Abstract
Lymph node involvement in pancreatic adenocancer is one of the strongest predictors of prognosis. However, the extent of lymph node dissection is still a matter of debate and number of dissected nodes varies widely among patients. In order to homogenize this diverse group of patients and more accurately predict their prognosis, we aimed to analyze the effect of metastatic lymph node ratio as an independent prognostic factor. We retrospectively analyzed medical recordings of 326 patients with pancreatic cancer who were treated in a tertiary medical oncology center over a 10-year period. Both in univariate and multivariate analyses, metastatic lymph node ratio proved to be a strong predictor of prognosis which was unaffected from heterogeneity of our patient population and can be used to facilitate predict prognosis of patients who underwent lymph node dissection to various extents and with future studies it can emerge as a successful tool for creating prognostic subgroups of the disease.
Keywords: Prognostic value, Metastatic lymph node ratio, Pancreatic cancer
Introduction
Pancreatic cancer (PC) is one of the most aggressive cancers and with 200,000 annual new cases, it is the fourth most common cause of cancer-related deaths worldwide [1, 2]. It has a very poor prognosis with reported overall 5-year survival rates between 1 and 5% that increases up to 15–25% with radical resection [1–3]. Despite advances in chemotherapeutics, surgery maintains its major role in treatment and more radical surgical approaches are being offered in the literature [4, 5].
Lymph node metastasis (LNM) being a major prognostic determinant even in the setting of radical resection [6–8], literature still lacks a generally applicable rule on the extent of lymph node dissection [9]. There is limited number of randomized controlled trials on the subject which all failed to show any survival benefit of extended lymph node dissection [10–13]. A recent consensus statement by International Study Group on Pancreatic Surgery (ISGPS) concluded that extended lymphadenectomy is not recommendable and suggested resection of standardized lymph node stations [14].
Prognostic value of the ratio of metastatic lymph nodes to the resected nodes has been studied for different kinds of cancer with some significant results. A good example of this is gastric cancer and accumulating data on the topic suggests that the metastatic lymph node ratio (MLR) is emerging as an independent prognostic factor [15, 16]. However, prognostic significance of MLR in PC is still controversial and recent research suggests its significance similar to the patterns in gastric cancer [17].
Using ratio, rather than the number of metastatic lymph nodes for predicting prognosis, may help to standardize this heterogeneous group of patients who undergo lymph node dissection to various extents. This study aims to evaluate the prognostic value of MLR in PC with a brief review of the literature.
Material and Methods
Medical recordings of patients who were admitted to a tertiary medical oncology center for a span of 10 years were retrospectively searched based on International Classification of Diseases-10 (ICD-10) codes. A total of 427 patients labeled with a diagnosis of pancreatic adeno cancer were enrolled in the study and their recordings were further analyzed. Recordings of 90 patients that lacked proper histopathologic results were immediately discarded and a further 11 patients who were labeled as adeno cancer but actually had different types of pancreatic malignancies also were excluded. Thus, data from a net total of 326 patients with pancreatic adeno cancer were statistically analyzed.
Qualitative Properties of the Patient Population
The database that the patients were recorded belonged to a tertiary medical oncology center that served to all western region of Turkey. High volume and large geographic coverage of the center provided advantages of a multicenter study. However, this heterogeneity made it impossible to analyze some prognostic parameters suggested by the literature such as extent of lymph node dissection and topographic mapping of the metastases.
Quantitative Analysis of the Data
A receiver operating characteristic (ROC) analysis failed to define a cut off value for MLR in our population (area under the curve was 0.605, p = 0.275). Thus, we tested 5, 10, and 15% cutoff values which were determined by previous large studies [18–22].
Results
Descriptive Analysis of the Patients
Average age of the population was 59.3 ± 10.4 ranging from 21 to 87. Majority of the patients were male (68.7% n = 224) and 38.3% (n = 125) patients were operated. Only 16 patients were alive at the time the study was conducted and 45 patients were lost to follow-up. Overall 5-year survival from the time of diagnosis was 4.4% and average survival time was 458 days. However, 5-year survival rate for the patients who underwent surgery was 7.9% with an average survival time of 651 days. Estimated survival times for the whole patient population and the operated group were 500 and 805 days respectively.
Majority of the tumors were located in the head of the pancreas (60.4%, n = 197) followed by body (27%, n = 88) and tail (12.6%, n = 41). The distribution of the surgical procedure types was consistent with tumor location distribution. Whipple’s procedure was the most common type of operation (60%, n = 75) followed by pylorus protective pancreaticoduodenectomy (PPPD) (18.4%, n = 23), distal pancreatectomy (20%, n = 25), and total pancreatectomy (1.6%, n = 2).
Of the 125 patients who were operated on, there was microscopic evidence of tumor on the resection border (R1 resection) in 23 patients (18.4%).
Average number of dissected lymph nodes was 12.6 ± 7.4 ranging from one to 44.
Majority of the tumors were moderately differentiated (61.4%) in the whole patient population. This was also valid for the operated patient group in which 54.3% of the tumors were moderately differentiated. Table 1 summarizes descriptive statistics for the patient population and the group of operated patients.
Table 1.
Descriptive statistics of the patients
| Population | Operated group | |
|---|---|---|
| Age (average) | 59.3 | 57.6 |
| Sex | ||
| Male | 224 (68.7%) | 84 (67.2%) |
| Female | 102 (31.3%) | 41 (32.8%) |
| Tumor location | ||
| Head | 197 (60.4%) | 99 (79.2%) |
| Body | 88 (27%) | 17 (13.6%) |
| Tail | 41 (12.6%) | 9 (7.2%) |
| Tumor differentiation | ||
| Poor | 60 (18.6%) | 18 (14.7%) |
| Moderate | 201 (61.4%) | 68 (54.3%) |
| Well | 65 (20%) | 39 (31%) |
| 5-year survival (%) | 4.4% | 7.9% |
| Average time of survival (days) | 458 | 651 |
| Type of surgery | ||
| Whipple | NA | 75 (60%) |
| PPPD | NA | 23 (18.4%) |
| Distal Pancreatectomy | NA | 25 (20%) |
| Total Pancreatectomy | NA | 2 (1.6%) |
| Resection accuracy | ||
| R0 | NA | 102 |
| R1 | NA | 23 |
| Number of dissected lymph nodes | NA | 12.6 ± 7.4 (range 1–44) |
NA, not applicable
Survival Analysis
A logistic regression analysis was employed to test the effect of, tumor size, resection status, number of resected and metastatic lymph nodes, histologic differentiation and the presence of vascular and local invasion on survival. Among these variables, tumor size being larger than 4 cm, poor histologic differentiation and presence of vascular invasion had significant effect on survival (p values 0.03, 0.01 and 0.01 respectively). Interestingly, positive surgical margins (R1 resection) (p = 0.081) and presence of local invasion (p = 0.097) did not significantly altered survival.
As a proven negative prognostic factor, LNM was observed 69% of the operated patients and this proved to be a strong predictor of survival (p = 0.008).
Number of dissected lymph nodes varied widely among the patients ranging from one to 44 suggesting an inhomogeneity in the extent of lymph node dissection among different surgeons.
We tested the cut of values of 5, 10, and 15% for MLR separately, using tumor size as a covariant as it was the only significant prognostic factor for the operated group.
All these cutoff values proved significant for predicting survival. Among the tested MLR, there was a correlation between the percentage of involved lymph nodes and life expectancy. The less percent of involved nodes was associated with more estimated survival time.
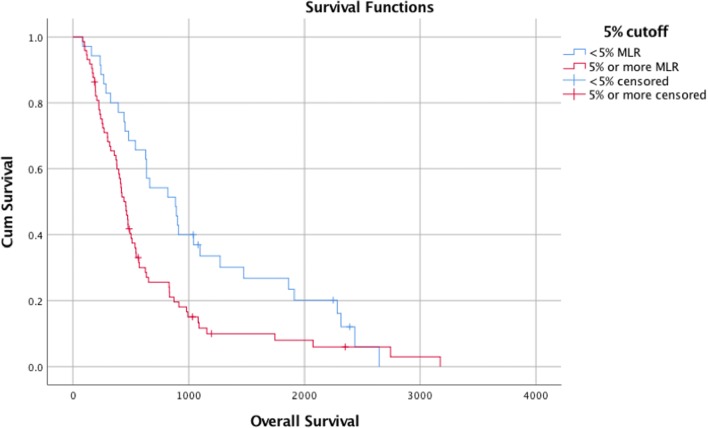
For example, life expectancy for the patients with less than 5 % MLR was 1090 days. However, estimated survival for the patients with more than 5 % MLR was 665 days. The p value for this difference in 95% confidence interval was 0.01 and having less than 5 % MLR was found to be a strong predictor of survival (Fig. 1).
Fig. 1.
Survival plot for 5% cutoff for MLR
Similar results were found for the patients with less than 10 % MLR. Estimated survival time dropped from 975 to 649 days for the patients having more than 10 % MLR (p = 0.016). As an independent prognostic factor, tumor size being more than 4 cm did not did not alter prognostic relevance of 10 % MLR when tested as a covariate (p = 0.007).
Lastly, life expectancy for the patients with less than 15% MLR was 912, which was significantly longer when compared to 616 days for the patients with more than 15% MLR (p = 0.04).
Discussion
Pancreatic cancer is one of the deadliest type of cancers with reported 5-year survival rates between 1 and 5% [2]. Radical surgery remains the best treatment option, rising five-year survival rates up to 15–25% in some series [1, 3].
The literature almost agrees on the important effect of LNM on prognosis [23, 24]. Despite our failure to demonstrate significance of some other classic prognostic markers of PC in our patient population, LNM still proved a strong predictor of survival.
Extent of lymph node dissection is very well established for some malignancies such as gastric and rectal cancer [25, 26]. However, there are only few randomized prospective studies evaluating the effect of extended lymph node dissection on the prognosis of PC and even these studies lack some qualities such as covering only periampullary region tumors or having limited number of patients to accurately show survival advantage [4, 10, 11]. Moreover, it is postulated that very large number of cohorts are needed to determine subgroups that would benefit from extended dissection [27].
The number of evaluated lymph nodes is shown to be associated with improved survival by multiple studies [28–30]. Current opinion in the literature suggests the removal of at least 10 lymph nodes to avoid under staging. Concordantly, having less than 10 nodes removed was associated with poor prognosis in our patient group (p < 0.005). However, MLR proved to be a significant predictor of survival even in this subgroup of patients. Nevertheless, this should not be interpreted as a discouragement for proper lymph node dissection but rather taken as an opportunity to more accurately predict the prognosis of patients with low number of dissected nodes. Additionally, MLR provides the opportunity to homogenize the adjuvant treatment of PC patients operated by a heterogeneous group of surgeons coming from diverse disciplines.
Another factor that could have affected prognosis of our patients is adjuvant chemotherapy. Since all the data was collected from the archives of a medical oncology center, all the patients received adjuvant therapies. However, those therapies were not homogenous neither by means of regiment nor duration. This probably have some effect on our results but we expect this effect to be minimal because, despite our failure to demonstrate the effect of even classical prognostic factors of PC in our population, LNM and MLR proved to be strong predictors of prognosis even for our heterogeneous group of patients.
In conclusion, MLR offers a feasible solution for homogeneously predicting prognosis of PC patients who underwent heterogeneous lymph node dissection procedures and with future studies, it can emerge as a successful tool for creating prognostic subgroups of the disease.
References
- 1.Buchler MW, Kleeff J, Friess H. Surgical treatment of pancreatic cancer. J Am Coll Surg. 2007;205(4 Suppl):S81–S86. doi: 10.1016/j.jamcollsurg.2007.06.332. [DOI] [PubMed] [Google Scholar]
- 2.Loos M, Kleeff J, Friess H, Buchler MW. Surgical treatment of pancreatic cancer. Ann N Y Acad Sci. 2008;1138:169–180. doi: 10.1196/annals.1414.024. [DOI] [PubMed] [Google Scholar]
- 3.Adams RB, Allen PJ. Surgical treatment of resectable and borderline resectable pancreatic cancer: expert consensus statement by Evans et al. Ann Surg Oncol. 2009;16(7):1745–1750. doi: 10.1245/s10434-009-0410-z. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13(9):1189–1200. doi: 10.1245/s10434-006-9016-x. [DOI] [PubMed] [Google Scholar]
- 5.Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21(2):195–200. doi: 10.1007/s002689900215. [DOI] [PubMed] [Google Scholar]
- 6.Cameron JL, Crist DW, Sitzmann JV, Hruban RH, Boitnott JK, Seidler AJ, Coleman J. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991;161(1):120–124. doi: 10.1016/0002-9610(91)90371-J. [DOI] [PubMed] [Google Scholar]
- 7.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165(1):68–72. doi: 10.1016/S0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa O, Ohhigashi H, Sasaki Y, Kabuto T, Fukuda I, Furukawa H, Imaoka S, Iwanaga T. Practical usefulness of lymphatic and connective tissue clearance for the carcinoma of the pancreas head. Ann Surg. 1988;208(2):215–220. doi: 10.1097/00000658-198808000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eskander MF, de Geus SW, Kasumova GG, Ng SC, Al-Refaie W, Ayata G, Tseng JF (2016) Evolution and impact of lymph node dissection during pancreaticoduodenectomy for pancreatic cancer. Surgery doi:10.1016/j.surg.2016.09.032, 161, 968, 976 [DOI] [PubMed]
- 10.Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, Kloppel G, Dhaene K, Michelassi F. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg. 1998;228(4):508–517. doi: 10.1097/00000658-199810000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, Coleman J, Abrams RA, Hruban RH (2002) Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 236 (3):355–366; discussion 366-358. doi:10.1097/01.SLA.0000027272.08464.0B [DOI] [PMC free article] [PubMed]
- 12.Nimura Y NM, Kato H (2004) Regional versus extended lymph node dissection in radical pancreaticoduodenectomy for pancreatic cancer: a multicenter, prospective, randomized controlled trial HPB 6 (2)
- 13.Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Burgart LJ, Dahl TR, Foster N, Sargent DJ. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138(4):618–628. doi: 10.1016/j.surg.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 14.Tol JA, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M, Andren-Sandberg A, Asbun HJ, Bockhorn M, Buchler MW, Conlon KC, Fernandez-Cruz L, Fingerhut A, Friess H, Hartwig W, Izbicki JR, Lillemoe KD, Milicevic MN, Neoptolemos JP, Shrikhande SV, Vollmer CM, Yeo CJ, Charnley RM, International Study Group on Pancreatic S Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS) Surgery. 2014;156(3):591–600. doi: 10.1016/j.surg.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SY, Hwang I, Park YS, Gardner J, Ro JY. Metastatic lymph node ratio in advanced gastric carcinoma: a better prognostic factor than number of metastatic lymph nodes? Int J Oncol. 2010;36(6):1461–1467. doi: 10.3892/ijo_00000632. [DOI] [PubMed] [Google Scholar]
- 16.Alatengbaolide LD, Li Y, Xu H, Chen J, Wang B, Liu C, Lu P. Lymph node ratio is an independent prognostic factor in gastric cancer after curative resection (R0) regardless of the examined number of lymph nodes. Am J Clin Oncol. 2013;36(4):325–330. doi: 10.1097/COC.0b013e318246b4e9. [DOI] [PubMed] [Google Scholar]
- 17.Zhan HX, Xu JW, Wang L, Zhang GY, Hu SY. Lymph node ratio is an independent prognostic factor for patients after resection of pancreatic cancer. World J Surg Oncol. 2015;13:105. doi: 10.1186/s12957-015-0510-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger AC, Watson JC, Ross EA, Hoffman JP (2004) The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg 70 (3):235–240; discussion 240 [PubMed]
- 19.House MG, Gonen M, Jarnagin WR, D'Angelica M, DeMatteo RP, Fong Y, Brennan MF, Allen PJ. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11(11):1549–1555. doi: 10.1007/s11605-007-0243-7. [DOI] [PubMed] [Google Scholar]
- 20.Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, Wolfgang C, Hruban RH, Schulick RD, Yeo CJ, Choti MA. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141(5):610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Riediger H, Keck T, Wellner U, zur Hausen A, Adam U, Hopt UT, Makowiec F. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg. 2009;13(7):1337–1344. doi: 10.1007/s11605-009-0919-2. [DOI] [PubMed] [Google Scholar]
- 22.Robinson SM, Rahman A, Haugk B, French JJ, Manas DM, Jaques BC, Charnley RM, White SA. Metastatic lymph node ratio as an important prognostic factor in pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2012;38(4):333–339. doi: 10.1016/j.ejso.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS, Schulick RD, Choti MA, Lillemoe KD, Yeo CJ. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10(9):1199–1210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27(3):324–329. doi: 10.1007/s00268-002-6659-z. [DOI] [PubMed] [Google Scholar]
- 25.Havenga K, Enker WE, Norstein J, Moriya Y, Heald RJ, van Houwelingen HC, van de Velde CJ. Improved survival and local control after total mesorectal excision or D3 lymphadenectomy in the treatment of primary rectal cancer: an international analysis of 1411 patients. Eur J Surg Oncol. 1999;25(4):368–374. doi: 10.1053/ejso.1999.0659. [DOI] [PubMed] [Google Scholar]
- 26.Kodera Y, Schwarz RE, Nakao A. Extended lymph node dissection in gastric carcinoma: where do we stand after the Dutch and British randomized trials? J Am Coll Surg. 2002;195(6):855–864. doi: 10.1016/S1072-7515(02)01496-5. [DOI] [PubMed] [Google Scholar]
- 27.Pawlik TM, Abdalla EK, Barnett CC, Ahmad SA, Cleary KR, Vauthey JN, Lee JE, Evans DB, Pisters PW. Feasibility of a randomized trial of extended lymphadenectomy for pancreatic cancer. Arch Surg. 2005;140(6):584–589. doi: 10.1001/archsurg.140.6.584. [DOI] [PubMed] [Google Scholar]
- 28.Valsangkar NP, Bush DM, Michaelson JS, Ferrone CR, Wargo JA, Lillemoe KD, Fernandez-del Castillo C, Warshaw AL, Thayer SP. N0/N1, PNL, or LNR? The effect of lymph node number on accurate survival prediction in pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2013;17(2):257–266. doi: 10.1007/s11605-012-1974-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hellan M, Sun CL, Artinyan A, Mojica-Manosa P, Bhatia S, Ellenhorn JD, Kim J. The impact of lymph node number on survival in patients with lymph node-negative pancreatic cancer. Pancreas. 2008;37(1):19–24. doi: 10.1097/MPA.0b013e31816074c9. [DOI] [PubMed] [Google Scholar]
- 30.Slidell MB, Chang DC, Cameron JL, Wolfgang C, Herman JM, Schulick RD, Choti MA, Pawlik TM. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15(1):165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]