Abstract
PET/CT has made significant inroads into routine oncological practice in recent times. In our study, we aim to determine its value in preoperative assessment of endometrial carcinoma. A retrospective study between January 2011 and March 2016 was conducted; we included all cases of carcinoma endometrium with a preoperative PET/CT scan. PET/CT images were analyzed and correlated with histological findings after surgical staging. A total of 46 cases were analyzed, mean age was 59.8 years, BMI 30.8 kg/m2, and most common histology endometrioid type (69.5%). We correlated PET/CT findings with histopathology as reference standard. PET/CT had a sensitivity of 40%, moderate specificity (75%) and accuracy (71.7%), good NPV (91.2%), but poor PPV (16.7%) for lymph node involvement. A total of 10 (21.7%) cases were detected to have distant metabolically active lesions on PET/CT, seven out of these were positive for malignancy. And 90% of them were either non-endometrioid type or grade two and higher. We found that SUV of primary tumor was significantly higher in patients with deep myometrial invasion (p = 0.018), and high-risk histological type of tumor (p = 0.022), though not statistically significant when lymph nodal involvement (p = 0.9), cervical involvement (p = 0.56), or histological grade (p = 0.84) were considered. Sensitivity and specificity of PET/CT in staging endometrial cancer is not high enough to reliably tailor lymphadenectomy. Although SUV of the primary tumor was significantly higher in patients with deep myometrial invasion and high-risk histological type, it’s usefulness in classifying patients into predefined risk groups seems to be limited. However, it is useful in detecting distant metastasis especially in high-grade and non-endometrioid type of tumors. Thus, implementation of PET/CT as a surrogate for surgical staging of endometrial cancer remains enigmatic and is open to further research.
Keywords: PET/CT, Positron emission tomography/computed tomography, Endometrial carcinoma, Lymphadenectomy
Introduction
Endometrial carcinoma is the second most common gynecological malignancy with an incidence of 5.9 per 100,000 women in most developing countries. In India, the incidence is 4.3 per 100,000 women [1] with a cumulative risk for a diagnosis of 1.71% [2]. Most of these cases occur in the postmenopausal age group with a median age at diagnosis of 63 years [3]. Overall, 5-year survival of endometrial carcinomas is about 81.7% [4]. The majority of these cancers are diagnosed early (80% in stage I), with 5-year survival rates of over 95%. However, 5-year survival rates are much lower if there is regional spread or distant disease (68 and 17%, respectively) [5].
Historically, endometrial carcinoma has been classified into two main clinicopathological and molecular types: type I is the much more common endometrioid adenocarcinoma (80–90%); and type II comprises non-endometrioid subtypes, such as serous, clear cell, and undifferentiated carcinomas, as well as carcinosarcoma/malignant-mixed Mullerian tumor (10–20%), which have worse prognosis compared to type I tumors [6].
Staging of this malignancy is based on surgicopathologic International Federation of Gynecologic Oncology (FIGO) criteria [7, 8].
The surgicopathologic staging system uses findings from exploratory laparotomy/laparoscopy, peritoneal washings for cytology, total abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic and paraaortic lymphadenectomy.
Adjuvant treatment in the form of radiation therapy with or without chemotherapy is indicated for locally advanced and metastatic disease [3].
Though surgical staging is the standard management, cross-sectional imaging is commonly used to aid in preoperative evaluation. Potential advantages of presurgical imaging include assessment of the depth of myometrial invasion, which in turn may predict the likelihood of lymph node involvement and also determine the surgical procedure required (lymphadenectomy may be omitted in grade 1 or 2 lesions < 2 cm with < 50% myoinvasion); determination of cervical invasion which again dictates the procedure to be planned (type II radical hysterectomy); the identification of suspicious lymph nodes suggestive of metastatic disease and the detection of distant metastasis itself which may require a different modality of treatment altogether.
There is substantial evidence that addresses the diagnosis and management of endometrial carcinoma. A pelvic examination and pelvic ultrasonography are mandatory components of the clinical staging of endometrial cancer in order to establish a tentative FIGO staging before definitive pathology [3]. Ultrasonography is an essential tool to evaluate abnormal uterine bleeding. In cases of carcinoma endometrium, it offers the possibility of evaluating the size of tumor, ruling out ovarian disease and assessing myometrial invasion and cervical stromal involvement [9].
Many other imaging techniques have been tried and tested to aid in the preoperative evaluation of endometrial carcinoma, including computerized tomography (CT), magnetic resonance imaging (MRI), and most recently PET/CT. Additional imaging has been considered optional according to the clinical situation. Computed tomography scan and/or positron emission tomography (PET/CT) have been recommended as options in clinically advanced endometrial cancer [9]. MRI may be useful mainly in stage I tumors to assess the depth of myometrial invasion, cervical invasion, tumor size, and also the lymph node status to tailor the lymphadenopathy. Most of these, including MRI, use a short-axis diameter of 10 mm or greater to identify suspicious lymph nodes. However, there is significant overlap between the sizes of metastatic and reactive lymph nodes. PET/CT, as we hypothesize, may be able to bridge this gap, which allows for simultaneous acquisition of anatomic and metabolic information.
Hence, we undertook this study to evaluate the role of PET/CT in the preoperative assessment of depth of myometrial invasion, cervical extension, and lymph nodal status, and in cases of endometrial carcinoma, to correlate the SUV (SUVmax) of primary tumor on PET/CT with the lymph nodal, myometrial, cervical involvements, histopathological grade, and type of the tumor.
Patients and Methods
A retrospective study was conducted in a tertiary oncology referral Centre between January 2011 and March 2016. The study was approved by the institutional ethics committee.
All cases of carcinoma endometrium with a preoperative PET/CT scan were included in the study. All the patients irrespective of the PET/CT findings underwent a comprehensive surgical staging as described earlier. PET/CT images were analyzed and correlated with histological findings of surgical staging.
Data was analyzed using SPSS, Ver.10.0.5. The results were averaged (mean + standard deviation) for continuous data and percentage for dichotomous data. Proportions were compared using chi-square (χ2) test of significance. The mean values between the groups were compared using the one-way ANOVA. Diagnostic accuracy of PET/CT with lymph nodal, myometrial, and cervical involvements was assessed by calculating the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy. In all the above tests, “p” value of less than 0.05 was accepted as indicating statistical significance.
Results
There were total 46 cases of carcinoma endometrium in these study periods which were analyzed. Mean age was 59.8 years, ranging between 33 and 76 years. Mean BMI was 30.8 kg/m2, ranging between 20.6 and 53.4 kg/m2. Most common histology was endometrioid type of adenocarcinoma (69.5%). Majority of the patients were postmenopausal (84.8%). When coexistent medical conditions were considered, 54.3% were hypertensive, 47.8% diabetic, and 8.7% had hypothyroidism.
Out of the total 46 carcinoma endometrium patients, 19/46 (41.3%) were FIGO stage IA, 11/46(23.9%) were stage IB, 4/46(8.6%) were stage II, 6/46 (13.04%) were stage III, and the rest 6/46 (13.04%) belonged to stage IVB.
We correlated PET/CT findings based on increased tracer uptake, independent of size with histopathology as reference standard (Table 1). PET/CT in our study had a sensitivity of 40%, specificity of 75% and accuracy of 71.7% for lymph nodes. The ability to correctly identify metastatic lymph nodes is, however, largely affected by lymph node size as well. Size-based sensitivities of 80 and 20% in metastatic nodes with positive tracer uptake and sizes ≥ 10 and < 10 mm, respectively, were noted in our study. With respect to myometrial involvement, PET/CT had a sensitivity of 72.2%, specificity of 75%, and accuracy of 72.5% with a good PPV (96.3%). Although a specificity of 94.1% was noted when cervical involvement was considered, results were precluded by sensitivity of 44.4% and PPV of 66.7%.
Table 1.
Sensitivity, specificity, PPV, NPV and accuracies of PET/CT for lymph nodal involvement, myometrial, and cervical involvement
| PET/CT | Sensitivity | Specificity | Positive predictive value (PPV) | Negative predictive value (NPV) | Accuracy |
|---|---|---|---|---|---|
| Lymph nodal involvement | 40% | 75% | 16.7% | 91.2% | 71.7% |
| Myometrial involvement | 72.2% | 75% | 96.3% | 23.1% | 72.5% |
| Cervical involvement | 44.4% | 94.1% | 66.7% | 86.5% | 83.7% |
A total of 10 (21.7%) cases were detected to have distant metabolically active lesions on PET/CT, seven of these were positive for malignancy. one had a metabolically active cervical lymph node (SUV: 24) with a synchronous papillary carcinoma thyroid. Histology of the lymph node proved to be a metastasis from thyroid. A metabolically active adrenal mass (SUV 10) was seen in another patient, who underwent a partial adrenalectomy, which was histologically normal. Two other patients had mild metabolically active pretracheal and hilar LNs which were histologically proven to be benign. Six other patients were histologically proven to have distant metastasis from primary endometrial carcinoma, and they received neoadjuvant chemotherapy (NACT). Following this, response to therapy was evaluated with a repeat PET/CT, and good response was noted in all six cases, who then underwent surgery. Ninety percent of these patients with stage IV cancer, either had non-endometrioid type of cancer or grade two and higher disease.
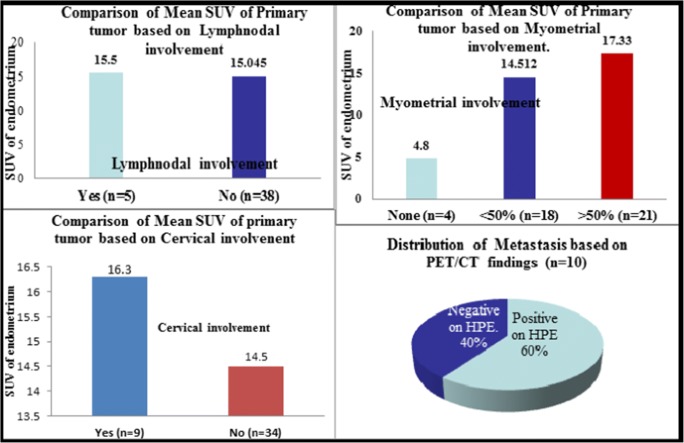
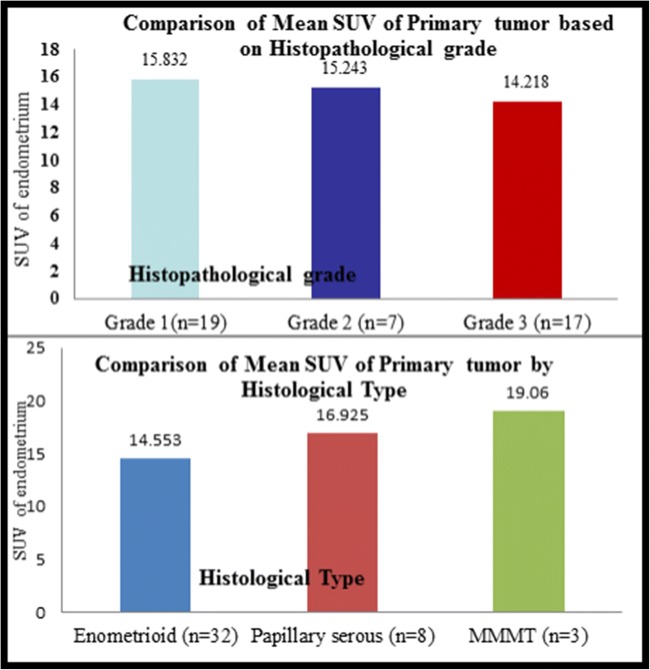
We further analyzed if there is any correlation between the SUV of the primary tumor and certain factors (Figs. 1 and 2). Of the 46 patients, 3 were referred to our institute following the detection of endometrial carcinoma on a hysterectomy specimen; hence, we analyzed the rest 43 cases. The mean SUV was 4.8 when there was no myometrial involvement (n = 4), 14.5 with < 50% myometrial involvement (n = 18) and 17.3 when > 50% myometrium was involved (n = 21). When cervical involvement was considered, there 9 positive cases in which mean SUV recorded was 16.3 and 34 negative cases with SUV of 14.5. With respect to lymph node involvement, it was positive in 5 cases and negative in 38 cases. These had a SUV of 15.04 and 15.09 respectively. There were 33 cases of endometrioid carcinoma with mean SUV of 14.5, 8 cases of papillary serous carcinoma with SUV of 16.9, and three carcinosarcoma cases with SUV of 19.06. We also analyzed the correlation of SUV with grade of the tumor and noted that it was 15.8, 15.2, and 14.2 respectively for grade 1, 2, and 3 tumors (n = 19, 7, 17 respectively). Thus, SUV of primary tumor was significantly higher in patients with deep myometrial invasion (p = 0.018) and high-risk histological type of the tumor (p = 0.022), though not statistically significant when lymph nodal involvement (p = 0.9), cervical involvement (p = 0.56), or histological grade (p = 0.84) were considered.
Fig. 1.
Charts representing Comparison of SUV of primary tumor based on lymh nodal, myometrial, and cervical involvements. Chart depicting the distribution of metastasis correlating with PET/CT findings
Fig. 2.
Charts representing comparison of SUV of primary tumor. Based on histopathological grade and type
Discussion
Lymphadenectomy presently is one of the prerequisites of FIGO surgical staging for endometrial cancer despite an unproven survival benefit [10, 11] and associated morbidity. However, an accurate noninvasive nodal staging for endometrial cancer may help us in staging the disease without adding onto the morbidity. Since endometrial cancer cells demonstrate increased tumor glucose metabolism and glycolysis rate, FDG PET/CT imaging could prove to be a useful preoperative adjunct.
A literature review conducted by Peterson et al. in 2016, suggested the usefulness of PET/CT in lung, lymphoma, melanoma, head and neck, and colorectal cancers, whereas evidence was sparse in gynecological cancers [12]. Following this in December 2016, Bollineni et al. [13] did a systematic review and meta-analysis of 13 studies for the role of PET/CT in detecting lymph nodal involvement in endometrial cancer which showed a pooled sensitivity of 72%, much higher than our study which stood at 40%. Thus, there are still a majority of cases which in which nodal involvement is missed. One of the explanations could be the necessity of a minimum number of tumor cells which exhibit increased glucose metabolism. In an attempt to increase the sensitivity, even though the spatial resolution of PET/CT is not very high, we included the size criteria and metabolically active nodes > 1 cm were considered. This increased the sensitivity to 80%. Literature quotes node-based sensitivities of 13%, 67%, and 100% in metastatic lymph nodes of 4 mm or smaller, 5–9 mm, and of 10 mm or larger, respectively, in endometrial cancer [14]. Specificity, as stated by the meta-analysis was 94%, and in our study, it was 75% [13]. Again, this is not sufficiently high to omit the complete surgical staging based on PET/CT. This is also supported by a low PPV (16.9%) which again does not correlate with the literature which states a high pooled PLR of 10.9 [13].
When we consider PET/CT for myometrial involvement, Antonsen et al. [15] reported a sensitivity of 93% while our study showed 72.2% sensitivity, although, specificity, PPV, and accuracy were higher in our study. Dynamic contrast-enhanced MRI has a pooled sensitivity and specificity of 81 and 72%, respectively [16], and T2-weighted imaging has a pooled sensitivity and specificity of 87 and 58%, respectively, in the assessment of myometrial invasion [17]. Dynamic contrast-enhanced images and T2WI together have an accuracy of 98% for assessing myometrial invasion [18].
With respect to cervical involvement, our study showed comparable values for sensitivity, specificity, PPV, NPV, and accuracy as stated by Antonsen et al., all of which stood in moderation. The sensitivity, specificity, and diagnostic accuracy of MRI imaging assessment of cervical invasion have been reported to be 100%, 87%, and 90%, respectively, on T2WI; 100%, 95%, and 96%, respectively, on post-contrast T1WI; and 100%, 100%, and 100%, respectively, on dynamic MRI [19].
With the existent evidence, when myometrial and cervical invasions are considered, diagnostically, PET/CT has been outdone by MRI. Studies in uterine cancer have shown the power of PET/CT for the detection of local relapse and distant metastases, as this may cause a change in management in 22–35% of cases [20, 21]. In our study, 6/46 (13%) were proven to have distant metastasis and had an altered course of treatment with NACT. Ninety percent of these patients with stage IV cancer, either had non-endometrioid type of cancer or grade two and higher disease. ESMO states FDG PET/CT to be generally superior to CECT and MRI for detecting distant metastasis in advanced stage patients and has recommended PET/CT for detecting distant metastases in current clinical practice guidelines [17, 22]. Thus, our study also supports this recommendation that on suspicion of advanced or high-risk uterine cancer PET/CT is recommended for the choice between surgical or systemic treatment [23]. Table 2 summarizes various studies including ours.
Table 2.
Sensitivity, specificity, PPV, NPV of PET/CT in various studies
| Name | Year | Type of study | Parameter | Sensitivity | Specificity | Accuracy | PPV | NPV | PLR | NLR |
|---|---|---|---|---|---|---|---|---|---|---|
| Bollineni et al. [13] | 2016 | Systematic review and meta-analysis | LNM | 72 | 94 | 88 | – | – | 10.9 | 0.36 |
| Chang et al. [24] | 2012 | Systematic review and meta-analysis | LNM | 63 | 94.7 | – | – | – | 10.46 | 0.39 |
| Kim et al. [25] | 2016 | Monocentric prospective study | LNM | 70 | 95.4 | – | – | 94.3 | – | – |
| Husby et al. [26] | 2015 | Monocentric prospective study | LNM | 74–85% | 91–96% | 89–93% | ||||
| CI | 25–33% | 74–87% | ||||||||
| Signorelli et al. [27] | 2015 | Monocentric prospective study | LNM | 73.3% | 98.7% | 93.6% | 93.3% | 93.6% | – | – |
| Crivellaro et al. [28] | 2013 | Monocentric prospective study | LNM | 78.6% | 98.4% | 94.7% | 91.7% | 95.3% | – | – |
| Antonsen et al. [15] | 2012 | Multicetric prospective comparative study | LNM | 74.2 | 92.8 | 91% | 59%, | 96% | – | – |
| MI | 93% | 49% | 61% | 41% | 95% | |||||
| CI | 43% | 94% | 83% | 69% | 85% | |||||
| Katijima et al. [14] | 2009 | Prospective monocentric study | LNM | 53.3 | 99.6 | 97.8 | – | – | – | – |
| Current study | 2016 | Retrospective analysis | LNM | 40% | 75% | 71.7% | 16.7% | 91.2% | ||
| MI | 72.2% | 75% | 72.5% | 96.3% | 23.1% | |||||
| CI | 44.4% | 94.1% | 83.7% | 66.7% | 86.5% |
Several recent studies in endometrial cancer have attempted to correlate the preoperative primary tumor metabolic parameters with lymph node metastasis, myometrial and cervical involvements, and histological type and grade. All of these have found significant higher SUVmax of the endometrial tumor with nodal metastasis, myometrial and cervical involvements, and higher grade and high-risk histological types. These have been summarized in Table 3. However, our study showed a significantly higher SUVmax of primary tumor only with respect to myometrial involvement (p = 0.018) and high-risk histological type (p = 0.022) but failed to give similar results when lymph nodal metastasis (p = 0.9), cervical involvement (p = 0.56), and histological grade (p = 0.56) were considered. In this regard, our study does not uphold the usefulness of preoperative PET/CT imaging of primary endometrial carcinomas with keen interest on SUVmax of the primary tumor as a tool for prognostication and lymph nodal metastasis detection that facilitate personalized patient care.
Table 3.
SUV of primary tumor when correlated with different parameters in various studies and their ‘p’ values
| Name | Year | LNM | MI | CI | Grade | Type |
|---|---|---|---|---|---|---|
| Husby et al. [26] | 2016 | Yes, 0.025 | Yes, 0.015 | – | Yes, 0.015 | – |
| Yahata et al. [29] | 2016 | Yes | Yes | – | Yes | Yes |
| Crivellaro et al. [28] | 2013 | Yes, 0.038 | – | – | – | – |
| Walentowicz-Sadlecka et al. [16] | 2013 | – | Yes | – | Yes | – |
| Antonsen et al. [30] | 2013 | Yes, 0.04 | Yes, 0.002 | Yes, 0.04 | – | Yes, 0.003 |
| Katijima et al. [31] | 2015 | – | Yes, 0.0001 | – | – | – |
| Lee et al. [32] | 2011 | – | Yes, 0.020 | – | Yes, 0.001 | Yes, 0.024 |
| Current study | 2016 | No, 0.9 | Yes, 0.018 | No, 0.56 | No, 0.84 | Yes, 0.022 |
Conclusion
Sensitivity and specificity of PET/CT in staging endometrial cancer reported by our study is not high enough to reliably tailor lymphadenectomy. Although SUV of the primary tumor was significantly higher in patients with deep myometrial invasion and high-risk histological type, it’s usefulness in classifying patients into predefined risk groups seems to be limited. However, it is useful in detecting distant metastasis especially in high grade and non-endometrioid type of tumors. Considering the limitations of our study, such as the retrospective nature, inadequately powered study due to low sample size, we recommend that the potential added value from this novel radiological technique should be explored further with better-designed studies. Also, the assessment of costs and benefits of PET/CT in clinical practice should be done. To conclude, implementation of PET/CT as a surrogate for surgical staging of endometrial cancer remains enigmatic and is open to further research.
Contributor Information
Rohini Kulkarni, Phone: +91-8281376824, Email: dr.rohini.vk@gmail.com.
Rani Akhil Bhat, Email: rani.oncology@gmail.com.
Vibhawari Dhakharia, Email: vibhawari9dr@gmail.com.
Kumar Kallur, Email: kumarkallur@yahoo.com.sg.
Aparna Gangoli, Email: draparnagangoli@yahoo.co.in.
References
- 1.Balasubramaniam G, Sushama S, Rasika B, Mahantshetty U. Hospital-based study of endometrial cancer survival in Mumbai, India. Asian Pac J Cancer Prev. 2013;14:977–980. doi: 10.7314/APJCP.2013.14.2.977. [DOI] [PubMed] [Google Scholar]
- 2.WHO (2012) GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. Accessed date 3 Apr 2015
- 3.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza MR & Sessa C (2015) Ann Oncol 2015; 00: 1–26 [DOI] [PMC free article] [PubMed]
- 4.SEER Cancer Statistics Factsheets: Endometrial cancer. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/statfacts/html/corp.html. Accessed Aug 2016
- 5.National Cancer Institute (2015) Endometrial cancer treatment Physician Data Query (PDQ). http://www.cancer.gov/cancertopics/pdq/treatment/endometrial/ healthprofessional. Accessed date 1 Apr 2015
- 6.ACOG ACOG practice bulletin, clinical management guidelines for obstetriciangynecologists, number 65, August 2005: management of endometrial cancer. Obstet Gynecol. 2005;106:413–425. doi: 10.1097/00006250-200508000-00050. [DOI] [PubMed] [Google Scholar]
- 7.Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer. American Joint Committee on Cancer Staging Manual. 7. New York: Springer; 2010. pp. 403–418. [Google Scholar]
- 9.Epstein E, Blomqvist L. Imaging in endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2014;28:721–739. doi: 10.1016/j.bpobgyn.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 11.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomized study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(09)60678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen H, Holdgaard PC, Madsen PH, et al. FDG PET/CT in cancer: comparison of actual use with literature-based recommendations. Eur J Nucl Med Mol Imaging. 2016;43:695–706. doi: 10.1007/s00259-015-3217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bollineni VR, Ytre-Hauge S, Bollineni-Balabay O, Salvesen HB, Haldorsen IS. High diagnostic value of 18F-FDG PET/CT in endometrial cancer: systematic review and meta-analysis of the literature. J Nucl Med. 2016;57:879–885. doi: 10.2967/jnumed.115.170597. [DOI] [PubMed] [Google Scholar]
- 14.Kitajima K, Murakami K, Yamasaki E, Kaji Y, Sugimura K. Accuracy of integrated FDG-PET/contrast-enhanced CT in detecting pelvic and paraaortic lymph node metastasis in patients with uterine cancer. Eur Radiol. 2009;19:1529–1536. doi: 10.1007/s00330-008-1271-8. [DOI] [PubMed] [Google Scholar]
- 15.Antonsen SL, Jensen LN, Loft A, Berthelsen AK, Costa J, Tabor A, Qvist I, Hansen MR, Fisker R, Andersen ES, Sperling L, Nielsen AL, Asmussen J, Høgdall E, Fagö-Olsen CL, Christensen IJ, Nedergaard L, Jochumsen K, Høgdall C. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer - a multicenter prospective comparative study. Gynecol Oncol. 2013;128(2):300–308. doi: 10.1016/j.ygyno.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Wu WJ, Yu MS, Su HY, Lin KS, Lu KL, Hwang KS. The accuracy of magnetic resonance imaging for preoperative deep myometrium assessment in endometrial cancer. Taiwan J Obstet Gynecol. 2013;52:210–214. doi: 10.1016/j.tjog.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Colombo N, Carinelli S, Colombo A, Marini C, Rollo D, Sessa C, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii27–vii32. doi: 10.1093/annonc/mds268. [DOI] [PubMed] [Google Scholar]
- 18.Peungjesada S, Bhosale PR, Balachandran A, Iyer RB. Magnetic resonance imaging of endometrial carcinoma. J Comput Assist Tomogr. 2009;33:601–608. doi: 10.1097/RCT.0b013e31818d4279. [DOI] [PubMed] [Google Scholar]
- 19.Murakami T, Kurachi H, Nakamura H, Tsuda K, Miyake A, Tomoda K, Hori S, Kozuka T. Cervical invasion of endometrial carcinoma-evaluation by parasagittal MR imaging. Acta Radiol. 1995;36:248–253. doi: 10.1177/028418519503600307. [DOI] [PubMed] [Google Scholar]
- 20.Pelikan HM, Trum JW, Bakers FC, Beets-Tan RG, Smits LJ, Kruitwagen RF. Diagnostic accuracy of preoperative tests for lymph node status in endometrial cancer: a systematic review. Cancer Imaging. 2013;13(3):314–322. doi: 10.1102/1470-7330.2013.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadkhodayan S, Shahriari S, Treglia G, Yousefi Z, Sadeghi R. Accuracy of 18-F-FDG PET imaging in the follow up of endometrial cancer patients: systematic review and meta-analysis of the literature. Gynecol Oncol. 2013;128(2):397–404. doi: 10.1016/j.ygyno.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi33–vi38. doi: 10.1093/annonc/mdt353. [DOI] [PubMed] [Google Scholar]
- 23.Dalla Palma M, Gregianin M, Fiduccia P, Evangelista L, Cervino AR, Saladini G, et al. PET/CT imaging in gynecologic malignancies: a critical overview of its clinical impact and our retrospective single center analysis. Crit Rev Oncol Hematol. 2012;83(1):84–98. doi: 10.1016/j.critrevonc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Chang MC, Chen JH, Liang JA, Yang KT, Cheng KY, Kao CH. 18F-FDG PET or PET/CT for detection of metastatic lymph nodes in patients with endometrial cancer: a systematic review and metaanalysis. Eur J Radiol. 2012;81(11):3511–3517. doi: 10.1016/j.ejrad.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 25.Kim HJ, Cho A, Yun M, Kim YT, Kang WJ. Comparison of FDG PET/CT and MRI in lymph node staging of endometrial cancer. Ann Nucl Med. 2016;30:104–113. doi: 10.1007/s12149-015-1037-8. [DOI] [PubMed] [Google Scholar]
- 26.Husby JA, Reitan BC, Biermann M, Trovik J, Bjørge L, Magnussen IJ, Salvesen ØO, Salvesen HB, Haldorsen IS. Metabolic tumor volume on 18F-FDG PET/CT improves preoperative identification of high-risk endometrial carcinoma patients. J Nucl Med. 2015;56(8):1191–1198. doi: 10.2967/jnumed.115.159913. [DOI] [PubMed] [Google Scholar]
- 27.Signorelli M, Crivellaro C, Buda A, Guerra L, Fruscio R, Elisei F, Dolci C, Cuzzocrea M, Milani R, Messa C. Staging of high-risk endometrial cancer with PET/CT and sentinel lymph node mapping. Clin Nucl Med. 2015;40(10):780–785. doi: 10.1097/RLU.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 28.Crivellaro C, Signorelli M, Guerra L, De Ponti E, Pirovano C, Fruscio R, Elisei F, Montanelli L, Buda A, Messa C. Tailoring systematic lymphadenectomy in high-risk clinical early stage endometrial cancer: the role of 18F-FDG PET/CT. Gynecol Oncol. 2013;130(2):306–311. doi: 10.1016/j.ygyno.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Yahata T, Yagi S, Mabuchi Y, Tanizaki Y, Kobayashi A, Yamamoto M, Mizoguchi M, Nanjo S, Shiro M, Ota N, Minami S, Terada M, Ino K. Prognostic impact of primary tumor SUVmax on preoperative 18F-fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography in endometrial cancer and uterine carcinosarcoma. Mol Clin Oncol. 2016;5:467–474. doi: 10.3892/mco.2016.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antonsen SL, Loft A, Fisker R, Nielsen AL, Andersen ES, Høgdall E, Tabor A, Jochumsen K, Fagö-Olsen CL, Asmussen J, Berthelsen AK, Christensen IJ, Høgdall C. SUVmax of 18FDG PET/CT as a predictor of high-risk endometrial cancer patients. Gynecol Oncol. 2013;129(2):298–303. doi: 10.1016/j.ygyno.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Kitajima K, Suenaga Y, Ueno Y, Maeda T, Ebina Y, Yamada H, Okunaga T, Kubo K, Sofue K, Kanda T, Tamaki Y, Sugimura K. Preoperative risk stratification using metabolic parameters of 18F FDG PET/CT in patients with endometrial cancer. Eur J Nucl Med Mol Imaging. 2015;42:1268–1275. doi: 10.1007/s00259-015-3037-2. [DOI] [PubMed] [Google Scholar]
- 32.Lee HJ, Ahn BC, Hong CM, Song BI, Kim HW, Kang S, Jeong SY, Lee SW, Lee J. Preoperative risk stratification using (18)F-FDG PET/CT in women with endometrial cancer. Nuklearmedizin. 2011;50(5):204–213. doi: 10.3413/nukmed-0375-10-12. [DOI] [PubMed] [Google Scholar]