Abstract
Peritoneal metastases may occur from a majority of cancers that occur within the abdomen or pelvis. When cancer spread to the peritoneal surfaces is documented, a decision regarding palliation versus an aggressive approach using cytoreductive surgery (CRS) and hyperthermic perioperative intraperitoneal chemotherapy (HIPEC) must be made. This decision is dependent on a well-defined group of prognostic indicators. In addition to treatment, prevention of peritoneal metastases may be an option. The clinical and pathologic features of a primary cancer can be used to select perioperative treatments that may prevent cancer cells within the abdomen and pelvis from progressing to established peritoneal metastases. In some clinical situations with appendiceal and colorectal cancers, the clinical or histopathologic features may indicate that second-look surgery plus perioperative chemotherapy should occur. Peritoneal metastases should always be considered by the multidisciplinary team for treatment or prevention.
Keywords: Peritoneal metastases, Appendiceal cancer, Malignant peritoneal mesothelioma, Colon cancer, Recurrent ovarian cancer, Gastric cancer, Cancer prevention
Introduction
An increasing concern for improved management of peritoneal dissemination and local recurrence of cancers that occur within the abdomen and pelvis has been expressed by both surgeons and medical oncologists. This condition was, in the past, regarded as a universally fatal manifestation of cancer dissemination. It has been associated with early death and a miserable quality of life in those patients manifesting peritoneal dissemination and the progression of peritoneal metastases. In the past 30 years, a marked conceptual change in the possibilities to prevent or treat peritoneal metastases has occurred. Currently, management strategies for this condition from a large number of abdominal and pelvic cancers exist. It has become imperative for the multidisciplinary team (MDT) to consider options for prevention and treatment of peritoneal metastases. Table 1 lists the primary and recurrent cancers that must have special attention by the MDT if peritoneal metastases occur [1–6]. Systemic chemotherapy alone is not optimal management of selected patients with peritoneal metastases; also sometimes peritoneal metastases can be prevented.
Table 1.
Diseases with peritoneal metastases that may be recommended by the multidisciplinary team for definitive treatment of selected patients by cytoreductive surgery and intraperitoneal chemotherapy
| Disease | Reference |
|---|---|
| Appendiceal cancer | Sugarbaker [1] |
| Malignant peritoneal mesothelioma | Sugarbaker et al. [2] |
| Colon cancer | [3] |
| Ovarian cancer | Tewari et al. [4] |
| Gastric cancer | Sugarbaker and Kwong [5] |
| Unusual abdominal/pelvic malignancies with dissemination limited to peritoneal surfaces | Goere et al. [6] |
Treatment of Peritoneal Metastases Has Been Defined as an Oncologic Necessity
Why is it that a large number of world opinion leaders in gastrointestinal cancer and gynecologic malignancy have focused such great time and effort on peritoneal dissemination? Carcinomatosis has been a diagnosis treated by palliation for many decades. It is important to identify the stimulus for concerted efforts to improve the management of peritoneal metastases. I suggest that the origins for this new attitude had two beginnings. First, clinical research showed that the dissemination of cancer on peritoneal surfaces and at the surgical resection site was a terrible ongoing problem in gastrointestinal and gynecologic oncology. Something needed to be done. Chu and colleagues at the University of Arkansas in 1989 published a prospective study on peritoneal carcinomatosis from nongynecologic malignancy [7]. They studied 100 patients with peritoneal metastases including colorectal cancer (45), pancreas cancer (20), gastric cancer (6), and a variety of other less common causes of peritoneal metastases. The mean survival of patients with colorectal metastases was 8.5 months, pancreas cancer 2.4 months, and gastric cancer 2.2 months. Chu and coworkers pointed out that these patients developed severe adverse symptoms such as bowel obstruction in over half of their patients, perforated viscus, enterocutaneous fistula, and debilitating ascites. Surgical procedures to alleviate these conditions were uniformly unsuccessful. The only prognostic factor that was associated with a disease-free survival following surgery was the disease-free interval (p = 0.04).
Another pioneer investigating the prognosis of patients with peritoneal carcinomatosis from nongynecologic malignancy was Sadeghi and coworkers. They presented the results of a French multicenter prospective study known as EVOCAPE 1 [8]. Again, the survival of patients with peritoneal metastases was very limited. Gastric cancer patients showed a mean survival of 6.5 months, colorectal carcinoma patients a mean survival of 6.9 months, and pancreatic carcinoma patients a mean survival of 2.9 months. Sadeghi and colleagues quantitated the extent of carcinomatosis in a staging system that assessed both the size and distribution of “malignant granulations.” This staging system for the total of 370 patients treated significantly was associated with survival (p = 0.001). Those patients who had liver metastases in addition to peritoneal dissemination had a reduced prognosis (p = 0.0009).
Jayne and colleagues at Singapore General Hospital in 2002 had 349 patients (13%) in their database identified as patients with peritoneal metastases [9]. Two hundred fourteen patients had synchronous disease and 135 had metachronous carcinomatosis. The survival of these patients was limited with a median survival of patients with synchronous disease of 7 months and a median survival for patients with metachronous carcinomatosis of 28 months from the initial diagnosis of colorectal cancer. These authors again noted that the extent of disease was a significant factor in predicting survival (p = 0.009). In the multivariate analysis, cancer differentiation and presence versus absence of liver metastases were not significant clinical predictors of survival.
Pharmacologic Data from Intraperitoneal Administration of Anticancer Drugs Showed the Potential for Control of Small Peritoneal Nodules and a Reduced Systemic Toxicity
The rationale for the use of chemotherapy administration directly into the peritoneal space may have emanated from pharmacologic research in chronic ambulatory peritoneal dialysis [10]. The efficacy of this novel method for intraperitoneal drug delivery for peritoneal metastases patients has been slow to develop. Karnofsky and colleagues in 1948 used nitrogen mustard for the palliative treatment of carcinomatous ascites. The efficacy was such that FDA approval of nitrogen mustard for intraperitoneal administration was granted and remains in effect until this day [11]. However, the rationale for intraperitoneal chemotherapy administration came from pharmacologic research in patients who had cancer spread to peritoneal surfaces. It was recognized that some drugs would be especially appropriate for prolonged retention within the peritoneal space based on their molecular structure [12]. It was Dedrick and colleagues at the American National Institutes of Health who called attention to the potential benefits of intraperitoneal chemotherapy administration of cancer chemotherapy agents especially in ovarian cancer [13]. The studies of Speyer and colleagues clearly identified 5-fluorouracil as an agent with high concentrations within the peritoneal space after intraperitoneal administration as compared to drug levels within the plasma [14]. The rapid metabolism of the 5-fluorouracil after absorption of this drug by the visceral peritoneum within the liver parenchyma resulted in a markedly enhanced exposure of cancer nodules on peritoneal surfaces [15]. Jones and colleagues recognized that a high volume of intraperitoneal chemotherapy solution (belly bath technique) was necessary to adequately distribute the drugs [16]. Ozols and colleagues investigated the pharmacokinetics of doxorubicin and McVee and colleagues the possible benefits of intraperitoneal cisplatin [17, 18]. With continued efforts to identify drugs appropriate for intraperitoneal chemotherapy administration, an extended list of possible chemotherapy agents and their pharmacologic advantage following intraperitoneal administration has been defined [19].
Because of a large molecular size and hydrophobic surface, cancer chemotherapy agents were shown to have a slow clearance from the peritoneal compartment through the lining of the abdomen and pelvis to the body compartment. Also, metabolism of the cancer chemotherapy in the body compartment was at all points in time faster than clearance from the peritoneal space. This resulted in a much greater concentration times time (area under the curve) of the drug in the peritoneal space as compared to concentration times time measured in the blood. This results in an increased therapeutic effect on cancer nodules on the peritoneal surface and a reduced systemic toxicity. Figure 1 shows a pharmacokinetic study of the cancer chemotherapy agent, mitomycin C, administered into the peritoneal space [20].
Fig. 1.
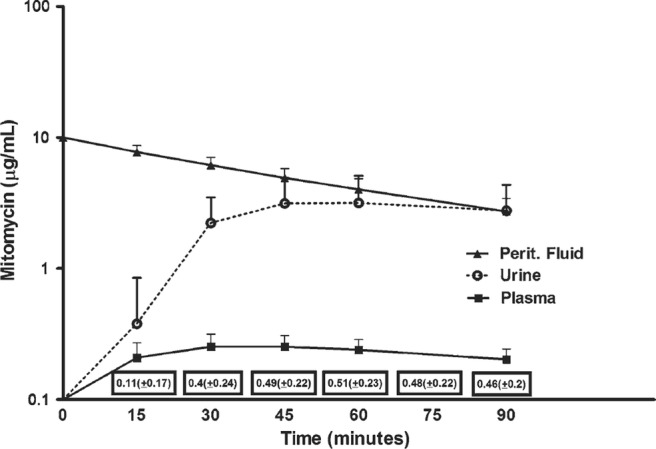
Pharmacokinetic study of mitomycin C
Augmentation of Intraperitoneal Chemotherapy Cytotoxicity by Moderate Heat
The effects of intraperitoneal heat by itself were shown by Shiu and Fortner in an experimental animal to have a potential for application as an adjunct to cancer surgery [21]. It had been shown many times in the past that cancer chemotherapy could be augmented by heat [22]. However, it was left to Spratt and colleagues to first combine intraperitoneal chemotherapy with intraperitoneal heat in an attempt to maximize the control of peritoneal metastases as part of a treatment plan for gastrointestinal and gynecologic malignancy [23]. In 1980, these efforts in Louisville, Kentucky, by Spratt and colleagues were the first applications of hyperthermic intraperitoneal chemotherapy (HIPEC). In summary, the modern approach to the prevention and treatment of peritoneal metastases arises out of a well-described oncologic need for treatment of this condition. Favorable pharmacologic studies suggested increased responses of peritoneal nodules combined with reduced systemic toxicity following intraperitoneal chemotherapy delivery. The application of heat within the peritoneal space along with a large volume of chemotherapy solution would increase the uniformity of treatment and augment its efficacy throughout the peritoneal space.
Prevention of Peritoneal Metastases as an Initial Successful Application of HIPEC
The case report by Spratt and colleagues regarding the use of HIPEC did not gain attention within the USA or Europe. It was the Japanese under the direction of Koga at Totori University who first recognized the potential application of HIPEC for the prevention of peritoneal metastases in patients with gastric cancer. Koga and colleagues introduced a new Japanese drug, mitomycin C, as the chemotherapy agent to be instilled in a large volume of fluid to prevent or to treat peritoneal metastases. They took this concept to the laboratory and demonstrated that heat alone could reduce the progression of an intraperitoneal rat ascites hepatoma; mitomycin C alone was also capable of improving survival in this rat model. However, far and away, the longest median survivals of rats inoculated with an intraperitoneal tumor were those who were treated by mitomycin C with the presence of 41.5 °C heat for 60 min [24].
The group at Totori University in 1988 reported on a study of prophylaxis for peritoneal recurrence of gastric cancer using HIPEC with mitomycin C. In a group of patients matched to historical controls (n = 38) and in a randomized controlled study of 47 patients, there was an improvement in survival in those patients receiving HIPEC mitomycin C. The results, because of small numbers, were of borderline significance. These investigators noted that anastomotic leak, postoperative ileus, and possible chemical peritonitis were not induced by HIPEC mitomycin C. Their conclusion was that their results demonstrated a simple, safe, and readily applicable prophylactic therapy for peritoneal recurrence in patients with serosal positive gastric cancer [25]. Studies at Kanazawa University by Yonemura and colleagues and studies in Chiba, Japan, by Fujimoto and colleagues showed positive results with HIPEC mitomycin C or HIPEC mitomycin C plus cisplatin for the prevention of peritoneal recurrence of gastric cancer. These were positive randomized controlled studies [26, 27]. To the great credit of these early investigators, a systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resected gastric cancer shows benefit. Yan and colleagues in 2007 found 13 reports of randomized controlled trials on adjuvant intraperitoneal chemotherapy for resected gastric cancer for appraisal and data analysis. Ten reports were judged to be of fair quality and subjected to their meta-analysis. There was a significant improvement in survival associated with HIPEC with a hazard ratio of 0.60 (95% CI = 0.43 to 0.83, p = 0.002) [28]. Recently, Feingold et al. performed a systematic review and random effect analysis to analyze current literature regarding the role of adjuvant intraperitoneal chemotherapy [29]. One of the randomized controlled trials using HIPEC in patients at risk for gastric cancer peritoneal metastases was reported by Yonemura et al. [30]. There were improved outcomes with HIPEC with a 5-year overall survival of 61% for surgery plus HIPEC versus 42% for surgery alone. This was significant with a p value of 0.019. By multivariate analysis, the relative risk for surgery alone was 3.075 with 95% confidence interval of 1.483 to 6.422. This remains the single most important study confirming HIPEC for adjuvant treatment of resected gastric cancer. Currently, a Western randomized controlled trial, GastriCHIP, is accruing patients [31].
Prevention of Peritoneal Metastases with Colon Cancer
Recent improvements in the surgical technology of colorectal cancer resection have decreased the incidence of treatment failures, both at the resection site or at a distance from the primary. The benefits of total mesorectal excision have been established and the survival benefit published [32, 33]. This survival advantage has been a result of the absence of tumor contamination within the confines of the pelvis because of a meticulous dissection which maintains a layer of tissue between the primary malignancy and the margins of resection [33]. Also, the benefits of colon cancer resection using wide excision, generous lymphadenectomy, and an intact mesocolic resection have been demonstrated [34]. These improvements in surgical technology and therefore in survival are the result of decreased tumor cell contamination resulting from the surgical event itself. A complete absence of tumor cell contamination with primary colorectal cancer surgery has become an absolute requirement of treatment. Any dependence upon systemic chemotherapy to manage resection site disease or peritoneal metastases must be abandoned.
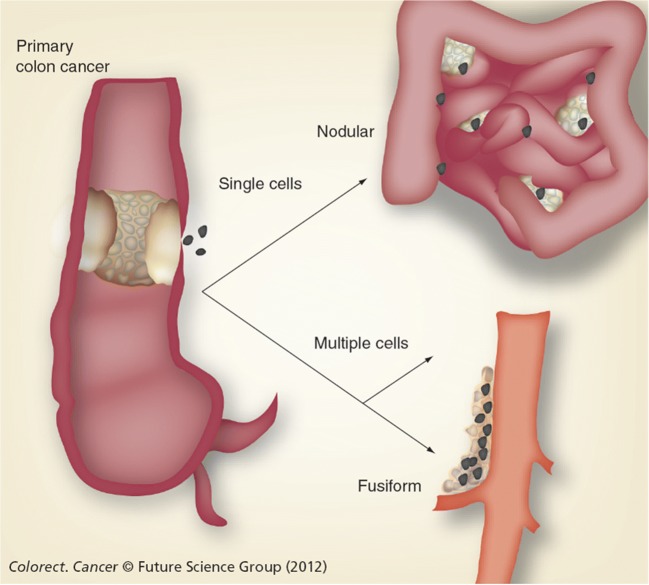
It is important to establish that the mechanism of resection site recurrence and peritoneal metastases is the same. Cancer cells are disseminated either prior to or at the time of the cancer resection. The cancer cells at high density will layer out within the bed of the resection site. Because the surgery has disrupted the peritoneum and created a “sticky surface,” a high metastatic efficiency is expected. Single cells disseminated at a distance from the anatomic site of primary cancer resection will progress as peritoneal metastases [35]. Figure 2 illustrates anatomic sites of right colon cancer progression by the dissemination of cancer cells or minute cancer nodules at the time of surgery. The mechanism whereby cancer cells recur within the abdominal incision, within the resection site along the superior mesenteric vessels, or on peritoneal surfaces is similar.
Fig. 2.
Anatomic sites of right colon cancer progression
In approximately 20% of primary colorectal cancer patients, there are clinical findings present at the time of primary cancer resection that indicate that there is a high likelihood of intraperitoneal cancer cell dissemination [36]. These clinical findings indicate that the primary colorectal cancer surgery, even performed in its most perfect manner with or without systemic chemotherapy, is not a sufficient management strategy. These patients at special risk for local–regional recurrence and peritoneal metastases are listed in Table 2. In groups 1–4 in Table 2, patients can be considered to have 50–100% incidence of local–regional recurrence and/or peritoneal metastases in the absence of special treatments. Peritoneal metastases discovered and resected at the time of primary colorectal cancer resection will show progression with follow-up in 75% of patients. This occurs even if these metastases are completely removed with the primary intervention [37]. Ovarian metastases have over 60% incidence of other sites of peritoneal dissemination in follow-up. Perforation through the primary cancer at the time of primary cancer resection and a positive margin of resection, usually a lateral margin, indicates a likelihood of local–regional or peritoneal progression in 30 and near 100% of patients, respectively.
Table 2.
Patients with primary colorectal cancer identified to be at high risk for local–regional recurrence and/or peritoneal metastases. Groups 1–10 are candidates for prophylactic HIPEC or EPIC as part of the primary colorectal cancer resection. Groups 1–4 are candidates for proactive second-look surgery
| 1. Visible evidence of peritoneal metastases | |
| 2. Ovarian cysts showing adenocarcinoma suggested to be of gastrointestinal origin | |
| 3. Perforated cancer | |
| 4. Positive margins of excision | |
| 5. Positive cytology either before or after cancer resection | |
| 6. Adjacent organ involvement of cancer-induced fistula | |
| 7. T3 mucinous cancer | |
| 8. T4 cancer or positive “imprint cytology” of the primary cancer | |
| 9. Cancer mass ruptured with the excision | |
| 10. Obstructed cancer |
The other clinical findings (nos. 5–10 listed in Table 2) have been shown to place the patient at a lesser risk for local–regional recurrence or peritoneal metastases. Positive peritoneal cytology either before or after colorectal cancer resection, adjacent organ involvement or a cancer-induced fistula, T3 mucinous cancers, T4 cancers or a positive imprint cytology from the primary malignancy, rupture of the cancerous mass, or obstruction at the time of presentation all would have an elevated incidence of local–regional recurrence and peritoneal metastases.
Data Showing Benefit from Perioperative Chemotherapy in Patients with Primary Colorectal Cancer with Peritoneal Seeding or at High Risk for Peritoneal Seeding
Local–regional recurrence and peritoneal metastases occupy a prominent role in the natural history of gastrointestinal cancer. Intraperitoneal chemotherapy used as a planned part of a surgical intervention to control local–regional recurrence and peritoneal dissemination from colorectal cancer was proposed by Sugarbaker and colleagues [38–40]. In a phase I/II study, 5-fluorouracil and mitomycin C were administered directly into the peritoneal cavities in the early postoperative period before adhesions had progressed. There was a marked pharmacokinetic advantage of perioperative intraperitoneal chemotherapy with single cancer cells on peritoneal surfaces as the targets of this treatment [41].
Experience with patients demonstrating peritoneal metastases recognized at the time of primary colon cancer resection came from Washington, DC, and was reported by Pestieau and Sugarbaker [42]. They identified five patients who had definitive treatment of peritoneal metastases from colon cancer concomitant with the resection of the primary tumor. At the time of writing this paper, the median disease-free survival of these patients had not been reached and their 5-year survival was 100%. The statistical difference between patients who had perioperative treatment of their peritoneal metastases as compared to those who had delayed management with cytoreductive surgery and early postoperative intraperitoneal chemotherapy (EPIC) was statistically significant (p < 0.0001).
Tentes has reported their experience on the use of hyperthermic perioperative chemotherapy in patients at high risk for local–regional recurrence. These were patients with locally advanced T3 or T4 colorectal cancer. Only patients with R-0 resection were randomly assigned to receive HIPEC plus systemic chemotherapy versus conventional treatments. The 5-year survival for the HIPEC group was 100% and 72% for the conventional group (p = 0.0938). During follow-up, two patients in the HIPEC group and eight patients in the conventional group were recorded with recurrence (p = 0.002). It is important to note that no local–regional recurrence or peritoneal metastases was recorded in the HIPEC group. By contrast, the group treated in a conventional manner showed three patients with local–regional recurrence. These data suggest that perioperative chemotherapy had no effect on the development of distant metastases but exhibited an advantage in eradicating viable cancer cells that were disseminated local–regionally at the time or prior to the colorectal cancer resection [43].
Noura and colleagues reported on colorectal cancer patients with a positive peritoneal lavage cytology. Thirty-one of 52 patients with positive cytology were treated by mitomycin C instillation through catheters after abdominal closure. Patients receiving perioperative chemotherapy had a significantly improved survival rate (p < 0.05). In a multivariate analysis, perioperative chemotherapy remained an independent prognostic factor for peritoneal recurrence-free survival [44].
Braam and colleagues reported on a total of 72 patients with synchronous peritoneal metastases from colorectal cancer. In 20 patients (27.8%), the primary tumor was resected simultaneously with HIPEC (early referral). In the other 52 patients (72.2%), the primary tumor was resected prior to a reoperative surgery with HIPEC (late referral). During CRS plus HIPEC following late referral, 22 (59.5%) of the 37 anastomoses of the earlier operation were resected, revealing malignancy in 12 patients (54.5%). In 20 late referral (27.8%) patients, a permanent colostomy was constructed after HIPEC. The relaparotomy rate was higher in patients after a resection of a previous anastomosis (36.4%) compared to 12% in the rest of the patients (p = 0.02). Resection of the primary tumor simultaneously with HIPEC in patients with synchronous peritoneal metastases from colorectal cancer may prevent extended bowel resections and permanent colostomy. These data support early referral of patients with peritoneal metastases from colorectal cancer [45].
Sammartino and colleagues studied colon cancer patients with clinical T3/T4, any N, M0 stage, and mucinous histology or signet ring histology [46]. Twenty-five patients in the experimental group underwent carcinomatosis prevention strategies including complete omentectomy, bilateral salpingo-oophorectomy, hepatic round ligament resection, and appendectomy. At the end of the colorectal cancer resection plus carcinomatosis prevention resections, hyperthermic intraperitoneal chemotherapy using intraperitoneal oxaliplatin with intravenous fluorouracil was administered. These experimental patients were compared with 50 matched controlled patients. All patients had an R-0 resection. The morbidity of the two groups of patients was the same. At 48 months, after the study ended, fewer patients in the proactive group than in the control group had recurrent disease (28 versus 42%). Peritoneal metastases and local recurrence developed significantly less often in the proactive group than in the control group (4 versus 28%, p < 0.03). Median survival was 59.5 months among the patients included in the proactive treatment and 52 months in the control group. The disease-free survival in the two groups was different with p < 0.04. The overall survival in the two groups was different with p < 0.03.
To date, the optimal perioperative chemotherapy treatment for prevention of local–regional recurrence and peritoneal metastases has not been determined. It is possible that the best choice is the early postoperative intraperitoneal chemotherapy. This was used by Pestieau and Sugarbaker to achieve good results [42]. Also, in the prevention of peritoneal metastases in gastric cancer, EPIC was shown by Yu et al. to be very successful in a prospective randomized controlled study [47]. From a logistical perspective, EPIC may be favored in that patients with unexpected peritoneal metastases who have not signed an informed consent for HIPEC can be treated with full consent in the early postoperative period. It is possible that a single dose of intraoperative chemotherapy (HIPEC) is not as effective as the 5-day intraperitoneal lavage used postoperatively (EPIC). However, EPIC has been shown to be associated with a higher incidence of adverse events but not a higher incidence of mortality [48].
Morbidity/Mortality for the Prevention of Colon Cancer Peritoneal Metastases by HIPEC
Of course, a new initiative for the comprehensive management of peritoneal metastases should not be implemented without strong evidence that it does not add to the complications that occur in this group of patients. In the 80 randomized patients presented by Tentes, there was one in-hospital mortality in the HIPEC group and three in the conventionally treated group. There was a 32% morbidity in the HIPEC group and a 22% morbidity in the conventionally treated group. The incidence of complications was statistically and significantly higher in the HIPEC group with a p value less than 0.05 [43]. In the manuscript presented by Sammartino and colleagues, there was a 4% combined grade III and IV toxicity. In the control group, there was an 8% incidence of grade III and IV adverse events. There were no deaths in either group [46]. In a recent review of morbidity/mortality in colorectal and appendiceal patients who have had extensive cytoreduction combined with perioperative chemotherapy, Sugarbaker and colleagues showed a 0.6% mortality and a 12% grade IV morbidity [49]. These data taken together suggest that once the learning curve has been ascended in patients who have cytoreductive surgery combined with perioperative chemotherapy, the morbidity and mortality compares favorably and is perhaps even lower than in patients who undergo advanced surgery for gastrointestinal malignancy.
Establishing Perioperative Chemotherapy as a Standard of Care for Selected Patients
Prior reviews of proactive management of primary colorectal cancer to eradicate minimal residual disease in the perioperative period have been published [50, 51]. To bring these concepts into the standard of care, there are three randomized controlled trials active in Europe to test the efficacy of HIPEC in patients with colon cancer at high risk for the progression of peritoneal metastases. The COLOPEC trial from the Netherlands uses clinical features to select patients for prophylactic HIPEC with oxaliplatin [52]. The PROMENADE trial emanating from Rome, Italy, uses CT to identify locally advanced colon cancers [53]. These patients are then randomized to either receive or not receive HIPEC. The prophylactic trial from Spain identifies patients with T4 colon cancer and randomizes them to receive HIPEC as a part of their primary resection of colon or rectal cancer (Arjona Sanchez A, Prophylactic HIPEC for cT4 colon cancer, personal communication).
Current Data Regarding Benefits Expected with Proactive Second-Look Surgery
In patients treated for primary CRC in institutions where cytoreductive surgery and HIPEC are not available, a second strategy for proactive management of patients at high risk for progression of peritoneal metastases must be formulated. The inclusion criteria for the patients included in this clinical pathway are those listed in Table 2. Patients in groups 1–4 are those who may be recommended for a repeat surgical intervention (proactive second-look surgery) if a high likelihood of long-term survival as a result of optimal treatment is expected. Patients in the high-risk groups 5–10 need to be carefully monitored; laparoscopy rather than laparotomy may be recommended for a planned second-look intervention.
In the USA, a long history of efforts to use second-look surgery to improve the survival rate of colorectal cancer patients has been accumulated in the surgical literature. Griffen and colleagues first organized a planned approach of reoperative surgery in asymptomatic gastrointestinal cancer patients [54]. Minton and colleagues published data on second-look surgery suggesting that it should be initiated by patients’ symptoms (symptomatic second-look) or a progressive increase in serial carcinoembryonic antigen (CEA) assays obtained in follow-up [55].
The concept of second-look surgery and its application has been recently reviewed [56]. Two important modifications in the second-look strategy have occurred. First, patients selected for a repeat intervention in the absence of signs or symptoms of progressive disease are those patients listed in groups 1–4 of Table 2. The second important modification is that this second look would be combined with cytoreductive surgery and HIPEC.
The evaluation of this revised strategy for the use of second-look surgery must be prospective and thorough. The primary endpoint for the study is the percentage of patients who have a positive second look with an R-0 resection and as a result of the repeat surgical intervention enjoy long-term survival. To use Wangensteen terminology, these are patients “converted” from disease documented at the time of second-look surgery to a 5-year survival [54]. A second endpoint would be the percentage of patients who had a negative second-look. This would provide an estimate of patients who had “unnecessary surgery” as result of the elective reintervention. Of course, a third endpoint would be a comprehensive morbidity and mortality assessment of both positive and negative second-look procedures.
Elias and colleagues from Villejuif, France, published their experience with second-look surgery for colorectal cancer patients at high risk for progression [57]. This was a highly selected group of patients who had biopsy-proven peritoneal metastases, ovarian peritoneal metastases, or perforation confirmed at the time of primary colorectal cancer resection. The second-look surgery was performed within 1 year after the first surgery and after the completion of systemic adjuvant chemotherapy. The patients treated by Elias were asymptomatic with a completely negative workup. The authors detected additional peritoneal metastases in 63% of patients who had synchronous peritoneal metastases, 75% of patients with ovarian metastases, and 33% of patients with a perforated primary tumor. Patients with macroscopic peritoneal metastases were treated with cytoreductive surgery plus HIPEC with no mortality, a low morbidity, and a 2-year disease-free survival rate exceeding 50%. Patients without macroscopic peritoneal metastases received prophylactic peritoneal metastases surgery with or without HIPEC. It is interesting to note that, in this subgroup with no macroscopic peritoneal metastases, 17% who received HIPEC showed recurrence versus 43% showed recurrence who did not receive HIPEC.
Delhorme et al. from Strasbourg have published data on a mandatory second-look surgery for the treatment of histologically confirmed peritoneal metastases present with the primary colon cancer resection. At the time of their proactive second-look surgery, 71% of patients were found to have persistent or progressive disease and the median peritoneal carcinomatosis index was 10. There was no postoperative mortality and there was a 7% incidence of grade III/IV complications. The 2-year overall survival and disease-free survival rates were 91 and 38%, respectively. Following proactive second-look surgery with HIPEC, peritoneal recurrence was observed in only 8% of patients versus 100% of the patients treated in a standardized fashion [58].
Patients with colon or rectal cancer are not only at risk for tumor cell entrapment. After a potentially curative resection of a pancreas cancer, disease recurrence has been recorded in the local and regional area in 50% of patients and on peritoneal surfaces in 40–60% of patients [59]. Also, in gastric cancer patients who did not have optimal surgical resection of the primary disease, 54% will progress with peritoneal metastases [5]. In colorectal cancer, the local and regional failure rate is less frequent but still exists in around 30% of those patients who do have successful surgical treatment [37].
Surgical Treatment Strategies for Peritoneal Metastases Diagnosed in Follow-Up
There are multiple reasons why the surgery to resect peritoneal metastases must be as complete as possible. Data from all studies thus far clearly establish that the outcomes of treatment are dependent on a complete cytoreduction. There are some exceptions to this rule but they are few. Complete visible removal of all abdominal and pelvic tumor is necessary because of limited penetration of the intraperitoneal chemotherapy solution. Estimates of one to several cell layers have been published. Gross nodules will only respond minimally or not at all. However, single cancer cells or minute nodules may be eradicated by an effective chemotherapy regimen. In order for the treatment of established peritoneal metastases to be treated, a new surgical technology was needed. That new surgical intervention was the peritonectomy procedure [60].
Peritonectomy
In order to make a transition between preoperative intraperitoneal chemotherapy for prevention to treatment, a surgical technology to reduce the extent of abdominal and peritoneal cancer to a microscopic level was necessary. This requirement was necessary because of the very limited penetration of intraperitoneal chemotherapy [17, 18, 61]. The invention of the peritonectomy procedures was the necessary link between success with prevention and success with treatment of established peritoneal metastases [60]. When peritonectomy procedures are combined with visceral resections, a complete visible clearing of the abdomen and pelvis is possible in selected patients. Peritonectomy procedures include right upper quadrant peritonectomy, left upper quadrant peritonectomy, pelvic peritonectomy, lesser omentectomy with omental bursectomy, and anterior parietal peritonectomy. Normal appearing peritoneal surfaces are not resected. Visceral resections include greater omentectomy–splenectomy, right colectomy, rectosigmoid colon resection, and occasionally partial gastrectomy. Again, only structures coated by disease are resected. Perioperative chemotherapy occurs after the cytoreductive surgery and usually precedes bowel reconstruction and closure of the abdomen.
Rationale for a Combined Treatment for Peritoneal Metastases Utilizing CRS plus Perioperative Chemotherapy
Success in the control of peritoneal metastases from gastrointestinal or ovarian cancer never occurred if CRS alone or intraperitoneal chemotherapy alone were used separately. Success was first recognized when the CRS with peritonectomy was combined with perioperative intraperitoneal chemotherapy as a planned surgical procedure [62]. In resecting abdominal or pelvic deposits of cancer using peritonectomy in a patient with known peritoneal metastases, contamination of the dissected surfaces is unavoidable. This combination of cancer surgery and resection site plus peritoneal progression of disease has been called “tumor cell entrapment” [63]. Interruption of contamination of the surgical resection sites with cancer cells requires that these implants be destroyed prior to their entrapment within the scar tissue that is part of the healing process. Sugarbaker et al. hypothesized that attempts to eliminate cancer cells from peritoneal surfaces were limited chemotherapy lavage administered within the first postoperative week [38]. These treatments would then occur before fibrosis sets in as part of the healing of the surfaces of the abdomen and pelvis. In an ideal situation, in order to prevent entrapment of cancer cells within tissues that are sutured together, the chemotherapy solutions must be used in the operating room after the cytoreduction but prior to making an intestinal anastomosis and prior to the closing of the abdominal wall [64].
The simultaneous use of cancer chemotherapy and heat strongly contributes to the control of peritoneal metastases. The heat significantly increases the cytotoxicity of a select number of chemotherapy agents [22]. Also, the hyperthermia should always be applied while the chemotherapy is present within the peritoneal space. Knowledge of the proper length of time for HIPEC requires a comprehension of the pharmacologic parameters established for the intraperitoneal administration of the chemotherapy agent.
Quantitative Prognostic Indicators for Knowledgeable Patient Selection
There can be no doubt that definitive treatment of peritoneal metastases is a major intervention involving a large commitment from the patient, his/her family, and health-care providers. In order to avoid a major intervention with limited benefit, knowledgeable patient selection is essential. This selection process includes a histologic assessment; review of chest, abdomen, and pelvic CT for concerning radiologic features; peritoneal cancer index (PCI) assessment at the time of abdominal exploration; and determination of the completeness of cytoreduction score (CC score) at the completion of the cancer resection [65]. In patients who have had prior surgery to resect peritoneal metastases, the prior surgical score (PSS) needs to be considered [65].
The principles of management to select patients were initially described for use with appendiceal and colorectal peritoneal metastases patients [62]. However, they apply not only to appendiceal and colorectal malignancy, but they have been used to more knowledgeably select patients with colorectal cancer [66], gastric cancer [67], ovarian cancer [68], and mesothelioma [69, 70]. A validation of these quantitative prognostic indicators in a large number of malignancies with peritoneal metastases allows knowledgeable selection of patients with rare abdominal and pelvic neoplasms that have peritoneal metastases for successful treatment by CRS and perioperative chemotherapy.
Histologic Criteria
The grade of a malignancy is not usually considered in the TNM staging of a malignancy. However, the invasive versus noninvasive character of peritoneal metastases is an estimate of the biology of the cancerous process. In appendiceal malignancy, malignant peritoneal mesothelioma, and ovarian cancer peritoneal dissemination, there is a wide variation in the biologic aggressiveness of the peritoneal dissemination. A minimally invasive cancer will be more effectively removed by peritonectomy with less involvement of subperitoneal lymphatics. With more complete CRS, the more effective perioperative chemotherapy will be in the eradication of microscopic residual disease. Even though the low-grade appendiceal malignancy may be extensive, with complete CRS, the prognosis is excellent [71]. Likewise, cystic mesothelioma may be of huge proportions and yet have long-term disease-free status expected after CRS plus HIPEC [72].
For gastric cancer and colorectal cancer, the histologic parameters are often less meaningful as a prognostic variable. However, at the extreme upper grades of malignancy, the prognosis with CRS and perioperative chemotherapy is guarded. For appendiceal cancer, poorly differentiated and signet ring carcinoma has a markedly reduced outcome with treatment [73].
Not only is there a larger risk of incomplete cytoreduction and more invasion of subperitoneal lymphatics with high-grade malignancy, the incidence of cancer progression at other sites such as liver metastases and retroperitoneal or pelvic lymph node metastases is greater. With the high-grade cancers, sometimes the peritoneal metastases may be controlled, but the patient succumbs to disease beyond the peritoneal surfaces.
As might be expected, other sites of metastatic disease in addition to peritoneal metastases confer a reduced prognosis after CRS and perioperative chemotherapy. For appendiceal malignancy of peritoneal mucinous carcinoma grade, lymph node involvement caused a small reduction in long-term survival. Sugarbaker’s data on 967 appendiceal malignancy patients with peritoneal metastases showed 50% overall survival at 10 years in the absence of lymph node metastases and 25% when lymph nodes were involved (p = 0.003) [69]. For 156 colon cancer patients with complete cytoreduction, 50% showed overall survival at 5 years in the absence of lymph node metastases and 15% when lymph nodes were involved (p = 0.03) [64]. In patients with colorectal peritoneal metastases, limited resectable liver metastases are sometimes not an absolute contraindication to treatment with CRS and HIPEC. Elias et al. in 24 patients with colorectal peritoneal metastases reported a 5-year overall survival in the absence of liver metastases of 41.5% and if liver metastases were present 23.6% [74]. These data may be interpreted to show that other sites of metastatic disease reduce the prognosis when CRS and HIPEC are used to treat peritoneal metastases. However, they are not considered an absolute contraindication to an attempt at curative treatment.
Concerning Radiologic Features Seen on Preoperative CT
At the consensus conference for management of peritoneal metastases held in Milan in 2006, CT was declared the standard preoperative radiologic test from which the selection of patients for CRS plus perioperative chemotherapy should occur [75]. Other radiologic studies such as MRI or PET would be used to supplement CT of the chest, abdomen, and pelvis. The contribution of CT to patient selection is limited by the high false negative percent reported when small peritoneal implants are imaged [76, 77]. If nodules are 0.5 cm or less, 72% of nodules are not detected. If the size of the implant is between 0.5 and 5.0 cm, the false negative percentage is 28%. Nodules greater than 5 cm are reliably imaged with a false negative percentage of 10% [76]. In all size categories, the false positive percentage was low. With CT detection of peritoneal metastases, if nodules are identified, they are very likely to indicate peritoneal involvement at that anatomic site. If nodules are not imaged, many small nodules are not ruled out.
Recently, another strategy for interpreting CT findings on peritoneal metastases patients uses the concerning CT features [78]. A series of 15 CT images describing a particular abdominal or pelvic pathology entity was described by Sugarbaker et al. In none of these 15 CT features existed, complete cytoreduction should be the outcome. If a single concerning radiologic feature was identified, the cytoreductive surgery should be more extensive and considered a greater risk for adverse events. If two concerning radiologic features are present, complete cytoreduction is unlikely [79–81]. Yan et al. published this concept for malignant peritoneal mesothelioma [80], Jacquet for mucinous appendiceal and colorectal cancer [79], and Rivard et al. for colorectal cancer [81].
Suidan and colleagues retrospectively reviewed abdominal and pelvic CT on 669 patients with ovarian cancer [82]. Their goal was to radiologically identify preoperative features that would suggest a suboptimal result for CRS. In the multivariate analysis, suprarenal retroperitoneal lymph nodes > 1 cm (p < 0.001); diffuse small bowel adhesions/thickening (p < 0.001); and lesions > 1 cm in the small bowel mesentery (p = 0.03), root of the superior mesenteric artery (p = 0.003), perisplenic area (p < 0.001), and lesser sac (p < 0.001) correlated with suboptimal cytoreduction. These authors suggested neoadjuvant chemotherapy should be used prior to cytoreductive surgery in patients with these concerning CT features.
Peritoneal Cancer Index
The PCI provides an estimate of the extent of peritoneal metastases present within the abdomen or pelvis. It is determined at the time of complete abdominal and pelvic exploration in the early phases of a cytoreductive surgery. Both the distribution and size of peritoneal nodules are used to determine PCI. For many different diseases with peritoneal dissemination, the extent of disease as determined by PCI has a profound effect on prognosis in patients treated by CRS and perioperative chemotherapy. This has been shown to be true for appendiceal cancer [71], colorectal cancer [66], ovarian cancer [68], sarcoma, and malignant peritoneal mesothelioma. For colorectal cancer, peritoneal metastases treated in patients with a PCI ≤ 10 are expected to show a 50% long-term benefit from treatment [66]. Patients with a PCI > 20 rarely achieve more than palliative benefit [83]. In contrast, patients with a low biologic grade of malignancy (similar to a low-grade appendiceal mucinous neoplasm) can benefit if the CRS is complete despite a very high PCI. For example, patients who had an appendiceal mucinous neoplasm with adenomucinosis and PCI > 20 have a 10-year survival of 50% [71].
Completeness of Cytoreduction Score
The most important prognostic variable is the completeness of cytoreduction score (CC score). A complete cytoreduction (CC-0) is greatly preferred and indicates that no visible tumor remains at the completion of the cytoreductive surgery. CC-1 indicates residual tumor nodules less than 2.5 mm. A CC-2 score indicates residual tumor nodules between 2.5 and 5 mm. A CC-3 score indicates residual tumor nodules greater than 5 mm or a layering of malignancy on peritoneal surfaces with few exceptions [65]. Until more effective perioperative treatments become available, to select a patient for elective CRS and HIPEC, the end result of the surgery should be predicted to be a complete CRS.
However, even if the cytoreduction is complete (CC-0 or CC-1), the extent of disease recorded by the PCI has a profound effect on prognosis after CRS and HIPEC. Aggressive treatment with CRS and HIPEC is restricted to those with a PCI of ≤ 20 if the malignancy is high grade.
Some exceptions to the statement that the only CRS of high value is a CC-0 or CC-1 may exist. Ovarian cancer and peritoneal mesothelioma frequently have bowel and bowel mesentery involved with multiple small cancer nodules that cannot be reduced to CC-0/CC-1 status. Using peritonectomy procedures and visceral resections, all other sites of disease can be resected to no visible evidence of disease. These patients with a limited extent of CC-2 residual disease can be treated by CRS and perioperative chemotherapy with prolonged benefit [84, 85]. Also, pseudomyxoma peritonei patients with CC-2 cytoreduction may profit from CRS plus perioperative chemotherapy [86]. For gastric cancer, colorectal cancer and peritoneal mucinous carcinoma from appendiceal cancer, a CC-0 cytoreduction must be the goal for CRS.
Prior Surgical Score
Less emphasis has been placed on prior surgical score than on other prognostic variables. However, from a theoretical and now clinical perspective, it may have profound implications for outcome. The PSS estimates the extent of tumor cell entrapment that may have occurred as a result of prior surgical interventions performed when peritoneal metastases were present [65]. To calculate the PSS, the composite of surgical dissections within the nine abdominopelvic regions is determined from previous operative interventions. If no prior interventions were performed, the PSS is 0. If 1 or 2 regions were dissected, the PSS = 1; 3–5 regions, the PSS = 2; and more than 5 regions, PSS = 3. With a PSS of 3, the patient must have had an attempt at a prior CRS.
Assuming that the peritoneum acts as a “first line of defense” against peritoneal metastases, the greater the disruption of peritoneum prior to CRS and perioperative chemotherapy, the less perfect the cancer implant resection. Reseeding of cancer cells into a retroperitoneal dissection or into a surface stripped of its peritoneum is not likely to be resected at the definitive CRS. Also, perioperative chemotherapy is not likely to eradicate cancer cells trapped in adhesions or scar tissue.
Prior surgical score has been shown to be an important prognostic variable in appendiceal cancer [71], ovarian cancer [87], sarcoma [88], and most recently, colorectal cancer [89]. These data for colon cancer, as well as rectal cancer, may have implications for treating the 5–10% of colorectal cancer patients who are found to have peritoneal metastases at the same time the primary cancer is discovered [35].
Perioperative Chemotherapy
The important factors for performing perioperative chemotherapy are (1) a proper selection of chemotherapy agents, (2) the appropriate duration of HIPEC as part of the combined treatment for peritoneal metastases, (3) a rational level of heat for hyperthermia appropriate for a particular chemotherapy agent, (4) selection of a methodology for HIPEC delivery, (5) selection of an appropriate commercial hyperthermia pump, and (6) realization that an important aspect of HIPEC by the open technologies is commercially available table-mounted retractors.
Proper Selection of Chemotherapy Agents for HIPEC
Perhaps the most crucial aspect of an optimal HIPEC is the selection of a chemotherapy agent and its proper dose for use within the peritoneal space. To select a chemotherapy agent, one must know the response expected with this drug in patients with metastatic disease. The area under the curve (AUC) ratio is important in that it estimates the dose intensity expected in the treatment of peritoneal metastases as compared to the toxicity experienced as a result of systemic effects of the drug.
Those drugs that are used in the operating room with heat are acute phase drugs that can exert their effects in the absence of cell proliferation [90]. Those drugs that are used for EPIC are selected because they are not augmented by heat and they require cell division for their optimal effects. Such drugs are 5-fluorouracil and paclitaxel [91]. Bakrin and colleagues presented data suggesting that the combination of hyperthermia with a drug shown to have developed systemic drug resistance may be effective with hyperthermia when used within the peritoneal space [92]. These data showed that cisplatin-resistant ovarian cancer patients had the same benefits from CRS and HIPEC with cisplatin as the group of patients who were judged to be cisplatin-sensitive.
The AUC ratio of an intraperitoneal chemotherapy agent estimates the exposure of peritoneal metastases to drug as compared to the exposure of the body compartment. As shown in Table 3, many of the drugs selected for HIPEC have a large AUC ratio [91]. The heat-augmented drugs with the most favorable AUC ratios are mitomycin C, doxorubicin, gemcitabine, and pegylated liposomal doxorubicin.
Table 3.
Chemotherapy agents used for perioperative intraperitoneal chemotherapy
| Drug | Molecular weight | Type | AUC ratio | t1/2 (min) | T80% (min) | Dose | Carrier solution | Incompatibility in solution | Heat synergy | Heat stability (°C) | Depth of penetration |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Doxorubicin | 579.99 | Antitumor antibiotic | 230 | 20 | 80 | 15 mg/m2 | 1.5% dextrose dialysis solution | Heparin, fluorouracil | Yes | 42 | 4–6 cell layers |
| DOXIL (liposomal doxorubicin) | 579.99 | Antitumor antibiotic | 1040 | 180 | NA | 100 mg/m2 | 1.5% dextrose dialysis solution | Heparin, fluorouracil | Yes | 42 | 4–6 cell layers |
| Etoposide | 588.58 | Antitumor antibiotic | 65 | NA | NA | 25–350 mg/m2 | 5% dextrose | Plastic devices; acrylics; antibiotics | Yes | 42 | NA |
| 5-Fluorouracil | 130.08 | Antimetabolite | 280 | 30 | 75 | 650 mg/m2 (× 5 days) | 0.9% sodium chloride; 1.5% dextrose dialysis solution; icodextrin | Idarubicin, cisplatin, diazepam, cytarabine | Minimal | 43 | 0.2 mm |
| Floxuridine (FUDR) | 246.2 | Antimetabolite | 75 | NA | NA | 500 mg/m2 twice daily (×3 days) | 0.9% sodium chloride | NA | Minimal | 43 | NA |
| Gemcitabine | 299.5 | Pyrimidine antagonist | 205 | 40 | 75 | 1000 mg/m2 | 0.9% sodium chloride | NA | At 48 h | 42.5 | NA |
| Irinotecan | 677.19 | Antitumor antibiotic | NA | NA | NA | 200 mg/m2 | 1.5% dextrose dialysis solution | NA | No | 44 | NA |
| Melphalan | 305.2 | Alkylator | 56 | 33 | 69 | 70 mg/m2 | 0.9% sodium chloride | NA | Marked | 42 | NA |
| Mitomycin C | 334.3 | Antitumor antibiotic | 27 | 40 | 90 | 15 mg/m2 | 1.5% dextrose dialysis solution | Bleomycin | Yes | 42.5 | 2000 μm |
| Mitoxantrone | 517.41 | Antitumor antibiotic | 115–255 | NA | NA | 28 mg/m2 | 0.9% sodium chloride; lactated Ringer’s solution | Heparin | Yes | 43 | 5–6 cell layers |
| Pemetrexed | 471.4 | Multitargeted antifolate | 70 | 90 | 260 | 500 mg/m2 | 1.5% dextrose dialysis solution | NA | NA | NA | NA |
| Carboplatin | 371.25 | Alkylator | 10 | NA | NA | 300 mg/m2 | 0.9% sodium chloride | NA | Yes | 41.5 | 0.5 mm |
| Cisplatin | 300.1 | Alkylator | 10 | 30 | 90 | 90 mg/m2 | 0.9% sodium chloride | NA | Yes | 41.5 | 1–3 mm |
| Oxaliplatin | 397.3 | Alkylator | 16 | 40 | 60 | 460 mg/m2 | 5% dextrose | Aluminum alkaline or NaCl solutions | Yes | 46 | 1–2 mm |
| Paclitaxel | 853.9 | Antimitotic | 1000 | NA | NA | 120–180 mg/m2 | 1.5% dextrose dialysis solution; 6% hetastarch | Plastic containers and tubes | No | 42.5 | > 80 cell layers |
| Docetaxel | 861.9 | Antimitotic | 552 | NA | NA | 45 mg/m2 | 0.9% sodium chloride | Plastic containers and tubes | No | NA | NA |
AUC, area under the curve; T80%, time for 80% clearance of the drug from peritoneal space; NA, data not available
A prolonged dwell of the intraperitoneal chemotherapy agent is important in drug selection because distribution of the peritoneal metastasis is dependent upon a sufficient time over which a drug is present at the surface of the cancer nodule. Slow clearance of the intraperitoneal drug combined with prolonged heat would cause a maximal response. Two heat-augmented drugs which remain long term in the peritoneal space are gemcitabine and pegylated liposomal doxorubicin.
Another strategy for prolonged exposure of peritoneal nodules to chemotherapy comes by continuous intravenous infusion of a heat-augmented drug. The best studied intravenous chemotherapy agent targeted to heated peritoneal surfaces is ifosfamide. Melphalan is another drug that can be heat targeted. Continuous infusion of ifosfamide during HIPEC will result in cytotoxic levels of this drug at the surface of the peritoneal nodule over 90 min of HIPEC [93]. Also, 5-fluorouracil has been used as a bolus intravenous infusion to augment the effects of hyperthermic intraperitoneal and sensitize cancer cells to oxaliplatin [94].
A third mechanism that has been used to increase drug retention within the peritoneal space during HIPEC is repeated dosing of the chemotherapy agents. Van Ruth and colleagues used a triple dosing schedule for mitomycin C in order to increase the intraperitoneal exposure of this drug. This regimen requires half the drug dose at the initiation of HIPEC, then one quarter of it at 30 min, and finally another one quarter of the dose at 60 min for a total of 90 min HIPEC. By their calculations, this increased the exposure of peritoneal nodules to mitomycin C [95].
The chemotherapy agents frequently used for HIPEC are listed in Table 3 [19]. The table presents the intraperitoneal half-life, the time at which 80% of the drug has cleared from the peritoneal space, and the AUC of peritoneal concentration times time divided by the intravenous concentration times time. One of the most rapidly cleared drugs is oxaliplatin. Its t½ within the peritoneal space is approximately 40 min and 80% of the drug leaves the peritoneal space within 60 min. For most groups, the duration of hyperthermia for intraperitoneal oxaliplatin is 30 min [94]. For mitomycin C, the t½ is 40 min and 80% of the drug is gone from the peritoneal space within 90 min. For most groups, the duration of HIPEC for mitomycin C is 90 min [20]. Similar pharmacologic parameters exist for doxorubicin. At 90 min, 80% of the drug is cleared from the peritoneal space [96]. In contrast, for liposomal doxorubicin, there is a profound retention of the drug within the peritoneal space. This drug is a nanoparticle with a large molecular size as compared to free doxorubicin. The t½ for pegylated liposomal doxorubicin is estimated at 180 min. The time for 80% clearance has not been determined. The duration of HIPEC when pegylated liposomal doxorubicin is used is 3 h (unpublished data).
Level of Hyperthermia
The mechanisms whereby hyperthermia will increase the tumor response to cancer chemotherapy are threefold. First, heat alone has a small direct antitumor effect. Although potentially important, because of blood flow, the extent of the temperature elevation within the core of a tumor nodule is limited. Selective cytotoxicity of malignant cells by heat is related to impaired DNA repair, increased protein denaturation, increased acidity, lysosomal activation, and increased apoptotic cell death [97].
The second and perhaps more important mechanism whereby hyperthermia increases chemotherapy effect is an increased heat. The synergy between heat and cancer chemotherapy drugs is a complex and poorly understood pharmacologic event. Augmented effects have been demonstrated for doxorubicin, cisplatin, mitomycin C, melphalan, oxaliplatin, and gemcitabine [98].
The third mechanism for increased cell kill of peritoneal metastases with hyperthermia is related to increased penetration of the cancer chemotherapy into tumor nodules. Jacquet et al. showed increased tissue penetration of doxorubicin when this drug was administered intraperitoneally at 43 °C. This increase in tissue concentration did not decrease the pharmacokinetic advantages of the intraperitoneal administration [99]. It has been postulated that the elevated interstitial fluid pressure in tumor nodules compared to normal tissue causes decreased chemotherapy penetration [100]. A thermal dose-dependent decrease in interstitial fluid pressure in experimental solid tumors in an animal model has been reported by Leunig et al. [101].
The extent of intraperitoneal heat must be matched to the intraperitoneal cancer chemotherapy agent. With cisplatin, the higher the temperature, the greater the increase in cytotoxicity. In contrast, those chemotherapy agents that function as prodrugs may have a temperature threshold for maximal augmentation of cytotoxicity. Mitomycin C and gemcitabine are included in this category. It has been shown that gemcitabine with 43 °C heat is decreased in its cytotoxicity. It is postulated that the intracellular conversion to gemcitabine triphosphate (the active agent) may be inhibited with high heat. Therefore, with gemcitabine, intraperitoneal heat should be limited to 41–42 °C [40]. The same situation may exist with mitomycin C.
Urano and colleagues in a mouse model of delay of cancer growth identified the cancer chemotherapy agents that are augmented by moderate hyperthermia of 41 °C. The drugs most increased in their cytotoxicity were cisplatin, melphalan, ifosfamide, and cyclophosphamide [102]. However, these “super drugs” for hyperthermia are not all appropriate for intraperitoneal administration. Ifosfamide and cyclophosphamide are prodrugs which are expected to show little cytotoxicity when present with cancer cells in a chemotherapy solution. However, cisplatin and melphalan have a direct action on the peritoneal metastases and are augmented by 41–42 °C hyperthermia with a marked therapeutic effect expected.
Technologies for HIPEC
As might be expected, different apparatus for administering HIPEC have been developed in institutions experienced in the management of peritoneal surface malignancy. The open technique with a vapor barrier created by smoke evacuators has been used extensively at the Washington Cancer Institute [103]. The coliseum technique is with the open abdominal incision covered by a plastic sheet. Access for manipulation of the intra-abdominal contents is by a cruciate incision within the plastic cover. A closed technique that has open access has been described by Benoit and colleagues and is referred to as the Landager technique [104]. In contrast, some groups close the abdomen prior to the HIPEC administration, and then after HIPEC is complete, they open the abdomen to perform anastomoses, repair seromuscular tears, and then close the abdominal incision. In this closed technique, the skin only is closed in a watertight fashion so that all of the structures of the anterior abdominal wall are thoroughly treated by the chemotherapy solution. Finally, some use a totally closed technique. In this methodology, the CRS is performed, and the abdomen is irrigated prior to the performance of intestinal anastomoses and the closure of the abdominal incision. Tubes and drains are positioned prior to the definitive closure of the abdominal incision. The cancer chemotherapy is then administered in the operating room as a final step prior to the patient being taken to the surgical intensive care unit.
Table 4 lists the credits and debits of the open versus closed abdomen technique [19].
Table 4.
Credits and debits of two different technologies for hyperthermic intraperitoneal chemotherapy (from reference [19] with permission)
| Features | Open abdomen manually distributed | Closed abdomen |
|---|---|---|
| Efficiency | Allows continued cytoreduction of bowel and mesenteric surfaces | No surgery possible during chemotherapy |
| Environmental hazard | No aerosols detected | Perception of increased safety |
| Distribution | Uniform distribution of heat and chemotherapy solutions, tissues close to skin edge not immersed | Possible poor distribution to dependent sites and closed spaces |
| Pressure | No increased intra-abdominal pressure | Increased intra-abdominal pressure may increase chemotherapy penetration into the tissue |
| Pharmacology | Allows pharmacokinetic monitoring of tumor and normal tissue | Tissue uptake of chemotherapy cannot be determined |
| Abdominal incision and suture lines | Treated prior to performing the suturing | Risk of recurrence in abdominal incision and suture lines |
| Diaphragm perforation with peritonectomy | Pleural space treated by hyperthermic chemotherapy may prevent seeding of the pleural space | Diaphragm closed prior to hyperthermic intraperitoneal chemotherapy so pleural space is not treated |
| Intestinal perforation | Detected by observing immersed bowel loops | Not detected |
| Hyperthermia | Increased heat necessary to maintain 42 °C | Less heat required to maintain 42 °C |
An absence of risk to the environment using the open technique has been repeatedly demonstrated. At the levels of detection possible, no chemotherapy aerosols have been found to be present within the operating room environment. However, from a theoretical perspective, some drugs may be recommended only for the closed technique. One of these drugs is melphalan. Melphalan is nitrogen mustard and is an aromatic compound which may escape into the operating room environment. Melphalan is usually recommended for use using a closed technique.
Commercially Available Hyperthermia Pumps
To date, ten different commercial groups are manufacturing hyperthermia pumps. All of the devices are capable of heating the intraperitoneal fluid to 44 °C. They are monitored at several sites within the abdomen and pelvis with thermister probes. Variable maximal rates of flow will influence the rate at which the intraperitoneal fluid can be heated to the desired 42 °C temperature. Some apparatus may be more appropriate for open administration (Belmont, SunChip, Euromedical). Others are more appropriate for the closed system (RanD Performer, Hyperthermic Solutions, Cavitherm).
Approximately 20% of the institutions in the USA that perform HIPEC still use a “homemade” machine. Most often this is a cardiopulmonary bypass machine with a water bath at the inflow so that the desired temperatures can be reached within the peritoneal space. Inflow temperatures need to be 45–46 °C in order to reach appropriate temperatures within the peritoneal space in a reasonable time period.
Diseases
In this review, we have selected six diseases or disease categories that merit emphasis regarding disease-specific treatments and a summary of expected outcomes.
Appendiceal Mucinous Neoplasms
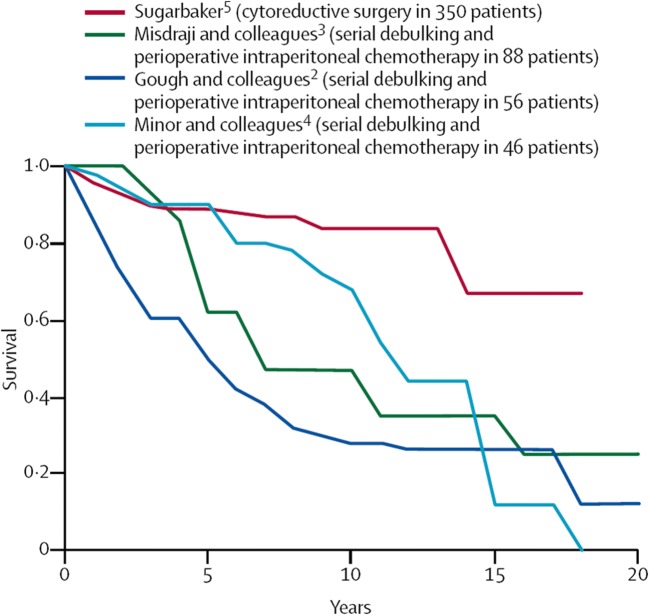
Appendiceal mucinous neoplasms have been treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for over 30 years [71]. As the cytoreductive surgical procedures improved and the HIPEC became more refined, this new treatment option for mucinous appendiceal neoplasms was accepted as a new standard of care for this disease [1]. It replaces serial debulking and multiple cycles of systemic chemotherapy for management; however, to see the survival differences in these patients, a long-term (10-year) follow-up is necessary. Figure 3 compares the results of cytoreduction plus HIPEC to serial debulking procedures plus systemic chemotherapy or delayed intraperitoneal chemotherapy at four prominent cancer centers [1]. With long-term follow-up, cytoreductive surgery plus HIPEC is the only treatment associated with cure of this disease process.
Fig. 3.
Results of cytoreduction plus HIPEC compared to serial debulking procedures plus systemic chemotherapy or delayed intraperitoneal chemotherapy
A systematic review evaluated the results from the world’s literature in the treatment of appendiceal mucinous neoplasms. Yan and colleagues concluded that cytoreductive surgery plus HIPEC showed promising long-term results as compared to historical control [105]. Because of the many difficulties in performing a phase III study in this rare disease that requires long-term follow-up to show survival benefits, this is the best data available.
Peritoneal Mesothelioma
Peritoneal mesothelioma is another rare disease process in which cytoreductive surgery combined with perioperative intraperitoneal chemotherapy has emerged as a new standard of care. Four groups have now reported on approximately 300 malignant peritoneal mesothelioma patients: The National Cancer Institute in Bethesda, MD [106]; The Washington Cancer Institute in Washington, DC [85]; The Columbia Mesothelioma Center in New York [107]; and the National Cancer Institute in Milan, Italy [108]. All groups presented data with cytoreductive surgery and HIPEC with treatment results markedly improved over those reported in the past with conservative management by palliative surgery and systemic chemotherapy. Each group presented their experience with between 50 and 100 patients. With current treatment, all the groups report a median survival of 5 years or better. The median survival in the past was approximately 1 year. Despite the fact that these are nonrandomized data, this apparent major improvement in survival with a new treatment strategy has been regarded as a new standard of care to which other options should be compared [2].
For hyperthermic intraoperative intraperitoneal chemotherapy, all groups advocate a cisplatin-based hyperthermic intraperitoneal chemotherapy. The doses were different at all four institutions. The heat, approximately 42.5 °C, was the same at all institutions. The drugs combined with cisplatin were either doxorubicin or mitomycin C. At the National Cancer Institute, USA, high-dose cisplatin with systemic thiosulfate has been used.
Colorectal Cancer
Colorectal cancer peritoneal carcinomatosis is a result of transcoelomic invasion by the primary cancer or intraperitoneal seeding during surgical manipulation. In contrast to lymphatic, liver, and pulmonary dissemination, colorectal cancer peritoneal metastases may be regarded as a local–regional extension of disease rather than a manifestation of systemic metastasis.
Management of Peritoneal Metastases Diagnosed in Follow-Up
Survival benefits for peritoneal metastases from colon and rectal cancer using cytoreductive surgery and perioperative chemotherapy began to appear in publications in the 1990s. Although a small percentage of these patients had synchronous peritoneal metastases (less than 5%), a great majority had peritoneal metastases diagnosed in follow-up. In 1995, Sugarbaker and Jablonski showed a 3-year survival of 35% in patients with peritoneal metastases from colon cancer treated with cytoreductive surgery plus intraperitoneal mitomycin C and fluorouracil [62]. In 2003, Verwaal and colleagues from Amsterdam published a 3-year projected survival of 38% in 54 patients treated by cytoreductive surgery and hyperthermic intraperitoneal mitomycin C with adjuvant systemic 5-fluorouracil [109]. Shen and colleagues accumulated patients between 1991 and 2002 [110]. Seventy-seven patients with nonappendiceal colorectal cancer underwent the combined treatment. These investigators concluded that one third of patients with complete resection have long-term survival and that systemic chemotherapy did not contribute to the control of peritoneal metastases. These studies performed in the absence of modern systemic colorectal cancer chemotherapy (oxaliplatin and irinotecan) document the efficacy of cytoreductive surgery and perioperative chemotherapy to rescue approximately one third of patients with peritoneal metastases.
Since that time, multiple publications confirming the efficacy of the combination of cytoreductive surgery and perioperative chemotherapy to benefit patients with established colorectal peritoneal metastases have been published. Glehen and colleagues, in a multi-institutional retrospective study of 506 patients from 28 institutions, reported an overall median survival of 19.2 months in patients with peritoneal metastases from colorectal cancer treated with the combined approach [48]. Patients in whom the cytoreductive surgery was complete had a median survival of 32.4 months compared with 8.4 months in patients in whom cytoreduction was not completed (p < 0.001). The morbidity was 22.9% and the mortality was 4%. These investigators concluded that the therapeutic approach of combining cytoreductive surgery with perioperative intraperitoneal chemotherapy achieved long-term survival in a selected group of patients with peritoneal metastases of colorectal origin with acceptable morbidity and mortality. The complete cytoreduction was the most important prognostic indicator.
Elias and colleagues reported on colorectal peritoneal metastases in a retrospective analysis of 523 patients from 23 French-speaking centers [111]. The overall median survival was 30.1 months and the 5-year overall survival was 27%. Eighty-four percent of the patients had a complete cytoreduction, with a median survival of 33 months. These investigators concluded that cytoreductive surgery and perioperative chemotherapy are now considered the gold standard in the French guidelines for the management of peritoneal metastases. The survival of 562 patients at 10 years was 37%.
At the top of the list regarding evidence-based medicine for this treatment strategy is the phase 3 study reported by Verwaal and colleagues in 2003 [112]. The Dutch trial compared 105 patients with colorectal peritoneal metastases who were randomly assigned to receive either standard treatment with systemic 5-fluorouracil and leucovorin compared with an aggressive cytoreductive surgery with perioperative chemotherapy using hyperthermic mitomycin C. The patients in the experimental therapy arm also had systemic 5-fluorouracil chemotherapy. After a median follow-up of 21.6 months, the median survival was 12.6 months with systemic chemotherapy and 22.3 months with cytoreduction and perioperative chemotherapy (p = 0.032). These investigators reported that a complete cytoreduction and a limited extent of disease were important determinants of benefit. The durability of the benefit of cytoreductive surgery and perioperative chemotherapy was confirmed in a follow-up article in 2008 [113]. Currently, this treatment strategy is the standard of care in Holland and there are five regional centers of excellence open for peritoneal metastases patients.
Yan and colleagues performed a systematic review to estimate the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for patients with peritoneal carcinomatosis from colorectal carcinoma [114]. Two randomized controlled trials, one comparative study, one multi-institutional registry study, and ten most recent case-series studies were evaluated. The level of evidence was low in 13 of the 14 eligible studies. The median survival varied from 13 to 29 months, and the 5-year survival rates ranged from 11 to 19%. Patients who received complete cytoreduction benefited most, with median survival varying from 28 to 60 months and 5-year survival ranging from 22 to 49%. The overall morbidity rate varied from 23 to 44%, and the mortality rate ranged from 0 to 12%.
In the 2017 National Comprehensive Cancer Network Guidelines, cytoreductive surgery and perioperative chemotherapy were included as an approved treatment option. “The panel currently believes that complete cytoreductive surgery and/or intraperitoneal chemotherapy can be considered in experienced centers for selected patients with limited peritoneal metastases for whom R0 resection can be achieved. The panel recognizes the need for (additional) randomized clinical trials that will address the risks and benefits associated with each of these modalities” [3].
If hyperthermic intraperitoneal chemotherapy is effective in treating selected patients with carcinomatosis from colorectal cancer, one would suspect that prevention of local–regional recurrence on peritoneal surfaces or the resection site may be possible. This causes a dilemma that cancer surgeons must currently answer for themselves on numerous patients. Until information from clinical trials becomes available, it is my opinion that patients with primary cancer that demonstrate a high risk of dissemination in the peritoneal cavity should be treated. This includes patients with T4 lesions, positive peritoneal cytology, and involvement of the ovaries and patients whose cancer is disrupted with resection. Several phase III trials in this group of patients are currently in progress.
Gastric Cancer
Combined peritoneal carcinomatosis and resection site disease occurs in as high as 50% of patients who recur following gastrectomy. This pattern of recurrence is most prominent in patients who have stage III or resectable stage IV disease. Xu and colleagues performed a meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for advanced gastric cancer [115]. Eleven trials involving 1161 patients were included for data extraction. A significant improvement in survival was associated with hyperthermic intraoperative intraperitoneal chemotherapy. The meta-analysis indicated that hyperthermic intraperitoneal chemotherapy after resection of advanced primary gastric cancer is associated with an improved overall survival. An adequately powered trial called GastriCHIP in Western gastric cancer patients is currently in progress [116].
Ovarian Cancer
Ovarian cancer is the fifth leading cause of cancer-related deaths among females in the USA with 15,310 deaths projected in 2015. The majority of cases are diagnosed with peritoneal metastases present and malignant ascites as the presenting sign. Despite a 60–80% response rate to platinum-based systemic chemotherapy, only approximately 60% of patients die due to a high rate of recurrence. Because epithelial ovarian cancer has a marked propensity for peritoneal spread, it is suitable for aggressive local–regional therapies. Consequently, the combination of intravenous and intraperitoneal route of administration for chemotherapeutic agents in ovarian cancer has been extensively investigated. Despite convincing data, intraperitoneal chemotherapy treatment is still not universally accepted due to the increased rate of complications associated with intraperitoneal drug delivery. Cytoreductive surgery combined with heated intraoperative intraperitoneal chemotherapy is a comprehensive treatment modality directed at the whole abdomen and pelvis. Its use of intraperitoneal chemotherapy at the time of surgery and/or in the immediate postoperative period facilitates uniform drug delivery and may avoid some of the complications of prolonged peritoneal access.
Bijelic and colleagues performed a systematic review to critically evaluate cytoreductive surgery combined with heated intraoperative intraperitoneal chemotherapy in the treatment of ovarian cancer [117]. This was a systematic review of all manuscripts published in the English literature that met predetermined inclusion criteria. Fourteen studies were analyzed. A wide variety of drug doses, methods of intraperitoneal chemotherapy administration, and volume of chemotherapy solution were used. Seven studies showed that patients with complete cytoreduction had the greatest benefit. The median overall survival for primary and recurrent disease ranged from 22 to 54 months and the median disease-free survival from 10 to 26 months. The rates of significant morbidity associated with the combined treatment were low, ranging from 5 to 36%. The mortality did not exceed 10%. These authors concluded that cytoreductive surgery combined with heated intraoperative intraperitoneal chemotherapy is a treatment option for patients with ovarian cancer that is worthy of further investigation. Selection criteria for patients most likely to benefit need to be defined.
Future Prospects
Very often, the first step in finding new treatments for cancer involves studies in patients with advanced disease. A response that results in improved or prolonged life is then taken to patients with less advanced disease. Usually, this second step in development involves randomized trials. To date, cytoreductive surgery plus HIPEC has resulted in a survival benefit in numerous phase II and in a single phase III trial in colorectal cancer with carcinomatosis. Benefit is strongly suggested in ovarian cancer. In appendiceal cancer and peritoneal mesothelioma, it is a new standard of care, and for ethical and statistical reasons, phase III studies will probably never be performed.
Currently, limited application of this treatment is occurring worldwide. An inadequate number of treatment centers are operational. The practicing oncologist is usually content to give intravenous chemotherapy as compared to the more technologically demanding intraperitoneal chemotherapy. Also, the practicing surgeon is not eager to have his time in the operating room extended approximately 2 h. Nevertheless, the number of patients who could profit from this new treatment option can be estimated. Nearly all of the 1500 new appendiceal mucinous neoplasms that occur annually in the USA should be treated. Also, a majority of the 300 new peritoneal mesothelioma patients should be treated. Approximately half of the 20,000 patients with peritoneal dissemination of colorectal cancer should be treated. Most of the 30,000 new ovarian cancer patients are eligible for this treatment option.
Of course, if this application of hyperthermia and intraperitoneal chemotherapy is extended to the adjuvant treatment of primary disease, there will be a much larger number of patients eligible. Of the 150,000 new colorectal cancer patients each year, approximately 30,000 will be at high risk for subsequent local recurrence or peritoneal carcinomatosis. These patients are excellent candidates for this treatment option. Also, at least half of the 30,000 new gastric cancer patients in the USA are stage III or resectable stage IV and are candidates for this treatment option. Many other diseases such as small bowel adenocarcinoma, endometrial cancer, and gallbladder cancer may be considered for treatment because of the high risk for resection site recurrence and peritoneal carcinomatosis.
Of course, such dramatic changes in practice patterns are not going to occur quickly. Numerous additional prospective clinical trials will be necessary [118]. Perhaps the most direct way to bridge the gap between current practice of intravenous chemotherapy only to bidirectional chemotherapy using both intraperitoneal and intravenous chemotherapy is through the initiation of studies with hyperthermic intraoperative intraperitoneal chemotherapy. In this treatment modality, the effects of the intraperitoneal treatment are likely to be most profound. Also, the technical problems of intraperitoneal drug delivery are absent. The technological requirements for this treatment option are low and its morbidity and mortality is very limited. It seems to be a good place in which to initiate the multiple changes which may be necessary in the practice of oncology.
Compliance with Ethical Standards
Conflict of Interest
The author declares that he has no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sugarbaker PH. New standard of care for appendiceal epithelial malignancies and pseudomyxoma peritonei syndrome. Lancet Oncol. 2006;7(1):69–76. doi: 10.1016/S1470-2045(05)70539-8. [DOI] [PubMed] [Google Scholar]
- 2.Sugarbaker PH, Turaga KK, Alexander HR, Jr, Deraco M, Hesdorffer M. Management of malignant peritoneal mesothelioma using cytoreductive surgery and perioperative chemotherapy. J Oncol Pract. 2016;12(10):928–935. doi: 10.1200/JOP.2016.011908. [DOI] [PubMed] [Google Scholar]
- 3.Colon cancer. Clinical practice guidelines in oncology (NCCN guidelines). Version 2.2017—March 13, 2017. Accessed at https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- 4.Tewari D, Java JJ, Salani R, Armstrong DK, Markman M, Herzog T, Monk BJ, Chan JK. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2015;33:1460–1466. doi: 10.1200/JCO.2014.55.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugarbaker PH, Kwong ML. Regional chemotherapy: possibilities for prevention and treatment of peritoneal metastases from gastric cancer. (In) Management of gastric cancer. SMGroup at www.smgbooks.com
- 6.Goere D, Passot G, Gelli M, Sugarbaker PH, Glehen O. Complete cytoreductive surgery plus HIPEC for peritoneal metastases from unusual cancer sites of origin: results from a worldwide analysis of the Peritoneal Surface Oncology Group International (PSOGI) Int J Hyperth. 2017;33:528–533. doi: 10.1080/02656736.2017.1301576. [DOI] [PubMed] [Google Scholar]
- 7.Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy: a prospective study of prognostic factors. Cancer. 1989;63:364–367. doi: 10.1002/1097-0142(19890115)63:2<364::aid-cncr2820630228>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, Porcheron J, Peix JL, François Y, Vignal J, Gilly FN. Peritoneal carcinomatosis from non-gynecologic malignancies. Results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 10.Popovich RP, Moncrief JW, Nolph KD, Ghods AJ, Twardowski ZJ, Pyle WK. Continuous ambulatory peritoneal dialysis. Ann Intern Med. 1978;88:449–456. doi: 10.7326/0003-4819-88-4-449. [DOI] [PubMed] [Google Scholar]
- 11.Karnofsky DA, Abelmann WH, Craver LF, et al. The use of the nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;40:634–656. [Google Scholar]
- 12.Torres IJ, Litterst CL, Guarino AM. Transport of model compounds across the peritoneal membrane in the rat. Pharmacology. 1978;17:330–340. doi: 10.1159/000136874. [DOI] [PubMed] [Google Scholar]
- 13.Dedrick RL, Myers CE, Bungay PM, de Vita V., Jr Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978;62:1–11. [PubMed] [Google Scholar]
- 14.Speyer JL, Collins JM, Dedrick RL, Brennan MF, Buckpitt AR, Londer H, DeVita VT Jr, Myers CE. Phase 1 and pharmacologic studies of 5-fluorouracil administered intraperitoneally. Cancer Res. 1980;40:567–572. [PubMed] [Google Scholar]
- 15.Speyer JL, Sugarbaker PH, Collins JM, Dedrick RL, Klecker RW Jr, Myers CE. Portal levels and hepatic clearance of 5-fluorouracil after intraperitoneal administration in humans. Cancer Res. 1981;41:1916–1922. [PubMed] [Google Scholar]
- 16.Jones RB, Myers CE, Guarino AM, et al. High volume intraperitoneal chemotherapy (“belly bath”) for ovarian cancer. Cancer Chemother Pharmacol. 1978;1:161–166. doi: 10.1007/BF00253116. [DOI] [PubMed] [Google Scholar]
- 17.Ozols RF, Locker GY, Doroshow JH, Grotzinger KR, Myers CE, Young RC. Pharmacokinetics of adriamycin and tissue penetration in murine ovarian cancer. Cancer Res. 1979;39:3209–3214. [PubMed] [Google Scholar]
- 18.McVie JG, Dikhoff T, Van der Heide J, et al. Tissue concentration of platinum after intraperitoneal cisplatinum in patients. Proc Am Assoc Cancer Res. 1985;26:162. [Google Scholar]
- 19.Sugarbaker PH, van der Speeten K. Surgical technology and pharmacology of hyperthermic perioperative chemotherapy. J Gastrointest Oncol. 2016;7(1):29–44. doi: 10.3978/j.issn.2078-6891.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van der Speeten K, Stuart OA, Chang D, Mahteme H, Sugarbaker PH. Changes induced by surgical and clinical factors in the pharmacology of intraperitoneal mitomycin C in 145 patients with peritoneal carcinomatosis. Cancer Chemother Pharmacol. 2011;68:147–156. doi: 10.1007/s00280-010-1460-4. [DOI] [PubMed] [Google Scholar]
- 21.Shiu MH, Fortner JG. Intraperitoneal hyperthermic treatment of implanted peritoneal cancer in rats. Cancer Res. 1980;40:4081–4084. [PubMed] [Google Scholar]
- 22.Meyer JL, Kapp DS, Fessenden P, Hahn GH. Hyperthermic oncology: current biology, physics and clinical results. Pharmac Ther. 1989;42:251–288. doi: 10.1016/0163-7258(89)90038-7. [DOI] [PubMed] [Google Scholar]
- 23.Spratt JS, Adcock RA, Muskovin M, Sherrill W, McKeown J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40:256–260. [PubMed] [Google Scholar]
- 24.Koga S, Hamazoe R, Maeta M, Shimizu N, Kanayama H, Osaki Y. Treatment of implanted peritoneal cancer in rats by continuous hyperthermic peritoneal perfusion in combination with an anticancer drug. Cancer Res. 1980;44:1840–1842. [PubMed] [Google Scholar]
- 25.Koga S, Hamazoe R, Maeta M, Shimizu N, Murakami A, et al (1988) Prophylactic therapy for peritoneal recurrence of gastric cancer by continuous hyperthermic peritoneal perfusion with mitomycin C. 61:232–237 [DOI] [PubMed]
- 26.Fujimura T, Yonemura Y, Muraoka K, Takamura H, Hirono Y, Sahara H, Ninomiya I, Matsumoto H, Tsugawa K, Nishimura GI, Sugiyama K, Miwa K, Miyazaki I. Continuous hyperthermic peritoneal perfusion for the prevention of peritoneal recurrence of gastric cancer: randomized controlled study. World J Surg. 1994;18:150–155. doi: 10.1007/BF00348209. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer. 1998;85:529–534. [PubMed] [Google Scholar]
- 28.Yan TD, Black D, Sugarbaker PH, Zhu J, Yonemura Y, Petrou G, Morris DL. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol. 2007;14(10):2702–2713. doi: 10.1245/s10434-007-9487-4. [DOI] [PubMed] [Google Scholar]
- 29.Feingold PL, Kwong MLM, Sabesan A, Sorber R, Rudloff U. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for gastric cancer and other less common disease histologies: is it time? J Gastrointest Oncol. 2016;7:87–98. doi: 10.3978/j.issn.2078-6891.2015.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yonemura Y, de Aretxabala X, Fujimura T, Fushida S, Katayama K, Bandou E, Sugiyama K, Kawamura T, Kinoshita K, Endou Y, Sasaki T. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology. 2001;48:1776–1782. [PubMed] [Google Scholar]
- 31.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2013 June 13. Identifier NCT01882933, D2 Resection and HIPEC (Hyperthermic Intraperitoneal Chemoperfusion) in Locally Advanced Gastric Carcinoma (GASTRICHIP); [cited 2017 April 22]; Available from: https://clinicaltrials.gov/ct2/show/NCT01882933
- 32.Quirke P, Sebag-Montefiore D, Steele R et al (2006) Local recurrence after rectal cancer resection is strongly related to the plane of the surgical dissection and is further reduced by pre-operative short course radiotherapy. Preliminary results of the Medical Research Council (MRC) CR07 trial. J Clin Oncol vol. 24, article 3512
- 33.Heald RJ, Ryall RDH. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1(8496):1479–1482. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 34.West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol. 2010;28(2):272–278. doi: 10.1200/JCO.2009.24.1448. [DOI] [PubMed] [Google Scholar]
- 35.Sugarbaker PH. Colorectal cancer metastases, a surgical perspective. Surg Oncol Clin N Am. 2013;22(2):289–298. doi: 10.1016/j.soc.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Honore C, Gelli M, Francoul J, et al. Ninety percent of the adverse outcomes in 10% of patients: can we identify the populations at high risk of developing peritoneal metastases after curative surgery for colorectal cancer? Int J Hyperth. 2017;33(5):505–510. doi: 10.1080/02656736.2017.1306119. [DOI] [PubMed] [Google Scholar]
- 37.Honore C, Goere D, Souadka A, Dumont F, Elias D. Definition of patient presenting a high risk of developing peritoneal carcinomatosis after curative surgery for colorectal cancer: a systematic review. Ann Surg Oncol. 2013;20:183–192. doi: 10.1245/s10434-012-2473-5. [DOI] [PubMed] [Google Scholar]
- 38.Cunliffe WJ, Sugarbaker PH. Gastrointestinal malignancy: rationale for adjuvant therapy using early postoperative intraperitoneal chemotherapy (EPIC) Br J Surg. 1989;76:1082–1090. doi: 10.1002/bjs.1800761030. [DOI] [PubMed] [Google Scholar]
- 39.Sugarbaker PH, Graves T, DeBruijn EA, Cunliffe WJ, Mullins RE, Hull WE, Oliff L, Schlag P. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacologic studies. Cancer Res. 1990;50:5790–5794. [PubMed] [Google Scholar]
- 40.Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol. 1999;43(Suppl):S15–S25. doi: 10.1007/s002800051093. [DOI] [PubMed] [Google Scholar]
- 41.Jacquet P, Averbach A, Stephens AD, Stuart OA, Chang D, Sugarbaker PH. Heated intraoperative intraperitoneal mitomycin C and early postoperative intraperitoneal 5-fluorouracil: pharmacokinetic studies. Oncology. 1998;55:130–138. doi: 10.1159/000011847. [DOI] [PubMed] [Google Scholar]
- 42.Pestieau SR, Sugarbaker PH. Treatment of primary colon cancer with peritoneal carcinomatosis: a comparison of concomitant versus delayed management. Dis Colon Rectum. 2000;43:1341–1348. doi: 10.1007/BF02236627. [DOI] [PubMed] [Google Scholar]
- 43.Tentes AA, Spiliotis ID, Korakianitis OS, Vaxevanidou A, Kyziridis D. Adjuvant perioperative intraperitoneal chemotherapy in locally advanced colorectal carcinoma: preliminary results. ISRN Surg. 2011;2011:529876. doi: 10.5402/2011/529876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noura S, Ohue M, Shingai T, Kano S, Ohigashi H, Yano M, Ishikawa O, Takenaka A, Murata K, Kameyama M. Effects of intraperitoneal chemotherapy with mitomycin C on the prevention of peritoneal recurrence in colorectal cancer patients with positive peritoneal lavage cytology findings. Ann Surg Oncol. 2011;18:396–404. doi: 10.1245/s10434-010-1319-2. [DOI] [PubMed] [Google Scholar]
- 45.Braam HJ, Boerma D, Wiezer MJ, Ramshorst v. Hyperthermic intraperitoneal chemotherapy during primary tumour resection limits extent of bowel resection compared to two-stage treatment. Eur J Surg Oncol. 2013;39:988–993. doi: 10.1016/j.ejso.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Sammartino P, Sibio S, Accarpio F, Di Giorgio A. Long-term results after proactive management for locoregional control in patients with colonic cancer at high risk of peritoneal metastases. Int J Color Dis. 2014;29:1081–1089. doi: 10.1007/s00384-014-1929-4. [DOI] [PubMed] [Google Scholar]
- 47.Yu W, Whang I, Chung HY, Averbach A, Sugarbaker PH. Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: results of a prospective randomized trial. World J Surg. 2001;25:985–990. doi: 10.1007/s00268-001-0067-7. [DOI] [PubMed] [Google Scholar]
- 48.Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, de Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F, Gutman M, Tentes AA, Lorimier G, Bernard JL, Bereder JM, Porcheron J, Gomez-Portilla A, Shen P, Deraco M, Rat P. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22(16):3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Sugarbaker PH, van der Speeten K, Stuart OA, Chang D, Mahteme H. Patient- and treatment-related variables, adverse events and their statistical relationship for treatment of peritoneal metastases. In: Sugarbaker PH, editor. Cytoreductive surgery & perioperative chemotherapy for peritoneal surface malignancy. Textbook and video atlas. Woodbury: Cine-Med Publishing; 2012. pp. 183–206. [Google Scholar]
- 50.Tanis PJ, Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA). Adjuvant HIPEC in High Risk Colon Cancer (COLOPEC). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [cited 2017 September 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT02231086 NLM Identifier: NCT02231086
- 51.Sugarbaker PH, Sammartino P, Tentes AA. Proactive management of peritoneal metastases from colorectal cancer: the next logical step toward optimal locoregional control. Colorect Cancer. 2012;1(2):115–123. [Google Scholar]
- 52.Sammartino P, Tentes AA, Sugarbaker PH. Eradication of minimal residual disease in the perioperative period in primary colorectal cancer. Colorectal Cancer. 2015;4:157–166. [Google Scholar]
- 53.Sammartino P, Societa Italiana di Chirurgia Oncologica (SICO). PROMENADE Trial (PROactive Management of ENdoperitoneal spreAD in colonic cancer). [cited 2017 September 19]
- 54.Griffen WO, Humphrey L, Sosin H. The prognosis and management of recurrent abdominal malignancies. Curr Probl Surg. 1969;6:1–43. [PubMed] [Google Scholar]
- 55.Minton JP, Hoehn JL, Gerber DM, Horsley JS, Connolly DP, Salwan F, Fletcher WS, Cruz AB, Gatchell FG, Oviedo M, Meyer KK, Leffall LD, Berk RS, Stewart PA, Kurucz SE. Results of a 400-patient carcinoembryonic antigen second-look colorectal cancer study. Cancer. 1985;55:1284–1290. doi: 10.1002/1097-0142(19850315)55:6<1284::aid-cncr2820550622>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 56.Sugarbaker PH. Second-look surgery for colorectal cancer: revised selection factors and new treatment options for greater success. Int J Surg Oncol Volume. 2011;2011:Article ID 915078. doi: 10.1155/2011/915078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elias D, Goere D, Di Pietrantonio D, et al. Results of systematic second-look surgery in patients at high risk of developing colorectal peritoneal carcinomatosis. Ann Surg. 2008;247(3):445–450. doi: 10.1097/SLA.0b013e31815f0113. [DOI] [PubMed] [Google Scholar]
- 58.Delhorme JB, Triki E, Romain B, Meyer N, Rohr S, Brigand C. Routine second-look after surgical treatment of colonic peritoneal carcinomatosis. J Visc Surg. 2015;152(3):149–154. doi: 10.1016/j.jviscsurg.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Sugarbaker PH, Stuart OA, Bijelic L. Intraperitoneal gemcitabine chemotherapy treatment for patients with resected pancreatic cancer, rationale and report of early data. Int J Surg Oncol Volume 2011, Article ID 161862, 7 pages, 10.1155/2011/161862 [DOI] [PMC free article] [PubMed]
- 60.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Los G, Mutsaers PH, Lenglet WJ, Baldew GS, McVie JG. Platinum distribution in intraperitoneal tumors after intraperitoneal cisplatin treatment. Cancer Chemother Pharmacol. 1990;25:389–394. doi: 10.1007/BF00686048. [DOI] [PubMed] [Google Scholar]
- 62.Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221(2):124–132. doi: 10.1097/00000658-199502000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sethna KS, Sugarbaker PH. New prospects for the control of peritoneal surface dissemination of gastric cancer using perioperative intraperitoneal chemotherapy. Cancer Therapy. 2004;2:79–84. [Google Scholar]
- 64.Sugarbaker PH. Strategies for the prevention and treatment of peritoneal carcinomatosis from gastrointestinal cancer. Cancer Investig. 2005;23:155–172. [PubMed] [Google Scholar]
- 65.Jacquet P, Sugarbaker PH. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res. 1996;15(1):49–58. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 66.da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg. 2006;203(6):878–886. doi: 10.1016/j.jamcollsurg.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 67.Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17(9):2370–2377. doi: 10.1245/s10434-010-1039-7. [DOI] [PubMed] [Google Scholar]
- 68.Lampe B, Kroll N, Piso P, Forner DM, Mallman P. Prognostic significance of Sugarbaker’s peritoneal cancer index for the operability of ovarian carcinoma. Int J Gynecol Cancer. 2015;25:135–144. doi: 10.1097/IGC.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 69.Sugarbaker PH, Yan TD, Stuart OA, Yoo D. Comprehensive management of diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol. 2006;32(6):686–691. doi: 10.1016/j.ejso.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 70.Sugarbaker PH, Chang D. Long-term regional chemotherapy for patients with epithelial malignant peritoneal mesothelioma results in improved survival. Eur J Surg Oncol. 2017;43(7):1228–1235. doi: 10.1016/j.ejso.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 71.Sugarbaker PH. Pseudomyxoma peritonei and peritoneal metastases from appendiceal malignancy. In: Sugarbaker PH, editor. Cytoreductive surgery & perioperative chemotherapy for peritoneal surface malignancy. Textbook and video atlas (second edition) Woodbury: Cine-Med Publishers; 2017. pp. 109–132. [Google Scholar]
- 72.Gonzalez-Moreno S, Yan H, Alcorn KW, Sugarbaker PH. Malignant transformation of “benign” cystic mesothelioma of the peritoneum. J Surg Oncol. 2002;79(4):243–251. doi: 10.1002/jso.10081. [DOI] [PubMed] [Google Scholar]
- 73.Ihemelandu C, Sugarbaker PH. Clinicopathologic and prognostic features in patients with peritoneal metastasis from mucinous adenocarcinoma, adenocarcinoma with signet ring cells, and adenocarcinoid of the appendix treated with cytoreductive surgery and perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2016;23(5):1474–1480. doi: 10.1245/s10434-015-4995-0. [DOI] [PubMed] [Google Scholar]
- 74.Elias D, Benizri E, Pocard M, Ducreux M, Boige V, Lasser P. Treatment of synchronous peritoneal carcinomatosis and liver metastases from colorectal cancer. Eur J Sur Oncol. 2006;32(6):632–636. doi: 10.1016/j.ejso.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 75.Yan TD, Morris DL, Kusamura S, Baratti D, Deraco M. Preoperative investigations in the management of peritoneal surface malignancy with cytoreductive surgery and perioperative intraperitoneal chemotherapy: expert consensus statement. J Surg Oncol. 2008;98:224–227. doi: 10.1002/jso.21069. [DOI] [PubMed] [Google Scholar]
- 76.Jacquet P, Jelinek JS, Steves MA, Sugarbaker PH. Evaluation of computer tomography in patients with peritoneal carcinomatosis. Cancer. 1993;72(5):1631–1636. doi: 10.1002/1097-0142(19930901)72:5<1631::aid-cncr2820720523>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 77.Koh JL, Yan TD, Glenn D, Morris DL. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2009;16:327–333. doi: 10.1245/s10434-008-0234-2. [DOI] [PubMed] [Google Scholar]
- 78.Sugarbaker PH, Sardi A, Brown G, Dromain C, Rousset P, Jelinek JS. Concerning CT features used to select patients for treatment of peritoneal metastases, a pictoral essay. Int J Hyperth. 2017;33(5):497–504. doi: 10.1080/02656736.2017.1317368. [DOI] [PubMed] [Google Scholar]
- 79.Jacquet P, Jelinek JS, Chang D, Koslowe P, Sugarbaker PH. Abdominal computed tomographic scan in the selection of patients with mucinous peritoneal carcinomatosis for cytoreductive surgery. J Am Coll Surg. 1995;181:530–538. [PubMed] [Google Scholar]
- 80.Yan TD, Haveric N, Carmignani P, Chang D, Sugarbaker PH. Abdominal computed tomography scans in the selection of patients with malignant peritoneal mesothelioma for comprehensive treatment with cytoreductive surgery and perioperative intraperitoneal chemotherapy. Cancer. 2005;15:839–849. doi: 10.1002/cncr.20836. [DOI] [PubMed] [Google Scholar]
- 81.Rivard JD, Temple WJ, McConnell YJ, et al. Preoperative computed tomography does not predict resectability in peritoneal carcinomatosis. Am J Surg. 2014;207:760–765. doi: 10.1016/j.amjsurg.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 82.Suidan RS, Ramirez PT, Sarasohn DM, Teitcher JB, Mironov S, Iyer RB, Zhou Q, Iasonos A, Paul H, Hosaka M, Aghajanian CA, Leitao MM, Jr, Gardner GJ, Abu-Rustum NR, Sonoda Y, Levine DA, Hricak H, Chi DS. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol Oncol. 2014;134(3):455–461. doi: 10.1016/j.ygyno.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goere D, Souadka A, Faron M, et al. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol. 2015;22:2958–2964. doi: 10.1245/s10434-015-4387-5. [DOI] [PubMed] [Google Scholar]
- 84.Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, Guile MW, Bristow RE, Aghajanian C, Barakat RR. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114:26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 85.Sugarbaker PH, Welch L, Mohamed F, Glehen O. A review of peritoneal mesothelioma at the Washington Cancer Institute. Surg Oncol Clin N Am. 2003;12(3):605–621. doi: 10.1016/s1055-3207(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 86.Glehen O, Mohamed F, Sugarbaker PH. Incomplete cytoreduction in 174 patients with peritoneal carcinomatosis from appendiceal malignancy. Ann Surg. 2004;240(2):275–285. doi: 10.1097/01.sla.0000133183.15705.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Look M, Chang D, Sugarbaker PH. Long-term results of cytoreductive surgery for advanced and recurrent epithelial ovarian cancers and papillary serous carcinoma of the peritoneum. Int J Gynecol Cancer. 2004;14(1):35–41. doi: 10.1111/j.1048-891x.2004.14008.x. [DOI] [PubMed] [Google Scholar]
- 88.Berthet B, Sugarbaker TA, Chang D, Sugarbaker PH. Quantitative methodologies for selection of patients with recurrent abdominopelvic sarcoma for treatment. Eur J Cancer. 1999;35(3):413–419. doi: 10.1016/s0959-8049(98)00375-x. [DOI] [PubMed] [Google Scholar]
- 89.Paul BK, Ihemelandu C, Sugarbaker PH Prior surgical score: an analysis of the prognostic significance of an initial non-definitive surgical intervention in patients with peritoneal carcinomatosis of a colorectal origin undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Abstract in diseases of colon and rectum, March 2016 [DOI] [PubMed]
- 90.Van der Speeten K, Stuart OA, Sugarbaker PH. Pharmacology of perioperative intraperitoneal and intravenous chemotherapy in patients with peritoneal surface malignancy. Surg Oncol Clin N Am. 2012;21(4):577–597. doi: 10.1016/j.soc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 91.Mohamed F, Sugarbaker PH. Intraperitoneal taxanes. Surg Oncol Clin N Am. 2003;12(3):825–833. doi: 10.1016/s1055-3207(03)00038-3. [DOI] [PubMed] [Google Scholar]
- 92.Bakrin N, Bereder JM, Decullier E, et al. Peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol. 2013;22:1570–1575. doi: 10.1016/j.ejso.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 93.Van der Speeten K, Stuart OA, Mahteme H, Sugarbaker PH Pharmacokinetic study of perioperative intravenous ifosfamide. Int J Surg Oncol 2011:Article ID 185092, 9 pages. 10.1155/2011/185092 [DOI] [PMC free article] [PubMed]
- 94.Elias D, Bonnay M, Puizillou JM, Antoun S, Demirdjian S, el Otmany A, Pignon JP, Drouard-Troalen L, Ouellet JF, Ducreux M. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol. 2002;13:267–272. doi: 10.1093/annonc/mdf019. [DOI] [PubMed] [Google Scholar]
- 95.Van Ruth S, Verwaal VJ, Zoetmulder FAN. Pharmacokinetics of intraperitoneal mitomycin C. Surg Oncol Clin N Am. 2003;12:771–780. doi: 10.1016/s1055-3207(03)00031-0. [DOI] [PubMed] [Google Scholar]
- 96.Sugarbaker PH, Van der Speeten K, Stuart OA, et al. Impact of surgical and clinical factors on the pharmacology of intraperitoneal doxorubicin in 145 patients with peritoneal carcinomatosis. Eur J Surg Oncol. 2011;37:719–726. doi: 10.1016/j.ejso.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 97.Sticca RP, Dach BW. Rationale for hyperthermia with intraoperative intraperitoneal chemotherapy agents. Surg Oncol Clin N Am. 2003;12:689–701. doi: 10.1016/s1055-3207(03)00029-2. [DOI] [PubMed] [Google Scholar]
- 98.Sugarbaker PH. Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int J Hyperth. 2007;23:431–442. doi: 10.1080/02656730701455318. [DOI] [PubMed] [Google Scholar]
- 99.Jacquet P, Averbach A, Stuart OA, Chang D, Sugarbaker PH. Hyperthermic intraperitoneal doxorubicin: pharmacokinetics, metabolism, and tissue distribution in a rat model. Cancer Chemother Pharmacol. 1998;41:147–154. doi: 10.1007/s002800050721. [DOI] [PubMed] [Google Scholar]
- 100.Young JS, Lumsden CE, Stalker AL. The significance of the tissue pressure of normal testicular and of neoplastic (Brown-Pearce carcinoma) tissue in the rabbit. J Pathol Bacteriol. 1950;62:313–333. doi: 10.1002/path.1700620303. [DOI] [PubMed] [Google Scholar]
- 101.Leunig M, Goetz AE, Dellian M, Zetterer G, Gamarra F, Jain RK, Messmer K. Interstitial fluid pressure in solid tumors following hyperthermia: possible correlation with therapeutic response. Cancer Res. 1992;52:487–490. [PubMed] [Google Scholar]
- 102.Urano M, Kuroda M, Nishimura Y. Invited review for the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperth. 1999;15:79–107. doi: 10.1080/026567399285765. [DOI] [PubMed] [Google Scholar]
- 103.Sugarbaker PH, van der Speeten K. An overview of peritonectomy, visceral resection, and therapeutic laparoscopy for peritoneal surface malignancy. In: Sugarbaker PH, editor. Cytoreductive surgery & perioperative chemotherapy for peritoneal surface malignancy. Textbook and video atlas (second edition) Woodbury: Cine-Med Publishers; 2017. pp. 17–46. [Google Scholar]
- 104.Benoit L, Cheynel N, Ortega-Deballon P, Giacomo GD, Chauffert B, Rat P. Closed hyperthermic intraperitoneal chemotherapy with open abdomen: a novel technique to reduce exposure of the surgical team to chemotherapy drugs. Ann Surg Oncol. 2008;15:542–546. doi: 10.1245/s10434-007-9635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yan TD, Black D, Savady R, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2007;14(2):484–492. doi: 10.1245/s10434-006-9182-x. [DOI] [PubMed] [Google Scholar]
- 106.Feldman AL, Libutti SK, Pingpank JF, Bartlett DL, Beresnev TH, Mavroukakis SM, Steinberg SM, Liewehr DJ, Kleiner DE, Alexander HR. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol. 2003;21(24):4560–4567. doi: 10.1200/JCO.2003.04.150. [DOI] [PubMed] [Google Scholar]
- 107.Hesdorffer ME, Chabot JA, Keohan ML, Fountain K, Talbot S, Gabay M, Valentin C, Lee SM, Taub RN. Combined resection, intraperitoneal chemotherapy, and whole abdominal radiation for the treatment of malignant peritoneal mesothelioma. Am J Clin Oncol. 2008;31(1):49–54. doi: 10.1097/COC.0b013e3180684181. [DOI] [PubMed] [Google Scholar]
- 108.Deraco M, Nonaka D, Baratti D, Casali P, Rosai J, Younan R, Salvatore A, Cabras AD, Kusamura S. Prognostic analysis of clinicopathologic factors in 49 patients with diffuse malignant peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol. 2006;13(2):229–237. doi: 10.1245/ASO.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 109.Verwaal VJ, van Ruth S, Witkamp A, Boot H, van Slooten G, Zoetmulder FAN. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2005;12(1):65–71. doi: 10.1007/s10434-004-1167-z. [DOI] [PubMed] [Google Scholar]
- 110.Shen P, Hawksworth J, Lovato J, Loggie BW, Geisinger KR, Fleming RA, Levine EA. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol. 2004;11(2):178–186. doi: 10.1245/aso.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 111.Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: restrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28(1):63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 112.Verwaal VJ, van Ruth S, de Bree E, van Slooten GW, van Tinteren H, Boot H, Zoetmulder FAN. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 113.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-Year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 114.Yan TD, Black D, Savady R, Sugarbaker PH. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol. 2006;24(24):4011–4019. doi: 10.1200/JCO.2006.07.1142. [DOI] [PubMed] [Google Scholar]
- 115.Xu DZ, Zhan YQ, Sun XW, Cao SM, Geng QR. Meta-analysis of intraperitoneal chemotherapy for gastric cancer. World J Gastroenterol. 2004;10:2727–2730. doi: 10.3748/wjg.v10.i18.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2013 June 13. Identifier NCT01882933, D2 Resection and HIPEC (Hyperthermic Intraperitoneal Chemoperfusion) in Locally Advanced Gastric Carcinoma (GASTRICHIP); [cited 2017 September 20]; Available from: https://clinicaltrials.gov/ct2/show/NCT01882933
- 117.Bijelic L, Jonson A, Sugarbaker PH. Systematic review of cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis in primary and recurrent ovarian cancer. Ann Oncol. 2007;18(12):1943–1450. doi: 10.1093/annonc/mdm137. [DOI] [PubMed] [Google Scholar]
- 118.Elias D, Raynard B, Bonnay M, Pocard M. Heated intra-operative intraperitoneal oxaliplatin alone and in combination with intraperitoneal irinotecan: pharmacologic studies. Eur J Surg Oncol. 2006;32:607–613. doi: 10.1016/j.ejso.2006.03.004. [DOI] [PubMed] [Google Scholar]