Abstract
Background
The Seven Countries study in the 1960s showed that populations in the Mediterranean region experienced lower coronary heart disease (CHD) mortality probably as a result of different dietary patterns. Later observational studies have confirmed the benefits of adherence to a Mediterranean dietary pattern on cardiovascular disease (CVD) risk factors but clinical trial evidence is more limited.
Objectives
To determine the effectiveness of a Mediterranean‐style diet for the primary and secondary prevention of CVD.
Search methods
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 9); MEDLINE (Ovid, 1946 to 25 September 2018); Embase (Ovid, 1980 to 2018 week 39); Web of Science Core Collection (Thomson Reuters, 1900 to 26 September 2018); DARE Issue 2 of 4, 2015 (Cochrane Library); HTA Issue 4 of 4, 2016 (Cochrane Library); NHS EED Issue 2 of 4, 2015 (Cochrane Library). We searched trial registers and applied no language restrictions.
Selection criteria
We selected randomised controlled trials (RCTs) in healthy adults and adults at high risk of CVD (primary prevention) and those with established CVD (secondary prevention). Both of the following key components were required to reach our definition of a Mediterranean‐style diet: high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts) and a high intake of plant‐based foods, including fruits, vegetables and legumes. Additional components included: low to moderate red wine consumption; high consumption of whole grains and cereals; low consumption of meat and meat products and increased consumption of fish; moderate consumption of milk and dairy products. The intervention could be dietary advice, provision of relevant foods, or both. The comparison group received either no intervention, minimal intervention, usual care or another dietary intervention. Outcomes included clinical events and CVD risk factors. We included only studies with follow‐up periods of three months or more defined as the intervention period plus post intervention follow‐up.
Data collection and analysis
Two review authors independently assessed studies for inclusion, extracted data and assessed risk of bias. We conducted four main comparisons:
1. Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention;
2. Mediterranean dietary intervention versus another dietary intervention for primary prevention;
3. Mediterranean dietary intervention versus usual care for secondary prevention;
4. Mediterranean dietary intervention versus another dietary intervention for secondary prevention.
Main results
In this substantive review update, 30 RCTs (49 papers) (12,461 participants randomised) and seven ongoing trials met our inclusion criteria. The majority of trials contributed to primary prevention: comparisons 1 (nine trials) and 2 (13 trials). Secondary prevention trials were included for comparison 3 (two trials) and comparison 4 (four trials plus an additional two trials that were excluded from the main analyses due to published concerns regarding the reliability of the data).
Two trials reported on adverse events where these were absent or minor (low‐ to moderate‐quality evidence). No trials reported on costs or health‐related quality of life.
Primary prevention
The included studies for comparison 1 did not report on clinical endpoints (CVD mortality, total mortality or non‐fatal endpoints such as myocardial infarction or stroke). The PREDIMED trial (included in comparison 2) was retracted and re‐analysed following concerns regarding randomisation at two of 11 sites. Low‐quality evidence shows little or no effect of the PREDIMED (7747 randomised) intervention (advice to follow a Mediterranean diet plus supplemental extra‐virgin olive oil or tree nuts) compared to a low‐fat diet on CVD mortality (hazard ratio (HR) 0.81, 95% confidence interval (CI) 0.50 to 1.32) or total mortality (HR 1.0, 95% CI 0.81 to 1.24) over 4.8 years. There was, however, a reduction in the number of strokes with the PREDIMED intervention (HR 0.60, 95% CI 0.45 to 0.80), a decrease from 24/1000 to 14/1000 (95% CI 11 to 19), moderate‐quality evidence). For CVD risk factors for comparison 1 there was low‐quality evidence for a possible small reduction in total cholesterol (‐0.16 mmol/L, 95% CI ‐0.32 to 0.00) and moderate‐quality evidence for a reduction in systolic (‐2.99 mmHg (95% CI ‐3.45 to ‐2.53) and diastolic blood pressure (‐2.0 mmHg, 95% CI ‐2.29 to ‐1.71), with low or very low‐quality evidence of little or no effect on LDL or HDL cholesterol or triglycerides. For comparison 2 there was moderate‐quality evidence of a possible small reduction in LDL cholesterol (‐0.15 mmol/L, 95% CI ‐0.27 to ‐0.02) and triglycerides (‐0.09 mmol/L, 95% CI ‐0.16 to ‐0.01) with moderate or low‐quality evidence of little or no effect on total or HDL cholesterol or blood pressure.
Secondary prevention
For secondary prevention, the Lyon Diet Heart Study (comparison 3) examined the effect of advice to follow a Mediterranean diet and supplemental canola margarine compared to usual care in 605 CHD patients over 46 months and there was low‐quality evidence of a reduction in adjusted estimates for CVD mortality (HR 0.35, 95% CI 0.15 to 0.82) and total mortality (HR 0.44, 95% CI 0.21 to 0.92) with the intervention. Only one small trial (101 participants) provided unadjusted estimates for composite clinical endpoints for comparison 4 (very low‐quality evidence of uncertain effect). For comparison 3 there was low‐quality evidence of little or no effect of a Mediterranean‐style diet on lipid levels and very low‐quality evidence for blood pressure. Similarly, for comparison 4 where only two trials contributed to the analyses there was low or very low‐quality evidence of little or no effect of the intervention on lipid levels or blood pressure.
Authors' conclusions
Despite the relatively large number of studies included in this review, there is still some uncertainty regarding the effects of a Mediterranean‐style diet on clinical endpoints and CVD risk factors for both primary and secondary prevention. The quality of evidence for the modest benefits on CVD risk factors in primary prevention is low or moderate, with a small number of studies reporting minimal harms. There is a paucity of evidence for secondary prevention. The ongoing studies may provide more certainty in the future.
Keywords: Adult; Humans; Diet, Mediterranean; Blood Pressure; Cardiovascular Diseases; Cardiovascular Diseases/blood; Cardiovascular Diseases/mortality; Cardiovascular Diseases/prevention & control; Cholesterol; Cholesterol/blood; Cholesterol, HDL; Cholesterol, HDL/blood; Cholesterol, LDL; Cholesterol, LDL/blood; Primary Prevention; Primary Prevention/methods; Randomized Controlled Trials as Topic; Secondary Prevention; Secondary Prevention/methods
Plain language summary
Mediterranean‐style diet for the prevention of cardiovascular disease
It is well established that diet plays a major role in cardiovascular disease risk. The traditional Mediterranean dietary pattern is of particular interest because of observations from the 1960s that populations in countries of the Mediterranean region, such as Greece and Italy, had lower mortality from cardiovascular disease compared with northern European populations or the US, probably as a result of different eating habits.
This review assessed the effects of providing dietary advice to follow a Mediterranean‐style diet or provision of foods relevant to the diet (or both) to healthy adults, people at increased risk of cardiovascular disease and those with cardiovascular disease, in order to prevent the occurrence or recurrence of cardiovascular disease and reduce the risk factors associated with it. Definitions of a Mediterranean dietary pattern vary and we included only randomised controlled trials (RCTs) of interventions that reported both of the following key components: a high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts) and a high intake of plant‐based foods, including fruits, vegetables and legumes. Additional components included: low to moderate red wine consumption; high consumption of whole grains and cereals; low consumption of meat and meat products and increased consumption of fish; moderate consumption of milk and dairy products. The control group was no intervention or minimal intervention, usual care or another dietary intervention. We found 30 RCTs (49 papers) that met these criteria. The trials varied enormously in the participants recruited and the different dietary interventions. We grouped studies to look at the effects of following a Mediterranean‐style diet into the following four categories to help us with our interpretation of the results:
1. Mediterranean dietary intervention compared to no intervention or a minimal intervention to prevent the onset of cardiovascular disease;
2. Mediterranean dietary intervention compared to another dietary intervention to prevent the onset of cardiovascular disease;
3. Mediterranean dietary intervention compared to usual care for people with cardiovascular disease to prevent recurrence;
4. Mediterranean dietary intervention compared to another dietary intervention for people with cardiovascular disease to prevent recurrence.
Few trials reported on the occurrence of cardiovascular disease either in those with or without disease to begin with. A large trial in people at high risk of cardiovascular disease found a benefit of the Mediterranean dietary intervention compared to a low‐fat diet on the risk of having a stroke, but not on heart attacks, death from heart disease or other causes. A further study in people with cardiovascular disease found a benefit of the Mediterranean dietary intervention on death from heart disease or other causes. We rated these two studies as providing low to moderate‐quality evidence. We had to exclude two studies from our analyses as concerns had been raised that the data were unreliable. The other trials in the review measured risk factors for cardiovascular disease. There was low to moderate‐quality evidence for some beneficial changes in lipid levels and blood pressure with a Mediterranean‐style diet in people without disease. In people with cardiovascular disease already there was very low to low‐quality evidence that there was no effect of a Mediterranean‐style diet on risk factors. Two trials reported side effects of the diet that were either absent or minor.
The review concludes that, despite the large number of included trials, there is still uncertainty regarding the effects of a Mediterranean‐style diet on cardiovascular disease occurrence and risk factors in people both with and without cardiovascular disease already. We did find seven studies that are still ongoing and when we have the results from these we will incorporate them into the review to help reduce the uncertainty.
Summary of findings
Summary of findings for the main comparison. Mediterranean dietary intervention compared to no intervention or minimal intervention for the primary prevention of cardiovascular disease.
| Mediterranean dietary intervention compared to no intervention or minimal intervention for the primary prevention of cardiovascular disease | ||||||
| Patient or population: adults without cardiovascular disease Setting: community Intervention: Mediterranean dietary intervention Comparison: no intervention or minimal intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no intervention or minimal intervention | Risk with Mediterranean dietary intervention | |||||
| CVD mortality | — | — | — | — | — | Not reported |
| Total mortality | — | — | — | — | — | Not reported |
| Total cholesterol (mmol/L), change from baseline Follow‐up: range 3 months to 24 months | The mean total cholesterol change from baseline ranged from ‐0.003 to ‐0.2 mmol/L | MD 0.16 mmol/L lower (0.32 lower to 0.00) | — | 569 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | — |
| LDL cholesterol (mmol/L), change from baseline Follow‐up: range 3 months to 6 months | The mean LDL cholesterol change from baseline ranged from ‐0.2 to 0.05 mmol/L | MD 0.08 mmol/L lower (0.26 lower to 0.09 higher) | — | 389 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | — |
| HDL cholesterol (mmol/L), change from baseline Follow‐up: range 3 months to 24 months | The mean HDL cholesterol change from baseline ranged from ‐0.07 to 0.03 mmol/L | MD 0.02 mmol/L higher (0.04 lower to 0.08 higher) | — | 569 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 2 4 | — |
| Triglycerides (mmol/L), change from baseline | See comment | See comment | — | 480 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Studies were not pooled statistically due to substantial heterogeneity (I2 = 92%) |
| Systolic blood pressure (mmHg), change from baseline Follow‐up: range 3 months to 24 months | The mean systolic blood pressure change from baseline ranged from ‐1 to 1.4 mmHg | MD 2.99 mmHg lower (3.45 lower to 2.53 lower) | — | 269 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | — |
| Diastolic blood pressure (mmHg), change from baseline Follow‐up: range 3 months to 24 months | The mean diastolic blood pressure change from baseline ranged from ‐1 to 1.7 mmHg | MD 2.00 mmHg lower (2.29 lower to 1.71 lower) | — | 269 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | — |
| Adverse events | — | — | — | — | — | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVD: cardiovascular disease; HDL: high‐density lipoprotein; LDL: low‐density lipoprotein; MD: mean difference; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by one level for risk of bias. Majority of studies were at unclear risk of selection bias or attrition bias, or both.
2Downgraded by one level for inconsistency. Forest plot shows different directions of effect and I2 value is very high.
3Downgraded by one level for imprecision due to small number of participants (< 400).
4Downgraded one level for inconsistency. Studies could not be pooled due to very high heterogeneity, and forest plots show different directions of effect.
Summary of findings 2. Mediterranean dietary intervention compared to another dietary intervention for the primary of cardiovascular disease.
| Mediterranean dietary intervention compared to another dietary intervention for the primary of cardiovascular disease | ||||||
| Patient or population: adults without cardiovascular disease Setting: community Intervention: Mediterranean dietary intervention Comparison: another dietary intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with another dietary intervention | Risk with Mediterranean dietary intervention | |||||
| CVD mortality Follow‐up: mean 4.8 years | Study population | HR 0.81 (0.50 to 1.32) | 7447 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 12 per 1000 | 10 per 1000 (6 to 16) | |||||
| Total mortality Follow‐up: mean 4.8 years | Study population | HR 1.00 (0.81 to 1.24) | 7447 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 47 per 1000 | 47 per 1000 (38 to 57) | |||||
| Myocardial infarction Follow‐up: mean 4.8 years | Study population | HR 0.79 (0.57 to 1.10) | 7447 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — | |
| 16 per 1000 | 12 per 1000 (9 to 17) | |||||
| Stroke Follow‐up: mean 4.8 years | Study population | HR 0.60 (0.45 to 0.80) | 7447 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | — | |
| 24 per 1000 | 14 per 1000 (11 to 19) | |||||
| Peripheral arterial disease | Study population | HR 0.42 (0.28 to 0.61) | 7447 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | — | |
| 18 per 1000 | 8 per 1000 (5 to 11) | |||||
| Total cholesterol (mmol/L), change from baseline Follow‐up: range 3 months to 4.8 years | The mean total cholesterol change from baseline was ‐0.29 to 0.51 mmol/L | MD 0.13 mmol/L lower (0.3 lower to 0.04 higher) | — | 939 (7 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | — |
| LDL cholesterol (mmol/L), change from baseline Follow‐up: range 3 months to 4.8 years | The mean LDL cholesterol change from baseline ranged from ‐0.18 to 0.27 mmol/L | MD 0.15 mmol/L lower (0.27 lower to 0.02 lower) | — | 947 (7 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | — |
| HDL cholesterol (mmol/L), change from baseline Follow‐up: range 3 months to 4.8 years | The mean HDL cholesterol change from baseline ranged from ‐0.02 to 0.16 mmol/L | MD 0.02 mmol/L higher (0.01 lower to 0.04 higher) | — | 891 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | — |
| Triglycerides (mmol/L), change from baseline Follow‐up: range 3 months to 4.8 years | The mean triglycerides change from baseline ranged from ‐0.44 to 1.32 mmol/L | MD 0.09 mmol/L lower (0.16 lower to 0.01 lower) | — | 939 (7 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | — |
| Systolic blood pressure (mmHg), change from baseline Follow‐up: range 3 months to 12 months | The mean systolic blood pressure change from baseline ranged from ‐10.4 to 6.9 mmHg | MD 1.5 mmHg lower (3.92 lower to 0.92 higher) | — | 448 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | — |
| Diastolic blood pressure (mmHg), change from baseline Follow‐up: range 3 months to 12 months | The mean diastolic blood pressure change from baseline ranged from ‐8.1 to 5.3 mmHg | MD 0.26 mmHg lower (2.41 lower to 1.9 higher) | — | 448 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | — |
| Adverse events | Adverse effects were reported by only one RCT ‐ no adverse events were noted for either dietary intervention in the PREDIMED trial. | — | 7447 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | — | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVD: cardiovascular disease; HDL: high‐density lipoprotein;HR: hazard ratio; LDL: low‐density lipoprotein; MD: mean difference; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by one level for imprecision. Confidence interval is wide enough to include both an important increase or decrease in the outcome.
2Downgraded by one level for risk of bias. The only included study was the PREDIMED trial, which was retracted due to methodological issues with randomisation, re‐analysed and republished.
3Downgraded by one level for risk of bias. Majority of studies are at unclear risk of selection bias, attrition bias, or both.
4Downgraded by one level for inconsistency. High I2 and forest plots shows different directions of effect.
Summary of findings 3. Mediterranean dietary intervention compared to usual care for secondary prevention of cardiovascular disease.
| Mediterranean dietary intervention compared to usual care for secondary prevention of cardiovascular disease | ||||||
| Patient or population: adults with established cardiovascular disease Setting: community Intervention: Mediterranean dietary intervention Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with Mediterranean dietary intervention | |||||
| CVD mortality Follow‐up: mean 46 months | Study population | RR 0.35 (0.15 to 0.82) | 605 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | — | |
| 63 per 1000 | 22 per 1000 (9 to 51) | |||||
| Total mortality Follow‐up: mean 4 years | Study population | RR 0.44 (0.21 to 0.92) | 605 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | — | |
| 79 per 1000 | 35 per 1000 (17 to 73) | |||||
| Total cholesterol (mmol/L), change from baseline Follow‐up: range 1 year to 4 years | The mean total cholesterol change from baseline ranged from ‐0.22 to ‐0.31 mmol/L | MD 0.07 mmol/L higher (0.19 lower to 0.33 higher) | — | 441 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 | — |
| LDL cholesterol (mmol/L), change from baseline Follow‐up: range 1 year to 4 years | The mean LDL cholesterol change from baseline ranged from ‐0.26 to ‐0.41 | MD 0.11 higher (0.09 lower to 0.31 higher) | — | 441 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 | — |
| HDL cholesterol (mmol/L), change from baseline Follow‐up: range 1 year to 4 years | The mean HDL cholesterol change from baseline ranged from 0 to 0.15 mmol/L | MD 0.01 mmol/L lower (0.08 lower to 0.07 higher) | — | 441 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 | — |
| Triglycerides (mmol/L), change from baseline Follow‐up: range 1 year to 4 years | The mean triglycerides change from baseline ranged from ‐0.02 to ‐0.08 mmol/L | MD 0.14 mmol/L lower (0.38 lower to 0.1 higher) | — | 441 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 | — |
| Systolic blood pressure (mmHg), change from baseline Follow‐up: 4 years | The mean systolic blood pressure change from baseline was 9 mmHg | MD 2 mmHg lower (5.29 lower to 1.29 higher) | — | 339 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | — |
| Diastolic blood pressure (mmHg), change from baseline Follow‐up: 4 years | The mean diastolic blood pressure change from baseline was 5 mmHg | MD 1 mmHg lower (4.29 lower to 2.29 higher) | — | 339 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | — |
| Adverse events | Adverse effects were reported in only one RCT. Two of 302 CHD patients noted margarine‐related side effects of colitis and diarrhoea in The Lyon Diet Heart Study. | — | 605 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | — | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVD: cardiovascular disease; HDL: high‐density lipoprotein;LDL: low‐density lipoprotein; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by two levels for risk of bias. The only included study had an unclear randomisation method and the modified Zelen design may have introduced other biases, although the study was at low risk of bias for allocation concealment and attrition.
2Downgraded by two levels for risk of bias as both included studies were at unclear risk of selection bias or attrition bias, or both, and the majority weight in the meta‐analysis was for the study with a modified Zelen design.
3Downgraded by one level for imprecision due to small number of participants (N < 400).
4Downgraded by two levels for imprecision due to small number of participants and wide CI that includes both important increases and decreases in the outcome.
Summary of findings 4. Mediterranean dietary intervention compared to another dietary intervention for the secondary prevention of cardiovascular disease.
| Mediterranean dietary intervention compared to another dietary intervention for the secondary prevention of cardiovascular disease | ||||||
| Patient or population: adults with established cardiovascular disease Setting: community Intervention: Mediterranean dietary intervention Comparison: another dietary intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with another dietary intervention | Risk with Mediterranean dietary intervention | |||||
| Total cardiac endpoints (all‐cause and cardiac deaths, myocardial infarction, hospital admissions for heart failure, unstable angina or stroke, unadjusted) Follow‐up: 2 years | Study population | RR 0.98 (0.40 to 2.41) | 101 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | Total cardiac endpoints was used instead of the 2 individual outcomes cardiovascular mortality and total mortality because this was the format used in the only trial reporting this. | |
| 160 per 1000 | 157 per 1000 (64 to 386) | |||||
| Total cholesterol (mmol/L), change from baseline (sensitivity analysis without Singh studies) | See comment | See comment | — | (0 RCTs) | — | None of the included studies measured this outcome when Singh studies were removed in sensitivity analyses. |
| LDL cholesterol (mmol/L), change from baseline (sensitivity analysis without Singh studies) Follow‐up: 2 years | The mean LDL cholesterol change from baseline was 0.13 mmol/L | MD 0.08 mmol/L higher (0.26 lower to 0.42 higher) | — | 71 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | — |
| HDL cholesterol (mmol/L), change from baseline (sensitivity analysis without Singh studies) Follow‐up: 2 years |
The mean HDL cholesterol change from baseline was 0.10 mmol/L | MD 0.05 mmol/L lower (0.17 lower to 0.06 higher) | — | 71 (1 RCT) | ⊕⊕⊝⊝ LOW 1 3 | — |
| Triglycerides (mmol/L), change from baseline (sensitivity analysis without Singh studies) Follow‐up: 2 years |
The mean triglycerides change from baseline was ‐0.63 mmol/L | MD 0.46 mmol/L higher (0.24 lower to 1.16 higher) | — | 71 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | — |
| Systolic blood pressure (mmHg), change from baseline (sensitivity analysis without Singh studies) Follow‐up range: 12 weeks to 2 years |
The mean systolic blood pressure change from baseline ranged from 4 to ‐9.33 mmHg | MD 1.76 mmHg higher (2.8 lower to 6.33 higher) | — | 150 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 4 | — |
| Diastolic blood pressure (mmHg), change from baseline (sensitivity analysis without Singh studies) Follow‐up range: 12 weeks to 2 years |
The mean diastolic blood pressure change from baseline ranged from 1 to ‐9.23 mmHg | MD 0.98 mmHg higher (1.97 lower to 3.93 higher) | — | 150 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 4 | — |
| Adverse events | — | — | — | — | — | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HDL: high‐density lipoprotein;LDL: low‐density lipoprotein; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by one level for risk of bias. Only included study had unclear random sequence generation and unclear attrition.
2Downgraded by two levels for imprecision due to small sample size and wide confidence interval that crosses the null.
3Downgraded by one level for imprecision due to small sample size. Although CI includes the null, it is reasonably narrow.
4Downgraded by one level for risk of bias. Both studies had unclear randomisation method, although allocation was concealed. One study was at low risk of attrition bias, the other at unclear risk of attrition bias.
Background
Description of the condition
Cardiovascular disease (CVD) is currently the leading cause of mortality worldwide, causing one‐third of deaths globally (Roth 2017). In 2015, there were more than 400 million individuals living with CVD and nearly 18 million CVD deaths worldwide, based on the most recent estimates from the Global Burden of Disease (GBD) consortium (Roth 2017). Importantly, data suggest that CVD mortality trends are no longer declining in high‐income regions, whereas low‐ and middle‐income countries are experiencing an increasing burden from CVD‐related deaths (Roth 2017). According to World Health Organization's estimates, over 80% of CVD deaths occur in low‐ and middle‐income countries and the number of CVD deaths is expected to increase to 23.3 million by 2030, with CVD remaining the single leading cause of mortality globally (Mathers 2006; WHO 2011).
In Europe, more than 85 million people currently (2015) live with CVD, which causes nearly 4 million deaths annually, accounting for 45% of the overall mortality burden. Death rates from both ischaemic heart disease (IHD) and stroke are generally higher in Central and Eastern Europe than in Northern, Southern and Western Europe (European Heart Network 2017).
The societal burden of CVD is substantial, in terms of both direct health care costs and indirect costs, such as productivity losses and informal care of people living with CVD. For example, it is estimated that CVD costs the European Union economy €210 billion a year (European Heart Network 2017).
In addition to the role of genetic, demographic and socioeconomic characteristics, modifiable risk factors for CVD, such as high blood pressure, high cholesterol, tobacco smoking, obesity and poor diet are now widespread throughout the world, accounting for a large proportion of the overall CVD burden (Roth 2017). This calls for cost‐effective preventive strategies to address these risk factors in the first place.
Specifically, there is a longstanding recognition that diet plays a major role in the aetiology of many chronic diseases, thereby contributing to significant geographic variations in morbidity and mortality rates from chronic disease across different countries and populations worldwide (WHO 2003). For example, it is estimated that dietary factors are responsible for the largest contribution, among all behavioural risk factors, to the risk of CVD mortality at the population level across Europe (European Heart Network 2017).
In particular, the Mediterranean dietary pattern has been long investigated for its potential beneficial effects on a range of chronic disease outcomes, starting from ecological data in the context of the Seven Countries study in the 1960s (Keys 1986). Several observational studies have shown greater longevity and quality of life, as well as reduced mortality and morbidity from CVD, cancer and other nutrition‐related diseases with greater adherence to a Mediterranean dietary pattern (Benetou 2008; Buckland 2009; Feart 2009; Fung 2009; Knoops 2004; Lagiou 2006; Mitrou 2007; Trichopoulou 1995; Trichopoulou 2003; Trichopoulou 2007). Systematic reviews of observational prospective studies have confirmed that greater adherence to a Mediterranean diet is associated with a significant improvement in health status and a significant reduction in overall mortality, as well as in morbidity and mortality from CVD and other major chronic diseases (Dinu 2018; Grosso 2017; Rosato 2017; Sofi 2008; Sofi 2010; Sofi 2014). For example, in a comprehensive meta‐analysis of observational prospective studies including 4,172,412 participants, a two‐point increase in adherence score to the Mediterranean diet was associated with an 8% reduction in overall mortality and a 10% reduced risk of CVD (Sofi 2014). These results were further corroborated by a recent overview of the evidence from meta‐analyses of both observational studies and randomised clinical trials (Dinu 2018). This latest review provides robust evidence supporting beneficial effects of a greater adherence to the Mediterranean diet on a range of health outcomes, including overall mortality, CVD, coronary heart disease and myocardial infarction (Dinu 2018). Furthermore, the Mediterranean diet has been associated with favourable effects on major CVD risk factors. For example, studies have documented a decreased incidence of hypertension, diabetes mellitus and metabolic syndrome as a whole with a greater adherence to a Mediterranean dietary pattern (Martinez‐Gonzalez 2008; Nunez‐Cordoba 2009; Psaltopoulou 2004; Rumawas 2009; Sánchez‐Taínta 2008). These findings have been corroborated by systematic reviews supporting beneficial effects of the Mediterranean diet on the metabolic syndrome and its individual components (Buckland 2008; Kastorini 2011).
Against the large body of epidemiological observational studies, there is less evidence from well‐conducted and adequately powered randomised controlled trials (RCTs), especially with regard to the potential efficacy of the Mediterranean diet in the primary prevention of CVD (Serra‐Majem 2006). Most of the RCTs have addressed the effect of a Mediterranean type of diet on the occurrence of complications and recurrent events in people with existing CVD, showing favourable effects in CVD secondary prevention (Barzi 2003; de Lorgeril 1994; de Lorgeril 1996; de Lorgeril 1999; de Lorgeril 2011; Panagiotakos 2016). There is also considerable variability in the definition of, and duration of, the interventions evaluated.
Recent evidence from the PREDIMED (Prevención con Dieta Mediterránea) study, a large primary prevention trial (N = 7447) among high‐risk individuals in Spain, showed that a modified Mediterranean diet supplemented with extra‐virgin olive oil or nuts was associated with major cardiovascular benefits (Estruch 2013). Specifically, both interventions groups experienced an approximately 30% reduction in the rate of major cardiovascular events (myocardial infarction, stroke or death from cardiovascular causes) compared to the control diet group (advice to reduce dietary fat), after a median follow‐up of 4.8 years (Estruch 2013). This trial has recently been retracted and re‐analysed as methodological issues concerning randomisation came to light for 2 of the 11 sites, and the inclusion of non‐randomised second household members. The new publication controlled for these in the analyses and has conducted a series of sensitivity analyses excluding these sites where they have found similar results for clinical endpoints (Estruch 2018).
Description of the intervention
The original Mediterranean type of diet reflects the common dietary pattern of communities in countries of the Mediterranean region in the early 1960s (Keys 1986), which was an expression of common cultural and historical roots, and a shared set of lifestyle and eating habits rather than a mere assortment of specific micro‐ and macro‐nutrients (Trichopoulou 1997). The Mediterranean diet has been defined (Helsing 1989; Nestle 1995; Serra‐Majem 1993; Willett 1995), and includes the following dietary factors: a high intake of plant foods comprising mainly fruits and vegetables, cereals and whole‐grain breads, beans, nuts and seeds; locally grown, fresh and seasonal, unprocessed foods; large quantities of fresh fruit consumed daily whereas concentrated sugars or honey are consumed a few times per week in smaller quantities; olive oil as a main cooking ingredient and source of fat; low to moderate amounts of cheese and yogurt; low quantities of red meat and higher quantities of fish; and low to moderate amounts of red wine often accompanying main meals.
The intervention under investigation for the current review was dietary advice to follow a Mediterranean‐style diet or provision of foods relevant to the Mediterranean diet or both. At least two key components were required to reach our definition of a Mediterranean‐style diet (Helsing 1989; Nestle 1995; Serra‐Majem 1993; Willett 1995).
These are the following:
1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts);
2. high intake of plant‐based foods, including fruits, vegetables and legumes.
The rationale for this definition is based on recent work (Grosso 2017; Martínez‐González 2017), which emphasises that protective effects of the diet appear to be most attributable to olive oil, fruits, vegetables and legumes. We chose at least two of the key active components as our definition of a Mediterranean‐style diet as one component does not constitute a dietary pattern.
Additional components include:
3. low to moderate red wine consumption;
4. high consumption of whole grains and cereals;
5. low consumption of meat and meat products and increased consumption of fish;
6. moderate consumption of milk and dairy products.
The traditional Mediterranean diet is not low in fat but is characterised by a relative increase in monounsaturated fats in the form of olive oil and tree nuts compared to saturated fats.
How the intervention might work
There is a large quantity of observational and experimental evidence supporting potential mechanisms to explain the beneficial effect of the Mediterranean diet on cardiovascular health (Serra‐Majem 2006). For example, there is evidence of favourable effects of the Mediterranean diet on insulin resistance and endothelium‐dependent vasoreactivity, as well as of the antioxidant and anti‐inflammatory effects of the Mediterranean diet and its individual components such as fruits and vegetables, olive oil, nuts, whole grains, fish and red wine (Chrysohoou 2004; Dai 2008; Estruch 2010; Pitsavos 2005; Ryan 2000). In addition, the Mediterranean dietary pattern has been associated with beneficial effects on many cardiovascular risk factors, including lipoproteins, obesity, diabetes mellitus and hypertension (Buckland 2008; Kastorini 2011; Martinez‐Gonzalez 2008; Nunez‐Cordoba 2009; Psaltopoulou 2004; Rumawas 2009; Sánchez‐Taínta 2008). There is additionally a large body of consistent epidemiological evidence supporting the notion that light to moderate red wine intake (one or two drinks/day), and moderate alcohol consumption in general, is associated with reduced all‐cause and cardiovascular mortality and morbidity, and has beneficial effects on cardiovascular risk factors, when compared with both abstention and heavy drinking (Brien 2011; Corrao 2000; Di Castelnuovo 2002; Di Castelnuovo 2006; Ronksley 2011). In contrast, excess alcohol consumption is associated with an increased risk of cardiovascular mortality and morbidity, primarily through an increased risk of hypertension and stroke (Stranges 2004; Taylor 2009).
Recent trial evidence also suggests anti‐inflammatory effects of the Mediterranean diet, with potential benefits on endothelial function as well (Estruch 2010; Schwingshackl 2014). Overall, the protective effects of the Mediterranean diet on health outcomes are likely derived from synergistic interactions among different components as a whole dietary pattern rather than from relative effects of specific food groups (Grosso 2017).
Why it is important to do this review
Modification of dietary factors forms an integral part of the primary prevention of cardiovascular diseases, as well as of their clinical management (secondary prevention). A Mediterranean‐style dietary pattern is likely to produce a beneficial effect on the occurrence of several chronic diseases, primarily CVD, which are closely linked to lifestyle and eating habits. This notion is corroborated by the dietary recommendations of several scientific associations for the prevention of major chronic disease (AHA 2006; WHO 2003). We aim to update and expand our previous systematic review (New Reference), to examine the effectiveness of a Mediterranean‐style diet in both the primary and secondary prevention of CVD, so that the findings are of use to a broader audience, and to explore heterogeneity further with an increased number of included studies. We will include participants at risk as well as those with established CVD to inform guidelines for both prevention and management of CVD. We will also consider any control group and stratify results based on this.
Objectives
To determine the effectiveness of a Mediterranean‐style diet for the primary and secondary prevention of CVD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Adults of all ages (18 years or more) without established CVD to examine the effects of a Mediterranean‐style diet on the primary prevention of CVD, and those with established CVD to determine the effects of the intervention on secondary prevention. Established CVD was defined as people who had experienced a previous myocardial infarction (MI), stroke, revascularisation procedure (coronary artery bypass grafting (CABG) or percutaneous transluminal coronary angioplasty (PTCA)), people with angina, or angiographically defined CHD, cerebrovascular disease (stroke) and peripheral arterial disease. For participants without established CVD we included both those from the general population and those at increased risk of CVD. We excluded studies that were conducted exclusively in patients with type 2 diabetes (T2DM) as whilst having T2DM is a major risk factor for CVD, patients with T2DM form a specific group and interventions for diabetes are covered specifically by the Cochrane Metabolic and Endocrine Disorders review group. We performed stratified analyses to examine the effects of a Mediterranean‐style diet on those with and without established CVD.
Types of interventions
The intervention under investigation for the current review was dietary advice to follow a Mediterranean‐style diet or a provision of foods relevant to the Mediterranean diet, or both. At least two key components were required to reach our definition of a Mediterranean‐style diet (Helsing 1989; Nestle 1995; Serra‐Majem 1993; Willett 1995).
These are the following:
1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts);
2. high intake of plant‐based foods, including fruits, vegetables and legumes.
The rationale for this definition is based on recent work (Grosso 2017; Martínez‐González 2017), which emphasises that protective effects of the diet appear to be most attributable to olive oil, fruits, vegetables and legumes. We chose at least two of the key active components as our definition of a Mediterranean‐style diet as one component does not constitute a dietary pattern.
Additional components include:
3. low to moderate red wine consumption;
4. high consumption of whole grains and cereals;
5. low consumption of meat and meat products and increased consumption of fish;
6. moderate consumption of milk and dairy products.
The traditional Mediterranean diet is not low in fat but is characterised by a relative increase in monounsaturated fats in the form of olive oil and tree nuts compared to saturated fats.
We were interested in studying the effects of a Mediterranean‐style diet and so excluded studies with multi component interventions including other dietary interventions or lifestyle interventions such as exercise unless the effects of the Mediterranean‐style diet were reported separately.
We included only studies with follow‐up periods of three months or more defined as the intervention period plus post intervention follow‐up. We considered trials where the comparison group was no intervention or minimal intervention (e.g. leaflet to follow a dietary pattern with no person‐to‐person intervention or reinforcement) and also other dietary interventions.
In the main analysis we did not combine primary and secondary prevention studies and different comparator groups as this would have made interpretation of the results difficult due to heterogeneity; instead we conducted four main analyses:
Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention;
Mediterranean dietary intervention versus another dietary intervention for primary prevention;
Mediterranean dietary intervention versus usual care for secondary prevention;
Mediterranean dietary intervention versus another dietary intervention for secondary prevention.
Types of outcome measures
Endpoints were measured using validated measures.
Primary outcomes
Cardiovascular mortality.
All‐cause mortality.
Non‐fatal endpoints such as MI, CABG, PTCA, angina or angiographically defined CHD, stroke, carotid endarterectomy or peripheral arterial disease (PAD).
Secondary outcomes
Changes in blood lipids (total cholesterol, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol, triglycerides) and blood pressure (systolic and diastolic blood pressure).
Occurrence of type 2 diabetes as a major CVD risk factor.
Health‐related quality of life.
Adverse effects (as defined by the authors of the included trials).
Costs.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 9) in theCochrane Library (searched 26 September 2018);
MEDLINE Daily and MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations (Ovid, 1946 to 25 September 2018) (searched 26 September 2018);
Embase (Ovid, 1980 to 2018 week 39) (searched 26 September 2018);
Web of Science Core Collection (Thomson Reuters, 1900 to 26 September 2018) (searched 26 September 2018);
DARE Issue 2 of 4, 2015 (Cochrane Library) – no longer updated (searched 26 June 2017);
HTA Issue 4 of 4, 2016 (Cochrane Library) – no longer updated (searched 26 June 2017);
NHS EED Issue 2 of 4, 2015 (Cochrane Library) – no longer updated (searched 26 June 2017).
We used medical subject headings (MeSH) or equivalent and text word terms and the Cochrane sensitivity‐maximising RCT filter for MEDLINE (Lefebvre 2011), and adaptations of it for Embase and Web of Science. We applied no language restrictions. We tailored searches to individual databases (Appendix 1).
Searching other resources
In addition, we checked reference lists of reviews for additional studies.
We searched ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) for ongoing trials. The the metaRegister of controlled trials (mRCT) (www.controlled‐trials.com/mrct) is no longer available and was searched last for the previous review publication (Rees 2013).
We contacted authors where necessary for additional information. We will continue to monitor retraction statements for included studies.
Data collection and analysis
Selection of studies
Two review authors (of KR, NM, AT, LE, DW, AV, AD) independently screened titles and abstracts for inclusion of all the potential studies identified as a result of the searches and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We combined the responses from each of the two review authors and retrieved the full‐text study reports/publication. Two review authors (of KR, NM, AT, LE, DW, AV, AD, LH) independently screened the full text and identified studies for inclusion and exclusion using the pre‐specified inclusion criteria. In the case of any disagreements, a third author arbitrated (KR). We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram.
Data extraction and management
We used a data collection form for study characteristics and outcome data, which we had piloted. Two review authors (of KR, LE, DW, AV, AD, LH) extracted the following characteristics from included studies:
Methods: study design, total duration of study, number of study centres and location, study setting and date of study.
Participants: N randomised, N lost to follow‐up/withdrawn, N analysed, mean age, age range, gender, primary or secondary prevention (at increased risk of CVD, or established CVD), inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant treatments/medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Additional notes, e.g. conflicts of interest of trial authors.
Disagreements were resolved by consensus or by involving a third person (KR). One review author (KR) transferred data into the Review Manager (RevMan 2014) file (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the data extraction form.
Assessment of risk of bias in included studies
Two review authors (of KR, LE, DW, AV, AD, LH) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another author (KR). We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. We expected blinding of participants and personnel to be difficult to achieve and unlikely for trials of dietary interventions and so we have not recorded this as high risk but unclear.
For cluster‐randomised trials we intended to follow the guidance in section 16.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and to explore the following: recruitment bias, baseline imbalance, loss of clusters, incorrect analysis and comparability with individually randomised trials. However, no cluster‐randomised trials met our inclusion criteria.
When considering treatment effects, we took into account the risk of bias for the studies that contribute to that outcome.
Measures of treatment effect
We processed data in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We expressed dichotomous outcomes as risk ratios (RR) with 95% confidence intervals (CI). Where available we have used adjusted estimates of treatment effect as hazard ratios, and used the inverse variance method to pool these statistically. For continuous variables, we compared net changes (i.e. intervention group minus control group differences) and calculated mean differences (MD) and 95% CIs for each study. We intended to use standardised mean difference (SMD) where different scales had been used to measure the same outcome (e.g. quality of life) and to test the robustness of using this and MD using sensitivity analyses. However, none of the included studies reported these outcomes. We narratively described skewed data reported as medians and interquartile ranges.
Unit of analysis issues
We intended to analyse cluster‐randomised trials in accordance with guidance in section 16.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), however no cluster‐RCTs met the inclusion criteria. For trials with multiple arms we divided the control group N by the number of arms to avoid double‐counting in meta‐analyses. We analysed outcomes at the longest period of follow‐up where multiple measurements had been taken unless there was significant (> 30%) attrition.
Dealing with missing data
Where standard deviations (SD) for outcomes were not reported, other variance measures such as standard errors and confidence intervals were not available to derive SDs from and we were unable to obtain information from study authors, we imputed these following the methods presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where studies did not report results as change from baseline for continuous outcomes, we calculated these and the SD differences following the methods presented in the Cochrane Handbook for Systematic Reviews of Interventions for imputing these (Section 16.1.3.2 Imputing standard deviations for changes from baseline; Higgins 2011), and assumed a correlation of 0.5 between baseline and follow‐up measures as suggested by Follman 1992.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. When we identified substantial heterogeneity (50% to 90%) we reported it and explored possible causes by prespecified subgroup analysis. Where heterogeneity was considerable (75% to 100%), we did not pool studies statistically but presented them in forest plots and suppressed the summary effect estimate.
Assessment of reporting biases
For outcomes where we were able to pool more than 10 trials, we created and examined a funnel plot to explore possible publication bias and these fed into the GRADE assessment (see below).
Data synthesis
We undertook meta‐analyses only where this was meaningful, i.e. if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense. We used a random‐effects model as we cannot assume that all studies in the meta‐analysis are estimating the same intervention effect, but rather are estimating intervention effects that follow a distribution across studies.
'Summary of findings' table
We created a 'Summary of findings' tables using the following outcomes:
Cardiovascular mortality.
All‐cause mortality.
Non‐fatal endpoints such as MI, CABG, PTCA, angina or angiographically defined CHD, stroke, carotid endarterectomy or peripheral arterial disease (PAD).
Changes in blood lipids (total cholesterol, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol, triglycerides) and blood pressure (systolic and diastolic blood pressure).
Adverse events.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro software (https://gradepro.org/). We created a separate 'Summary of findings' table for each comparison:
Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention;
Mediterranean dietary intervention versus another dietary intervention for primary prevention;
Mediterranean dietary intervention versus usual care for secondary prevention;
Mediterranean dietary intervention versus another dietary intervention for secondary prevention.
We justified all decisions to downgrade the quality of studies using footnotes and made comments to aid the reader's understanding of the review where necessary.
Two review authors (AT, NM) working independently made judgements about evidence quality, with disagreements resolved by discussion or involving a third author (KR). We justified, documented and incorporated the judgements into reporting of results for each outcome.
Subgroup analysis and investigation of heterogeneity
We have stratified the main analyses for the following comparisons, to address heterogeneity and aid interpretation of findings:
Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention;
Mediterranean dietary intervention versus another dietary intervention for primary prevention;
Mediterranean dietary intervention versus usual care for secondary prevention;
Mediterranean dietary intervention versus another dietary intervention for secondary prevention.
We have also performed subgroup analyses to examine the effect of interventions described as the Mediterranean diet or style of diet or those including both of the core components of increased fruit and vegetable consumption and exchange of saturated fat for monounsaturated fat, compared with other interventions meeting our criteria.
Sensitivity analysis
We excluded two studies from the main analysis in sensitivity analyses where concerns have been publicly made as to the reliability of the data (Singh 1992; Singh 2002).
We intended to conduct sensitivity analyses including only studies at low risk of bias in the domains of random sequence generation, allocation concealment and incomplete outcome data, but for the majority of studies these domains were rated as unclear.
Reaching conclusions
We based our conclusions only on findings from the quantitative and narrative synthesis of included studies for this review. We avoided making recommendations for practice and our implications for research suggest priorities for future research and outline what the remaining uncertainties are in the area.
Results
Description of studies
Results of the search
The original review explored the effects of a Mediterranean‐style diet compared to no intervention or minimal intervention for the primary prevention of CVD and included 11 RCTs (New Reference). The current review represents a substantive update and expansion in scope to include also secondary prevention in those with established CVD and other dietary interventions as comparison groups.
The previous review, New Reference, identified 11 RCTs and one ongoing trial and six of these RCTs are included in the current review. Five studies in the previous review were excluded from this update as the definition of a Mediterranean‐style diet has been refined further following expert review and recent evidence suggesting the most likely active components (see Types of interventions). Searching to September 2018 identified a further 12,133 references, which reduced to 9483 after de‐duplication. We also re‐screened the database from the original review given the expansion in scope in terms of both participants and comparison groups. From the updated searching we shortlisted 187 studies and these went forward for formal inclusion and exclusion. From re‐screening the original database we shortlisted 77 studies and these went forward for formal inclusion and exclusion. Following full‐text review and collation of multiple papers for individual studies 30 RCTs (49 papers) and seven ongoing trials met the inclusion criteria. The flow of studies throughout the review is presented in the PRISMA diagram in Figure 1.
1.
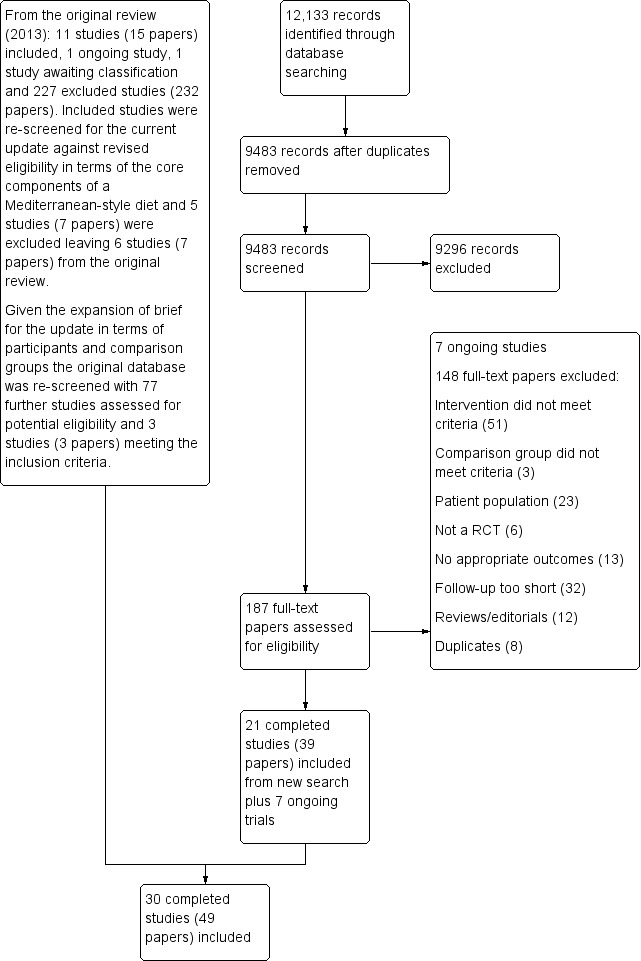
Study flow diagram.
Included studies
Details of the methods, participants, intervention, comparison group and outcome measures for each of the studies included in the review are shown in the Characteristics of included studies table. A summary of the description of included studies is presented below for each comparison group for clarity.
1. Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention
Nine trials (11 papers) were included with 1337 participants randomised.
The health status of participants varied between studies. The majority of participants were classified as healthy and were recruited by three of the trials (Castagnetta 2002; Djuric 2009; Konstantinidou 2010), with two further trials recruiting elderly people (Clements 2017; Davis 2017). The remaining four trials recruited previously untreated hypercholesteraemic participants (Wardle 2000), elderly participants with long‐standing hypercholesterolaemia (Lindman 2004), and sedentary people with metabolic syndrome (Esposito 2004) or metabolic disease (Chasapidou 2014). Two trials recruited only women: one recruited only postmenopausal women (Castagnetta 2002), and the other trial recruited women aged 25 to 65 years (Djuric 2009). In contrast, one trial recruited only men (Lindman 2004), and the remaining six recruited both men and women (Chasapidou 2014; Clements 2017; Davis 2017; Esposito 2004; Konstantinidou 2010; Wardle 2000). The trials were conducted in the US (Djuric 2009), Italy (Castagnetta 2002; Esposito 2004), Spain (Konstantinidou 2010), Greece (Chasapidou 2014), Norway (Lindman 2004), Australia (Davis 2017) and the UK (Clements 2017; Wardle 2000). The duration of the intervention and follow‐up periods varied: three months (Konstantinidou 2010; Wardle 2000), six months (Castagnetta 2002; Chasapidou 2014; Davis 2017; Djuric 2009; Lindman 2004), one year (Clements 2017), and two years (Esposito 2004).
We identified four ongoing trials (Hardman 2015; NCT03053843; NCT03129048; Sotos‐Prieto 2017) (see Characteristics of ongoing studies table). All describe the intervention as a Mediterranean diet. Three will report CVD risk factors in an elderly Australian population (Hardman 2015), older obese adults from the US (NCT03129048), and firefighters from the US (Sotos‐Prieto 2017), and one will report quality of life in patients with atrial fibrillation (NCT03053843).
2. Mediterranean dietary intervention versus another dietary intervention for primary prevention
Thirteen trials (25 papers) were included with 8687 participants randomised. The majority of participants were enrolled in one large multicentre trial (7747 participants, PREDIMED).
The health status of participants varied between studies. The majority of participants were described as at increased risk of CVD (Dinu 2017; PREDIMED; Sofi 2018; Vincent‐Baudry 2005), with specific diagnoses of hypertension (Lapetra 2018), central obesity (Bajerska 2018), hypercholesterolaemia (Athyros 2011), non‐alcoholic fatty liver disease (NAFLD) (Misciagna 2017; Properzi 2018), HIV (Ng 2011; Stradling 2018), and heart or lung transplant recipients (Entwistle 2018). One study recruited women with breast cancer (Skouroliakou 2017). Two trials recruited only women (Bajerska 2018; Skouroliakou 2017), the remainder recruiting both men and women. The trials were conducted in Spain (Lapetra 2018; PREDIMED), Italy (Dinu 2017; Misciagna 2017; Sofi 2018), Greece (Athyros 2011; Skouroliakou 2017), France (Vincent‐Baudry 2005), the UK (Entwistle 2018; Stradling 2018), Poland (Bajerska 2018), Australia (Properzi 2018), and China (Ng 2011). The duration of the intervention and follow‐up periods varied: three months (Dinu 2017; Properzi 2018; Sofi 2018; Vincent‐Baudry 2005), four months (Athyros 2011; Bajerska 2018), six months (Misciagna 2017; Skouroliakou 2017), one year (Entwistle 2018; Ng 2011; Stradling 2018), two years (Lapetra 2018), and up to five years (PREDIMED).
The dietary interventions in the comparison group varied, including low‐fat (Athyros 2011; Entwistle 2018; Lapetra 2018; Ng 2011; PREDIMED; Properzi 2018; Stradling 2018; Vincent‐Baudry 2005), the traditional diet of that country (Bajerska 2018), national recommendations/disease‐specific guidance (Misciagna 2017; Skouroliakou 2017), and vegetarian (Dinu 2017; Sofi 2018).
We identified one ongoing trial (Papamiltiadous 2016) (see Characteristics of ongoing studies table) looking at the effects of a Mediterranean diet compared to a low‐fat moderate carbohydrate diet on CVD risk factors in NAFLD.
3. Mediterranean dietary intervention versus usual care for secondary prevention
Two trials (four papers) were included with 706 participants randomised.
Both trials recruited patients with CVD, one in men and women with CHD (Michalsen 2006), and the other in men and women who had experienced a myocardial infarction within six months (The Lyon Diet Heart Study). Participants were recruited from Germany (Michalsen 2006) and France (The Lyon Diet Heart Study). The duration of the intervention and follow‐up periods varied from 12 months (Michalsen 2006) to 24 and 46 months (The Lyon Diet Heart Study).
No ongoing trials have been identified to date for this comparison group.
4. Mediterranean dietary intervention versus another dietary intervention for secondary prevention
Six trials (10 papers) were included with 1731 participants randomised. An expression of concern has been published about the reliability of two of the studies in this comparison group (Singh 1992; Singh 2002), and we have conducted sensitivity analyses excluding these studies from all analyses. These were also the trials with the majority of participants (1406 participants, Singh 1992; Singh 2002).
All trials recruited patients with CVD. Three trials recruited men and women with CHD (Colquhoun 2000; Mayr 2018; Weber 2012), one after a first myocardial infarction (Tuttle 2008) and one with acute myocardial infarction or unstable angina (Singh 1992). One trial recruited patients with established CHD or those at high risk of CHD, although the majority of participants had established disease (58% in the intervention group and 59% in the comparison group) so this study has been analysed as a secondary prevention study (Singh 2002). Participants were recruited from Australia (Colquhoun 2000; Mayr 2018), the US (Tuttle 2008), Brazil (Weber 2012), and India (Singh 1992; Singh 2002). The duration of the intervention and follow‐up periods varied: three months (Colquhoun 2000; Weber 2012), six months (Mayr 2018), and two years (Singh 1992; Singh 2002; Tuttle 2008).
In a pilot trial, the comparison group comprised foods typical of the Mediterranean diet and the intervention was a Brazilian cardioprotective diet following the principles of the Mediterranean dietary pattern but with local foods to enhance adherence (Weber 2012). We have used the Mediterranean diet as the intervention group in our analyses.
The dietary interventions in the comparison group varied, including low‐fat (Colquhoun 2000; Mayr 2018; Tuttle 2008) and national recommendations/disease‐specific guidance (Singh 1992; Singh 2002; Weber 2012).
We identified two ongoing trials (Delgado‐Lista 2016; Itsiopoulos 2018) (see Characteristics of ongoing studies table) in patients with CHD and all will report on clinical endpoints.
Excluded studies
Details and reasons for exclusion for the studies that most closely missed the inclusion criteria are presented in the Characteristics of excluded studies table. The majority of studies were excluded on the basis of the intervention not meeting the two core criteria of a Mediterranean‐style diet (see Types of interventions) or studies were short‐term (less than 12 weeks).
Risk of bias in included studies
Details are provided for each of the included studies in the 'Risk of bias' section of the Characteristics of included studies table and summaries are presented in Figure 2 and Figure 3. We assessed risk of bias as 'low', 'high' or 'unclear'. A summary of the risk of bias of the included studies is presented below for each comparison group for clarity.
2.
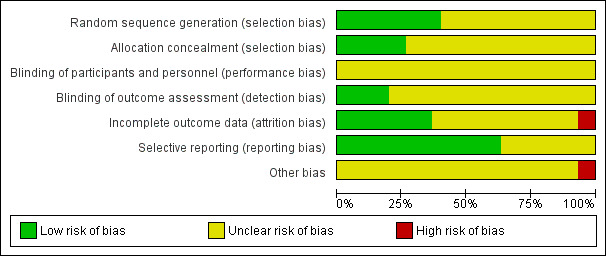
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
1. Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention
The methods of random sequence generation were unclear in six of the nine included studies (Castagnetta 2002; Chasapidou 2014; Clements 2017; Djuric 2009; Lindman 2004; Wardle 2000). In the three studies where this was clear, we judged the methods used to be at low risk of bias (Davis 2017; Esposito 2004; Konstantinidou 2010). The methods of allocation concealment were unclear in seven of the nine included studies. Where this was clear, we judged the methods used to be at low risk of bias (Esposito 2004; Wardle 2000).
2. Mediterranean dietary intervention versus another dietary intervention for primary prevention
The methods of random sequence generation were unclear in six of the 13 included studies (Athyros 2011; Lapetra 2018; Dinu 2017; Properzi 2018; Skouroliakou 2017; Vincent‐Baudry 2005). In the seven studies where this was clear, we judged the methods used to be at low risk of bias (Bajerska 2018; Entwistle 2018; Misciagna 2017; Ng 2011; PREDIMED; Sofi 2018; Stradling 2018). The methods of allocation concealment were unclear in 10 of the 13 included studies. Where this was clear, we judged the methods used to be at low risk of bias (Entwistle 2018; Sofi 2018; Stradling 2018).
3. Mediterranean dietary intervention versus usual care for secondary prevention
The methods of random sequence generation were unclear in one of the two included studies (The Lyon Diet Heart Study), and in the other we judged the methods used to be at low risk of bias (Michalsen 2006). The methods of allocation concealment were unclear in one study (Michalsen 2006) and in the other we judged the methods used to be at low risk of bias (The Lyon Diet Heart Study).
4. Mediterranean dietary intervention versus another dietary intervention for secondary prevention
The methods of random sequence generation were unclear in five of the six included studies (Colquhoun 2000; Singh 1992; Singh 2002; Tuttle 2008; Weber 2012), and in the one study where this was clear, we judged the methods used to be at low risk of bias (Mayr 2018). The methods of allocation concealment were unclear in four of the six included studies. Where this was clear, we judged the methods used to be at low risk of bias (Tuttle 2008; Weber 2012).
Blinding
The blinding of participants and personnel for behavioural interventions is difficult, if not impossible, in most cases and so we have not judged this as a high risk of bias. We rated this domain as unclear for all trials in all four comparison groups.
1. Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention
The blinding of participants and personnel was unclear in all nine trials. Blinding of outcome assessment was unclear in eight of the nine trials (Castagnetta 2002; Chasapidou 2014; Clements 2017; Davis 2017; Djuric 2009; Konstantinidou 2010; Lindman 2004; Wardle 2000). In the remaining trial, outcome assessments were made blind to the group assignment and we judged this to be at low risk of bias (Esposito 2004).
2. Mediterranean dietary intervention versus another dietary intervention for primary prevention
The blinding of participants and personnel was unclear in all 13 trials. Blinding of outcome assessment was unclear in 10 of the 13 trials (Athyros 2011; Entwistle 2018; Lapetra 2018; Dinu 2017; Ng 2011; PREDIMED; Properzi 2018; Skouroliakou 2017; Stradling 2018; Vincent‐Baudry 2005). In the remaining three trials, outcome assessments were made blind to the group assignment and we judged this to be at low risk of bias (Bajerska 2018; Misciagna 2017; Sofi 2018).
3. Mediterranean dietary intervention versus usual care for secondary prevention
The blinding of participants and personnel was unclear in both trials. Blinding of outcome assessment was unclear in one trial (Michalsen 2006). In the remaining trial, outcome assessments were made blind to the group assignment and we judged this to be at low risk of bias (The Lyon Diet Heart Study).
4. Mediterranean dietary intervention versus another dietary intervention for secondary prevention
The blinding of participants and personnel was unclear in all six trials. Blinding of outcome assessment was unclear in five of the six trials (Colquhoun 2000; Mayr 2018; Singh 1992; Tuttle 2008; Weber 2012). In the remaining trial, outcome assessments were made blind to the group assignment and we judged this to be at low risk of bias (Singh 2002).
Incomplete outcome data
1. Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention
We judged three of the nine trials to be at low risk of bias as loss to follow‐up was low and reasons provided or intention‐to‐treat (ITT) analyses were performed, or both (Esposito 2004; Konstantinidou 2010; Wardle 2000). We judged one study to be at high risk of bias as there was differential loss to follow‐up that exceeded 20% in the intervention group (Djuric 2009). For the remaining trials, we judged the risk of bias as unclear.
2. Mediterranean dietary intervention versus another dietary intervention for primary prevention
We judged six of the 13 trials to be at low risk of bias as loss to follow‐up was absent or low and reasons provided or ITT analyses were performed, or both (Athyros 2011; Bajerska 2018; Entwistle 2018; Misciagna 2017; PREDIMED; Sofi 2018). We judged one study to be at high risk of bias for attrition due to differential loss to follow‐up between the intervention and comparison groups with loss to follow‐up at 36% in the comparison diet (Vincent‐Baudry 2005). For the remaining trials, we judged the risk of bias as unclear.
3. Mediterranean dietary intervention versus usual care for secondary prevention
We judged both trials to be at low risk of bias as loss to follow‐up was low and reasons provided or ITT analyses were performed (Michalsen 2006; The Lyon Diet Heart Study).
4. Mediterranean dietary intervention versus another dietary intervention for secondary prevention
For all six trials (Colquhoun 2000; Mayr 2018; Singh 1992; Singh 2002; Tuttle 2008; Weber 2012), we judged the risk of attrition bias as unclear.
Selective reporting
1. Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention
For four studies we judged the risk of bias associated with selective reporting as unclear (Castagnetta 2002; Chasapidou 2014; Clements 2017; Lindman 2004). The remaining five studies clearly stated the primary and secondary outcomes and reported the results for these and were therefore judged to be of low risk of bias in this domain (Davis 2017; Djuric 2009; Esposito 2004; Konstantinidou 2010; Wardle 2000).
2. Mediterranean dietary intervention versus another dietary intervention for primary prevention
For four studies we judged the risk of bias associated with selective reporting as unclear (Dinu 2017; Lapetra 2018; Properzi 2018; Stradling 2018). The remaining nine studies clearly stated the primary and secondary outcomes and reported the results for these and were therefore judged to be of low risk of bias in this domain (Athyros 2011; Bajerska 2018; Entwistle 2018; Misciagna 2017; Ng 2011; PREDIMED; Skouroliakou 2017; Sofi 2018; Vincent‐Baudry 2005).
3. Mediterranean dietary intervention versus usual care for secondary prevention
Both studies clearly stated the primary and secondary outcomes and reported the results for these and were therefore judged to be of low risk of bias (Michalsen 2006; The Lyon Diet Heart Study).
4. Mediterranean dietary intervention versus another dietary intervention for secondary prevention
For three studies we judged the risk of bias associated with selective reporting as unclear (Colquhoun 2000; Mayr 2018; Singh 1992). The remaining three studies clearly stated the primary and secondary outcomes and reported the results for these and were therefore judged to be of low risk of bias in this domain (Singh 2002; Tuttle 2008; Weber 2012).
Other potential sources of bias
1. Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention
There was insufficient information to judge the risk of other sources of bias and we categorised all nine studies as unclear (Castagnetta 2002; Chasapidou 2014; Clements 2017; Davis 2017; Djuric 2009; Esposito 2004; Konstantinidou 2010; Lindman 2004; Wardle 2000).
2. Mediterranean dietary intervention versus another dietary intervention for primary prevention
There was insufficient information to judge the risk of other sources of bias and we categorised all 13 studies as unclear (Athyros 2011; Bajerska 2018; Dinu 2017; Entwistle 2018; Lapetra 2018; Misciagna 2017; Ng 2011; PREDIMED; Properzi 2018; Skouroliakou 2017; Sofi 2018; Stradling 2018; Vincent‐Baudry 2005).
3. Mediterranean dietary intervention versus usual care for secondary prevention
There was insufficient information to judge the risk of other sources of bias and we categorised both studies as unclear (Michalsen 2006; The Lyon Diet Heart Study).
4. Mediterranean dietary intervention versus another dietary intervention for secondary prevention
An expression of concern has been published about the reliability of two of the studies in this comparison group (Singh 1992; Singh 2002). We have conducted sensitivity analyses excluding these studies from all analyses. We regarded these two studies as at high risk of other bias. We judged the remaining four studies as at unclear risk of other sources of bias as there was insufficient information to make a judgement (Colquhoun 2000; Mayr 2018; Tuttle 2008; Weber 2012).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
See: Table 1; Table 2; Table 3; Table 4.
Data are presented in the analyses by primary and secondary prevention of CVD and by comparison group ‐ no intervention/usual care/minimal intervention versus another dietary intervention.
As an expression of concern has been published about the reliability of the studies Singh 1992 and Singh 2002, we conducted sensitivity analyses excluding these studies. This affects the following outcomes in the Mediterranean dietary intervention versus another dietary intervention for secondary prevention comparisons: non‐fatal MI, fatal MI, sudden cardiac death, total cardiac endpoints, lipid levels and blood pressure.
Clinical events (primary outcomes: cardiovascular mortality, all‐cause mortality and other non‐fatal endpoints)
1. Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention
None of the nine included studies reported on clinical events. Trials were relatively small (numbers randomised ranged from 60 to 384) and short‐term (three months to two years).
2. Mediterranean dietary intervention versus another dietary intervention for primary prevention
The PREDIMED trial was the only trial reporting clinical events for this comparison. PREDIMED comprised two dietary interventions: the PREDIMED intervention plus supplementation with extra‐virgin olive oil and the PREDIMED intervention plus supplementation with tree nuts, and compared these to a low‐fat diet. The trial included 7447 men and women from 11 sites in Spain at increased risk of CVD. The trial was stopped early as clear benefits of the Mediterranean diet over the low‐fat diet were seen for the primary outcome at 4.8 years. The original trial, Estruch 2013, was retracted and re‐analysed when methodological issues concerning randomisation came to light for two sites, and the inclusion of non‐randomised second household members. The new publication controlled for these in the analyses and conducted a series of sensitivity analyses excluding these sites (Estruch 2018).
The new publication reports on the composite clinical outcome, CVD and total mortality, MI and stroke where an effect of the PREDIMED intervention compared to a low‐fat diet on composite clinical endpoints was found (hazard ratio (HR) 0.70, 95% confidence interval (CI) 0.58 to 0.85) (Analysis 2.1). In sensitivity analyses, the hazard ratio for this outcome in 6405 participants compared to control was 0.65 (95% CI 0.50 to 0.85) when excluding participants from site D and second household members, and 0.69 (95% CI 0.53 to 0.92) in 5859 participants when excluding participants also from site B.
2.1. Analysis.
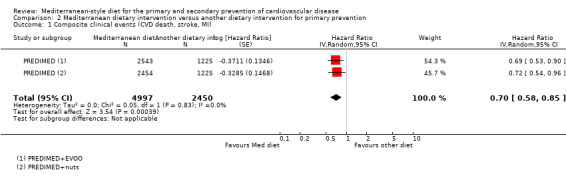
Comparison 2 Mediterranean dietary intervention versus another dietary intervention for primary prevention, Outcome 1 Composite clinical events (CVD death, stroke, MI).
The re‐analysed paper also reports clinical endpoints separately where there was little or no effect of the PREDIMED intervention compared to a low‐fat diet on total mortality (HR 1.0, 95% CI 0.81 to 1.24, low‐quality evidence) (Analysis 2.3), CVD mortality (HR 0.81, 95% CI 0.50 to 1.32, low‐quality evidence) (Analysis 2.2) or myocardial infarction (HR 0.79, 95% CI 0.57 to 1.10, low‐quality evidence) (Analysis 2.4), but moderate‐quality evidence for a reduction in the number of strokes with the intervention (HR 0.60, 95% CI 0.45 to 0.80) (Analysis 2.5). Reductions in the numbers of participants experiencing peripheral arterial disease were also observed with the PREDIMED intervention (HR 0.42, 95% CI 0.28 to 0.61, moderate‐quality evidence) (Analysis 2.6), but these data are less certain as they were not re‐analysed in the recent paper (Estruch 2018), but come from earlier reports of the trial.
2.3. Analysis.

Comparison 2 Mediterranean dietary intervention versus another dietary intervention for primary prevention, Outcome 3 Total mortality.
2.2. Analysis.

Comparison 2 Mediterranean dietary intervention versus another dietary intervention for primary prevention, Outcome 2 CVD mortality.
2.4. Analysis.
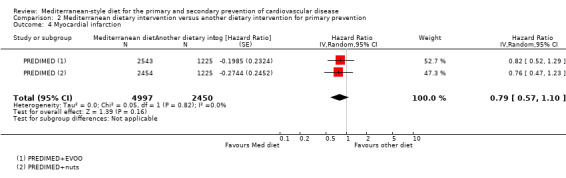
Comparison 2 Mediterranean dietary intervention versus another dietary intervention for primary prevention, Outcome 4 Myocardial infarction.
2.5. Analysis.
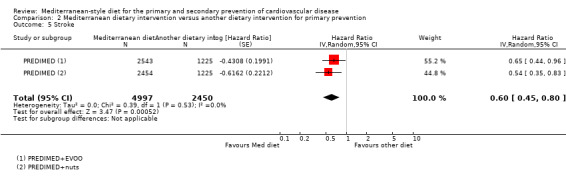
Comparison 2 Mediterranean dietary intervention versus another dietary intervention for primary prevention, Outcome 5 Stroke.
2.6. Analysis.
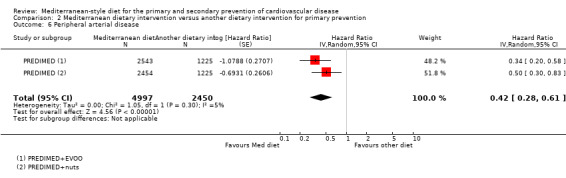
Comparison 2 Mediterranean dietary intervention versus another dietary intervention for primary prevention, Outcome 6 Peripheral arterial disease.
One small trial (N = 180) comparing the Mediterranean diet to a low‐fat diet in hypertensive patients reported unadjusted estimates for stroke of risk ratio (RR) 0.33, 95% CI 0.04 to 3.14) over two years of follow‐up (Analysis 2.8) (Lapetra 2018).
2.8. Analysis.
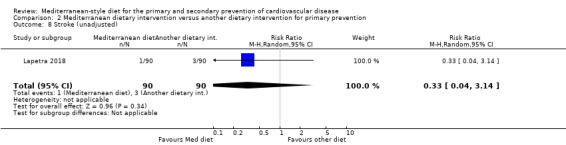
Comparison 2 Mediterranean dietary intervention versus another dietary intervention for primary prevention, Outcome 8 Stroke (unadjusted).
3. Mediterranean dietary intervention versus usual care for secondary prevention
One study reports clinical endpoints for this comparison group (The Lyon Diet Heart Study). This study recruited 605 patients within six months of a myocardial infarction, aged less than 70 years, the majority of whom were men (90%) from secondary care in France (The Lyon Diet Heart Study).
The Lyon Diet Heart Study examined the effect of a Mediterranean diet compared to usual care over 46 months and found reductions in adjusted estimates for a composite endpoint of CVD deaths and non‐fatal myocardial infarction (HR 0.28, 95% CI 0.15 to 0.52) (Analysis 3.3), CVD mortality (HR 0.35, 95% CI 0.15 to 0.82, low‐quality evidence) (Analysis 3.2) and total mortality (HR 0.44, 95% CI 0.21 to 0.92, moderate‐quality evidence) (Analysis 3.1) with the intervention (The Lyon Diet Heart Study).
3.3. Analysis.
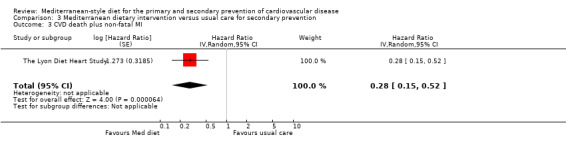
Comparison 3 Mediterranean dietary intervention versus usual care for secondary prevention, Outcome 3 CVD death plus non‐fatal MI.
3.2. Analysis.
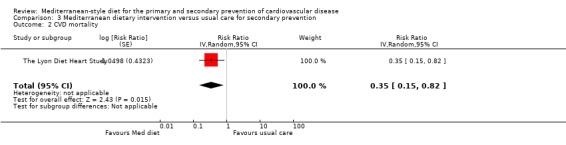
Comparison 3 Mediterranean dietary intervention versus usual care for secondary prevention, Outcome 2 CVD mortality.
3.1. Analysis.

Comparison 3 Mediterranean dietary intervention versus usual care for secondary prevention, Outcome 1 Total mortality.
4. Mediterranean dietary intervention versus another dietary intervention for secondary prevention
Three studies report clinical endpoints for this comparison group (Singh 1992; Singh 2002; Tuttle 2008), and two of these have been excluded in sensitivity analyses from all main analyses due to published concerns regarding the reliability of the data (Singh 1992; Singh 2002). For all analyses for comparison 4, forest plots are provided including and excluding these two studies, and we report in the text the results of sensitivity analyses excluding these studies. For the adjusted outcomes non‐fatal myocardial infarction, fatal myocardial infarction, sudden cardiac death, total cardiac endpoints, total mortality and CVD mortality, no other studies were identified after removing the Singh 1992 and Singh 2002 studies so these forest plots are empty and could not be shown.
One small study from the US in 101 patients randomised six weeks post myocardial infarction, following a Mediterranean diet or low‐fat diet, provided unadjusted estimates for total cardiac endpoints (all‐cause and cardiac deaths, myocardial infarction, hospital admissions for heart failure, unstable angina or stroke (RR 0.98, 95% CI 0.40 to 2.41, very low‐quality evidence) (Analysis 4.13), showing considerable uncertainty in the effect size (Tuttle 2008).
4.13. Analysis.
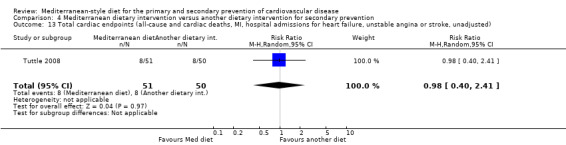
Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 13 Total cardiac endpoints (all‐cause and cardiac deaths, MI, hospital admissions for heart failure, unstable angina or stroke, unadjusted).
Two further ongoing trials will report clinical endpoints in CHD patients randomised to the Mediterranean dietary intervention compared to other dietary interventions (Delgado‐Lista 2016; Itsiopoulos 2018). One is conducted in Spain and randomising 1002 patients with an estimated completion date of September 2019 (Delgado‐Lista 2016). The other is conducted in Australia and randomising 1032 patients with anticipated last enrolment in October 2018 (Itsiopoulos 2018).
Cardiovascular risk factors (secondary outcomes: changes in blood lipids and blood pressure, and occurrence of type 2 diabetes)
Lipid levels
Total cholesterol
1. Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention
Five trials (569 participants randomised) measured total cholesterol levels and reported data that could be used in meta‐analyses (Davis 2017; Djuric 2009; Esposito 2004; Konstantinidou 2010; Wardle 2000). We assessed the overall quality of evidence as low and it showed a possible reduction in total cholesterol of ‐0.16 mmol/L (95% CI ‐0.32 to 0.00, 5 trials, 569 participants, I² = 73%) (Analysis 1.1).
1.1. Analysis.
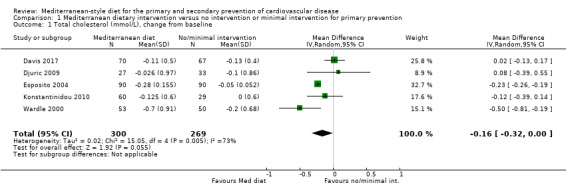
Comparison 1 Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention, Outcome 1 Total cholesterol (mmol/L), change from baseline.
Two trials measured total cholesterol but did not provide data in a useable format for meta‐analyses (Castagnetta 2002; Clements 2017). One trial reported a significant reduction in total cholesterol levels with the dietary intervention (Castagnetta 2002), and the other reported that total cholesterol was unaffected by both the Mediterranean diet and minimal dietary intervention (Clements 2017).
2. Mediterranean dietary intervention versus another dietary intervention for primary prevention
Seven trials (939 participants randomised) measured total cholesterol and provided data that could be pooled in a meta‐analysis (Athyros 2011; Bajerska 2018; Ng 2011; PREDIMED; Skouroliakou 2017; Sofi 2018; Vincent‐Baudry 2005). For the PREDIMED trial data on lipids were reported for two study sites rather than all 11 sites, but these were not the two sites where methodological issues arose. There was low‐quality evidence that the Mediterranean diet produced a possible small reduction in total cholesterol (mean difference (MD) ‐0.13 mmol/L, 95% CI ‐0.30 to 0.04, I² = 70%) (Analysis 2.9).
2.9. Analysis.
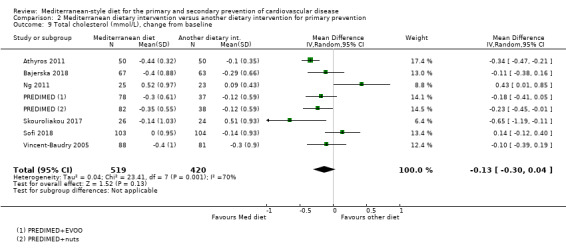
Comparison 2 Mediterranean dietary intervention versus another dietary intervention for primary prevention, Outcome 9 Total cholesterol (mmol/L), change from baseline.
Two further trials measured total cholesterol but did not provide data in a useable format for meta‐analyses. Preliminary results from the CARDIOVEG study showed that the vegetarian diet was more effective in reducing total cholesterol (‐2.9%) with no significant change in the Mediterranean group (Dinu 2017). In a preliminary report of a trial comparing the Mediterranean diet and a low‐fat diet in patients with NAFLD to reduce CVD risk, significant within‐group improvements were seen for total cholesterol in the Mediterranean diet group but not the low‐fat diet group (P < 0.05) (Properzi 2018).
3. Mediterranean dietary intervention versus usual care for secondary prevention
Two trials (441 participants randomised) measured total cholesterol and provided data that could be pooled in a meta‐analysis (Michalsen 2006; The Lyon Diet Heart Study). There was low‐quality evidence that the Mediterranean diet produced little or no effect on total cholesterol levels (MD 0.07 mmol/L, 95% CI ‐0.19 to 0.33, I² = 19%) (Analysis 3.4).
3.4. Analysis.
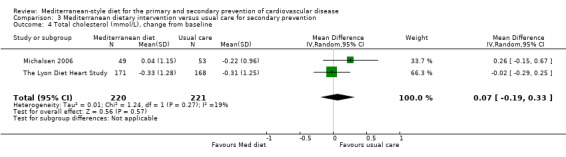
Comparison 3 Mediterranean dietary intervention versus usual care for secondary prevention, Outcome 4 Total cholesterol (mmol/L), change from baseline.
4. Mediterranean dietary intervention versus another dietary intervention for secondary prevention
Two studies with a published expression of concern report total cholesterol for this comparison group with data in a useable format for meta‐analyses (Singh 1992; Singh 2002). Both of these studies have published concerns regarding the reliability of the data and have been excluded in sensitivity analyses from all main analyses (Singh 1992; Singh 2002). No other studies were identified after removing the Singh 1992 and Singh 2002 studies so these forest plots are empty and could not be shown.
Two further studies reported on lipid levels overall. One study reported as a conference proceeding compared effects of the Mediterranean diet with a low‐fat diet on lipid levels in CHD patients on statin therapy (Colquhoun 2000). We were unable to pool these data statistically as no measures of variance were available. The authors found no differences between the two diets at three months follow‐up. In a preliminary analysis of the AUSMED trial the authors report that compared to the low‐fat diet, the MedDiet did not change the lipid profile (P > 0.05) (Mayr 2018). The variables were not measured in a later analysis of the full cohort.
Low‐density lipoprotein cholesterol
1. Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention
Four trials (389 participants randomised) measured LDL cholesterol and provided data that could be pooled in a meta‐analysis (Davis 2017; Djuric 2009; Konstantinidou 2010; Wardle 2000). There was very low‐quality evidence that the Mediterranean diet produced little or no effect on levels of LDL cholesterol (MD ‐0.08 mmol/L, 95% CI ‐0.26 to 0.09, I² = 54%) (Analysis 1.2).
1.2. Analysis.
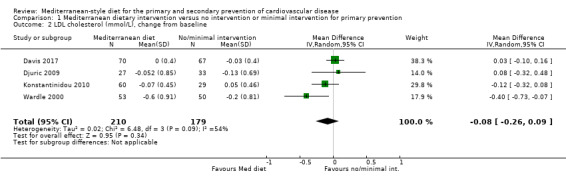
Comparison 1 Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention, Outcome 2 LDL cholesterol (mmol/L), change from baseline.
Two trials measured LDL cholesterol but did not provide data in a useable format for meta‐analyses. Preliminary analysis of an ongoing study reported a change in LDL cholesterol levels of 0.39 mmol/L between baseline and follow‐up of six months in 181 patients with metabolic disease following Mediterranean dietary advice, with a difference between the intervention and control group who received no advice of ‐7.9% (P = 0.05) (Chasapidou 2014). Another trial reported that LDL cholesterol was unaffected by both the Mediterranean diet and minimal dietary intervention (Clements 2017).
2. Mediterranean dietary intervention versus another dietary intervention for primary prevention
Seven trials (947 participants randomised) measured LDL cholesterol and provided data that could be pooled in a meta‐analysis (Athyros 2011; Bajerska 2018; PREDIMED; Skouroliakou 2017; Sofi 2018; Stradling 2018; Vincent‐Baudry 2005). For the PREDIMED trial data on lipids were reported for two study sites rather than all 11 sites, but these were not the two sites where methodological issues arose. There was moderate‐quality evidence that the Mediterranean diet produced a small reduction in LDL cholesterol (MD ‐0.15 mmol/L, 95% CI ‐0.27 to ‐0.02, I² = 46%) (Analysis 2.10).
2.10. Analysis.
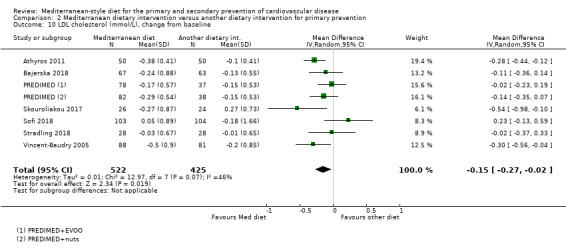
Comparison 2 Mediterranean dietary intervention versus another dietary intervention for primary prevention, Outcome 10 LDL cholesterol (mmol/L), change from baseline.
One further trial measured LDL cholesterol but did not provide data in a useable format for meta‐analyses. Preliminary results from the CARDIOVEG study show that the vegetarian diet was more effective in reducing LDL cholesterol (‐5.1%) with no significant change in the Mediterranean diet group (Dinu 2017).
3. Mediterranean dietary intervention versus usual care for secondary prevention
Two trials (441 participants randomised) measured LDL cholesterol and provided data that could be pooled in a meta‐analysis (Michalsen 2006; The Lyon Diet Heart Study). There was low‐quality evidence that the Mediterranean diet produced little or no effect on LDL cholesterol levels (MD 0.11 mmol/L, 95% CI ‐0.09 to 0.31, I² = 0%) (Analysis 3.5).
3.5. Analysis.

Comparison 3 Mediterranean dietary intervention versus usual care for secondary prevention, Outcome 5 LDL cholesterol (mmol/L), change from baseline.
4. Mediterranean dietary intervention versus another dietary intervention for secondary prevention
Three studies report LDL cholesterol for this comparison group with data in a useable format for meta‐analyses (Singh 1992; Singh 2002; Tuttle 2008). Two of the studies have been excluded in sensitivity analyses from all main analyses due to published concerns regarding the reliability of the data (Singh 1992; Singh 2002). For all analyses for comparison 4, forest plots are provided including and excluding these two studies, and we report in the text the results of the sensitivity analyses excluding these studies. In the remaining study, Tuttle 2008, there was very low‐quality evidence of little or no effect of the Mediterranean diet on LDL cholesterol levels (MD 0.08 mmol/L, 95% CI ‐0.26 to 0.42) (Analysis 4.17).
4.17. Analysis.
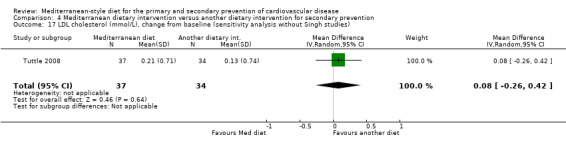
Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 17 LDL cholesterol (mmol/L), change from baseline (sensitivity analysis without Singh studies).
High‐density lipoprotein cholesterol
1. Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention
Five trials (569 participants randomised) measured HDL cholesterol levels and reported data that could be used in meta‐analyses (Davis 2017; Djuric 2009; Esposito 2004; Konstantinidou 2010; Wardle 2000). There was low‐quality evidence of little or no effect of the intervention on HDL levels (MD 0.02 mmol/L, 95% CI ‐0.04 to 0.08, I² = 70%) (Analysis 1.3).
1.3. Analysis.
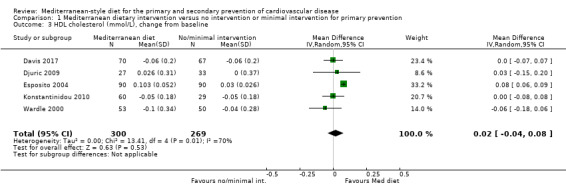
Comparison 1 Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention, Outcome 3 HDL cholesterol (mmol/L), change from baseline.
One trial measured HDL cholesterol but did not provide data in a useable format for meta‐analyses (Clements 2017). This trial reported that HDL cholesterol was unaffected by both the Mediterranean diet and minimal dietary intervention (Clements 2017).
2. Mediterranean dietary intervention versus another dietary intervention for primary prevention
Six trials (891 participants randomised) measured HDL cholesterol and provided data that could be pooled in a meta‐analysis (Athyros 2011; Bajerska 2018; PREDIMED; Skouroliakou 2017; Sofi 2018; Vincent‐Baudry 2005). For the PREDIMED trial data on lipids were reported for two study sites rather than all 11 sites, but these were not the two sites where methodological issues arose. There was moderate‐quality evidence showing little or no effect of the Mediterranean diet on HDL cholesterol levels (MD 0.02 mmol/L, 95% CI ‐0.01 to 0.04), I² = 0%) (Analysis 2.11).
2.11. Analysis.
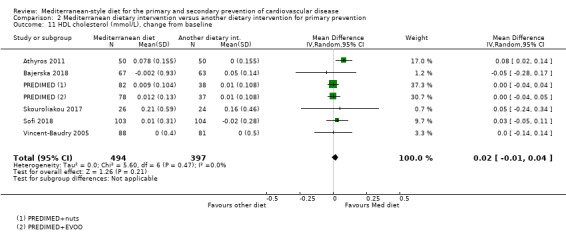
Comparison 2 Mediterranean dietary intervention versus another dietary intervention for primary prevention, Outcome 11 HDL cholesterol (mmol/L), change from baseline.
One study in patients with NAFLD reported lipid levels at baseline and follow‐up as normal or altered rather than actual values and variance. They found that lower levels of HDL cholesterol were observed only in the low glycaemic Mediterranean diet group after six months (Misciagna 2017).
3. Mediterranean dietary intervention versus usual care for secondary prevention
Two trials (441 participants randomised) measured HDL cholesterol and provided data that could be pooled in a meta‐analysis (Michalsen 2006; The Lyon Diet Heart Study). There was low‐quality evidence that the Mediterranean diet produced little or no effect on HDL cholesterol levels (MD ‐0.01 mmol/L, 95% CI ‐0.08 to 0.07, I² = 13%) (Analysis 3.6).
3.6. Analysis.

Comparison 3 Mediterranean dietary intervention versus usual care for secondary prevention, Outcome 6 HDL cholesterol (mmol/L), change from baseline.
4. Mediterranean dietary intervention versus another dietary intervention for secondary prevention
Three studies report HDL cholesterol for this comparison group with data in a useable format for meta‐analyses (Singh 1992; Singh 2002; Tuttle 2008). Two of the studies have been excluded in sensitivity analyses from all main analyses due to published concerns regarding the reliability of the data (Singh 1992; Singh 2002). For all analyses for comparison 4, forest plots are provided including and excluding these two studies, and we report in the text the results of the sensitivity analyses excluding these studies. In the remaining study, Tuttle 2008, there was low‐quality evidence of little or no effect of the Mediterranean diet on HDL cholesterol levels (MD ‐0.05 mmol/L, 95% CI ‐0.17 to 0.06) (Analysis 4.19).
4.19. Analysis.
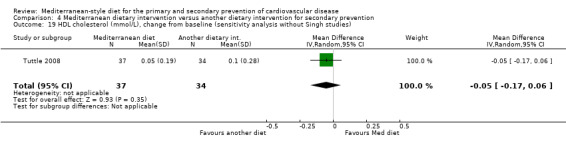
Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 19 HDL cholesterol (mmol/L), change from baseline (sensitivity analysis without Singh studies).
Triglycerides
1. Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention
Four trials (480 participants randomised) measured triglyceride levels and reported data that could be used in meta‐analyses (Davis 2017; Djuric 2009; Esposito 2004; Wardle 2000). There was considerable heterogeneity between trials (I² = 92%) and so we did not pool the studies statistically (Analysis 1.4). Two trials reported beneficial effects of the Mediterranean diet (Davis 2017; Esposito 2004), one reported no effect (Djuric 2009), and the other favoured the control (Wardle 2000).
1.4. Analysis.

Comparison 1 Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention, Outcome 4 Triglycerides (mmol/L), change from baseline.
Three trials measured triglyceride levels but did not provide data in a useable format for meta‐analyses (Clements 2017), or provided data as medians (with 25th and 75th percentiles) (Konstantinidou 2010; Lindman 2004). One trial reported that triglyceride levels were unaffected by both the Mediterranean diet and minimal dietary intervention (Clements 2017). In the two trials reporting medians, no effect of the diet on triglyceride levels was observed (Konstantinidou 2010; Lindman 2004).
2. Mediterranean dietary intervention versus another dietary intervention for primary prevention
Seven trials (939 participants randomised) measured triglyceride levels and provided data that could be pooled in a meta‐analysis (Athyros 2011; Bajerska 2018; Ng 2011; PREDIMED; Skouroliakou 2017; Sofi 2018; Vincent‐Baudry 2005). For the PREDIMED trial data on lipids were reported for two study sites rather than all 11 sites, but these were not the two sites where methodological issues arose. There was moderate‐quality evidence that the Mediterranean diet produced a possible small reduction in triglyceride levels (MD ‐0.09 mmol/L, 95% CI ‐0.16 to ‐0.01, I² = 15%) (Analysis 2.12).
2.12. Analysis.

Comparison 2 Mediterranean dietary intervention versus another dietary intervention for primary prevention, Outcome 12 Triglycerides (mmol/L), change from baseline.
Four further trials measured triglyceride levels but did not provide data in a useable format for meta‐analyses. In a study of Mediterranean diet versus low‐fat diet in heart and lung transplant recipients, the serum triglycerides levels declined in both groups over 12 months: Mediterranean diet −0.17 mmol/L (mean −9%, 95% CI –20 to 4); low‐fat diet −0.44 mmol/L (mean −21%, 95% CI –33 to −7) (Entwistle 2018). In a preliminary report of a trial comparing the Mediterranean diet and a low‐fat diet in patients with NAFLD to reduce CVD risk, significant within‐group improvements were seen for serum triglycerides in the Mediterranean diet group but not the low‐fat diet group (P < 0.05) (Properzi 2018). Preliminary results from the CARDIOVEG study comparing the effects of a Mediterranean diet and vegetarian diet on CVD risk factors found a significant reduction in triglycerides (‐8.9%) only after the Mediterranean period (Dinu 2017). Another study in patients with NAFLD reported lipid levels at baseline and follow‐up as normal or altered rather than actual values and variance. The authors found lower levels of triglycerides in both the intervention and control groups after six months (Misciagna 2017).
3. Mediterranean dietary intervention versus usual care for secondary prevention
Two trials (441 participants randomised) measured triglyceride levels and provided data that could be pooled in a meta‐analysis (Michalsen 2006; The Lyon Diet Heart Study). There was low‐quality evidence that the Mediterranean diet produced little or no effect on triglyceride levels (MD ‐0.14 mmol/L, 95% CI ‐0.38 to 0.10, I² = 0%) (Analysis 3.7).
3.7. Analysis.
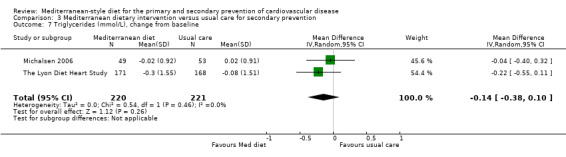
Comparison 3 Mediterranean dietary intervention versus usual care for secondary prevention, Outcome 7 Triglycerides (mmol/L), change from baseline.
4. Mediterranean dietary intervention versus another dietary intervention for secondary prevention
Three studies reported triglyceride levels for this comparison group with data in a useable format for meta‐analyses (Singh 1992; Singh 2002; Tuttle 2008). Two of the studies have been excluded in sensitivity analyses from all main analyses due to published concerns regarding the reliability of the data (Singh 1992; Singh 2002). For all analyses for comparison 4, forest plots are provided including and excluding these two studies, and we report in the text the results of the sensitivity analyses excluding these studies. In the remaining study, Tuttle 2008, there was very low‐quality evidence of little or no effect of the Mediterranean diet on triglyceride levels (MD 0.46 mmol/L, 95% CI ‐0.24 to 1.16) (Analysis 4.21).
4.21. Analysis.
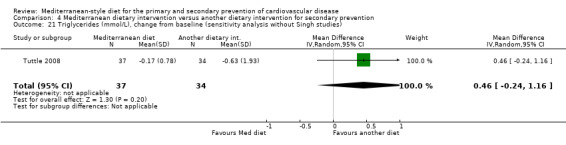
Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 21 Triglycerides (mmol/L), change from baseline (sensitivity analysis without Singh studies).
Blood pressure
1. Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention
Two trials (269 participants randomised) measured systolic blood pressure and reported data that could be used in meta‐analyses (Davis 2017; Esposito 2004). There was moderate‐quality evidence of a reduction in systolic blood pressure with the intervention (MD ‐2.99 mmHg, 95% CI ‐3.45 to ‐2.53, I² = 0%) (Analysis 1.5).
1.5. Analysis.
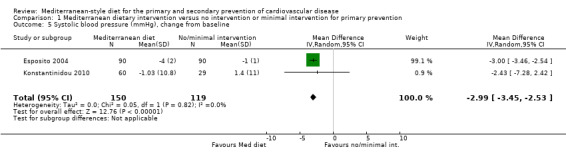
Comparison 1 Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention, Outcome 5 Systolic blood pressure (mmHg), change from baseline.
One trial measured systolic blood pressure but did not provide data in a useable format for meta‐analyses (Chasapidou 2014). Preliminary analysis of an ongoing study reported a change in systolic blood pressure of 2.6 mmHg between baseline and follow‐up of six months in 181 patients with metabolic disease following Mediterranean dietary advice, with a difference between the intervention and control group who received no advice of ‐5.1% (P < 0.05) (Chasapidou 2014).
Two trials (269 participants randomised) measured diastolic blood pressure and reported data that could be used in meta‐analyses (Davis 2017; Esposito 2004). There was moderate‐quality evidence of a reduction in diastolic blood pressure with the intervention (MD ‐2.0 mmHg, 95% CI ‐2.29 to ‐1.71, I² = 0%) (Analysis 1.6).
1.6. Analysis.
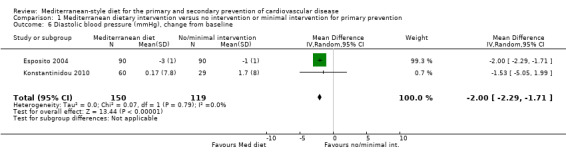
Comparison 1 Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention, Outcome 6 Diastolic blood pressure (mmHg), change from baseline.
2. Mediterranean dietary intervention versus another dietary intervention for primary prevention
Four trials (448 participants randomised) measured systolic blood pressure and provided data that could be pooled in a meta‐analysis (Athyros 2011; Bajerska 2018; Stradling 2018; Vincent‐Baudry 2005). For the PREDIMED trial, blood pressure was analysed in multivariate analyses and these are reported separately below. There was low‐quality evidence that the Mediterranean diet had little or no effect on systolic blood pressure levels (MD ‐1.5 mmHg, 95% CI ‐3.92 to 0.92, I² = 16%) (Analysis 2.13).
2.13. Analysis.
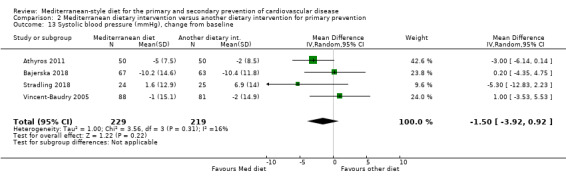
Comparison 2 Mediterranean dietary intervention versus another dietary intervention for primary prevention, Outcome 13 Systolic blood pressure (mmHg), change from baseline.
Four trials (448 participants randomised) measured diastolic blood pressure and provided data that could be pooled in a meta‐analysis (Athyros 2011; Bajerska 2018; Stradling 2018; Vincent‐Baudry 2005). For the PREDIMED trial, blood pressure was analysed in multivariate analyses and these are reported separately below. There was low‐quality evidence that the Mediterranean diet had little or no effect on diastolic blood pressure levels (MD ‐0.26 mmHg, 95% CI ‐2.41 to 1.9, I² = 37%) (Analysis 2.14).
2.14. Analysis.

Comparison 2 Mediterranean dietary intervention versus another dietary intervention for primary prevention, Outcome 14 Diastolic blood pressure (mmHg), change from baseline.
The PREDIMED study used multivariate adjusted analyses controlling for centre, age, sex and diabetes, baseline blood pressure and antihypertensive drugs. Mean differences in systolic blood pressure changes (mmHg) in the two intervention groups versus the control group after a median follow‐up of 3.8 years were 0.39 (‐0.48 to 1.26) for PREDIMED + extra virgin olive oil (EVOO) versus control (P=0.38) and ‐ 0.72 (‐1.58 to 0.13) for PREDIMED + nuts versus control (P = 0.10). Mean differences in diastolic blood pressure changes (mmHg) in the two intervention groups versus the control group after a median follow‐up of 3.8 years were ‐1.53 (‐2.01 to ‐1.04) for PREDIMED + EVOO versus control (P < 0.001) and ‐0.65 (‐1.15 to ‐0.15) for PREDIMED + nuts versus control (P = 0.01).
3. Mediterranean dietary intervention versus usual care for secondary prevention
One trial (556 participants randomised) measured blood pressure (The Lyon Diet Heart Study). There was very low‐quality evidence that the Mediterranean diet produced little or no effect on either systolic (MD ‐2.00 mmHg, 95% CI ‐5.29 to 1.29) (Analysis 3.8) or diastolic blood pressure (MD ‐1.00 mmHg, 95% CI ‐4.29 to 2.29) (Analysis 3.9).
3.8. Analysis.
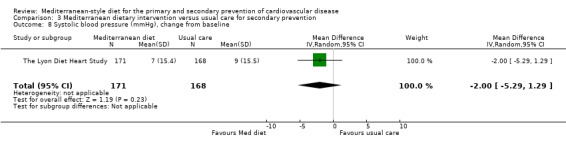
Comparison 3 Mediterranean dietary intervention versus usual care for secondary prevention, Outcome 8 Systolic blood pressure (mmHg), change from baseline.
3.9. Analysis.
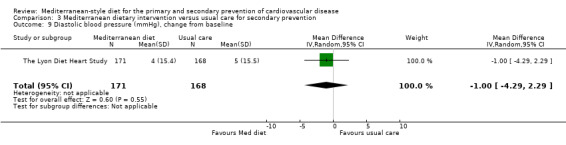
Comparison 3 Mediterranean dietary intervention versus usual care for secondary prevention, Outcome 9 Diastolic blood pressure (mmHg), change from baseline.
4. Mediterranean dietary intervention versus another dietary intervention for secondary prevention
Four studies report blood pressure for this comparison group with data in a useable format for meta‐analyses (Singh 1992; Singh 2002; Tuttle 2008; Weber 2012). Two of the studies have been excluded in sensitivity analyses from all main analyses due to published concerns regarding the reliability of the data (Singh 1992; Singh 2002). For all analyses for comparison 4, forest plots are provided including and excluding these two studies, and we report in the text the results of the sensitivity analyses excluding these studies. In the remaining two studies, Tuttle 2008 and Weber 2012, there was very low‐quality evidence of little or no effect of the Mediterranean diet on systolic blood pressure levels (MD 1.76 mmHg, 95% CI ‐2.80 to 6.33, I² = 0%) (Analysis 4.23) or diastolic blood pressure levels (MD 0.98 mmHg, 95% CI ‐1.97 to 3.93, I² = 0%) (Analysis 4.25).
4.23. Analysis.
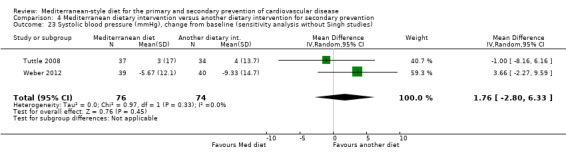
Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 23 Systolic blood pressure (mmHg), change from baseline (sensitivity analysis without Singh studies).
4.25. Analysis.

Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 25 Diastolic blood pressure (mmHg), change from baseline (sensitivity analysis without Singh studies).
In a further study and preliminary analysis of the AUSMED trial the authors report that compared to the low‐fat diet, the MedDiet did not change the blood pressure profile (P > 0.05) (Mayr 2018). The variables were not measured in a later analysis of the full cohort.
Type 2 diabetes
One study, which examined the effect of the Mediterranean dietary pattern for primary prevention, reported on incident diabetes (PREDIMED).
The PREDIMED trial reports on incident diabetes over 4.8 years of follow‐up in an earlier publication (Salas‐Salvado 2014), before the re‐analysis of the main paper (Estruch 2018). However, a recent report states that data for the incidence of type 2 diabetes has been re‐analysed to take account of the clustering and shows very similar estimates to the original analysis (Anonymous 2018). The PREDIMED intervention is described as a Mediterranean diet supplemented with extra‐virgin olive oil or tree nuts compared to a low‐fat diet control group. The authors found a statistically significant reduction in the incidence of type 2 diabetes with the PREDIMED intervention (HR 0.71, 95% CI 0.52 to 0.96).
Health‐related quality of life, adverse effects or costs
None of the trials in any of the four main comparison groups reported on health‐related quality of life or costs.
Adverse effects were reported in only two trials where no adverse events were noted for either dietary intervention in the PREDIMED trial (Ros 2014), and two of 302 CHD patients noted margarine‐related side effects of colitis and diarrhoea in The Lyon Diet Heart Study.
Discussion
The aim of this review was to evaluate the effectiveness of dietary advice to follow a Mediterranean‐style diet or the provision of foods relevant to the Mediterranean diet for both the primary and secondary prevention of CVD. As well as clinical endpoints, we also examined the effects of a Mediterranean‐style diet on major cardiovascular risk factors including blood lipids, blood pressure and occurrence of type 2 diabetes in both participants with and without established CVD.
Summary of main results
In this substantive review update, 30 RCTs (49 papers) and seven ongoing trials met our inclusion criteria. Four pre‐specified comparison groups were used to analyse the data to address both heterogeneity between participants and comparison groups and aid interpretation of findings. The comparison groups and number of trials and participants contributing to each are presented below:
Comparison 1: Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention, nine trials (1337 participants randomised).
Comparison 2: Mediterranean dietary intervention versus another dietary intervention for primary prevention, 13 trials (8687 participants randomised, 7747 of whom were from the PREDIMED trial).
Comparison 3: Mediterranean dietary intervention versus usual care for secondary prevention, two trials (706 participants randomised).
Comparison 4: Mediterranean dietary intervention versus another dietary intervention for secondary prevention, six trials (1731 participants randomised, 1406 of whom contributed to two trials excluded in sensitivity analyses from the main analyses due to published concerns regarding the reliability of the data) (Singh 1992; Singh 2002).
Clinical endpoints were measured in only one large primary prevention trial (PREDIMED), and a small trial reporting unadjusted estimates for stroke in hypertensive patients (Lapetra 2018). The PREDIMED trial contributed to comparison 2 examining dietary advice to follow a Mediterranean dietary pattern plus supplemental extra‐virgin olive oil or tree nuts compared to a low‐fat diet for primary prevention of CVD. The trial was conducted in Spain and randomised 7747 men and women at increased risk of CVD and observed them over 4.8 years of follow‐up. The original report of the PREDIMED trial, Estruch 2013, was retracted and re‐analysed when methodological issues came to light. The recent publication adjusts for these and the re‐analysed data are reported here (Estruch 2018). The PREDIMED intervention compared to a low‐fat diet shows an effect on composite clinical endpoints (HR 0.70, 95% CI 0.58 to 0.85). The re‐analysed paper also reports clinical endpoints separately where there was low‐quality evidence of little or no effect of the PREDIMED intervention compared to a low‐fat diet on total mortality, CVD mortality or myocardial infarction, but moderate‐quality evidence of a reduction in the number of strokes was seen with the intervention (HR 0.60, 95% CI 0.45 to 0.80). Reductions in the numbers of participants experiencing PAD were also observed with the PREDIMED intervention (HR 0.42, 95% CI 0.28 to 0.61, moderate‐quality evidence), but these data are less certain as they were not re‐analysed in the recent paper (Estruch 2018), but come from earlier reports of the trial.
Clinical endpoints were measured in secondary prevention trials contributing to comparisons 3 and 4. One trial contributed to comparison 3 (The Lyon Diet Heart Study). The Lyon Diet Heart Study examined the effect of advice to follow a Mediterranean diet plus supplemental canola margarine compared to usual care in 605 CHD patients over 46 months and found reductions in adjusted estimates for a composite endpoint of CVD deaths and non‐fatal myocardial infarction (HR 0.28, 95% CI 0.15 to 0.52), CVD mortality (HR 0.35, 95% CI 0.15 to 0.82, low‐quality evidence) and total mortality (HR 0.44, 95% CI 0.21 to 0.92, moderate‐quality evidence) with the intervention (The Lyon Diet Heart Study). For comparison 4, three studies report clinical endpoints (Singh 1992; Singh 2002; Tuttle 2008). Two of these have been excluded in sensitivity analyses from all main analyses due to published concerns regarding the reliability of the data (Singh 1992; Singh 2002). One small study from the US in 101 post myocardial infarction patients, following a Mediterranean diet or low‐fat diet, provided unadjusted estimates for total cardiac endpoints, with very low‐quality evidence showing considerable uncertainly of the effect size. Two further ongoing trials will report clinical endpoints in CHD patients randomised to the Mediterranean dietary intervention compared to other dietary interventions (Delgado‐Lista 2016; Itsiopoulos 2018), which will add to the evidence base.
CVD risk factors including lipid levels and blood pressure were reported in all four comparison groups. For comparison 1 there was low‐quality evidence for a possible small reduction in total cholesterol (‐0.16 mmol/L, 95% CI ‐0.32 to 0.00) and moderate‐quality evidence for a reduction in systolic (‐2.99 mmHg, 95% CI ‐3.45 to ‐2.53) and diastolic blood pressure (‐2.0 mmHg, 95% CI ‐2.29 to ‐1.71), with low or very low‐quality evidence of little or no effect of the intervention on LDL or HDL cholesterol or triglycerides. For comparison 2 there was moderate‐quality evidence of a possible small reduction in LDL cholesterol (‐0.15 mmol/L, 95% CI ‐0.27 to ‐0.02) and triglycerides (‐0.09 mmol/L, 95% CI ‐0.16 to ‐0.01) with moderate or low‐quality evidence of little or no effect of the intervention on total or HDL cholesterol or blood pressure. For comparison 3 there was low‐quality evidence of little or no effect of the Mediterranean diet on lipid levels and very low‐quality evidence for little or no effect on blood pressure. Similarly, for comparison 4 where only two trials contributed to the analyses there was low or very low‐quality evidence of little or no effect of the intervention on lipid levels or blood pressure.
The largest trial reported on the incidence of type 2 diabetes in primary prevention (PREDIMED), where there was a reduction in the incidence with the PREDIMED intervention (HR 0.71, 95% CI 0.52 to 0.96). Two trials reported on adverse events where these were absent (Ros 2014) or minor (The Lyon Diet Heart Study). No trials reported on health‐related quality of life or costs.
Overall completeness and applicability of evidence
In this substantive update we broadened the inclusion criteria of the original review, which focused only on primary prevention and no/minimal interventions as comparison groups to the Mediterranean‐style diet (New Reference). The expansion in scope was designed to make the review of relevance to secondary prevention but also allow comparisons of the Mediterranean diet with other dietary patterns for cardiovascular health. We have also refined our definition of the core components of a Mediterranean‐style diet based on extensive review and recent reports of the most likely active components (Grosso 2017; Martínez‐González 2017, see Types of interventions). We have stratified our analyses by primary and secondary prevention and by comparison group in an attempt to address heterogeneity and aid interpretation of findings to make the review as useful as possible.
There are now a larger number of included trials (30 trials, 12,461 participants randomised), but few report on clinical endpoints, our primary outcome, and the majority of trials report on CVD risk factors for primary prevention.
Definitions of the Mediterranean diet differed but all comprised at least the two core components of a high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts) and high intake of plant‐based foods, including fruits, vegetables and legumes.
Similarly, the dietary comparison groups differed across trials. The majority of comparison diets were, however, low‐fat diets or cardiac health guidance with notable exceptions of vegetarian diets. We have not explored the effect of different dietary comparison groups formally due to an insufficient number of studies to do so.
As noted above there were limited data on clinical endpoints, our primary outcome. Two studies were excluded from all main analyses in sensitivity analyses due to published concerns regarding the reliability of the data (Singh 1992; Singh 2002). Only one trial reported clinical endpoints for primary prevention and this study experienced methodological issues regarding randomisation with the report subsequently being retracted and re‐analysed (PREDIMED). The findings in secondary prevention are based on one older trial reporting very large effect estimates using a modified Zelen design (The Lyon Diet Heart Study). In addition, both the PREDIMED trial and The Lyon Diet Heart Study supplied supplemental foods as well as dietary advice to follow a Mediterranean‐style diet so the policy implications of the findings of these trials are unclear (Appel 2013).
The number of trials reporting primary and secondary outcomes for secondary prevention was limited, however a number of ongoing trials are exploring the effects of the Mediterranean diet on clinical endpoints in patients with CVD so these will add to the evidence base. No effects were seen on CVD risk factors in the limited number of trials reporting these, but this may be due to optimal pharmacological treatment where further improvements in lipid levels and blood pressure may be unlikely, particularly in more recent trials. We have not explored the effects of medication on outcomes in secondary prevention due to the low number of included studies, or in those at high risk in primary prevention, but we will explore this in future updates.
Adherence to dietary patterns both in the intervention and comparison groups will have an impact on their effectiveness. We did not measure adherence or compliance to the dietary interventions in this review. Other systematic reviews have shown that a greater adherence to a Mediterranean‐style diet is associated with a significant improvement in health status and a significant reduction in overall mortality, as well as in morbidity and mortality from CVD and other major chronic diseases (Sofi 2008; Sofi 2010). In a meta‐analysis of prospective cohort studies, a two‐point increase (scale from 0 to 7‐9 points) in adherence to a Mediterranean dietary pattern was associated with an 8% reduction in all‐cause mortality and a 10% reduction in CVD incidence or mortality (Sofi 2010).
The duration of the intervention and follow‐up periods varied widely across studies, ranging from short‐term trials (three to six months) to long‐term interventions (up to five years). Both short‐ and long‐term health effects of dietary interventions are plausible in terms of cardiovascular health, given the relatively quick response of cardiovascular risk factors such as blood lipids and blood pressure to lifestyle and dietary modifications (AHA 2006; Appel 1997; Appel 2001; Appel 2006). However, it is likely that potential beneficial effects of dietary interventions for the prevention of major chronic disease endpoints, such as mortality, CVD and type 2 diabetes, should represent the outcome of a long‐term process linked to the interplay of dietary patterns with genetic and environmental factors. In addition, the sustainability of long‐term lifestyle and dietary modifications is challenging. Therefore, the public health relevance of trials with extremely short‐term dietary interventions or follow‐up periods in this context is questionable.
Quality of the evidence
Due to the breadth of the review question, heterogeneity in terms of participants, interventions and comparators was high and we have attempted to reduce this by conducting the main analyses in four comparison groups for primary and secondary prevention and different comparators, and also explored the heterogeneity of the interventions in subgroup analyses.
The majority of studies included in this review were at unclear risk of bias for many of the risk of bias domains so results should be interpreted cautiously. We noted high risk of bias for differential attrition rates between the intervention and control groups in two trials (Djuric 2009; Vincent‐Baudry 2005), and high risk of other bias in two trials where there are published concerns regarding the reliability of the data (Singh 1992; Singh 2002). These two studies have been excluded from the main analyses and GRADE assessment. The 'Summary of findings' tables provide GRADE assessment of overall study quality for each of the four comparison groups:
For comparison 1, GRADE assessment of the outcomes has led to trials being downgraded for unclear risk of selection bias or attrition bias for the majority of studies, inconsistency due to high heterogeneity where studies were not pooled and imprecision due to low sample size.
For comparison 2, GRADE assessment of the outcomes has led to trials being downgraded for unclear risk of selection bias or attrition bias for the majority of studies, imprecision where a wide confidence interval includes both an important increase or decrease in the outcome, and inconsistency where forest plots show different levels of effect. The PREDIMED study has been downgraded for methodological issues regarding randomisation and retraction of the original report, which was then subsequently re‐analysed and republished.
For comparison 3, GRADE assessment of the outcomes has led to trials being downgraded for unclear risk of selection bias or attrition bias or both, and imprecision due to low sample size and wide confidence intervals that include both an important increase or decrease in the outcome. The Lyon Diet Heart Study has been downgraded for having an unclear randomisation method and use of the modified Zelen method, which may have introduced other biases.
For comparison 4, GRADE assessment of the outcomes has led to trials being downgraded for having an unclear method of randomisation and attrition and imprecision.
Potential biases in the review process
We conducted a comprehensive search across major databases for interventions involving the Mediterranean diet. Two review authors independently selected and assessed trials for inclusion using pre‐specified criteria, extracted data and assessed the quality of trials to minimise potential biases in the review processes.
There was a high degree of heterogeneity between trials from different sources (participants, nature and duration of intervention, comparison groups, follow‐up, outcome data), which precluded statistical pooling for some outcomes. We pre‐specified four main comparison groups for analysis to address the likely heterogeneity that we would encounter by broadening out the scope of the review, by primary and secondary prevention and by comparison groups.
Not all data from all studies were reported in a useable format to contribute to meta‐analyses. We have attempted to contact authors where possible to obtain these data and many report preliminary findings in conference proceedings. Data have been reported narratively where we were unable to pool these.
We took the decision to exclude two trials from the main analyses and GRADE assessment where concerns have been publicly raised about the integrity and reliability of the data (Singh 1992; Singh 2002). These two trials reported on 1406 participants and report clinical endpoints and CVD risk factors relevant to secondary prevention (Comparison 4) so their exclusion limited the findings. The PREDIMED trial was retracted due to methodological issues concerning randomisation for two of the 11 study sites, and the inclusion of non‐randomised second household members, but these data have been re‐analysed adjusting for these and republished. The new publication has conducted a series of sensitivity analyses excluding these sites where they have found similar results for clinical endpoints (Estruch 2018). The new publication reports on the composite clinical outcome, CVD and total mortality, myocardial infarction and stroke. Other reports of PREDIMED have been used for CVD risk factors and PAD, which were not reported in the new publication (Estruch 2018), and therefore have not been adjusted.
Our decision to restrict this review to interventions that only focused on the effectiveness of a Mediterranean‐style diet per se avoided the potential confounding effects of other behavioural interventions on our outcomes, for example, those involving increased exercise or weight loss in the context of multifactorial trials. Our decision to exclude trials in people with diabetes who are at increased risk for CVD also missed relevant studies, but interventions for the management of diabetes are covered by the Cochrane Metabolic and Endocrine Disorders Group and are not within the remit of the Cochrane Heart Group.
The definition of the Mediterranean dietary pattern is not homogeneous, and may vary across different geographical and cultural contexts (Helsing 1989; Nestle 1995; Serra‐Majem 1993; Serra‐Majem 2006; Willett 1995). Our choice to use a classification system rather than include only those studies describing the intervention as a Mediterranean diet attempted to address this heterogeneity, and given sufficient studies would allow further exploration of active components. The components required to meet our definition of a Mediterranean dietary pattern were based on previous definitions (Helsing 1989; Nestle 1995; Serra‐Majem 1993; Serra‐Majem 2006; Willett 1995), and required at least the following two core components: high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts) and high intake of plant‐based foods, including fruits, vegetables and legumes. The rationale for this definition is based on recent work (Grosso 2017; Martínez‐González 2017), which emphasises that the protective effects of the diet appear to be most attributable to olive oil, fruits, vegetables and legumes.
Agreements and disagreements with other studies or reviews
Several recent systematic reviews and overviews of reviews have reported on the effects of the Mediterranean diet on cardiovascular health.
A recent narrative overview of both prospective observational studies and RCTs concludes that the Mediterranean diet has some beneficial effects for CVD prevention but the effects are inconsistent between studies with few studies reported in meta‐analyses and calls for more high‐quality trials to address the inconsistencies (Salas‐Salvado 2018). This is in line with the findings of the current review reporting on RCT evidence. An umbrella review of systematic reviews reports on 13 meta‐analyses of observational studies and 16 meta‐analyses of RCTs investigating the association between the adherence to the Mediterranean diet and a number of different health outcomes (Dinu 2018). The authors found robust evidence for a greater adherence to the Mediterranean diet and a reduced risk of overall mortality, cardiovascular diseases, coronary heart disease, myocardial infarction and diabetes with no evidence for LDL cholesterol levels. Adherence to the Mediterranean diet was not specifically measured in the current review, which has been recorded as a potential limitation. With further updates of this review we will consider exploring the effect of adherence on outcomes.
A recent systematic review included both primary and secondary prevention trials and pooled clinical endpoints for these (Liyanage 2016). The trial selection differed from the current review within the search period for both, in that we excluded trials in type 2 diabetes (Toobert 2003), and did not report on total mortality in a trial of HIV patients where deaths were associated with AIDS‐related complications (Ng 2011). Sensitivity analyses were similarly conducted excluding a study with unreliable data (Singh 2002). A further trial that met our inclusion criteria reporting clinical endpoints was also excluded from their analyses (Tuttle 2008), as well as another trial with unreliable data (Singh 1992). Pooling their studies for primary and secondary prevention showed beneficial effects for major vascular events (risk ratio (RR) 0.69, 95% CI 0.55 to 0.86) and stroke (RR 0.66, 95% CI 0.48 to 0.92) (Liyanage 2016).
A systematic review comparing the effects of a Mediterranean diet with low‐fat diets on CVD risk factors in those at high risk or with established disease found favourable but modest effects of the Mediterranean diet on a wide range of cardiovascular risk factors and inflammatory markers, such as body weight, systolic and diastolic blood pressure, fasting plasma glucose, total cholesterol and high‐sensitivity C‐reactive protein (Nordmann 2011). Other systematic reviews have pooled together the evidence from both observational studies and RCTs on the effects of the Mediterranean dietary pattern on metabolic syndrome and individual cardiovascular risk factors, supporting favourable effects of the Mediterranean diet on cardio‐metabolic risk factors (Buckland 2008; Kastorini 2011). The results of the current review in RCTs show inconsistencies between studies but where meta‐analyses were possible there were small beneficial effects on some CVD risk factors for primary prevention.
Authors' conclusions
Implications for practice.
Despite the large number of trials included in the review there is still uncertainty regarding the effects of a Mediterranean‐style diet on clinical endpoints and cardiovascular disease (CVD) risk factors for both primary and secondary prevention from current clinical trial evidence. However, based on supportive observational evidence, positive findings from early clinical trials and the biological plausibility of several mechanisms to explain the beneficial effect of the Mediterranean diet, it has become a popular dietary pattern.
Indeed, some aspects and components of a Mediterranean‐style diet are already included in scientific and clinical guidelines to promote healthy eating and prevent cardiovascular disease, such as the DASH diet (AHA 2006; AHA/ASA 2011; Appel 2006; Locke 2018), the 2015 Dietary Guidelines for Americans, the Healthy Eating Plate (Locke 2018), and the Eatwell guide (Public Health England 2018).
Implications for research.
There remains uncertainty regarding the effects of a Mediterranean‐style diet on clinical endpoints and CVD risk factors for both primary and secondary prevention. Two trials reporting clinical endpoints for secondary prevention were excluded because of concerns regarding the reliability of the data, so the available evidence is restricted to one large trial and a small trial reporting unadjusted estimates of effect. Several ongoing trials have been identified, particularly reporting clinical endpoints in secondary prevention, which will add to the evidence base. Evidence for primary prevention on clinical endpoints is limited to one large trial with methodological issues (although these have now been addressed in a recent re‐analysis) and a small trial reporting unadjusted effects for stroke. Further adequately powered primary prevention trials are needed to confirm findings on clinical endpoints to date. Many trials reported on CVD risk factors, particularly in primary prevention, but heterogeneity precluded meta‐analyses for some outcomes. With the accrual of further evidence, the heterogeneity observed between trials in terms of both the nature and duration of the intervention, the comparators and the range of participants recruited can be explored further and its impact on outcomes examined.
What's new
| Date | Event | Description |
|---|---|---|
| 1 March 2019 | New search has been performed | Evidence is up to date to 26 September 2018. |
| 29 October 2018 | New citation required and conclusions have changed | Substantive update and expansion in scope. Included patients with established CVD as well as those from the general population and at high risk of CVD to examine the effects of the Mediterranean diet on both the primary and secondary prevention of CVD. Included other diets as comparators, not just no intervention or minimal intervention. Main analysis now has 4 comparisons to aid interpretation and reduce heterogeneity: 1. Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention 2. Mediterranean dietary intervention versus another dietary intervention for primary prevention 3. Mediterranean dietary intervention versus usual care for secondary prevention 4. Mediterranean dietary intervention versus another dietary intervention for secondary prevention |
History
Protocol first published: Issue 4, 2012 Review first published: Issue 8, 2013
| Date | Event | Description |
|---|---|---|
| 3 July 2014 | Amended | Minor error in figure corrected |
Acknowledgements
We are grateful to Clare Stradling for providing additional data from her trial (Stradling 2018). We would also like to acknowledge the Trials Search Co‐ordinators for constructing and running searches for the original review and substantive update, Lina Mattsson for translating a paper written in Norwegian, and William Tigbe for translating a paper written in Polish. Thanks also to the original authors of the review who did not continue as authors in the review update: Nadine Flowers, Margaret Thorogood, Aileen Clarke and Lee Hooper. We would also like to thank the editorial and peer reviewers for their detailed and helpful feedback, which has improved the clarity of this review.
Appendices
Appendix 1. Search strategies
CENTRAL, DARE, HTA and NHS EED
#1 MeSH descriptor: [Fruit] explode all trees
#2 fruit*
#3 MeSH descriptor: [Vegetables] explode all trees
#4 MeSH descriptor: [Vegetable Proteins] this term only
#5 vegetable*
#6 MeSH descriptor: [Fabaceae] explode all trees
#7 fabaceae
#8 bean*
#9 legume*
#10 MeSH descriptor: [Lycopersicon esculentum] this term only
#11 lycopersicon next esculent*
#12 tomato*
#13 solanum next lycopersicum
#14 MeSH descriptor: [Nuts] this term only
#15 nut or nuts
#16 MeSH descriptor: [Bread] this term only
#17 bread*
#18 MeSH descriptor: [Edible Grain] explode all trees
#19 cereal*
#20 grain*
#21 MeSH descriptor: [Solanum tuberosum] this term only
#22 solanum next tuberosum
#23 potato*
#24 MeSH descriptor: [Seeds] this term only
#25 seed or seeds
#26 olive next oil
#27 MeSH descriptor: [Fatty Acids, Monounsaturated] this term only
#28 monounsaturated next fat*
#29 mono‐unsaturated next fat*
#30 MeSH descriptor: [Seafood] explode all trees
#31 MeSH descriptor: [Fish Oils] explode all trees
#32 fish
#33 seafood*
#34 shellfish
#35 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10
#36 #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20
#37 #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30
#38 #31 or #32 or #33 or #34
#39 #35 or #36 or #37 or #38
#40 (high or more or increase* or elevat* or much or rais*) near/6 (intake or consumption or consume or eat* or amount*)
#41 #39 and #40
#42 MeSH descriptor: [Dairy Products] explode all trees
#43 MeSH descriptor: [Milk Proteins] explode all trees
#44 milk*
#45 marg?rine*
#46 butter*
#47 dairy
#48 cheese*
#49 red next meat*
#50 processed next meat*
#51 yog?urt*
#52 red near/4 wine*
#53 #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52
#54 (low or little or medium or moderate or less or decrease* or reduc* or restrict*) near/6 (intake or consumption or consume or eat* or amount*)
#55 #53 and #54
#56 MeSH descriptor: [Diet, Mediterranean] this term only
#57 mediterranean near/3 diet*
#58 mediterranean near/6 food*
#59 mediterranean near/6 nutrition*
#60 mediterranean near/6 eat*
#61 (diet* or food* or nutrit* or eat*) near/2 (pattern* or habit*)
#62 MeSH descriptor: [Feeding Behavior] this term only
#63 #56 or #57 or #58 or #59 or #60 or #61 or #62
#64 #41 or #55 or #63
#65 MeSH descriptor: [Cardiovascular Diseases] explode all trees
#66 cardio*
#67 cardia*
#68 heart*
#69 coronary*
#70 angina*
#71 ventric*
#72 myocard*
#73 pericard*
#74 isch?em*
#75 MeSH descriptor: [Stroke] explode all trees
#76 stroke or stokes
#77 cerebrovasc*
#78 apoplexy
#79 brain near/2 accident*
#80 (brain* or cerebral or lacunar) near/2 infarct*
#81 MeSH descriptor: [Hypertension] explode all trees
#82 hypertensi*
#83 peripheral next arter* next disease*
#84 (high or increased or elevated) near/2 (blood next pressure)
#85 MeSH descriptor: [Hyperlipidemias] explode all trees
#86 hyperlipid*
#87 hyperlip?emia*
#88 hypercholesterol*
#89 hypercholester?emia*
#90 hyperlipoprotein?emia*
#91 hypertriglycerid?emia*
#92 emboli*
#93 arrhythmi*
#94 thrombo*
#95 atrial next fibrillat*
#96 tachycardi*
#97 endocardi*
#98 sick next sinus
#99 MeSH descriptor: [Diabetes Mellitus] explode all trees
#100 diabet*
#101 MeSH descriptor: [Hyperglycemia] explode all trees
#102 hyperglycemi*
#103 glucose near/2 intoleran*
#104 MeSH descriptor: [Insulin Resistance] explode all trees
#105 metabolic near/3 syndrome near/3 x
#106 metabolic next cardiovascular next syndrome
#107 dysmetabolic next syndrome next x
#108 insulin next resistan*
#109 MeSH descriptor: [Arteriosclerosis] explode all trees
#110 MeSH descriptor: [Cholesterol] explode all trees
#111 cholesterol
#112 "coronary risk factor*"
#113 MeSH descriptor: [Blood Pressure] this term only
#114 "blood pressure"
#115 #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74
#116 #75 or #76 or #77 or #78 or #79 or #80 or #81 or #82 or #83 or #84
#117 #85 or #86 or #87 or #88 or #89 or #90 or #91 or #92 or #93 or #94
#118 #95 or #96 or #97 or #98 or #99 or #100
#119 #101 or #102 or #103 or #104 or #105 or #106 or #107 or #108 or #109 or #110 or #111 or #112 or #113 or #114
#120 #115 or #116 or #117 or #118 or #119
#121 #64 and #120 Publication Year from 2012 to 2018
MEDLINE Ovid
1. exp Fruit/
2. fruit*.tw.
3. exp Vegetables/
4. Vegetable Proteins/
5. vegetable*.tw.
6. exp Fabaceae/
7. fabaceae.tw.
8. bean*.tw.
9. legume*.tw.
10. Lycopersicon esculentum/
11. lycopersicon esculent*.tw.
12. tomato*.tw.
13. solanum lycopersicum.tw.
14. Nuts/
15. (nut or nuts).tw.
16. Bread/
17. bread*.tw.
18. exp Cereals/
19. cereal*.tw.
20. grain*.tw.
21. Solanum tuberosum/
22. solanum tuberosum.tw.
23. potato*.tw.
24. Seeds/
25. (seed or seeds).tw.
26. olive oil.tw.
27. Fatty Acids, Monounsaturated/
28. monounsaturated fat*.tw.
29. mono‐unsaturated fat*.tw.
30. exp Seafood/
31. exp Fish Oils/
32. fish.tw.
33. seafood*.tw.
34. shellfish.tw.
35. or/1‐34
36. ((high or more or increase* or elevat* or much or rais*) adj6 (intake or consumption or consume or eat* or amount*)).tw.
37. 35 and 36
38. exp Dairy Products/
39. exp Milk Proteins/
40. milk*.tw.
41. marg?rine*.tw.
42. butter*.tw.
43. dairy.tw.
44. cheese*.tw.
45. red meat*.tw.
46. processed meat*.tw.
47. yog?urt*.tw.
48. red wine*.tw.
49. or/38‐48
50. ((low or little or medium or moderate or less or decrease* or reduc* or restrict*) adj6 (intake or consumption or consume or eat* or amount*)).tw.
51. 49 and 50
52. Diet, Mediterranean/
53. (mediterranean adj3 diet*).tw.
54. (mediterranean adj6 food*).tw.
55. (mediterranean adj6 nutrition*).tw.
56. (mediterranean adj6 eat*).tw.
57. ((diet* or food* or nutrit* or eat*) adj2 (pattern* or habit*)).tw.
58. Food Habits/
59. or/52‐58
60. 37 or 51 or 59
61. exp Cardiovascular Diseases/
62. cardio*.tw.
63. cardia*.tw.
64. heart*.tw.
65. coronary*.tw.
66. angina*.tw.
67. ventric*.tw.
68. myocard*.tw.
69. pericard*.tw.
70. isch?em*.tw.
71. exp Stroke/
72. (stroke or stokes).tw.
73. cerebrovasc*.tw.
74. apoplexy.tw.
75. (brain adj2 accident*).tw.
76. ((brain* or cerebral or lacunar) adj2 infarct*).tw.
77. exp Hypertension/
78. hypertensi*.tw.
79. peripheral arter* disease*.tw.
80. ((high or increased or elevated) adj2 blood pressure).tw.
81. exp Hyperlipidemias/
82. hyperlipid*.tw.
83. hyperlip?emia*.tw.
84. hypercholesterol*.tw.
85. hypercholester?emia*.tw.
86. hyperlipoprotein?emia*.tw.
87. hypertriglycerid?emia*.tw.
88. isch?emi*.tw.
89. emboli*.tw.
90. arrhythmi*.tw.
91. thrombo*.tw.
92. atrial fibrillat*.tw.
93. tachycardi*.tw.
94. endocardi*.tw.
95. (sick adj sinus).tw.
96. exp Diabetes Mellitus/
97. diabet*.tw.
98. exp Hyperglycemia/
99. hyperglycemi*.tw.
100. (glucose adj2 intoleran*).tw.
101. exp Insulin Resistance/
102. (metabolic adj3 syndrome adj3 x).tw.
103. metabolic cardiovascular syndrome.tw.
104. dysmetabolic syndrome x.tw.
105. insulin resistan*.tw.
106. exp Arteriosclerosis/
107. exp Cholesterol/
108. cholesterol.tw.
109. "coronary risk factor*".tw.
110. Blood Pressure/
111. blood pressure.tw.
112. or/61‐111
113. 60 and 112
114. randomized controlled trial.pt.
115. controlled clinical trial.pt.
116. randomized.ab.
117. placebo.ab.
118. clinical trials as topic.sh.
119. randomly.ab.
120. trial.ti.
121. 114 or 115 or 116 or 117 or 118 or 119 or 120
122. exp animals/ not humans.sh.
123. 121 not 122
124. 113 and 123
125. limit 124 to ed=20121015‐20180926
Embase Ovid
1. exp fruit/
2. fruit*.tw.
3. exp vegetable/
4. exp vegetable protein/
5. vegetable*.tw.
6. fabaceae.tw.
7. bean*.tw.
8. legume*.tw.
9. lycopersicon esculent*.tw.
10. tomato*.tw.
11. solanum lycopersicum.tw.
12. exp nut/
13. (nut or nuts).tw.
14. bread*.tw.
15. cereal*.tw.
16. grain*.tw.
17. exp grain/
18. solanum tuberosum.tw.
19. potato*.tw.
20. exp plant seed/
21. (seed or seeds).tw.
22. olive oil/
23. olive oil.tw.
24. monounsaturated fatty acid/
25. monounsaturated fat*.tw.
26. mono‐unsaturated fat*.tw.
27. sea food/
28. fish oil/
29. fish meat/
30. fish.tw.
31. seafood*.tw.
32. sea food*.tw.
33. shellfish.tw.
34. or/1‐33
35. ((high or more or increase* or elevat* or much or rais*) adj6 (intake or consumption or consume or eat* or amount*)).tw.
36. 34 and 35
37. exp dairy product/
38. milk*.tw.
39. marg?rine*.tw.
40. butter*.tw.
41. dairy.tw.
42. cheese*.tw.
43. red meat*.tw.
44. processed meat*.tw.
45. exp red meat/
46. yog?urt*.tw.
47. red wine*.tw.
48. or/37‐47
49. ((low or little or medium or moderate or less or decrease* or reduc* or restrict*) adj6 (intake or consumption or consume or eat* or amount*)).tw.
50. 48 and 49
51. Mediterranean diet/
52. (mediterranean adj3 diet*).tw.
53. (mediterranean adj6 food*).tw.
54. (mediterranean adj6 nutrition*).tw.
55. (mediterranean adj6 eat*).tw.
56. ((diet* or food* or nutrit* or eat*) adj2 (pattern* or habit*)).tw.
57. eating habit/
58. or/51‐57
59. 36 or 50 or 58
60. exp cardiovascular disease/
61. cardio*.tw.
62. cardia*.tw.
63. heart*.tw.
64. coronary*.tw.
65. angina*.tw.
66. ventric*.tw.
67. myocard*.tw.
68. pericard*.tw.
69. isch?em*.tw.
70. exp cerebrovascular disease/
71. (stroke or stokes).tw.
72. cerebrovasc*.tw.
73. apoplexy.tw.
74. (brain adj2 accident*).tw.
75. ((brain* or cerebral or lacunar) adj2 infarct*).tw.
76. exp hypertension/
77. hypertensi*.tw.
78. peripheral arter* disease*.tw.
79. ((high or increased or elevated) adj2 blood pressure).tw.
80. exp hyperlipidemia/
81. hyperlipid*.tw.
82. hyperlip?emia*.tw.
83. hypercholesterol*.tw.
84. hypercholester?emia*.tw.
85. hyperlipoprotein?emia*.tw.
86. hypertriglycerid?emia*.tw.
87. emboli*.tw.
88. arrhythmi*.tw.
89. thrombo*.tw.
90. atrial fibrillat*.tw.
91. tachycardi*.tw.
92. endocardi*.tw.
93. (sick adj sinus).tw.
94. exp diabetes mellitus/
95. diabet*.tw.
96. diabet*.tw.
97. diabet*.tw.
98. hyperglycemia/
99. hyperglycemi*.tw.
100. (glucose adj2 intoleran*).tw.
101. insulin resistance/
102. (metabolic adj3 syndrome adj3 x).tw.
103. metabolic cardiovascular syndrome.tw.
104. dysmetabolic syndrome x.tw.
105. insulin resistan*.tw.
106. exp Arteriosclerosis/
107. exp Cholesterol/
108. cholesterol.tw.
109. "coronary risk factor*".tw.
110. Blood Pressure/
111. blood pressure.tw.
112. or/60‐111
113. random$.tw.
114. factorial$.tw.
115. crossover$.tw.
116. cross over$.tw.
117. cross‐over$.tw.
118. placebo$.tw.
119. (doubl$ adj blind$).tw.
120. (singl$ adj blind$).tw.
121. assign$.tw.
122. allocat$.tw.
123. volunteer$.tw.
124. crossover procedure/
125. double blind procedure/
126. randomized controlled trial/
127. single blind procedure/
128. 113 or 114 or 115 or 116 or 117 or 118 or 119 or 120 or 121 or 122 or 123 or 124 or 125 or 126 or 127
129. (animal/ or nonhuman/) not human/
130. 128 not 129
131. 59 and 112 and 130
132. limit 131 to embase
133. limit 132 to dd= 20121015‐20180926
Web of Science
#25 #24 AND #23 Publication date 2012‐2018
#24 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)
#23 #22 AND #10
#22 #21 OR #18 OR #15
#21 #20 OR #19
#20 TS= ((diet* or food* or nutrit* or eat*) SAME (pattern* or habit*))
#19 TS=((mediterranean) SAME (diet* or food* or nutrition* or eat*))
#18 #17 AND #16
#17 TS=((low or little or medium or moderate or less or decrease* or reduc* or restrict*) SAME (intake or consumption or consume or eat* or amount*))
#16 TS=(milk* or marg?rine* or butter* or dairy or cheese* or "red meat*" or "processed meat*" or "red wine*")
#15 #14 AND #13
#14 TS=((high or more or increase* or elevat* or much or rais*) SAME (intake or consumption or consume or eat* or amount*))
#13 #12 OR #11
#12 TS=("solamun tuberosum" or potato* or seed or seeds or "olive oil" or "monounsaturated fat*" or "mono‐unsaturated fat*" or fish or seafood* or shellfish)
#11 TS=(fruit* or vegetable* or fabaceae or bean* or legume* or "lycopersicon esculent*" or tomato* or "solanum lycopersicum" or nut or nuts or bread* or cereal* or grain*)
#10 #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
#9 TS=(arteriosclerosis or cholesterol or "coronary risk factor*" or "blood pressure") #8 TS=diabet*
#7 TS=(hyperlipid* or hyperlip?emia* or hypercholesterol* or hypercholester?emia* or hyperlipoprotein?emia* or hypertriglycerid?emia*)
#6 TS=("high blood pressure")
#5 TS=(hypertensi* or "peripheral arter* disease*")
#4 TS=(stroke or strokes or cerebrovasc* or cerebral or apoplexy or (brain SAME accident*) or (brain SAME infarct*))
#3 TS=("artrial fibrillat*" or tachycardi* or endocardi*)
#2 TS=(pericard* or isch?em* or emboli* or arrhythmi* or thrombo*)
Data and analyses
Comparison 1. Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total cholesterol (mmol/L), change from baseline | 5 | 569 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.32, 0.00] |
| 2 LDL cholesterol (mmol/L), change from baseline | 4 | 389 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.26, 0.09] |
| 3 HDL cholesterol (mmol/L), change from baseline | 5 | 569 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.04, 0.08] |
| 4 Triglycerides (mmol/L), change from baseline | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Systolic blood pressure (mmHg), change from baseline | 2 | 269 | Mean Difference (IV, Random, 95% CI) | ‐2.99 [‐3.45, ‐2.53] |
| 6 Diastolic blood pressure (mmHg), change from baseline | 2 | 269 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐2.29, ‐1.71] |
Comparison 2. Mediterranean dietary intervention versus another dietary intervention for primary prevention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Composite clinical events (CVD death, stroke, MI) | 1 | 7447 | Hazard Ratio (Random, 95% CI) | 0.70 [0.58, 0.85] |
| 2 CVD mortality | 1 | 7447 | Hazard Ratio (Random, 95% CI) | 0.81 [0.50, 1.32] |
| 3 Total mortality | 1 | 7447 | Hazard Ratio (Random, 95% CI) | 1.00 [0.81, 1.24] |
| 4 Myocardial infarction | 1 | 7447 | Hazard Ratio (Random, 95% CI) | 0.79 [0.57, 1.10] |
| 5 Stroke | 1 | 7447 | Hazard Ratio (Random, 95% CI) | 0.60 [0.45, 0.80] |
| 6 Peripheral arterial disease | 1 | 7447 | Hazard Ratio (Random, 95% CI) | 0.42 [0.28, 0.61] |
| 7 Incidence type 2 diabetes | 1 | 3541 | Hazard Ratio (Random, 95% CI) | 0.71 [0.52, 0.96] |
| 8 Stroke (unadjusted) | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.14] |
| 9 Total cholesterol (mmol/L), change from baseline | 7 | 939 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.30, 0.04] |
| 10 LDL cholesterol (mmol/L), change from baseline | 7 | 947 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.27, ‐0.02] |
| 11 HDL cholesterol (mmol/L), change from baseline | 6 | 891 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.01, 0.04] |
| 12 Triglycerides (mmol/L), change from baseline | 7 | 939 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.16, ‐0.01] |
| 13 Systolic blood pressure (mmHg), change from baseline | 4 | 448 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐3.92, 0.92] |
| 14 Diastolic blood pressure (mmHg), change from baseline | 4 | 448 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐2.41, 1.90] |
2.7. Analysis.

Comparison 2 Mediterranean dietary intervention versus another dietary intervention for primary prevention, Outcome 7 Incidence type 2 diabetes.
Comparison 3. Mediterranean dietary intervention versus usual care for secondary prevention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 1 | Risk Ratio (Random, 95% CI) | 0.44 [0.21, 0.92] | |
| 2 CVD mortality | 1 | Risk Ratio (Random, 95% CI) | 0.35 [0.15, 0.82] | |
| 3 CVD death plus non‐fatal MI | 1 | Hazard Ratio (Random, 95% CI) | 0.28 [0.15, 0.52] | |
| 4 Total cholesterol (mmol/L), change from baseline | 2 | 441 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.19, 0.33] |
| 5 LDL cholesterol (mmol/L), change from baseline | 2 | 441 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.09, 0.31] |
| 6 HDL cholesterol (mmol/L), change from baseline | 2 | 441 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.08, 0.07] |
| 7 Triglycerides (mmol/L), change from baseline | 2 | 441 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.38, 0.10] |
| 8 Systolic blood pressure (mmHg), change from baseline | 1 | 339 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐5.29, 1.29] |
| 9 Diastolic blood pressure (mmHg), change from baseline | 1 | 339 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐4.29, 2.29] |
Comparison 4. Mediterranean dietary intervention versus another dietary intervention for secondary prevention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐fatal MI | 1 | Risk Ratio (Random, 95% CI) | 0.47 [0.28, 0.79] | |
| 2 Non‐fatal MI (sensitivity analysis without Singh studies) | 0 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Fatal MI | 2 | Risk Ratio (Random, 95% CI) | 0.66 [0.61, 0.71] | |
| 4 Fatal MI (sensitivity analysis without Singh studies) | 0 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Sudden cardiac death | 2 | Risk Ratio (Random, 95% CI) | 0.48 [0.37, 0.63] | |
| 6 Sudden cardiac death (sensitivity analysis without Singh studies) | 0 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Total cardiac endpoints (fatal and non‐fatal MI, sudden cardiac death) | 2 | Risk Ratio (Random, 95% CI) | 0.59 [0.44, 0.80] | |
| 8 Total cardiac endpoints (fatal and non‐fatal MI, sudden cardiac death) (sensitivity analysis without Singh studies) | 0 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Total mortality | 1 | Risk Ratio (Random, 95% CI) | 0.59 [0.51, 0.68] | |
| 10 Total mortality (sensitivity analysis without Singh studies) | 0 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 CVD mortality | 1 | Risk Ratio (Random, 95% CI) | 0.50 [0.42, 0.60] | |
| 12 CVD mortality (sensitivity analysis without Singh studies) | 0 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Total cardiac endpoints (all‐cause and cardiac deaths, MI, hospital admissions for heart failure, unstable angina or stroke, unadjusted) | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.40, 2.41] |
| 14 Total cholesterol (mmol/L), change from baseline | 2 | 1283 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.61, ‐0.39] |
| 15 Total cholesterol (mmol/L), change from baseline (sensitivity analysis without Singh studies) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 LDL cholesterol (mmol/L), change from baseline | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17 LDL cholesterol (mmol/L), change from baseline (sensitivity analysis without Singh studies) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.26, 0.42] |
| 18 HDL cholesterol (mmol/L), change from baseline | 3 | 1354 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.01, 0.12] |
| 19 HDL cholesterol (mmol/L), change from baseline (sensitivity analysis without Singh studies) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.17, 0.06] |
| 20 Triglycerides (mmol/L), change from baseline | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21 Triglycerides (mmol/L), change from baseline (sensitivity analysis without Singh studies) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.46 [‐0.24, 1.16] |
| 22 Systolic blood pressure (mmHg), change from baseline | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23 Systolic blood pressure (mmHg), change from baseline (sensitivity analysis without Singh studies) | 2 | 150 | Mean Difference (IV, Random, 95% CI) | 1.76 [‐2.80, 6.33] |
| 24 Diastolic blood pressure (mmHg), change from baseline | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 25 Diastolic blood pressure (mmHg), change from baseline (sensitivity analysis without Singh studies) | 2 | 150 | Mean Difference (IV, Random, 95% CI) | 0.98 [‐1.97, 3.93] |
4.1. Analysis.
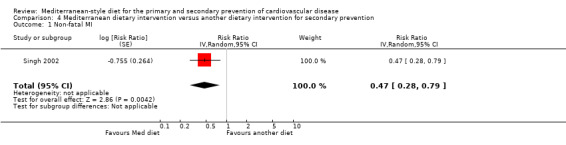
Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 1 Non‐fatal MI.
4.3. Analysis.
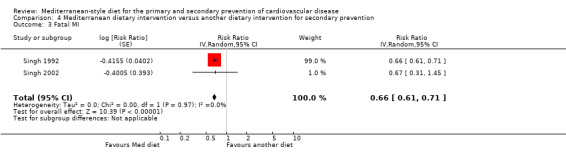
Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 3 Fatal MI.
4.5. Analysis.

Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 5 Sudden cardiac death.
4.7. Analysis.
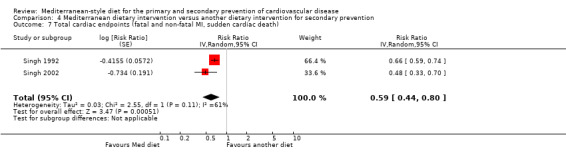
Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 7 Total cardiac endpoints (fatal and non‐fatal MI, sudden cardiac death).
4.9. Analysis.

Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 9 Total mortality.
4.11. Analysis.
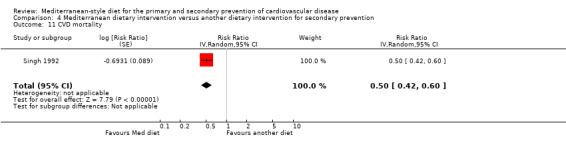
Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 11 CVD mortality.
4.14. Analysis.
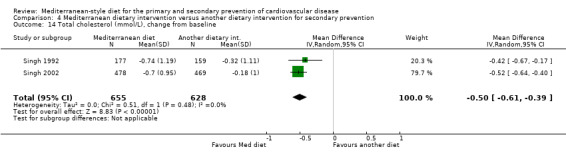
Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 14 Total cholesterol (mmol/L), change from baseline.
4.16. Analysis.
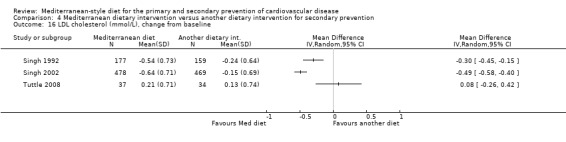
Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 16 LDL cholesterol (mmol/L), change from baseline.
4.18. Analysis.
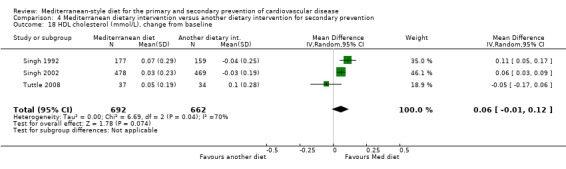
Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 18 HDL cholesterol (mmol/L), change from baseline.
4.20. Analysis.

Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 20 Triglycerides (mmol/L), change from baseline.
4.22. Analysis.
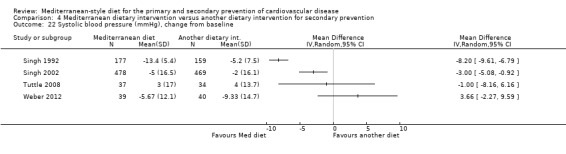
Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 22 Systolic blood pressure (mmHg), change from baseline.
4.24. Analysis.
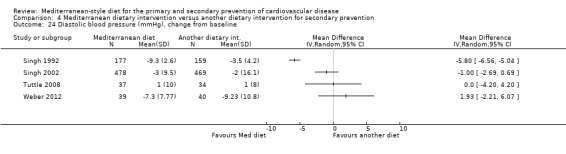
Comparison 4 Mediterranean dietary intervention versus another dietary intervention for secondary prevention, Outcome 24 Diastolic blood pressure (mmHg), change from baseline.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Athyros 2011.
| Methods | RCT of parallel‐group design | |
| Participants | 150 men and women with mild hypercholesterolaemia (5.2 to 6.4 mmol/L) Patients with established CVD, diabetes, metabolic syndrome, those with chronic diseases, malignancies, who are pregnant, on any drug treatment or unwilling to participate were excluded All patients had an initial 4‐week run‐in period where they were advised by trained dieticians to follow a step 1 hypolipidaemic diet (NCEP). Patients were then randomly assigned to 3 groups: plant stanol esters (2 g/day spread), a placebo spread and advice to adhere to a Mediterranean diet Only the Mediterranean diet and placebo spread groups were analysed in this review: 100 patients randomised; mean age 54.7 years; 49% men |
|
| Interventions | Patients were encouraged by trained dieticians to adhere to a Mediterranean dietary pattern with efforts to increase adherence and 7‐day menu plans with food that incorporated the salient characteristics of the Mediterranean diet The placebo group continued with the hypolipidaemic diet throughout the 16‐week intervention period |
|
| Outcomes | Total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, SBP and DBP | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported loss to follow‐up during the 16‐week intervention |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes as stated |
| Other bias | Unclear risk | Insufficient information to judge |
Bajerska 2018.
| Methods | RCT of parallel‐group design | |
| Participants | 144 centrally obese postmenopausal women recruited in 2014 through advertisements in Poland Inclusion criteria: non‐smoking, postmenopausal women (with absence of menses of over 12 months or serum follicle‐stimulating hormone > 30 IU/mL) with central obesity (waist circumference; WC ≥ 80 cm), plus at least one other criterion of the metabolic syndrome, who wished to lose weight Exclusion criteria: women with type 2 diabetes; monogenic dyslipidaemia; a history of cardiovascular disease; use of hypoglycaemic, hypolipidaemic, anti‐inflammatory or weight loss agents, as well as any drug known to influence liver function; with endocrine disorders or on hormonal replacement therapy. The exclusion criteria also included significant weight change in the 6 months prior to the current study, intolerance or food allergy to key components of the intervention diets and excessive alcohol consumption (> 2 drinks/day). Mean age 60.5 years |
|
| Interventions | The 2 supervised dietary intervention arms induced a caloric deficit of ˜2.93 MJ/day, based on individual energy requirements calculated from indirect calorimetry and physical activity (PA) adjustment Mediterranean diet group (MED) Followed a food plan designed on the basis of the Mediterranean dietary recommendations released in 2010 by the Mediterranean Diet Foundation. To build this menu, typical Mediterranean food products were used providing approximately 37% energy from total fat, 20% from MUFAs, 9% from PUFAs, 8% from SFAs, 18% from protein and 45% energy from carbohydrates. Olive oil was used in every meal and 5 to 7 nuts were served once a day. Central European diet group (CED) Based on the recommendations of the NCEP and the AHA, and was designed to provide 27% energy from total fat, 10% from MUFAs, 9% from PUFAs, 8% from SFAs, 18% from protein and 55% energy from carbohydrate, with a special emphasis on high levels of dietary fibre derived from food items typical of the central European region: cereals (oatmeal and barley), pulses (peas and beans), vegetables (root vegetables, cruciferous vegetables) and fruits (apples, plums). The proportion of soluble to insoluble dietary fibre in the CED was 35% to 65%; in the MED this was 20% to 80%. Added salt and refined fats, as well as sugar, were excluded from both diets. 14‐day cyclic dietary plans were formulated for both diets. During the entire 16‐week intervention period, study participants picked up packaged main meals (covering ˜35% daily energy requirements) prepared according to dietician’s recipes by a catering company. Others meals were prepared by the study participants themselves, according to the prescribed dietary plan, including recipes and written instructions to facilitate preparation of meals at home. Throughout the intervention, volunteers were advised to maintain their usual level of PA and keep other lifestyle factors unchanged. |
|
| Outcomes | Total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, SBP, DBP | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer program was used to generate the block randomisation sequence (block size 4), using body mass index as the stratification factor |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was performed by study staff who had not been involved in selection of the participants |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were blinded to all laboratory data. All study personnel (except the dieticians) were blinded to the dietary allocation of the participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study personnel (except the dieticians) were blinded to the dietary allocation of the participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/72 and 9/72 lost to follow‐up in MED and CED groups respectively with reasons provided |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as stated |
| Other bias | Unclear risk | Insufficient information to judge |
Castagnetta 2002.
| Methods | RCT of parallel‐group design | |
| Participants | Healthy postmenopausal female volunteers aged 44 to 71 years recruited by press campaign from Palermo (Southern Italy) Inclusion criteria: postmenopausal for at least 2 years, no history of bilateral ovariectomy, no HRT within the previous year, no history of cancer, no adherence to a vegetarian or macrobiotic diet, no treatment for diabetes, thyroid disease or chronic bowel disease 230 fulfilled these eligibility criteria and 115 women were enrolled in the study based on serum testosterone levels equal to or greater than the median population level (0.14 μg/mL). 58 women were randomised to the intervention group, 55 women to the control group. |
|
| Interventions | MEDIET project ‐ the intervention group were invited to a weekly cooking course and to a social dinner with chefs addressing the principles of the traditional Mediterranean diet. The proposed recipes were based on a traditional Sicilian diet including whole cereals, legumes, seeds, fish, fruits, vegetables, olive oil and red wine. Women were asked to avoid refined carbohydrates, salt and additional animal fat. The intervention ran for 6 months from January to June 2000, then from 3 months from October to December 2000. Women were instructed to consume the same foods on a daily basis at home. The comparison group followed their usual diet The follow‐up period was at 6 and 12 months |
|
| Outcomes | Plasma cholesterol | |
| Notes | The primary publication (Castagnetta 2002) stated that the comparison group was advised to increase the consumption of fruits and vegetables as recommended by the WHO. However, other reports of the study stated that women in the control group followed their usual diets (Carruba 2006, secondary reference for this study). No data were provided on cholesterol levels in the paper but simply a statement that they had reduced. We have contacted the authors several times to request the data to include in our analyses but, unfortunately, to date this has not been forthcoming. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation stratified for baseline parameters |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT analysis; < 20% loss to follow‐up in both groups but no reasons provided |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Insufficient information to judge |
Chasapidou 2014.
| Methods | RCT of parallel‐group design | |
| Participants | Greek adults with known cardio metabolic diseases recruited from 50 randomly selected municipalities in Greece From the preliminary report of 384 participants, 79.9% were obese, 19% had T2DM, 55.1% had hyperlipidaemia, 50.6% were hypertensive and 14.6% had established CVD |
|
| Interventions | The intervention group received a Mediterranean healthy diet personalised in calories and nutrients according to the patient's diseases, and was followed monthly by a dietitian The control group did not receive any dietary counselling 6 months follow‐up |
|
| Outcomes | LDL cholesterol, SBP | |
| Notes | Preliminary results for 384 patients from a total of 8000 estimated to finally participate in the study, recruited from 50 randomly selected municipalities in Greece (Food4Health study) Data are reported narratively in text as no variance is provided for the intervention group or values for the control group only the percentage difference between groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 17.7% lost to follow‐up; unclear if this is balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Unclear as preliminary report in abstract form but DBP was missing as was total cholesterol, HDL cholesterol and triglyceride levels |
| Other bias | Unclear risk | Insufficient information to judge |
Clements 2017.
| Methods | RCT of parallel‐group design | |
| Participants | 120 elderly participants aged 65 to 79 years were recruited to the Nu‐AGE project via the Clinical Research and Trials Unit at the University of East Anglia, UK. All were apparently healthy and free from current or recent (3 months) chronic disease. Exclusion criteria: recent changes to medications, type 1 diabetes, using steroids or taking antibiotics currently or within the previous 2 months Mean age 70 years; 39% men |
|
| Interventions | The Nu‐AGE project is a multicentre European dietary study specifically addressing the needs of the elderly. Across 5 countries, 60 participants were randomised to the control or MED‐diet groups, for 1 year. MED‐diet group The participants within the intervention group were provided with dietary advice sheets and individual dietary advice by members of the study team to achieve the quantitative requirements for the Nu‐AGE dietary intervention: Whole grains: 6 servings per day (1 serving = 25 g bread, 50 g breakfast cereal) Fruits: 2 servings per day (1 serving = 1 apple, 1 banana, 8 small plums) Vegetables and legumes: 330 g per day, once per week 200 g legumes Dairy and cheese: 500 mL dairy per day (of which 30 g cheese) Fish and other seafood: 2 times per week; 1 portion = 125 g Meat and poultry: 4 times per week; 1 portion = 125 g Nuts: 2 times per week; 20 g portion Potatoes, pasta and rice: 150 g per day; 80 g (raw weight) whole grain rice or pasta at least twice a week Eggs: 2 to 4 times per week Oil or fat: 20 g oil per day, 30 g margarine per day; maximum of 50 g fat per day. Should be olive oil and low‐fat margarine rich in MUFA and PUFA. Alcohol: maximum of 1 to 2 glasses per day for men and 1 glass per day for women. Preferably red wine, if not abstain. Fluid: 1.5 litre per day, including milk Salt: reduce added salt and intake of ready meals (soups, gravy, sauce) Sugar: limit consumption of sugar and sweetened drinks (replace with fruit or yogurt, no/reduce sugar in tea or coffee) This advice was based on the information provided within the 7‐day food records collected at baseline. Study participants in the MED‐diet group were given extra‐virgin olive oil, whole grain pasta and low‐fat margarine rich in MUFA and PUFA freely throughout the study. The study team distributed these products at baseline and 4 and 8 months, when the participants attended for appointments. Control group The control group were provided with a standard healthy living advice leaflet from the British Dietetic Association and asked to maintain their habitual dietary intake Follow‐up at 1 year |
|
| Outcomes | Total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides | |
| Notes | Study focused on effects of diets on dendritic cell function. Lipid levels are shown pre and post for each group as box and whisker plots in supplementary figure 1. We have contacted the authors to get the data for these but so far no response. In the report it states that blood pressure was measured at appointments but data are not shown. The effects of the dietary interventions on lipid levels have been described narratively in text. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10/120 participants dropped out of the study with no reasons given |
| Selective reporting (reporting bias) | Unclear risk | Blood pressure data not shown |
| Other bias | Unclear risk | States there were no conflicts of interest in relation to this study |
Colquhoun 2000.
| Methods | RCT of parallel‐group design | |
| Participants | 68 patients with CHD documented by coronary angiography were randomised to a Mediterranean diet or low‐fat diet. All patients were on statin therapy. | |
| Interventions | Mediterranean diet: 35% to 40% energy from fat with > 50% of fat being monounsaturated Low‐fat diet: 20% to 25% energy from fat with 8% to 10% saturated Follow‐up at 3 months |
|
| Outcomes | Total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides | |
| Notes | Few details ‐ reported as a conference proceeding No variance reported so results could not be pooled in meta‐analysis All patients were on statins and lipid levels were the only relevant outcomes for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details |
| Selective reporting (reporting bias) | Unclear risk | No details |
| Other bias | Unclear risk | No details |
Davis 2017.
| Methods | RCT of parallel‐group design | |
| Participants | 166 Australian men and women recruited from Adelaide aged greater than 64 years and free of any cardiovascular, liver, kidney, respiratory or gastrointestinal disease, cognitive impairment, type 1 or 2 diabetes, malignancy in the past 6 months, major recent head trauma or a significant psychiatric disorder Participants with blood pressure above 160/100 mmHg were excluded Mean age 71 years; 44% men |
|
| Interventions | The intervention diet was based on a traditional Mediterranean diet, with small adaptations to the Australian food supply. The diet comprised extra‐virgin olive oil, vegetables, fruit, nuts, whole grains, legumes and fish as core foods. It was moderate in red wine and dairy foods and contained small amounts of red meat. Participants attended the clinic biweekly to meet with a dietitian to ensure high adherence to the dietary protocol. Resources were provided that included a recipe book, guidelines for eating out, serving sizes and the recommended number of servings, and participants also received foods (olive oil, nuts, legumes, tuna and Greek yogurt) to increase the likelihood of adherence. The following recommendations were given: abundant use of extra‐virgin olive oil (≥ 1 tbsp/day), 5 to 6 servings of vegetables/day, ≥ 2 servings of fresh fruit/day, 4 to 6 servings of whole grain cereals/day, 4 to 6 servings of nuts/week, 3 servings legumes/week, 3 servings of fish (1 oily)/week, less than 1 serving of red meat/week, limit consumption of discretionary foods to ≤ 3 times/week. The control group were told to consume a regular diet without change (seasonal variation permitted) and received a voucher to buy regularly consumed foods from supermarkets Both groups were required to maintain their physical activities and medication and dietary supplement use throughout the intervention 6 months intervention and follow‐up |
|
| Outcomes | Total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides | |
| Notes | The MedDiet for cardiovascular and cognitive health in the elderly (MedLey) study: primary outcome was cognitive function, CVD risk factors were secondary outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Volunteers were randomly allocated to either the control group or the intervention group stratified by gender, BMI and age by the process of minimisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The researcher who administered the cognitive test battery and assessed and scored cognitive outcomes was blind to group assignment and will remain blind until after data analysis to reduce bias. No information regarding CVD risk factors. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The researcher who administered the cognitive test battery and assessed and scored cognitive outcomes was blind to group assignment and will remain blind until after data analysis to reduce bias. No information regarding CVD risk factors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for withdrawal or missing data were associated with the assigned treatment in 2 participants only. Therefore, missing data for participants who were not included in the final analysis were assumed to be missing at random. Overall attrition over 6 months was 17%. |
| Selective reporting (reporting bias) | Low risk | Report includes all specified outcomes |
| Other bias | Unclear risk | Insufficient information to judge |
Dinu 2017.
| Methods | Cross‐over RCT (3 months each phase) | |
| Participants | 117 participants with a low‐to‐medium cardiovascular risk profile, characterised by being overweight and by the presence of at least an additional metabolic risk factor, but free from medications, were included Mean age 51 years; 15% men |
|
| Interventions | All the participants were randomly allocated to Mediterranean or vegetarian diets lasting 3 months each, and then crossed over. The 2 diets were isocaloric between them and of 3 different sizes (1400, 1600, 1800 Kcal/day), according to specific energy requirements. 3 months follow‐up. |
|
| Outcomes | Total cholesterol, LDL cholesterol, triglycerides | |
| Notes | Few details as reported as a conference proceeding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States that an open cross‐over design was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 99 participants (85%) completed the study. States that the final analysis was performed in adherent participants with outliers removed. |
| Selective reporting (reporting bias) | Unclear risk | Unclear as study is reported as a conference proceeding only |
| Other bias | Unclear risk | Insufficient information to judge |
Djuric 2009.
| Methods | RCT of parallel‐group design | |
| Participants | Healthy, non‐obese women aged 25 to 65 years recruited from adverts in community newsletters, health fairs, flyers and employee newsletters in Michigan, US. Women completed 7‐day food diaries. Eligibility criteria: fat intake was at least 23% of calories with no more than 48% from MUFA and fruit and vegetable intake was < 5.5 servings per day. This was to reflect a typical American intake. Women had to have good general health, be current non‐smokers and be in the normal to overweight range (BMI 18 to 30). Exclusion criteria: chronic diseases such as diabetes, autoimmune disease, hypertension, being on medically prescribed diets, taking dietary supplements > 150% RDA, pregnant or lactating and being treated with therapies or supplements that could obscure the results 69 women were randomised; mean age 44 years (range 25 to 59) and mean BMI 24 (19 to 30) |
|
| Interventions | The intervention was a Greek Mediterranean exchange list diet with exchange goals determined by dieticians at baseline and focused on increasing fruit and vegetable intake and variety and increasing MUFA intake while maintaining the baseline energy intake and total fat intake. The fruit and vegetable goal was 7 to 9 servings/day depending on baseline calorie intake and maintaining baseline energy intake was achieved by substituting fruit and vegetables for other carbohydrates. Variety was achieved using exchange lists. The fat intake goal was PUFA:SFA:MUFA ratio of 1:2:5. This was achieved by reducing usual fat intakes by half using low‐fat food and then adding in olive oil or other high MUFA to the diet to keep energy and total fat intake at baseline levels. Participants were given 3 L of extra‐virgin olive oil at baseline and at 3 months. 7‐day food records were taken at baseline, 3 months and 6 months. Counselling by the dieticians occurred weekly by telephone for the first 3 months and twice weekly thereafter. Face‐to‐face counselling occurred at baseline and 3 months. The intervention period was 6 months. Women were counselled on home eating patterns, restaurant eating, eating at work and special occasions. The comparison group followed their usual diets. They did not receive counselling, but were given the National Cancer Institutes Action guide to healthy eating and written materials on nutritional deficiencies if below 67% RDA. Follow‐up was at 6 months after the end of the intervention period. |
|
| Outcomes | Total cholesterol, LDL cholesterol, HDL cholesterol and triacylglycerol | |
| Notes | Body weight increased by 0.24 kg in the control group and decreased by 1.21 kg in the intervention group after the 6‐month intervention period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated. Participants stratified by race and menopausal status prior to randomisation using a block design of 6. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential loss to follow‐up of 23% in the intervention group compared with 3% in the control group. No reasons for loss to follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes as stated |
| Other bias | Unclear risk | Insufficient information to judge |
Entwistle 2018.
| Methods | RCT of parallel‐group design | |
| Participants | Heart and lung transplant recipients who are at a substantially increased risk CVD. Eligible participants were clinically stable, aged ≥16 years, and a minimum 6 months post‐transplant Exclusion criteria included acute rejection, infection, prevalent cancer, diabetes or chronic kidney disease (estimated glomerular filtration rate ≤ 30). Patients with any competing dietary issues (i.e. food allergies and following medically prescribed diets that conflicted with the interventions) were also excluded. Study participants were identified through hospital records at the transplant outpatient clinic and recruitment commenced in February 2014 and ended in October 2014. The study was conducted at the University Hospital of South Manchester, UK. 116 patients were assessed for eligibility, 75 were excluded and 41 randomised (20 heart, 21 lung) Mean age 58; 70% men |
|
| Interventions | The Assessment of the MEditerraneaN Diet In heart and lung Transplantation (AMEND‐IT) study was a single‐centre parallel‐randomised study designed to assess the feasibility and acceptability of 2 dietary interventions, the Mediterranean diet and low‐fat diet among heart and lung transplant recipients. All participants received a printed booklet containing advice about shopping, food preparation, hygiene, storage, dining out and recipes. Additional advice and support were provided at 6‐ and 12‐month outpatient visits, and during six 15‐minute telephone consultations spaced evenly through the intervention period, when participants could raise any questions or concerns and when key dietary recommendations (e.g. plant‐based diet, consume minimally processed food) were reinforced. SMS messaging was also used to remind patients of clinic study requirements. Several 5‐hour group education sessions were conducted for each diet group (with an accompanying family member if desired) on specified dates outside routine outpatient visits. Mediterranean diet Received information and encouragement to follow an eating pattern representative of a traditional Mediterranean diet. The key dietary recommendations were: daily mixed consumption of a range of vegetables, fruit, whole grains, fish/seafood, raw nuts and legumes; abundant use of extra‐virgin olive oil (a free 5L container of extra‐virgin olive oil was provided to each participant); moderate consumption of dairy products and red wine; low intake of red and processed meats, of sweets, sweet‐baked pastries and sweetened beverages. Low‐fat diet Advised to follow modified British Heart Foundation low‐fat guidelines with an emphasis on consuming mainly plant‐based whole foods similar to the Mediterranean diet, with advice to minimise high‐fat foods such as processed meats, commercially baked pastries and desserts, and vegetable oils and spreads. Advice was given on how to identify and avoid different types of fat. Each participant received a low‐fat recipe book. The main difference between the 2 diets was the intake of oil and fat, which was encouraged to a moderate degree in the Mediterranean diet but discouraged in the low‐fat diet 12‐month follow‐up |
|
| Outcomes | Triglycerides | |
| Notes | Data reported narratively in text as variance reported for percentage change from baseline only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were stratified according to organ type and transplant date, and then randomly assigned to either a Mediterranean diet or a low‐fat diet intervention using a computerised system with random block size and an equal 1:1 allocation ratio |
| Allocation concealment (selection bias) | Low risk | To blind the investigator during recruitment, randomised codes were sent to a third person who then allocated the randomised interventions to patients per protocol |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/41 patients lost to follow‐up with reasons given |
| Selective reporting (reporting bias) | Low risk | All outcomes stated are reported |
| Other bias | Unclear risk | Insufficient information to judge |
Esposito 2004.
| Methods | RCT of parallel‐group design | |
| Participants | Men and women were recruited from June 2001 to January 2004 among those attending the outpatient department of the Division of Metabolic Diseases at the Second University of Naples, Naples, Italy 180 adults (99 men and 81 women); mean age 44.3 years (intervention diet) and 43.5 years (control diet) with metabolic syndrome were enrolled in the study Inclusion criteria: ≥ 3 of the following: (1) abdominal adiposity (defined as waist circumference 102 cm (men) or 88 cm (women)); (2) low levels of serum HDL cholesterol (40 mg/dL (men) or 50 mg/dL (women)); (3) hypertriglyceridaemia (triglycerides level of ≥ 150 mg/dL); (4) elevated blood pressure (≥ 130/85 mmHg); and (5) impaired glucose homeostasis (fasting plasma glucose concentration ≥ 110 mg/dL) Exclusion criteria: CVD, psychiatric problems, a history of alcohol abuse (alcohol consumption 500 g/week in the last year), if they smoked, or if they took any medication |
|
| Interventions | Intervention diet: 90 participants were given detailed advice about the usefulness of a Mediterranean‐style diet. Through a series of monthly small‐group sessions, participants received education in reducing dietary calories (if needed), personal goal‐setting and self‐monitoring using food diaries. Behavioural and psychological counselling was also offered. Dietary advice was tailored to each participant on the basis of 3‐day food records. The recommended composition of the dietary regimen was carbohydrates, 50% to 60%; proteins, 15% to 20%; total fat, < 30%; saturated fat, < 10%; and cholesterol consumption, < 300 mg/day. Participants were advised to consume at least 250 g to 300 g of fruits, 125 g to 150 g of vegetables, 25 g to 50 g of walnuts, 400 g of whole grains (legumes, rice, maize and wheat) daily and to increase their consumption of olive oil. Participants were in the programme for 24 months and had monthly sessions with the nutritionist for the first year and twice monthly sessions for the second year. Compliance with the programme was assessed by attendance at the meetings and completion of diet diaries. Control diet: 90 participants were given general oral and written information about healthy food choices at baseline and at subsequent visits. The general recommendation for macro‐nutrient composition of the diet was similar to that for the intervention group (carbohydrates, 50% to 60%; proteins, 15% to 20% and total fat, 30%). Participants had bimonthly sessions with study personnel. Participants in both groups also received guidance on increasing their level of physical activity, mainly by walking for a minimum of 30 minutes/day but also by swimming or playing aerobic ball games Trial was conducted from June 2001 to January 2004. Follow‐up period was 2 years. |
|
| Outcomes | Total cholesterol, HDL cholesterol, triglycerides, SBP, DBP | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Stored in sealed study folders and held in a central, secured location until informed consent obtained |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Staff members involved in the intervention had to be aware of the group assignment; thus, the study was only partly blinded. Blinding of participants and personnel for behavioural interventions is difficult and often not possible, so we have not judged this as at high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory staff did not know to which group the participants were assigned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes stated are reported |
| Other bias | Unclear risk | Insufficient information to judge |
Konstantinidou 2010.
| Methods | RCT of parallel‐group design | |
| Participants | From October 2007 to October 2008, 90 eligible community‐dwelling adults (26 men and 64 women, aged 20 to 50 years) were recruited from primary care centres in Spain. They were considered healthy on the basis of a physical examination and routine biochemical and haematological laboratory determinations. Exclusion criteria: intake of antioxidant supplements; intake of aceto salicylic acid or any other drug with established antioxidative properties; high levels of physical activity (3000 kcal/week in leisure‐time physical activity); obesity (BMI 30 kg/m2); hypercholesterolaemia (total cholesterol 8.0 mM or dyslipidaemia therapy); diabetes (glucose 126 mg/dL or diabetes treatment); hypertension (SBP ≥ 140 mmHg) or (DBP ≥ 90 mmHg), or both or antihypertensive treatment; multiple allergies; coeliac or other intestinal diseases; any condition that could limit the mobility of the participant, making study visits impossible; life‐threatening illnesses or other diseases or conditions that could worsen adherence to the measurements or treatments; vegetarianism or a need for other special diets; and alcoholism or other drug addiction |
|
| Interventions | Participants were assigned to 1 of 2 interventions or a control group as follows: 1. Traditional Mediterranean diet with virgin olive oil (30 participants) 2. Traditional Mediterranean diet with washed virgin olive oil (30 participants) The dietician gave personalised advice during a 30‐minute session to each participant following the traditional Mediterranean diets, with recommendations on the desired frequency of intake of specific foods. Participants were instructed to use olive oil for cooking and dressing; increase consumption of fruit, vegetables and fish; consume white meat instead of red or processed meat; prepare homemade sauce with tomato, garlic, onion, aromatic herbs and olive oil to dress vegetables, pasta, rice and other dishes; and, for alcohol drinkers, moderate consumption of red wine. 3. Control group (30 participants): participants were advised by a dietician to maintain their habitual lifestyle Intervention period and follow‐up was 3 months |
|
| Outcomes | Total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, SBP, DBP | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant dropped out of the trial |
| Selective reporting (reporting bias) | Low risk | All outcomes stated are reported |
| Other bias | Unclear risk | Not stated |
Lapetra 2018.
| Methods | RCT of parallel‐group design | |
| Participants | Multicentre trial in primary care in Spain CFAMED ‐ Insuficiencia Cardiaca (Heart Failure), Fibrilación Auricular (Atrial Fibrillation) and dieta MEDiterránea (MEDiterranean diet) 180 hypertensive patients between 55 and 75 years of age at high CVD risk were randomised to a Mediterranean diet or low‐fat diet; 92% men Exclusion criteria: previous history of CVD (CHD, stroke, HF or AF), BMI > 40, severe chronic disease with poor prognosis, illegal drug use or chronic alcoholism, physical limitations, mental or intellectual barriers to participation in the trial, low predicted likelihood of changing dietary habits, any condition that may affect the development of the trial |
|
| Interventions | Mediterranean‐style diet (N = 90) Low‐fat diet according to American Heart Association guidelines (N = 90) Both groups received dietary advice (individual and group) every 3 months for at least 2 years. Participants attended educational talks about hypertension and healthy eating and were given a booklet that included essential information from the talks and a seasonal menu, tailored for each group. 2 years follow‐up |
|
| Outcomes | Stroke | |
| Notes | Conference proceeding so few details given. Further details taken from trial registration ‐ ISRCTN27497769. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear ‐ states "simple blind" in abstract (presume this should read single blind) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear ‐ states "simple blind" in abstract (presume this should read single blind) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes reported as listed on trial registry but it was a conference proceeding presenting clinical events |
| Other bias | Unclear risk | Insufficient information to judge |
Lindman 2004.
| Methods | RCT of parallel‐group design (2 x 2 factorial design) | |
| Participants | 219 older men with long‐standing hypercholesterolaemia were recruited from the Diet and Omega‐3 Intervention trial on atherosclerosis (DOIT) study, Norway Mean age 69.7 years for both genotypes |
|
| Interventions | Men were randomised into 3 intervention groups or the control group as follows:
Diet counselling was given individually by a clinical nutritionist based on a food frequency questionnaire. The food frequency questionnaire was also answered by the participants at the end of the main study (36 months). Energy content and nutrient composition of the diet were calculated from the questionnaires at baseline and 36 months. Dietary advice was given during 30 to 45 minutes at time of randomisation, and for 30 minutes after 3 months. Participants were supported with a margarine rich in PUFA and vegetable oils free of cost. Advice was given to increase intake of vegetables, fruit and fish, and decrease consumption of meat and target energy percents at 27% to 30% fat, 15% to 18% protein and 50% to 55% carbohydrate. To fulfil these goals participants were recommended to use rapeseed or olive oil for cooking; use leafy vegetables daily; include fruits, berries and nuts in the diet; eat fish 3 times per week; use wholemeal bread, skimmed milk and reduced‐fat cheese. 2 capsules were taken twice daily corresponding to 2.4 g VLC n‐3 capsules or 2.4 g corn oil (placebo capsules). Follow‐up period was 6 months |
|
| Outcomes | Triglycerides | |
| Notes | Only data from the usual care and placebo capsules (control group) (n = 51) and dietary advice ('Mediterranean type' diet) and placebo capsules (n = 47). (The focus of the study was to investigate the effect of long‐term diet and VLC n‐3 fatty acids intervention on plasma coagulation factor VII (FVII), choline‐containing phospholipids and triglycerides, especially relating to the R353Q polymorphism of the FVII gene). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Insufficient information to judge |
Mayr 2018.
| Methods | RCT of parallel‐group design | |
| Participants | AUSMED Heart Trial ‐ secondary prevention trial in CHD patients recruited from 2 hospitals in Melbourne, Australia between 2014 and 2106 Patients were eligible if they had documented CHD including at least one of the following: acute MI, angiographically confirmed angina, revascularisation Exclusion criteria: malignant tumour, symptomatic chronic heart failure, chronic inflammatory disease, chronic kidney disease, decompensated liver disease, pregnancy, breastfeeding, history of allergy to olive oil or nuts or current participation in another trial Mean age 62 years; 84% men |
|
| Interventions | 73 patients were randomised to a Mediterranean diet or low‐fat diet. For both diets advice was tailored to the individual through dieticians using client‐centred counselling and goal setting. Different dieticians advised for the 2 groups to prevent contamination. Face‐to‐face meetings with dieticians occurred at baseline, 3 months and 6 months, and phone calls at weeks 3, 6, 9 and months 4 and 5. The number of contacts and intensity of the intervention was the same for both diets. Mediterranean diet Based on a traditional Cretan Mediterranean diet. Modelled a 2‐week meal plan incorporating key dietary components of a Mediterranean diet with a mix of traditional and modified recipes considered to be realistic options for multi‐ethnic Australians. Target macronutrient intakes were: 42% total fat (at least 50% MUFA, 25% PUFA), < 10% SFA, 35% carbohydrates, 15% protein. Patients received a recipe book, shopping lists, a food pyramid, weekly dietary intake checklists and label reading information. Food recommendations were: daily intake of extra‐virgin olive oil, nuts, fruit and vegetables, whole grains, regular intake of fish legumes and yogurt and limited intake of red and processed meat and sweets and pastries. Hampers were provided at baseline and 3 months to aide adherence (6 L extra‐virgin olive oil, 1.2 kg nuts, tinned fish and legumes and Greek yogurt). Low‐fat diet Followed the standard diet recommendations for cardiac patients in Australia at the time (2014). Target recommended macronutrient intakes were: < 30% total fat with less than 10% saturated fat, 45% to 65% carbohydrate, 15% to 25% protein. Food recommendations included daily intake of grains and cereals (mostly whole grain 5 to 7 servings per day), fruits (2 servings per day) and vegetables (5 to 6 servings per day), protein foods (2 to 3 servings per day) and low‐fat dairy food (2 servings per day). A one week meal plan was provided, resources for label reading, low‐fat cooking and recommended food group serving sizes. To aid compliance patients were provided with a supermarket voucher at the 3 face‐to‐face meetings. 6 months follow‐up |
|
| Outcomes | Lipid levels, blood pressure | |
| Notes | Lipid levels and blood pressure were not reported in the full paper but only in a preliminary analysis as a conference proceeding in a subset of the cohort. No data were provided and the authors findings are reported narratively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation tables were developed by the trial statistician using a computer‐generated stratified approach |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 27% loss to follow‐up in the intervention group and 17% in the control group over 6 months. Reasons for dropout provided. Those who dropped out had a higher dietary inflammatory index and lower intake of fibre at baseline but were otherwise similar to the completers. |
| Selective reporting (reporting bias) | Unclear risk | Blood pressure and lipids not reported in the main paper, only in a preliminary analysis |
| Other bias | Unclear risk | Insufficient information to judge |
Michalsen 2006.
| Methods | RCT of parallel‐group design | |
| Participants | Patients with established coronary artery disease as verified by coronary angiography within 3 months. Recruited from 2 hospitals in Germany and the national press Exclusion criteria: an acute coronary syndrome or coronary artery bypass graft within the previous 3 months, diabetes mellitus type I, manifest cardiac arrhythmias, heart failure, life‐threatening comorbidity and a BMI > 33 101 patients; mean age 59 years; 77% men |
|
| Interventions | The study was inspired by the Lyon Diet Heart Study and the Lifestyle Heart Trial, and aimed to combine the nutritional approach of the traditional Mediterranean diet with a group‐supported comprehensive lifestyle modification program in order to ensure maximum adherence with the diet in a non‐Mediterranean country, Germany. Eligible participants were assigned either to: Intervention A lifestyle modification group with an intensive 100 hour/1‐year programme and the focus on Mediterranean diet. The nutritional therapy did not include any supplements or free delivered food items, but participants had to adopt the recommended diet strictly by themselves after intensive instructions and education. The programme began with a 3‐day non‐residential retreat, followed by weekly 3‐hour meetings for 10 weeks. Thereafter, 2‐hour meetings took place every other week for 9 months. The meetings were held in groups of 10 to 13 participants. The lifestyle programme addressed diet and stress management. Participants were extensively informed about the Mediterranean diet by nutritional information, repetitive group discussions, cooking classes and group meals, and dietary instructions were tailored to individuals where necessary. The aim of the dietary instructions was to provide a diet rich in alinolenic acid (ALA), marine n‐3 polyunsaturated fatty acids (PUFA), monounsaturated fats (MUFA) and phytochemicals, and low in saturated fats (SFA). The instructions were to consume at least 5 portions of fruits and vegetables daily, with an emphasis on root and green vegetables with a high content of ALA, and more than 2 portions of fatty fish per week, to consume preferably whole‐grain bread, pasta and rice, the intake of flaxseed and walnuts was strongly recommended whereas the intakes of meat and sausage should be limited to three servings per week, and beef, lamb and pork were to be replaced by poultry, fish or vegetarian dishes. Both olive oil and canola oil, and, for some dishes, walnut and flaxseed oil, were strongly recommended. Modest regular alcohol consumption in the form of red wine with the meals was recommended. Control Patients in the control group received only written and less detailed information about the dietary principles of the Mediterranean diet, and some general advice about stress reduction by means of leaflets that were mailed shortly after randomisation Follow‐up at 1 year |
|
| Outcomes | Total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides | |
| Notes | 82% were taking statins at the beginning of the study. During the study, the dose of statins was non‐significantly more reduced in the intervention patients and increased in control patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised assignments were made centrally by a computer program. Assignments were stratified by age, sex and status of revascularisation. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/105 patients dropped out with reasons given |
| Selective reporting (reporting bias) | Low risk | All outcomes stated are reported |
| Other bias | Unclear risk | Insufficient information to judge |
Misciagna 2017.
| Methods | RCT of parallel‐group design | |
| Participants | Participants with non‐alcoholic fatty liver disease (NAFLD) were identified during the NutriEp survey enrolment process (Puglia, Italy). Eligible participants were those with moderate or severe NAFLD (N = 203). Exclusion criteria included: overt cardiovascular disease and revascularisation procedures; stroke; clinical peripheral artery disease; T2DM; more than 20 g/daily of alcohol intake; severe medical condition that may impair the person participating in a nutritional intervention study; people following a special diet or involved in a programme for weight loss, or who had experienced recent weight loss and inability to follow a Mediterranean diet for religious or other reasons 98 participants randomised; 50% men |
|
| Interventions |
Intervention Low glycaemic index Mediterranean diet (LGIMD). Foods in LGIMD have all a low glycaemic index (GI) and no more than 10% of total daily calories coming from saturated fats. The LGIMD was high in monounsaturated fatty acids from olive oil and contained also omega 3 polyunsaturated fatty acids, from both plant and marine sources. Adherence to the LGIMD as measured by Mediterranean Adequacy Index. Control Italian National Research Institute for Foods and Nutrition (INRAN) guidelines. The recommended diets were provided in brochure format, with graphical explanations organised according to a traffic light system: with a list of foods that can be consumed frequently (green foods), sometimes (yellow foods) and never (red foods). The brochure also contained a dietary record, where participants daily indicated the code of each food consumed at breakfast, lunch, dinner and during snack time. Monthly follow‐up visits in both groups included a face‐to‐face interview with the dietician in order to assess the diet followed by the subject and to give, if needed, personal recommendations to achieve the "group assigned" goal. 6 months follow‐up |
|
| Outcomes | Total cholesterol, HDL cholesterol, triglycerides | |
| Notes | Data provided as number and percentage of participants with normal and altered levels rather than mean and SD at baseline and follow‐up so these cannot be used in meta‐analyses. Findings are reported narratively in text. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned, according to a computerised random number sequence |
| Allocation concealment (selection bias) | Unclear risk | With the exception of the dietitians, investigators and staff were unaware of the participants' diet assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States dieticians were aware of group assignment. States blinding and equipoise were strictly maintained by emphasising to the intervention staff and participants that each diet adhered to healthy principles. Blinding of participants and personnel for behavioural interventions is difficult and often not possible so we have not judged this as at high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Staff members who obtained outcome measurements were not informed about diet assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The primary analysis was intention‐to‐treat. 6/50 individuals were lost in the follow‐up in the intervention group and 2/48 in a control group. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated are reported |
| Other bias | Unclear risk | Insufficient information to judge |
Ng 2011.
| Methods | RCT of parallel‐group design (pilot study) | |
| Participants | 48 patients with HIV were recruited from the Queen Elizabeth Hospital in Hong Kong (People’s Republic of China) Inclusion criteria: (1) HIV‐positive, (2) 18 years old or above, (3) considered to be physically well by an experienced nurse specialising in HIV and stable within the context of their HIV diagnosis with no current illness concerns, (4) not pregnant and (5) had not previously received dietary advice on lipid lowering |
|
| Interventions | Participants in both groups were given both verbal and written instructions regarding the particular diet that they had been assigned, which they were required to adhere to for a period of 1 year. The dietitian designed an individualised meal plan for each participant, taking into account any specific requirements related to their HIV status. Patients were educated as to the necessary adjustments to their eating habits required to meet the criteria of their assigned diet group. Modified Mediterranean diet The Mediterranean diet was based on the basic principle of the low cholesterol diet with emphasis on avoiding foods rich in saturated fat and cholesterol, modified slightly to suit the local eating culture. In order to increase the consumption of mono and polyunsaturated fats, the diet also included one serving per day of 3 items from the following list: * 100 g of white meat (fish or chicken) to replace a serving of red meat * 10 mL of canola, rapeseed or olive oil to be used as cooking oil to replace saturated fats * 17 g of canola margarine per day in place of butter or other margarine * 100 g of dried legumes, including soy beans, chick peas and lentils, or 100 g of tofu to replace meat as a protein source * 30 g nuts including peanuts, almonds and hazelnuts * 237 mL of low‐fat dairy or soy drink instead of full fat dairy * 5 servings of fruits and vegetables Low‐fat, low‐cholesterol diet The low‐fat and low‐cholesterol diet was prescribed according to the NCEP Adult Treatment Panel III guidelines. It involves reducing the intake of saturated fat (< 7% of total calories) and cholesterol (< 200 mg per day). Up to 10% of calories can be derived from polyunsaturated fat and up to 20% from monounsaturated fat. Total fat should make up 25% to 35% of the total calories, carbohydrates 50% to 60% and protein ˜15%. Intake of 20 g to 30 g of fibre per day is encouraged, as are weight reduction and physical activity. 12 months follow‐up |
|
| Outcomes | Total cholesterol, triglycerides | |
| Notes | Rationale: HIV and highly active antiretroviral therapies have been associated with changes in individuals' lipid profiles and fat distribution (lipodystrophy). This pilot RCT study was conducted for future larger RCT to evaluate whether lipodystrophy in HIV patients can be controlled by adopting a low‐fat and low‐cholesterol diet or the modified Mediterranean diet. The authors point out that there were several procedural and methodological issues identified, which must be rectified before a similar large‐scale trial taking place (see other biases below). The standard deviation difference for changes from baseline in total cholesterol and triglycerides was calculated from P values following guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated into the 2 different diet streams, using computer‐generated randomisation. Blinding was not used in this study. |
| Allocation concealment (selection bias) | Unclear risk | The dietitian allocated patients into different diets according to the next available diet type on entry into the trial. Although the dietitian ran the computer‐generated randomisation, bias was minimised by hiding the allocation of diet groups until the participant was recruited; the randomised diet group was then revealed to the dietitian and the participant. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not used in this study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not used in this study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The data were analysed on the basis of intention‐to‐treat, including the 12/48 participants for whom baseline samples were available but who dropped out of the study at later stages. 1/23 patients in the low‐fat diet group dropped out compared to 7/25 following the Mediterranean diet. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as stated |
| Other bias | Unclear risk | Several difficulties were identified with respect to the procedures utilised, primarily related to recruitment of participants. Despite appearing physically well, as assessed by experienced HIV nurses and physicians, 4 participants died during the study. More strict inclusion and exclusion criteria should be set if any similar, large‐scale study were to be undertaken. Similarly, the reasonably large change in CD4 counts exhibited by our participants suggests that some were not in a stable phase of their HIV treatment: given the effect of highly active antiretroviral treatment on lipid levels, this makes it difficult to draw conclusions as to whether the diet or treatment regime was affecting the level of lipids measured here. In addition, several participants were "lost to follow‐up", with the majority of those ceasing participation coming from the Mediterranean diet group. Some of these participants were known regularly to miss scheduled appointments at the clinic, and perhaps greater attention should be paid to participant attendance at regular clinical visits when recruiting. |
PREDIMED.
| Methods | RCT of parallel‐group design | |
| Participants | PREDIMED is a multicentre trial conducted over 11 sites (169 clinics) in Spain to examine the effects of the Mediterranean diet supplemented with extra‐virgin olive oil or nuts compared to a low‐fat diet in participants at increased risk of CVD Inclusion criteria: community‐dwelling with high risk of CVD but with no CVD at enrolment, aged 55 to 80 for men and 60 to 80 for women with either T2DM or 3 or more risk factors (current smoker, HTN, hypercholesterolaemia (LDL > 160 mg/dL or on hypolipidaemic drugs), HDL < 40 mg/dl, overweight or obesity (BMI > 25), family history of premature CHD) Exclusion criteria: previous history of CVD. Any severe chronic illness. Immunodeficiency or HIV status. Illegal drug use or chronic alcoholism. History of allergy to olives or nuts. Low predicted likelihood of changing dietary habits according to the Prochaska and DiClemente stages of change model. Recruitment took place between 25 June 2003 and 30 June 2009. 8713 screened for eligibility, 973 refused to participate, 293 did not meet the inclusion criteria, 7447 participants were randomised 1:1:1 to each of the 3 groups. 42% men; mean age 67 |
|
| Interventions | 2 intervention groups followed a Mediterranean dietary pattern with supplemental extra‐virgin olive oil or tree nuts, and the control group followed a low‐fat diet. Initially the control group received tailored advice at baseline and a leaflet and yearly follow‐up with trained dieticians, and 3 years into the trial this was amended so the intensity of the low‐fat intervention matched that of the Mediterranean diet intervention groups where there were tailored individual visits to dieticians and group sessions every 3 months. During these sessions behavioural change techniques employed included goal‐setting, self‐monitoring, feedback and reinforcement, self‐efficacy enhancement, incentives, problem‐solving, relapse prevention and motivational interviewing. Group sessions included informative talks and discussion with review of dietary goals, menu planning and shopping lists appropriate for each dietary intervention and provision of supplemental extra‐virgin olive oil or nuts or non‐food incentives for the control group. Energy restriction was not specifically advised nor was physical activity promoted in any of the 3 groups. Mediterranean diet groups: In these 2 groups a 14‐item questionnaire of adherence to the Mediterranean diet was used in each session and personalised advice given to increase the score. General dietary advice to follow a Mediterranean diet included the following: a) Abundant use of olive oil for cooking and dressing dishes b) Consumption of ≥ 2 daily servings of vegetables (at least one of them as fresh vegetables in a salad) c) ≥ 2 to 3 daily servings of fresh fruits (including natural juices) d) ≥ 3 weekly servings of legumes e) ≥ 3 weekly servings of fish or seafood (at least one serving of fatty fish) f) ≥ 1 weekly serving of nuts or seeds g) Select white meats (poultry without skin or rabbit) instead of red meats or processed meats h) Cook regularly (at least twice a week) with tomato, garlic and onion with abundant olive oil to dress vegetables, pasta, rice and other dishes i) For usual drinkers, the dietitian's advice was to use wine as the main source of alcohol (maximum 300 mL per day) j) Two main meals per day should be eaten (seated at a table, lasting more than 20 minutes) Negative recommendations are also given to eliminate or limit the consumption of cream, butter, margarine, cold meat, pate, duck, carbonated and/or sugared beverages, pastries, industrial bakery products and desserts, french fries or potato chips Depending on group allocation, either a 15‐litre (4 tablespoons per day) supply of extra‐virgin olive oil (Hojiblanca and Fundación Patrimonio Comunal Olivarero, both from Spain) or 3‐month allowances of nuts consisting of 1350 g (15 g per day) sachets of walnuts (California Walnut Commission, Sacramento, CA), 675 g (7.5 g per day) sachets of almonds (Borges SA, Reus, Spain) and 675 g (7.5 g per day) sachets of hazelnuts (La Morella Nuts, Reus, Spain) were provided at each 3‐month group session. Quantities were sufficient for each family unit. The rationale for the 2 Mediterranean diet groups was as follows: extra‐virgin olive oil is a rich source of monounsaturated fatty acids and a good source of phenolic antioxidants. Walnuts make up half the allowance of nuts in the other intervention group and are a good source of polyunsaturated fatty acids, particularly linoleic acid and alpha‐linolenic acid, the plant‐derived omega‐3 fatty acid, in addition to polyphenols. Almonds and hazelnuts are both rich in monounsaturated fatty acids and polyphenols. Thus the 2 intervention arms of the study differed in the intake of 2 foods (extra‐virgin olive oil and nuts) and 2 nutrients (monounsaturated fatty acids and polyunsaturated fatty acids, including alpha‐linolenic acid) that are all felt to be important in cardiovascular prevention and might have differential beneficial effects. Low‐fat diet group The focus in the control group was to reduce all types of fat, with particular emphasis on recommending the consumption of lean meats, low‐fat dairy products, cereals, potatoes, pasta, rice, fruits and vegetables. The use of olive oil for cooking and dressing and consumption of nuts and fatty fish were discouraged. A 9‐item quantitative score of compliance with the low‐fat control diet was constructed as an instrument for dietitians to assess and modify the participant’s dietary pattern to upgrade the score. Cooking instructions were also given to participants in the control group about the preparation of foods to avoid frying and using instead steaming, broiling, or microwaving. Follow‐up was 4.8 years |
|
| Outcomes | Primary outcome was a composite clinical outcome (CVD deaths, stroke, MI). Other clinical events included CVD mortality, total mortality, MI, stroke, PAD, T2DM. CVD risk factors included blood pressure and lipid levels. | |
| Notes | The original trial (Estruch 2013) was retracted and re‐analysed when methodological issues concerning randomisation came to light for 2 sites, and the inclusion of non‐randomised second household members. The new publication (Estruch 2018) controls for these in the analyses and has conducted a series of sensitivity analyses excluding these sites where they have found similar results for clinical endpoints. The new publication reports on the composite clinical outcome, CVD and total mortality, MI and stroke. Other reports of PREDIMED have also been used for CVD risk factors and PAD which were not reported in the main 2018 paper and therefore have not been adjusted. Data for the incidence of T2DM has been re‐analysed to take account of the clustering and shows very similar estimates to the original analysis (Correction ‐ Annals Internal Medicine 2018;169(4): 270‐2). Data on lipids are reported for 2 study sites rather than all 11 sites, but these were not the 2 sites where methodological issues arose. Follow‐up periods vary for different outcomes ‐ these are 4.8 years for clinical events and incidence of T2DM and PAD, 4 years for blood pressure and 1 year for lipids. Blood pressure has been analysed in multivariate analyses in the Toledo paper and is reported narratively in text. An earlier abstract reports unadjusted values but the addition of these to the meta‐analyses created significant heterogeneity. There is currently no re‐analysis of blood pressure data to take account of the methodological issues with this trial. The trial was stopped early as clear benefits of the Mediterranean diet over the low‐fat diet were seen for the primary outcome at 4.8 years. Drug treatment regimens were similar for the 3 groups at baseline and continued to be similar throughout the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random‐number sequence provided randomisation tables for the 11 participating sites. These tables included 4 strata (men < 70 years of age, men ≥ 70 years of age, women < 70 years of age and women ≥ 70 years of age) and were initially generated for 1000 participants (250 per stratum) for each site. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was concealed with sealed envelopes for the pilot phase of the study but not thereafter |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients were informed of their treatment allocation. Blinding of participants and personnel for behavioural interventions is difficult and often not possible so we have not judged this as at high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary analyses used ITT |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as detailed in the protocol |
| Other bias | Unclear risk | Insufficient rationale or evidence that an identified problem will introduce bias (see notes section above) |
Properzi 2018.
| Methods | RCT of parallel‐group design | |
| Participants | 56 patients with non‐alcoholic fatty liver disease (NAFLD) who are at increased CVD risk recruited in Australia; 49 participants completed the intervention and 48 were included in the analysis | |
| Interventions | 2 ad libitum isocaloric diets: Mediterranean (MD) versus low‐fat (LF) 12‐week intervention and follow‐up |
|
| Outcomes | Total cholesterol, triglycerides | |
| Notes | Conference proceeding so few details and effects of the 2 diets on lipid levels reported narratively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States blinded dietary intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | States blinded dietary intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 48/56 analysed |
| Selective reporting (reporting bias) | Unclear risk | Conference proceeding reporting preliminary findings |
| Other bias | Unclear risk | Insufficient information to judge |
Singh 1992.
| Methods | RCT of parallel‐group design | |
| Participants | Those with definite or possible acute myocardial infarction and unstable angina based on World Health Organization criteria were assigned to diet A (N = 204) or diet B (N = 202) within 24 to 48 hours of infarction Mean age 51 years; 90% men |
|
| Interventions | In both diets meat, eggs, hydrogenated oils, butter and clarified butter were replaced with vegetarian meat substitutes and soya bean, sunflower and ground nut oils so as to provide a prudent diet reflecting the recommendations of the American Heart Association. Group A patients were also advised to eat fruit, vegetables, pulses, nuts and fish. The goal was for patients to provide at least 400 g/day of fruits and vegetables. Other health‐related advice, such as stopping smoking, reducing alcohol intake, counselling to reduce mental stress and on physical activity, was given to both groups. Patients in group A had the advice regularly reinforced, whereas those in group B were left to usual care after the initial advice. | |
| Outcomes | Clinical endpoints at 2 years follow‐up (2012 and 2017 papers): total cardiac mortality, fatal MI, sudden cardiac death, total CVD mortality, total mortality. Total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, SBP, DBP at 1 year follow‐up (1992 paper). | |
| Notes |
BMJ has published concerns about research fraud in relation to this study. https://doi.org/10.1136/bmj.331.7511.281. This study has also been discussed in the expression of concern published in the Lancet about Singh 2002. Consequently other risk of bias is rated as high and sensitivity analyses have been performed excluding this study. Several reports of this trial: 2‐year follow‐up data from the 1992 trial published in 2012 and 2017. 2‐year clinical endpoints were used. Blood pressure and lipid levels are reported at baseline with variance and mean change from baseline with no variance. In all cases the baseline variance has been used to impute the SD difference for change from baseline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Individually randomised by the dietitian and pharmacists and assigned a diet by blindly selecting a pre‐coded sequence of cards designated diet A or diet B from a stack with an equal number in each |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States the doctor was blind to the assigned diet and the dietician was not, and that it is a single‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | States the doctor was blind to the assigned diet |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were analysed by intention‐to‐treat for clinical endpoints. 27/204 and 43/202 were lost to follow‐up in diet A and B respectively for CVD risk factors. |
| Selective reporting (reporting bias) | Unclear risk | Several clinical endpoints reported in 1992 paper at 1‐year follow‐up not reported in subsequent papers at 2‐year follow‐up (2012, 2017) |
| Other bias | High risk | BMJ has published concerns about research fraud in relation to this study. https://doi.org/10.1136/bmj.331.7511.281. Concerns about Singh 1992 have also been discussed in the expression of concern published in the Lancet about Singh 2002. |
Singh 2002.
| Methods | RCT of parallel‐group design | |
| Participants | Participants with risk factors for coronary artery disease (CAD) were recruited through advertisements in newspapers and local service clubs in India from 17 centres over 4 years for free medical advice about diagnosis and treatment of their disorders. The recruitment criterion > 25 years of age and having one or more of the major risk factors for CAD (hypertension, hypercholesterolaemia, diabetes mellitus, angina pectoris or a previous myocardial infarction) in the absence or presence of other risk factors. Of 1650 people who responded to advertisements, 1066 volunteered to participate in the trial. For patients without a documented history, exercise electrocardiography was used to detect CAD. Exclusion criteria were: absence of major risk factors for CVD, cancer, chronic diarrhoea or dysentery, a blood urea of more than 6.6 mmol/L, arthritis, dislike of the intervention diet, refusal of laboratory testing and death before randomisation. 66 participants did not meet the inclusion criterion and 1000 participants were randomised; mean age 48.5 years; 90% men |
|
| Interventions | Participants in both groups were advised to eat food substitutes that would provide a dietary intake similar to that recommended by the National Cholesterol Education Program (NCEP) in the step I prudent diet. This diet recommends that less than 30% of energy comes from total fat, less than 10% from saturated fat, and that less than 300 mg of cholesterol is consumed per day. Additionally, patients in the intervention group (Indo‐Mediterranean diet) were advised to consume at least 400 to 500 g of fruits, vegetables and nuts per day, (i.e. 250 g to 300 g of fruit, 125 g to 150 g of vegetables, and 25 g to 50 g of walnuts or almonds). This group were also encouraged to eat 400 g to 500 g of whole grains, legumes, rice, maize and wheat) daily, as well as mustard seed or soy bean oil, in 3 to 4 servings per day, which is consistent with recommendations from the Indian Consensus Group. Patients with diabetes mellitus, angina pectoris, a history of myocardial infarction or hypertension who visited the physician frequently, received more frequent dietary advice during the 2 years of follow‐up than those who did not. No details provided regarding the number of contacts for these patients or the intervention group as a whole, although food diaries were completed at 4, 8, 12 and 24 weeks, then at 12‐week intervals. Measurements were taken at baseline, at 12 weeks, 24 weeks and 2 years. Control patients were given an information sheet on the step I prudent diet at each visit. Intervention group patients were given a thorough explanation of the usefulness of the experimental diet, and the types of food that are rich in n‐3 fatty‐acids. At all meetings, dieticians provided additional motivation to both groups to adhere to the advice about diet and exercise. Both groups received the same advice to exercise. Smoking and alcohol consumption were discouraged, and mental relaxation through yoga, meditation techniques and breathing exercises were encouraged in both groups. Appropriate drugs for angina pectoris, arrhythmias, raised blood pressure, diabetes and other complications were provided to both groups. 2 years follow‐up |
|
| Outcomes | Non‐fatal MI, fatal MI, sudden cardiac death, total cardiac endpoints, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, SBP, DBP | |
| Notes | An expression of concern was published about the reliability of this work by the Lancet journal editor Richard Horton in 2005: https://doi.org/10.1016/S0140‐6736(05)67006‐7. Consequently other risk of bias is high and we conducted sensitivity analyses excluding this study. The majority of participants have confirmed CAD (58% and 59% in the intervention and comparison groups) so this study has been analysed as a secondary prevention study. No details regarding the number of people assessed at 2 years follow‐up for CVD risk factors. Have taken the number randomised minus those who dropped out and cardiac and non‐cardiac deaths as the N in meta‐analyses so 478 for the intervention group and 469 for the comparison group. Singh 2014: same study from the same institution reporting total mortality and weight loss in a conference proceeding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were assigned to either the intervention or control group, by selection of a card from a pile of equal numbers of cards for each group |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States single‐blinded study and outcome assessors were blinded. Blinding of participants and personnel for behavioural interventions is difficult and often not possible so we have not judged this as at high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In both groups, clinical data, drug intake, adverse events, coronary events, hospital admission, blood pressure, blood glucose and blood lipids were recorded by a physician unaware of patient diet |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low dropout rate: 9 in the intervention group and 11 in the control group of 1000 patients randomised. All dropouts occurred within first 12 weeks and no reasons were provided. |
| Selective reporting (reporting bias) | Low risk | Reported outcomes as stated |
| Other bias | High risk | An expression of concern has been published about the reliability of the data reported (see notes section above) |
Skouroliakou 2017.
| Methods | RCT of parallel‐group design | |
| Participants | 70 females suffering from breast cancer with a histological confirmed diagnosis of invasive breast cancer stage I–IIIA (diagnosed up to 3 months before recruitment) recruited from a maternity clinic in Athens, Greece Exclusion criteria: multivitamin or simple vitamin supplementation; a previous or current history of a second cancer; active infection; other severe coexisting medical conditions; symptomatic brain metastases; malabsorption; refusal to comply with the nutritional programme and physical activity recommendations |
|
| Interventions | Eligible participants were randomly allocated to: Mediterranean Diet The intervention group were treated with a personalised dietary intervention based on the Mediterranean diet, conducted by 2 trained registered dietitians. The diet was enriched with olive oil and foods with specific health benefits for breast cancer survivors. Recommendations: (1) 1 tablespoon of flaxseed oil or 4 tablespoons grounded flaxseed per day, (2) 3 cups of green tea or Greek Mountain Tea per day, (3) seasonal fruits and vegetables with high antioxidant capacity. They received a personalised dietary programme via e‐mail as well as face‐to‐face appointments every 15 days for the first 3 months and phone calls at the end of months 4 and 5 with in‐person meetings at the end of the study at 6 months. Specific meals, products, recipes and food portions, educational booklets, food diaries and individual nutritional advice was provided. Control diet Received the updated American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention and ad libitum diet. Patients were contacted by phone every 15 days for the first 3 months, then at months 4 and 5 and in‐person meetings at baseline, 3 and 6 months. Recommendations from the American Cancer Society regarding physical activity were also provided to both groups 6 months follow‐up |
|
| Outcomes | Total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides | |
| Notes | Breast cancer patients. Rationale for study was that the Mediterranean diet may modify patients serum antioxidant capacity, body composition and biochemical parameters. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Randomised by odd or even numbers (stated in figure, nothing in text) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall, the withdrawal rate from the study was 35.7% (25 women) and this was significantly associated with BMI |
| Selective reporting (reporting bias) | Low risk | All outcomes stated are reported |
| Other bias | Unclear risk | Insufficient information to judge |
Sofi 2018.
| Methods | RCT of cross‐over design (cross‐over at 3 months) | |
| Participants | Clinically healthy participants (18 to 75 years of age) with a low‐to‐moderate cardiovascular risk profile (< 5% at 10 years according to the European Society of Cardiology) recruited through advertisements in local media, newspapers, social media and websites from the Clinical Nutrition Unit of Careggi University Hospital, Florence, Italy, from March 2014 to June 2015. Eligibility criteria included being overweight (BMI ≥ 25) and the simultaneous presence of ≥ 1 of the following criteria: total cholesterol levels > 190 mg/dL, LDL cholesterol levels > 115 mg/dL, triglyceride levels > 150 mg/dL and glucose levels > 110 but < 126 mg/dL Participants were excluded if they were taking medications for any reason, had a serious illness or an unstable condition, were pregnant or nursing, were participating or had participated in a weight loss treatment programme in the last 6 months, or were following or had followed a food profile which, to a certain extent, excluded meat, poultry or fish in the last 6 months Median age 50 (range 21 to 75); 22% men; 118 participants randomised |
|
| Interventions | 2 dietary interventions: Mediterranean diet and lacto‐ovo vegetarian diet Interventions were delivered through face‐to‐face, individual counselling sessions at the Clinical Nutrition Unit. Participants were provided with a detailed, 1‐week menu plan as well as tips and information on the food groups that could be included and those that could not. Both of the diets were low‐calorie in nature and acted as dietary interventions to reduce body weight or the risk parameters for cardiovascular disease. The vegetarian diet included recipes for preparing meals. Both diets were hypo caloric with respect to the energy requirements of the participants, but isocaloric between them, and consisted of ≈50% to 55% of energy from carbohydrate, 25% to 30% from total fat (≤ 7% of energy from saturated fat, < 200 mg/day of cholesterol) and 15% to 20% from protein. The vegetarian diet was characterised by abstinence from the consumption of meat and meat products, poultry, fish and seafood, and the flesh of any other animal. It included eggs and dairy products, as well as all the other food groups. The Mediterranean diet was characterised by the consumption of all the food groups, including meat and meat products, poultry and fish. There were no substantial differences in the frequency of servings per week for cereals, fruits and vegetables, potatoes, sweets and olive oil between the diets. As expected, a higher frequency of consumption, per week, of legumes (5 versus 2.5 servings), nuts (2 versus 1), eggs (2 versus 1), and dairy products (21.5 versus 18.5) was reported for the vegetarian diet compared to the Mediterranean diet. Follow‐up at 3 months |
|
| Outcomes | Total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides | |
| Notes | The 2 diets are very similar in terms of the components of the Mediterranean diet with the exception of low consumption of meat and meat products and increased consumption of fish for the Mediterranean diet Analysed as a parallel‐group design for the first 3‐month phase |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a web‐based online randomisation procedure |
| Allocation concealment (selection bias) | Low risk | Used a centralised service and it was not possible for the investigators to know the allocation sequence in advance |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States that blinding of participants and dieticians is not possible because of obvious differences between the intervention diets. Blinding of participants and personnel for behavioural interventions is difficult and often not possible so we have not judged this as at high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial personnel who enrolled participants, outcome assessors and data analysts were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% and 10% loss to follow‐up in the intervention and comparison group respectively with reasons given |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as detailed in the protocol |
| Other bias | Unclear risk | Insufficient information to judge |
Stradling 2018.
| Methods | RCT of parallel‐group design (pilot) | |
| Participants | Adults with stable HIV infection on anti‐retroviral treatment for > 6 months and LDL cholesterol > 3mmol/L from 3 UK centres in the West Midlands were recruited Exclusion criteria: planning pregnancy in next 6 months; current use of lipid‐lowering agents (any interfering drug or diet); secondary causes of dyslipidaemia (renal or liver disease, diabetes, hypothyroidism, familial hyperlipidaemia); known nut allergy; unstable psychiatric disorder (including eating disorders); current participation in a weight loss programme or other dietary intervention; and inability to understand printed materials |
|
| Interventions | 60 patients were randomised to Diet 1: low saturated fat or Diet 2: Mediterranean Portfolio. Both groups attended 3 individual consultations with the research dietitian, and received further telephone reinforcement and support during the 6‐month intervention period. This was followed by a 6‐month maintenance period, with routine clinic visits only. The same research dietitian, experienced in HIV nutritional care, provided all consultations. Diet 1: low saturated fat Focus on reduction of saturated fat to < 10% of energy intake, in line with UK guidelines. Resources were provided, such as written information, recipes and online videos, covering various topics including sources of saturated fat, food swaps, food labelling, cooking methods, cheese facts and margarine types. On completion of the 12‐month outcome measurements, participants in group 1 received the dietary information from Diet 2 (Mediterranean Portfolio). Diet 2: Mediterranean Portfolio In addition to the information provided to group 1, participants allocated to Diet 2 received advice and support to adopt the Mediterranean diet supplemented by additional functional foods with cholesterol‐lowering properties. This was embedded within a motivational interviewing style consultation to include assessing readiness to change, utilising decisional balance, reflective listening and open‐ended questions, to identify needs, motivators and barriers to changing their diet. The diet was not prescriptive; goals were negotiated individually with each participant during their first session and reviewed at each visit. Daily consumption of 57 g tree nuts and 2 g plant stanols was encouraged in the form of 2 handfuls of unsalted mixed nuts (almonds, cashew nuts, peanuts, Brazil nuts, hazelnuts, pecans, walnuts, pistachios, macadamia nuts) and a 50 mL cholesterol‐lowering drink at randomisation and subsequent sessions. Participants were encouraged to continue with the nuts and stanols, while also aiming to eat 15 g/day soy protein as soya milk, yogurt or dessert, tofu and meat substitutes, and adopt a Mediterranean‐style diet, with more vegetables and fruit, olive oil and approximately 15 g to 20 g/day soluble fibre from oats, pearl barley, lentils, beans and flaxseed. Supplies of the functional foods (nuts, soy protein, plant stanols, oats and pulses) were given to participants to offset the additional cost of making dietary changes. Follow‐up 12 months |
|
| Outcomes | LDL cholesterol, SBP, DBP | |
| Notes | 12‐month follow‐up data kindly provided by the authors. ISRCTN32090191. Protocol paper published and conference abstracts with 6‐month follow‐up data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician produced a computer‐generated allocation sequence using random block sizes of 2 and 4, stratified by gender and smoking status |
| Allocation concealment (selection bias) | Low risk | The research dietitian allocated participants according to the diet number concealed in the next sequentially numbered, opaque, sealed envelope, relevant for their gender and smoking status |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | As this is a complex intervention, it was not possible to blind the participants, nor is it possible to blind the healthcare professionals. The terms Diet 1 and Diet 2 were used with the aim of achieving participant blinding to the exact content of the diet and type of foods included, to prevent Internet searching of diet titles and potential contamination between groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | At 12 months 6/31 and 5/29 missing data for some outcomes for Diet 1 and Diet 2 respectively. No further details at this stage as the full paper is not yet published. |
| Selective reporting (reporting bias) | Unclear risk | Results reported as conference proceedings only so cannot be determined |
| Other bias | Unclear risk | Insufficient information to judge |
The Lyon Diet Heart Study.
| Methods | RCT of parallel‐group design. Modified Zelen design where during hospital stay, patients were asked to participate in a cohort study with a follow‐up of 5 years and to sign a first informed consent. They were not fully informed about the design of the study, especially regarding the comparison of 2 diets. Patients assigned to the experimental group were asked to comply with a Mediterranean‐type diet and had to sign a second consent form. | |
| Participants | Men and women less than 70 years old, who survived a myocardial infarction within 6 months of enrolment were eligible Exclusion criteria included heart failure (stage III and IV NYHA), hypertension (systolic > 180 mmHg, diastolic > 110 mmHg) and inability to complete an exercise test due to recurrent angina, ventricular arrhythmias or atrioventricular block. Among patients who had coronary angioplasty or bypass, only those who were clinically stable were eligible. Patients were also excluded if they had any other conditions thought to limit survival or ability to participate in a long‐term trial. 605 patients randomised; mean age 53.5; 90% men |
|
| Interventions |
Diet: Patients in the experimental group were advised by the research cardiologist and dietician, during a 1‐hour‐long session, to adopt a Mediterranean‐type diet: more bread, more root vegetables and green vegetables, more fish, less meat (beef, lamb and pork to be replaced with poultry), no day without fruit, and butter and cream to be replaced with margarine supplied by the study. The patients would not accept olive oil as the only fat, therefore a rapeseed (canola) oil‐based margarine was supplied free for the whole family to all experimental participants. This margarine had a composition comparable to olive oil but was higher in linoleic (16‐4 versus 8% to 6%) alpha‐linolenic acid (4‐8 versus 0% to 6%). The oils recommended for salads and food preparation were rapeseed and olive oils exclusively. Moderate alcohol consumption in the form of wine was allowed at meals. Advice was tailored to individuals. At each subsequent visit of the experimental patients, a dietary survey and further counselling were done by the research dietician. Comparison group: Control patients received no dietary advice apart from that of hospital dieticians or attending physicians as usual care After the randomisation visit, patients from both groups were scheduled to be seen 2 months later and then annually at the Research Unit. These visits did not replace their regular visits to the attending physicians, who were responsible for all aspects of treatment, including use of medication and of invasive diagnostic and therapeutic procedures. Follow‐up at 24 and 46 months |
|
| Outcomes | CVD mortality, total mortality, composite clinical endpoints CVD death and non‐fatal MI at 46 months. Total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, SBP, DBP at 24 months. | |
| Notes | Clinical events reported in 1999 paper detail extended follow‐up (mean 46 months). 2 additional composite clinical endpoints that include additional outcomes not listed as primary outcomes in our review have not been used. Original 1994 paper reports clinical events and CVD risk factors at 27 months and 24 months respectively. "An intermediate analysis was proposed by the Scientific Committee to be performed in March 1993, clinical data being frozen after a minimum follow‐up of 1 year for each patient. Because of a statistically significant result, the decision was made to stop the trial. The first report was published in June 1994. For ethical, medical, and scientific reasons, all patients were invited to come to the Research Unit for a final visit, during which they were fully informed about the main results of the trial. Hence, given the delay after the clinical status of the 2 groups in March 1993, the decision to invite the patients to a new assessment, and the time needed to see each patient, an additional follow‐up of '19 months was available in the 2 groups to perform the final analyses. This offered the opportunity to evaluate the long‐term (mean, 4 years) effect of the diet tested in the trial and whether the patients continued to comply with it." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded study. Modified Zelen design so patients assigned to the intervention group are fully aware of their assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assignment of patients was not known by the attending physicians. Mortality and morbidity outcomes were validated and classified by an independent committee that worked only on the blinded data from hospital files concerning outcomes that involved hospital admission. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analyses were done based on the intention‐to‐treat principle |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as stated |
| Other bias | Unclear risk | To avoid between‐group contamination, with the approval of the Ethical and Scientific Committees, patients were not fully informed of the design of the study, especially of the comparison between 2 diets. To be included in the study, they had to come to the outpatient clinic, 2 weeks after discharge, and be randomised. Patients assigned to the experimental group had to sign a second informed consent in which they agreed to modify their diets. |
Tuttle 2008.
| Methods | RCT of parallel‐group design | |
| Participants | The Heart Institute of Spokane Diet Intervention and Evaluation Trial (THIS‐DIET) was designed to actively compare a conventional heart‐healthy low‐fat diet with a Mediterranean‐style diet for effects on cardiovascular events and survival after first myocardial infarction. Patients were recruited < 6 weeks after first MIs by referrals from their attending physicians in the US. Patients were excluded for New York Heart Association class III or IV heart failure, ventricular arrhythmias requiring medication or a defibrillator, or uncontrolled hypertension 705 patients were screened, 333 did not meet the criteria, 271 refused and 101 were randomised Mean age 58; 80% men in the intervention group, 68% in the control group |
|
| Interventions | Participants were randomised to a Mediterranean‐style diet (intervention) or a low‐fat diet (the American Heart Association Step II diet) (control) The main goals of the low‐fat dietary intervention were to reduce saturated fat calories to ≤ 7% and cholesterol intake to ≤ 200 mg/day.The Mediterranean‐style diet shared these goals, with additional goals of increasing the intake of omega‐3 fatty acids (> 0.75% of calories) and monounsaturates (20% to 25% of calories). The 2 diets recommended the increased intake of fresh fruits and vegetables (≥ 5 servings/day) and whole grains. The Mediterranean‐style diet was distinguished by an emphasis on the increased consumption of cold‐water fish (3 to 5 times/week) and oils from olives, canola and soybeans. Participants procured and prepared their own meals. Although not a weight‐loss intervention, participants who were overweight or obese were encouraged to reduce calories to facilitate weight loss. Exercise and smoking cessation were encouraged but were not specific intervention targets. Participants in both groups received 2 individual dietary counselling sessions from study dietitians within the first month, followed by additional individual sessions at months 3, 6, 12, 18 and 24. In separate classes for each diet conducted by study dietitians, participants attended 6 different group sessions focused on behavioural modification and practical aspects of their assigned diets, including recipes, grocery shopping and dining out. After completing 6 classes, participants were invited but not required to continue attending group sessions. 2 years follow‐up |
|
| Outcomes | Composite of endpoints including all‐cause and cardiac deaths, MI, hospital admissions for heart failure, unstable angina or stroke, HDL cholesterol, LDL cholesterol, triglycerides, SBP, DBP | |
| Notes | Both diets were combined and compared to a non‐randomised control group as well as directly compared with one another. Only the randomised comparisons are reported here. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes concealing the allocation sequence were prepared by a research co‐ordinator. Assignment was stratified by diabetes mellitus status using 10‐envelope blocks. Envelopes were selected in the prepared order from a locked drawer by a study dietitian to assign interventions. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States that neither the intervention team nor participants could be blinded to dietary assignment. Blinding of participants and personnel for behavioural interventions is difficult and often not possible so we have not judged this as at high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The principal investigator was blinded for the purpose of adjudicating clinical endpoints and adverse events by the removal of identifiers from records used for review |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analyses used for clinical endpoints. 3/51 patients dropped out of the intervention group, 5/50 patients dropped out of the control group. At 2 years 27% and 28% data missing for the intervention and control group for CVD risk factors. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as stated |
| Other bias | Unclear risk | Insufficient information to judge |
Vincent‐Baudry 2005.
| Methods | RCT of parallel‐group design | |
| Participants | Medi‐RIVAGE study conducted in France. Participants recruited from Center for Detection and Prevention of Arteriosclerosis at La Timone University Hospital. 232 were invited and 212 were randomised. Inclusion criteria: at least 1 of the following criteria: fasting plasma cholesterol concentration of 6.5 to 7.7 mmol/L; triacylglycerol concentration of 2.1 to 4.6 mmol/L; glucose concentration of 6.1 to 6.9 mmol/L; SBP and DBP between 140 to 180 and 90 to 105 mmHg respectively; BMI > 27; smoking; sedentary; or family history of CVD Participants treated by hypolipaemic or hypoglycaemic drugs were excluded 102 participants were randomised to the Mediterranean diet group; mean age 50.8; 42% men 110 participants were randomised to the low‐fat diet group; mean age 51.6; 39.5% men |
|
| Interventions | The Mediterranean diet recommended nuts, wholemeal bread, cereals and a variety of raw or cooked, fresh or dried fruit and vegetables and legumes, with up to 35% to 38% of total energy intake as fat. Olive oil was recommended as the main source of added fat, and 50% of the energy provided by fat was to come from MUFAs, 25% from PUFAs and 25% from SFAs. Fish was recommended 4 times/week and red meat only 1 time/week. The recommended fibre intake was 25 g/day. The suggested red wine intake was 1 to 2 glasses per day. Dairy intake was limited by giving participants a calcium limit of 800 mg/day. The target for carotenoid intake was at 7 mg/day as a marker of fruit and vegetable intake. Dietary advice was given by physicians and dieticians and participants received a booklet with nutritional recommendations. In addition, participants were provided with oat‐bran enriched pasta, tomato sauce and olive oil. A commonly prescribed low‐fat American Heart Association–type diet was adapted for the low‐fat diet group. Recommendations were to eat more poultry than mammal meat, to avoid offal and saturated fat–rich animal products, and to eat fish 2 to 3 times/week. The consumption of raw and cooked fruit and vegetables, low‐fat dairy products and vegetable oils was recommended. Low‐fat diet recommendations limited fat intake to 30% of total energy, with 33% of energy from MUFAs, PUFAs and SFAs. The recommended fibre intake was 20 g/day and alcohol was to be avoided, especially for hypertriglyceridaemic participants. Cholesterol was restricted to 200 to 300 mg/day in both diets. To ensure adequate compliance with dietary recommendations, 3‐day food records (at inclusion and after 3 months) and 24‐hour unscheduled dietary recalls (once a month) were used by dieticians.The physical activity of the participants was recorded on questionnaires and did not differ at inclusion or at 3 months between the 2 groups. Follow‐up was at 3 months |
|
| Outcomes | Total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, SBP, DBP | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 43 participants dropped during 3 months. The characteristics of the dropouts were not significantly different from those of the other participants, but there was differential dropout, with 15.9% in the Mediterranean diet group and 35.8% in the low‐fat diet group. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated are reported |
| Other bias | Unclear risk | Insufficient information to judge |
Wardle 2000.
| Methods | RCT of parallel‐group design | |
| Participants | Participants were adults with mild‐to‐moderate hypercholesterolaemia with serum cholesterol levels above 5.2 mmol/L, not current or previous (within 3 months) users of lipid‐lowering medication and with no serious illness Participants were recruited from dietetic clinics, hospital physicians and general practitioners in London and the South East, UK 117 participants were randomised; mean age 53.5 years; 43.5% men |
|
| Interventions | The intervention (Mediterranean diet) was delivered in 8 sessions during the 12‐week intervention period using a combination of individual and group sessions with a dietician and psychologist. Dietary advice was to increase intake of fruit and vegetables, and oily fish and to reduce fat to 30% of energy with substitution of predominantly monounsaturated fat for saturated fat. All participants received individualised advice to implement dietary changes based on their lifestyle and food preferences and group support in maintaining changes. Intervention participants were also given free spreading fats and oils high in monounsaturated fats. The comparison group was a wait‐list control. Participants were told it was necessary to wait for treatment but that they would be seen at 6‐week intervals. They were not given any specific dietary advice but were not discouraged from making changes and some participants did so. 12 weeks follow‐up |
|
| Outcomes | Total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides | |
| Notes | Focus of the study was the effect of the Mediterranean diet on cognitive function | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided but the control group was a wait‐list control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessment was done by a member of the research team who was blinded (in most cases) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT but details of attrition provided and reasons |
| Selective reporting (reporting bias) | Low risk | All of the outcomes stated were reported |
| Other bias | Unclear risk | Insufficient information to judge |
Weber 2012.
| Methods | RCT of parallel‐group design (pilot trial) | |
| Participants | Included outpatients who were over 45 years of age with established or previous atherothrombotic CVD occurring in the past 10 years and who were at high CVD risk. The patients also had to have at least one of the following risk factors: diabetes mellitus, hypertension, smoking, dyslipidaemia family coronary artery disease history, asymptomatic carotid disease or BMI > 25 Exclusion criteria: neurocognitive or psychiatric conditions, pregnant or lactating women, patients with hepatic impairment or renal insufficiency, and patients with a life expectancy of less than 6 months (e.g. those with metastatic malignancies) 122 patients randomised; mean age 63 years; 66% men |
|
| Interventions | Pilot of the BALANCE trial Patients were randomised in a 1:1:1 ratio to receive one of 3 dietary interventions (A, B or C) Group A Joined the Brazilian Cardioprotective Diet Program, which involves a Brazilian version of an accessible dietary therapy for cardiovascular diseases and weekly counselling with dieticians. The main difference between the Brazilian Cardioprotective Diet Program and the usual dietary therapy (groups B and C) was the consideration of energy density. The Brazilian Cardioprotective Diet Program helped the patients to avoid high energy density foods (> 1 kcal/g), thus allowing them to eat more and consume fewer calories. As they made the right food choices, they felt less restricted, aiding in the improvement of adherence. The Brazilian Cardioprotective Diet Program features nutritional recommendations that are feasible for the Brazilian population, allowing for the easy access and full use of foods, in addition to the prioritisation of regional foods that are culturally accepted by the patients (rice, bean, soy oil, and Brazilian fruits and vegetables). Patients in Group A attended weekly in‐person sessions with dietitians either by phone or in a gourmet shop. During attendance at the gourmet shop, the patients received tips for eating in restaurants, instructions on label reading and a list of typical Brazilian recipes that were adjusted for nutrients and energy densities. Group B Received the dietary therapy that was proposed by the Brazilian guidelines for cardiovascular diseases and also attended weekly counselling sessions with dietitians. This diet had the same nutrient profile as that which was presented in Group A but was customised by the integration of typical Mediterranean foods (e.g. olives, olive oil, chestnuts, walnuts, almonds, hazelnuts, peanuts and cold water fish). Group B received weekly sessions that were conducted in person or by telephone. Group C Received the same dietary intervention as Group B, but the patients were counselled monthly in person The nutrient profiles of the 3 diets were based on the Brazilian guidelines for cardiovascular disease treatment. The diets contained 50% to 60% of energy from carbohydrates, 15% from proteins and 25% to 35% from fats. In addition, 20 g to 30 g/day of fibre and 2000 mg/day of sodium were recommended. The concentrations of saturated, monounsaturated and polyunsaturated fatty acids were 7%, 20% and 10%, respectively. The total dietary energy intake was adjusted only for patients with a baseline BMI > 25 kg/m². The first nutritional session lasted for 60 minutes. The follow‐up counselling sessions lasted for 30 minutes once the teaching and nutrition goals were reviewed. The phone interviews lasted approximately 15 minutes and included just the time that was necessary to assess the 24‐hour dietary recall. Follow‐up 12 weeks |
|
| Outcomes | SBP, DBP | |
| Notes | Used Groups A and B as comparators in this review due to the same number of contacts, Group B representing the Mediterranean diet and intervention group, Group A the comparison diet. Change in blood pressure and SD difference were provided in graphs. Values have been estimated from these to use in meta‐analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was guaranteed by using sealed and opaque envelopes that were numbered sequentially |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/42 and 2/41 lost to follow‐up in Groups A and B respectively with reasons provided |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as stated |
| Other bias | Unclear risk | Insufficient information to judge |
AF: Atrial Fibrillation; BMI: body mass index; CVD: cardiovascular disease; DBP: diastolic blood pressure; HDL: high‐density lipoprotein; HF: Heart Failure; HRT: hormone replacement therapy; HTN: Hypertension; IHD: ischaemic heart disease; ITT: intention‐to‐treat; LDL: low‐density lipoprotein; MI: myocardial infarction; MUFA: monounsaturated fatty acid; PAD: peripheral arterial disease; PUFA: polyunsaturated fatty acid; RCT: randomised controlled trial; RDA: recommended daily allowance; SBP: systolic blood pressure; SD: standard deviation; SFA: saturated fatty acid; T2DM: type 2 diabetes mellitus; VLC: very‐long‐chain; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abedi 2010 | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
| Azadbakht 2005 | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
| Berrino 2001 | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
| Bruno 2018 | Intervention included a physical activity element |
| Burr 2003 | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
| Conlin 2000 | Follow‐up < 12 weeks |
| CRESSIDA | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
| de la Iglesia 2013 | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
| ENCORE | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
| Fuentes 2001 | Follow‐up < 12 weeks |
| Jula 2002 | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
| Lankinen 2014 | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
| Lanza 2001 | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
| Lima 2013 | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
| Lindeberg 2007 | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
| Mayneris‐Perxachs 2014 | Sub‐study of the PREDIMED trial |
| Mezzano 2003 | Not all participants were randomised |
| Papadaki 2008 | Not an RCT |
| Poulsen 2014 | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
| Sondergaard 2003 | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
| SYSDIET | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
| Thomazella 2011 | To maximise adherence, diet allocation was not randomised |
| Wade 2017 | Follow‐up period too short at 8 weeks |
| Weber 2016 | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. The pilot for the BALANCE trial is included in the review Weber 2012 as there is an arm with foods typical of the traditional Mediterranean diet tested against the new intervention being developed. |
| WHI | Intervention did not comprise both core components of: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes. |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Delgado‐Lista 2016.
| Trial name or title | CORonary Diet Intervention with Olive oil and cardiovascular PREVention study (the CORDIOPREV study) [NCT00924937] |
| Methods | RCT of parallel‐group design of 2 dietary interventions |
| Participants | 1002 patients with CHD from Spain aged 20 to 75 years Inclusion criteria: Informed consent Clinical: unstable coronary disease with documented vessel/myocardial damage, acute myocardial infarction, revascularisation Exclusion criteria: Age < 20 or > 75 years (or life expectancy lower than 5 years) Patients already planned for revascularisation Patients submitted to revascularisation in the last 6 months Grade II‐IV heart failure Left ventricle dysfunction with ejection fraction lower than 35% Patients unable to follow a protocol Patients with severe uncontrol of diabetes mellitus, or those with renal insufficiency with plasma creatinine higher than 2 mg/dl, or cerebral complications of diabetes mellitus Other chronic diseases: psychiatric diseases, renal insufficiency, chronic hepatopathy, active malignancy, chronic obstructive pulmonary disease, diseases of the digestive tract, endocrine disorders Patients participating in other clinical trials (in the enrolment moment or 30 days prior) |
| Interventions | 1) Mediterranean diet, with a minimum 35% of calories as fat (22% MUFA fat, 6% PUFA fat and < 10% saturated fat), 15% proteins and a maximum of 50% carbohydrates 2) Low‐fat high complex carbohydrate diet recommended by the National Cholesterol Education Program and the American Heart Association, comprising of < 30% total fat (< 10% saturated fat, 12% to 14% MUFA fat and 6% to 8% PUFA fat), 15% protein and a minimum 55% carbohydrates The objective was to compare the dietary pattern of the Mediterranean diet food pyramid versus the dietary pattern recommended by the American Heart Association. Both therapeutic diets should provide a wide variety of foods, including vegetables, fruit, cereals, potatoes, legumes, dairy products, meat and fish. Participants in both intervention groups receive the same intensive dietary counselling. Dietitians administered personalised individual interviews at inclusion and every 6 months, and quarterly group education sessions with up to 20 participants per session and separate sessions for each group. These sessions consisted of informative talks accompanied by written information with detailed descriptions of typical foods for each dietary pattern, seasonal shopping lists, meal plans and recipes. For those randomised to the Mediterranean diet, on the basis of the initial assessment of individual scores of adherence using a 14‐item questionnaire, dietitians gave personalised dietary advice with instructions directed to increasing the score, by including, among others, 1) abundant use of olive oil for cooking and dressing, 2) increased consumption of fruit, vegetables, legumes and fish, 3) reduction in total meat consumption, with white meat recommended instead of red or processed meat, 4) preparation of homemade sauces with tomato, garlic, onion and spices with olive oil to dress vegetables, pasta, rice and other dishes, 5) avoidance of butter, cream, fast food, sweets, pastries and sugar‐sweetened beverages, and 6) in alcohol drinkers, a moderate consumption of red wine. The participants assigned to the Mediterranean diet were given free extra‐virgin olive oil (1 litre/week). The participants randomised to the low‐fat diet received recommendations focused on limiting all types of fat, from both animal and vegetable sources, and on increasing the intake of complex carbohydrates. The participants also received free food packs incorporating the main food components of this dietary pattern. No energy restriction was administered, nor was physical activity promoted specifically by the study team. Follow‐up 7 years |
| Outcomes | Primary outcome: combined cardiovascular events (myocardial infarction, revascularisation, ischaemic stroke, documented peripheral artery disease or cardiovascular death) over 7‐year time frame Pre‐specified secondary outcomes are: incidence of intermittent claudication; concentration of LDL cholesterol; lipid‐related atherogenic ratios: total cholesterol/HDL and LDL/HDL; metabolic control of carbohydrates (assessed by glycaemic and insulin responses to tolerance tests to glucose); metabolic control of lipids and postprandial lipaemia; blood pressure; incidence of malignancy; incidence of type 2 diabetes mellitus; incidence of metabolic syndrome; arrhythmias; an extended composite of heart events (cardiac death, myocardial infarction, unstable angina, revascularisation, heart failure, heart transplantation and cardiac arrest), an extended composite of cardiovascular disease progression (cardiac death, myocardial infarction, unstable angina, revascularisation, heart failure, heart transplantation, cardiac arrest, stroke and peripheral artery disease), progression of cognitive decline and changes in gut microbiota |
| Starting date | November 2009 |
| Contact information | Francisco Perez Jimenez, Chief of Internal Medicine Unit, Hospital Universitario Reina Sofia de Cordoba, Spain |
| Notes | Estimated study completion date September 2019. NCT00924937 accessed 7 October 2018. |
Hardman 2015.
| Trial name or title | The Lifestyle Intervention in Independent Living Aged Care (LIILAC) study [ACTRN12614001133628] |
| Methods | Factorial design, participants individually randomised to one of the following groups: Group 1: Diet change to reflect a greater adherence to a Mediterranean diet Group 2: Exercise change to walk up to 30 minutes every second day Group 3: Combined diet and exercise change Group 4: Control group with no diet or exercise change |
| Participants | Inclusion criteria: currently living independently or supported accommodation within an aged care facility in Australia, ability to walk and be ambulatory for at least 30 minutes, free from major physical ailments, willing to provide blood samples, men and women aged 60 to 90 years Exclusion criteria: cognitive impairment as defined as a score below 24 on the Mini Mental State Examination, clinical diagnosis of depression, or score of 8 or above on the long form Geriatric Depression Scale, diagnosis of dementia or Alzheimer's disease, history of stroke or head trauma, colour blindness Participants were recruited over a minimum of 15 sites by posters and bulletins for an information event and opportunity to participate following informed consent |
| Interventions | Diet group: those allocated to the diet change group will be required to score their diet in relation to a Mediterranean diet sheet and achieve at least an 85% adherence to the diet. Instruction on how to self‐assess the diet sheets and further guidance will be given to participants to inform them of food choices via a specifically designed Mediterranean Healthier living diet recipe booklet created by a dietician. Participants will cook for themselves and be issued with recipes and meal ideas. Each person in the diet change group will be allocated 6 X 750 mL bottles of premium extra‐virgin olive oil for the study (allowing for an average usage of 46 ml/day). An ongoing interaction with the participants will take place at 6 weeks (10 to 20‐minute telephone call) after the initiation of the trial by a member of the research team and then again at the 3‐month (20 to 30‐minute face‐to‐face discussion) intervention and then again at 4.5 months (10 to 20‐minute telephone call) to ensure their adherence to the diet guide and also to ensure their enthusiasm and participation is maintained. Control group: no intervention |
| Outcomes | Cognitive function as primary outcomes, CVD risk factors as secondary outcomes (blood pressure and lipid levels) and quality of life. Follow‐up at 6 months and ethical approval for longer term follow‐up. |
| Starting date | First participant enrolment 21 May 2014 |
| Contact information | A/Prof Andrew Pipingas Head of Neurocognitive Ageing Research, Centre for Human Psychopharmacology (CHP) Faculty of Health, Arts & Design Swinburne University of Technology P.O. Box 218 Hawthorn, Victoria 3122 Australia Phone: +61 3 9214 5215 Email: apipingas@swin.edu.au |
| Notes | Trial registry shows as completed with last data collection 18 January 2016. 152 recruited from target of 208. For the current review only 2 arms of the trial are relevant ‐ diet only and the no intervention control group. ACTRN12614001133628 accessed 7 October 2018. |
Itsiopoulos 2018.
| Trial name or title | AUSMED: AUStralian MEDiterranean Diet Heart Trial |
| Methods | RCT of parallel‐group design |
| Participants | Inclusion criteria: "Eligible patients will be aged ≥ 18 years, English speaking, and within 1 year of acute presentation of AMI to the recruitment sites, as defined by the Cardiac Society Guidelines: a type 1 MI: ST‐segment elevation MI (STEMI) or non‐STEMI presenting with angina pectoris confirmed with elevated cardiac enzymes (troponin levels) or coronary angiography or balloon angioplasty (with or without stent) as defined by the third universal definition of MI (UDMI). Patients with type 2 Diabetes will be included." Exclusion criteria: "Patients will be excluded if they have active malignancy; symptomatic chronic heart failure (New York Heart Association Functional Classification II, III, and IV); chronic inflammatory disease (e.g., inflammatory bowel disease or rheumatoid arthritis treated with anti‐inflammatory or immunomodulating medications); chronic kidney disease stage 3 or above, decompensated liver disease or taking medications that cause hepatosteatosis; immunodeficiency or HIV‐positive status; body mass index > 40; are currently breastfeeding, pregnant, or trying to fall pregnant; are currently participating in an intervention trial targeting CVD, diet, or exercise; or are unable to attend all study appointments. Patients with serious food allergies will be managed appropriately by the dietitians on the team to ensure allergens are avoided." Target recruitment 1032 patients |
| Interventions | Mediterranean diet: "Participants who are randomized to the intervention group (MedDiet) will receive nutrition assessment and intensive education on the Mediterranean diet. [...] Participants will be asked to complete a 7‐day food diary in household measures during the week before the baseline appointment to determine habitual diet. Individuals will then be interviewed by an accredited practicing dietitian (APD) and will receive a 14‐day meal plan which incorporates the key principles of the Mediterranean diet and is consistent with the participant's cultural and religious dietary requirements. [...] Meal plans will be designed to meet current energy requirements for weight maintenance and will be consistent with the macronutrient composition of the Mediterranean diet (15%‐20% protein, 35%‐40% fat [18%‐20% of total energy intake as monounsaturated fatty acids], 40%‐45% carbohydrate). Food group recommendations will include daily intake of EVOO, nuts, vegetables, fruit and whole‐grain cereals, regular intake of legumes, fish, and yoghurt, and limited intake of commercial sweets or pastries and red or processed meat. Poultry, eggs, and feta cheese will be recommended in moderation." Low‐fat diet: "Participants following the standard care low‐fat diet will receive assessment and education according to the standard protocol that is consistent with the dietetic service of the participating hospitals, that is, a diet based on the National Heart Foundation Guidelines and the Australian Dietary Guidelines. [...] In terms of contribution to total energy consumption, target macronutrient intakes will be < 30% total fat, < 7% saturated fat, 45%‐65% carbohydrate, 15%‐25% protein, and ≤ 5% alcohol. Food group recommendations will include daily intake of grains and cereals (mostly whole grains, 5‐7 serves per day), vegetables (5‐6 serves per day), fruit (2 serves per day), protein foods (2‐3 serves per day) and low‐fat dairy foods (2 serves per day). [...] The low‐fat diet group will have the same number of appointments and support as the intervention group to control for the level of attention received by both groups." |
| Outcomes | Primary: cardiovascular events Secondary: cardiovascular clinical biomarkers, arterial stiffness, immune and inflammatory markers, platelet activity, body composition, cost‐effectiveness |
| Starting date | Enrolment started 1 October 2014. Anticipated last enrolment 1 October 2018. |
| Contact information | Prof Catherine Itsiopoulos Health Sciences Building 1, Allied Health Executive Office, Room 256 Department of Rehabilitation, Nutrition and Sport School of Allied Health College of Science, Health and Engineering La Trobe University Bundoora VIC 3086 Email: c.itsiopoulos@latrobe.edu.au |
| Notes | ACTRN12616000156482 |
NCT03053843.
| Trial name or title | PREDIMAR |
| Methods | RCT of parallel‐group design |
| Participants | "Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: "Mediterranean diet plus extra virgin olive oil. The patients in the intervention group will receive 1 liter of EVOO per week free of charge and dietary advice on how to follow a Mediterranean diet with contacts every two months. Dietary intervention will be carried out by nutritionists with previous experience in the PREDIMED study. All of them were registered, trained and certified for developing the PREDIMED intervention protocol that is similar to the one to be carried out in this study. The theoretical sessions with patients about dietary education shall be conducted in telephone form, using the internet and sending comprehensive written material to their homes that includes recipes, shopping lists, menus and explanations of typical food in the Mediterranean diet." Control: "no specific diet. The control group will be assigned to the usual care and patients assigned to this group will not receive any special intervention to follow a particular diet, as occurs in the current clinical practice." |
| Outcomes | Primary: atrial tachyarrhythmias Secondary: atrial fibrillation, inflammatory markers, quality of life |
| Starting date | Start: 6 March 2017. Estimated completion: 6 March 2020. |
| Contact information | Teresa Barrio‐López Email: terebarriol@gmail.com |
| Notes | — |
NCT03129048.
| Trial name or title | Mediterranean diet, weight loss and cognition in obese older adults |
| Methods | RCT of parallel‐group design |
| Participants | "Inclusion criteria:
Exclusion criteria:
|
| Interventions | "The MedDiet‐A group will learn about and how to adhere to the Mediterranean Diet. Over the course of 8 months, they will receive twenty‐two classes 60‐minute in length. The MedDiet‐WL group will learn about the Mediterranean Diet, how to adhere to is and engage in lifestyle choices like exercising and eating fewer calories so that they will lose weight. Over the course of 8 months they will receive 22 classes, each 90 minutes in length. The Typical Diet Control group will be asked to maintain current eating and activity patterns over the course of the 14 month study." MedDiet‐A and the Typical Diet Control groups are of interest to this review only |
| Outcomes | Primary: cognitive function Secondary: "CVD/metabolic risk factors, systemic inflammation, OxStress, and body weight/composition" |
| Starting date | 1 September 2016. Estimated completion date 1 March 2021. |
| Contact information | Dr. Fitzgibbon, Professor Department of Pediatrics, University of Illinois at Chicago Email: mlf@uic.edu |
| Notes | — |
Papamiltiadous 2016.
| Trial name or title | MEDINA: Mediterranean Dietary Intervention Study in Nonalcoholic Fatty Liver Disease (NAFLD) patients |
| Methods | RCT of parallel‐group design |
| Participants | Inclusion criteria: participants will be included if they are > 18 years, BMI 20 to 40 kg/m2; patients must have had at least one elevated serum aminotransferase (ALT) level (> 20U/L female, > 30 U/L male) during the past 6 months and at screening have a level between > 1.5 and < 5 times upper limit of normal (ULN) in the absence of another cause of liver disease. Diagnosis of NAFLD upon u/s. Exclusion criteria: participants will be excluded if: they are non‐English speaking; refusal or inability to give informed consent; average weekly alcohol ingestion > 140 g males or females; a current or past history of cardiovascular, cerebrovascular or peripheral vascular disease; presence of clinically relevant pulmonary, gastro‐intestinal, renal, haematological, neurological, psychiatric, systemic or any acute infectious disease or signs of acute illness; women who are pregnant or currently breastfeeding; psychosocial or gastrointestinal (malabsorptive conditions e.g. coeliac disease) contraindications included bulimia nervosa, substance abuse, clinically significant depression or current psychiatric care. Recent (within 3 months of screening visit) change in dose/regimen or introduction of vitamin E, vitamin C or high‐dose vitamin D, fish oil or probiotics. Participation in any other clinical study targeting diet and lifestyle factors. |
| Interventions | Mediterranean dietary intervention versus standard low‐fat moderate carbohydrate diet An accredited practising dietitian will provide a dietary consultation for intervention patients to follow a Mediterranean diet protocol or comparator patients to follow a standard protocol (low‐fat, Australian Guide to Healthy Eating and the Heart Foundation's Guidelines of a low‐fat, moderate carbohydrate diet). A food hamper representing typical Mediterranean diet foods (for example olive oil, nuts, natural Greek yoghurt, legumes) will be provided to the intervention group and a supermarket voucher will be provided for the standard group to purchase low‐fat, moderate carbohydrate food products to achieve the dietary goals. These will be provided at baseline, 6 weeks and 12 weeks. Meal plans and recipe books are all provided at baseline to the relevant diet group. The intervention will be run over a 12‐week period with a further 6‐ and 12‐month follow‐up to assess duration of effect and feasibility of sustaining the diet. The dietary intervention and standard diet consultations will be delivered by an dietician through a face‐to‐face consultation initially and face‐to‐face consultations at mid‐intervention (6 weeks) and post‐intervention time points. There are regular phone call reviews to check dietary compliance at weeks 2, 4 and 9. A dietitian will administer the intervention and monitor adherence via food diaries and a food frequency questionnaire, and plasma fatty acids and urinary hydroxytyrosol will be used as measures for compliance. |
| Outcomes | Primary outcome ‐ insulin resistance Secondary outcomes ‐ hepatic steatosis, liver function tests, inflammatory markers, blood lipid levels, liver stiffness, anthropometric measures, blood pressure, quality of life, body composition |
| Starting date | 14 April 2015 first participant recruited, anticipated last recruited participant 11 April 2017 but no actual figure provided when accessed. Recruitment target N = 94. |
| Contact information | A/Prof Audrey Tierney Health Sciences Building 3, Room 438 Discipline of Dietetics and Human Nutrition Department of Rehabilitation, Nutrition and Sport School of Allied Health College of Science, Health and Engineering La Trobe University Bundoora Victoria 3086 Australia Phone: +61 (0) 3 9479 5253 Fax: +61 (0) 3 9479 5768 Email: a.tierney@latrobe.edu.au |
| Notes | ACTRN12615001010583 accessed 11 October 2018 |
Sotos‐Prieto 2017.
| Trial name or title | Feeding America's Bravest: Mediterranean Diet‐Based Interventions to Change Firefighters' Eating Habits |
| Methods | A prospective, cluster‐randomised trial, with cross‐over of the control group after 1 year to compare a Mediterranean diet nutrition intervention (MDNI) versus usual care (control) in career firefighters within the Indianapolis Fire Department (IFD) |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Mediterranean diet intervention: educational materials (online learning) will be provided via the study website; group educational sessions and written materials (brochure with Mediterranean diet recommendations, shopping list recommendations and sample recipes, specific Mediterranean diet pyramid); videos; educational sessions; in‐person chef‐led, Mediterranean cooking demonstrations. Peer‐education and support. Discounted food access: The investigators have partnered with Kroger supermarkets, a large national chain with numerous stores in the Indianapolis area, to provide discounted access to key Mediterranean foods for both participating firefighters and their families. Email or text message encouragement and reminders during the intervention. Control group: usual care, consisting of existing IFD health and wellness activities, with no investigator‐provided interventions. In phase I, Group 1 will receive the MDNI for 12 months. Phase II: Group 1 fire houses will cross‐over to "self‐sustained continuation," a less intense, self‐directed, maintenance phase for 12 months to examine longer‐term persistence of behaviour change after the active 12‐month MDNI. During self‐sustained continuation access to some environmental changes: such as discounted food access, peer education/support and online learning will remain; however, the stations will not receive investigator‐led educational sessions. In Phase II, Group 2 fire houses will cross‐over to receive the full active MDNI for 6 months. The Group 2 MDNI will test the efficacy of a shorter, but otherwise identical MDNI. It will be followed by a final 6 months of "self‐sustained continuation" (as described above) to examine the shorter MDNI's effect on persistence of adherence. |
| Outcomes | Primary outcome measures: Changes in Mediterranean diet scale (time Frame: 6, 12 and 24 months) 12 months change in the MD scores as well as 12‐ and 24‐month change in group 1; and 6‐ and 12‐month change from baseline to follow‐up in group 2 Secondary outcome measures: changes in BMI (m²/kg) (time frame: 24 months) Changes in weight (kg) (time frame: 24 months) Changes in waist circumference (cm) (time frame: 24 months) Changes in lipids (time frame: 24 months) LDL (mg/dl), HDL (mg/dl), total cholesterol Changes in inflammatory markers (time frame: 24 months) CRP (mg/L) Changes in biomarkers (time frame: 6 months) (tyrosol, hydroxytyrosol and plasma fatty acids) |
| Starting date | October 2016, estimated completion date September 2019 |
| Contact information | Harvard TH Chan School of Public Health Boston, Massachusetts United States, 02115 Contact: Stefanos N Kales, MD, MPH 617‐665‐1580 (skales@hsph.harvard.edu) Contact: Mercedes Sotos Prieto, PhD 6178608979 (msotosp@hsph.harvard.edu) |
| Notes | NCT02941757 |
BMI: body mass index; CHD: coronary heart disease; CVD: cardiovascular disease; HDL: high‐density lipoprotein; LDL: low‐density lipoprotein; NAFLD: non‐alcoholic fatty liver disease; NICE: National Institute for Health and Care Excellence; RCT: randomised controlled trial
Differences between protocol and review
Differences between the previous version of this review (2013) and this update (2018):
Authors: altered. The Acknowledgements section recognises authors of the previous version who chose not to participate in this update.
Background: updated.
Objectives: altered from "To determine the effectiveness of dietary advice to follow a Mediterranean‐style dietary pattern or the provision of foods relevant to the Mediterranean diet for the primary prevention of CVD" to "To determine the effectiveness of a Mediterranean‐style diet for the primary and secondary prevention of CVD".
Types of participants: we have broadened out the scope of the review to include also patients with established CVD as well as healthy participants and those at increased risk of CVD so that we can examine the effect of the Mediterranean diet on secondary as well as primary prevention of CVD.
Types of interventions: we have refined the definition of the two core components to be: 1. high monounsaturated/saturated fat ratio (use of olive oil as main cooking ingredient and/or consumption of other traditional foods high in monounsaturated fats such as tree nuts); 2. high intake of plant‐based foods, including fruits, vegetables and legumes.
Types of comparators: we have broadened out the scope of the review to include studies where the comparator is another dietary intervention as well as no intervention or minimal intervention.
Main comparisons: there are now four main comparisons to aid interpretation and address heterogeneity for the different participant and comparator groups. These are: Mediterranean dietary intervention versus no intervention or minimal intervention for primary prevention, Mediterranean dietary intervention versus another dietary intervention for primary prevention, Mediterranean dietary intervention versus usual care for secondary prevention and Mediterranean dietary intervention versus another dietary intervention for secondary prevention.
Sensitivity analyses: we have excluded studies where the reliability of the data has been publicly questioned.
GRADE: we created a 'Summary of findings' table using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes.
Contributions of authors
All authors of the original review contributed to the protocol development. The expansion of scope for the update was conceived and led by KR and SS, and KR, LH and SS were authors on the original review. KR, NM, AT, LE, DW, AV and AD screened titles and abstracts. KR, NM, AT, LE, DW, AV, AD and LH assessed full‐text papers for inclusion; KR, NM, AT and AD located full texts and KR managed collation of studies. KR, LE, DW, AV, AD and LH abstracted data and assessed risk of bias. KR entered data into RevMan and conducted the analyses. NM and AT led on the GRADE assessment and interpretation with input from KR. KR drafted the review with input from SS for the introduction and discussion sections. All authors critically read and commented on the final draft and agreed on it for submission.
Sources of support
Internal sources
Warwick Medical School, University of Warwick, UK.
-
Cochrane Heart Group, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Heart Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK
External sources
-
NIHR Cochrane Programme Grant, UK.
Funding for the original review published in 2013. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK
-
NIHR Cochrane Incentive Grant, UK.
Funding for the substantive update and expansion in scope 2018. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK
Declarations of interest
KR: none known.
AT: none known.
NM: none known.
LE: none known.
DW: none known.
AV: none known.
AD: none known.
LH: none known.
SS: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Athyros 2011 {published data only}
- Athyros VG, Kakafika AI, Papageorgiou AA, Tziomalos K, Peletidou A, Vosikis C, et al. Effect of a plant stanol ester‐containing spread, placebo spread, or Mediterranean diet on estimated cardiovascular risk and lipid, inflammatory and haemostatic factors. Nutrition, Metabolism & Cardiovascular Diseases 2011;21:213‐21. [DOI] [PubMed] [Google Scholar]
Bajerska 2018 {published data only}
- Bajerska J, Chmurzynska A, Muzsik A, Krzyzanowska P, Madry E, Malinowska AM, et al. Weight loss and metabolic health effects from energy‐restricted Mediterranean and Central‐European diets in postmenopausal women: a randomized controlled trial. Scientific Reports 2018;8:11170. [DOI: 10.1038/s41598-018-29495-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzsik A, Bajerska J, Krzyzanowska P, Walkowiak J, Chmurzynska A. The CED‐MED project: the effect of a dietary intervention on inflammatory cytokines and lipid metabolism in women with metabolic syndrome: preliminary results. Annals of Nutrition and Metabolism. 2015; Vol. 67:231‐2.
Castagnetta 2002 {published data only}
- Carruba G, Granata OM, Pala V, Campisi I, Agostara B, Cusimano R, et al. A traditional Mediterranean diet decreases endogenous estrogens in healthy postmenopausal women. Nutrition and Cancer 2006;56(2):253‐9. [DOI] [PubMed] [Google Scholar]
- Castagnetta L, Granata OM, Cusimano R, Ravazzolo B, Liquori M, Polito L, et al. The Mediet Project. Annals of the New York Academy of Sciences 2002;963:282‐9. [DOI] [PubMed] [Google Scholar]
Chasapidou 2014 {published data only}
- Chasapidou M, Tzotzas T, Pagkalos I, Tziomalos K, Papadimitriou K. A Mediterranean‐type nutrition intervention program in Greek municipalities for patients with cardio metabolic diseases (food 4health study). Diabetes. 2014; Vol. 63:A386.
Clements 2017 {published data only}
- Clements SJ, Maijo M, Ivory K, Nicoletti C, Carding SR. Age‐ associated decline in dendritic cell function and the impact of Mediterranean diet intervention in elderly subjects. Frontiers in Nutrition 2017;4:65. [DOI: 10.3389/fnut.2017.00065] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colquhoun 2000 {published data only}
- Colquhoun D, Glasziou P, Horsley P, Somerset S, Gallagher B. Comparison of a Mediterranean diet to low fat diet on lipids in patients with coronary heart disease. Atherosclerosis 2000;151(1):237. [Google Scholar]
Davis 2017 {published data only}
- Davis CR, Bryan J, Hodgson JM, Woodman R, Murphy KJ. A Mediterranean diet reduces F2‐isoprostanes and triglycerides among older Australian men and women after 6 months. Journal of Nutrition 2017;147(7):1348‐55. [DOI] [PubMed] [Google Scholar]
Dinu 2017 {published data only}
- Dinu M, Pagliai G, Mangino A, Cesari F, Giusti B, Marcucci R, et al. Comparison between Mediterranean and vegetarian diets for cardiovascular prevention: the CARDIVEG study. European Journal of Preventive Cardiology 2017;24(1 Suppl 1):S136. [Google Scholar]
Djuric 2009 {published data only}
- Djuric Z, Ren J, Blythe J, VanLoon G, Sen AA. Mediterranean dietary intervention in healthy American women changes plasma carotenoids and fatty acids in distinct clusters. Nutrition Research 2009;29(3):156‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuric Z, Vanloon G, Radakovich K, Dilaura NM, Heilbrun LK, Sen A. Design of a Mediterranean exchange list diet implemented by telephone counseling. Journal of the American Dietetic Association 2008;108(12):2059‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Entwistle 2018 {published data only}
- Entwistle TR, Green AC, Fildes JE, Miura K. Adherence to Mediterranean and low‐fat diets among heart and lung transplant recipients: a randomized feasibility study. Nutrition Journal 2018;17:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esposito 2004 {published data only}
- Esposito K, Marfella R, Ciotola M, Di PC, Giugliano F, Giugliano G, et al. Effect of a Mediterranean‐style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 2004;292(12):1440‐6. [DOI] [PubMed] [Google Scholar]
Konstantinidou 2010 {published data only}
- Konstantinidou V, Covas MI, Munoz‐Aguayo D, Khymenets O, Torre R, Saez G, et al. In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: a randomized controlled trial. FASEB Journal 2010;24(7):2546‐57. [DOI] [PubMed] [Google Scholar]
Lapetra 2018 {published data only}
- ISRCTN27497769. A Mediterranean diet for preventing heart failure and atrial fibrillation in hypertensive patients. http://www.isrctn.com/ISRCTN27497769 (first received 12 July 2012).
- Lapetra J, Lozano‐Rodriguez JM, Miro‐Moriano L, Ortega‐Calvo M, Santos‐Lozano JM, Garcia‐Corte FJ, et al. Effect of a Mediterranean diet on the primary prevention of atrial fibrillation and major cardiovascular events in hypertensive patients with high cardiovascular risk: results of ICFAMED randomized trial. European Journal of Clinical Investigation. 2018; Vol. 48:182‐3.
Lindman 2004 {published data only}
- Lindman AS, Pedersen JI, Hjerkinn EM, Arnesen H, Veierod MB, Ellingsen I, et al. The effects of long‐term diet and omega‐3 fatty acid supplementation on coagulation factor VII and serum phospholipids with special emphasis on the R353Q polymorphism of the FVII gene. Thrombosis & Haemostasis 2004;91(6):1097‐104. [DOI] [PubMed] [Google Scholar]
Mayr 2018 {published data only}
- Mayr H, Bendall C, Tierney A, Kingsley M, Radcliffe J, Itsiopoulos C, et al. A multi‐ethnic Australian cohort with coronary heart disease adhere well to a Mediterranean diet intervention and improve plasma adiponectin levels. Annals of Nutrition and Metabolism 2017;17:906‐7. [Google Scholar]
- Mayr HL, Thomas CJ, Tierney AC, Kucianski T, George ES, Ruiz‐Canela M, et al. Randomization to 6‐month Mediterranean diet compared with a low‐fat diet leads to improvement in Dietary Inflammatory Index scores in patients with coronary heart disease: the AUSMED Heart Trial. Nutrition Research 2018;55:94‐107. [DOI] [PubMed] [Google Scholar]
Michalsen 2006 {published data only}
- Michalsen A, Lehmann N, Pithan C, Knoblauch NTM, Moebus S, Kannenberg F, et al. Mediterranean diet has no effect on markers of inflammation and metabolic risk factors in patients with coronary artery disease. European Journal of Clinical Nutrition 2006;60(4):478‐85. [DOI] [PubMed] [Google Scholar]
Misciagna 2017 {published data only}
- Misciagna G, Diaz MD, Caramia DV, Bonfiglio C, Franco I, Noviello MR, et al. Effect of a low glycemic index Mediterranean diet on non‐alcoholic fatty liver disease. A randomized controlled clinici trial. Journal of Nutrition Health & Aging 2017;21(4):404‐12. [DOI] [PubMed] [Google Scholar]
Ng 2011 {published data only}
- Ng GWB, Chan UMS, Li PCK, Wong WCW. Can a Mediterranean diet reduce the effects of lipodystrophy syndrome in people living with HIV? A pilot randomised controlled trial. Sexual Health 2011;8:43–51. [DOI] [PubMed] [Google Scholar]
PREDIMED {published data only}
- Anonymous. Correction: Effects of a Mediterranean‐style diet on cardiovascular risk factors. Annals of Internal Medicine 2018;169(4):270‐2. [DOI] [PubMed] [Google Scholar]
- Doménech M, Roman P, Lapetra J, García de la Corte FJ, Sala‐Vila A, Torre R, et al. Mediterranean diet reduces 24‐hour ambulatory blood pressure, blood glucose, and lipids one‐year randomized, clinical trial. Hypertension 2014;64:69‐76. [DOI] [PubMed] [Google Scholar]
- Estruch R, Ros E, Salas‐Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra‐virgin olive oil or nuts. New England Journal of Medicine 2018;378:e34. [DOI: 10.1056/NEJMoa1800389] [DOI] [PubMed] [Google Scholar]
- Ros E, Martínez‐González MA, Estruch R, Salas‐Salvadó J, Fitó M, Martínez JA, et al. Mediterranean diet and cardiovascular health: teachings of the PREDIMED study. Advances in Nutrition 2014;5:330S–6S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Canela M, Estruch R, Corella D, Salas‐Salvadó J, Martínez‐González MA. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA 2014;311(4):415. [DOI] [PubMed] [Google Scholar]
- Salas‐Salvadó J, Bulló M, Estruch R, Ros E, Covas MI, Ibarrola‐Jurado N, et al. Prevention of diabetes with Mediterranean diets a subgroup analysis of a randomized trial. Annals of Internal Medicine 2014;160:1‐10. [DOI] [PubMed] [Google Scholar]
- Toledo E, Hu FB, Estruch R, Cosiales PB, Salas‐Salvado J, Corella D, et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: results from a randomized controlled trial. BMC Medicine 2013;11:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo E, Hu FB, Estruch R, Salas‐Salvado J, Corella D, Covas MI, et al. Association between adherence to the Mediterranean diet and blood pressure: 4‐year follow‐up in the PREDIMED trial. European Journal of Epidemiology 2012;27 Suppl 1:S46. [Google Scholar]
Properzi 2018 {published data only}
- Properzi C, Sullivan T, Sherriff J, Adams L, Jeffrey G, Ching H, et al. Ad libitum Mediterranean and low fat diets both significantly reduce hepatic steatosis: a randomized controlled trial. Hepatology International 2018;12 Suppl 2:S232. [DOI] [PubMed] [Google Scholar]
Singh 1992 {published data only}
- Horton R. Expression of concern: Indo‐Mediterranean Diet Heart Study. Lancet 2005;366:354‐6. [DOI] [PubMed] [Google Scholar]
- Singh RB, Fedacko J, Vargova V, Pella D, Niaz MA, Ghosh S. Effect of low W‐6/W‐3 fatty acid ratio Paleolithic style diet in patients with acute coronary syndromes: a randomized, single blind, controlled trial. World Heart Journal 2012;4(1):71‐84. [Google Scholar]
- Singh RB, Rastogi SS, Verma R, Laxmi B, Singh R, Ghosh S, et al. Randomised controlled trial of cardioprotective diet in patients with recent acute myocardial infarction: results of one year follow up. BMJ 1992;304:1015‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RB, Saboo B, Mahashwari A, Bharatdwaj K, Verma N, Hristova K, et al. Effects of Indo‐Mediterranean style diet and low fat diet on incidence of diabetes in acute coronary syndromes. World Heart Journal 2017;9(1):25‐36. [Google Scholar]
- White C. Suspected research fraud: difficulties of getting at the truth. BMJ 2005;331:281. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2002 {published data only}
- Horton R. Expression of concern: Indo‐Mediterranean Diet Heart Study. Lancet 2005;366:354‐6. [DOI] [PubMed] [Google Scholar]
- Singh RB, Dubnov G, Niaz MA, Ghosh S, Singh R, Rastogi SS, et al. Effect of an Indo‐Mediterranean diet on progression of coronary artery disease in high risk patients (Indo‐Mediterranean Diet Heart Study): a randomised single‐blind trial. Lancet 2002;360(9344):1455‐61. [DOI] [PubMed] [Google Scholar]
- Singh S, Darlenska T, Handjiev S, Hristova K. Effect of weight loss due to Mediterranean style diet in patients with acute coronary syndromes. Obesity Facts. 2014; Vol. 7:140.
Skouroliakou 2017 {published data only}
- Skouroliakou M, Grosomanidis D, Massara P, Kostara C, Papandreou P, Ntountaniotis D, et al. Serum antioxidant capacity, biochemical profile and body composition of breast cancer survivors in a randomized Mediterranean dietary intervention study. European Journal of Nutrition 2017 June 20 [Epub ahead of print]. [DOI: 10.1007/s00394-017-1489-9] [DOI] [PubMed]
Sofi 2018 {published data only}
- Sofi F, Dinu M, Pagliai G, Cesari F, Gori AM, Sereni A, et al. Low‐ calorie vegetarian versus Mediterranean diets for reducing body weight and improving cardiovascular risk profile: CARDIVEG study (Cardiovascular Prevention With Vegetarian Diet). Circulation 2018;137(11):1103‐13. [DOI: 10.1161/CIRCULATIONAHA.117.030088] [DOI] [PubMed] [Google Scholar]
- Sofi F, Dinu M, Pagliai G, Cesari F, Marcucci R, Casini A. Mediterranean versus vegetarian diet for cardiovascular disease prevention (the CARDIVEG study): study protocol for a randomized controlled trial. Trials 2016;17(1):233. [10.1186/s13063‐016‐1353‐x. Erratum in: Trials. 2016;17(1):253.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stradling 2018 {published and unpublished data}
- Stradling C, Taylor S, Thomas GN, Taheri S, Ross J, Das S. Mediterranean diet can improve cardiovascular risk in HIV dyslipidaemia: a randomised controlled dietary intervention trial. HIV Medicine 2017;18:41. [Google Scholar]
- Stradling C, Thomas GN, Hemming K, Frost G, Garcia‐Perez I, Redwood S, et al. Randomised controlled pilot study to assess the feasibility of a Mediterranean portfolio dietary intervention for cardiovascular risk reduction in HIV dyslipidaemia: a study protocol. BMJ Open 2016;6:e010821. [DOI: 10.1136/bmjopen-2015-010821] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stradling C, Thomas GN, Hemming K, Taheri S, Taylor S, Ross J, et al. The Mediterranean portfolio diet in HIV dyslipidaemia: a randomized controlled trial. Topics in Antiviral Medicine 2018;26:306s. [Google Scholar]
The Lyon Diet Heart Study {published data only}
- Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, et al. Mediterranean alpha‐linolenic acid‐rich diet in secondary prevention of coronary heart disease. Lancet 1994;343:1454‐9. [DOI] [PubMed] [Google Scholar]
- Lorgeril M, Salen P, Martin JL, Mamelle N, Monjaud I, Touboul P, et al. Effect of a Mediterranean type of diet on the rate of cardiovascular complications in patients with coronary artery disease. Insights into the cardioprotective effect of certain nutriments. Journal of the American College of Cardiology 1996;28(5):1103‐8. [DOI] [PubMed] [Google Scholar]
- Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999;99(6):779‐85. [DOI] [PubMed] [Google Scholar]
Tuttle 2008 {published data only}
- Tuttle KR, Shuler LA, Packard DP, Milton JE, Daratha KB, Bibus DM, et al. Comparison of low‐fat versus Mediterranean‐style dietary intervention after first myocardial infarction. American Journal of Cardiology 2008;101(11):1523‐30. [DOI] [PubMed] [Google Scholar]
Vincent‐Baudry 2005 {published data only}
- Vincent S, Gerber M, Bernard MC, Defoort C, Loundou A, Portugal H, et al. The Medi‐RIVAGE study (Mediterranean Diet, Cardiovascular Risks and Gene Polymorphisms): rationale, recruitment, design, dietary intervention and baseline characteristics of participants. Public Health Nutrition 2004;7(4):531‐42. [DOI] [PubMed] [Google Scholar]
- Vincent‐Baudry S, Defoort C, Gerber M, Bernard MC, Verger P, Helal O, et al. The Medi‐RIVAGE study: reduction of cardiovascular disease risk factors after a 3‐mo intervention with a Mediterranean‐type diet or a low‐fat diet. American Journal of Clinical Nutrition 2005;82(5):964‐71. [DOI] [PubMed] [Google Scholar]
Wardle 2000 {published data only}
- Wardle J, Rogers P, Judd P, Taylor MA, Rapoport L, Green M, et al. Randomized trial of the effects of cholesterol‐lowering dietary treatment on psychological function. American Journal of Medicine 2000;108(7):547‐53. [DOI] [PubMed] [Google Scholar]
Weber 2012 {published data only}
- Weber B, Galante AP, Bersch‐Ferreira AC, Torreglosa CR, Carvalho VO, Victor Eda S, et al. Effects of Brazilian Cardioprotective Diet Program on risk factors in patients with coronary heart disease: a Brazilian Cardioprotective Diet randomized pilot trial. Clinics 2012;67(12):1407‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abedi 2010 {published data only}
- Abedi P, Lee MHS, Kandiah M, Yassin Z, Shojaeezade D, Hosseini M, et al. Diet intervention to improve cardiovascular risk factors among Iranian postmenopausal women. Nutrition Research and Practice 2010;4(6):522‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azadbakht 2005 {published data only}
- Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi T, Azizi F. Beneficial effects of a dietary approaches to stop hypertension eating plan on features of the metabolic syndrome. Diabetes Care 2005;28(12):2823‐31. [DOI] [PubMed] [Google Scholar]
Berrino 2001 {published data only}
- Berrino F, Bellati C, Secreto G, Camerini E, Pala V, Panico S, et al. Reducing bioavailable sex hormones through a comprehensive change in diet: the Diet and Androgens (DIANA) Randomized Trial. Cancer Epidemiology, Biomarkers and Prevention 2001;10:25–33. [PubMed] [Google Scholar]
Bruno 2018 {published data only}
- Bruno E, Manoukian S, Venturelli E, Oliverio A, Rovera F, Iula G, et al. Adherence to Mediterranean diet and metabolic syndrome in BRCA mutation carriers. Integrative Cancer Therapies 2018;17(1):153‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burr 2003 {published data only}
- Burr ML, Ashfield‐Watt PAL, Dunstan FDJ, Fehily AM, Breay P, Ashton T, et al. Lack of benefit of dietary advice to men with angina: results of a controlled trial. European Journal of Clinical Nutrition 2003;57:193–200. [DOI] [PubMed] [Google Scholar]
Conlin 2000 {published data only}
- Conlin PR, Chow D, Miller IER, Svetkey LP, Lin PH, Harsha DW, et al. The effect of dietary patterns on blood pressure control in hypertensive patients: results from the Dietary Approaches to Stop Hypertension (DASH) trial. American Journal of Hypertension 2000;13(9):949‐55. [DOI] [PubMed] [Google Scholar]
CRESSIDA {published data only}
- Reidlinger DP, Darzi J, Hall WL, Maniou Z, Govoni V, Seed P, et al. The effectiveness of an integrated dietary intervention compared with an average UK diet in reducing cardiovascular disease risk factors in older men and women aged 40 to 70 years: the CRESSIDA study. Proceedings of the Nutrition Society. 2013; Vol. 72:E249.
- Reidlinger DP, Darzi J, Hall WL, Seed PT, Chowienczyk PJ, Sanders TAB. How effective are current dietary guidelines for cardiovascular disease prevention in healthy middle‐aged and older men and women? A randomized controlled trial. American Journal of Clinical Nutrition 2015;101(5):922‐30. [DOI] [PubMed] [Google Scholar]
- Reidlinger DP, Sanders TAB, Goff LM. How expensive is a cardioprotective diet? Analysis from the CRESSIDA study. Public Health Nutrition 2017;20(8):1423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
de la Iglesia 2013 {published data only}
- Iglesia R, Lopez‐Legarrea P, Abete I, Navas Carrtero S, Martinez JA, Zulet MA. The beneficial effects of the RESMENA dietary pattern on oxLDL in patients with metabolic syndrome. Annals of Nutrition and Metabolism 2013;63 Suppl 1:171. [Google Scholar]
- Iglesia R, Lopez‐Legarrea P, Celada P, Sanchez‐Muniz FJ, Martinez JA, Zulet MA. Beneficial effects of the RESMENA dietary pattern on oxidative stress in patients suffering from metabolic syndrome with hyperglycemia are associated to dietary TAC and fruit consumption. International Journal of Molecular Sciences 2013;14(4):6903‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
ENCORE {published data only}
- Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Archives of Internal Medicine 2010;170(2):126‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Sherwood A, Craighead L, Lin PH, Johnson J, et al. Effects of the dietary approaches to stop hypertension diet alone and in combination with exercise and caloric restriction on insulin sensitivity and lipids. Hypertension 2010;55(5):1199‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fuentes 2001 {published data only}
- Fuentes F, Lopez‐Miranda J, Sanchez E, Sanchez F, Paez J, Paz‐Rojas E, et al. Mediterranean and low‐fat diets improve endothelial function in hypercholesterolemic men. Annals of Internal Medicine 2001;134(12):1115‐9. [DOI] [PubMed] [Google Scholar]
Jula 2002 {published data only}
- Jula A, Marniemi J, Huupponen R, Virtanen A, Rastas M, Ronnemaa T, et al. Effects of diet and simvastatin on serum lipids, insulin, and antioxidants in hypercholesterolemic men: a randomized controlled trial. JAMA 2002;287(5):598‐605. [DOI] [PubMed] [Google Scholar]
Lankinen 2014 {published data only}
- Lankinen M, Kolehmainen M, Jaaskelainen T, Paananen J, Joukamo L, Kangas AJ, et al. Effects of whole grain, fish and bilberries on serum metabolic profile and lipid transfer protein activities: a randomized trial (Sysdimet). PloS One 2014;9(2):e90352. [DOI: 10.1371/journal.pone.0090352] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lanza 2001 {published data only}
- Lanza E, Schatzkin A, Daston C, Corle D, Freedman L, Ballard‐Barbash R, et al. Implementation of a 4‐y, high‐fibre, high‐fruit‐and‐vegetable, low‐fat dietary intervention: results of dietary changes in the Polyp Prevention Trial. American Journal of Clinical Nutrition 2001;74(3):387‐401. [DOI] [PubMed] [Google Scholar]
Lima 2013 {published data only}
- Lima ST, Silva Nalin de Souza B, Franca AK, Salgado Filho N, Sichieri R. Dietary approach to hypertension based on low glycaemic index and principles of DASH (Dietary Approaches to Stop Hypertension): a randomised trial in a primary care service. British Journal of Nutrition 2013;110(8):1472‐9. [DOI] [PubMed] [Google Scholar]
Lindeberg 2007 {published data only}
- Lindeberg S, Jonsson T, Granfeldt Y, Borgstrand E, Soffman J, Sjostrom K, et al. A Palaeolithic diet improves glucose tolerance more than a Mediterranean‐like diet in individuals with ischaemic heart disease. Diabetologia 2007;50(9):1795‐807. [DOI] [PubMed] [Google Scholar]
- Manheimer EW, Zuuren EJ, Fedorowicz Z, Pijl H. Paleolithic nutrition for metabolic syndrome: systematic review and meta‐analysis. American Journal of Clinical Nutrition 2015;102(4):922–32. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayneris‐Perxachs 2014 {published data only}
- Mayneris‐Perxachs J, Sala‐Vila A, Chisaguano M, Castellote A, Estruch R, Covas M, et al. Effects of 1‐year intervention with a Mediterranean diet on plasma fatty acid composition and metabolic syndrome in a population at high cardiovascular risk. PloS One 2014;9(3):e85202. [DOI: 10.1371/journal.pone.0085202] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mezzano 2003 {published data only}
- Mezzano D, Leighton F. Haemostatic cardiovascular risk factors: differential effects of red wine and diet on healthy young. Pathophysiology of Haemostasis & Thrombosis 2003;33(5‐6):472‐8. [DOI] [PubMed] [Google Scholar]
Papadaki 2008 {published data only}
- Papadaki A, Scott JA. Follow‐up of a web‐based tailored intervention promoting the Mediterranean diet in Scotland. Patient Education and Counseling 2008;73(2):256‐63. [DOI] [PubMed] [Google Scholar]
Poulsen 2014 {published data only}
- Larsen TM. Nordic diet in obese subjects: results from the SHOPUS study. Annals of Nutrition and Metabolism 2015;67:52. [Google Scholar]
- Larsen TM. The Nordic diet. Obesity Facts. 2014; Vol. 7:13‐4.
- Poulsen SK, Due A, Jordy AB, Kiens B, Stark KD, Stender S, et al. Health effect of the New Nordic Diet in adults with increased waist circumference: a 6‐mo randomized controlled trial. American Journal of Clinical Nutrition 2014;99(1):35‐45. [DOI] [PubMed] [Google Scholar]
Sondergaard 2003 {published data only}
- Søndergaard E, Møller JE, Egstrup K. Effect of dietary intervention and lipid lowering treatment on brachial vasoreactivity in patients with ischemic heart disease and hypercholesterolemia. American Heart Journal Volume 2003;145(5):903. [DOI] [PubMed] [Google Scholar]
SYSDIET {published data only}
- Brader L, Uusitupa M, Dragsted LO, Hermansen K. Effects of an isocaloric healthy Nordic diet on ambulatory blood pressure in metabolic syndrome: a randomized SYSDIET sub‐study. European Journal of Clinical Nutrition 2014;68(1):57‐63. [DOI] [PubMed] [Google Scholar]
- Lankinen M, Schwab U, Kolehmainen M, Paananen J, Nygren H, Seppanen‐Laakso T, et al. A healthy Nordic diet alters the plasma lipidomic profile in adults with features of metabolic syndrome in a multicenter randomized dietary intervention. Journal of Nutrition 2016;146(4):662‐72. [DOI] [PubMed] [Google Scholar]
- Uusitupa M, Hermansen K, Savolainen MJ, Schwab U, Kolehmainen M, Brader L, et al. Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome ‐‐ a randomized study (SYSDIET). Journal of Internal Medicine 2013;274(1):52‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomazella 2011 {published data only}
- Thomazella MC, Goes MF, Andrade CR, Debbas V, Barbeiro DF, Correia RL, et al. Effects of high adherence to Mediterranean or low‐fat diets in medicated secondary prevention patients. American Journal of Cardiology 2011;108(11):1523‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wade 2017 {published data only}
- ACTRN12616000309482. The effect of a Mediterranean Diet, with adequate dairy foods, to meet Australian recommendations, on cardiometabolic and cognitive health outcomes in men and women at high risk of cardiovascular disease. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000309482 (first received 17 December 2015).
- Wade AT, Davis CR, Dyer KA, Hodgson JM, Woodman RJ, Keage HAD, et al. A Mediterranean diet to improve cardiovascular and cognitive health: protocol for a randomised controlled intervention study. Nutrients 2017;9(2):E145. [DOI: 10.3390/nu9020145] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weber 2016 {published data only}
- Weber B, Bersch‐Ferreira AC, Torreglosa CR, Ross‐Fernandes MB, Silva JT, Galante AP, et al. The Brazilian Cardioprotective Nutritional Program to reduce events and risk factors in secondary prevention for cardiovascular disease: study protocol (The BALANCE Program Trial). American Heart Journal 2016;171(1):73‐81.e1‐2. [DOI] [PubMed] [Google Scholar]
WHI {published data only}
- Allison MA, Aragaki A, Eaton C, Li W, Horn L, Daviglus ML, el al. Effect of dietary modification on incident carotid artery disease in postmenopausal women results from the women's health initiative dietary modification trial. Stroke 2014;45:1748‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison MA, Aragaki AK, Ray RM, Margolis KL, Beresford SAA, Kuller L, et al. A randomized trial of a low‐fat diet intervention on blood pressure and hypertension: tertiary analysis of the WHI dietary modification trial. American Journal of Hypertension 2016;28(8):959‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard BV, Van HL, Hsia J, Manson JE, Stefanick ML, Wassertheil‐Smoller S, et al. Low‐fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295(6):655‐66. [DOI] [PubMed] [Google Scholar]
- Tinker LF, Bonds DE, Margolis KL, Manson JE, Howard BV, Larson J, et al. Low‐fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women: the Women's Health Initiative randomized controlled dietary modification trial. Archives of Internal Medicine 2008;168(14):1500‐11. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Delgado‐Lista 2016 {published data only}
- Delgado‐Lista J, Perez‐Martinez P, Garcia‐Rios A, Alcala‐Diaz JF, Perez‐Caballero AI, Gomez‐Delgado F, et al. CORonary Diet Intervention with Olive oil and cardiovascular PREVention study (the CORDIOPREV study): Rationale, methods, and baseline characteristics: a clinical trial comparing the efficacy of a Mediterranean diet rich in olive oil versus a low‐fat diet on cardiovascular disease in coronary patients [NCT00924937]. American Heart Journal 2016;177:42‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00924937. Coronary diet intervention with olive oil and cardiovascular prevention (CORDIOPREV). clinicaltrials.gov/ct2/show/NCT00924937 (first received 19 June 2009).
Hardman 2015 {published data only}
- Hardman RJ, Kennedy G, Macpherson H, Scholey AB, Pipingas A. A randomised controlled trial investigating the effects of Mediterranean diet and aerobic exercise on cognition in cognitively healthy older people living independently within aged care facilities: the Lifestyle Intervention in Independent Living Aged Care (LIILAC) study protocol [ACTRN12614001133628]. Nutrition Journal 2015;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Itsiopoulos 2018 {published data only}
- ACTRN12616000156482. The AUSMED Heart Trial: the Australian Mediterranean diet trial for secondary prevention of heart disease [The effects of a Mediterranean dietary intervention in modifying cardiovascular risk factors in high‐risk individuals who have experienced a cardiac event]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=369896 (first received 12 January 2016).
- Itsiopoulos C. The Australian Mediterranean diet heart trial (AUSMED Heart Trial): a randomized clinical trial in secondary prevention of coronary heart disease in a multiethnic Australian population: study protocol. American Heart Journal 2018;203:4‐11. [DOI] [PubMed] [Google Scholar]
NCT03053843 {published data only}
- NCT03053843. Mediterranean diet plus extravirgin olive oil in the prevention of recurrent arrhythmias [PREvención Con DIeta Mediterránea de Arritmias Recurrentes Trial (PREDIMAR)]. clinicaltrials.gov/ct2/show/nct03053843 (first received 15 February 2017).
NCT03129048 {published data only}
- NCT03129048. Mediterranean diet, weight loss, and cognition in obese older adults. clinicaltrials.gov/ct2/show/nct03129048 (first received 26 April 2017).
Papamiltiadous 2016 {published data only}
- Papamiltiadous ES, Roberts SK, Nicoll AJ, Ryan MC, Itsiopoulos C, Salim A, et al. A randomised controlled trial of a Mediterranean dietary intervention for adults with non alcoholic fatty liver disease (MEDINA): study protocol [ACTRN12615001010583]. BMC Gastroenterology 2016;16:14. [DOI: 10.1186/s12876-016-0426-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sotos‐Prieto 2017 {published data only}
- Sotos‐Prieto M, Cash SB, Christophi C, Folta S, Moffatt S, Muegge C, et al. Rationale and design of feeding America's bravest: Mediterranean diet‐based intervention to change firefighters' eating habits and improve cardiovascular risk profiles. Contemporary Clinical Trials 2017;61:101‐7. [DOI] [PubMed] [Google Scholar]
Additional references
AHA 2006
- American Heart Association Nutrition Committee, Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006;114:82‐96. [DOI] [PubMed] [Google Scholar]
AHA/ASA 2011
- Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:517‐84. [DOI] [PubMed] [Google Scholar]
Anonymous 2018
- Anonymous. Correction: Effects of a Mediterranean‐style diet on cardiovascular risk factors. Annals of Internal Medicine 2018;169(4):270‐2. [DOI] [PubMed] [Google Scholar]
Appel 1997
- Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. New England Journal of Medicine 1997;336:1117‐24. [DOI] [PubMed] [Google Scholar]
Appel 2001
- Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Non‐pharmacologic Interventions in the Elderly (TONE). Archives of Internal Medicine 2001;161:685‐93. [DOI] [PubMed] [Google Scholar]
Appel 2006
- Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Frank FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006;47:296‐308. [DOI] [PubMed] [Google Scholar]
Appel 2013
- Appel LJ, Horn L. Did the PREDIMED trial test a Mediterranean diet?. New England Journal of Medicine 2013;368(14):1353‐4. [DOI] [PubMed] [Google Scholar]
Barzi 2003
- Barzi F, Woodward M, Marfisi RM, Tavazzi L, Valagussa F, Marchioli R, et al. Mediterranean diet and all‐causes mortality after myocardial infarction: results from the GISSI‐Prevenzione trial. European Journal of Clinical Nutrition 2003;57:604‐11. [DOI] [PubMed] [Google Scholar]
Benetou 2008
- Benetou V, Trichopoulou A, Orfanos P, Naska A, Lagiou P, Boffetta P, et al. Conformity to traditional Mediterranean diet and cancer incidence: the Greek EPIC cohort. British Journal of Cancer 2008;99:191‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brien 2011
- Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta‐analysis of interventional studies. BMJ 2011;342:d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buckland 2008
- Buckland G, Bach A, Serra‐Majem L. Obesity and the Mediterranean diet: a systematic review of observational and interventional studies. Obesity Review 2008;9:582‐93. [DOI] [PubMed] [Google Scholar]
Buckland 2009
- Buckland G, González CA, Agudo A, Vilardell M, Berenguer A, Amiano P, et al. Adherence to the Mediterranean diet and risk of coronary heart disease in the Spanish EPIC Cohort Study. American Journal of Epidemiology 2009;170:1518‐29. [DOI] [PubMed] [Google Scholar]
Chrysohoou 2004
- Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: the ATTICA study. Journal of the American College of Cardiology 2004;44:152‐8. [DOI] [PubMed] [Google Scholar]
Corrao 2000
- Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Alcohol and coronary heart disease: a meta‐analysis. Addiction 2000;95:1505‐23. [DOI] [PubMed] [Google Scholar]
Dai 2008
- Dai J, Jones DP, Goldberg J, Ziegler TR, Bostick RM, Wilson PW, et al. Association between adherence to the Mediterranean diet and oxidative stress. American Journal of Clinical Nutrition 2008;88:1364‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Lorgeril 1994
- Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, et al. Mediterranean alpha‐linolenic acid‐rich diet in secondary prevention of coronary heart disease. Lancet 1994;343:1454‐9. [DOI] [PubMed] [Google Scholar]
de Lorgeril 1996
- Lorgeril M, Salen P, Martin JL, Mamelle N, Monjaud I, Touboul P, et al. Effect of a Mediterranean type of diet on the rate of cardiovascular complications in patients with coronary artery disease. Insights into the cardioprotective effect of certain nutriments. Journal of the American College of Cardiology 1996;28:1103‐8. [DOI] [PubMed] [Google Scholar]
de Lorgeril 1999
- Lorgeril M, Salen P, Martin J, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon diet heart study. Circulation 1999;99:799‐85. [DOI] [PubMed] [Google Scholar]
de Lorgeril 2011
- Lorgeril M, Salen P. Mediterranean diet in secondary prevention of CHD. Public Health Nutrition 2011;14(12A):2333‐7. [DOI] [PubMed] [Google Scholar]
Di Castelnuovo 2002
- Castelnuovo A, Rotondo S, Iacoviello L, Donati MB, Gaetano G. Meta‐analysis of wine and beer consumption in relation to vascular risk. Circulation 2002;105:2836‐44. [DOI] [PubMed] [Google Scholar]
Di Castelnuovo 2006
- Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta‐analysis of 34 prospective studies. Archives of Internal Medicine 2006;166:2437‐45. [DOI] [PubMed] [Google Scholar]
Dinu 2018
- Dinu M, Pagliai G, Casini A, Sofi F. Mediterranean diet and multiple health outcomes: an umbrella review of meta‐analyses of observational studies and randomised trials. European Journal of Clinical Nutrition 2018;72(1):30‐43. [DOI] [PubMed] [Google Scholar]
Estruch 2010
- Estruch R. Anti‐inflammatory effects of the Mediterranean diet: the experience of the PREDIMED study. Proceedings of the Nutritional Society 2010;69(3):333‐40. [DOI] [PubMed] [Google Scholar]
Estruch 2013
- Estruch R, Ros E, Salas‐Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. New England Journal of Medicine 2013;368:1279‐90. [DOI: 10.1056/NEJMoa1200303] [DOI] [PubMed] [Google Scholar]
Estruch 2018
- Estruch R, Ros E, Salas‐Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra‐virgin olive oil or nuts. New England Journal of Medicine 2018;378:e34. [DOI] [PubMed] [Google Scholar]
European Heart Network 2017
- European Heart Network. European Cardiovascular Disease Statistics 2017 edition. http://www.ehnheart.org/cvd‐statistics.
Feart 2009
- Féart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009;302:638‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Follman 1992
- Follman D, Elliot P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [DOI] [PubMed] [Google Scholar]
Fung 2009
- Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009;119:1093‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grosso 2017
- Grosso G, Marventano S, Yang J, Micek A, Pajak A, Scalfi L, et al. A comprehensive meta‐analysis on evidence of Mediterranean diet and cardiovascular disease: are individual components equal?. Critical Review of Food Science and Nutrition 2017;57(15):3218‐32. [DOI] [PubMed] [Google Scholar]
Helsing 1989
- Helsing E, Trichopoulou A. The Mediterranean diet and food culture: a symposium. European Journal of Clinical Nutrition 1989;43 Suppl 1:1‐92. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kastorini 2011
- Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta‐analysis of 50 studies and 534,906 individuals. Journal of the American College of Cardiology 2011;57:1299‐313. [DOI] [PubMed] [Google Scholar]
Keys 1986
- Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, et al. The diet and 15‐year death rate in the Seven Countries Study. American Journal of Epidemiology 1986;124:903‐15. [DOI] [PubMed] [Google Scholar]
Knoops 2004
- Knoops KT, Groot LC, Kromhout D, Perrin AE, Moreiras‐Varela O, Menotti A, et al. Mediterranean diet, lifestyle factors, and 10‐year mortality in elderly European men and women. JAMA 2004;292:1433‐9. [DOI] [PubMed] [Google Scholar]
Lagiou 2006
- Lagiou P, Trichopoulos D, Sandin S, Lagiou A, Mucci L, Wolk A, et al. Mediterranean dietary pattern and mortality among young women: a cohort study in Sweden. British Journal of Nutrition 2006;96:384‐92. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Liyanage 2016
- Liyanage T, Ninomiya T, Wang A, Neal B, Jun M, Wong MG, et al. Effects of the Mediterranean diet on cardiovascular outcomes—a systematic review and meta‐analysis. PloS One 2016;11(8):e0159252. [DOI: 10.1371/journal.pone.0159252] [DOI] [PMC free article] [PubMed] [Google Scholar]
Locke 2018
- Locke A, Schneiderhan J, Zick SM. Diets for health: goals and guidelines. American Family Physician 2018;97(11):721‐8. [PubMed] [Google Scholar]
Martinez‐Gonzalez 2008
- Martínez‐González MA, Fuente‐Arrillaga C, Nunez‐Cordoba JM, Basterra‐Gortari FJ, Beunza JJ, Vazquez Z, et al. Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ 2008;336:1348‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martínez‐González 2017
- Martínez‐González MA, Hershey MS, Zazpe I, Trichopoulou A. Transferability of the Mediterranean diet to non‐Mediterranean countries. what is and what is not the Mediterranean diet. Nutrients 2017;9:1226. [DOI: 10.3390/nu9111226] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathers 2006
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine 2006;3(11):e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitrou 2007
- Mitrou PN, Kipnis V, Thiébaut AC, Reedy J, Subar AF, Wirfält E, et al. Mediterranean dietary pattern and prediction of all‐cause mortality in a US population. Archives of Internal Medicine 2007;167:2461‐8. [DOI] [PubMed] [Google Scholar]
Nestle 1995
- Nestle E. Mediterranean diets: science and policy implications. American Journal of Clinical Nutrition 1995;61 Suppl 6:1313‐427. [DOI] [PubMed] [Google Scholar]
Nordmann 2011
- Nordmann AJ, Suter‐Zimmermann K, Bucher HC, Shai I, Tuttle KR, Estruch R, et al. Meta‐analysis comparing Mediterranean to low‐fat diets for modification of cardiovascular risk factors. American Journal of Medicine 2011;124:841‐51. [DOI] [PubMed] [Google Scholar]
Nunez‐Cordoba 2009
- Nunez‐Cordoba JM, Valencia‐Serrano F, Toledo E, Alfonso A, Martinez‐Gonzalez MA. The Mediterranean diet and incidence of hypertension: the Seguimiento Universidad de Navarra (SUN) Study. American Journal of Epidemiology 2009;169:339‐46. [DOI] [PubMed] [Google Scholar]
Panagiotakos 2016
- Panagiotakos DB, Notara V, Kouvari M, Pitsavos C. The Mediterranean and other dietary patterns in secondary cardiovascular disease prevention: a review. Current Vascular Pharmacology 2016;14(5):442‐51. [DOI] [PubMed] [Google Scholar]
Pitsavos 2005
- Pitsavos C, Panagiotakos DB, Tzima N, Chrysohoou C, Economou M, Zampelas A, et al. Adherence to the Mediterranean diet is associated with total antioxidant capacity in healthy adults: the ATTICA study. American Journal of Clinical Nutrition 2005;82:694‐9. [DOI] [PubMed] [Google Scholar]
Psaltopoulou 2004
- Psaltopoulou T, Naska A, Orfanos P, Trichopoulos D, Mountokalakis T, Trichopoulou A. Olive oil, the Mediterranean diet, and arterial blood pressure: the Greek European Prospective Investigation into Cancer and Nutrition (EPIC) study. American Journal of Clinical Nutrition 2004;80:1012‐8. [DOI] [PubMed] [Google Scholar]
Public Health England 2018
- Public Health England. The Eatwell guide. https://www.gov.uk/government/publications/the‐eatwell‐guide (accessed 11 November 2018).
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ronksley 2011
- Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta‐analysis. BMJ 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ros 2014
- Ros E, Martínez‐González MA, Estruch R, Salas‐Salvadó J, Fitó M, Martínez JA, et al. Mediterranean diet and cardiovascular health: teachings of the PREDIMED study. Advances in Nutrition 2014;5:330S–6S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosato 2017
Roth 2017
- Roth GA, Johnson C, Abajobir A, Abd‐Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. Journal of the American College of Cardiology 2017;70(1):1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rumawas 2009
- Rumawas ME, Meigs JB, Dwyer JT, McKeown NM, Jacques PF. Mediterranean‐style dietary pattern, reduced risk of metabolic syndrome traits, and incidence in the Framingham Offspring Cohort. American Journal of Clinical Nutrition 2009;90:1608‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 2000
- Ryan M, McInerney D, Owens D, Collins P, Johnson A, Tomkin GH. Diabetes and the Mediterranean diet: a beneficial effect of oleic acid on insulin sensitivity, adipocyte glucose transport and endothelium‐dependent vasoreactivity. QJM 2000;93:85‐91. [DOI] [PubMed] [Google Scholar]
Salas‐Salvado 2014
- Salas‐Salvadó J, Bulló M, Estruch R, Ros E, Covas MI, Ibarrola‐Jurado N, et al. Prevention of diabetes with Mediterranean diets a subgroup analysis of a randomized trial. Annals of Internal Medicine 2014;160(1):1‐10. [DOI] [PubMed] [Google Scholar]
Salas‐Salvado 2018
- Salas‐Salvado J, Becerra‐Tomas N, Garcia‐Gavilan JF, Bullo M, Barrubes L. Mediterranean diet and cardiovascular disease prevention: what do we know?. Progress in Cardiovascular Diseases 2018;61:62–7. [DOI] [PubMed] [Google Scholar]
Schwingshackl 2014
- Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta‐analysis of intervention trials. Nutrition Metabolism and Cardiovascular Diseases 2014;24(9):929‐39. [DOI] [PubMed] [Google Scholar]
Serra‐Majem 1993
- Serra‐Majem L, Helsing E. Changing patterns of fat intake in Mediterranean countries. European Journal of Clinical Nutrition 1993;47 Suppl 1:1‐100. [PubMed] [Google Scholar]
Serra‐Majem 2006
- Serra‐Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutrition Review 2006;64:S27‐47. [DOI] [PubMed] [Google Scholar]
Sofi 2008
- Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta‐analysis. BMJ 2008;337:a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sofi 2010
- Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta‐analysis. American Journal of Clinical Nutrition 2010;92:1189‐96. [DOI] [PubMed] [Google Scholar]
Sofi 2014
- Sofi F, Macchi C, Abbate R, Gensini G, Casini A. Mediterranean diet and health status: an updated meta‐analysis and a proposal for a literature‐based adherence score. Public Health Nutrition 2014;17(12):2769‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stranges 2004
- Stranges S, Wu T, Dorn JM, Freudenheim JL, Muti P, Farinaro E, et al. Relationship of alcohol drinking pattern to risk of hypertension: a population‐based study. Hypertension 2004;44:813‐9. [DOI] [PubMed] [Google Scholar]
Sánchez‐Taínta 2008
- Sánchez‐Taínta A, Estruch R, Bulló M, Corella D, Gómez‐Gracia E, Fiol M, et al. Adherence to a Mediterranean‐type diet and reduced prevalence of clustered cardiovascular risk factors in a cohort of 3,204 high‐risk patients. European Journal of Cardiovascular Prevention and Rehabilitation 2008;15:589‐93. [DOI] [PubMed] [Google Scholar]
Taylor 2009
- Taylor B, Irving HM, Baliunas D, Roerecke M, Patra J, Mohapatra S, et al. Alcohol and hypertension: gender differences in dose‐response relationships determined through systematic review and meta‐analysis. Addiction 2009;104:1981‐90. [DOI] [PubMed] [Google Scholar]
Toobert 2003
- Toobert DJ, Glasgow RE, Strycker LA, Barrera M, Radcliffe JL, Wander RC, et al. Biologic and quality‐of‐life outcomes from the Mediterranean Lifestyle Program: a randomized clinical trial. Diabetes Care 2003;26:2288–93. [DOI] [PubMed] [Google Scholar]
Trichopoulou 1995
- Trichopoulou A, Kouris‐Blazos A, Wahlqvist ML, Gnardellis C, Lagiou P, Polychronopoulos E, et al. Diet and overall survival in elderly people. BMJ 1995;311:1457‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trichopoulou 1997
- Trichopoulou A, Lagiou P. Healthy traditional Mediterranean diet: an expression of culture, history, and lifestyle. Nutrition Review 1997;55:383‐9. [DOI] [PubMed] [Google Scholar]
Trichopoulou 2003
- Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. New England Journal of Medicine 2003;348:2599‐608. [DOI] [PubMed] [Google Scholar]
Trichopoulou 2007
- Trichopoulou A, Bamia C, Norat T, Overvad K, Schmidt EB, Tjønneland A, et al. Modified Mediterranean diet and survival after myocardial infarction: the EPIC‐Elderly Study. European Journal of Epidemiology 2007;22:871‐81. [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization Study Group. Diet, Nutrition and the Prevention of Chronic Diseases. WHO Technical Report Series 916. Geneva: WHO, 2003. [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. Global status report on noncommunicable diseases 2010. www.who.int/nmh/publications/ncd_report2010/en/ (accessed 6 August 2013).
Willett 1995
- Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro‐Luzzi A, Helsing E, et al. Mediterranean diet pyramid: a cultural model for healthy eating. American Journal of Clinical Nutrition 1995;61 Suppl 6:1402‐6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Rees 2013
- Rees K, Hartley L, Flowers N, Clarke A, Hooper L, Thorogood M, et al. 'Mediterranean' dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD009825.pub2] [DOI] [PubMed] [Google Scholar]


