Abstract
Trigger factor (TF) has a known cytoplasmic function as a chaperone. In a previous study we showed that pneumococcal TF is also cell-wall localized and this finding combined with the immunogenic characteristic of TF, has led us to determine the vaccine potential of TF and decipher its involvement in pneumococcal pathogenesis. Bioinformatic analysis revealed that TF is conserved among pneumococci and has no human homologue. Immunization of mice with recombinant (r)TF elicited a protective immune response against a pneumococcal challenge, suggesting that TF contributes to pneumococcal pathogenesis. Indeed, rTF and an anti-rTF antiserum inhibited bacterial adhesion to human lung derived epithelial cells, indicating that TF contributes to the bacterial adhesion to the host. Moreover, bacteria lacking TF demonstrated reduced adhesion, in vitro, to lung-derived epithelial cells, neural cells and glial cells. The reduced adhesion could be restored by chromosomal complementation. Furthermore, bacteria lacking TF demonstrated significantly reduced virulence in a mouse model. Taken together, the ability of rTF to elicit a protective immune response, involvement of TF in bacterial adhesion, conservation of the protein among pneumococcal strains and the lack of human homologue, all suggest that rTF can be considered as a future candidate vaccine with a much broader coverage as compared to the currently available pneumococcal vaccines.
Introduction
The commensal bacterium Streptococcus pneumoniae continues to cause morbidity and mortality worldwide1. Since the implementation of pneumococcal capsular polysaccharide vaccines2,3 a substantial reduction in disease burden has been reported. While the 23 valent unconjugated pneumococcal polysaccharide vaccine (PPSV23) was found to be 45–65% effective in immunocompetent adult patients2, this vaccine, unfortunately, does not elicit an immune response in the group with the highest rate of pneumococcal disease burden, i.e., children younger than two years of age4,5. The first commercial version of the pneumococcal conjugate vaccine (PCV), which included 5 capsular serotypes, has evolved over the last three decades to include up to 15 capsular polysaccharide serotypes6,7. PCVs induce immune memory and a protective immune response in infants, but only protect against serotypes that are included in the vaccine8,9. Limitations of the currently available polysaccharide vaccines and the continuous increase in antibiotic resistance to S. pneumoniae underscore the urgency of the need for pneumococcal vaccines with broader coverage9–12.
Toward the development of protein-based vaccines, candidate pneumococcal immunogenic surface proteins are identified by proteomics- and bioinformatics-based analyses13–17. The identified proteins are screened for low or no homology to human proteins and then tested for their vaccine potential. Using this working scheme several immunogenic proteins have been identified and shown to elicit protective immune responses in mouse models. Among these proteins are: pneumococcal surface protein A (PspA)18, the histidine triad motif (Pht) A, B, D and E proteins19, fructose bisphosphate aldolase (FBA)20, glutamyl tRNA synthetase (GtS)21, pneumococcal serine-rich repeat protein (PsrP)22, PcsB and StkP23 and nucleoside ABC transporter component)24. The presumed function of these proteins in pneumococcal pathogenicity and physiology were then studied further. For example, PhtD controls Zinc homeostasis in the bacterium25 and GtS, PsrP and FBA were found to function as adhesins21,22,26.
We have previously determined that the S. pneumoniae trigger factor (TF) in addition to its known cytoplasmic function, is immunogenic in mice and that it is cell-wall (CW) localized27,28. TF is a heat shock protein that binds the ribosome in the cytoplasm with its N terminal domain and encounters, co-translationally, the nascent protein chain emerging from the ribosome by its C terminal chaperone domain. This function protects the newly synthesized protein from degradation and assists in its proper folding and maturation29. In addition, the central part of TF catalyzes peptidyl-prolyl cis-trans isomerization, which further contributs to the proper folding of proteins30. TF can be found in all eubacteria, with variable degrees of homology, but not in yeast or mammalian cells30. In S. pyogenes TF belongs to the highly conserved anchorless CW proteins with no cross reactivity to human proteins31,32. The intentation of the current study was to determiner whether S. pneumoniae-derived TF is capable of eliciting protective immune responses in a mouse model of S. pneumoniae infection and reveal the function of the CW-localized TF in pathogenesis.
Results
Bioinformatic analysis of trigger factor
Characterization of Trigger factor
TF is a unique bacterial protein with no homologue in the human genome. Blast analysis demonstrated that TF is highly conserved (98% identity) among the available S. pneumoniae sequenced strains in the NCBI database. BlastP of S. pneumoniae TF against other bacteria identified a TF protein with 96% homology in S. mitis, 76% in S. pyogenes, 66–76% in S. agalactiae and 38% in Clostridium difficile. Notably, the homology to several strains of E. coli varied between 31–76%.
The S. pneumoniae TF protein and its Streptococcus orthologs produced an un-gapped multiple sequence alignment, except for an “extruding” sequence in the N terminus of one of the proteins, which was removed from the alignment. A histogram of the conservation score per position, calculated by Jalview, was added below the alignment. B-cell linear epitopes were predicted in silico by four different prediction programs, however there was only partial agreement between the programs. Epitopes were found both in highly conserved and in less conserved regions of the alignment (Supplementary Fig. S1). We cannot conclude whether putative antigenicity regions of the S. pneumoniae TF are preferably located in S. pneumonia conserved or in unique regions. Thus, further studies are necessary to determine whether an immunization with TF may affect other Streptococcus species which may be present in the natural microbiota.
Recombinant (r)TF characteristics
A custom-designed and commercially produced rTF was separated on SDS-PAGE and a band corresponding to 55 kDa was observed (Fig. 1a). The discrepancies between the expected (47190 Da) and the observed molecular weight may result from the altered electrophoretic mobility of rTF. A mass spectrometry (MS) analysis (Fig. 1b) identified a major peak with molecular mass of 46536 Da, which is different from the theoretical molecular weight of 47190 Da and this discrepancy may result from a N or C terminal truncation of the protein. MS analysis of the major peak peptide composition had 73% sequence coverage of trigger factor from S. pneumoniae TIGR4 strain (gi: 15900319, queried on October, 2009).
Figure 1.
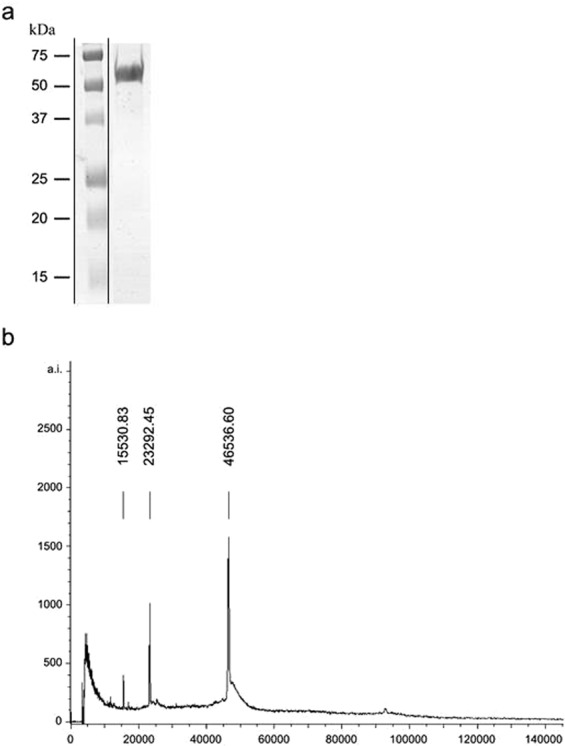
rTF characteristics. Commercially produced and purified rTF was analyzed on SDS-PAGE. A band corresponding to 55 kDa was identified by (a) Coomassie brilliant blue staining and (b) Mass spectroscopy analysis.
Cell wall localization of TF
Previously we found TF in the CW fraction of S. pneumoniae27,28. Notably, we recently showed that a bona fide cytoplasmic protein, malonyl-CoA:ACP transacylase (FabD), which is involved in lipid metabolism could not be found in the CW protein extract28,33. To further verify that TF is indeed CW-localized, a monoclonal anti-rTF antibody was incubated with a live unencapsulated, serotype 2-derived, R6 strain and flow cytometry analysis was performed. TF was detected on the surface of approximately 50% of the bacteria that had been tested, confirming the surface localization of TF (Fig. 2a). Of note, antibodies cannot penetrate the cytoplasmic membrane to access the cytoplasm-localized TF and thus can detect only the CW-localized TF.
Figure 2.
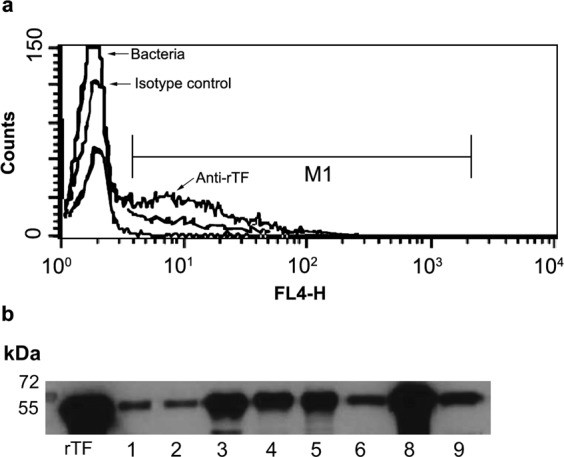
CW localization of TF. (a) R6 bacteria were incubated with either: i. anti-rTF mAb or ii. isotype control mouse serum (as indicated) or iii. phosppate buffered seline. All were stained with Alexa Fluor 647®-conjugated goat-anti-mouse-IgG as a secondary antibody and then analyzed by flow cytometry. (b) Thirty micrograms of protein of CW fractions from 60S. pneumoniae clinical strains (see Supplementary Table S2) were loaded per lane, subjected to SDS-PAGE, and immunoblotted. Purified untagged rTF (0.01 μg) was loaded as a positive control. A representative blot is shown cropped from the 15 min exposed blot is presented.
A CW protein fraction from 60S. pneumoniae clinical strains (Supplementary Table S2) was extracted and separated by SDS-PAGE. Immunoblotting was performed with a rabbit anti-rTF antiserum. If TF band was not detected, increased loading or exposure time were performed and resulted in positive signal (Supplementary Fig. S2). All tested strains demonstrated a band corresponding to the molecular weight of untagged rTF (Fig. 2b, Supplementary Table S2).
TF immunogenicity
In a previous study we showed that TF is immunogenic in mice27. To determine the extent of antibody production following immunization with rHis-TF, ELISA assays were performed. Sera that were obtained following the second and third immunizations (TF II and TF III, respectively) were analyzed on rHis-TF coated plates. The antibody titer in both TF II and TF III was found to be 1:121,500 (see Supplemetary Fig. S3a), demonstrating the ability of rTF to elicit a substantial immune response. TF II and TF III antibody responses were significantly higher than those observed with serum obtained from mice that were immunized with another pneumococcal recombinant His tagged protein, namely rPtsA (PtsA III; See Supplementary Fig. S3a; one way ANOVA with the Dunnett post-hoc test, p = 0.0014 and p = 0.0008, respectively). PtsA III, (but not TF II and TF III), successfully reacted with rHis-PtsA coated plates (see Supplementary Fig. S3b; one way ANOVA, with the Dunnett post-hoc test, p = 0.0001). Taken together, these results establish the immunogenicity of TF and the specificity of the anti-rTF antiserum.
rTF elicits a protective immune response in mice
The findings that TF is (a) immunogenic, (b) lacks homology to human proteins, (c) is CW localized and (d) conserved within S. pneumoniae strains, prompted us to test the ability of rTF to elicit a protective immune response. Immunizing BALB/c mice with rHis-TF significantly reduced mortality following a lethal intranasal challenge with the WU2 wild type (WT) strain, as compared to immunization with the adjuvant only [Fig. 3; n = 45 in each group, Log Rank (Mantel-Cox) test, p = 0.0005]. These findings highlight the vaccine potential of rTF.
Figure 3.
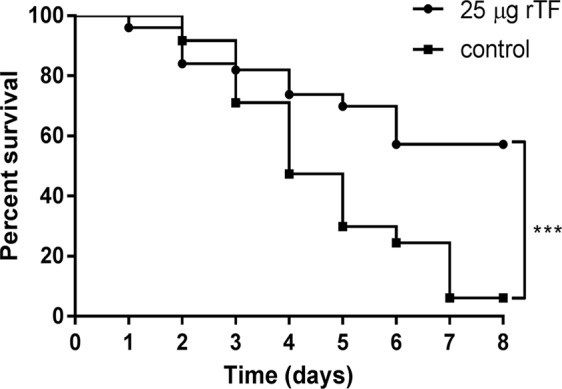
rTF elicits a protective immune response in mice. BALB/c mice were immunized with rTF emulsified with CFA and subsequently boosted with rTF emulsified in IFA. Control mice were immunized with the adjuvant only. Two weeks following the final immunization, mice were challenged intranasally with a lethal dose (1 × 108 CFU) of WU2 and survival was monitored daily. The figure is a summary of three independent experiments. The extent of survival was determined by using the Log-rank (Mantel-Cox) test (n = 45 in each group; ***p = 0.0005).
Cell wall-localized TF contributes to S. pneumoniae adhesion to the host
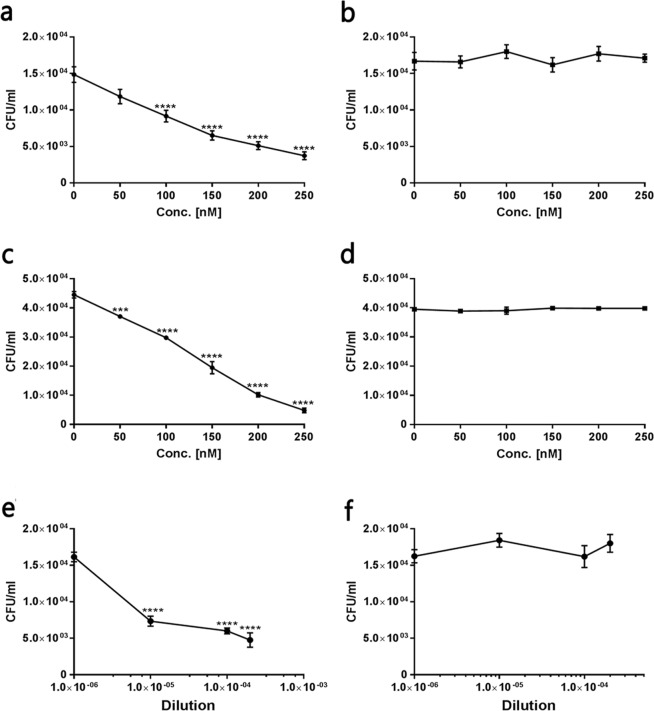
The ability of rTF to elicit a protective immune response suggests that TF contributes to bacterial virulence. Many cell wall-localized proteins were found to be involved as adhesins in S. pneumoniae interaction with the host21,22,26,34. Thus we tested the ability of rHis-Trx-TF to interfere in bacterial adhesion to cultured human lung derived epithelial cells (A549 cells)35–37. Indeed, rHis-Trx-TF significantly inhibited the adhesion of WU2 WT to the cells in a dose dependent manner (Fig. 4a; Spearman’s correlation p = 0.0027; r = −1). Substantial reduction relative to the adhesion in the absence of rHis-Trx-TF was observed upon the addition of as little as 50 nM of rHis-Trx-TF and this reduction became statistically significant at 100 nM or higher concentrations (Fig. 4a; one way ANOVA, with the Dunnett post-hoc test p < 0.0001). Purified rHis-Trx served as the negative control and, indeed, did not interfere in the adhesion of WU2 WT to A549 cells (Fig. 4b). Similar results were obtained with the His-rTF protein (data not shown) but, we present here only the results of rHis-Trx-TF, which provided us with an internal negative control as the expressed and purified rHis-rTrx protein that excluded the possibility that either rHis or rTrx are involved in adhesion.
Figure 4.
Cell wall-localized TF contributes to the adhesion of S. pneumoniae to the host. A549 cells were cultured in 96-well plates. Twenty four hours later, the cells. (~105 cells/well) were incubated for 1 h with rHis-TRX-TF (0–500 nM). Excess protein was removed and S. pneumoniae were added at a MOI of ~100:1 for an additional 1 h incubation. Unattached bacteria were removed and cells were released with trypsin and plated onto blood agar plates for bacterial enumeration. rHis-Trx was used as a negative control. Values are the mean of three independent experiments, conducted in triplicates. (a) Extent of WU2 adhesion in the presence of rHis-Trx-TF (Spearman’s correlation p = 0.0027; r = −1; one-way ANOVA with the Dunnett post-hoc test, ****p < 0.0001 for 100–250 nM). (b) Extent of WU2 adhesion in the presence of r-His-Trx. (c) Extent of R6 adhesion in the presence of rHis-Trx-TF (Spearman’s correlation p = 0.0027; r = −1; one way ANOVA, Dunnett post-hoc test 50 nM: ***p = 0.0001; 100–250 nM: ****p < 0.0001). (d) Extent of R6 adhesion in the presence of rHis-Trx. (e) Extent of WU2 adhesion in the presence of anti rHis-Trx (Spearman’s correlation r = −1, p = 0.083; one-way ANOVA with the Dunnett post-hoc test, ****p < 0.0001). (f) Extent of WU2 adhesion in the presence of pre-immune serum.
Our analysis also revealed that rHis-Trx-TF significantly inhibited, in a dose dependent manner, the adhesion of the unencapsulated R6 strain to A549 cells, whereas rHis-Trx had no effect on the adhesion (Fig. 4c; Spearman’s correlation p = 0.0027; r = −1, one-way ANOVA with the Dunnett post-hoc test: p < 0.0001, Fig. 4d, respectivly). A significant reduction in adhesion, relative to adhesion in the absence of rHis-Trx-TF, was observed in all tested concentrations (Fig. 4c; one way ANOVA, Dunnett post-hoc test 50 nM: p = 0.0001; 100–250 nM: p < 0.0001). Next we tested the ability of sera obtained from rHis-TF immunized mice to inhibit the adhesion of S. pneumoniae to A549 cells. A post-immune serum significantly inhibited WU2 WT adhesion to A549 cells in a dose-dependent manner but a pre-immune serum did not inhibit the adhesion of the bacteria to the cells (Fig. 4e; Spearman’s correlation r = −1, p = 0.083; one way ANOVA, with the Dunnett post-hoc test p < 0.0001, Fig. 4f, respectively). Taken together, these results demonstrate the contribution of CW-localized TF to the adhesion of S. pneumoniae to host cells.
Lack of TF reduces S. pneumoniae adhesion to host cells
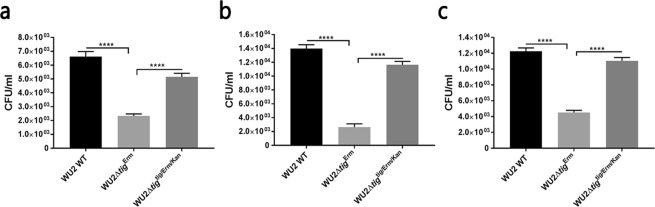
To confirm that TF contributes to adhesion of S. pneumoniae to the host, we created a mutant that lacks TF (WU2ΔtigErm) and a tig-complemented strain (WU2Δtigtig/Erm/Kan). We tested the extent of the adhesion of these strains to A549 cells35–37, to the murine motor neuron NSC 3438 cells and to the human glioblastoma cells U251 cells39. The extent of adhesion of WU2ΔtigErm to A549 cells was significantly reduced compared to both WU2 WT and the complemented strain WU2Δtigtig/Erm/Kan (Fig. 5a; one way ANOVA, with the Dunnett post-hoc test p < 0.0001), suggesting that TF contributes to S. pneumoniae adhesion to host cells. A similarly reduced adhesion to A549 cells was observed with an additional tig mutant WU2Δtigkan compared to WU2 WT (data not shown). The finding that WU2Δtigtig/Erm/Kan regained most of its capacity to adhere to the A549 cells indicates that the reduced adhesion observed in WU2ΔtigErm is not the result of a polar effect. To exclude the possibility that differences in growth rates accounted for the altered extent of adhesion we compared the growth rates of WU2ΔtigErm, WU2 WT and WU2Δtigtig/Erm/Kan under anaerobic conditions and found them to be similar during the midlog stage, with estimated doubling times of 60, 78 and 72 minutes respectively, differences deemed insignificant. Following the midlog stage the growth of mutant strain was slightly attenuated in comparison to the wild type and complemented strains, reaching final OD600 of only 0.758, as opposed to final OD600 of 0.807 and 0.798 recorded for wild type and complemented strain, respectively (see Supplementary Fig. S4). Notably the growth rate of WU2Δtigkan mutant was similar to that of the WU2 WT WU2, in both aerobic (74.8 minutes and 69.8 minutes, respectively) and anaerobic (65 minutes and 61 minutes, respectively) conditions, with no significant variances. Significantly, we found that the mutated WU2ΔtigErm, and the complemented strain WU2Δtigtig/Erm/Kan were identical to the WU2 WT strain in terms of colony morphology (data not shown).
Figure 5.
The lack of TF reduces the adhesion of S. pneumoniae to host cells. (a) The extent of adhesion of WU2ΔtigErm compared to that of the WU2 WT strain and the complemented strain WU2Δtigtig/Erm/Kan to A549 cells (one-way ANOVA with the Dunnett post-hoc test, ****p < 0.0001). (b) The extent of adhesion of the WU2ΔtigErm strain, compared to that of the WU2 WT and WU2Δtigtig/Erm/Kan strains, to NSC 34 cells (one-way ANOVA with the Dunnett post-hoc test, ****p < 0.0001). (c) The extent of adhesion of the WU2ΔtigErm strain, compared to that of the WU2 WT and to WU2Δtigtig/Erm/Kan strains, to U251 cells (one-way ANOVA with the Dunnett post-hoc test, ****p < 0.0001).
The extent of adhesion of WU2ΔtigErm to NSC 34 cells was also significantly reduced as compared to that of WU2 WT and WU2Δtigtig/Erm/Kan (Fig. 5b; one way ANOVA, with the Dunnett post-hoc test p < 0.0001). The extent of adhesion of WU2ΔtigErm to U251 cells was significantly lower compared to WU2 WT and WU2Δtigtig/Erm/Kan (Fig. 5c; one way ANOVA, with the Dunnett post-hoc test p < 0.0001).
TF contributes to S. pneumoniae virulence in vivo
To assess the involvement of TF in virulence in vivo, BALB/c mice were inoculated intranasally with a sublethal dose of either WU2 WT or WU2Δtigkan. The lack of TF resulted in reduced bacterial loads in both the nasopharynx and the lungs of mice, as compared to inoculation with WU2 WT (Fig. 6; n = 5 in each group, Student t-test, p = 0.0001 and p = 0.0031, respectively). These results confirm the involvement of TF in S. pneumoniae virulence. The growth rate and colony morphology of the WU2Δtigkan strain were similar to those of its parental WU2 WT strain (data not shown), as described for WU2ΔtigErm. To reduce animal suffering we did not repeat the virulence test with the complemented strain.
Figure 6.
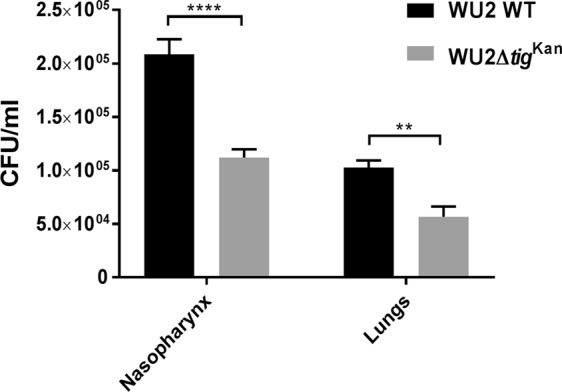
TF contributes to S. pneumoniae virulence in vivo. BALB/c mice were inoculated intranasally with a sublethal dose (5 × 107 CFU) of the WU2 WT or WU2Δtigkan strains. Forty-eight hours following the inoculation, the mice were euthanized and their nasopharynx and lungs were excised, homogenized, and plated onto blood agar plates for enumeration. Results are a summary of three independent experiments (n = 15 in each group; Student t-test, ****p = 0.0001 and **p = 0.0031, respectively).
Discussion
The aim of this study was to determine the vaccine potential of TF and elucidate the involvement of TF in S. pneumoniae pathogenesis. Initially, we confirmed our previous findings that TF is localized to the CW of S. pneumoniae, although it does not have a signal peptide or any other known export sequences. TF lacks a human homologue and is conserved among all the clinical strains of S. pneumoniae tested, in addition to all of the sequenced pneumococcal strains available in the NCBI database. We show that mice immunized with rTF are protected against a lethal challenge by S. pneumoniae. We hypothesized that TF contributes to S. pneumoniae pathogenesis by facilitating bacterial adhesion to host cells. Indeed, we found that rTF and the anti-rTF antiserum inhibited S. pneumoniae adhesion to lung derived epithelial cells. Moreover, bacteria lacking TF showed reduced adhesion to cultured lung derived epithelial cells, as well as to neuronal and glial cells in vitro. A deletion of TF reduced virulence in a mouse model, as compared with WU2 WT in vivo. These findings indicate that TF may serve as a candidate vaccine whose coverage is much broader than that of the currently available pneumococcal vaccines.
Yang et al., (2005 and 2007) showed that the immunization of mice with Brucella melitensis-derived tig DNA or rTF elicited a protective immune response40,41. Similarly, the immunization of mice with S. pyogenes-derived rTF elicited a protective immune response against a lethal challenge31,32. Yet, the ability of a certain protein to elicit a protective immune response against a specific bacterium does not necessarily indicate an ability to elicit protection against another bacteria. For example, fructose bis phosphate aldolase (FBA) was found to be localized to the bacterial CW in both S. pneumoniae and S. pyogenes. However, albeit the high homology between the two proteins (79–89%), rFBA elicited a protective immune response against S. pneumoniae challenge but not against S. pyogenes challenges in mice immunized with the respectively derived rFBA20,31. Similarly, rDnaK from S. pyogenes elicited a protective immune response against a challenge in mice31, while the homologous protein, derived from S. pneumoniae, failed to protect mice from an S. pneumoniae challenge20. Therefore, the vaccine potential of a protein should be tested in the context of the relevant bacterium species.
DnaK is a chaperon involved in nascent protein folding42, which functions downstream of TF. The deletion of either tig or dnaK in E. coli did not affect either protein aggregation or growth phenotype under standard laboratory conditions43–45. However, the deletion of both tig and dnaK resulted in a strain that demonstraed increased protein aggregation and was lethal at temperatures higher than 30 °C46,47. These studies imply that the function of TF in protein folding can be replaced by that of DnaK46. Notably, DnaK is also localized to the CW of S. pneumoniae, S. pyogenes, Clostridium thermocellum, Mycobacterium tuberculosis and Listeria monocytogenes20,27,31,48–50.
CW localized TF may contribute indirectly to S. pneumoniae pathogenesis via the alteration in folding other proteins, thus affecting their function. Our result, that immunization with rTF protected mice from a S. pneumoniae challenge, albeit the presence of DnaK in the CW, which should have replaced the TF function in protein folding51, suggests that CW-associated TF is not involved in the folding of extracellular proteins but rather in bacterial pathogenicity.
TF in E. coli was demonstrated to affects the maturation, membrane binding and translocation through the membrane of pro-OmpA, in addition to being involved in the protection and folding of the nascent protein extruding from the ribosome52. The maturation of pro-OmpA is mediated, among others, by the peptidyl-prolyl isomerization domain of TF52. Streptococcal lipoprotein rotamase A (SlrA), which is a functional peptidyl-prolyl isomerase, was demonstrated to be indirectly involved in the adhesion of S. pneumoniae to the host53. Hermans et al.53, have shown that the adhesion and penetration of an S. pneumoniae D39ΔslrA mutant to A549 cells and to the human nasopharyngeal epithelial cell line Detroit 562 were significantly reduced compared to the parental D39 WT strain. However, neither the rSlrA protein nor an anti-rSlrA antiserum inhibited bacterial adhesion to the cells. Hence, the authors concluded that the involvement of SlrA in adhesion is indirect, possibly by affecting the structure/function of as yet unknown protein(s)53. In the current study, we show that an rTF and an anti-rTF antiserum inhibited the adhesion of S. pneumoniae to A549 cells, suggesting that TF directly contributes S. pneumoniae adhesion to the host. The fact that rTF can compete only with CW TF and that anti-rTF antibodies can react only with CW TF but cannot penetrate the cytoplasmic membrane and react with the cytoplasmic TF highlights the different roles that TF may plays in each of the cellular locations.
We also tested the extent of adhesion of S. pneumoniae to A54921, NSC 3438 and U25139 cells. A549 cells were found to retain the morphological, biochemical and immunological characteristics of human type II lung epithelial cells and are widely used as a model to study pneumococcal interaction with human cells21,35–37. NSC 3438 and U25139 cell-lines were used due to their neural origin. S. pneumoniae, Neisseria meningitidis and Haemophilus influenzae are the major bacterial pathogens causing meningitis, with S. pneumoniae being responsible for two thirds of meningitis cases in the developed world54,55. NSC 34 is a mouse neuroblastoma-spinal cord hybrid immortalized cell-line38, which has been used to study differentiation and characteristics of motor neuron56,57. The glioblastoma multiforme derived cell-line U25139 has been used to study the nature of glioblastoma multiforme and its susceptibility to anti-cancerous drugs58,59. To our knowledge, the current study is the first to use NCS 34 and U251 cell-lines as targets for S. pneumoniae adhesion. The deletion of tig significantly reduced bacterial adhesion to A549, NCS 34 and U251 cells, establishing that TF contributes to S. pneumoniae adhesion to various targets in the host. Moreover, the tig deletion significantly reduced bacterial loads in both the nasopharynx and the lungs of mice following a challenge, further validating the involvement of TF in S. pneumoniae pathogenesis. Importantly, the deletion of tig did not affect the bacterial growth rate at midlog, thus excluding the possibility that the observed differences in the extent of adhesion resulted from attenuated growth of the mutant. Similarly, the deletion of tig did not significantly affect the growth of Bacillus subtilis60,61, Sinorhizobium meliloti62, L. monocytogenes63, S. mutans64,65 or S. pyogenes66.
Taken together the ability of rTF to elicit a protective immune response against a lethal challenge, the contribution of TF to S. pneumoniae adhesion, the conservation of the protein among S. pneumoniae strains and the lack of a human homologue, all suggest that rTF can be considered as a candidate vaccine with a broad coverage.
Methods
Ethics statement
Experiments involving animals were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of the Ben-Gurion University of the Negev, Beer Sheva, Israel (Permit number: 53.08.08).
Mouse strains
Seven-week-old BALB/cOlaHsd (BALB/c) female mice (Harlan Laboratories, Israel) were used in this study. The mice were housed in sterile conditions under 12-h light/dark cycles and fed Purina Chow and tap water ad libitum.
Immunization of mice
BALB/c mice were immunized subcutaneously (SC) with 25 µg of rHis-TF emulsified with complete Freund’s adjuvant (CFA) and subsequently boosted (days 14 and 28) with 25 µg of rHis-TF emulsified in incomplete Freund’s adjuvant (IFA). Control mice were immunized with the adjuvant only.
Lethal challenge of mice
Following immunization on day 42, the mice were challenged intranasally, under deep anesthesia using isoflurane (Piramal Critical Care Inc., PA, USA), with a lethal dose (1 × 108) of S. pneumoniae serotype 3 strain WU267. Mice were humanely euthanized by CO2 asphyxiation, as recommended by the AVMA Guidelines for Euthanasia in Animals: 2013 Edition (https://www.avma.org/KB/Policies/Documents/euthanasia.pdf), if they became moribund or showed evidence of distress. The following criteria were considered sufficient evidence of distress to warrant such intervention and minimize pain and suffering to animals: severe weight loss (20% body weight); reluctance or inability to move freely; the appearance of bristle fur; social disengagement; refusal or inability to eat or drink. No analgesic treatment was provided as such treatment could alter the immune response and therfore independently affect the outcome of the experiments68. Morbidity and mortality were monitored daily.
Intranasal inoculation and bacterial load determination
Six to seven weeks old BALB/c mice were inoculated with a sublethal dose (5 × 107) of either WU2 WT (n = 5 in each experiment) or WU2ΔtigKan (n = 5 in each experiment). Mice were humanely sacrificed 48 h later by CO2 asphyxiation. The nasopharynx and lungs were excised, homogenated and plated onto blood agar plates for enumeration. The results presented are a summary of three experiments performed on different occasions.
Preparation of rabbit and mouse anti-rTF antisera
Three-month-old white albino rabbits (Harlan Laboratories, Israel) or 6-week-old BALB/c mice were immunized SC with 200 µg or 25 µg rHis-TF, respectively, emulsified with CFA (1:1) in the first immunization or with IFA in booster immunizations in 2 week intervals. Two weeks after the final immunization, sera were obtained from blood collected from the rabbits’ marginal ear vein and by cardiac puncture following deep anesthesia from the mice.
Reagents
Unless otherwise stated, all chemicals and biochemical materials were of the highest purity available and were purchased from Sigma-Aldrich (St. Louis, MS, USA).
Bioinformatic analysis of trigger factor
“The amino acid sequence of Trigger Factor protein from Streptococcus pneumoniae serotype 4 (strain ATCC BAA-334/TIGR4) was retrieved from UniProt, ID Q97SG9 (TIG_STRPN). Orthologous proteins of Q97SG9 were retrieved from EggNOG (ID ENOG4105DEA), a database of orthologous groups and functional annotation, and filtered to only include proteins of the genus Streptococcus (n = 52). Amino acid sequences of Q97SG9 and all Streptococcus orthologs from EggNOG (n = 53) were multiply aligned using Muscle and visualized in Jalview (Supplementary Table S1 and Supplementary Fig. S1). The leading 33 amino acids of protein 888048.HMPREF8577_1918 (S. parasanguinis ATCC 903) were trimmed since they were not homologous to any of the other protein sequences. Below the multiple sequence alignment Jalview adds an automatically calculated conservation track, which is visualized as a histogram of the conservation score for each column. Conserved columns are indicated by ‘*’, and columns with mutations where all properties are conserved are marked with a ‘+’ (Supplementary Fig. S1)”.
B-cell epitope prediction analysis of Q97SG9 was conducted using four different web tools: 1. ABCpred Prediction Server (http://crdd.osdd.net/raghava/abcpred/), where epitopes with ranks 1–5 were retrieved; 2. LBtope: Linear B-cell Epitope Prediction Server (http://crdd.osdd.net/raghava/lbtope/), where epitopes with probabilities 61–100% were retrieved; 3. BCPreds: B-cell epitope prediction server (http://ailab.ist.psu.edu/bcpred/); and 4. Bepipred Linear Epitope Prediction 2.0 (http://tools.iedb.org/bcell/).
All programs were run using default parameters. Prediction was done using the linear amino acid sequence of Q97SG9, since (according to UniProt) solved structures of Q97SG9 are currently unavailable. The predicted epitopes from the four web tools were added as annotations below the conservation track in Jalview. In cases where epitopes were ranked by the prediction program, ranks were indicated in the display (with 1 designating the best predictions and higher numbers designating lower ranks); otherwise, the epitopes were indicated as 0.
Bacterial strains and growth conditions
The S. pneumoniae encapsulated serotype 3 WU267 strain and the unencapsulated serotype 2 D39-derived, R6 strain (ATCC, MD, USA) were used. Pneumococci were grown in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY) and growth was monitored at O.D620 or on blood agar plates, as previously described20. In addition, 60 S. pneumoniae clinical strains were obtained as a courtesy of GSK, Belgium (Supplementary Table S2). Two Escherichia coli strains were used as cloning vectors: DH5α UltraMAX (DH5α; Invitrogen Corp, CA, USA) and BL21 (DE3) pLysS (BL21; Promega Corp, WI, USA) and were grown in Luria broth (LB) Lennox.
Cloning, expression and purification of rTF
The nucleotide sequence of the locus SP_RS01985, coding for the WP_000116479.1 protein was amplified by PCR from the genomic DNA of WU2 pneumococcal strain, according to the NCBI published sequence of TIGR4 serotype 4 strain. PCR was performed with the coding region for TF (tig) and tig pET32+ forward and reverse primers (see Supplementary Table S3). The amplified product was digested with BamHI and XhoI (Takara Biomedicals, Japan) and cloned into the pET32a+ expression vector (Novagen, China) prior or following the removal of the thioredoxin reductase (Trx) domain, flanked with NdeI using the NdeI restriction enzyme (Takara Biomedicals, Japan). pET32a+trx+tig and pET32a+tig were transformed into E. coli DH5α cells. Verification of sequence identity was performed by plasmid insert sequencing (data not shown) and found to be in frame. The vector was purified using the Qiagen High-Speed Plasmid Maxi Kit and transformed into E. coli BL21. Bacteria were grown overnight, and expression of rTF was induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 5 h. The cells were harvested and lysed, and the protein was purified under native conditions and then dialyzed against PBS for imidazole removal. r-His-Trx-TF was used to test S. pneumoniae adhesion to cultured cells (as described below) and rHis-TF was used in immunization experiments. Both recombinant proteins were ~95% pure (data not shown). We also purified rHis-Trx from pET32a+ 35,36 to serve as a negative control for the adhesion experiments.
The untagged rTF protein was commercially produced by Protein laboratories, Ltd. (Israel), which amplified tig from TIGR4 genome69, and cloned, expressed and purified the protein as previously described28,33. Validation was also performed by the company using MS.
Anti-rTF antibody titers in immunized mice
Microtiter plates (F96 Maxisorp Nunc, ThermoFisher) were coated with 1 µg/ml solution of rHis-TF in bicarbonate buffer pH 9.6. ELISA was performed as previously described33. Sera obtained from mice after the second (TF II) and third (TF III) rHis-TF immunizations served as primary antibodies. Serum obtained following the third immunization of mice with rHis-PtsA28 (PtsA III) served as a negative control.
Immunoblot analysis
rHis-TF (1 µg) or CW protein fraction (30, 40, 45 or 60 μg) were separated by SDS-PAGE under reduced conditions and transferred to nitrocellulose membranes (Bio-Rad, CA, USA), as previously described28. Detection of TF was performed using rabbit anti-rHis-TF antiserum and peroxidase-conjugated AffiniPure F(ab9)2 fragment goat anti-rabbit IgG (H + L; Jackson Laboratories, ME, USA). Membranes were developed using MicroChemi 4.2 (DNR, Israel), and thereafter the molecular weight markers were added to the chemiluminescent image (Markers overlay). Either pre-immune serum or exclusion of the primary antibody were used as negative controls.
Anti rTF monoclonal entibody (mAb) production
BALB/c mice were immunized 4 times, in 2 week intervals, with 25 µg rHis-TF in the presence of CFA in primary immunization and IFA in booster immunizations. Two weeks after the last immunization splenocytes were harvested for fusion with NSO cells by standard techniques. The hybridomas were tested for reactivity with rHis-TF by ELISA. Positive clones were further subcloned, and the clone with the highest reactivity to the protein was adapted to the serum-free media. The cells were expanded, and the mAb was purified from the culture supernatant by Protein G affinity chromatography. The reactivity of the purified mAb was reconfirmed by ELISA (data not shown) before sorting by FACS. The purified mAb preparations were in the range of 0.6–1.3 mg/mL−1. Purified IgG from naive mice was used as a control. An additional control was the exclusion of the primary antibody (data not shown).
Flow cytometry of S. pneumoniae
Flow cytometry was performed as previously described28. Briefly, R6 bacteria were first stained with 5(6)-Carboxyfluorescein diacetate N-succinimidyl ester (CFSE), then washed, incubated with anti-rHis-TF mAb or isotype control mouse serum, or phosphate buffer seline (PBS), washed again, and stained with Alexa Fluor 647®-conjugated goat-anti-mouse-IgG (Jackson ImmunoResearch, PA, USA). Flow cytometry was performed using a FACSCalibur flow cytometer (Becton Dickinson (BD), CA, USA). CFSE staining was visualized by excitation with an argon laser (488 nm) and detection through FL1 (530/30 BP). Cells positive for CFSE were gated as live cells and evaluated for their 647 nm staining intensity (excitation by red diode laser 633/635 nm and emission detected through FL4 661/12 BP). The cytometer collected 30,000 events (FL1) and 10,000 CFSC positive by FL1 were gated and analyzed by FL4 at 647 nm. Software used for collection was CellQuest™ (BD and Company, NJ, USA). For analysis of the data, we used FlowJo software version 9.2 for Macintosh (Tree Star, Inc., Ashland, OR, USA).
Cell lines
A549 cells (lung adenocarcinoma cells; ATCC, MD, USA)21,35–37 and NSC 34 cells (murine motor-neuron like cells)38 were grown in Dulbecco’s modified Eagle medium (DMEM; Biological Industries, Israel). U251 cells (human glioblastoma multiforme derived cells)39 were grown in RPMI 1640 (Biological Industries, Israel). All media were supplemented with 10% fetal calf serum, penicillin and streptomycin (100 µg/ml each) and cells grown at 37 °C in a humidified incubator.
Evaluation TF contribution to pneumococci adhesion to cultured human cell-lines
A549 cells were cultured on 96-well plates, as previously described25. The extent of S. pneumoniae adhesion to the cells in the presence of r-His-Trx-TF (0–500 nM) was tested using MOI of ~100:1. rHis-Trx served as a negative control. The extent of S. pneumoniae adhesion to A549 cells in the presence of anti-rTF antiserum was tested as previously described25,28. Similar results were obtained with the His-rTF protein (data not shown) but, we present here only the results of rHis-Trx-TF, which provided us with an internal negative control as the expressed and purified rHis-rTrx protein that excluded the possibility that either rHis or rTrx are involved in adhesion. Evaluation of WU2 WT, WU2ΔtigErm mutant and WU2Δtigtig/Erm/Kan complemented strains adhesion was performed with A54921, U251 and NSC 34 cells. The results presented are the summation of data obtained from three independent experiments, each performed in triplicates.
Preparation of WU2Δtig mutant (kanamycin or erythromycin resistance)
To create null mutants of the tig gene, either the kanamycin (Kan) or erythromycin (Erm) resistance cassette was inserted into the coding sequence of tig via homologous recombination, as previously described70, with minor modifications. The upstream and downstream flanking regions of tig were amplified by PCR from the DNA of WU2 using primers designed according to the TIGR4 sequence. Primer combinations included upwing-F/upwing-R (see Supplementary Table S3, Upwing WU2Δtig), for the upstream region of 800 bp and downwing-F/downwing-R, for the downstream region of 702 bp (Supplementary Table S3, Downwing WU2Δtig). The Kan cassette was amplified by PCR from the genome of CP125071 using the primer combination Kan AB-F/Kan AB-R (see Supplementary Table S3, Kan AB cassette). The Erm cassette was amplified by PCR from the genome of WU2ΔnoxErm 72 using the primer combination Erm AM-F/Erm AM-R (see Supplementary Table S3, Erm AM cassette). The PCR products were digested with the corresponding restriction nucleases, as specified in Supplementary Table S3, purified, ligated and transformed into WU2 in the presence of the competence stimulating factor CSP1 and CaCl2. Transformants were selected on THY plates solidified with 1.5% agar, containing kanamycin (80 µg/ml) or erythromycin (125 µg/ml). Verification of tig deletion was done by PCR using specific primers (see Supplementary Table S2, Kan AB cassette for Kan resistance and Erm AM cassette for Erm resistance).
Cis-complementation of WU2ΔtigErm
To confirm that mutation of tig introduced no polar effects, WU2ΔtigErm was complemented with an intact copy of the gene using pCEP, which is a non-replicative plasmid that allows controlled gene expression under its native promoter, following ectopic integration into the chromosome73,74. Briefly, tig was amplified from WU2 with tig pCEP-F and tig pCEP-R primers (see Supplementary Table S3, tig pCEP complementation), which introduce NcoI and PstI sites. The amplicons were then ligated into NcoI and PstI digested vector. An aliquot of ligation mixture was transformed into Stellar™ competent cells (Clonetech, France), as described by the manufacturer. The transformants were selected for kanamycin resistance (500 μg/ml). Successful ligation was determined by colony PCR using Mal-F and pCEP-R primers (see Supplementary Table S3, tig pCEP verification), whose recognition sites are localized immediately upstream and downstream of the cloning site, respectively. These primers amplified a 263 bp product in an empty vector, while they produce a product of 1747 bp in the recombinant clones (additional 1484 bp represents the cloned fragment containing tig of 1284 bp). The recombinant plasmid was purified using a commercial kit (Qiagen) and an aliquot was transformed into WU2ΔtigErm, as described previously75, to produce the complemented strain WU2Δtigtig/Erm/Kan. The transformants were selected on blood agar plates supplemented with erythromycin (125 μg/ml) and kanamycin (500 μg/ml). Integration of tig into the genome was confirmed by PCR (see Supplementary Table S3, Kan resistance in pCEP verification).
Preparation of S. pneumoniae CW protein fraction
The isolation of the CW proteins was performed as previously described27,76.
Statistical analysis
Normal distribution and sufficient sample size of small sample sized data sets were verified using the Shapiro-Wilk test and post-hoc statistical power analysis, respectively, to justify the use of one-way ANOVA for parametric data, followed by the Dunnett test for multiple comparisons. A one-tailed Student’s t-test with Welch’s correction was used for bacterial load comparisons between the two groups. Data are reported as the mean ± SEM, unless stated otherwise. Spearman correlations were used to assess the significance of change in adhesion assays. Survival of S. pneumoniae-inoculated mice was determined using the Log-rank (Mantel-Cox) test. Differences were considered significant at p < 0.05. All statistical analyses were performed with the software package in GraphPad Prism version 7 (La Jolla, CA, USA).
Supplementary information
Acknowledgements
This work was supported by grants from the Israeli Ministry of Health numbers 4776 and 5540, 3000003867, from the Center of Emerging Diseases #2506, from the Israel Academy of Science 613/04, The Israel Ministry of Commerce and Industry to NasVax Ltd., European Community 7th FP: CAREPNEUMO 223111, from BGNegev Biotechnology to YMN.
Author Contributions
Y.M.N. designed the study. A.C., S.T., S.D., H.N., H.Y., T.K., M.S., M.P., and H.N. performed the experiments. S.D., T.K., R.B., M.T., R.E., R.D., V. C.C. and Y.M.N. analyzed the data. R.B. and Y.M.N. wrote the manuscript. All authors discussed and approved of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-40779-0.
References
- 1.Feldman C, Anderson R. Epidemiology, virulence factors and management of the pneumococcus. F1000Res. 2016;5:2320. doi: 10.12688/f1000research.9283.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falkenhorst G, et al. Effectiveness of the 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23) against Pneumococcal Disease in the Elderly: Systematic Review and Meta-Analysis. PLoS One. 2017;12:e0169368. doi: 10.1371/journal.pone.0169368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagan R, Poolman J, Siegrist CA. Glycoconjugate vaccines and immune interference: A review. Vaccine. 2010;28:5513–5523. doi: 10.1016/j.vaccine.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Heilmann C. Human B and T lymphocyte responses to vaccination with pneumococcal polysaccharides. APMIS Suppl. 1990;15:1–23. [PubMed] [Google Scholar]
- 5.Berical AC, Harris D, Dela Cruz CS, Possick JD. Pneumococcal Vaccination Strategies. An Update and Perspective. Ann Am Thorac Soc. 2016;13:933–944. doi: 10.1513/AnnalsATS.201511-778FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segal N, Greenberg D, Dagan R, Ben-Shimol S. Disparities in PCV impact between different ethnic populations cohabiting in the same region: A systematic review of the literature. Vaccine. 2016;34:4371–4377. doi: 10.1016/j.vaccine.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 7.McFetridge R, et al. Safety, tolerability, and immunogenicity of 15-valent pneumococcal conjugate vaccine in healthy adults. Vaccine. 2015;33:2793–2799. doi: 10.1016/j.vaccine.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Hicks LA, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis. 2007;196:1346–1354. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 9.Dagan R. Serotype replacement in perspective. Vaccine. 2009;27(Suppl 3):C22–24. doi: 10.1016/j.vaccine.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Blasi F, Mantero M, Santus P, Tarsia P. Understanding the burden of pneumococcal disease in adults. Clin Microbiol Infect. 2012;18(Suppl 5):7–14. doi: 10.1111/j.1469-0691.2012.03937.x. [DOI] [PubMed] [Google Scholar]
- 11.Ash SY, Sheffield JV. Pneumococcus. Med Clin North Am. 2013;97(647–666):x–xi. doi: 10.1016/j.mcna.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Daniels CC, Rogers PD, Shelton CM. A Review of Pneumococcal Vaccines: Current Polysaccharide Vaccine Recommendations and Future Protein Antigens. J Pediatr Pharmacol Ther. 2016;21:27–35. doi: 10.5863/1551-6776-21.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barocchi MA, Censini S, Rappuoli R. Vaccines in the era of genomics: the pneumococcal challenge. Vaccine. 2007;25:2963–2973. doi: 10.1016/j.vaccine.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 14.Kaushik DK, Sehgal D. Developing antibacterial vaccines in genomics and proteomics era. Scand J Immunol. 2008;67:544–552. doi: 10.1111/j.1365-3083.2008.02107.x. [DOI] [PubMed] [Google Scholar]
- 15.Morsczeck C, et al. Streptococcus pneumoniae: proteomics of surface proteins for vaccine development. Clin Microbiol Infect. 2008;14:74–81. doi: 10.1111/j.1469-0691.2007.01878.x. [DOI] [PubMed] [Google Scholar]
- 16.Mahdi LK, Wang H, Van der Hoek MB, Paton JC, Ogunniyi AD. Identification of a novel pneumococcal vaccine antigen preferentially expressed during meningitis in mice. J Clin Invest. 2012;122:2208–2220. doi: 10.1172/JCI45850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wizemann TM, et al. Use of a whole genome approach to identify vaccine molecules affording protection against Streptococcus pneumoniae infection. Infect Immun. 2001;69:1593–1598. doi: 10.1128/IAI.69.3.1593-1598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDaniel LS, Scott G, Kearney JF, Briles DE. Monoclonal antibodies against protease-sensitive pneumococcal antigens can protect mice from fatal infection with Streptococcus pneumoniae. J Exp Med. 1984;160:386–397. doi: 10.1084/jem.160.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adamou JE, et al. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect Immun. 2001;69:949–958. doi: 10.1128/IAI.69.2.949-958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling E, et al. Glycolytic enzymes associated with the cell surface of Streptococcus pneumoniae are antigenic in humans and elicit protective immune responses in the mouse. Clin Exp Immunol. 2004;138:290–298. doi: 10.1111/j.1365-2249.2004.02628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizrachi Nebenzahl Y, et al. Streptococcus pneumoniae surface-exposed glutamyl tRNA synthetase, a putative adhesin, is able to induce a partially protective immune response in mice. J Infect Dis. 2007;196:945–953. doi: 10.1086/521028. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez CJ, et al. The pneumococcal serine-rich repeat protein is an intra-species bacterial adhesin that promotes bacterial aggregation in vivo and in biofilms. PLoS Pathog. 2010;6:e1001044. doi: 10.1371/journal.ppat.1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giefing C, et al. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J Exp Med. 2008;205:117–131. doi: 10.1084/jem.20071168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxena S, Khan N, Dehinwal R, Kumar A, Sehgal D. Conserved surface accessible nucleoside ABC transporter component SP0845 is essential for pneumococcal virulence and confers protection in vivo. PLoS One. 2015;10:e0118154. doi: 10.1371/journal.pone.0118154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eijkelkamp BA, et al. The First Histidine Triad Motif of PhtD Is Critical for Zinc Homeostasis in Streptococcus pneumoniae. Infect Immun. 2015;84:407–415. doi: 10.1128/IAI.01082-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blau K, et al. Flamingo cadherin: a putative host receptor for Streptococcus pneumoniae. J Infect Dis. 2007;195:1828–1837. doi: 10.1086/518038. [DOI] [PubMed] [Google Scholar]
- 27.Portnoi M, Ling E, Feldman G, Dagan R, Mizrachi-Nebenzahl Y. The vaccine potential of Streptococcus pneumoniae surface lectin- and non-lectin proteins. Vaccine. 2006;24:1868–1873. doi: 10.1016/j.vaccine.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 28.Mizrachi Nebenzahl Y, et al. Streptococcus pneumoniae Cell-Wall-Localized Phosphoenolpyruvate Protein Phosphotransferase Can Function as an Adhesin: Identification of Its Host Target Molecules and Evaluation of Its Potential as a Vaccine. PLoS One. 2016;11:e0150320. doi: 10.1371/journal.pone.0150320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Sakai H, Wiedmann M. NAC covers ribosome-associated nascent chains thereby forming a protective environment for regions of nascent chains just emerging from the peptidyl transferase center. J Cell Biol. 1995;130:519–528. doi: 10.1083/jcb.130.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henningham A, et al. Conserved anchorless surface proteins as group A streptococcal vaccine candidates. J Mol Med (Berl) 2012;90:1197–1207. doi: 10.1007/s00109-012-0897-9. [DOI] [PubMed] [Google Scholar]
- 32.Rivera-Hernandez, T. et al. Differing Efficacies of Lead Group A Streptococcal Vaccine Candidates and Full-Length M Protein in Cutaneous and Invasive Disease Models. MBio7, 10.1128/mBio.00618-16 (2016). [DOI] [PMC free article] [PubMed]
- 33.Morozov GI, et al. Flavin Reductase Contributes to Pneumococcal Virulence by Protecting from Oxidative Stress and Mediating Adhesion and Elicits Protection Against Pneumococcal Challenge. Sci Rep. 2018;8:314. doi: 10.1038/s41598-017-18645-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniely D, et al. Pneumococcal 6-phosphogluconate-dehydrogenase, a putative adhesin, induces protective immune response in mice. Clin Exp Immunol. 2006;144:254–263. doi: 10.1111/j.1365-2249.2006.03047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17:62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- 36.Balis JU, Bumgarner SD, Paciga JE, Paterson JF, Shelley SA. Synthesis of lung surfactant-associated glycoproteins by A549 cells: description of an in vitro model for human type II cell dysfunction. Exp Lung Res. 1984;6:197–213. doi: 10.3109/01902148409109248. [DOI] [PubMed] [Google Scholar]
- 37.Asano K, et al. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc Natl Acad Sci USA. 1994;91:10089–10093. doi: 10.1073/pnas.91.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cashman NR, et al. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn. 1992;194:209–221. doi: 10.1002/aja.1001940306. [DOI] [PubMed] [Google Scholar]
- 39.Osborn M, et al. Expression of glial and vimentin type intermediate filaments in cultures derived from human glial material. Differentiation. 1981;19:161–167. doi: 10.1111/j.1432-0436.1981.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 40.Yang X, Hudson M, Walters N, Bargatze RF, Pascual DW. Selection of protective epitopes for Brucella melitensis by DNA vaccination. Infect Immun. 2005;73:7297–7303. doi: 10.1128/IAI.73.11.7297-7303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Walters N, Robison A, Trunkle T, Pascual DW. Nasal immunization with recombinant Brucella melitensis bp26 and trigger factor with cholera toxin reduces B. melitensis colonization. Vaccine. 2007;25:2261–2268. doi: 10.1016/j.vaccine.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Yang F, et al. Single-molecule dynamics of the molecular chaperone trigger factor in living cells. Mol Microbiol. 2016;102:992–1003. doi: 10.1111/mmi.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teter SA, et al. Polypeptide flux through bacterial Hsp70: DnaK cooperates with trigger factor in chaperoning nascent chains. Cell. 1999;97:755–765. doi: 10.1016/S0092-8674(00)80787-4. [DOI] [PubMed] [Google Scholar]
- 44.Deuerling E, Schulze-Specking A, Tomoyasu T, Mogk A, Bukau B. Trigger factor and DnaK cooperate in folding of newly synthesized proteins. Nature. 1999;400:693–696. doi: 10.1038/23301. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann A, Bukau B, Kramer G. Structure and function of the molecular chaperone Trigger Factor. Biochim Biophys Acta. 2010;1803:650–661. doi: 10.1016/j.bbamcr.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Genevaux P, et al. In vivo analysis of the overlapping functions of DnaK and trigger factor. EMBO Rep. 2004;5:195–200. doi: 10.1038/sj.embor.7400067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vorderwulbecke S, et al. Low temperature or GroEL/ES overproduction permits growth of Escherichia coli cells lacking trigger factor and DnaK. FEBS Lett. 2004;559:181–187. doi: 10.1016/S0014-5793(04)00052-3. [DOI] [PubMed] [Google Scholar]
- 48.Yu T, Xu X, Peng Y, Luo Y, Yang K. Cell wall proteome of Clostridium thermocellum and detection of glycoproteins. Microbiol Res. 2012;167:364–371. doi: 10.1016/j.micres.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Hickey TB, Thorson LM, Speert DP, Daffe M, Stokes RW. Mycobacterium tuberculosis Cpn60.2 and DnaK are located on the bacterial surface, where Cpn60.2 facilitates efficient bacterial association with macrophages. Infect Immun. 2009;77:3389–3401. doi: 10.1128/IAI.00143-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaumburg J, et al. The cell wall subproteome of Listeria monocytogenes. Proteomics. 2004;4:2991–3006. doi: 10.1002/pmic.200400928. [DOI] [PubMed] [Google Scholar]
- 51.Castanie-Cornet MP, Bruel N, Genevaux P. Chaperone networking facilitates protein targeting to the bacterial cytoplasmic membrane. Biochim Biophys Acta. 2014;1843:1442–1456. doi: 10.1016/j.bbamcr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Crooke E, Wickner W. Trigger factor: a soluble protein that folds pro-OmpA into a membrane-assembly-competent form. Proc Natl Acad Sci USA. 1987;84:5216–5220. doi: 10.1073/pnas.84.15.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hermans PW, et al. The streptococcal lipoprotein rotamase A (SlrA) is a functional peptidyl-prolyl isomerase involved in pneumococcal colonization. J Biol Chem. 2006;281:968–976. doi: 10.1074/jbc.M510014200. [DOI] [PubMed] [Google Scholar]
- 54.VanDemark M. Acute bacterial meningitis: current review and treatment update. Crit Care Nurs Clin North Am. 2013;25:351–361. doi: 10.1016/j.ccell.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Prager O, Friedman A, Nebenzahl YM. Role of neural barriers in the pathogenesis and outcome of Streptococcus pneumoniae meningitis. Exp Ther Med. 2017;13:799–809. doi: 10.3892/etm.2017.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maier O, et al. Differentiated NSC-34 motoneuron-like cells as experimental model for cholinergic neurodegeneration. Neurochem Int. 2013;62:1029–1038. doi: 10.1016/j.neuint.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Sabitha KR, Sanjay D, Savita B, Raju TR, Laxmi TR. Electrophysiological characterization of Nsc-34 cell line using Microelectrode Array. J Neurol Sci. 2016;370:134–139. doi: 10.1016/j.jns.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 58.Hegge B, Sjottem E, Mikkola I. Generation of a PAX6 knockout glioblastoma cell line with changes in cell cycle distribution and sensitivity to oxidative stress. BMC Cancer. 2018;18:496. doi: 10.1186/s12885-018-4394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valtorta S, et al. Metformin and temozolomide, a synergic option to overcome resistance in glioblastoma multiforme models. Oncotarget. 2017;8:113090–113104. doi: 10.18632/oncotarget.23028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reyes DY, Yoshikawa H. DnaK chaperone machine and trigger factor are only partially required for normal growth of Bacillus subtilis. Biosci Biotechnol Biochem. 2002;66:1583–1586. doi: 10.1271/bbb.66.1583. [DOI] [PubMed] [Google Scholar]
- 61.Gothel SF, Scholz C, Schmid FX, Marahiel MA. Cyclophilin and trigger factor from Bacillus subtilis catalyze in vitro protein folding and are necessary for viability under starvation conditions. Biochemistry. 1998;37:13392–13399. doi: 10.1021/bi981253w. [DOI] [PubMed] [Google Scholar]
- 62.Miller-Williams M, Loewen PC, Oresnik IJ. Isolation of salt-sensitive mutants of Sinorhizobium meliloti strain Rm1021. Microbiology. 2006;152:2049–2059. doi: 10.1099/mic.0.28937-0. [DOI] [PubMed] [Google Scholar]
- 63.Bigot A, Botton E, Dubail I, Charbit A. A homolog of Bacillus subtilis trigger factor in Listeria monocytogenes is involved in stress tolerance and bacterial virulence. Appl Environ Microbiol. 2006;72:6623–6631. doi: 10.1128/AEM.00624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crowley PJ, et al. Requirements for surface expression and function of adhesin P1 from Streptococcus mutans. Infect Immun. 2008;76:2456–2468. doi: 10.1128/IAI.01315-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wen ZT, Suntharaligham P, Cvitkovitch DG, Burne RA. Trigger factor in Streptococcus mutans is involved in stress tolerance, competence development, and biofilm formation. Infect Immun. 2005;73:219–225. doi: 10.1128/IAI.73.1.219-225.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lyon WR, Gibson CM, Caparon MG. A role for trigger factor and an rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J. 1998;17:6263–6275. doi: 10.1093/emboj/17.21.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Briles DE, et al. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stables MJ, et al. Priming innate immune responses to infection by cyclooxygenase inhibition kills antibiotic-susceptible and -resistant bacteria. Blood. 2010;116:2950–2959. doi: 10.1182/blood-2010-05-284844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tettelin H, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 70.Piotrowski A, Luo P, Morrison DA. Competence for genetic transformation in Streptococcus pneumoniae: termination of activity of the alternative sigma factor ComX is independent of proteolysis of ComX and ComW. J Bacteriol. 2009;191:3359–3366. doi: 10.1128/JB.01750-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pestova EV, Havarstein LS, Morrison DA. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol Microbiol. 1996;21:853–862. doi: 10.1046/j.1365-2958.1996.501417.x. [DOI] [PubMed] [Google Scholar]
- 72.Muchnik L, et al. NADH oxidase functions as an adhesin in Streptococcus pneumoniae and elicits a protective immune response in mice. PLoS One. 2013;8:e61128. doi: 10.1371/journal.pone.0061128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hajaj B, et al. Thiol peroxidase is an important component of Streptococcus pneumoniae in oxygenated environments. Infect Immun. 2012;80:4333–4343. doi: 10.1128/IAI.00126-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guiral S, et al. Construction and evaluation of a chromosomal expression platform (CEP) for ectopic, maltose-driven gene expression in Streptococcus pneumoniae. Microbiology. 2006;152:343–349. doi: 10.1099/mic.0.28433-0. [DOI] [PubMed] [Google Scholar]
- 75.Dworschak E, et al. Medical activities of Aesculus hippocastaneum (horse-chestnut) saponins. Adv Exp Med Biol. 1996;404:471–474. doi: 10.1007/978-1-4899-1367-8_37. [DOI] [PubMed] [Google Scholar]
- 76.Siegel JL, Hurst SF, Liberman ES, Coleman SE, Bleiweis AS. Mutanolysin-induced spheroplasts of Streptococcus mutants are true protoplasts. Infect Immun. 1981;31:808–815. doi: 10.1128/iai.31.2.808-815.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.