Abstract
Oncological interventions in thoracic cavity have some important problems such as choice of correct operative approaches depending on the tumor, size, extension, and location. In sarcoma surgery, wide resection should be aimed for the curative surgery. Purpose of this study was to evaluate pre-operative planning of patient-specific thoracic cavity model made by multidisciplinary surgeon team for complex tumor mass for oncological procedures. Patient’s scans showed a large mass encroaching on the mediastinum and heart, with erosion of the adjacent ribs and vertebral column. Individual model of this case with thoracic tumor was reconstructed from the DICOM file of the CT data. Surgical team including six interdisciplinary surgeons explained their surgical experience of the use of 3D life-size individual model for guiding surgical treatment. Before patients consented to surgery, each surgeon explained the surgical procedure and perioperative risks to her. A questionnaire was applied to 10 surgical residents to evaluate the 3D model’s perception. 3D model scans were useful in determining the site of the lesion, the exact size, extension, attachment to the surrounding structures such as lung, aorta, vertebral column, or vascular involvement, the number of involved ribs, whether the diaphragm was involved also in which order surgeons in the team enter the surgery. 3D model’s perception was detected statistical significance as < 0.05. Viewing thoracic cavity with tumor model was more efficient than CT imaging. This case was surgically difficult as it included vital structures such as the mediastinal vessels, aorta, ribs, sternum, and vertebral bodies. A difficult pathology for which 3D model has already been explored to assist anatomic visualization was mediastinal osteosarcoma of the chest wall, diaphragm, and the vertebral column. The study helped to establish safe surgical line wherever the healthy tissue was retained and enabled osteotomy of the affected spinal corpus vertically with posterior-anterior direction by preserving the spinal cord and the spinal nerves above and distal the tumor. 3D tumor model helps to transfer complex anatomical information to surgeons, provide guidance in the pre-operative planning stage, for intra-operative navigation and for surgical collaboration purposes. Total radical excision of the bone tumor and reconstructions of remaining structures using life-size model was the key for successful treatment and better outcomes. The recent explosion in popularity of 3D printing is a testament to the promise of this technology and its profound utility in orthopedic oncological surgery.
Keywords: Osteosarcoma; Thoracic neoplasms; 3D printing model; Preoperative planning; Personalized medicine; Orthopedic oncology, surgical specialties; Computer-aided design
Introduction
Primary tumors of the bony in mediastinal localization with extension to chest wall, abdomen, and vertebral column are uncommon, although a wide variety of both benign and malignant tumors arises within the chest wall [1–3]. Preoperative evaluation of patients is aimed at diagnosis and assessment of the extent of the tumor [4, 5]. The keys to successful treatment are early recognition and excision with adequate margins and immediate reconstruction because bone tumors are relatively resistant to radiotherapy and conventional cytotoxic chemotherapy [1–4, 6]. Complete excision with widely negative microscopic margins at the initial operation is of the utmost importance; local recurrence portends systemic metastasis and eventual tumor-related mortality [5, 7].
Oncological surgery for this case, wide resection of tumor, and reconstructions and fixation are highly demanding surgical procedures, consisting of thorax vessels, pulmo, heart, and spinal cord. Due to the tumor location, the presence of neurovascular structures and vital organs are high risk of bleeding, infection, and paralysis. Partial osteotomy of the affected spinal corpus should be performed vertically with posterior-anterior direction that is associated with possibility of compromising important motor and sensorial function of spinal cord. Unfortunately, it is correlated with loss of important functions mentioned above depending on the resection level of the spine and related spinal cord injury. Because it is unpredictable remained corpus after osteotomy of the spine, it is not known exactly the level and type of fixation and fusion of the spine.
Consequently, resection of huge tumor localized in thoracic cavity required patient-specific anatomical and pathological features. Imaging plays a major role in the diagnosis and characterization of thoracic lesions, with imaging appearance and radiological reporting outcomes being utilized to direct patient-specific treatment planning [8, 9]. Nevertheless, there is not enough data about this anatomical site to provide insight for clinical practice currently. 3D computed tomography (CT) has much improved imaging; complete understanding of the tumors behavior’s and affects is still difficult [10, 11]. Another problem is that, when manipulating vital organs, they are also being moved on the side that is often not visible during surgical procedure.
The development of imaging modalities and computer technology provides new approaches for surgical planning. The 3D printing technique has been adopted in oncosurgeons recently. It allows rapid construction of accurate, full-scale individual tumor model so that surgeons can observe, take measurements, and even practice surgery on the models [12, 13]. In recent years, with the development of digital medicine and imaging modalities, a 3D tumor model can be generated to plan the resection and instrumentation position of screws to adapt complex thoracic tumors [14, 15].
The newest developments in intraoperative stereotactic navigation have added to the accuracy and preciseness for addressing tumor surgery.
We hypothesize that using 3D models as a surgical cutting guide would significantly improve the surgical outcomes in planning for huge mediastinal tumor. They have been used to aid in localization of tumor borders, visualize operative margins, plan osteotomies, and restore postoperative structures in order to optimize surgical intervention. A fundamental aspect of surgical planning in large resections and osteotomies is the identification of mediastinal structures and spinal cord—roots to preserve healthy muscle, bone, spinal cord—roots and tissue while fully resecting the tumor. This creates significant cognitive load, especially for collaborators, who participates rarely in these difficult operations as it relies on image interpretation, anatomical and surgical knowledge, experience, and spatial sense.
The purpose of this study was to evaluate if creation of preoperative 3D patient-specific life-size osteosarcoma model will assist interprofessional level surgeons in making operative plans including its presentation, diagnostic tools, surgical guide, and surgical outcome.
Materials and Methods
A 17-year-old girl presented with a large swelling in her left thorax was transferred to our center. The patient underwent radiological investigations in the form of chest radiography and CT (Fig. 1). A large-sized mass lesion was observed in the hemorrhagic high-density areas extending to the paravertebral space of the left hemithorax, T12 and the left side of the posterior elements of affected vertebrae, filling the entire left lower lung lobe. The characteristic appearance was a well-defined, lobulated soft tissue mass with mediastinal, paravertebral, or thoracic cavity involvement. The preoperative diagnosis was a large sarcoma of the left chest wall possibly osteosarcoma.
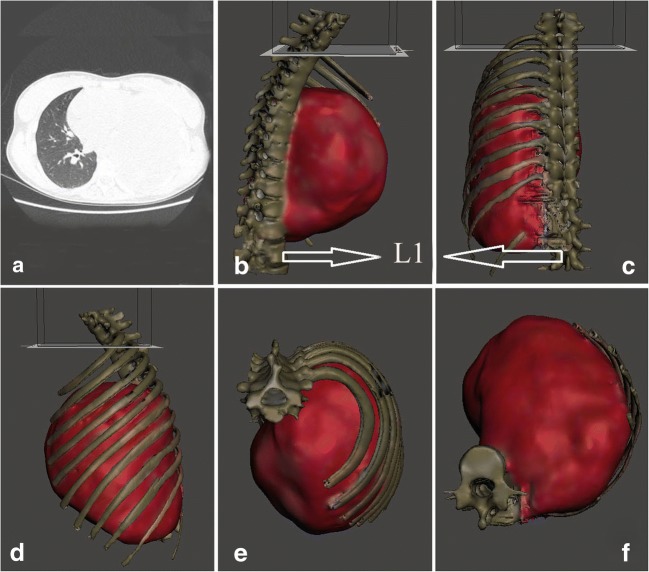
Fig. 1.
Computed tomography images of an irregular mass of high-density in the left mediastinum in a. Model tumor with standard tessellation language files in b, c, d, e and f, as tracking tumor at all angles
The study was approved by the suitably constituted Ethical Committee at Researches Department of Ege University (17-6/19), within which the work was undertaken, and the study conforms to the Declaration of Helsinki. The patient provided written informed consent.
The case that would require significantly different preoperative plans was based on key features identifiable in the preoperative CT imaging. After the consultation of multidisciplinary surgical team for malignancy, including the experts of oncologic thoracic, oncologic orthopedic, spine, flap general, and cardiovascular surgeons, it was decided to remove the huge tumor in one operation. A questionnaire was applied to 10 surgical residents to evaluate the 3D model’s perception. An Objective Structured Assessment of Technical Skills (OSATS) scale was used in the questionnaire to provide information about the competence and sensitivity of the anatomical model as a specialty training tool.
Pearson correlation coefficients and kappa statistics were used to assess the rating for OSATS questions. SPSS (18.0) program was used for statistical analysis and the threshold value for statistical significance was calculated as 0.05.
Results
Patient-Specific 3D Modeling
CT images (GE Discovery CT750 HD) were processed in DICOM (Digital Imaging and Communications in Medicine) format. The data images were processed and converted to a standard tessellation language (STL) file for printing (Fig. 1a–f). The mean time required for successful 3D reconstruction was 120 min. 3D model scans were useful in determining the site of the lesion, exact size, extension, attachment to the surrounding structures such as the lung, aorta, vertebral column, or vascular involvement, the number of involved ribs, and whether the diaphragm was involved. (Fig. 1b–f).
Printing of Individual Thorax with Osteosarcoma
Proposed tumor model and the thoracic cavity in relation to the topographic neighbors were printed with Mass Portal. The model took approximately 10 h to print. There was a high degree of correlation between STL file dimensions and measurements made on the 3D tumor model. We used the manipulated 3D image to print a patient-specific thoracic cavity model that has the same size (1:1 model) and the same geometric features as the patient’s thorax (Fig. 2a, b). The life-size 3D model of the tumors was essential to the success of the operation (Figs. 3, 4, 5, 6, 7, 8, and 9). With the help of the model, surgical cutting line of the osteotomy, the number of vertebra to conduct laminectomy, reconstructive regions to be cleared with was planned (Fig. 3). The preoperative planning and postoperative controlling of posterior instrumentation including screw positions, orientation, and number level of screws and intervals of screws were determined with 3D life-size models (Figs. 2, 3, and 8c, d).
Fig. 2.
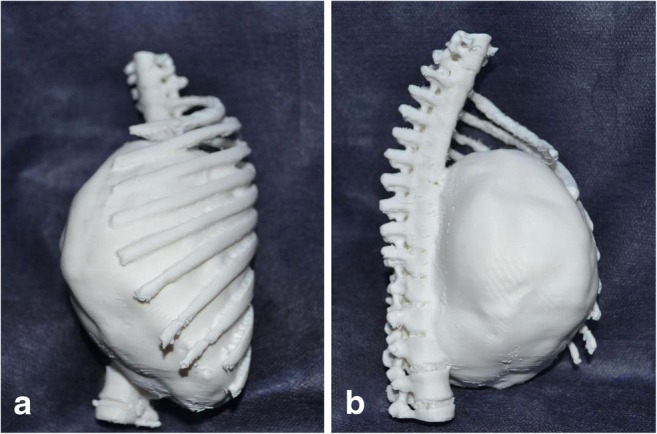
a, b Showing the site, size, and extension of the tumor as vertebral column, diaphragm, and ribs
Fig. 3.
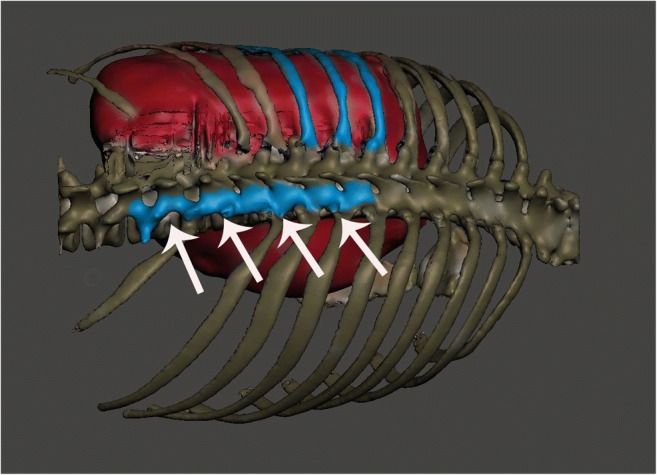
Multiplanar resection planning of the tumor through fused preoperative 3D reconstructive images and intra-operative paired-point matching. The blue areas indicate the planned resection planes and white arrows for paired-point matching and surface-point matching
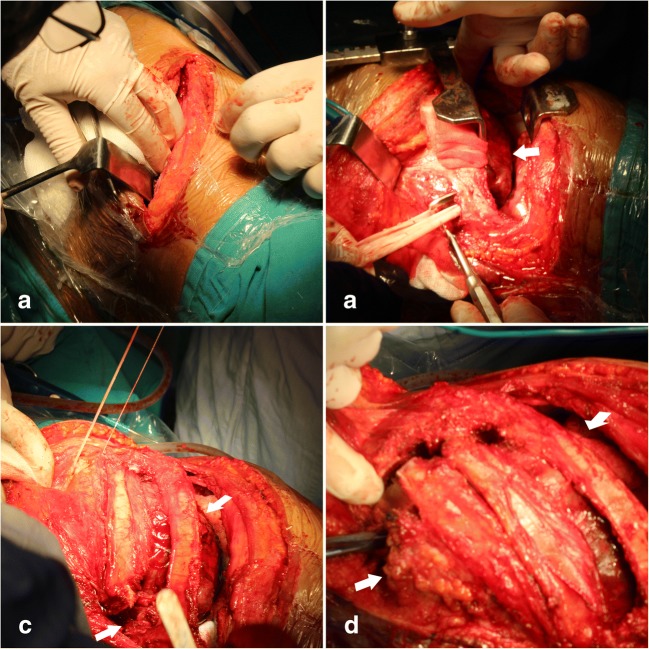
Fig. 4.
a–d Operative photographs show the tumor (white arrow) and its relationship with the surrounding structures
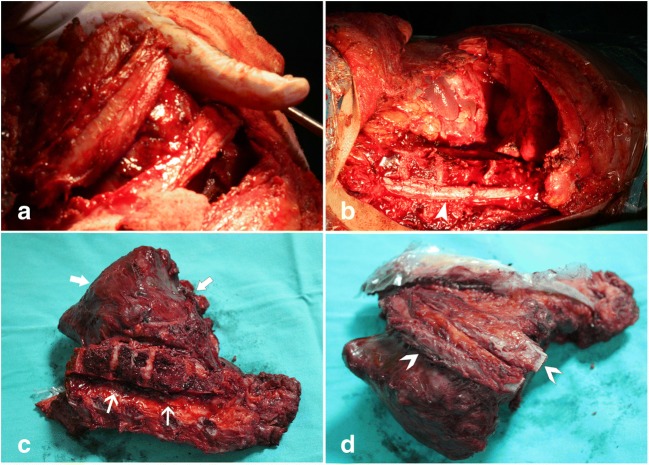
Fig. 5.
Application of partial osteotomy in a and b and tumor resection in c and d. ˃ribs, ►spinal cord, → tumor
Fig. 6.
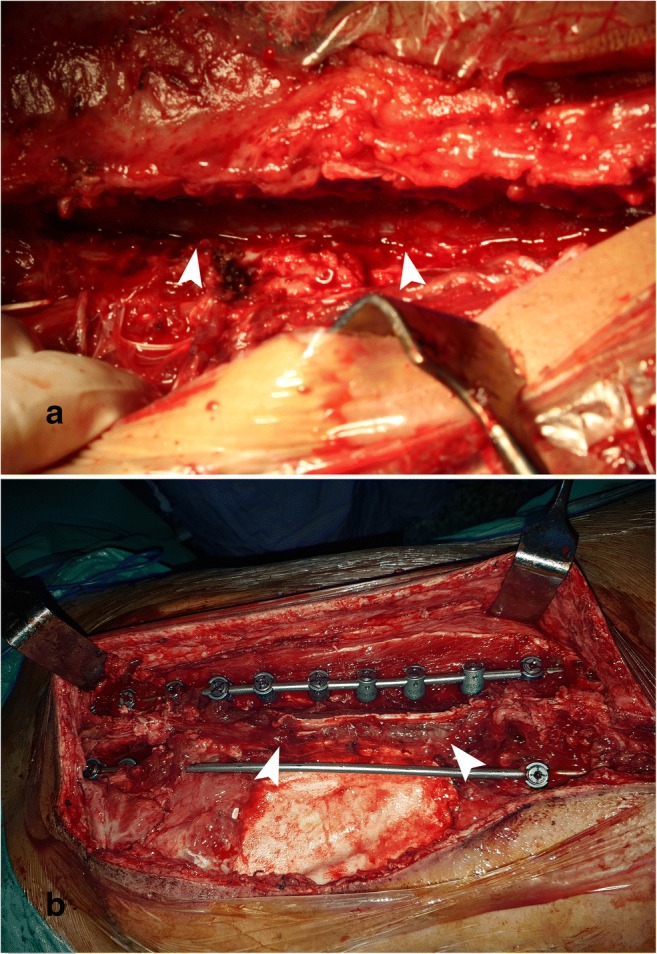
Intraoperative vascular control of the spinal cord (►) in a. Posterior instrumentation and undamaged spinal cord after laminectomy in b
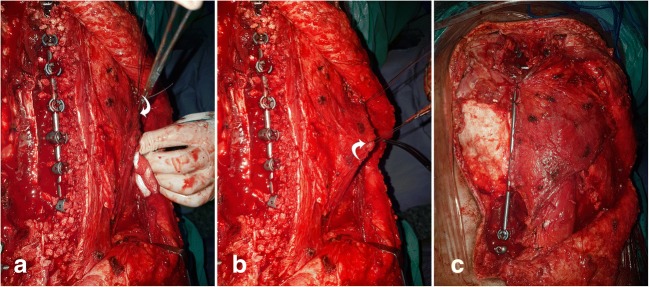
Fig. 7.
a–cThe residual defect after excision and latissimus dorsi flap (arrow) repair of the defect
Fig. 8.
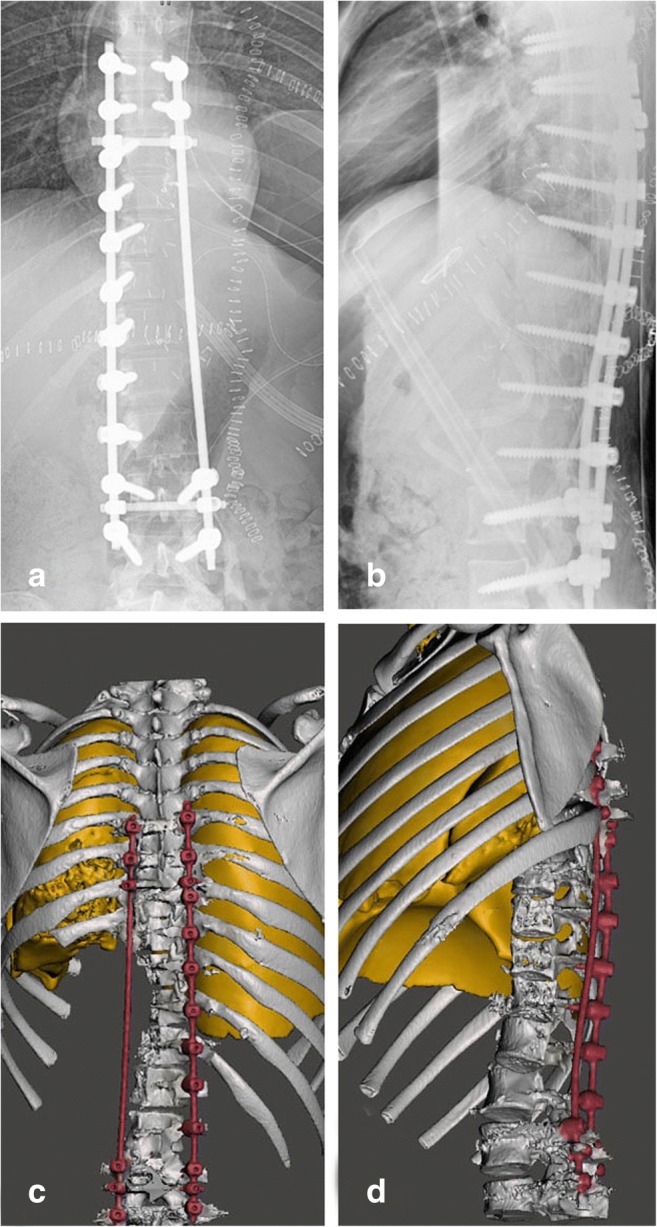
Postoperative controlling of posterior fixation including positions, locations, and numbers of screws and intervals of screws in radiograms in a, b and reconstructions demonstrate a complete thoracolumbal construct in c, d
Fig. 9.
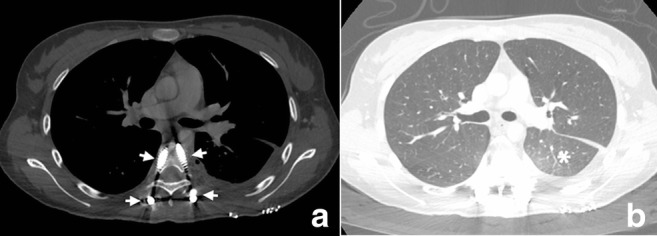
a, b Postoperative CT image demonstrating as screw position in pedicule (arrow) and new lobe ventilation (star)
Patient Positioning
Case was placed to in right lateral position on an operating table. Anatomical landmarks were selected on the skin surface and orientation of the entire procedure was provided. First, the thorax surgery team enters the surgery and the patients underwent a thoracotomy on the affected side with excision of the ribs of ex biopsy tracks (Figs. 3 and 4a–d). After printing the 3D model, surgical approaches were evaluated by rehearsal, and the lateral thoracotomy was finally selected rather than median sternotomy. In the intercostal space, a 20 cm incision was made on the posterior axillary incision line extending from the line to the back (Fig. 4a). Open thoracotomy under controlled thorax under thorax.
Oncologic Surgery
During surgery, the tumor mass in the chest was found to be large and it involved most of the anterolateral chest wall (Fig. 4 b, c). It was separated with a sharp and blunt dissection from the lung, the lateral portion of the chest wall, site of mediastinal adhesion diaphragm, and 8–12 ribs (Fig. 4d). By the cardiovascular surgeon team, the left segmental intercostal arteries (T8-L1) of the aorta in the close proximity of the tumor tissue were ligated and cut and removed from the mass (Fig. 5a–d). After the orthopedic spine and oncology surgeon team, since the tumor was on the left side of the thoracic cavity, osteotomy and laminectomy were performed on the right side of the T8-L1 (Fig. 5b, c). After laminectomy, the spinal cord was revealed and nerve roots were ligated on left side (T8-T12) and 1/3 corpus of the spine was ostetomized vertically with posterior-anterior direction on affected left side (Figs. 5b and 6a). Using combined sharp and blunt dissection, the mass was resected completely (Fig. 5b–d). Wide resection of the tumor included the ribs immediately above and below, the adjacent muscles (with at least a 4-cm safety margin) and the underlying pleura, diaphragm and vertebral column to prevent the local recurrence.
The border of the tumor was checked regularly to prevent and minimize the neurovascular damage (Figs. 5b and 6a). Nerve roots were also checked during the intervention in case of neuromotor nerve damage. The left roots emerging from the spinal cord between T8 and T12 were ligated. Tumor mass was removed safely without damaging the neighboring nerves (Fig. 4b, c).
The serratus anterior muscle was partially taken. The histopathology report showed the axillary lymph nodes to be tumor-free. Once the thoracic cavity, abdomen, and spinal canal were exposed, depending on the type of resection (intraregional, wide resection, and partial osteotomies), the margins of the desired resection were marked for tumor removal (Figs. 4, 5, and 6). Case of malignant neoplasms of bone and articular cartilage has taken the diagnosis of osteosarcoma (05.16.2018).
Reconstructive Surgery
When resection of the tumor was completed, the patient’s retroperitoneum, abdomen, thorax, and spinal cavities should be opened. First, diaphragmatic defect was closed with a 10 × 15 PTFE. The thorax defect was closed with a 15 × 20 PTFE graft. The thorax wall graft was attached to the spinal muscles at the back. The spinal muscles and thorax muscles were approached to a great extent. The skin flap between the vertebral sulcus and the thoracotomy line was appropriately closed.
Posterior Instrumentation and Flap Surgery
The position of the patient changed to prone after closure of the thorax and abdomen. The second step was performed with posterior instrumentation and closure of the big operative defect (the implants, spinal cord, fusion area) with the flap (Figs. 6b and 7a–c). By the orthopedic spine surgeon team, posterior instrumentation was performed on right side between T8 and L2 since after the osteotomy, the left side pedicles are removed together with the mass. At the T6, T7, L3, L4 level, pedicle screws were put bilaterally so that the rigidity of the posterior instrumentation is provided. The bone grafts were put on the right side for fusion. All pedicular screws were put both with the 3D model and scopy guidances.
Depending on the residual defect after resection, latissimus dorsi fleb were used for closure of the spine and the instrumentations. With a pedicled latissimus dorsi flap because the case was wide enough to provide adequate coverage, in the same operating region (opened spinal cord, instrumentation, and fusion area). There was no additional donor site morbidity, no need for microsurgical anastomosis, and no longer need for prolonged surgery. There was no infection or void leakage. The left chest wall mass was an intramedullary osteosarcoma, most probably of rib origin.
Post-operative Period
No operative complications were reported in the case. Patient stays one night in intensive care. Chest exercise started immediately after the operation. Chest tube taken 3 days after the operation. On the third day after the operation, the surgical wound was examined and pressure dressing was re-applied. Any wound complications were identified and recorded. Neurological signs were re-evaluated. Plain radiograph and CT after the operation was carried out to determine the osteotomy site (Fig. 8a–d). All stitches were removed at the end of the second week after the operation. Physical examination including neurological examination of the lower limbs, plain radiograph of chest, and instrumented vertebrae were carried out at each follow-up. During the first years after the operation, the patient was followed up every 3 months. At last control (postoperative 6 months), there was no problem at surgical site and neurologic status. Postoperative chest computed tomography, radiography, and bone scintigraphy showed no evidence of a metastatic disease (Figs. 8a, b and 9a, b).
Surgical intervention was found in all stages of the responsibility of the most experienced surgeon in sarcoma (DS). Surgical team head evaluated surgical assets of personalized 3D models, postoperatively.
The 3D model of the surgery during the planning of the whole team can easily bring the tumor in 3D. With 2D images, it has not been easy for anyone. Team members’ expectations from each other were much easier to understand. Thoracic, cardiovascular, general surgeon, spine, oncologic, and flap surgeons were involved in this study.
Benefits of Modeling
3D model’s perception was detected statistical significance as < 0.05.
*Primer osteosarcoma spreads to the posterior wall of chest wall, involves the chest wall by direct invasion from intrathoracic cavity, diaphragm, and vertebral column. The patient had expanding masses in different dimensions in the vertebral column, thoracic cavity, and abdomen.
*The records of the case were reviewed with regard to symptoms, location, and diameter of the tumor, tumor grade, and extent of invasion, evidence of distant metastases, surgery, and methods of reconstruction, time and site of recurrence, mortality, and subsequent follow-up. All details concerning the extent of surgical resection were carefully documented.
*3D model has been used to aid complex oncological surgery planning and operating individual cases with complicated situations, which has achieved satisfying results by making operation safer and easier, showing a great potential in the era of precision medicine.
*Study team has planned the course of the surgery collaboratively together with the surgeon team. They considered 3D models to be useful in assisting interprofessional collaboration. One participant commented on the model’s ability to allow for a better appreciation of the tumor’s anatomical orientation in space within the thoracic cavity.
*Team surgeons believed that the 3D personalized model allowed for a better perception of information related to structural depth and spatial relationships of the tumor when compared to the corresponding 3D reconstructed image provided.
*Both surgeons believed that 3D life-size model would be useful in the process of identifying a safe surgical pathway, in addition to intraoperative navigation and orientation in this complex case. It was also suggested that the actual-size model might be a useful supplementary tool for surgical team when learning how to interpret multi-planar medical images.
*The 3D model allows easy visualization of the location and size of the tumor as well as identifying the relationship of the tumor to key anatomical structures such as the aorta, diaphragm, heart, lung, vertebral column, and thoracic roots. Specifically, pre-operative 3D thoracic mass models could potentially promote total resection, laminectomy-osteotomy sparing surgery, and preservation of healthy tissue, as surgeons gain a better understanding of the size and location of a tumor in relation to normal tissue and oncological mass (Figs. 3, 4, and 5).
*In the field of mediastinal oncological surgery, the limit of resectable length has always been the biggest obstacle. The team of surgeons focuses more on the reconstruction rather than destruction.
*That is, the surgical team successfully worked in complicated onco-surgical even as three body cavities are opened at the same time, the tumor was removed before disintegration, and the closure procedure was successfully performed. The assets of personalized 3D models were evaluated postoperatively.
*It is also enabled to designate safe excisions and osteotomy corridor while preserving the neurovascular structures and the spinal cord. Personalized models also revealed the course of the coroner and sagittal plane angles that can extend within dissection and bone osteotomy border. This provided right and safe guidance and anatomical landmarks for the intervention.
*Without the use of 3D modeling, there would not be a guiding method, so the operation would last longer and there would be more blood loss and severe complications.
Since the surgical team will not be so selective, it would be a surgical procedure to experience the trouble like walking in the cloudy water.
*Spinal cord would not have been protected and the patient would have suffered from paraplegia. With the help of 3D modeling, preserving the vertebral column and thoracic cavity in this intervention avoided restraints and provided a lifetime comfort to the patient in work and social life.
*Models with life size tactile and visual characteristics offer multisensory inputs that can enrich and aid in spatial cognition and learning. This is especially true in the context of a complex tumor involved with highly variable anatomy. It is an innovative navigational tool allowing a convenient surgery to the surgeon while preserving the life quality of the patient. This study presents our first clinical experience within a huge tumor of a patient whom we have applied 3D model navigation-assisted cancer surgery.
*The surgical algorithm was to print the tumor with thoracic cavity model first, then perform onco-surgical procedures in planning, and finally conduct onco-surgical procedures; this was time-consuming, and radiation-free.
*Creating a 3D model requires close collaboration between the anatomist and onco-surgeons. Discussing the 3D thoracic model with the whole team improved our understanding of the structure damage. It provided a moment of reflection and the possibility to discuss suitable approaches and apply options.
Discussion
The surgical key to successful treatment of primary chest wall tumors remains with early diagnosis and complete surgical resection [9, 14]. Better survival has been documented in patients with complete resection of their tumor at the time of initial surgery [16, 17]. Wide resection of all thoracic tumors with appropriate margins, reconstruction of the bony chest wall to ensure preservation of respiratory mechanics, and soft tissue coverage of the reconstructive screws with healthy vascularized tissue are the treatment of choice [6, 18]. Resection of all sarcomas should include all of the involved soft tissue or bone and a normal tissue on all sides [7, 8, 19]. For neoplasms of the rib cage, this includes the removal of the involved ribs as 8–11 (Figs. 1c–f, 2a, b, 4d, and 5a, d) and several partial ribs above and below the neoplasm (Fig. 4c, d).
Complex resection is a procedure with the potential to influence quality of life drastically [15]. It is difficult to predict the association between thoracic nerve roots and the residual function due to several factors. These factors include pre-surgical neurological status (disease may have damaged the roots prior to the surgery), the presence of variable anastomoses above the roots, onset of post-operative complications, collateral damage, personal motivation during rehabilitation, and time spent for the follow-up (Figs. 1, 2, 3, 4, 5, 6, 7, 8, and 9).
In this study, we tested 3D model assisted with surgical procedure assessment for preoperative planning of complex osteosarcoma excision in thorax. Options for excision and reconstruction of the thoracic cavity include extensive surgical approaches with the possibility of morbidities such as infection, blood loss, and wound complications and spinal problems.
The great consistence of the cutting line guides, screw positions, and orientations applied in the actual surgery with the pre-operation designs and the efficient restoration of the remaining tissue provide convincing evidence that 3D printing techniques in preoperative planning allows for oncological surgery management providing shorter, less invasive, more precise, and more reliable surgeries [9, 19–21].
The 3D tumor model allows rapid and accurate construction of a full-scale individual model, which can facilitate the visualization of the patient pattern and complex thorax anatomy prior to the surgery. Surgeons with 3D modeling team can determine appropriate surgical incision line and approach. This life-size model has been successfully used in oncological surgery as they improve the surgeon’s efficiency, shorten surgical duration, and reduce iatrogenic complications. In this study, we have experienced pre-operative design of multidisciplinary approach of a complex case with the positions and orientations of the thoracic tumors.
In the case of huge osteosarcoma in the setting of prior posterior and inferior of chest wall with extension to diaphragm and vertebral column, the personalized thorax model enabled the selection of a targeted approach with the extension of the prior lateral thoracotomy to assess and excise the three cavities foci of tumor (Fig. 4a, b) with adequate margins, while reducing perioperative morbidity (Figs. 1, 2, 3, 4, 5, 6, 7, 8, and 9).
This study suggests that in tumor case, physical models of the anatomy produced by 3D printing may assist surgeons in visualizing the anatomy, and thus providing a net benefit for surgical planning. However, only a single study to date has systematically evaluated the utility of 3D printed thoracic cavity models in assisting surgical planning for complex oncologic cases.
The most powerful aspect of this study is that each surgical discipline can plan and perform its own work in the best way with a successful multidisciplinary surgical approach. By means of the 3D model, we have been informed in advance which fields need to be entered.
By offering a greater understanding of spatial relationships prior to the initiation of a surgical procedure, this technology may assist the surgeon to select the best surgical approach, identify high-risk areas, anticipate complications, and the need for consultation of other surgical specialties. The mainstay of our research findings is the nuances in treating this mediastinal tumor with patient-specific model guided navigation to achieve optimal oncological resection, to reduce neurological complications, and to minimize recurrence. [9, 14, 15]. Although point-to-point measurements were not different, 3D personalized models increased the understanding of shape, scale, and anatomy [15–21]. It enabled understanding significantly faster than other media. In difficult surgical cases such as the present case with complex anatomy and a need for efficient multidisciplinary coordination, 3D life-size models should be considered for surgical planning [22–26].
Surgical Outcome
In this sarcoma case surgery, removal of tumor mass without extensive excision is essential. This case was difficult because of the close proximity of the tumor mass to the main neurovascular structure, contact with the heart, lung, aorta and vertebrae, its large size, having high postoperative morbidity, mortality, and complications. On completion of tumor removal, the patient had to undergo progressive surgery oncologic thoracic, oncologic orthopedic, cardiovascular, general, orthopedic spine, and flap surgery, respectively. It is useful for crowded surgical branch physicians to decide and act together, in which sequence of operations, to start, continue, and terminate the interference algorithm.In addition, each branch was useful in terms of evaluating the complications and solutions that might occur in planning their own surgeries. It was also helpful to know how to resolve the closure of remaining defects after the mass removal of the team.
The 3D technology was superior to the current standard of care in assisting surgeons with visualizing the anatomy for surgical planning. Six interdisciplinary collaboration surgical team in identifying complex cases that could benefit from this technology may enhance the expert care provided to patients.
Conclusion
3D model-assisted surgery represents a safe and helpful tool for resection of mediastinal osteosarcoma and may influence surgical treatment plans in selected cases to enable resections that are more limited.
This technique can significantly improve the outcome of cancer surgery via providing a better pre-operation plan, and a collaborating platform for surgical teams to understand the surgical procedures completely.
Acknowledgments
Special thanks to Prof. Dr. Cemil Caliskan MD, Department of General Surgery and Dr. Serkan Ertugay MD, Department of Cardiovascular Surgery Faculty of Medicine, Ege University for sincere efforts and assistances.
Compliance with Ethical Standards
The study was approved by the suitably constituted Ethical Committee at Researches Department of Ege University (17-6/19), within which the work was undertaken, and the study conforms to the Declaration of Helsinki. The patient provided written informed consent.
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arndt CA, Rose PS, Folpe AL, Laack NN. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc. 2012;87(5):475–487. doi: 10.1016/j.mayocp.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gladish GW, Sabloff BM, Munden RF, Truong MT, Erasmus JJ, Chasen MH. Primary thoracic sarcomas. Radiographics. 2002;22(3):621–637. doi: 10.1148/radiographics.22.3.g02ma17621. [DOI] [PubMed] [Google Scholar]
- 3.Rocca M, Salone M, Galletti S, Balladelli A, Vanel D, Briccoli A. The role of imaging for the surgeon in primary malignant bone tumors of the chest wall. Eur J Radiol. 2013;82(12):2070–2075. doi: 10.1016/j.ejrad.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Athanassiadi K, Kalavrouziotis G, Rondogianni D, Loutsidis A, Hatzimichalis A, Bellenis I. Primary chest wall tumors: early and long-term results of surgical treatment. Eur J Cardiothorac Surg. 2001;19(5):589–593. doi: 10.1016/S1010-7940(01)00655-8. [DOI] [PubMed] [Google Scholar]
- 5.Hung JJ, Chou TY, Sun CH, Liu JS, Hsu WH. Primary synovial sarcoma of the posterior chest wall. Ann Thorac Surg. 2008;85(6):2120–2122. doi: 10.1016/j.athoracsur.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Anderson BO, Burt ME. Chest wall neoplasms and their management. Ann Thorac Surg. 1994;58(6):1774–1781. doi: 10.1016/0003-4975(94)91691-8. [DOI] [PubMed] [Google Scholar]
- 7.Krauel L, Fenollosa F, Riaza L, Pérez M, Tarrado X, Morale A, Gomà J, Mora J. Use of 3D prototypes for complex surgical oncologic cases. World J Surg. 2016;40(4):889–894. doi: 10.1007/s00268-015-3295-y. [DOI] [PubMed] [Google Scholar]
- 8.Kurenov SN, Ionita C, Sammons D, Demmy TL. Three dimensional printing to facilitate anatomic study, device development, simulation, and planning in thoracic surgery. J Thorac Cardiovasc Surg. 2015;149(4):973–979. doi: 10.1016/j.jtcvs.2014.12.059. [DOI] [PubMed] [Google Scholar]
- 9.Braham E, Aloui S, Aouadi S, Drira I, Kilani T, El Mezni F. Synovial sarcoma of the chest wall: a case report and literature review. Ann Transl Med. 2013;1(1):9. doi: 10.3978/j.issn.2305-5839.2013.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillaspie EA, Matsumoto JS, Morris NE, Downey RJ, Shen KR, Allen MS, Blackmon SH. From 3-dimensional printing to 5-dimensional printing: enhancing thoracic surgical planning and resection of complex tumors. Ann Thorac Surg. 2016;101(5):1958–1962. doi: 10.1016/j.athoracsur.2015.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Govsa F, Karakas AB, Ozer MA, Eraslan C. Development of life-size patient-specific 3D- printed dural venous models for preoperative planning. World Neurosurg. 2018;110:e141–e149. doi: 10.1016/j.wneu.2017.10.119. [DOI] [PubMed] [Google Scholar]
- 12.Cromeens BP, Ray WC, Hoehne B, Abayneh F, Adler B, Besner GE. Facilitating surgeon understanding of complex anatomy using a three-dimensional printed model. J Surg Res. 2017;216:18–25. doi: 10.1016/j.jss.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Hoang D, Perrault D, Stevanovic M, Ghiassi A. Surgical applications of three-dimensional printing: a review of the current literature & how to get started. Ann Transl Med. 2016;4(23):456. doi: 10.21037/atm.2016.12.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhury SK, Subbarao KS, Nachiappan M, Agrawal K. Primary neoplasm of the chest wall: surgical management. Asian Cardiovasc Thorac Ann. 2000;8:249–252. doi: 10.1177/021849230000800313. [DOI] [Google Scholar]
- 15.Cakir O, Topal U, Bayram AS, Tolunay S. Sarcomas: rare primary malignant tumors of the thorax. Diagn Interv Radiol. 2005;11(1):23–27. [PubMed] [Google Scholar]
- 16.Walsh GL, Davis BM, Swisher SG, Vaporciyan AA, Smythe WR, Willis-Merriman K, Roth JA, Jr, Putnam JB. A single-institutional, multidisciplinary approach to primary sarcomas involving the chest wall requiring full-thickness resections. J Thorac Cardiovasc Surg. 2001;121(1):48–60. doi: 10.1067/mtc.2001.111381. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Cao T, Li X, Huang L. Three-dimensional printing titanium ribs for complex reconstruction after extensive posterolateral chest wall resection in lung cancer. J Thorac Cardiovasc Surg. 2016;152(1):e5–e7. doi: 10.1016/j.jtcvs.2016.02.064. [DOI] [PubMed] [Google Scholar]
- 18.Staats K, Panotopoulos J, Tiefenboeck TM, Windhager R, Funovics PT. Computer navigation-assisted surgery for musculoskeletal tumors: a closer look into the learning curve. Eur J Orthop Surg Traumatol. 2017;27(6):851–858. doi: 10.1007/s00590-017-2004-y. [DOI] [PubMed] [Google Scholar]
- 19.Novoa N, Benito P, Jime’nez MF, de Juan A, Luis Aranda J, Varela G. Reconstruction of chest wall defects after resection of large neoplasms: ten-year experience. Interact Cardiovasc Thorac Surg. 2005;4(3):250–255. doi: 10.1510/icvts.2004.103432. [DOI] [PubMed] [Google Scholar]
- 20.George E, Liacouras P, Rybicki FJ, Mitsouras D. Measuring and establishing the accuracy and reproducibility of 3D-printed medical models. Radiographics. 2017;37(5):1424–1450. doi: 10.1148/rg.2017160165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto JS, Morris JM, Rose PS. 3-dimensional printed anatomic models as planning aids in complex oncology surgery. JAMA Oncol. 2016;2(9):1121–1122. doi: 10.1001/jamaoncol.2016.2469. [DOI] [PubMed] [Google Scholar]
- 22.George E, Barile M, Tang A, Wiesel O, Coppolino A, Giannopoulos A, Mentzer S, Jaklitsch M, Hunsaker A, Mitsouras D. Utility and reproducibility of 3-dimensional printed models in pre-operative planning of complex thoracic tumors. J Surg Oncol. 2017;116(3):407–415. doi: 10.1002/jso.24684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannopoulos A, Steigner M, George E, Barile M, Hunsaker AR, Rybicki FJ, Mitsouras D. Cardiothoracic applications of 3D printing. J Thorac Imaging. 2016;31(5):253–272. doi: 10.1097/RTI.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MP, Ta AH, Ellsworth WA, Marco RA, Gaur P, Miller JS. Three dimensional model for surgical planning in resection of thoracic tumors. Int J Surg Case Rep. 2015;16:127–129. doi: 10.1016/j.ijscr.2015.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Govsa F, Ozer MA, Biceroglu H, Karakas AB, Cagli S, Eraslan C, Alagoz AK. Creation of 3D life size: patient specific C1 fracture models for screw fixation. World Neurosurg. 2018;114:e173–e181. doi: 10.1016/j.wneu.2018.02.131. [DOI] [PubMed] [Google Scholar]
- 26.Andolfi C, Plana A, Kania P, Banerjee PP, Small S. Usefulness of three-dimensional modeling in surgical planning, resident training, and patient education. J Laparoendosc Adv Surg Tech A. 2017;27(5):512–515. doi: 10.1089/lap.2016.0421. [DOI] [PubMed] [Google Scholar]