensions.
Figure 6.
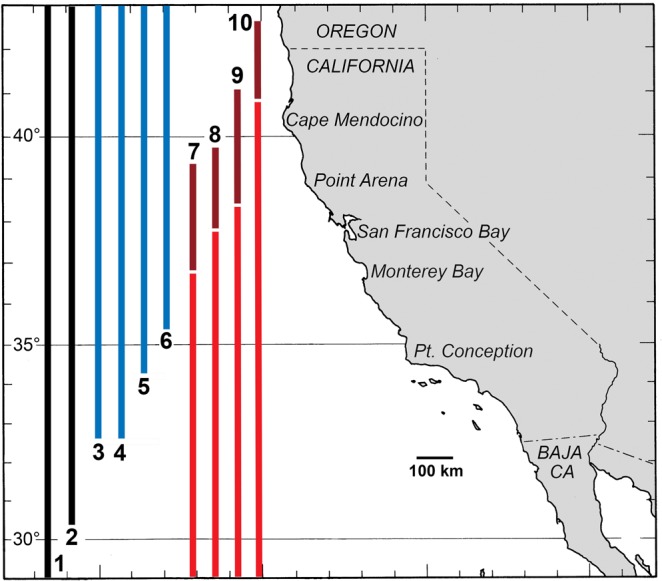
Geographic ranges of common intertidal/shallow subtidal barnacles of California (after Newman34). This assemblage includes species that are cosmopolitan (black bars), primarily northern (blue bars), and primarily southern (red bars). The highest species richness of intertidal barnacles occurs in central/north central California between Point Conception and Point Arena. For southern species, red bars indicate northern range limits during the 1970s34, and dark red bars indicate geographic range expansions to current poleward boundaries (see Supplementary Information). Species are coded as follows: (1) Pollicipes polymerus, (2) Balanus glandula, (3) Chthamalus dalli, (4) Balanus nubilus, (5) Balanus crenatus, (6) Semibalanus cariosus, (7) Paraconcavus pacificus, (8) Chthamalus fissus, (9) Tetraclita rubescens, (10) Megabalanus californicus. Note that southern range limits are those published by Newman34, as we are unaware of data that address whether these equatorward boundaries have retracted.
Episodic recruitment of southern species during El Niño events may also play a key role in facilitating geographic range expansions against a backdrop of ongoing climate change. In recent decades, the species composition of intertidal communities in the Bodega Bay region has shifted to include more southern fauna of the Montereyan biogeographic province, including the sea anemone Anthopleura sola, the barnacles Tetraclita rubescens and Megabalanus californicus, the porcelain crab Petrolisthes manimaculis, the owl limpet Lottia gigantea, and the vermetid tube snail Thylacodes squamigerus. These six species were historically rare or absent in the region during the 1970s (see Supplementary Information), but all have become more common in recent decades, including marked increases in abundance during 2014–2016. In addition, the geographic ranges of four of the six species expanded farther north during 2014–2016. Our results are thus consistent with a gradual shift in the poleward boundary of the Montereyan biogeographic province5 analogous to trends towards tropicalization seen in other regions of the world8,37,39.
Growing evidence suggests that gradual, long-term shifts in geographic distributions can be punctuated by rapid expansions where marginal populations are established during extreme events6. Meta-analyses suggest that marine range extensions have occurred at a mean rate (±SE) of 72.0 km (±13.5) per decade64. In contrast, the mean (±SE) range extension recorded in our study was 345.4 km (±53.9 ) occurring within a period of a few years or less. Thus, marine heatwaves provide a mechanism for the rapid poleward expansion of some species into new regions. While many of these northern populations might not persist, the establishment of such marginal populations may be essential if poleward range expansions proceed in a stepping-stone fashion71. Marginal populations established during extreme events might be more likely to persist if background levels of ocean warming make northern habitats more favorable for southern species during intervening periods between these events59. In addition, more frequent and longer heatwaves might also increase the persistence of marginal populations and accelerate shifts in species ranges37. For example, the prolonged duration of warm-water conditions and two years of successful larval recruitment might have allowed populations of volcano barnacles, owl limpets, and other southern species to reach a threshold density for effective reproduction (e.g., a minimum density required for fertilization success in broadcast spawning limpets or copulating barnacles). Our surveys of owl limpets in May 2018 indicate low levels of larval recruitment occurred in northern California in late 2016 after warm-water conditions had dissipated. This suggests the possibility that owl limpet populations in our study region have become sufficiently dense that they are starting to contribute larvae that may help sustain northern populations and perhaps facilitate a poleward shift in geographic distribution.
Whereas relatively short, high-intensity marine heatwaves can be sufficient to trigger mass mortality events1,7, we suggest that greatly prolonged heatwaves, like those that occurred along the California coast during 2014–2016, are especially likely to facilitate poleward dispersal and range extensions. Understanding the oceanographic, ecological, and evolutionary processes that mediate the success of such range expansions will be critical to predicting future shifts in the community composition of biogeographic transition zones in an era of accelerating climatic change.
Methods
Oceanographic Conditions
We considered two oceanographic parameters that were likely to influence poleward transport of southern fauna: sea surface temperatures (SST) and surface currents. We also reviewed wind data, which did not exhibit marked anomalies, seldom exceeding one standard deviation owing to high variability in winds.
Daily SST data for the period were obtained from NOAA/NDBC Buoys 46013 (off Bodega Bay) and 46014 (off Point Arena) located over the continental shelf (Fig. 1). Gaps in these data were filled with linear regressions with data from the Optimal Interpolation SST database V272. Climatological means and variances (SD) were calculated for the period 1981–2011, and daily anomalies as well as 90th percentiles were calculated from the climatological mean. Marine heatwaves during January 2014 to March 2017 were identified using previously published metrics1, and were defined as any event that lasted five days or longer with SST warmer than the 90th percentile based on the 30-year climatology; short periods of one or two days below the 90th percentile do not end a marine heatwave event. We used the Python scripts provided by E. C. J. Oliver (https://github.com/ecjoliver/marineHeatWaves) to identify these events (start and end dates) and to calculate the duration and intensity (maximum, mean, and cumulative) for each event.
Daily North-South surface flow was calculated from High Frequency (HF) Radar at three California coastal areas, where values were averaged within prescribed geographic units defined as: Bodega Bay, 38.0–38.2°N, 123.33–123.00°W; Año Nuevo, 36.9–37.4°N, 122.9–122.4°W; and Point Sur, 36.1–36.6°N, 122.4–121.9°W. Anomalies were calculated based on the climatological means and variances (SD) for the period 2002–2011. Detailed methods for the processing of HF Radar are provided elsewhere73.
We used the Multivariate Ocean Climate Indicator (MOCI)74 to compare oceanographic conditions during 2014–2016 to past patterns of interannual variability in the northern California region. MOCI has seasonal resolution and synthesizes local and regional data and indices (including the upwelling index and data on sea surface and air temperature, winds, sea level, atmospheric pressure at sea level), with climate indices including El Niño Southern Oscillation (ENSO), Pacific Decadal Oscillation, and the North Pacific Gyre Oscillation. Data included in MOCI and the methodology of calculation are described elsewhere74.
Field Surveys and Biological Observations
Our biological surveys were conducted primarily in the region between Point Reyes and Point Arena (Fig. 1). We include some survey data and records of geographic range extensions from regions farther north, ranging from northern California to British Columbia, Canada. Unusual biological records from south of Point Reyes (e.g., tropical and subtropical animals recorded for the first time in Baja California, southern California, or central California19,20) are beyond the scope of this study.
Sandy beach surveys
During this study period, E.S. and J.L.S. routinely searched for animals washed ashore along the southern end of Salmon Creek Beach and at Horseshoe Cove on the Bodega Marine Reserve. Surveys consisted of inspecting the beach wrack zone along 1.0 km at Salmon Creek Beach, and/or 0.25 km at Horseshoe Cove Beach, on approximately 229 dates between June 2014 and March 2017. Animals that were uncommon or unfamiliar to us (based on our 12 years of experience at these locations) were recorded and collected for further identification in the laboratory.
Rocky intertidal surveys
Between June 2014 and March 2017, E.S. and J.L.S. inspected the intertidal zones of rocky shores in the study region on approximately 118 dates. These visits were associated with monitoring efforts, other research projects, and field courses. Field sites included Bodega Marine Reserve, Van Damme State Park, and other rocky intertidal locations in northern California (Supplementary Information). During these visits, we recorded the presence of any uncommon or unfamiliar marine invertebrates (based on our 12 years of experience working at these locations), and collected specimens for further identification in the laboratory. When possible, voucher specimens of invertebrates were deposited in the collections at the California Academy of Sciences in San Francisco, California (Supplementary Information). For two southern species (owl limpets and volcano barnacles), we conducted field surveys to quantify changes in recruitment and abundance through time, as described in the following two sections.
Owl limpet surveys
In spring/summer of 2010, 2016, 2017, and 2018, we conducted targeted searches for owl limpets (Lottia gigantea) along 1.0 km of rocky intertidal shoreline within the Bodega Marine Reserve. Lottia gigantea are large grazing gastropods that maintain permanent territories on vertical and sloping surfaces in the mid and high intertidal zones69. The territories of owl limpets are easily recognized during visual surveys because these patches of rock remain free of sessile animals and support the growth of conspicuous diatom films. We measured the shell length of each owl limpet that was discovered and mapped and recorded its location within the same intertidal areas during each survey.
Volcano barnacle surveys
We quantified the size frequency distributions of volcano barnacles (Tetraclita rubescens) in the mid intertidal zone at Van Damme State Park in May/June 2006, 2015, 2016, 2017, and 2018. We placed five mid intertidal transects (~10 m long) parallel to the shoreline and randomly sampled 0.25 m2 quadrats placed along each transect (n = 30 quadrats total). Within each quadrat, we counted the number of live Tetraclita present and measured the basal diameter of each individual. In May 2006, we did not quantify density but instead measured the basal diameters of the first 88 individuals encountered.
Other records of unusual occurrences
In addition to our own field surveys, we collected records from several other sources. These included specimens that we identified in plankton tows that were conducted by colleagues within Bodega Marine Reserve and over Bodega Canyon (approximately 45 km offshore), primarily during Fall 2014. We verified and compiled additional observations made by other researchers at Bodega Marine Laboratory and observations reported via other resources20, including records from the online database iNaturalist (http://www.inaturalist.org/).
Analyses of ecological responses to warm-water events
We classified these observations into three categories of ecological responses19,67:
Geographic range extension: a new record of a species occurring beyond its previously recorded northern range limit.
Unusual northern occurrence: an observation of a southern species not normally found in our study region, but with some prior records (often in association with past El Niño events).
Increase in abundance: a primarily southern species that had been observed in the region during most years, but that underwent a large increase in abundance.
For each species in this study, we determined its previously documented northern range limit using a variety of resources, including published journal articles, reference guides, and taxonomic keys62,75. Published range records are often incomplete, so we also searched online museum databases and visited the California Academy of Sciences to search for uncatalogued northern records of these species. Although historical data are invaluable for the study of range shifts, we acknowledge that some species in our study that lacked prior records from poleward regions might in fact represent cases of non-detection arising from insufficient or unequal sampling76. Using the best estimates of prior range boundaries (Supplementary Information), we then calculated the distance along the coastline for each new geographic range extension (km) using Google Earth. These species were separated into three categories based on habitat and life history: (a) members of the pelagic community, (b) benthic species with planktonic, feeding larvae, or (c) benthic species with limited dispersal potential (i.e., direct development, lecithotrophic/non-feeding larvae, or algal spores). Two vertebrates (Oceanodroma tethys, Tursiops truncatus; Table 1) did not fit into these life history categories and were excluded from this analysis. We used an Analysis of Variance (ANOVA) to test whether the distance of range extensions varied among these three groups of taxa. Distances were log-transformed prior to analysis to meet model assumptions regarding homogeneity of variances. Analyses were conducted in JMP Pro v.12.0.1.
Supplementary information
Acknowledgements
We are especially grateful to James T. Carlton, Christina Piotrowski, and Molly Engelbrecht for their insights and assistance. For access to their collections and databases, we thank the California Academy of Sciences, the Smithsonian Museum of Natural History, the Los Angeles County Museum, the Royal British Columbia Museum, the University of Alaska Museum of the North, the Global Biodiversity Information Facility (GBIF), and iNaturalist. We are also grateful to numerous individuals who generously shared their observations and photographs of southern biota with us. A full list of these contributors is provided in the Supplementary Information. We thank Marcel Losekoot and Deedee Shideler for processing the HF Radar data associated with this study, and the University of California Natural Reserve System and California State Parks for access to field sites. HF Radar data were funded in part by NOAA through the United States Integrated Ocean Observing System (IOOS).
Author Contributions
E.S. and J.L.S. conceived the study. E.S., J.L.S. and J.H.R.G. collected and analyzed the biological data; M.G.R. and J.L.L. analyzed and interpreted the oceanographic data. E.S. wrote the first draft of the manuscript, with substantial contributions from M.G.R. and J.L.L. to writing the oceanographic sections. All authors reviewed and contributed to the final text.
Data Availability
Sea surface temperature (SST) data analyzed during this study are available from the U.S. National Data Buoy Center repository [http://www.ndbc.noaa.gov], and the Physical Oceanography Distributed Active Archive Center [https://podaac.jpl.nasa.gov/dataset/AVHRR_OI-NCEI-L4-GLOB-v2.0]. Surface flow data analyzed during this study are available from the Bodega Ocean Observing Node repository [http://boon.ucdavis.edu/flow_pointreyes.html] and [http://boon.ucdavis.edu/data/flow/boxes/]. Multivariate Ocean Climate Indicator (MOCI) data analyzed in this study are available from the Farallon Institute repository [http://www.faralloninstitute.org/moci]. All data regarding species’ distributions and occurrences (Tables 1–3) generated and analyzed during this study are included in this article and its Supplementary Information file. All remaining datasets generated and/or analyzed during the current study are available from the corresponding author on request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-40784-3.
References
- 1.Hobday AJ, et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016;141:227–238. doi: 10.1016/j.pocean.2015.12.014. [DOI] [Google Scholar]
- 2.Frölicher TL, Laufkötter C. Emerging risks from marine heat waves. Nat. Commun. 2018;9:650. doi: 10.1038/s41467-018-03163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Southward AJ, Hawkins SJ, Burrows MT. Seventy years’ observations of changes in distribution and abundance of zooplankton and intertidal organisms in the western English Channel in relation to rising sea temperature. J. Therm. Biol. 1995;20:127–155. doi: 10.1016/0306-4565(94)00043-I. [DOI] [Google Scholar]
- 4.Holbrook SJ, Schmitt RJ, Stephens JS. Changes in an assemblage of temperate reef fishes associated with a climate shift. Ecol. Appl. 1997;7:1299–1310. doi: 10.1890/1051-0761(1997)007[1299:CIAAOT]2.0.CO;2. [DOI] [Google Scholar]
- 5.Sagarin RD, Barry JP, Gilman SE, Baxter CH. Climate-related change in an intertidal community over short and long time scales. Ecol. Monogr. 1999;69:465–490. doi: 10.1890/0012-9615(1999)069[0465:CRCIAI]2.0.CO;2. [DOI] [Google Scholar]
- 6.Wethey DS, et al. Response of intertidal populations to climate: Effects of extreme events versus long term change. J. Exp. Mar. Biol. Ecol. 2011;400:132–144. doi: 10.1016/j.jembe.2011.02.008. [DOI] [Google Scholar]
- 7.Garrabou J, et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob. Change Biol. 2009;15:1090–1103. doi: 10.1111/j.1365-2486.2008.01823.x. [DOI] [Google Scholar]
- 8.Wernberg T, et al. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Chang. 2013;3:78–82. doi: 10.1038/nclimate1627. [DOI] [Google Scholar]
- 9.Wernberg T, et al. Climate-driven regime shift of a temperate marine ecosystem. Science. 2016;353:169–172. doi: 10.1126/science.aad8745. [DOI] [PubMed] [Google Scholar]
- 10.Hughes TP, et al. Global warming and recurrent mass bleaching of corals. Nature. 2017;543:373–377. doi: 10.1038/nature21707. [DOI] [PubMed] [Google Scholar]
- 11.Oliver ECJ, et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 2018;9:1324. doi: 10.1038/s41467-018-03732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Lorenzo E, Mantua N. Multi-year persistence of the 2014/15 North Pacific marine heatwave. Nat. Clim. Chang. 2016;6:1042–1047. doi: 10.1038/nclimate3082. [DOI] [Google Scholar]
- 13.Leising AW, et al. State of the California Current 2014–15: Impacts of the warm-water “Blob”. Calif. Coop. Ocean Fish. Invest. Rep. 2015;56:31–68. [Google Scholar]
- 14.Jacox MG, et al. Impacts of the 2015–2016 El Niño on the California Current System: Early assessment and comparison to past events. Geophys. Res. Lett. 2016;43:7072–7080. doi: 10.1002/2016GL069716. [DOI] [Google Scholar]
- 15.Gentemann CL, Fewings MR, García-Reyes M. Satellite sea surface temperatures along the West Coast of the United States during the 2014–2016 northeast Pacific marine heat wave. Geophys. Res. Lett. 2017;44:312–319. doi: 10.1002/2016GL071039. [DOI] [Google Scholar]
- 16.Peterson WT, et al. The pelagic ecosystem in the Northern California Current off Oregon during the 2014–2016 warm anomalies within the context of the past 20 years. J. Geophys. Res.-Oceans. 2017;122:7267–7290. doi: 10.1002/2017JC012952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ummenhofer CC, Meehl GA. Extreme weather and climate events with ecological relevance: a review. Phil. Trans. R. Soc. B. 2017;372:20160135. doi: 10.1098/rstb.2016.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers-Bennett, L., Kashiwada, J., Taniguchi, I. K., Kawana, S. K. & Catton, C. A. Using density-based fishery management strategies to respond to mass mortality events. J. Shellfish Res.38 (2019).
- 19.Cavole LM, et al. Biological impacts of the 2013–2015 warm-water anomaly in the Northeast Pacific. Oceanography. 2016;29:273–285. doi: 10.5670/oceanog.2016.32. [DOI] [Google Scholar]
- 20.Goddard, J. H. R. et al. Heterobranch sea slug range shifts in the northeast Pacific Ocean associated with the 2015–16 El Niño. Proceedings of the California Academy of Sciences Series 465, 107–131 (2018).
- 21.Radovich J. Relationships of some marine organisms of the northeast Pacific to water temperatures particularly during 1957 through 1959. Fish Bull. Sacramento. 1961;No. 112:1–62. [Google Scholar]
- 22.Pearcy WG, Schoener A. Changes in the marine biota coincident with the 1982–1983 El Niño in the northeastern subarctic Pacific Ocean. J. Geophys. Res.-Oceans. 1987;92:14417–14428. doi: 10.1029/JC092iC13p14417. [DOI] [Google Scholar]
- 23.Lluch-Belda D, Lluch-Cota DB, Lluch-Cota SE. Changes in marine faunal distributions and ENSO events in the California Current. Fisheries Oceanography. 2005;14:458–467. doi: 10.1111/j.1365-2419.2005.00347.x. [DOI] [Google Scholar]
- 24.Glynn PW. Southern Oscillation 1982–1983 — Nearshore population, community, and ecosystem responses. Annu. Rev. Ecol. Syst. 1988;19:309–345. doi: 10.1146/annurev.es.19.110188.001521. [DOI] [Google Scholar]
- 25.Lenarz WH, Ventresca DA, Graham WM, Schwing FB, Chavez F. Explorations of El Niño events and associated biological population dynamics off central California. Calif. Coop. Ocean Fish. Invest. Rep. 1995;36:106–119. [Google Scholar]
- 26.Pearcy WG. Marine nekton off Oregon and the 1997–98 El Niño. Prog. Oceanogr. 2002;54:399–403. doi: 10.1016/S0079-6611(02)00060-5. [DOI] [Google Scholar]
- 27.McGowan JA, Cayan DR, Dorman LM. Climate-ocean variability and ecosystem response in the northeast Pacific. Science. 1998;281:210–217. doi: 10.1126/science.281.5374.210. [DOI] [PubMed] [Google Scholar]
- 28.Fisher JL, Peterson WT, Rykaczewski RR. The impact of El Niño events on the pelagic food chain in the northern California Current. Glob. Change Biol. 2015;21:4401–4414. doi: 10.1111/gcb.13054. [DOI] [PubMed] [Google Scholar]
- 29.Paine RT. Benthic community-water column coupling during the 1982–1983 El Niño — Are community changes at high latitudes attributable to cause or coincidence? Limnol. Oceanogr. 1986;31:351–360. doi: 10.4319/lo.1986.31.2.0351. [DOI] [Google Scholar]
- 30.Dayton, P. K. & Tegner, M. J. Bottoms beneath trouble waters: benthic impacts of the 1982–1984 El Niño in the temperate zone. [ed. Glynn, P. W.]. Global Ecological Consequences of the 1982–1983 El Niño–Southern Oscillation. 433–472 (Elsevier Oceanography Series, 1990).
- 31.Montagne DE, Cadien DB. Northern range extensions into the southern California Bight of ten decapod Crustacea related to the 1991/92 and 1997/98 El Niño events. Bulletin of the Southern California Academy of Sciences. 2001;100:199–211. [Google Scholar]
- 32.Reed D, et al. Extreme warming challenges sentinel status of kelp forests as indicators of climate change. Nat. Commun. 2016;7:13757. doi: 10.1038/ncomms13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valentine JW. Numerical analysis of marine molluscan ranges on the extratropical northeastern Pacific shelf. Limnol. Oceanogr. 1966;11:198–211. doi: 10.4319/lo.1966.11.2.0198. [DOI] [Google Scholar]
- 34.Newman, W. A. Californian transition zone: Significance of short-range endemics. [eds Gray, J. & Boucot, A. J.]. Historical Biogeography, Plate Tectonics and the Changing Environment. 339–416 (Oregon State University Press, 1979).
- 35.Gaines, S. D. et al. Dispersal and geographic ranges in the sea. [eds Witman, J. D. & Roy, K.]. Marine Macroecology. 227–249 (University of Chicago Press, 2009).
- 36.Blanchette CA, et al. Biogeographical patterns of rocky intertidal communities along the Pacific coast of North America. J. Biogeogr. 2008;35:1593–1607. doi: 10.1111/j.1365-2699.2008.01913.x. [DOI] [Google Scholar]
- 37.Costa BHE, et al. Tropicalization of fish assemblages in temperate biogeographic transition zones. Mar. Ecol.-Prog. Ser. 2014;504:241–252. doi: 10.3354/meps10749. [DOI] [Google Scholar]
- 38.Vergés A, et al. The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B. 2014;281:20140846. doi: 10.1098/rspb.2014.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawkins SJ, et al. Consequences of climate-driven biodiversity changes for ecosystem functioning of North European rocky shores. Mar. Ecol.-Prog. Ser. 2009;396:245–259. doi: 10.3354/meps08378. [DOI] [Google Scholar]
- 40.Hiscock K, Southward A, Tittley I, Hawkins S. Effects of changing temperature on benthic marine life in Britain and Ireland. Aquatic Conserv: Mar. Freshw. Ecosyst. 2004;14:333–362. doi: 10.1002/aqc.628. [DOI] [Google Scholar]
- 41.Zacherl D, Gaines SD, Lonhart SI. The limits to biogeographical distributions: insights from the northward range extension of the marine snail, Kelletia kelletii (Forbes, 1852) J. Biogeogr. 2003;30:913–924. doi: 10.1046/j.1365-2699.2003.00899.x. [DOI] [Google Scholar]
- 42.Helmuth B, Mieszkowska N, Moore P, Hawkins SJ. Living on the edge of two changing worlds: Forecasting the responses of rocky intertidal ecosystems to climate change. Annu. Rev. Ecol. Evol. Syst. 2006;37:373–404. doi: 10.1146/annurev.ecolsys.37.091305.110149. [DOI] [Google Scholar]
- 43.Ling SD, Johnson CR, Ridgway K, Hobday AJ, Haddon M. Climate-driven range extension of a sea urchin: inferring future trends by analysis of recent population dynamics. Glob. Change Biol. 2009;15:719–731. doi: 10.1111/j.1365-2486.2008.01734.x. [DOI] [Google Scholar]
- 44.Schultz ST, et al. Climate-index response profiling indicates larval transport is driving population fluctuations in nudibranch gastropods from the northeast Pacific Ocean. Limnol. Oceanogr. 2011;56:749–763. doi: 10.4319/lo.2011.56.2.0749. [DOI] [Google Scholar]
- 45.Goddard JHR, et al. Nudibranch range shifts associated with the 2014 warm anomaly in the northeast Pacific. Bulletin of the Southern California Academy of Sciences. 2016;115:15–40. doi: 10.3160/soca-115-01-15-40.1. [DOI] [Google Scholar]
- 46.Kido JS, Murray SN. Variation in owl limpet Lottia gigantea population structures, growth rates, and gonadal production on southern California rocky shores. Mar. Ecol.-Prog. Ser. 2003;257:111–124. doi: 10.3354/meps257111. [DOI] [Google Scholar]
- 47.Hines, A. H. The comparative reproductive ecology of three species of intertidal barnacles. [ed. Stancyk, S. E.]. Reproductive Ecology of Marine Invertebrates. 213–234 (University of South Carolina Press, 1979).
- 48.Hubbs CL. Changes in the fish fauna of western North America correlated with changes in ocean temperature. J. Mar. Res. 1948;7:459–482. [Google Scholar]
- 49.Ruthrof, et al. Subcontinental heat wave triggers terrestrial and marine, multi-taxa responses. Sci. Rep. 2018;8:13094. doi: 10.1038/s41598-018-31236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliver ECJ, et al. The unprecedented 2015/16 Tasman Sea marine heatwave. Nat. Commun. 2017;8:16101. doi: 10.1038/ncomms16101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanford E, Holzman SB, Haney RA, Rand DM, Bertness MD. Larval tolerance, gene flow, and the northern geographic range limit of fiddler crabs. Ecology. 2006;87:2882–2894. doi: 10.1890/0012-9658(2006)87[2882:LTGFAT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 52.McLain DR, Thomas DH. Year-to-year fluctuations of the California Countercurrent and effects on marine organisms. Calif. Coop. Ocean Fish. Invest. Rep. 1983;24:165–181. [Google Scholar]
- 53.Davis JLD. Changes in a tidepool fish assemblage on two scales of environmental variation: Seasonal and El Niño Southern Oscillation. Limnol. Oceanogr. 2000;45:1368–1379. doi: 10.4319/lo.2000.45.6.1368. [DOI] [Google Scholar]
- 54.Largier JL. Considerations in estimating larval dispersal distances from oceanographic data. Ecol. Appl. 2003;13:S71–S89. doi: 10.1890/1051-0761(2003)013[0071:CIELDD]2.0.CO;2. [DOI] [Google Scholar]
- 55.Chavez, F. P. et al. Biological and chemical consequences of the 1997–1998 El Niño in central California waters. Prog. Oceanogr.54, 205–232 (2002).
- 56.Sanford, E. The biogeography of marine communities. [eds Bertness, M. D., Bruno, J. F., Silliman, B. R. & Stachowicz, J. J.]. Marine Community Ecology and Conservation. 131–163 (Sinauer Assoc, 2013).
- 57.O’Connor MI, et al. Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc. Natl. Acad. Sci. USA. 2007;104:1266–1271. doi: 10.1073/pnas.0603422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Byers, J. E. & Pringle, J. M. Going against the flow: retention, range limits and invasions in advective environments. Mar. Ecol.-Prog. Ser.313, 27–41 (2006).
- 59.Figueira WF, Booth DJ. Increasing ocean temperatures allow tropical fishes to survive overwinter in temperate waters. Glob. Change Biol. 2010;16:506–516. doi: 10.1111/j.1365-2486.2009.01934.x. [DOI] [Google Scholar]
- 60.Sorte CJB, Williams SL, Carlton JT. Marine range shifts and species introductions: comparative spread rates and community impacts. Glob. Ecol. Biogeogr. 2010;19:303–316. doi: 10.1111/j.1466-8238.2009.00519.x. [DOI] [Google Scholar]
- 61.Keith SA, Herbert RJH, Nortan PA, Hawkins SJ, Newton AC. Individualistic species limitations of climate-induced range expansions generated by meso-scale dispersal barriers. Diversity Distrib. 2011;17:275–286. doi: 10.1111/j.1472-4642.2010.00734.x. [DOI] [Google Scholar]
- 62.Morris, R. H., Abbott, D. P. & Haderlie, E. C. Intertidal Invertebrates of California. (Stanford University Press, 1980).
- 63.Dawson MN, Grosberg RK, Stuart YE, Sanford E. Population genetic analysis of a recent range expansion: mechanisms regulating the poleward range limit in the volcano barnacle Tetraclita rubescens. Mol. Ecol. 2010;19:1585–1605. doi: 10.1111/j.1365-294X.2010.04588.x. [DOI] [PubMed] [Google Scholar]
- 64.Poloczanska, et al. Global imprint of climate change on marine life. Nat. Clim. Chang. 2013;3:919–925. doi: 10.1038/nclimate1958. [DOI] [Google Scholar]
- 65.Angert, A. L. et al. Do species’ traits predict recent shifts at expanding range edges? Ecol. Lett.14, 677–698 (2011). [DOI] [PubMed]
- 66.Sunday JM, et al. Species traits and climate velocity explain geographic range shifts in an ocean-warming hotspot. Ecol. Lett. 2015;18:944–953. doi: 10.1111/ele.12474. [DOI] [PubMed] [Google Scholar]
- 67.Lonhart SI, Tupen JW. New range records of 12 marine invertebrates: The role of El Niño and other mechanisms in Southern and Central California. Bulletin of the Southern California Academy of Sciences. 2001;100:238–248. [Google Scholar]
- 68.Cowen, R. K. Large-scale pattern of recruitment by the labrid, Semicossyphus pulcher – Causes and implications. J. Mar. Res.43, 719–742 (1985).
- 69.Fenberg PB, Rivadeneira MM. Range limits and geographic patterns of abundance of the rocky intertidal owl limpet, Lottia gigantea. J. Biogeogr. 2011;38:2286–2298. doi: 10.1111/j.1365-2699.2011.02572.x. [DOI] [Google Scholar]
- 70.Arntz WE, et al. El Niño and similar perturbation effects on the benthos of the Humboldt, California, and Benguela Current upwelling ecosystems. Adv. Geosci. 2006;6:243–265. doi: 10.5194/adgeo-6-243-2006. [DOI] [Google Scholar]
- 71.Saura S, Bodin Ö, Fortin MJ. Stepping stones are crucial for species’ long-distance dispersal and range expansion through habitat networks. J. Appl. Ecol. 2014;51:171–182. doi: 10.1111/1365-2664.12179. [DOI] [Google Scholar]
- 72.Reynolds RW, et al. Daily high-resolution-blended analyses for sea surface temperature. J. Climate. 2007;20:5473–5496. doi: 10.1175/2007JCLI1824.1. [DOI] [Google Scholar]
- 73.Halle CM, Largier JL. Surface circulation downstream of the Point Arena upwelling center. Cont. Shelf Res. 2011;31:1260–1272. doi: 10.1016/j.csr.2011.04.007. [DOI] [Google Scholar]
- 74.García-Reyes M, Sydeman WJ. California Multivariate Ocean Climate Indicator (MOCI) and marine ecosystem dynamics. Ecol. Indic. 2017;72:521–529. doi: 10.1016/j.ecolind.2016.08.045. [DOI] [Google Scholar]
- 75.Carlton, J. T. (ed.) The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon. Fourth edition. (University of California Press, 2007).
- 76.Tingley MW, Beissinger SR. Detecting range shifts from historical species occurrences: new perspectives on old data. Trends Ecol. Evol. 2009;24:625–633. doi: 10.1016/j.tree.2009.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sea surface temperature (SST) data analyzed during this study are available from the U.S. National Data Buoy Center repository [http://www.ndbc.noaa.gov], and the Physical Oceanography Distributed Active Archive Center [https://podaac.jpl.nasa.gov/dataset/AVHRR_OI-NCEI-L4-GLOB-v2.0]. Surface flow data analyzed during this study are available from the Bodega Ocean Observing Node repository [http://boon.ucdavis.edu/flow_pointreyes.html] and [http://boon.ucdavis.edu/data/flow/boxes/]. Multivariate Ocean Climate Indicator (MOCI) data analyzed in this study are available from the Farallon Institute repository [http://www.faralloninstitute.org/moci]. All data regarding species’ distributions and occurrences (Tables 1–3) generated and analyzed during this study are included in this article and its Supplementary Information file. All remaining datasets generated and/or analyzed during the current study are available from the corresponding author on request.