Abstract
Osteoporosis is a major health problem in terms of fracture probability and disability. The aim of this ecological study is to identify the temporal trends in osteoporosis mortality in Spain from 1999 to 2015. Data on the Spanish population and number of deaths due to osteoporosis were obtained from the Spanish National Institute for Statistics. Age-adjusted mortality rates were estimated. Join point regression was used to identify the years when changes in mortality s and annual percentage change in mortality rates took place. Women presented a greater mortality rate decrease (p < 0.001), though this mortality difference by sex was reduced by half at the end of the period. The higher the age, the faster the mortality rate declined in women, while no clear pattern could be identified in men. In women, significant changes in trends were identified in three age groups (50–54, 60–64 and 80–84 years old). A sustained decrease in osteoporosis-associated mortality was found in women aged 75–79 and ≥85 years and men aged 60–64. In conclusion, mortality caused by osteoporosis in Spain is decreasing faster in the older age ranges especially in women.
Introduction
Osteoporosis is defined as a systemic skeletal disease characterized by low bone mineral density (BMD) and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to osteoporotic fractures which are a leading cause of morbidity and mortality worldwide1,2. Due to its prevalence, osteoporosis is considered a serious public health concern. It is estimated that over 200 million people suffer from this disease worldwide3. It affects about 27.6 million people in the European Union, the prevalence being 22.1% in women and 6.6% in men aged 50 years o over4. In Spain, the prevalence of osteoporosis is higher in women -reaching 26%- and lower in men -being 4.15%- and it is expected to increase with an aging population5. Men older than 50 present a 4.4% prevalence of hip osteoporosis and 4.8% of lumbar spine osteoporosis being much lower than in women5. In the USA, 2005–2008, the prevalence of osteoporosis of the lumbar spine ranges from 6.8% in women aged 50 to 596. The age group ≥75 years presents a considerable difference between hip and lumbar spine osteoporosis prevalence, being 24% and 40% respectively5. Osteoporotic fractures are widely considered to be the most serious outcome of osteoporosis and an increased risk of death is noticeably existent in both women and men, especially after hip fracture7. Regarding temporal trends of osteoporotic fracture repercussions, studies in western populations have reported increases in hip fracture incidence through the second half of the 20th century; in the last two decades, however, rates seem to have stabilized while age-adjusted rates have decreased3. Azagra et al. have described that in Spain, the trend of hip fractures according to age groups and gender is clearly down, warding only women aged between 65 and 80 while this remains rather stable in the 80–84-year-old group and presents a significant increase in the 85-year-old on groups. Nevertheless, the mortality rate dropped remarkably in both sexes8.
Changes in the osteoporotic fracture and mortality rates trend could be related to diagnosis and treatment improvements. Regarding the diagnosis, though Dual-energy X-ray absorptiometry (DXA) is the most widely used technique to assess bone mineral, there are others that include: quantitative ultrasound, quantitative computed tomography, peripheral DXA, digital X-ray radiogrammetry, radiographic absorptiometry9 and magnetic resonance imaging10.
The WHO fracture prediction tool FRAX was created in 2008 in order to estimate fracture risk by using risk factors11. According to FRAX, a patient fracture risk over 10 years can be classified as low (<10% in the next 10 years), moderate (10–20% in the next 10 years), or high (>20% in the next 10 years)12. Other fracture risk calculators are available online13.
Regarding pharmacological interventions, for many years the prevention of postmenopausal bone loss was marked by the use of hormone replacement therapy (HRT)12 until in 2001, when Women’s Health Initiative study finding the association between HT and several cancers was published14,15. The widespread use in the prescription of bisphosphonates in developed countries began in 2002. Since then, an increasing use has been observed16. Alendronate was the first oral bisphosphonate drug for treatment of osteoporosis in 1995, followed by risedronate in 1998, and ibandronate in 2005. The generic alendronate became available in 2008 and generic ibandronate in 201212,17.
The effect that the introduction of new diagnosis methods and drugs for treating osteoporosis would have had on mortality due to osteoporosis is uncertain. The main objective of this study was to evaluate trends in the mortality rate of osteoporosis in Spain from 1999 until 2015 and their relationship to changes in diagnosis and treatment in recent decades.
Methods
Data extraction
This ecological study used data on the number of people dying from osteoporosis divided or grouped according to sex and within a 5-year-diffeence, age groups from 50 years old on were obtained from the Spanish National Institute for Statistics, which obtained its data from national death certificates that listed osteoporosis as the cause of death. Such deaths were identified implementing, ninth revision (ICD-9) diagnosis codes, M.80 through M81.9 according to the International Classification of Diseases.
Statistical analysis
To identify changes in mortality rate trends, join point regression was estimated for every age and sex group by using the Join point Regression Program, Version 4.5.0.1 (Statistical Research and Applications Branch, National Cancer Institute).
In brief, by using mortality rates as inputs, this method identifies the year(s) when a trend change is produced, it calculates the annual percentage change (APC) in rates between trend-change points, and it also estimates the average annual percentage change (AAPC) in the whole period studied.
To estimate the APC, the following model is used:
, where log (Yx) is the natural logarithm of the rate in year x.
Then, the APC from year x to year x + 1 is:
When there are no join points (i.e., no changes in trend), APC is constant, so it equals the AAPC. Otherwise, the whole period is segmented by the points with trend change. Then, AAPC is estimated as a weighted average of the estimated APC in each segment by using the segment lengths as weights. For instance, in 50- to 54-year-old men, join point regression identifies two join points in 2005 and 2009, so the whole period is segmented in three periods: 1999–2005, 2005–2009, and 2009–2015, with APC equal to – 0.014, − 0.032, and – 0.012, respectively, and segment widths equal to 6, 4 and 6 years, respectively. Then, AAPC is estimated as:
An approximate 95% confidence interval for AAPC is: (AAPCL,AAPCU), where
and is the estimate of the variance of bx obtained from the fit of the join point model.
The number of join points is obtained using a permutation test via Monte Carlo resampling18. Once the number k of join points has been obtained, the different models with k join points are compared by estimating their Bayesian Information Criterion (BIC)19. This procedure is detailed in the Supplementary material.
To further explore changes in trends related to events linked to diagnoses or treatment of osteoporosis in our country we have developed a point regression analysis not allowing the program to estimate the years of trend change, but pre-specifying the join points in 2003 (the year bisphosphonates were introduced) and 2008 (the year generic bisphosphonates and FRAX were introduced). Therefore, this further analysis three set periods: (1) 1999–2003 release and implementation of the Guide of Diagnosis and Treatment of Osteoporosis, (2) 2003–2008 the period of the bisphosphonates and (3) 2008–2015 the period of the generic bisphosphonates and FRAX introduction. APC for a pre-specified period is obtained as the average of the APCs previously obtained in the regular join point analysis, weighted by the number of years included in each period. This analysis has no meaning in age groups without join points: Firstly, let us further explain how it works when there is one join point: consider an age group with a join point in 2005, having APC = −1.5% in 1999–2005 and APC = −3.0% in 2005–2015. In the analysis using pre-determined join points, we will have APC = −1.5% in 1999–2003 (as −1.5 is the APC for the whole period 1999–2005), APC = [2*(−1.5%) + 10*(−3.0%)]/12, where 2 is the number of years with APC = −1.5 (i.e., 2003–2005) and 10 is the number of years with APC = −3.0% (i.e., 2005–2015). Secondly, let us apply the same procedure when there is no join point: consider an age group without join points, having APC = −2.0% in 1999–2015. In the analysis with pre-determined points the result will always be the same: APC = −2.0% in 1999–2003, APC = −2.0% in 2003–2008 and APC = −2.0% in 2008–2015. The benefit of the analysis method used allow us to identify changes affecting different age groups in different years However, a more tradittional aproach (age- and sex-adjusted mortality rates) only would allow us to identify changes affecting the population altogether. Moreover, changes in younger age groups could be undetectable -had they existed- as the impact of older age groups on age-adjusted mortality is much higher.
To further analyze mortality due to osteoporosis in Spain, an age, period and cohort analysis was performed using Poisson regression with natural cubic splines with six knots for age, five knots for period (=year of death) and three knots for birth cohort20.
Results
General trend in mortality
Figure 1 displays the decreasing trend of the age-adjusted mortality rates by gender, which is more pronounced in women (p < 0.001). During the period under study, the highest osteoporosis mortality rate registered in Spain was in 1999, in both women and men (23.1/100.000 in women and 17.7/100 000 in men), decreasing around 38% in women and 33% in men at the end of the period (2015). As women presented a higher rate decrease, mortality rate differences among women and men in 2015 –at the end of the period- halved those in 1999 (5.4/10000 in 1999 vs. 2.5/100000 in 2015).
Figure 1.
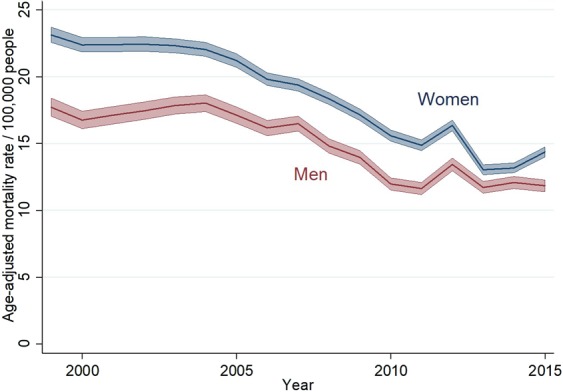
Age-adjusted mortality caused by Osteoporosis in Spain, 2000–2015; women (red line) and men (blue line).
Trend in mortality by age and sex according to the join points identified by the analysis
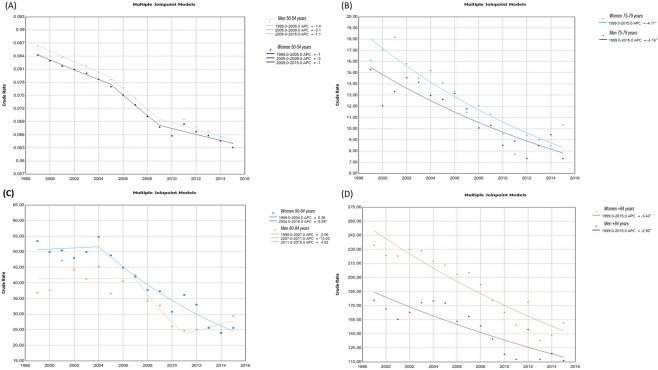
As osteoporosis is a disease influenced by age, in our analysis a specific analysis was made in age groups (five-year wide groups). Table 1 shows the trends in mortality caused by osteoporosis in Spain by gender, according to the join points identified by the analysis. Columns headed with AAPC indicate the trend in the whole period 1999–2015: mortality due to osteoporosis decreased in most age groups in both women and men, although such a trend was more pronounced in women over 70. Columns headed with APC display the mortality trend in each period identified by the join point regression; in five age groups (namely, 55–59, 65–69, 70–74, 75–79 and 85+), the analysis found no join point in either men or women, so APC equals AAPC. Two join points were identified in the 50–54 years old group in 2005 and 2009, leading to three periods with different trend; the fastest descendent trend in mortality was found in the intermediate period (2005/2009). Three periods (jointed in 2002 and 2009) were also identified in women, but not in men, aged 60–64; the intermediate period (2002/2009) showed a 3.4% annual decreased in mortality, while the trends were towards an increase in mortality in periods 1999/2002 and 2009/2015 (2.9% and 0.4% annual increase, respectively). Two periods were identified in the 80–84-year-old group; in women, there was an almost plain trend in 1999/2004 and a strong decrease in 2004/2015 (−6.5% annual change), while in men the decrease began in 2007 (−13% decrease in 2007/2011), but it was followed by an increase in 2011/2015. Results from selected age groups are displayed in Fig. 2.
Table 1.
Trends in mortality caused by Osteoporosis in Spanish women and men: Year of change of trend, annual percentage change, and annual average percentage change.
| Age group, yrs | Period | Women | Period | Men | ||||
|---|---|---|---|---|---|---|---|---|
| Change year | APC (95% CI) | AAPC (95% CI) | Change year | APC (95%CI) | AAPC (95% CI) | |||
| 50–54 | 1999–2015 | −1.7 (−2.3,−1.1) | 1999–2015 | −1.8 (−2.3, −1.3) | ||||
| 1999–2005 | 2005 | − 1.4 (−2.1,−0.7) | 1999–2005 | 2005 | −1.4 (−2.1, −0.8) | |||
| 2005–2009 | 2009 | − 3.2 (−5.2,−1.1) | 2005–2009 | 2009 | − 3.2 (−5.0, −1.3) | |||
| 2009–2015 | − 1.0 (−1.8,−0.3) | 2009–2015 | − 1.2 (−1.8, −0.5) | |||||
| 55–59 | 1999–2015 | None | +1.2 (−3.5, +6.2) | +1.2 (−3.5, +6.2) | 1999–2015 | None | − 1.5 (−3.8, +1.0) | −1.5 (−3.8, +1.0) |
| 60–64 | 1999–2015 | −0.8 (−1.3, −0.3) | 1999–2015 | None | −5.1 (−8.9, −1.2) | −5.1 (−8.9, −1.2) | ||
| 1999–2002 | 2002 | +2.9 (+0.6, +5.1) | ||||||
| 2002–2009 | 2009 | −3.4 (−4.1, −2.7) | ||||||
| 2009–2015 | +0.4 (−0.3, +1.2) | |||||||
| 65–69 | 1999–2015 | None | −2.5 (−7.7, +3.1) | −2.5 (−7.7, +3.1) | 1999–2015 | None | +0.5 (−5.5, +6.9) | +0.5 (−5.5, +6.9) |
| 70–74 | 1999–2015 | None | −4.8 (−10.8, +1.6) | −4.8 (−10.8, +1.6) | 1999–2015 | None | −0.4 (−4.3, +3.6) | −0.4 (−4.3, +3.6) |
| 75–79 | 1999–2015 | None | −4.7 (−5.7, −3.7) | −4.7 (−5.7, −3.7) | 1999–2015 | None | −4.2 (−5.2, −3.1) | −4.2 (−5.2, −3.1) |
| 0–84 | 1999–2015 | −4.4 (−5.9, −3.0) | 1999–2015 | −2.4 (−6.5, +1,8) | ||||
| 1999–2004 | 2004 | +0.4 (−4.0, +5.0) | 1999–2007 | 2007 | +0.1 (−3.4, 3.6) | |||
| 2004–2015 | −6.5 (−7.9, −5.2) | 2007–2011 | 2011 | −13.0 (−25.7, +1.8) | ||||
| 2011–2015 | +4.0 (−5.7, +14.7) | |||||||
| 85+ | 1999–2015 | None | −3.4 (−4.2, −2.7) | 1999–2015 | None | −2.90 (−3.8, −2.0) | ||
APC = annual percent change; CI = confidence interval. AAPC = annual average percent change; CI = confidence interval.
Figure 2.
(A) Join point models 50–54 years. (B) Join point models 75–79 years. (C) Joinpoint models 80–84 years. (D) Join point models +84 years.
Trend in mortality defined by age and sex in pre-specified periods
To explore the influence of changes in treatment or diagnosis on mortality trends, we have estimated the average APC in three predefined periods (Table 2): the first one (1999–2003, before bisphosphonate introduction) presented only a 1.4% decrease both in women and men in the 50–54 years age group. Nevertheless, in the second period (2003–2008, after bisphosphonates introduction) the same age group presented a more pronounced decline in both genders compared with the previous period (−2.5 95% CI −3.6 to −1.3 in women and −2.5% 95% CI −3.5 to −1.5 in men). In addition, women in the 80–84 years age group showed the higher significant decline (−5.2% (95% CI −6.4 to 4.0)). Finally, between 2008–2015 (third period, after generic bisphosphonates and FRAX introduction) in the 50–54 age group the decrease was 1.3% in women and 1.5% in men, the 6.5% decrease observed in women in the 80–84 years age group is worth highlighting.
Table 2.
Trends in mortality with pre-designed join point caused by Osteoporosis in Spanish men and women.
| Age group, yrs | Period | Women | Period | Men |
|---|---|---|---|---|
| AAPC (95% CI) | AAPC (95% CI) | |||
| 50–54 | 1999–2003 | −1.4 (−2.1, −0.7) | 1999–2003 | −1.4 (−2.1, −0.8) |
| 2003–2008 | −2.5 (−3.6, −1.3) | 2003–2008 | −2.5 (−3.5, −1.5) | |
| 2008–2015 | −1.3 (−1.9, −0.7) | 2008–2015 | −1.5 (−2.0, −0.9) | |
| 60–64 | 1999–2003 | +1.2 (− 0.2, +2,7) | ||
| 2003–2008 | − 3.4 (− 4.1, −2.7) | |||
| 2008–2015 | − 0.1 (− 0.7, +0.4) | |||
| 80–84 | 1999–2003 | +0.4 (−4.0, +5.0) | 1999–2003 | +0.1 (−3.4, +3.6) |
| 2003–2008 | −5.2 (−6.4, −4.0) | 2003–2008 | −2.7 (−6.2, +0.9) | |
| 2008–2015 | −6.5 (−7.9, −5.2) | 2008–2015 | −3.7 (−10.7, +3.9) |
AAPC = annual average percent change; CI = 95% confidence interval.
*p < 0.05.
Of note, this analysis could only be carried out on those age groups which are in Table 1 and identified at least one join point (i.e.: at least two periods in mortality trend).
The age-period-cohort analysis did not find any period or cohort effect in neither women nor men. Its results are displayed in Supplementary Table 1 and Supplementary Fig. 1.
Discussion
According to our results, mortality rates by osteoporosis are decreasing in both Spanish women and men, the decline being faster in women aged over 70. Although we identified different trend periods in some age groups (namely, 50–54, 60–64, 80–84 years old), it is unclear whether the introduction of new diagnosis/screening techniques such-as FRAX or DXA- or new drugs such-as bisphosphonates- could have played a role in the mortality trends we have described.
For a specific measure to be considered responsible for changes in mortality trend, one could have expected some age-related pattern emerging in the analysis. For instance, if alendronate had been generalized in women aged 70 onwards from, say, 2003, then a faster mortality decline could have been expected in women included in all age groups over 70. Our analysis, however, does not support the existence of any clear pattern, as only three non-consecutive age groups experienced trend changes, while most age groups had a sustained decrease in mortality throughout the whole studied period. The fact that 11 out of 16 groups, organized or defined by age and sex have no join points strongly suggests that there has been no change in the study period.
Several factors would explain this lack of accelerated declining pattern after implementing new diagnosis/treatments. Firstly, the low adherence to both osteoporotic treatment at the time of fracture occurrence and DXA testing21; secondly, a great variability observed in osteoporosis treatment among general practitioners, who prescribe medication to a high percentage of women without a high FRAX risk while keeping those women with a high FRAX risk untreated22. Lastly, in our analysis, we have not considered a lag period; i.e.: there could have been a delay between the introduction of new measures for reducing or treating osteoporosis and any effect of them on mortality.
Some results of ours are, however, noteworthy. The steepest decline observed was around 5% in specific age groups (75–79 years old women and 60–64 years old in men). Along the same lines, Azagra et al., studying hip fractures (the most serious cause of mortality) found that in women aged between 75–79 years, the incidence rate fell significantly by 7.7%8. In the same way, men in the 75–79 years old group presented a globally significant decrease that could be related to the recommendation of DXA in men from 70 years on23. Furthermore, screening rates were higher among men older than 75 years24.
In age groups where trend changes were identified, the largest decline in incidence rate was observed in women aged 80–84 years old in the 2004–2015 period. This trend is consistent with the decrease observed in osteoporosis diagnosis (from 73% to 69%) between 2002 and 201225. In this period, oral bisphosphonate initiation shifted towards older women and those with prior fractures26, which corresponds to the increasing focus on primary and secondary fracture prevention of patients at elevated fracture risk given by WHO’s FRAX introduction. In addition, this age group is likely to represent patients with polypharmacy which is associated with better treatment adherence27. On the other hand, the lack of significant changes in tendency observed in men could be related to the fact that men were less likely to receive osteoporosis treatment (8%) compared with women (23.3%) after a hip fracture, as observed in a study between 2000 and 201028. Our results are consistent with a meta-analysis published by Bolland et al., which shows a 10% decrease of the mortality risk associated with osteoporosis treatment in the older population29. However, an ecological studies developed in our country has failed to show correlation between the increasing use of antiresorptive therapy and the incidence of femoral fracture30.
On the other hand, in the youngest group (50–54 years) the greatest decrease in mortality was observed in the 2005–2009 period, which could be related to the decline of the prescription of the HRT14,15 and the increasing use of bisphosphonates16. On the contrary, in the period 2009–2015 a slow decrease in th mortality rate was observed. We can specula that these changes in trend might be associated with the release of generic bisphosphonates14,31. Regarding this point, in February 2008 the brand alendronate patent expired and generic alendronate became available; four years later (2012) ibandronate was marketed as generic17. At the same time, practice guidelines in the UK and elsewhere recommended that generic alendronate should be viewed as the first-line treatment and this currently dominates many European markets32. Since the introduction of generic bisphosphonates, reports have consistently concluded that its adherence is poorer than the original brand32–34, which could eventually lead to higher rates of gastro-intestinal intolerance35, lower increase of lumbar spine and total hip bone mineral density36,37. In addition, age group 50–64 years present high level of treatment and low prevalence of risk factors38,39. In Spain, primary treatment has been associated with lower adherences than secondary treatment27. Treatment adherence represents a common problem in the treatment of osteoporosis34 and it is responsible for an increased risk of fracture of approximately 30%32 and increases the cost-effectiveness ratio of osteoporosis screening strategies26.
Finally, the different trends observed in groups organized by sex in the 60–64 year olds group must be stressed. In men, a sharp decline of mortality trends was detected through the whole period, while women showed a less pronounced decrease. This finding does not seem to be related to changes in bisphosphonates treatment, given that men were less likely to receive osteoporosis treatment after a hip fracture compared with women28. However, the improvement of the evaluation and treatment of glucocorticoid-induced osteoporosis (the most common cause of secondary osteoporosis in men) could be related to this descendent trend40,41. On the other hand, two significant join points were detected in women between 60–64 years old, showing opposite trends: an increase of mortality rate at the early years (1999–2002) and a markedly subsequent decline (2002–2009). The initial increase was probably related to the fact that hormone therapy was the most commonly prescribed treatment in postmenopausal women before 200112, while the later decline could probably be due to the introduction of bisphosphonates from 2002 despite the decline of the proportion of women under 65 years old meeting treatment criteria applying FRAX since 200811.
In addition to the new diagnostic methods and treatments, some articles in the United States42, Canadá43, Sweden44, Denmark45, Portugal46 and Korea, suggest that trends in mortality from osteoporosis could be motivated by a birth cohort effect. For example, the economic or political situation of a country that leads to better or worse maternal nutrition and the nutrition of children47. However, few articles have evaluated cohort and period effects and their results are contradictory regarding the period effect43,44,48–50. However, recent studies suggest that the birth cohort effect and the period effect suppose a significant reduction in the incidence of hip fracture in each cohort or subsequent birth period and these effects are more marked among women than among men43,44. In our article, we have found no birth cohort effect on death due to osteoporosis.
Some reproductive factors observed in United States such as the increase in the average number of reproductive years51, are not sustained either, given that they do not explain the parallel changes in the decrease in hip fractures in men. Similarly, other authors consider other factors such as an increase in calcium and vitamin D intake52, a higher BMI53, greater physical activity, smoking cessation51 and the prevention of falls54 to explain the decreasing trends in the incidence of hip fracture. It may contribute to the decline of hip fractures and therefore the decline of mortality due to osteoporosis. New investigations are necessary to prove it. Despite the high prevalence of osteoporosis in older population, its impact on mortality has scarcely been studied. To the best of our knowledge, this is the first study evaluating trends on mortality caused by osteoporosis developed in Spain. However, our study also has some limitations. Firstly, Join point regression consists in an ecological study, so causal relationship cannot be established and our results require further confirmation with individual-level data. As such, we can only hypothesize about associations highlighted by our data and have strayed from making claims of causality. Secondly, databases of the Spanish National Institute of Statistics do not provide osteoporosis classification data so we are not able to determine which data corresponds to primary osteoporosis or secondary one. Thirdly, the scarce number of studies focused on osteoporosis mortality makes it difficult to compare our results and forces us to contrast them with studies focused on osteoporotic fractures. However, we consider osteoporotic fractures an acceptable proxy to mortality because it has been demonstrated that general fractures, and especially hip fractures, are related to reducing personal autonomy through disability and dependence55, influencing the quality of life56,57 and even mortality58,59.
In conclusion, osteoporosis mortality in Spain is decreasing faster in the older age cohorts especially in women; the causes of this are not clear, but it cannot be fully attributed to improvements in osteoporosis diagnosis or screening or in primary or secondary osteoporosis prevention via bisphosphonates. Further observational studies with individual-level data are needed to clarify if new diagnosis, preventative tools, or their combination could contribute to these changes on mortality due to osteoporosis.
Supplementary information
Author Contributions
I.D., T.D.S. and I.G.A. contributed substantially to the conception, design and acquisition of data. I.D. and T.D.S.: wrote the main manuscript text. I.D. and I.G.A. prepared figures. T.D.S., I.G.A. and J.L. contributed to the analysis and interpretation of the data. All authors participated in revising the manuscript and in the final approval of the version to be published.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Inés Gómez-Acebo and Trinidad Dierssen-Sotos jointly supervised this work.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-40806-0.
References
- 1.Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 94, 646–650 (1993). [DOI] [PubMed]
- 2.Osteoporosis prevention, diagnosis, and therapy. NIH Consens. Statement17, 1–45 (2000). [PubMed]
- 3.Cooper C, et al. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2011;22:1277–1288. doi: 10.1007/s00198-011-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanis JA, et al. SCOPE: a scorecard for osteoporosis in Europe. Arch. Osteoporos. 2013;8:144. doi: 10.1007/s11657-013-0144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sociedad Española de Medicina de Familia y Comunitaria & Grupo de Trabajo de Enfermedades Reumatológicas. Osteoporosis: manejo, prevención, diagnóstico y tratamiento. (SemFYC 2014).
- 6.Gourlay ML, Overman RA, Ensrud KE. Bone Density Screening and Re-screening in Postmenopausal Women and Older Men. Curr. Osteoporos. Rep. 2015;13:390–398. doi: 10.1007/s11914-015-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haentjens P, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann. Intern. Med. 2010;152:380–390. doi: 10.1059/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azagra R, et al. Changing trends in the epidemiology of hip fracture in Spain. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2014;25:1267–1274. doi: 10.1007/s00198-013-2586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanis JA, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2013;24:23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao SS, et al. Thoracic Quantitative Computed Tomography (QCT) Can Sensitively Monitor Bone Mineral Metabolism: Comparison of Thoracic QCT vs Lumbar QCT and Dual-energy X-ray Absorptiometry in Detection of Age-relative Change in Bone Mineral Density. Acad. Radiol. 2017;24:1582–1587. doi: 10.1016/j.acra.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Kanis JA, et al. A systematic review of intervention thresholds based on FRAX: A report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch. Osteoporos. 2016;11:25. doi: 10.1007/s11657-016-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J. Steroid Biochem. Mol. Biol. 2014;142:155–170. doi: 10.1016/j.jsbmb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanis JA, McCloskey E, Johansson H, Oden A, Leslie WD. FRAX(®) with and without bone mineral density. Calcif. Tissue Int. 2012;90:1–13. doi: 10.1007/s00223-011-9544-7. [DOI] [PubMed] [Google Scholar]
- 14.Bolton JL. Menopausal Hormone Therapy, Age, and Chronic Diseases: Perspectives on Statistical Trends. Chem. Res. Toxicol. 2016;29:1583–1590. doi: 10.1021/acs.chemrestox.6b00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baladé Martínez, L., Montero Corominas, D. & Macías Saint-Gerons, D. Uso del tratamiento hormonal sustitutivo en España: tendencias en el período 2000–2014. Med. Clínica 287–292, 10.1016/j.medcli.2016.05.023 (2018). [DOI] [PubMed]
- 16.Salgueiro ME, et al. Trends in the pharmacological treatment of osteoporosis in Spain from 2000 to 2008. Maturitas. 2013;74:74–78. doi: 10.1016/j.maturitas.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Wysowski DK, Greene P. Trends in osteoporosis treatment with oral and intravenous bisphosphonates in the United States, 2002-2012. Bone. 2013;57:423–428. doi: 10.1016/j.bone.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Zhang NR, Siegmund DO. A modified Bayes information criterion with applications to the analysis of comparative genomic hybridization data. Biometrics. 2007;63:22–32. doi: 10.1111/j.1541-0420.2006.00662.x. [DOI] [PubMed] [Google Scholar]
- 20.Sasieni, P. D. Age-period-cohort models in stata. 12 (2012).
- 21.Caeiro JR, et al. Burden of First Osteoporotic Hip Fracture in Spain: A Prospective, 12-Month, Observational Study. Calcif. Tissue Int. 2017;100:29–39. doi: 10.1007/s00223-016-0193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naranjo A, Rosas J, Ojeda S, Salas E. & CANAL group. Management of osteoporosis in primary care before and after the result of densitometry: treatments in real practice versus the recommended by guidelines. CANAL study. Reumatol. Clin. 2013;9:269–273. doi: 10.1016/j.reuma.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Lewiecki EM, et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone. 2008;43:1115–1121. doi: 10.1016/j.bone.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 24.Cheng, N. & Green, M. E. Osteoporosis screening for men: are family physicians following the guidelines? Can. Fam. Physician Med. Fam. Can. 54, 1140–1141, 1141.e1–5 (2008). [PMC free article] [PubMed]
- 25.Whitaker M, Guo J, Kehoe T, Benson G. Bisphosphonates for osteoporosis–where do we go from here? N. Engl. J. Med. 2012;366:2048–2051. doi: 10.1056/NEJMp1202619. [DOI] [PubMed] [Google Scholar]
- 26.Hiligsmann M, et al. Cost-effectiveness of osteoporosis screening followed by treatment: the impact of medication adherence. Value Health. J. Int. Soc. Pharmacoeconomics Outcomes Res. 2010;13:394–401. doi: 10.1111/j.1524-4733.2009.00687.x. [DOI] [PubMed] [Google Scholar]
- 27.García-Sempere A, et al. Primary and secondary non-adherence to osteoporotic medications after hip fracture in Spain. The PREV2FO population-based retrospective cohort study. Sci. Rep. 2017;7:11784. doi: 10.1038/s41598-017-10899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonelli M, Einstadter D, Magrey M. Screening and treatment of osteoporosis after hip fracture: comparison of sex and race. J. Clin. Densitom. Off. J. Int. Soc. Clin. Densitom. 2014;17:479–483. doi: 10.1016/j.jocd.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Bolland MJ, Grey AB, Gamble GD, Reid IR. Effect of osteoporosis treatment on mortality: a meta-analysis. J. Clin. Endocrinol. Metab. 2010;95:1174–1181. doi: 10.1210/jc.2009-0852. [DOI] [PubMed] [Google Scholar]
- 30.Arias LHM, et al. Hip fracture rates and bisphosphonate consumption in Spain. An ecologic study. Eur. J. Clin. Pharmacol. 2013;69:559–564. doi: 10.1007/s00228-012-1337-z. [DOI] [PubMed] [Google Scholar]
- 31.Lee DR, Ettinger B, Chandra M, Hui RL, Lo JC. Changing Patterns in Oral Bisphosphonate Initiation in Women between 2004 and 2012. J. Am. Geriatr. Soc. 2017;65:656–658. doi: 10.1111/jgs.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanis JA, et al. A reappraisal of generic bisphosphonates in osteoporosis. Osteoporos. Int. 2012;23:213–221. doi: 10.1007/s00198-011-1796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diez-Perez A, et al. International Osteoporosis Foundation and European Calcified Tissue Society Working Group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2017;28:767–774. doi: 10.1007/s00198-017-3906-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross S, et al. A meta-analysis of osteoporotic fracture risk with medication nonadherence. Value Health J. Int. Soc. Pharmacoeconomics Outcomes Res. 2011;14:571–581. doi: 10.1016/j.jval.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Rizzoli R, et al. Adverse reactions and drug-drug interactions in the management of women with postmenopausal osteoporosis. Calcif. Tissue Int. 2011;89:91–104. doi: 10.1007/s00223-011-9499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown JP, Davison KS, Olszynski WP, Beattie KA, Adachi JD. A critical review of brand and generic alendronate for the treatment of osteoporosis. SpringerPlus. 2013;2:550. doi: 10.1186/2193-1801-2-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ringe JD, Möller G. Differences in persistence, safety and efficacy of generic and original branded once weekly bisphosphonates in patients with postmenopausal osteoporosis: 1-year results of a retrospective patient chart review analysis. Rheumatol. Int. 2009;30:213–221. doi: 10.1007/s00296-009-0940-5. [DOI] [PubMed] [Google Scholar]
- 38.Sanfélix-Genovés J, et al. Prevalence of osteoporotic fracture risk factors and antiosteoporotic treatments in the Valencia region, Spain. The baseline characteristics of the ESOSVAL cohort. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2013;24:1045–1055. doi: 10.1007/s00198-012-2018-6. [DOI] [PubMed] [Google Scholar]
- 39.Sanfélix‐Gimeno G, Sanfélix‐Genovés J, Rodriguez‐Bernal CL, Peiró S, Hurtado I. Prevalence, determinants, and inappropriateness of calcium supplementation among men and women in a Spanish Mediterranean area: Cross-sectional data from the ESOSVAL cohort. J. Bone Miner. Res. 2013;28:2286–2294. doi: 10.1002/jbmr.1977. [DOI] [PubMed] [Google Scholar]
- 40.Korpi-Steiner N, Milhorn D, Hammett-Stabler C. Osteoporosis in men. Clin. Biochem. 2014;47:950–959. doi: 10.1016/j.clinbiochem.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 41.Newman ED, et al. Glucocorticoid-Induced Osteoporosis Program (GIOP): a novel, comprehensive, and highly successful care program with improved outcomes at 1 year. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2006;17:1428–1434. doi: 10.1007/s00198-006-0149-3. [DOI] [PubMed] [Google Scholar]
- 42.Nam H-S, et al. Race/ethnic differences in bone mineral densities in older men. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2010;21:2115–2123. doi: 10.1007/s00198-010-1188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jean S, et al. Trends in hip fracture rates in Canada: an age-period-cohort analysis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013;28:1283–1289. doi: 10.1002/jbmr.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosengren BE, et al. Secular trends in Swedish hip fractures 1987-2002: birth cohort and period effects. Epidemiol. Camb. Mass. 2012;23:623–630. doi: 10.1097/EDE.0b013e318256982a. [DOI] [PubMed] [Google Scholar]
- 45.Abrahamsen B, Heitmann BL, Eiken PA. Season of birth and the risk of hip fracture in danish men and women aged 65+ Front. Endocrinol. 2012;3:2. doi: 10.3389/fendo.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alves SM, Castiglione D, Oliveira CM, de Sousa B, Pina MF. Age-period-cohort effects in the incidence of hip fractures: political and economic events are coincident with changes in risk. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2014;25:711–720. doi: 10.1007/s00198-013-2483-6. [DOI] [PubMed] [Google Scholar]
- 47.Ballane G, Cauley JA, Luckey MM, Fuleihan GE-H. Secular Trends in Hip Fractures Worldwide: Opposing Trends East Versus West. J. Bone Miner. Res. 2014;29:1745–1755. doi: 10.1002/jbmr.2218. [DOI] [PubMed] [Google Scholar]
- 48.Evans JG, Seagroatt V, Goldacre MJ. Secular trends in proximal femoral fracture, Oxford record linkage study area and England 1968-86. J. Epidemiol. Community Health. 1997;51:424–429. doi: 10.1136/jech.51.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samelson EJ, Zhang Y, Kiel DP, Hannan MT, Felson DT. Effect of birth cohort on risk of hip fracture: age-specific incidence rates in the Framingham Study. Am. J. Public Health. 2002;92:858–862. doi: 10.2105/ajph.92.5.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langley J, Samaranayaka A, Davie G, Campbell AJ. Age, cohort and period effects on hip fracture incidence: analysis and predictions from New Zealand data 1974–2007. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2011;22:105–111. doi: 10.1007/s00198-010-1205-6. [DOI] [PubMed] [Google Scholar]
- 51.Nichols HB, et al. From menarche to menopause: trends among US Women born from 1912 to 1969. Am. J. Epidemiol. 2006;164:1003–1011. doi: 10.1093/aje/kwj282. [DOI] [PubMed] [Google Scholar]
- 52.Abrahamsson PA, Artibani W, Chapple CR, Wirth M. [European Association of Urology. Position statement on screening for prostate cancer] Actas Urol. Esp. 2010;34:221–222. [PubMed] [Google Scholar]
- 53.Looker AC, Melton LJ, Borrud LG, Shepherd JA. Changes in femur neck bone density in US adults between 1988-1994 and 2005-2008: demographic patterns and possible determinants. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2012;23:771–780. doi: 10.1007/s00198-011-1623-0. [DOI] [PubMed] [Google Scholar]
- 54.Gates S, Fisher JD, Cooke MW, Carter YH, Lamb SE. Multifactorial assessment and targeted intervention for preventing falls and injuries among older people in community and emergency care settings: systematic review and meta-analysis. BMJ. 2008;336:130–133. doi: 10.1136/bmj.39412.525243.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 56.Roux C, et al. Burden of non-hip, non-vertebral fractures on quality of life in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW). Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2012;23:2863–2871. doi: 10.1007/s00198-012-1935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zwart M, et al. Measuring health-related quality of life in men with osteoporosis or osteoporotic fracture. BMC Public Health. 2011;11:775. doi: 10.1186/1471-2458-11-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grønskag AB, Romundstad P, Forsmo S, Langhammer A, Schei B. Excess mortality after hip fracture among elderly women in Norway. The HUNT study. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2012;23:1807–1811. doi: 10.1007/s00198-011-1811-y. [DOI] [PubMed] [Google Scholar]
- 59.Librero J, et al. Timing of surgery for hip fracture and in-hospital mortality: a retrospective population-based cohort study in the Spanish National Health System. BMC Health Serv. Res. 2012;12:15. doi: 10.1186/1472-6963-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.