Abstract
Background
Observational studies suggest that some patients meeting criteria for ARDS no longer fulfill the oxygenation criterion early in the course of their illness. This subphenotype of rapidly improving ARDS has not been well characterized. We attempted to assess the prevalence, characteristics, and outcomes of rapidly improving ARDS and to identify which variables are useful to predict it.
Methods
A secondary analysis was performed of patient level data from six ARDS Network randomized controlled trials. We defined rapidly improving ARDS, contrasted with ARDS > 1 day, as extubation or a Pao2 to Fio2 ratio (Pao2:Fio2) > 300 on the first study day following enrollment.
Results
The prevalence of rapidly improving ARDS was 10.5% (458 of 4,361 patients) and increased over time. Of the 1,909 patients enrolled in the three most recently published trials, 197 (10.3%) were extubated on the first study day, and 265 (13.9%) in total had rapidly improving ARDS. Patients with rapidly improving ARDS had lower baseline severity of illness and lower 60-day mortality (10.2% vs 26.3%; P < .0001) than ARDS > 1 day. Pao2:Fio2 at screening, change in Pao2:Fio2 from screening to enrollment, use of vasopressor agents, Fio2 at enrollment, and serum bilirubin levels were useful predictive variables.
Conclusions
Rapidly improving ARDS, mostly defined by early extubation, is an increasingly prevalent and distinct subphenotype, associated with better outcomes than ARDS > 1 day. Enrollment of patients with rapidly improving ARDS may negatively affect the prognostic enrichment and contribute to the failure of therapeutic trials.
Key Words: acute lung injury, acute respiratory failure, epidemiology, ICUs
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ARDSNet, ARDS Network; BioLINCC, Biologic Specimen and Data Repository Information Coordinating Center; NHLBI, National Heart, Lung, and Blood Institute; PEEP, positive end-expiratory pressure; riARDS, rapidly improving ARDS
FOR EDITORIAL COMMENT, SEE PAGE 453
ARDS is a common and highly morbid condition in the ICU.1 Although there has been progress in supportive care of patients with ARDS,2, 3, 4 no targeted pharmacologic intervention has been proven beneficial.5, 6 The failure of clinical trials exploring pharmacologic therapies for ARDS has been attributed to the substantial heterogeneity of this syndrome.6, 7 ARDS can be divided into subphenotypes based on clinical (eg, underlying risk factor), physiological (eg, severity of hypoxemia), radiologic (eg, extension of pulmonary infiltrates), and biological (eg, biomarkers of lung and systemic injury) criteria, or a combination of them.6 Using a combination of clinical (presence of sepsis and shock), physiological (lower serum levels of bicarbonate), and biological (higher plasma levels of inflammatory biomarkers) criteria, researchers identified a subphenotype of ARDS associated with high mortality as opposed to a subphenotype associated with moderate mortality.8
At the other end of the spectrum, a subphenotype of ARDS associated with low mortality should exist. The Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure (LUNG SAFE) study,1 a worldwide analysis of the modern epidemiology of ARDS, reported that almost one-sixth of patients meeting criteria of the Berlin definition9 no longer fulfill these criteria after 24 h. Previous observational studies had shown that application of standardized ventilator settings may improve the measured Pao2 to Fio2 ratio (Pao2:Fio2) in some patients with ARDS, and therefore these patients may not continue to have Pao2:Fio2 ≤ 300 after 24 h.10, 11, 12, 13 This subphenotype, which we denote as rapidly improving ARDS (riARDS), has not been well characterized. Also, the rate of enrollment of patients with riARDS into therapeutic trials and whether one can identify them at the time of trial enrollment has not been explored. The present study analyzed the well-phenotyped clinical data from the ARDS Network (ARDSNet) randomized controlled trials, funded by the National Heart, Lung, and Blood Institute (NHLBI), to assess the prevalence, characteristics, and outcomes of riARDS and to identify which variables are useful to predict it.
Patients and Methods
Study Design and Patient Population
We performed a secondary analysis of data from 4,361 patients with ARDS enrolled in the following ARDSNet prospective therapeutic clinical trials: Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Low-Tidal-Volume (VT) Trial (ARMA),14 Assessment of Low Tidal Volume and Elevated End-expiratory Volume to Obviate Lung Injury (ALVEOLI),15 Fluid and Catheter Treatment Trial (FACTT),16 Albuterol for the Treatment of Acute Lung Injury (ALTA),17 Early vs Delayed Enteral Nutrition (EDEN),18 and Statins for Acutely Injured Lungs from Sepsis (SAILS).19 Subjects from the Omega Nutrition Supplement Trial (OMEGA)20 were included in this analysis as part of the EDEN trial18 because patients were enrolled in both studies. Subjects from the Late Steroid Rescue Study (LaSRS) were not considered for the present analysis because they needed to have late-phase ARDS.21 All patients had to receive positive-pressure mechanical ventilation through an endotracheal tube, had a Pao2:Fio2 ≤ 300, and had bilateral infiltrates on chest radiography that were consistent with pulmonary edema, with no of left atrial hypertension.22 Additional details on characteristics of the ARDSNet trials14, 15, 16, 17, 18, 19 are provided in e-Table 1. We were granted access to data collected in each trial through the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) of the NHLBI,23 following submission of a prospective protocol that is available in the Supplemental Material (e-Protocol, e-Appendix 1). Because the data would be received in de-identified form (non-human subjects research), the Institutional Review Board at Weill Cornell Medicine granted a waiver of the need for informed consent and approved the study (#1702018012).
Definition of riARDS
We defined riARDS by using the following criteria: (1) Pao2:Fio2 > 300 on the first study day following enrollment; and/or (2) achieving unassisted breathing on the first study day following enrollment and remaining free from assisted breathing for at least 48 h. Unassisted breathing was defined as extubated with face mask, nasal prong oxygen, or room air, T-tube breathing, tracheostomy collar breathing, or continuous positive airway pressure of ≤ 5 cm H2O without pressure support. All patients not explicitly meeting these criteria on the first study day following enrollment were considered to have ARDS > 1 day, including patients who remained intubated without an available Pao2:Fio2. A sensitivity analysis was performed after excluding intubated patients without an available Pao2:Fio2 on the first study day. Owing to the potential of Pao2:Fio2 measurements to depend on ventilator settings,10, 11, 12, 13 a strict definition of riARDS, which was independent of Pao2:Fio2 and was restricted to patients achieving unassisted breathing on the first study day following enrollment, was used in a sensitivity analysis.
Statistical Analysis
Continuous and categorical variables are presented for patients with riARDS vs patients with ARDS > 1 day using medians (interquartile range) and count (percentages) and testing for differences between groups with nonparametric Mann-Whitney U tests and χ2 tests, respectively.
The prevalence of riARDS was estimated across time via a study-level least squares linear regression, with time as the independent variable and within-study prevalence of riARDS as the dependent variable.24 To further assess whether changes in prevalence of riARDS across time could be explained by background ventilator practice or severity of illness, we performed multivariate logistic regression analysis. This analysis had year of study publication as its primary independent variable, and it controlled for study-wide ventilator practice (using median ventilator-free days among patients with ARDS > 1 day), for the number of days from meeting criteria for ARDS to enrollment, and for individual severity of illness (using Acute Physiology and Chronic Health Evaluation III [APACHE III] scores).
The primary outcome of the present study was 60-day mortality, with patients discharged from the hospital with unassisted breathing prior to 60 days considered to be alive at 60 days. Time to mortality was estimated for riARDS and ARDS > 1 day according to Kaplan-Meier analysis and compared by using log-rank tests. We further tested the association between riARDS and the primary outcome with a Cox proportional hazards regression, estimating the odds of mortality within 60 days and using riARDS status as the main covariate. The analysis corrected for severity of illness by using the APACHE III score, Pao2:Fio2 at enrollment, and individual trial assignment. Secondary outcomes included the number of days in the first 28 days that a patient was alive and not on a ventilator (ventilator-free days), not in the ICU (ICU-free days), or free of nonpulmonary organ failure (nonpulmonary organ failure-free days). These secondary outcomes have been consistently used in the literature.4, 5, 19, 25 For each individual trial, both the primary and secondary outcomes were also compared across experimental treatment groups among patients with riARDS and ARDS > 1 day.
We attempted to identify which variables are important to predict riARDS as early as the time of trial enrollment (when patients were ventilated according to a standardized ARDS Clinical Trials Network lower tidal-volume protocol using positive end-expiratory pressure [PEEP]:Fio2 tables).17, 18, 19 We randomly divided patients with full clinical data enrolled in the three most recently published ARDSNet trials (ALTA, EDEN, and SAILS) into a training and a validation dataset. Machine learning techniques were then used to analyze a large number of individual variables that were associated with riARDS in the derivation dataset. Random forests were used for the predictive variables based on level of importance for riARDS status, and the top variables were selected for further study. A final set of predictive variables was determined by using insights from the machine learning techniques as well as clinical expertise to optimize sensitivity and negative predictive value. A logistic regression model was then created based on these selected variables to quantify the independent effect of each variable in a parametric model. Multicollinearity of the model was explored by using correlation and variance inflation factors, with a variance inflation factor > 2 considered problematic. We measured accuracy of the logistic model with area under the receiver-operating curve, then dichotomized it at the Youden optimal point and estimated sensitivity, specificity, and negative and positive predicted values, with 95% CIs for each. The logistic model was then used to predict riARDS in the validation dataset, to this point unused. Further details are available in e-Appendix 1, Methods.
All statistical analyses were conducted by using R statistical software version 3.2.3 (R Foundation for Statistical Computing). All P values were two-sided, and statistical significance was considered at an α level of 0.05.
Results
Prevalence of riARDS
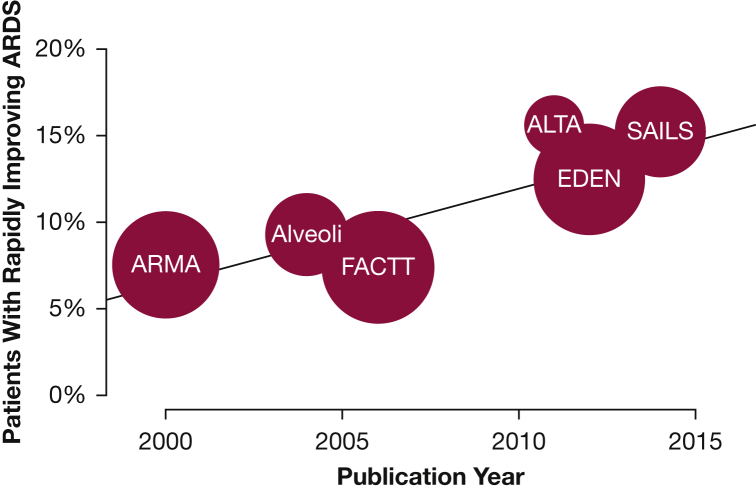
Of the 4,361 unique patients enrolled in the randomized controlled trials,14, 15, 16, 17, 18, 19 458 (10.5%) no longer met the criteria for ARDS on the first study day following enrollment. The proportion of enrolled subjects classified as riARDS increased over time, from a prevalence of 7.3% in ARMA14 to 15.2% in SAILS19 (r2 = 0.760; P = .024) (Fig 1). The association between year of study publication and riARDS status remained when accounting for study-wide ventilator practice, number of days from diagnosis of ARDS to trial enrollment, and patient-level APACHE III scores (adjusted OR, 1.08; 95% CI, 1.04-1.13; P = .0003) (e-Table 2).
Figure 1.
Prevalence of rapidly improving ARDS over time. Each circle represents an ARDS Network trial, and circle size is proportional to study sample size. Increase in prevalence of rapidly improving ARDS over time was statistically significant. ALTA = Albuterol for the Treatment of Acute Lung Injury; ALVEOLI = Assessment of Low Tidal Volume and Elevated End-expiratory Volume to Obviate Lung Injury; ARMA = Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Low-Tidal-Volume (VT) Trial; EDEN = Early vs Delayed Enteral Nutrition; FACTT = Fluid and Catheter Treatment Trial; SAILS = Statins for Acutely Injured Lungs from Sepsis.
Due to the increasing prevalence of riARDS over time (Fig 1) and to better reflect modern clinical practice, the remainder of our analyses considered only data from the three most recently published ARDSNet trials; namely, ALTA, EDEN, and SAILS (all published after 2010).17, 18, 19 Of the 1,909 patients in these trials, 197 (10.3%) were extubated on the first study day, and 265 (13.9%) patients in total met our definition of riARDS (Table 1).9
Table 1.
Baseline Characteristics of Patients With Rapidly Improving ARDS vs ARDS> 1 Day
| Characteristic | Rapidly Improving ARDS | ARDS > 1 Day | P Value |
|---|---|---|---|
| No. of patients | 265 (13.9) | 1,644 (86.1) | |
| Age, y | 54 (44-66) | 53 (42 to 64) | .228 |
| Male sex | 132 (49.8) | 834 (50.7) | .833 |
| Race | .534 | ||
| White | 218 (82.2) | 1,322 (80.4) | |
| Black | 40 (15.1) | 256 (15.6) | |
| Other | 7 (2.6) | 66 (4.0) | |
| BMI, kg/m2 | 28 (24 to 34) | 29 (24 to 35) | .369 |
| Comorbidity | |||
| Diabetes mellitus | 65 (24.5) | 421 (25.6) | .761 |
| Malignancy | 22 (8.3) | 116 (7.1) | .549 |
| Cirrhosis | 10 (3.8) | 85 (5.2) | .419 |
| End-stage renal disease | 3 (1.1) | 47 (2.9) | .999 |
| Immunosuppression | 39 (14.7) | 200 (12.2) | .287 |
| Usage of vasopressor agents | 98 (37.0) | 867 (52.7) | < .001 |
| APACHE III score | 80 (64 to 100) | 92 (73 to 112) | < .001 |
| Primary risk factor of ARDS | |||
| Pneumonia | 147 (55.5) | 1,066 (64.8) | .004 |
| Sepsis | 59 (22.3) | 286 (17.4) | .068 |
| Aspiration | 28 (10.6) | 158 (9.6) | .708 |
| Trauma | 9 (3.4) | 55 (3.3) | .999 |
| Multiple transfusions | 8 (3.0) | 17 (1.0) | .016 |
| Other | 16 (6.0) | 67 (4.1) | .197 |
| Nonpulmonary organ failure | |||
| Circulatory | 166 (62.6) | 1,188 (72.3) | .002 |
| Coagulation | 49 (18.5) | 294 (17.9) | .908 |
| Hepatic | 27 (10.2) | 246 (15.0) | .039 |
| Renal | 64 (24.2) | 400 (24.3) | .944 |
| Neurologic | 220 (83.0) | 1,480 (90.0) | .001 |
| Days from intubation to enrollment | 1 (1 to 2) | 1 (1 to 2) | .563 |
| Days from diagnosis of ARDS to enrollment | 1 (0 to 1) | 1 (0 to 1) | .543 |
| Severity of ARDS at screening | < .001 | ||
| Mild | 97 (36.6) | 251 (15.3) | |
| Moderate | 123 (46.4) | 816 (49.6) | |
| Severe | 45 (17.0) | 577 (35.1) | |
| Pao2:Fio2 at screening | 149 (99 to 205) | 118 (80 to 171) | < .001 |
| Change in Pao2:Fio2 from screening to enrollment | 80 (16 to 149) | 25 (–8 to 72) | < .001 |
| Driving pressure | 13 (11 to 16) | 14 (11 to 18) | .055 |
| Plateau pressure | 20 (17 to 25) | 24 (20 to 28) | < .001 |
| Positive end-expiratory pressure | 8 (5 to 10) | 10 (8 to 12) | < .001 |
| Minute ventilation | 10 (8 to 12) | 11 (9 to 13) | < .001 |
| Balance fluid | 93 (–1,419 to 1,900) | –37 (–2,371 to 2,051) | .160 |
| VT per kg of ideal body weight | 7 (6 to 8) | 6 (6 to 7) | .045 |
| VD/VT | 0.52 (0.39 to 0.64) | 0.49 (0.37 to 0.62) | .212 |
| Corrected minute ventilation | 9 (7 to 11) | 11 (9 to 13) | < .001 |
Data are presented as No. (%) or median (interquartile range). Severity of ARDS was categorized based on the Berlin definition.9 APACHE = Acute Physiology and Chronic Health Evaluation; VD/VT = the ratio of physiologic dead space over tidal volume; VT = tidal volume.
Baseline Characteristics
Baseline data are summarized in Table 1. Use of vasopressor agents was less common (98 of 265 [37.0%] vs 867 of 1,644 [52.7%]; P < .001), and APACHE III scores were lower (80 [64-100] vs 92 [73-112]; P < .001) in patients with riARDS compared with ARDS > 1 day. Pneumonia as the primary risk factor was less common in patients with riARDS than with ARDS > 1 day (147 of 265 [55.5%] vs 1,066 of 1,644 [64.8%]; P = .004]. Risk factors did not differ between mild, moderate, and severe ARDS (e-Table 3).
The compared groups differed in severity according to the Berlin definition,9 with patients with riARDS more likely to have mild or moderate disease than severe disease at screening. However, 45 (17.0%) of 265 patients with riARDS had severe hypoxemia at screening. At screening, Pao2:Fio2 was 149 (99 to 205) in patients with riARDS compared with 118 (80 to 171) in patients with ARDS > 1 day (P < .001). Patients with riARDS had a larger increase in Pao2:Fio2 from screening to enrollment than comparators (80 [16 to 149] vs 25 [–8 to 72]; P < .001].
Outcomes
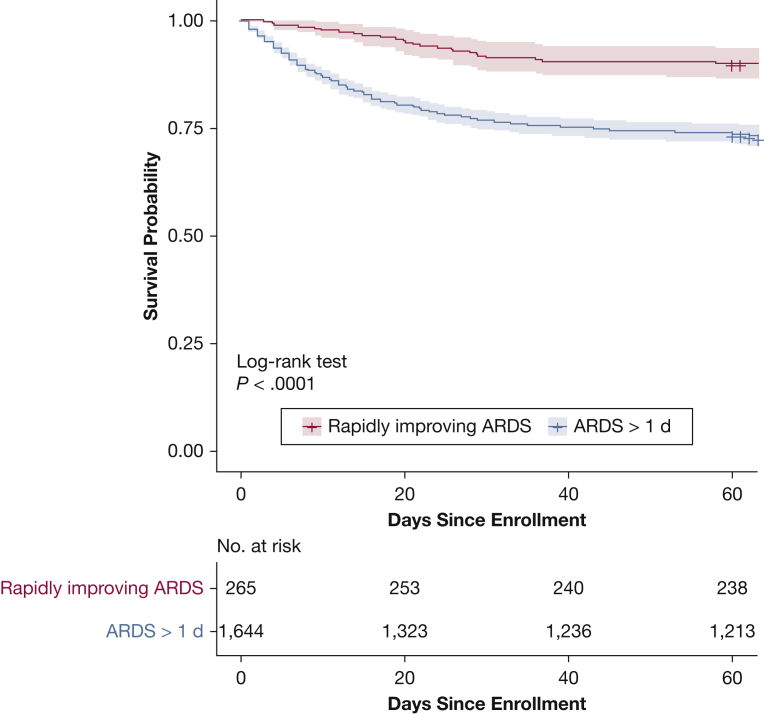
The probability of mortality was significantly lower in patients with riARDS compared with ARDS > 1 day (P < .0001 according to the log-rank test [Kaplan-Meier plot displayed in Fig 2 and e-Fig 1]). Mortality at 60 days was lower in patients with riARDS than in those with ARDS > 1 day (27 of 265 [10.2%] vs 433 of 1,644 [26.3%]; P < .0001] (Table 2). Total number of ventilator-free, ICU-free, and nonpulmonary organ failure-free days was greater in the riARDS group compared with the ARDS > 1 day group (P < .0001 for each comparison).
Figure 2.
Kaplan-Meier curves of mortality for rapidly improving ARDS and ARDS > 1 day. Patients discharged home considered alive at 60 days. Shaded area depicts 95% pointwise CIs for each curve.
Table 2.
Outcomes of Patients With Rapidly Improving ARDS vs ARDS> 1 Day
| Outcome | Rapidly Improving ARDS (n = 265) | ARDS > 1 Day (n = 1,644) | P Value |
|---|---|---|---|
| 60-d mortality | 27 (10.2) | 433 (26.3) | < .0001 |
| Ventilator-free days | 27 (24-27) | 18 (0-23) | < .0001 |
| ICU-free days | 24 (21-26) | 16 (0-21) | < .0001 |
| Nonpulmonary organ failure-free days | 25 (4-27) | 15 (0-25) | < .0001 |
Data are presented as No. (%) or median (interquartile range). Patients discharged from the hospital with unassisted breathing before 60 days were considered to be alive at 60 days. Ventilator-free days, ICU-free days, and nonpulmonary organ failure-free days were calculated by the number of days in the first 28 days that a patient was alive and not on a ventilator, not in the ICU, or free of nonpulmonary organ failure, respectively.
In each individual trial,17, 18, 19 classification as riARDS compared with ARDS > 1 day was associated with lower mortality (e-Table 4). This association between riARDS and mortality persisted even after correction for severity of illness, Pao2:Fio2 at enrollment, and individual trial assignment (e-Table 5). Consistently, in each individual trial, patients with riARDS had more ventilator-free, ICU-free, and nonpulmonary organ failure-free days compared with those with ARDS > 1 day (P < .01 for each comparison). In each trial,14, 15, 16, 17, 18, 19 the estimate of treatment effect was similar among patients with riARDS and patients with ARDS > 1 day (e-Table 6).
Sensitivity Analyses
The results of the sensitivity analyses were consistent with those of the main analysis (e-Tables 7, 8).
Prediction of riARDS at Enrollment
A predictive logistic regression model was built for riARDS by using machine learning techniques. The logistic regression model included Pao2:Fio2 at screening, change in Pao2:Fio2 from screening to enrollment, use of vasopressor agents, Fio2 at enrollment, and serum bilirubin levels. No multicollinearity among predictors was found (all variance inflation factors were < 1.5). The overall area under the receiver-operating curve of the model for predicting riARDS was 0.82 (95% CI, 0.78-0.85) in the derivation dataset and 0.76 (95% CI, 0.69-0.83) in the validation dataset (Table 3).
Table 3.
Logistic Regression Model for Predicting Rapidly Improving ARDS at Trial Enrollment
| Variable | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Pao2:Fio2 at screeninga | 1.05 (1.03-1.08) | < .0001 | 1.08 (1.04-1.12) | < .0001 |
| Change in Pao2:Fio2 from screening to enrollmenta | 1.10 (1.07-1.12) | < .0001 | 1.10 (1.07-1.13) | < .0001 |
| No use of vasopressor agents (vs yes) | 1.73 (1.19-2.52) | .004 | 1.60 (1.06-2.43) | .025 |
| Fio2 ≤ 0.45 | 5.56 (3.75-8.24) | < .0001 | 2.97 (1.90-4.64) | < .0001 |
| Bilirubin | 0.83 (0.71-0.98) | .024 | 0.81 (0.67-0.98) | .027 |
The area under the receiver-operating curve of the model for predicting rapidly improving ARDS was 0.82 (95% CI, 0.78-0.85) (negative predictive value, 97%; positive predictive value, 29%; specificity, 69%; sensitivity, 85%) in the derivation dataset and 0.76 (95% CI, 0.69-0.83) (negative predictive value, 93%; positive predictive value, 26%; specificity, 70%; sensitivity, 68%) in the validation dataset.
Reported as per 10 point difference.
Discussion
This secondary analysis of patient-level data from the ARDSNet trials suggests that not only is riARDS common but also that its prevalence has increased over time. It had distinct characteristics and was strongly and consistently associated with better outcomes compared with ARDS > 1 day, with differences in mortality and nonpulmonary organ failure-free days. Pao2:Fio2 at screening, change in Pao2:Fio2 from screening to enrollment, usage of vasopressor agents, Fio2 at enrollment, and serum bilirubin levels were useful variables for prediction of riARDS.
We found that riARDS was common. When estimating its prevalence, one should keep in mind that, given the lack of a gold standard, ARDS is a challenging diagnosis to make.7 One could therefore support that patients with riARDS had an alternate noninflammatory cause of hypoxemia and bilateral opacities (eg, atelectasis, cardiogenic pulmonary edema) that could be easily reversed.26, 27, 28, 29 This theory would explain both their rapid recovery and better overall outcomes (Fig 2, Table 2). However, all patients included in this secondary analysis met the consensus definition criteria of ARDS and were enrolled in high-quality, randomized controlled therapeutic trials.17, 18, 19
Our finding that the prevalence of riARDS increased over time is intriguing. One could wonder whether this finding is due to differences in exclusion criteria of ARDSNet trials.14, 15, 16, 17, 18, 19 For example, although in the ARMA and ALVEOLI trials14, 15 patients were excluded if clinicians-investigators were unwilling to use volume assist control for at least 12 h, this exclusion criterion was dropped in later trials,16, 17, 18, 19 and any mode of ventilation (including pressure support) was allowed. One could also attribute the temporal trends in prevalence of riARDS to the fact that optimal ICU practices, based in part on earlier ARDSNet studies,14, 16 were more likely applied in more recent than earlier trials. For example, lung protective ventilation and conservative fluid strategies, as well as sedation cessation policies and spontaneous breathing trials, may currently be more prevalent than previously, a fact that may help prevent ventilator-induced lung injury and decrease duration of mechanical ventilation.3, 16, 30 There has also been an increase in full-time intensivist staffing over time, which may have allowed for earlier extubation.31 However, increase in prevalence of riARDS over time remained even after adjustment for study ventilator practice (e-Table 2). Taken together, the increase in the prevalence of riARDS might be explained by differences in exclusion criteria of ARDSNet trials and by progress in supportive care of patients with ARDS.
Patients with riARDS had different baseline characteristics compared with those with ARDS > 1 day. Patients with riARDS had higher Pao2:Fio2 at screening than those with ARDS > 1 day (Table 1). One could argue that riARDS is not essentially different from mild ARDS given that mild ARDS (ie, Pao2:Fio2 > 200) is associated with rapid resolution of ARDS. The multivariate logistic regression model of Table 3 showed that Pao2:Fio2 at screening could indeed predict riARDS. However, as evidenced by the corresponding ORs, Pao2:Fio2 at screening was a less strong predictor of riARDS compared with change in Pao2:Fio2 from screening to enrollment, usage of vasopressor agents, Fio2 at enrollment, and serum bilirubin level. Also, most (63.4%) patients with riARDS had moderate or severe ARDS rather than mild ARDS at screening. Conversely, it is interesting that one in 14 patients with severe ARDS at screening had their ARDS resolved on the first study day. Taken together, riARDS is not simply mild ARDS, and severe hypoxemia at screening does not rule out the possibility that the patient may very soon be extubated.
Patients with riARDS had consistently better outcomes than those with ARDS > 1 day (although the 10.2% mortality of the riARDS group should not be considered inconsequential), regardless of treatment assignment. The estimate of treatment effect was similar among patients with riARDS and patients with ARDS > 1 day in each trial.14, 15, 16, 17, 18, 19 Taken together, these findings suggest that the prognostic enrichment (which refers to enrollment of individuals who are more likely to experience the outcome of interest) rather than the predictive enrichment (which refers to enrollment of individuals who are more likely to respond to a given treatment) of trials might have been negatively affected by the enrollment of patients with riARDS.6, 7, 32, 33 As shown in a recent study of vasopressin in septic shock,34 it is important to remember that prognostic and predictive enrichment do not always go in the same direction; that is, a lower likelihood of dying does not necessarily mean a lower likelihood of responding to a treatment.35
The predictive logistic regression model identifying clinical factors (that may possibly allow for prospective identification of riARDS) along with the prespecification of the statistical analysis plan may be considered among the strengths of the present study. This study also identifies individuals with riARDS across a 20-year horizon, offers the possibility of earlier identification (if validated prospectively), and has implications for the design and interpretation of future clinical trials.
The present study has limitations. First, it is a post hoc secondary analysis. However, we designed the statistical plan prospectively in our study protocol, which was given to BioLINCC (See e-Protocol in Supplemental Material online). Post hoc secondary analyses provide useful insights to better design trials of ARDS.36, 37 Second, we assumed that patients discharged from the hospital with unassisted breathing before 60 days were alive at 60 days. However, given the magnitude of difference between riARDS and ARDS > 1 day in terms of all outcomes examined (Table 2), it is unlikely that this assumption regarding 60-day mortality could undermine the main conclusions of our analysis. Third, although patients were ventilated according to a standardized ARDS Clinical Trials Network lower tidal-volume protocol using PEEP:Fio2 tables,17, 18, 19 one might consider that these tables do not represent a strict standardized approach for assessing Pao2:Fio2 because Pao2:Fio2 values were calculated on PEEP levels ranging from 5 to 12 cm H2O. Fourth, similar to other studies in the field,38 data on Pao2:Fio2 were missing in one-sixth of intubated patients on the first study day following trial enrollment. However, a sensitivity analysis was performed after excluding those patients, and we found similar results with our main analysis (e-Table 8). Finally, as it is well documented in the literature, there is a difference between patients enrolled in randomized controlled trials and those whom clinicians are obliged to treat.39 All patients included in our analysis had been enrolled in randomized controlled trials, which had strict exclusion criteria,14, 15, 16, 17, 18, 19 and may not be generalizable to all patients with ARDS. For example, in all ARDSNet trials, patients with advanced liver disease were excluded, and it is unclear how our predictive model (which considers serum bilirubin levels) would perform in patients with ARDS viewed in clinical practice. Similarly, our findings might not apply to individuals with chronic heart failure or lung disease, who represent an important percentage of patients with ARDS.1, 40, 41 Individuals enrolled in randomized controlled trials tend to have fewer comorbidities and accordingly lower mortality than those included in observational studies or seen in everyday clinical practice.
Conclusions
By using data from the large ARDSNet clinical trial population, this analysis showed that riARDS, mostly defined according to early extubation, is an increasingly prevalent and distinct subphenotype, associated with better outcomes than ARDS > 1 day. Enrollment of patients with riARDS may negatively affect the prognostic enrichment and contribute to the failure of therapeutic trials.
Acknowledgments
Author contributions: E. J. S. designed the study, participated in data cleaning, interpreted the data, wrote the first draft of the manuscript, and critically revised the manuscript. C. O. contributed to study design, conducted the data cleaning and analysis, developed the predictive model, contributed to data interpretation, and critically revised the manuscript. L. K. T., D. A. B., and A. M. K. C. contributed to data interpretation and critically revised the manuscript. I. I. S. conceived the study, designed the study, interpreted the data, critically revised the manuscript. I. I. S. is also is the guarantor, had full access to all the data in the study, and had final responsibility for the decision to submit for publication. All authors approved of the submission.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: A. M. K. C. is a co-founder of Proterris and serving as a consultant for an advisory board meeting of Teva Pharmaceutical Industries, July 2018. None declared (E. J. S., C. O., L. K. T., D. A. B., I. I. S.).
Role of the sponsor: The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Other contributions: This study was prepared by using ARMA, ALVEOLI, FACTT, ALTA, EDEN, and SAILS research materials obtained from the NHLBI BioLINCC. The article does not necessarily reflect the opinions or views of the researchers who performed these trials or the NHLBI. The authors acknowledge the incredible work by the ARMA, ALVEOLI, FACTT, ALTA, EDEN, and SAILS researchers, without which this study would not have been possible.
Additional information: The e-Appendix, e-Figure and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was supported by the National Institutes of Health [Grants KL2TR000458-10 to E. J. S., R01 HL055330 to A. M. K. C., and P01 HL108801 to A. M. K. C.].
Supplementary Data
References
- 1.Bellani G., Laffey J.G., Pham T. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 2.Fan E., Del Sorbo L., Goligher E.C. American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 3.Reade M.C., Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med. 2014;370(5):444–454. doi: 10.1056/NEJMra1208705. [DOI] [PubMed] [Google Scholar]
- 4.Guérin C., Reignier J., Richard J.C., PROSEVA Study Group Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 5.Kor D.J., Carter R.E., Park P.K. US Critical Illness and Injury Trials Group: Lung Injury Prevention with Aspirin Study Group (USCIITG: LIPS-A). Effect of aspirin on development of ARDS in at-risk patients presenting to the emergency department: the LIPS—a randomized clinical trial. JAMA. 2016;315(22):2406–2414. doi: 10.1001/jama.2016.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthay M.A., McAuley D.F., Ware L.B. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respir Med. 2017;5(6):524–534. doi: 10.1016/S2213-2600(17)30188-1. [DOI] [PubMed] [Google Scholar]
- 7.Pham T., Rubenfeld G.D. Fifty years of research in ARDS. The epidemiology of acute respiratory distress syndrome. A 50th birthday review. Am J Respir Crit Care Med. 2017;195(7):860–870. doi: 10.1164/rccm.201609-1773CP. [DOI] [PubMed] [Google Scholar]
- 8.Calfee C.S., Delucchi K., Parsons P.E., NHLBI ARDS Network Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D., Thompson B.T. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 10.Villar J., Pérez-Méndez L., López J., HELP Network An early PEEP/FIO2 trial identifies different degrees of lung injury in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;176(8):795–804. doi: 10.1164/rccm.200610-1534OC. [DOI] [PubMed] [Google Scholar]
- 11.Villar J., Pérez-Méndez L., Blanco J. Spanish Initiative for Epidemiology, Stratification, and Therapies for ARDS (SIESTA) Network. A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting—a prospective, multicenter validation study. Intensive Care Med. 2013;39(4):583–592. doi: 10.1007/s00134-012-2803-x. [DOI] [PubMed] [Google Scholar]
- 12.Villar J., Fernández R.L., Ambrós A. Acute Lung Injury Epidemiology and Natural History Network. A clinical classification of the acute respiratory distress syndrome for predicting outcome and guiding medical therapy. Crit Care Med. 2015;43(2):346–353. doi: 10.1097/CCM.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson N.D., Kacmarek R.M., Chiche J.D. Screening of ARDS patients using standardized ventilator settings: influence on enrollment in a clinical trial. Intensive Care Med. 2004;30(6):1111–1116. doi: 10.1007/s00134-004-2163-2. [DOI] [PubMed] [Google Scholar]
- 14.Acute Respiratory Distress Syndrome Network. Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 15.Brower R.G., Lanken P.N., MacIntyre N. National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(14):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 16.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 17.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized β₂-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Truwit J.D., Bernard G.R., Steingrub J. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370(23):2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice T.W., Wheeler A.P., Thompson B.T., de Boisblanc B.P., Steingrub J., Rock P. NIH NHLBI Acute Respiratory Distress Syndrome Network of Investigators. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinberg K.P., Hudson L.D., Goodman R.B. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354(16):1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 22.Bernard G.R., Artigas A., Brigham K.L. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 23.Coady S.A., Mensah G.A., Wagner E.L., Goldfarb M.E., Hitchcock D.M., Giffen C.A. Use of the National Heart, Lung, and Blood Institute data repository. N Engl J Med. 2017;376(19):1849–1858. doi: 10.1056/NEJMsa1603542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neter J., Kutner M.H., Nachtsheim C.J., Wasserman W. 4th ed. McGraw-Hill; Boston: 1996. Applied Linear Statistical Models. [Google Scholar]
- 25.Oromendia C., Siempos Reclassification of acute respiratory distress syndrome: a secondary analysis of the ARDS Network trials. Ann Am Thorac Soc. 2018;15(8):998–1001. doi: 10.1513/AnnalsATS.201803-192RL. [DOI] [PubMed] [Google Scholar]
- 26.Villar J., Schultz M.J., Kacmarek R.M. The LUNG SAFE: a biased presentation of the prevalence of ARDS! Crit Care. 2016;20(1):108. doi: 10.1186/s13054-016-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siempos, Berlin D.A. Incidence of acute respiratory distress syndrome. JAMA. 2016;316(3):346. doi: 10.1001/jama.2016.6465. [DOI] [PubMed] [Google Scholar]
- 28.Harrington J.S., Schenck E.J., Oromendia C., Choi A.M., Siempos Acute respiratory distress syndrome without identifiable risk factors: a secondary analysis of the ARDS Network trials. J Crit Care. 2018;47:49–54. doi: 10.1016/j.jcrc.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kacmarek R.M., Berra L. Prediction of ARDS outcome: what tool should I use? Lancet Respir Med. 2018;6(4):253–254. doi: 10.1016/S2213-2600(18)30098-5. [DOI] [PubMed] [Google Scholar]
- 30.Laffey J.G., Madotto F., Bellani G., LUNG SAFE Investigators; ESICM Trials Group Geo-economic variations in epidemiology, patterns of care, and outcomes in patients with acute respiratory distress syndrome: insights from the LUNG SAFE prospective cohort study. Lancet Respir Med. 2017;5(8):627–638. doi: 10.1016/S2213-2600(17)30213-8. [DOI] [PubMed] [Google Scholar]
- 31.Garland A., Gershengorn H.B. Staffing in ICUs: physicians and alternative staffing models. Chest. 2013;143(1):214–221. doi: 10.1378/chest.12-1531. [DOI] [PubMed] [Google Scholar]
- 32.Sjoding M.W., Hofer T.P., Co I., Courey A., Cooke C.R., Iwashyna T.J. Interobserver reliability of the Berlin ARDS definition and strategies to improve the reliability of ARDS diagnosis. Chest. 2018;153(2):361–367. doi: 10.1016/j.chest.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prescott H.C., Calfee C.S., Thompson B.T., Angus D.C., Liu V.X. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med. 2016;194(2):147–155. doi: 10.1164/rccm.201512-2544CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell J.A., Lee T., Singer J., Boyd J.H., Walley K.R. Vasopressin and Septic Shock Trial (VASST) Group. The Septic Shock 3.0 Definition and Trials: a vasopressin and septic shock trial experience. Crit Care Med. 2017;45(6):940–948. doi: 10.1097/CCM.0000000000002323. [DOI] [PubMed] [Google Scholar]
- 35.Thompson B.T. Greater treatment effect with lower disease severity: VASST insights. Crit Care Med. 2017;45(6):1094–1095. doi: 10.1097/CCM.0000000000002397. [DOI] [PubMed] [Google Scholar]
- 36.Villar J., Martínez D., Mosteiro F. Stratification and Outcome of Acute Respiratory Distress Syndrome (STANDARDS) Network. Is overall mortality the right composite endpoint in clinical trials of acute respiratory distress syndrome? Crit Care Med. 2018;46(6):892–899. doi: 10.1097/CCM.0000000000003022. [DOI] [PubMed] [Google Scholar]
- 37.Brown S.M., Grissom C.K., Moss M., NIH/NHLBI PETAL Network Collaborators Nonlinear imputation of Pao2/Fio2 from Spo2/Fio2 among patients with acute respiratory distress syndrome. Chest. 2016;150(2):307–313. doi: 10.1016/j.chest.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madotto F., Pham T., Bellani G. LUNG SAFE Investigators and the ESICM Trials Group. Resolved versus confirmed ARDS after 24 h: insights from the LUNG SAFE study. Intensive Care Med. 2018;44(5):564–577. doi: 10.1007/s00134-018-5152-6. [DOI] [PubMed] [Google Scholar]
- 39.Falagas M.E., Vouloumanou E.K., Sgouros K., Athanasiou S., Peppas G., Siempos Patients included in randomised controlled trials do not represent those seen in clinical practice: focus on antimicrobial agents. Int J Antimicrob Agents. 2010;36(1):1–13. doi: 10.1016/j.ijantimicag.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Linko R., Okkonen M., Pettilä V. FINNALI-study group. Acute respiratory failure in intensive care units. FINNALI: a prospective cohort study. Intensive Care Med. 2009;35(8):1352–1361. doi: 10.1007/s00134-009-1519-z. [DOI] [PubMed] [Google Scholar]
- 41.Villar J., Blanco J., Añón J.M., ALIEN Network The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37(12):1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.