Abstract
Worldwide, breast conservation has become increasingly accepted as the surgical management of breast cancer in clinical practice. Cancer care in India is also evolving tremendously with many cancer treatment centres following evidence-based practice hence the rates of breast conservation are expected to increase. Here, we are reporting the rate of breast-conserving surgery (BCS) at our centre. A retrospective study of 401 patients who underwent breast cancer surgery at a tertiary care centre in South India from January 2015 to August 2017 were analysed to study the rate of BCS. All early breast cancers (EBC) were offered BCS. For large operable breast cancer (LOBC) and locally advanced breast cancer (LABC), neoadjuvant chemotherapy (NACT) followed by BCS was offered to these patients who wish to conserve their breast. The mean age was 45 years. A total of 163 patients underwent BCS. Yearly, BCS rates were 38.8% in 2015, 36.7% in 2016 and 46.5% in 2017. Majority had EBC 310 (77.3%) of which 62.7% of T1 lesions (n = 51) had BCS, and 45.7% of T2 lesions (n = 258) had BCS of which 5 patients had to undergo NACT to preserve their breast whereas 100% Tis patient (n = 1) had mastectomy. Fifty patients had LOBC and only 2 (4%) patients had upfront BCS whereas 9 of them had to undergo NACT (18%). cT4 lesions had NACT followed by BCS in 2 patients. The rates of BCS have been increasing in India over the past few years. The majority of the women presented with EBC which makes them suitable for BCS.
Keywords: Breast-conserving surgery, Rates, Neoadjuvant chemotherapy, Early breast cancer
Introduction
The incidence of breast cancer has been rising steadily and for the first time in 2012, breast cancer was the most common cancer in women in India [1]. Breast cancer seems to be more common in the younger age group as a significant number of patients are below 30 years [2]. Surgical management of breast cancer has evolved tremendously after Sir Geoffrey Keynes, an English surgeon at St. Bartholomew Hospital in London first described breast-conserving surgery (BCS) for carcinoma in 1924 [3]. By the 1950s and 1960s, breast conservation became increasingly accepted in clinical practice, and in some institutions, this therapeutic approach was considered standard.
Breast conservation surgery (BCS) is the complete removal of the breast cancer with a margin of normal tissue surrounding the tumour. This is usually followed by radiation therapy (RT). In terms of loco-regional recurrences rates and overall survival rates, BCS is comparable to total mastectomy (TM) [4, 5]. Most reports indicate that the majority of women who present with breast cancer do not have contraindications to conservative surgery [6]. Reasons for underutilisation of breast conservation include patient preference, age and poor prognostic factors. Medical comorbidity is rarely a major factor in the underutilisation of breast-conserving surgery [7]. The major clinical factors to consider before performing BCS are tumour breast ratio, margin status and the presence of multifocal lesions [8].
When compared to the west, BCS is not popular among the surgeons in India (11–23% vs > 60–70% in west) [9]. At our institute, BCS is being offered to all early breast cancer (T1N0/N1, T2N0/N1) patients. For large operable breast cancer (LOBC, T3N0/N1) and locally advanced breast cancer (LABC, T4 lesions), neoadjuvant chemotherapy (NACT) followed by BCS was offered to these patients who wish to conserve their breast. At present, the cancer care in India is evolving tremendously with many cancer treatment centres following evidence-based practice especially in private sectors; hence, the rates of breast conservation are expected to increase. Here, we are reporting the rate of BCS at our centre.
Materials and Methods
This was a retrospective study. Electronic medical records of 401 patients who underwent surgery for breast cancer at a tertiary care centre in South India from January 2015 to August 2017 were collected and the data fed into an excel worksheet. Metastatic breast cancers were excluded from the study. All patients diagnosed with breast cancer were triple assessed with clinical examination, preoperative bilateral mammogram and core biopsy of the breast lump. They were clinically staged using the American Joint Committee on Cancer (AJCC) TNM staging. All early breast cancer patients irrespective of age were offered BCS. For large operable breast cancer (LOBC) and locally advanced breast cancer (LABC), neoadjuvant chemotherapy (NACT) followed by BCS was offered to these patients who wish to conserve their breast. Pre-chemotherapy ultrasound-guided metallic clips were placed routinely to localise the tumour to aid BCS in this setting. Breast-conserving surgery included wide local excision (also called lumpectomy), quadrantectomy, wire-guided localisation and excision of non-palpable lumps and revision of margins of previous lumpectomy done elsewhere. Patients were classified as ER+/PR+/HER2−, ER+/PR+/HER2+, ER-/PR-/HER2+ and triple negative based on the immunohistochemistry (IHC) analysis on core biopsy specimen preoperatively. Patient age, menstrual status, clinical stage, type of surgery, tumour site, tumour type, grade, nodal status and pathological stage were also assessed in this study. The collected data was analysed to study the rate of breast-conserving surgery (BCS) for breast cancer.
Rate of BCS was calculated by number of BCS done divided by the total number of breast cancer surgery done in that year. The association between molecular subtype and T stage was done using cross tabulation.
Results
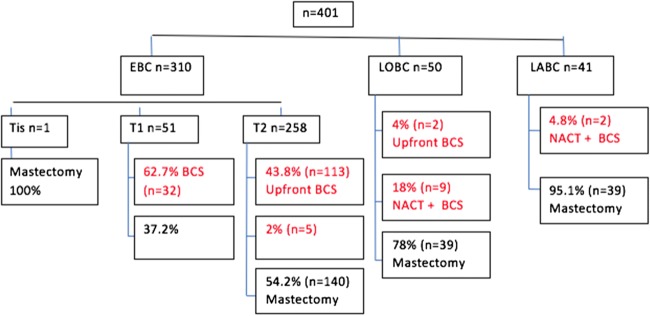
Out of 401 patients who underwent breast cancer surgery during the study period, the mean age was 45.1 years (SD 12.59), and the median age was 46 years with the youngest patient being 23 years old and oldest being 90 years old. Sixty percent were pre-/perimenopausal and 40% were postmenopausal. Right breast was involved in 50.4% and left in 49.6%. The most common tumour location was found to be at the upper outer quadrant 59.4% followed by upper inner quadrant 15.5%. pT2N0 stage IIA was the most common pathological staging found and histology was infiltrating ductal carcinoma, NOS type (82.3%) of which majority were grade 2. Twenty-one patients had invasive lobular carcinoma. Surgical margins for BCS were free for all of them except three which were identified on frozen section and a mastectomy could not be avoided in these patients. 64.6% of patients had SLNB and the sentinel lymph node harvested varies from 1 to 12. 49.4% underwent ALND and axillary lymph node harvested varied from 10 to 56. 31.9% had lympho-vascular invasion. 20.7% had extra-nodal spread. 28.2% had breast reconstruction in the form of volume displacement and 24% had volume replacement.
A total of 163 patients underwent BCS during the study period. The total number of mastectomy during the same period was 238 in the department. In 2015, 52 patients underwent BCS (38.8%) and in 2016, the numbers dipped to 50 (36.7%). But in 2017, 61 patients had BCS (46.5%) (Fig. 1).
Fig. 1.
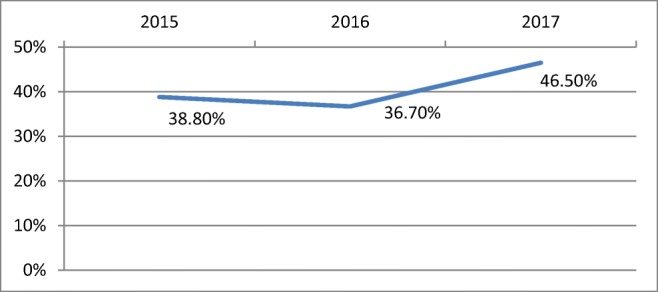
Increase in BCS rates from 2015 to 2017
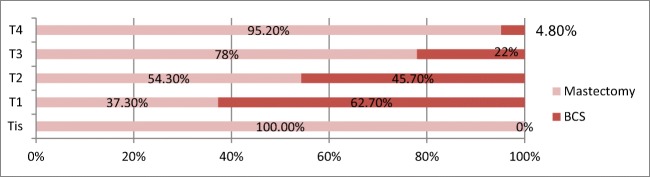
Total number of early breast cancer (EBC) was 310 (77.3%), large operable breast cancer LOBC 50 (12.5%) and locally advanced breast cancer LABC 41 (10.2%). Majority of early breast cancer were cT2 lesions (83.25). 62.7% of T1 lesions (n = 51) had breast-conserving surgery BCS and 45.7% of T2 lesions (n = 258) had BCS out of which five had to undergo neoadjuvant chemotherapy to be eligible for preserving their breast. Only one patient had Tis and underwent mastectomy. Fifty patients had large operable breast cancer (cT3 N0/N1) and only two (4%) patients had upfront BCS whereas nine of them had to undergo NACT (18%). cT4 lesions had NACT followed by BCS in 2 patients (Figs. 2 and 3).
Fig. 2.
BCS vs mastectomy rates for various T stages
Fig. 3.
BCS vs mastectomy rates among EBC, LOBC and LABC
In early breast cancer patients who underwent mastectomy (n = 159), 17.6% had history of previous lumpectomy done elsewhere with positive margins, bilateral breast cancer (3.1%), BRCA-positive (3.1%), previous contralateral breast cancer in 2.5%, ipsilateral breast cancer in 1%, and multi-centric (5%); 1 patient had Paget’s disease and 1 patient had wound infection with margins unknown. Types of BCS performed were lumpectomy (84%), quadrantectomy (11.6%), revision of positive or unknown margins post-lumpectomy done outside 3 (.6%) and wire-guided wide local excision of non-palpable lump (0.6%).
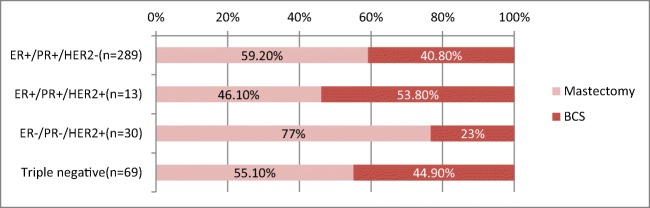
The most common molecular subtype among 401 patients were ER+/PR+/HER2-(72%) followed by triple negative (17.2%). ER+/PR+/HER2+ was 3.3% whereas ER−/PR−/HER2+ was 7.5%. Patients who underwent BCS (n = 163) had ER+/PR+/HER2− (72.3%) followed by triple negative (19%). Both ER+/PR+/HER2+ and ER−/PR−/HER2+ was 4.2%. All LABC and LOBC who had BCS were ER+/PR+/HER2−. Early breast carcinoma majority was ER+/PR+/HER2− followed by triple negative. In T1 lesions, the lowest BCS rate was found to be in ER+/PR+/HER2+ whereas in T2 lesions, it was ER−/PR−/HER2+ as seen in (Table 1 and Fig. 4).
Table 1.
Molecular subtypes of various T stages who underwent BCS (n = 163)
| Molecular subtype | T stage | |||||||
|---|---|---|---|---|---|---|---|---|
| T1 (n = 32) | T2 (n = 118) | T3 (n = 11) | T4 (n = 2) | |||||
| Count | % | Count | % | Count | % | Count | % | |
| ER+/PR+/HER2- | 14 | 43.8 | 91 | 77.1 | 11 | 100.0 | 2 | 100 |
| ER+/PR+/HER2+ | 3 | 9.3 | 4 | 3.3 | 0 | 0.00 | 0 | 0.00 |
| ER-/PR-/HER2+ | 4 | 12.5 | 3 | 2.5 | 0 | 0.00 | 0 | 0.00 |
| Triple negative | 11 | 34.3 | 20 | 16.9 | 0 | 0.00 | 0 | 0.00 |
Fig. 4.
BCS vs mastectomy rates across molecular subtypes
Discussion
The most common cancer in women is breast cancer with an estimated 1.67 million new cancer cases diagnosed worldwide in 2012 [10]. In India, there is a significant increase in the incidence and cancer-associated morbidity and mortality in Indian subcontinent as described in many Indian studies [11–13]. When compared to the west, Indian women diagnosed with breast cancer were a decade younger, many being premenopausal [14, 15]. In our study, the mean age was 45 years at diagnosis which is interesting to note as the youngest patient was 23 years old and oldest 90 years old with one fourth of the women being below 35 years of age.
The era of breast-conserving surgery was brought by NSABP B-06 study, published in the 1980s [16] after which breast-conserving surgery (BCS) has been the standard surgical treatment for early-stage breast cancer patients. BCS provides equivalent long-term survival and much better cosmetic outcomes than mastectomy. Trend to conserve breast has increased over years worldwide, which is also reflected in the present study which showed an increase in BCS rates (Fig. 1) from 2015 to 2017 (38.8 to 46.5%). But in India, breast-conserving surgery is offered only in few selected centres and the overall low rate of BCS may depend on late stage at presentation, non-availability of radiotherapy and low acceptance of the patient and her family [17–19]. Only 11.3% underwent BCS in a study conducted in Delhi which showed non-availability of radiotherapy was a major factor in overuse of mastectomies [19]. In Mumbai at Tata Memorial Hospital, the trends have been going upwards significantly from 12.6 to 59.3% over 5 years. Similar trends have been reported at SGPGIMS Lucknow in the recent 5 years, with more than 2/3 of EBC patients accepting BCS [13].
Present study showed that patients commonly presented with early breast cancer (77.3%) of which majority were cT2 lesions. All T1 lesions had upfront breast-conserving surgery. Large T2 lesions had to undergo neoadjuvant chemotherapy to be eligible for BCS. Lumpectomy was the preferred type of BCS performed (84%). Majority of patients who opted for mastectomy in early breast cancer had come with history of previous lumpectomy done elsewhere with positive margins, were BRCA mutated or had contralateral or ipsilateral breast cancer. The possible impact of inadequate or incomplete surgery in low-resource settings must be addressed as many of the patients presented to our centre with positive or unknown margins of lumpectomy done elsewhere (17.6%). The histopathology reports of lumpectomy were unavailable or inadequate to assess the marginal status. The possibility of undergoing surgery again, fear of residual breast cancer and recurrence in retained breast tissue could be major factors for these patients in choosing mastectomy over revision of margins. Such patients may be associated with worse outcomes when compared to the patients managed in protocol-based manner in the first place [20].
Neoadjuvant therapy can downstage large operable breast lesions and make them amendable to breast-conserving surgery [21]. It was noted in our study that large operable breast cancer had NACT followed by BCS in 18% and two patients opted for quadrantectomy after neoadjuvant chemotherapy for T4 lesions. Breast-conserving surgery in LABC after down staging with neoadjuvant chemotherapy was analysed by Parmar et al. [22] which showed local relapse rate was 8% after BCS and 10.7% after mastectomy on 30 months follow-up. Many innovations to make BCS practical and safer in large locally advanced breast cancers have been reported [23]. Pre-chemotherapy tumor localisation with metallic clips under ultrasound guidance can aid breast conservation in this setting [24].
A preoperative mammogram and core biopsy was mandatory for considering breast-conserving surgery at our setting. All 401 patients had confirmed histopathological diagnosis of malignancy by core biopsy of the breast lesions. Inconclusive mammogram was followed by additional imaging (spot compression, lateral views and MRI). Mammography is an important factor in planning for surgery and no BCS should be done without a preoperative mammogram.
Estrogen (ER) and progesterone receptors (PR) status of the patient are not commonly known in India according to a study in Delhi which showed only 35.5% of patients had receptor testing [19]. At our institute, all core biopsies were subjected to immunohistochemistry study and 100% patients had their receptor status known. The most common molecular subtype was ER+/PR+/HER2− (72%) followed by triple negative (17.2%). At SGPGIMS, only about 45% patients were ER+/PR+. At TMH Mumbai, the ER+ status was found in 33%, and PR+ in 46% [25].
The impact of receptor status on deciding the type surgery is less studied. Multifocal/multi-centric disease, extensive lymph node involvement and positive cavity margins are more associated with cancers that express HER2 [26]. HER2-positive and triple-negative cancers demonstrate increased risk of local recurrence than ER+/PR+ cancers [27]. These factors may render surgeons towards mastectomy for these patients. In this study, ER−/PR−/HER2+ had the highest mastectomy rate when compared to the other subgroups (Fig. 4). BCS rates vs mastectomy among ER+/PR+ and triple negative were almost the same meaning they had an equivalent chance of BCS when compared to ER−/PR−/HER2+ cancers. All LABC and LOBC who had BCS were ER+/PR+/HER2−. Early breast carcinoma majority was ER+/PR+/HER2− followed by triple negative. In T1 lesions, the lowest BCS rate was found to be in ER+/PR+/HER2+ whereas in T2 lesions, it was ER−/PR−/HER2+.
Whole breast radiation is the standard of care after BCS except in selected patients such as elderly women with ER+/PR+ tumors whereas radiation exposure can be limited or omitted [28]. Complications of radiotherapy such as dermatitis, edema, fibrosis and scarring can bring down the cosmetic outcome of BCS and can also interfere with the early detection of local recurrence. Whether the patient’s reluctance towards radiotherapy and its complications had any impact on BCS rates could not be studied due to the study being retrospective and inadequate data. Access to a radiotherapy facility was shown to influence the use of BCS in a study in North India [19].
Conclusion
Worldwide, the trend to conserve breast has increased over years, which is also reflected in India. Majority of the women presented with early breast cancer which makes them suitable for breast-conserving surgery. The need to evaluate these women at an appropriate protocol-based centre needs to be stressed upon.
Contributor Information
Shaziya Hassan Ali, Phone: 0044 7538778502, Email: dr.shaziya@hotmail.com.
Somashekhar S.P, Phone: 0091 9845712012, Email: somashekhar.sp@manipalhospitals.com.
Arun Kumar N, Phone: 0091 8050558443, Email: arun.biostat@gmail.com.
References
- 1.Asthana S, Chauhan S, Labani S. Breast and cervical cancer risk in India: an update. Indian J Public Health. 2014;58:5–10. doi: 10.4103/0019-557X.128150. [DOI] [PubMed] [Google Scholar]
- 2.Khokhar A. Breast cancer in India: where do we stand and where do we go? Asian Pac J Cancer Prev. 2012;13:4861–4866. doi: 10.7314/APJCP.2012.13.10.4861. [DOI] [PubMed] [Google Scholar]
- 3.Keynes G. Radium treatment of primary carcinoma of the breast. Lancet. 1928;2:108. doi: 10.1016/S0140-6736(00)83605-3. [DOI] [Google Scholar]
- 4.Lichter AS, Lippman ME, Danforth DN, Jr, d’Angelo T, Steinberg SM, deMoss E, MacDonald HD, Reichert CM, Merino M, Swain SM, et al. Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: a randomized trial at the National Cancer Institute. J Clin Oncol. 1992;10:976–983. doi: 10.1200/JCO.1992.10.6.976. [DOI] [PubMed] [Google Scholar]
- 5.Van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 6.Foster RS, Farwell ME, Costanza MC. Breast-conserving care for breast cancer: patterns of care in a geographic region and estimation of potential applicability. Ann Surg Oncol. 1995;2:275–280. doi: 10.1007/BF02307035. [DOI] [PubMed] [Google Scholar]
- 7.Morrow M, Bucci C, Rademaker A. Medical contraindications are not a major factor in the underutilization of breast conserving therapy. J Am Coll Surg. 1998;186:269–274. doi: 10.1016/S1072-7515(97)00153-1. [DOI] [PubMed] [Google Scholar]
- 8.Martin MA, Meyricke R, O’Neill T, et al. Mastectomy or breast conserving surgery? Factors affecting type of surgical treatment for breast cancer: a classification tree approach. BMC Cancer. 2006;6:98. doi: 10.1186/1471-2407-6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raina V, Bhutani M, Bedi R, Sharma A, Deo SV, Shukla NK, et al. Clinical features and prognostic factors of early breast cancer at a major centre in North India. Indian J Cancer. 2005;42:36–41. doi: 10.4103/0019-509x.15099. [DOI] [PubMed] [Google Scholar]
- 10.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 11.Babu GR, Lakshmi SB, Thiyagarajan JA. Epidemiological correlates of breast cancer in South India. Asian Pac J Cancer Prev. 2013;14:5077–5083. doi: 10.7314/APJCP.2013.14.9.5077. [DOI] [PubMed] [Google Scholar]
- 12.Ali I, Wani WA, Saleem K. Cancer scenario in India with future perspectives. Cancer Ther. 2011;8:56–70. [Google Scholar]
- 13.Rangarajan, Bharath et al. Breast cancer: an overview of published Indian data. South Asian J Cancer 2016. 5.3: 86–92 [DOI] [PMC free article] [PubMed]
- 14.Chopra B, Kaur V, Singh K, Verma M, Singh S, Singh A. Age shift: breast cancer is occurring in younger age groups—is it true? Clin Cancer Investig J. 2014;3:526–529. doi: 10.4103/2278-0513.142652. [DOI] [Google Scholar]
- 15.Narendra H, Ray S. Breast surgery for breast cancer: single institutional experience from Southern India. Indian J Cancer. 2011;48:415–422. doi: 10.4103/0019-509X.92260. [DOI] [PubMed] [Google Scholar]
- 16.Fisher B, Redmond C, Poisson R, Margolese R, Wolmark N, Wickerham L, Fisher E, Deutsch M, Caplan R, Pilch Y, Glass A, Shibata H, Lerner H, Terz J, Sidorovich L. Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1989;320:822–828. doi: 10.1056/NEJM198903303201302. [DOI] [PubMed] [Google Scholar]
- 17.Dinshaw KA, Budrukkar AN, Chinoy RF, Sarin R, Badwe R, Hawaldar R, Shrivastava SK. Profile of prognostic factors in 1022 Indian women with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2005;63:1132–1141. doi: 10.1016/j.ijrobp.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 18.Aziz Z, Sana S, Akram M, Saeed A. Socioeconomic status and breast cancer survival in Pakistani women. J Pak Med Assoc. 2004;54:448–453. [PubMed] [Google Scholar]
- 19.Raina V, Bhutani M, Bedi R, Sharma A, Deo SV, Shukla NK, Mohanti BK, Rath GK. Clinical features and prognostic factors of early breast cancer at a major cancer center in North India. Indian J Cancer. 2005;42:40–45. doi: 10.4103/0019-509X.15099. [DOI] [PubMed] [Google Scholar]
- 20.Tewari M, Pradhan S, Kumar M, Shukla HS. Effect of prevailing local treatment options of breast cancer on survival outside controlled clinical trials: experience of a specialist breast unit in North India. World J Surg. 2006;30:1794–1801. doi: 10.1007/s00268-006-0037-1. [DOI] [PubMed] [Google Scholar]
- 21.Hage JA, Velde CJ, Julien JP, Hulin MT, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 22.Parmar V, Krishnamurthy A, Hawaldar R, Nadkarni MS, Sarin R, Chinoy R, Nair R, Dinshaw KA, Badwe RA. Breast conservation treatment in women with locally advanced breast cancer – experience from a single centre. Int J Surg. 2006;4:106–114. doi: 10.1016/j.ijsu.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal V, Agarwal G, Lal P, Krishnani N, Mishra A, Verma AK, Mishra SK (2007, Nov 21) Feasibility study of safe breast conservation in large and locally advanced cancers with use of radiopaque markers to mark pre-neoadjuvant chemotherapy tumor margins. World J Surg [DOI] [PubMed]
- 24.Baron LF, Baron PL, Ackerman SJ, Durden DD, Pope TL. Sonographically guided clip placement facilitates localization of breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2000;174:539–540. doi: 10.2214/ajr.174.2.1740539. [DOI] [PubMed] [Google Scholar]
- 25.Desai SB, Moonim MT, Gill AK, Punia RS, Naresh KN, Chinoy RF. Hormone receptor status of breast cancer in India: a study of 798 tumors. Breast. 2000;9:267–270. doi: 10.1054/brst.2000.0134. [DOI] [PubMed] [Google Scholar]
- 26.Jia H, Jia W, Yang Y, Li S, Feng H, Liu J, Rao N, Jin L, Wu J, Gu R, Zhu L, Chen K, Deng H, Zeng Y, Liu Q, Song E, Su F. HER-2 positive breast cancer is associated with an increased risk of positive cavity margins after initial lumpectomy. World J Surg Oncol. 2014;12:289. doi: 10.1186/1477-7819-12-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrow M. Personalizing extent of breast cancer surgery according to molecular subtypes. Breast. 2013;22(Suppl 2):S106–S109. doi: 10.1016/j.breast.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Hughes KS, Schnaper LA, Berry D, Cirrincione C, McCormick B, Shank B, Wheeler J, Champion LA, Smith TJ, Smith BL, Shapiro C, Muss HB, Winer E, Hudis C, Wood W, Sugarbaker D, Henderson IC, Norton L, Cancer and Leukemia Group B. Radiation Therapy Oncology Group. Eastern Cooperative Oncology Group Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]