Abstract
Normal cystic fibrosis (CF) transmembrane regulator (CFTR) protein has multiple functions in health and disease. Many mutations in the CFTR gene produce abnormal or absent protein. CFTR protein dysfunction underlies the classic CF phenotype of progressive pulmonary and GI pathology but may underlie diseases not usually associated with CF. This review highlights selected extrapulmonary disease that may be associated with abnormal CFTR. Increasing survival in CF is associated with increasing incidence of diseases associated with aging. CFTR dysfunction in older individuals may have novel effects on glucose metabolism, control of insulin release, regulation of circadian rhythm, and cancer cell pathophysiology. In individuals who have cancers with acquired CFTR suppression, their tumors may more likely exhibit rapid expansion, epithelial-to-mesenchymal transformation, abnormally reduced apoptosis, and increased metastatic potential. The new modulators of CFTR protein synthesis could facilitate the additional exploration needed to better understand the unfolding clinical biology of CFTR in human disease, even as they revolutionize treatment of patients with CF.
Key Words: cancer, circadian rhythm, cystic fibrosis transmembrane regulator, hypoglycemia, sleep
Abbreviations: AgRP, agouti-related peptide; ATP, adenosine triphosphate; CF, cystic fibrosis; CFRD, cystic fibrosis-related diabetes; CFTR, cystic fibrosis transmembrane regulator; NPY, neuropeptide Y
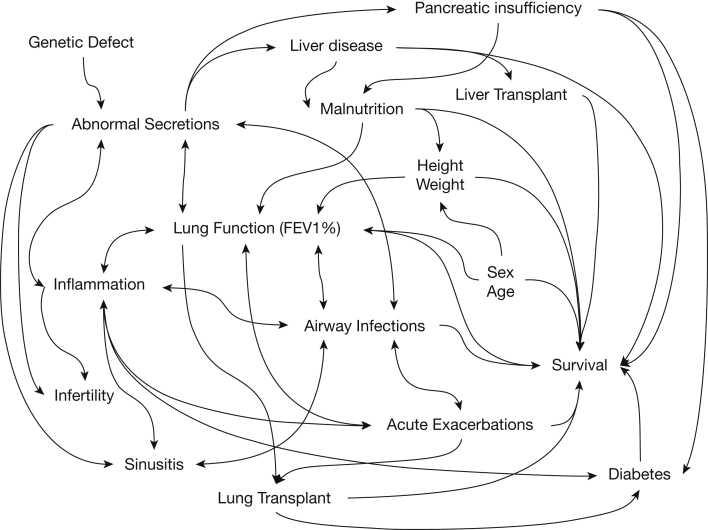
Biallelic mutations of the cystic fibrosis (CF) transmembrane regulator (CFTR) gene resulting in reduced or absent protein function cause CF.1 The observed clinical syndrome of CF centers on airway disease characterized by inflammation, chronic infections, inspissation of tenacious secretions, bronchiectasis, and eventually premature death. This syndrome has been extensively reviewed.2, 3 Beyond lung disease, CF involves multiple organs and encompasses many clinical characteristics. Interactions between organ-specific disease processes within the clinical syndrome create a complex web of likely causal relationships with many possible positive feedback loops (Fig 1).2, 4, 5, 6, 7, 8
Figure 1.
Causal inference diagram of cystic fibrosis clinical disease. Disease begins with allelic homozygosity of cystic fibrosis transmembrane regulator mutations and leads directly to abnormal secretions. Organ-specific disease ensues, directly affecting the lung, sinuses, pancreas, liver, and reproductive organs. Inflammation associated with airway infections and recurrent acute exacerbations of disease drive and are driven by worsening lung function.6 Factors intrinsic to patients such as age and sex alter the effects of cystic fibrosis on lung disease and survival.7 Malnutrition as a result of pancreatic and liver involvement affects the attainment of adult height and weight, and all three affect lung function and survival.7, 8 Sinusitis and infertility do not lead to decreased survival but cause substantial worsening of quality of life, as do other manifestations of disease.2, 4, 5 Various complications of disease include diabetes, which contributes to early death. Treatments such as lung transplantation and liver transplantation markedly change disease and treatment profiles and have effects that alter survival.
Increasing survival in CF allows identification of novel primary disease processes that may adversely affect glucose metabolism, control of insulin release, regulation of circadian rhythm, and cancer cell pathophysiology. Median observed survival has rapidly increased with new medications, treatments, and improvements in systematic care through rigorous accreditation processes by the Cystic Fibrosis Foundation in the United States,8, 9 but survival is improving in the rest of the world, sometimes at an apparently faster rate with equally rigorous attention to the details of care.10, 11, 12, 13 Older individuals are at greater risks of cognitive disorders, diabetes, sleep-wake disorders, cancer, and other chronic disorders.14, 15, 16, 17 Discrimination between processes specifically related to CFTR dysfunction and those related to other processes associated with advanced age may enable design and implementation of effective strategies to treat these diseases in the setting of CF.
Treatments for CF have improved steadily since its description in English in 1938.18 The most recently added therapies, modulators of the gene product, augment biosynthesis and function of the CFTR protein, producing sometimes dramatic clinical improvements in patients with the appropriate targeted mutations.19 Because multiple diseases often coexist in aging individuals and may increasingly appear in patients with CF, CFTR modulator therapy may become an increasingly important treatment with increasing age in CF.
The ability to directly address the underlying causes of progressive lung disease in patients with CF by using CFTR modulators may have unexpected but generally welcome effects on manifestations of abnormal CFTR in other organs. For patients without the classic clinical syndrome of CF, these same modulators may prove useful when CFTR mutations partially underlie pathophysiology. The present review examines the roles of CFTR and emerging data about the potential consequences of dysfunctional protein in multiple tissues and organs to explore some of the extent of CFTR-related disease. The new treatments have the potential to improve survival and quality of life in patients with CF as well as in others with more subtle or acquired defects in CFTR physiology.
These agents are intended to increase cell membrane CFTR protein activity. Corrector molecules increase CFTR protein delivery to the cell surface. Potentiators increase anion flow through CFTR chloride channels already at the cell surface. Amplifiers improve translation of CFTR messenger RNA to increase CFTR protein production, ultimately to facilitate the action of correctors. Other agents that potentially increase CFTR protein activity do not clearly fit in these categories,20, 21, 22, 23 or they have characteristics matching more than one category.
Cystic Fibrosis Transmembrane Regulator
The centrality of CFTR protein dysfunction in CF emphasizes its role in normal physiology. The CFTR protein is large, complex,24 and responsible for multiple functions that remain incompletely described. CFTR gene mutations are categorized according to the affected stage of protein production, activity, and disposal (Fig 2)2, 3, 25, 26, 27 and have recently been reviewed.2, 3 In epithelial cells in the airway and elsewhere, CFTR protein transports chloride28 and bicarbonate,26, 29 and regulates other ion channels (namely sodium, potassium, other chloride and calcium channels).30, 31, 32, 33 It also interacts with other membrane proteins to maintain epithelial tight-junctions and barriers to fluid flow.33, 34, 35 CFTR protein adjusts the levels of acidity in secretions that, along with fluid and chloride abnormalities, lead to some of the common manifestations of the CF clinical syndrome such as small airway disease and pancreatic insufficiency.26, 29 CFTR participates in transport of sphingosine-1 phosphate protein,36 a regulator of cell adhesion and a signaling molecule for inflammation.37 It helps maintain antioxidant defenses by transporting glutathione and accounts for 45% of glutathione efflux from human bronchial epithelial cells.38 At the subcellular level, CFTR maintains adequate internal acidification of several types of intracellular organelles,39 thus maintaining their normal functions for a wide variety of intracellular processes.40 The great variety of recently discovered cellular and intracellular activities suggests the potential for diverse manifestations of CFTR dysfunction.
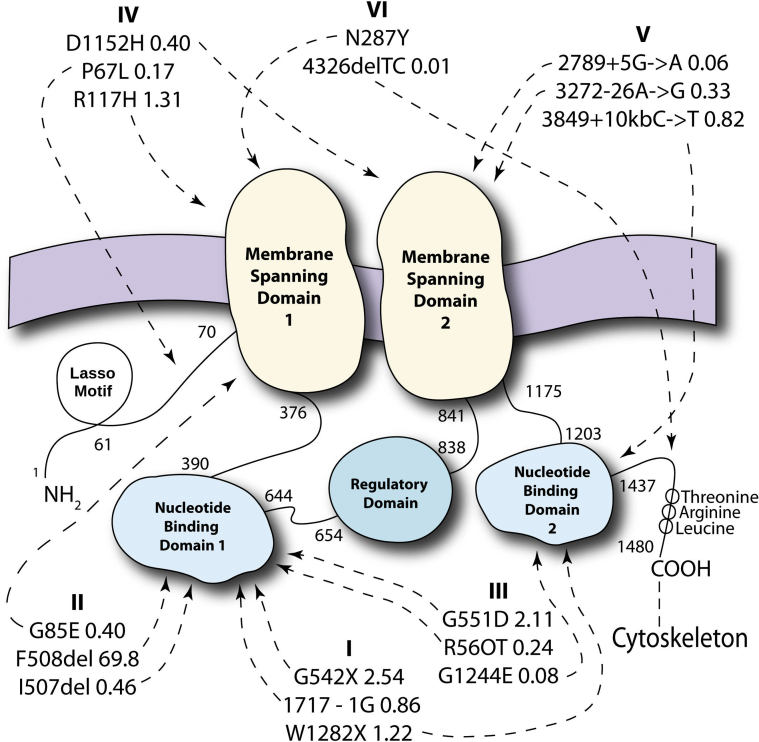
Figure 2.
Cystic fibrosis transmembrane regulator (CFTR) protein. CFTR protein consists of a 1480 amino acid chain.25 Starting from the amine end, there is a Lasso motif, a membrane-spanning domain (amino acids [AA] 70-376) with an associated nucleotide-binding domain (AA 391-644), a regulatory domain (AA 654-838), another membrane-spanning domain (AA 841-1175), another nucleotide-binding domain (AA 1203-1437) and a final threonine-arginine-leucine tail at the carboxyl end. The two similar membrane-spanning domains work together to transport anions; the two similar nucleotide-binding domains mediate adenosine triphosphate hydrolysis, and opening and closing of the actual anion channel; and a threonine-arginine-leucine carboxyl terminal anchors the protein to the cytoskeleton and mediates interactions with other proteins in the cell. The three most common alleles are shown for most of the six categories of mutations. Arrows from each mutation name point to the approximate regions in the full protein that are affected in the underlying DNA sequence mutation as indicated by either the legacy amino acid or modern nucleotide-based mutation nomenclatures. The most common allele world-wide is F508del, a class II gene mutation. Mutation class is associated with degree of clinical severity, and common mutations occurring following the regulatory domain, AA 838, are associated with preservation of CFTR-associated bicarbonate transport and pancreatic sufficiency.26 A regularly updated list of CFTR variants, allele frequencies, and disease-causing potential is available at www.cftr2.org, the source for the mutations noted in the figure.27 Details regarding mutation classifications have been reviewed and are available as published.2, 3
Clinical Consequences of CFTR Dysfunction
Consequences of CFTR protein dysfunction are well known in the lung2, 3 but may occur elsewhere. Pancreatic β-islet cell-related disease leads to CF-related diabetes (CFRD).41 Liver involvement frequently results in chronically elevated liver injury indicators in serum, and a minority of patients develop nonalcoholic steatohepatitis or cirrhosis, with a subset progressing to end-stage liver disease requiring transplantation.42 Abnormal secretions in the male reproductive tract lead to congenital absence of vas deferens and sterility; similar secretions in the female Fallopian tube impair passage of ova and sperm, reducing fertility.4, 43 Tenacious secretions in nasal sinuses lead to chronic sinusitis in most patients with CF, often requiring daily medical treatment and occasional surgical debridement.5 Renal manifestations of CF include abnormal clearance rates for antibiotics used to treat lung infections.44 Observed increased frequency of kidney stones seem to be due to unintended antibiotic-related alterations of the gut microbiome and increased systemic oxalic acid levels.45, 46 Abnormal sweat, perhaps the oldest recognized manifestation of CF,28, 47 leads to salt losses that may increase susceptibility to heat injury.48
The Unfolding Clinical Expression of CFTR Pathophysiology
With improving survivorship, patients with CF become more likely to experience diseases associated with older age.49 CFTR dysfunction may alter the expression of these disease states, create unusual presentations or possibly create unwelcome synergies to worsen age-related diseases.17 Cognitive dysfunction and diabetes related to aging are intertwined,50 and CFTR dysfunction may further complicate and increase morbidity. These disease processes, however, may provide additional treatment targets, especially with CFTR modulators, as well as new opportunities to measure clinically relevant outcomes that will require careful additional study. These modified presentations may provide insights for patients without CF experiencing similar disease states involving loss of CFTR function.51
CFTR and Modified Glucose Metabolism
Although the hypothesized core pathophysiology leading to CFRD is β-islet cell destruction during development of exocrine pancreatic insufficiency, some literature suggests that CFTR protein dysfunction directly causes glucose and insulin metabolic derangements that remain incompletely explored. In patients with CF, β-islet cells persist but exhibit morphologic and functional abnormalities.52, 53, 54 CFTR is normally expressed and active in pancreatic β-islet cells.55 However, dysfunctional CFTR protein reduces insulin release from β-islet cells through multiple mechanisms that may include direct intracellular effects and autoinduced effects via abnormal secretion of IL-6.56, 57 Failure of CFTR protein-mediated chloride transport alters membrane potential, reducing the ability of adenosine triphosphate (ATP)-sensitive potassium channels to depolarize the cell membrane in response to glucose,58 which reduces cellular influx of calcium, an important step prior to insulin release.59 Dysfunctional CFTR protein limits the function of other chloride channels via its regulatory activity, further limiting the effects of closure of ATP-sensitive potassium channels.55 CFTR protein dysfunction seems to act in yet another way that compromises calcium binding to insulin granules, preventing insulin release itself.56
CFTR protein dysfunction in the CNS60 and GI tract61 affects glucose metabolism independently of exocrine pancreas effects. In addition to its recently reviewed CNS effects,60 dysfunctional CFTR protein limits glucose-controlling mechanisms in the CNS. CFTR protein in glucose-inhibited neurons of the hypothalamus modulates release of neuropeptide Y (NPY) and agouti-related peptide (AgRP) in response to systemic glucose levels (Fig 3).53, 60, 62, 63, 64, 65, 66 In human neuronal cell models, hypoglycemia triggers an intracellular cascade of events leading to closure of the CFTR chloride channel, allowing secretion of AgRP.65 Dysfunctional CFTR is associated with defective release of insulin-like growth factor in the pituitary, another counter-regulatory response to hypoglycemia.67 Conversely, hyperglycemia normally results in a different cascade of events that promotes chloride conduction and reduces AgRP secretion and appetite, activities that are compromised by abnormal CFTR. Taken together, these data suggest that obesity and anorexia could be areas to investigate for a previously unknown heterozygous phenotype of CFTR dysfunction. For example, one possible hypothesis is that increased CFTR activity in the absence of hyperglycemia promotes chloride entry into hypoglycemia-sensing neurons, suppressing AgRP and NPY releases and thus suppressing appetite.
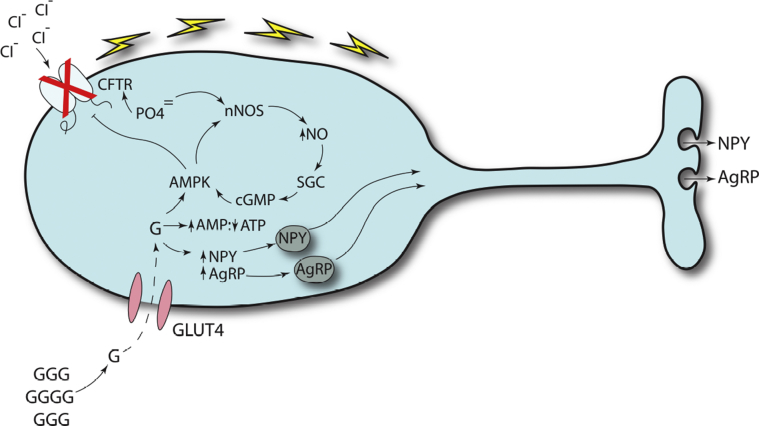
Figure 3.
Involvement of CFTR protein in hypoglycemia-sensing neurons. When circulating G levels drop (lower left), fewer molecules are transported by GLUT4 G-conducting channels into hypothalamic neurons specialized to respond to hypoglycemia.60, 62, 65 These neurons are located in a portion of the brain that has exposure to circulating G levels.66 The decreased G levels trigger an increase in synthesis of NPY and AgRP. AMP levels rise while ATP levels drop, triggering an increase in AMPK activity.64, 65 AMPK activity is amplified by a positive feedback cycle involving phosphorylation of nNOS, an increase in NO that binds SGC, which causes an increase in cyclic guanosine monophosphate that positively feeds back on AMPK.64 Increased AMPK blocks the action of CFTR protein, resulting in depolarization of the neuron which releases NPY and AgRP. Both peptides counter hypoglycemia by increasing appetite. CFTR protein dysfunction prevents normal depolarization and the release of NPY and AgRP, a mechanism consistent with the clinical observation that children with cystic fibrosis do not respond appropriately to hypoglycemia.53 AgRP = agouti-related peptide; AMP = adenosine monophosphate; AMPK = adenosine monophosphate-kinase; ATP = adenosine triphosphate; G = glucose; GLUT4 = glucose transporter type 4; nNOS = neuronal nitric oxide synthase; NO = nitric oxide; NPY = neuropeptide Y; SGC = soluble guanylyl cyclase. See Figure 2 legend for expansion of other abbreviation.
These recent mechanistic discoveries may explain differences between CFRD and diabetes reported in the general population. CFRD involves β-islet cell destruction similar to type 1 diabetes,68 but cell losses do not involve autoimmune mediation,52, 69 and patients rarely experience diabetic ketoacidosis.69 CFRD involves decreased insulin effects but unlike type 2 diabetes, the major issue involves decreased secretion, not increased resistance.59, 70 Dysfunctional CFTR-mediated neuronal effects on appetite-stimulating neuropeptides and growth hormone release may explain the reduced clinical responses to hypoglycemia, as well as the poor development and growth in patients with CF.53
CFTR and Circadian Rhythm
CFTR plays important roles in at least two cranial nerve pathways that help entrain the master circadian clock.71, 72 Normal olfaction73 and retinal epithelial function74, 75 require CFTR function. Relative to unaffected animals, nasal epithelial cells from rats and mice with CFTR deficiency deteriorate from normal morphology at birth to severely abnormal with aging.76 Chloride secretion abnormalities increase while olfactory neurons decrease in number, resulting in markedly decreased odor-evoked responses on electroolfactograms. These CFTR-associated abnormalities may underlie some of the clinical pathology seen in CF, a possibility that needs further confirmation, including increased incidence and prevalence of sinusitis and deficits in the sensation of smell. Decreased sensation of smell leads to severe quality of life impairments and disability.77
In retinal epithelium, incident light triggers impulses measurable by using electrooculograms. CFTR protein seems to be partially responsible for the movement of salt and fluid across the retinal pigment epithelium, which enables normal responses to light.74 However, alcohol- and light-evoked electrooculograms in patients with F508del heterozygous or homozygous mutations were abnormal compared with non-CF control subjects,75 most likely due to abnormal interactions between CFTR and other ion channels. The direct clinical consequence is unclear, but light reception is a critical step in normal entrainment of circadian rhythm within the hypothalamus.71 Measurement of electrooculograms in association with assessments of sleep-wake cycles may provide alternative methods of measuring the impact of treatment with CFTR modulators.
In animal models and humans, CFTR is expressed during embryonic brain development.60 CFTR messenger RNA and protein are expressed in the hypothalamus, thalamus, and arcuate and amygdaloid nuclei, areas that control homeostasis and energy metabolism.60, 62 CFTR messenger RNA expression occurs specifically in the anterior hypothalamus, the brain region in which the master circadian clock resides.78
Disease manifestations linked to dysfunction in these areas of the brain include conditions associated with circadian rhythm and homeostasis perturbations such as insomnia, sleep disorders, mood and affect disorders, abnormal energy metabolism, growth, and reproduction.78, 79, 80, 81, 82, 83 Many of these functions are difficult to discern as primary CFTR effects because of the sequelae of other features of CF, and they will require additional research to confirm. For example, although CF-associated female infertility could be related to neuron dysfunction leading to abnormal release of gonadotropin-releasing hormone as a primary effect of dysfunctional CFTR,84 it may also be due secondarily to abnormal secretions in the reproductive tract and low body weight associated with pancreatic insufficiency and malnutrition.4
The pattern of neuronal involvement suggests the possibility of circadian rhythm disturbance mediated by abnormal CFTR. Entrainment or resetting of the master circadian clock in mammals requires the ability to sense and process light, which is disturbed by abnormal CFTR.71, 74 Direct evidence shows that CFTR dysfunction compromises phase-shifting of the circadian rhythm,85 which could prevent clinically normal responses to changing daylight hours, travel, or changing personal schedules to accommodate work or school. A key function of daily circadian cycling is initiation of sleep. Acutely increased melatonin levels induce drowsiness and sleep, but neurons with deficient CFTR cannot respond normally to increased melatonin.86 Abnormal responses in some people heterozygous for CFTR mutations75 suggest that a far larger population may be affected. These findings predict that sleep phase may be delayed or otherwise altered in patients with CF, a conjecture consistent with recent clinical observations87 but which will need confirmation and more detailed basic science support.
Sleep phase delay is associated with multiple adverse outcomes. Difficulty sleeping at conventional times, which may be diagnosed as insomnia,88 can lead to chronic sleep deprivation because wake-up time is often fixed by morning responsibilities involving families, schools, and jobs. This form of circadian misalignment is similar to classic shift-work disorder89 and may lead to academic failures90, 91 and work-life difficulties.88, 92 Depressive symptoms and anxiety are associated with circadian misalignment and sleep phase delay.80, 93 Circadian disruption during pregnancy heightens the risk of adverse outcomes in humans.83 Circadian rhythm misalignment increases the risks of obesity, diabetes, cardiovascular disease, and neural degeneration,16, 81, 82, 94, 95, 96, 97 and it may compromise normal immune function and tumor suppression.98, 99 Many of these consequences overlap and potentially contribute to the extensive comorbidities of CF. A number of analyses explore disrupted sleep quality and latency in CF,100, 101, 102, 103 but sleep phase delay remains relatively unexplored.87 The potential for adverse outcomes suggests that studies in CF centered on sleep phase and circadian misalignment may find high-yielding, novel treatment opportunities for CFTR modulators.
CFTR and Cancer
Many cancer types rise in incidence with advancing age,15 and patients with CF are reaching older ages in rapidly growing numbers.8 Systematic investigations show that patients with CF have an increased risk of cancer.104 The majority of observed tumors originate in the GI tract, particularly from the ascending colon, but include gallbladder and small intestine tumors that occur uncommonly or rarely in the general population. Outside of the GI tract, lymphoid leukemia and testicular carcinomas occur more frequently than expected while the incidence of melanoma seems significantly decreased, possibly because patients with CF avoid sunlight to prevent heat injury.48
Genetic and Acquired Abnormal CFTR Functions in Cancer Cells
Observations reporting increased cancers in patients with CF cannot distinguish between a secondary effect of CF-associated chronic inflammation as seen in the colon or a primary effect of CFTR dysfunction. For example, intestinal-specific CFTR knockout mice had greatly increased development of colon and small intestine tumors compared with wild-type mice; however, the mouse GI tract had increased inflammation and dysregulated inflammatory response pathways.105 Nevertheless, investigations of human samples and cancer cell lines suggest that acquired CFTR dysfunction increases cancer aggressiveness, morbidity, and mortality independently of inflammation.106, 107, 108
Among patients with CF, tumors of the lung are too few to ascertain a difference in occurrence rate compared with the general population.104 However, CFTR suppression occurs commonly among lung tumors in patients without CF. Hypermethylation of the CFTR promoter region affected 41.7% of squamous cell cancers and 21.5% of adenocarcinomas in a study of 139 patients with lung cancer.108 Specific CFTR sequence mutations occurred in four of 17 patients with non-small cell lung cancer109 and in four of 22 patients with unspecified lung cancers.110
CFTR suppression may enhance deleterious cancer cell behaviors. In vitro studies of lung cancer cell lines revealed decreased adhesion, increased epithelial-to-mesenchymal transition, motility, and invasiveness of CFTR-deficient cells.111 Carefully controlled experiments with murine cancer models revealed more rapid tumor growth and increased metastases that were attenuated by increasing CFTR expression.105, 111 Clinically, patients with tumors with decreased CFTR expression presented with more advanced lung cancer stages. After correction for staging, these patients had decreased survival relative to patients with tumors with normal CFTR.108, 111 Although these findings suggest that CFTR may be more than a passenger gene in lung cancer, they require confirmation.
Other cancer types exhibit heterogeneous expression and function of CFTR protein. For example, in prostate cancer cell lines, CFTR suppression was associated with decreased adhesion, increased cell growth, and increased migration, findings that were reversed with restoration of CFTR function.107 CFTR suppression by hypermethylation of the promoter region occurred in 47% of prostate adenocarcinomas from 83 patients.112 These patients had decreased disease-free survival and decreased overall survival compared with those without CFTR-suppressed tumors. The frequency of sequence mutations in prostate cancer has not been explored.
Conversely, there is early evidence that CFTR suppression may improve some cancer treatment outcomes. CFTR is strongly associated with cyclic guanosine monophosphate-protein dependent kinase I in enrichment networks of tumor spheres for two glioma cell lines. Further analysis tentatively suggests that suppression of these associated genes may be helpful in the treatment of glioblastoma.113 Other early evidence suggests that CFTR overexpression may be associated with increased aggressiveness of ovarian cancer,114 a finding complementary to the suggestion of increased testicular cancer in people with CF.104
Unfortunately, no investigation has yet found a mechanism directly linking abnormal CFTR gene expression with modified cancer incidence or aggressiveness. Associations between CFTR protein and recognized cancer markers may generate plausible hypotheses suitable for testing (Table 1).35, 106, 107, 113, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125
Table 1.
Potential Involvement of CFTR With Other Proteins Implicated in Cancer
| Target Protein | Known Relationships With CFTR | Effect of CFTR Dysfunction | Potential Tumor Effects |
|---|---|---|---|
| AF-6/afadin | Colocalizes at cell-cell contacts and physically interacts with CFTR35 | Reduces epithelial tightness; reduces afadin35 | Increases metastatic potential35 |
| EGFR | CFTR suppresses EGFR signaling and IL-8 production115 | Activates EGFR pathway involving IL-1α and TNF-α converting enzyme115 | EGFR enhances VEGF effects in cancer116 Accelerates cell proliferation and tumor growth117 |
| p38 MAPK | CFTR inhibition increases p38 MAPK phosphorylation118, 119 | Increases p38 MAPK activity leading to increased inflammatory cytokines120 | Increases epithelial-to-mesenchymal transformation121 Decreases apoptosis122 |
| cGMP-PRKG1 | Strong linkage with CFTR in network analysis113 | May decrease PRKG1 activity113 | Decreased PRKG1 may reduce tumor growth113 |
| uPA | Suppresses uPA activity via suppression of NFκB106, 107 | Allows upregulation of NFκB and uPA activities | Increases epithelial-to-mesenchymal transformation106 Increases metastatic potential106 |
| VEGF | Elevated in sputum and serum with pulmonary exacerbations of CF123, 124, 125 | Induces VEGF synthesis124 | Enhances angiogenesis and tumor growth116 |
CF = cystic fibrosis; CFTR = cystic fibrosis transmembrane regulator; cGMP = cyclic guanosine monophosphate; EGFR = epidermal growth factor receptor; MAPK = mitogen-activated protein kinase; PRKG1 = protein-dependent kinase I; TNF-α = tumor necrosis factor α; uPA = urokinase plasminogen activator; VEGF = vascular endothelial growth factor.
Treatment With CFTR Modulators
Several approved CFTR modulators slow lung disease and reduce pulmonary exacerbation frequency in CF.19 However, the multisystem adverse effects of abnormal CFTR suggest that modulators may have other positive therapeutic outcomes. Some CF manifestations do not lead to rapid demise and thus have not been fully assessed as primary or even secondary outcomes in clinical trials.
CFTR modulators may prevent or delay the onset of CFRD in patients with treatable mutations by repairing CFTR function.126 Reduced CFTR dysfunction might improve associated β-islet cell dysfunction.55, 56, 60, 127 CFTR modulators could improve CNS regulation of glucose metabolism65, 67 depending on ability to reach glucose-sensing neurons.
CFTR modulators may also improve olfaction and light sensation. Restoration of olfaction has the potential to improve appetite, mood, and quality of life.128 Normalized processing of light signals coupled with improved CFTR protein function in the anterior hypothalamus might improve master circadian clock entrainment, a change that could have widespread positive effects. For example, improvement of CFTR functioning may decrease phase delay in sleep87 by shifting the increase in circulating melatonin closer to the end of the astronomical day and improving the response to melatonin to facilitate the onset of biological night.
In cancers in which acquisition of CFTR suppression seems to augment the severity and progression of the disease, modulating agents may improve outcomes. For example, in prostate cancer, reduction in aggression in some tumors may obviate invasive treatments, reducing costs and morbidities and improving quality of life and survival. However, caution is needed as there are other cell types in which the early data suggest that increased CFTR activity increases tumor aggressiveness.113, 114
Because CFTR suppression may be due to hypermethylation of the gene promoter or any one of a large number of sequence mutations, many different approaches will be needed to realize the potential benefits of modulator treatments. CFTR modulators may be useful in novel sequence mutations found in cancer cells for which knowledge of isolated CFTR dysfunctional effects are necessarily limited. Hypermethylation seems to be a common mechanism for CFTR suppression but might still allow synthesis of a certain number of protein molecules, and agents that improve trafficking and stability of F508del CFTR protein and slow scavenging of old proteins20, 51 could have a positive impact.
All the putative applications of CFTR modulators mentioned here and the potential effects must be more carefully defined than the present discussion. Carefully crafted new hypotheses must then undergo rigorous testing prior to deployment of these medications for novel, additional purposes.
Conclusions
The treatment of CF has been successful enough to allow the characterization of multiple manifestations of CFTR dysfunction beyond progressive airway and pancreas destruction. Glucose and insulin metabolism abnormalities due to abnormal CFTR seem to partially underlie malnutrition, poor growth, and CFRD; discoveries of the role of CFTR protein in these processes could lead to insights relevant to better understanding and possibly treatment of obesity and diabetes in people without CF. CFTR dysfunction in the nervous system may cause disordered energy homeostasis, abnormal pain perception, dysregulation of reproduction, and decreased plasticity of circadian rhythm entrainment. This finding in turn has implications for immune function, sleep phase, mood abnormalities, and cancer incidence. Successful studies in cancer biology have shown that frequently occurring CFTR suppression leads to cell behaviors that predict increased tumor growth, rapid metastasis, and earlier mortality. The progress in treatment of CF and the discoveries of CFTR involvement in the multiple cell types involved in a wide array of conditions suggest multiple new avenues for investigation at basic through clinical levels. The ongoing development of CFTR modulators promises to facilitate many of these studies and assist the design and implementation of novel treatment strategies for a diverse array of human diseases.
Acknowledgments
Financial/nonfinancial disclosures: The author has reported to CHEST the following: Dr Liou participates in clinical trials with support through the Cystic Fibrosis Foundation’s Therapeutic Development Network [Grant LIOU14Y0] and the Foundation for the National Institutes of Health. The Adult CF Center at the University of Utah receives research support through the NHLBI/NIH, the Cystic Fibrosis Foundation, the Ben B. and Iris M. Margolis Family Foundation of Utah and the Claudia Ruth Goodrich Stevens Endowment Fund. The University of Utah has received specific funding for clinical trials and other research in which Dr Liou and his research team participate. Sponsors of these projects within the last 3 years include Corbus Pharmaceuticals; Gilead Sciences, Inc; Laurent Pharmaceuticals; Nivalis Therapeutics, Inc; Novartis; Proteostasis, Inc; Savara Pharmaceuticals; Spiration, Inc; and Vertex Pharmaceuticals, Inc.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: A preliminary form of this review was presented at Internal Medicine Grand Rounds at the University of Utah on December 1, 2016. The author is grateful to Professor Sir David Cox, PhD, for strongly suggesting that the presentation should be presented as a formal review of this topic. Great thanks are also given to John Hoidal, MD, Barbara Cahill, MD, Fred Adler, PhD, Judy Jensen, MS, and Kristyn Packer, MAR, for reviews of several versions of the manuscript. Rebecca Liou kindly created Figure 2, Figure 3.
Footnotes
FUNDING/SUPPORT: The author receives financial support from the National Institutes of Health/National Heart, Lung, and Blood Institute [Grant R01 HL 125520], the Cystic Fibrosis Foundation [Grants LIOU14P0, LIOU13A0, LIOU14Y4, LIOU15Y4, LIOU14Y0, and CC132-16AD], the Ben B. and Iris M. Margolis Family Foundation of Utah, and the Claudia Ruth Goodrich Stevens Endowment Fund.
References
- 1.Sosnay P.R., Siklosi K.R., Van Goor F. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet. 2013;45(10):1160–1167. doi: 10.1038/ng.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elborn J.S. Cystic fibrosis. Lancet. 2016;388(10059):2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 3.Castellani C., Assael B.M. Cystic fibrosis: a clinical view. Cell Mol Life Sci. 2017;74(1):129–140. doi: 10.1007/s00018-016-2393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan H.C., Ruan Y.C., He Q. The cystic fibrosis transmembrane conductance regulator in reproductive health and disease. J Physiol. 2009;587(pt 10):2187–2195. doi: 10.1113/jphysiol.2008.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habib A.R., Buxton J.A., Singer J., Wilcox P.G., Javer A.R., Quon B.S. Association between chronic rhinosinusitis and health-related quality of life in adults with cystic fibrosis. Ann Am Thorac Soc. 2015;12(8):1163–1169. doi: 10.1513/AnnalsATS.201504-191OC. [DOI] [PubMed] [Google Scholar]
- 6.Liou T.G., Adler F.R., Keogh R.H. Sputum biomarkers and the prediction of clinical outcomes in patients with cystic fibrosis. PloS One. 2012;7(8):e42748. doi: 10.1371/journal.pone.0042748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liou T.G., Adler F.R., Fitzsimmons S.C., Cahill B.C., Hibbs J.R., Marshall B.C. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153(4):345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cystic Fibrosis Foundation Patient Registry 2016 Annual Data Report [Internet] Cystic Fibrosis Foundation; Bethesda, Maryland: 2017. http://www.cff.org/Our-Research/CF-Patient-Registry/ Accessed November 26, 2018. [Google Scholar]
- 9.Knapp E.A., Fink A.K., Goss C.H. The Cystic Fibrosis Foundation patient registry. Design and methods of a national observational disease registry. Ann Am Thorac Soc. 2016;13(7):1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 10.Keogh R.H., Szczesniak R., Taylor-Robinson D., Bilton D. Up-to-date and projected estimates of survival for people with cystic fibrosis using baseline characteristics: a longitudinal study using UK patient registry data. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2018;17(2):218–227. doi: 10.1016/j.jcf.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephenson AL, Sykes J, Stanojevic S Survival comparison of patients with cystic fibrosis in Canada and the United States: a population-based cohort study. Ann Intern Med. 2017;166(8):537–546. doi: 10.7326/M16-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castellani C., Duff A.J., Bell S.C. ECFS best practice guidelines: the 2018 revision. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2018;17(2):153–178. doi: 10.1016/j.jcf.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 13.MacKenzie T., Gifford A.H., Sabadosa K.A. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation patient registry. Ann Intern Med. 2014;161(4):233–241. doi: 10.7326/M13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes D.E., Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milholland B., Auton A., Suh Y., Vijg J. Age-related somatic mutations in the cancer genome. Oncotarget. 2015;6(28):24627–24635. doi: 10.18632/oncotarget.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singletary K.G., Naidoo N. Disease and degeneration of aging neural systems that integrate sleep drive and circadian oscillations. Front Neurol. 2011;2:66. doi: 10.3389/fneur.2011.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabbri E., Zoli M., Gonzalez-Freire M., Salive M.E., Studenski S.A., Ferrucci L. Aging and multimorbidity: new tasks, priorities, and frontiers for integrated gerontological and clinical research. J Am Med Dir Assoc. 2015;16(8):640–647. doi: 10.1016/j.jamda.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen D.H. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Child. 1938;56(2):344–399. [Google Scholar]
- 19.Elborn J.S. CFTR modulators: deciding what is best for individuals in an era of precision medicine. Ann Am Thorac Soc. 2018;15(3):298–300. doi: 10.1513/AnnalsATS.201712-951ED. [DOI] [PubMed] [Google Scholar]
- 20.Zaman K., Sawczak V., Zaidi A. Augmentation of CFTR maturation by S-nitrosoglutathione reductase. Am J Physiol Lung Cell Mol Physiol. 2016;310(3):L263–L270. doi: 10.1152/ajplung.00269.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerem E., Konstan M.W., De Boeck K. Ataluren for the treatment of nonsense-mutation cystic fibrosis: a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir Med. 2014;2(7):539–547. doi: 10.1016/S2213-2600(14)70100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norez C., Vandebrouck C., Bertrand J. Roscovitine is a proteostasis regulator that corrects the trafficking defect of F508del-CFTR by a CDK-independent mechanism. Br J Pharmacol. 2014;171(21):4831–4849. doi: 10.1111/bph.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norez C., Noel S., Wilke M. Rescue of functional delF508-CFTR channels in cystic fibrosis epithelial cells by the alpha-glucosidase inhibitor miglustat. FEBS Lett. 2006;580(8):2081–2086. doi: 10.1016/j.febslet.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z., Chen J. Atomic structure of the cystic fibrosis transmembrane conductance regulator. Cell. 2016;167(6):1586–1597.e9. doi: 10.1016/j.cell.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Zeitlin P.L. Pharmacologic restoration of ΔF508 CFTR-mediated chloride current. Kidney Int. 2000;57(3):832–837. doi: 10.1046/j.1523-1755.2000.00922.x. [DOI] [PubMed] [Google Scholar]
- 26.Choi J.Y., Muallem D., Kiselyov K., Lee M.G., Thomas P.J., Muallem S. Aberrant CFTR-dependent HCO3-transport in mutations associated with cystic fibrosis. Nature. 2001;410(6824):94–97. doi: 10.1038/35065099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welcome to CFTR2 | CFTR2 [Internet]. https://cftr2.org/. Accessed November 26, 2018.
- 28.Quinton P.M. Chloride impermeability in cystic fibrosis. Nature. 1983;301(5899):421–422. doi: 10.1038/301421a0. [DOI] [PubMed] [Google Scholar]
- 29.Shamsuddin A.K., Quinton P.M. Native small airways secrete bicarbonate. Am J Respir Cell Mol Biol. 2014;50(4):796–804. doi: 10.1165/rcmb.2013-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stutts M.J., Canessa C.M., Olsen J.C. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269(5225):847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 31.Lu M., Leng Q., Egan M.E. CFTR is required for PKA-regulated ATP sensitivity of Kir1.1 potassium channels in mouse kidney. J Clin Invest. 2006;116(3):797–807. doi: 10.1172/JCI26961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H., Zhang X., Zeng S. The cellular localization of Na(+)/H(+) exchanger 1, cystic fibrosis transmembrane conductance regulator, potassium channel, epithelial sodium channel γ and vacuolar-type H+-ATPase in human eccrine sweat glands. Acta Histochem. 2014;116(8):1237–1243. doi: 10.1016/j.acthis.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Kunzelmann K., Tian Y., Martins J.R. Airway epithelial cells—functional links between CFTR and anoctamin dependent Cl-secretion. Int J Biochem Cell Biol. 2012;44(11):1897–1900. doi: 10.1016/j.biocel.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Monterisi S., Favia M., Guerra L. CFTR regulation in human airway epithelial cells requires integrity of the actin cytoskeleton and compartmentalized cAMP and PKA activity. J Cell Sci. 2012;125(pt 5):1106–1117. doi: 10.1242/jcs.089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun T.T., Wang Y., Cheng H. Disrupted interaction between CFTR and AF-6/afadin aggravates malignant phenotypes of colon cancer. Biochim Biophys Acta. 2014;1843(3):618–628. doi: 10.1016/j.bbamcr.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Brown M.B., Hunt W.R., Noe J.E. Loss of cystic fibrosis transmembrane conductance regulator impairs lung endothelial cell barrier function and increases susceptibility to microvascular damage from cigarette smoke. Pulm Circ. 2014;4(2):260–268. doi: 10.1086/675989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proia R.L., Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J Clin Invest. 2015;125(4):1379–1387. doi: 10.1172/JCI76369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gould N.S., Min E., Martin R.J., Day B.J. CFTR is the primary known apical glutathione transporter involved in cigarette smoke-induced adaptive responses in the lung. Free Radic Biol Med. 2012;52(7):1201–1206. doi: 10.1016/j.freeradbiomed.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barasch J., Kiss B., Prince A., Saiman L., Gruenert D., al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991;352(6330):70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- 40.Bradbury N.A. Intracellular CFTR: localization and function. Physiol Rev. 1999;79(suppl 1):S175–S191. doi: 10.1152/physrev.1999.79.1.S175. [DOI] [PubMed] [Google Scholar]
- 41.Moran A., Dunitz J., Nathan B., Saeed A., Holme B., Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32(9):1626–1631. doi: 10.2337/dc09-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colombo C., Battezzati P.M., Crosignani A. Liver disease in cystic fibrosis: a prospective study on incidence, risk factors, and outcome. Hepatol Baltim Md. 2002;36(6):1374–1382. doi: 10.1053/jhep.2002.37136. [DOI] [PubMed] [Google Scholar]
- 43.di Sant’Agnese P.A. Guest editorial—fertility and the young adult with cystic fibrosis. N Engl J Med. 1968;279(2):103–105. doi: 10.1056/NEJM196807112790213. [DOI] [PubMed] [Google Scholar]
- 44.Rey E., Tréluyer J.M., Pons G. Drug disposition in cystic fibrosis. Clin Pharmacokinet. 1998;35(4):313–329. doi: 10.2165/00003088-199835040-00004. [DOI] [PubMed] [Google Scholar]
- 45.Katz S.M., Krueger L.J., Falkner B. Microscopic nephrocalcinosis in cystic fibrosis. N Engl J Med. 1988;319(5):263–266. doi: 10.1056/NEJM198808043190502. [DOI] [PubMed] [Google Scholar]
- 46.Knauf F., Thomson R.B., Heneghan J.F. Loss of cystic fibrosis transmembrane regulator impairs intestinal oxalate secretion. J Am Soc Nephrol. 2017;28(1):242–249. doi: 10.1681/ASN.2016030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quinton P.M. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev. 1999;79(suppl 1):S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- 48.Kessler W.R., Andersen D.H. Heat prostration in fibrocystic disease of the pancreas and other conditions. Pediatrics. 1951;8(5):648–656. [PubMed] [Google Scholar]
- 49.Parkins M.D., Parkins V.M., Rendall J.C., Elborn S. Changing epidemiology and clinical issues arising in an ageing cystic fibrosis population. Ther Adv Respir Dis. 2011;5(2):105–119. doi: 10.1177/1753465810386051. [DOI] [PubMed] [Google Scholar]
- 50.Biessels G.J., Reagan L.P. Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci. 2015;16(11):660–671. doi: 10.1038/nrn4019. [DOI] [PubMed] [Google Scholar]
- 51.Bodas M., Silverberg D., Walworth K., Brucia K., Vij N. Augmentation of S-nitrosoglutathione controls cigarette smoke-induced inflammatory-oxidative stress and chronic obstructive pulmonary disease-emphysema pathogenesis by restoring cystic fibrosis transmembrane conductance regulator function. Antioxid Redox Signal. 2017;27(7):433–451. doi: 10.1089/ars.2016.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iannucci A., Mukai K., Johnson D., Burke B. Endocrine pancreas in cystic fibrosis: an immunohistochemical study. Hum Pathol. 1984;15(3):278–284. doi: 10.1016/s0046-8177(84)80191-4. [DOI] [PubMed] [Google Scholar]
- 53.Moran A., Diem P., Klein D.J., Levitt M.D., Robertson R.P. Pancreatic endocrine function in cystic fibrosis. J Pediatr. 1991;118(5):715–723. doi: 10.1016/s0022-3476(05)80032-0. [DOI] [PubMed] [Google Scholar]
- 54.Couce M., O’Brien T.D., Moran A., Roche P.C., Butler P.C. Diabetes mellitus in cystic fibrosis is characterized by islet amyloidosis. J Clin Endocrinol Metab. 1996;81(3):1267–1272. doi: 10.1210/jcem.81.3.8772610. [DOI] [PubMed] [Google Scholar]
- 55.Edlund A., Esguerra J.L., Wendt A., Flodström-Tullberg M., Eliasson L. CFTR and anoctamin 1 (ANO1) contribute to cAMP amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. BMC Med. 2014;12:87. doi: 10.1186/1741-7015-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koivula F.N., McClenaghan N.H., Harper A.G., Kelly C. Islet-intrinsic effects of CFTR mutation. Diabetologia. 2016;59(7):1350–1355. doi: 10.1007/s00125-016-3936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun X., Yi Y., Xie W. CFTR influences beta cell function and insulin secretion through non-cell autonomous exocrine-derived factors. Endocrinology. 2017;158(10):3325–3338. doi: 10.1210/en.2017-00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miki T., Nagashima K., Tashiro F. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 1998;95(18):10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo J.H., Chen H., Ruan Y.C. Glucose-induced electrical activities and insulin secretion in pancreatic islet β-cells are modulated by CFTR. Nat Commun. 2014;5:4420. doi: 10.1038/ncomms5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reznikov L.R. Cystic fibrosis and the nervous system. Chest. 2017;151(5):1147–1155. doi: 10.1016/j.chest.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xue R., Gu H., Qiu Y. Expression of cystic fibrosis transmembrane conductance regulator in ganglia of human gastrointestinal tract. Sci Rep. 2016;6:30926. doi: 10.1038/srep30926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fioramonti X., Contié S., Song Z., Routh V.H., Lorsignol A., Pénicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes. 2007;56(5):1219–1227. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- 63.Murphy B.A., Fioramonti X., Jochnowitz N. Fasting enhances the response of arcuate neuropeptide Y-glucose-inhibited neurons to decreased extracellular glucose. Am J Physiol Cell Physiol. 2009;296(4):C746–C756. doi: 10.1152/ajpcell.00641.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy B.A., Fakira K.A., Song Z., Beuve A., Routh V.H. AMP-activated protein kinase and nitric oxide regulate the glucose sensitivity of ventromedial hypothalamic glucose-inhibited neurons. Am J Physiol Cell Physiol. 2009;297(3):C750–C758. doi: 10.1152/ajpcell.00127.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chalmers J.A., Jang J.J., Belsham D.D. Glucose sensing mechanisms in hypothalamic cell models: glucose inhibition of AgRP synthesis and secretion. Mol Cell Endocrinol. 2014;382(1):262–270. doi: 10.1016/j.mce.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 66.Canabal D.D., Song Z., Potian J.G., Beuve A., McArdle J.J., Routh V.H. Glucose, insulin, and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007;292(4):R1418–R1428. doi: 10.1152/ajpregu.00216.2006. [DOI] [PubMed] [Google Scholar]
- 67.Rogan M.P., Reznikov L.R., Pezzulo A.A. Pigs and humans with cystic fibrosis have reduced insulin-like growth factor 1 (IGF1) levels at birth. Proc Natl Acad Sci U S A. 2010;107(47):20571–20575. doi: 10.1073/pnas.1015281107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soejima K., Landing B.H. Pancreatic islets in older patients with cystic fibrosis with and without diabetes mellitus: morphometric and immunocytologic studies. Pediatr Pathol. 1986;6(1):25–46. doi: 10.3109/15513818609025923. [DOI] [PubMed] [Google Scholar]
- 69.Lanng S., Thorsteinsson B., Pociot F. Diabetes mellitus in cystic fibrosis: genetic and immunological markers. Acta Paediatr Oslo Nor. 1992. 1993;82(2):150–154. doi: 10.1111/j.1651-2227.1993.tb12628.x. [DOI] [PubMed] [Google Scholar]
- 70.Uc A., Olivier A.K., Griffin M.A. Glycaemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass. Clin Sci Lond Engl 1979. 2015;128(2):131–142. doi: 10.1042/CS20140059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freedman M.S., Lucas R.J., Soni B. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284(5413):502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 72.Abraham U., Saleh M., Kramer A. Odor is a time cue for circadian behavior. J Biol Rhythms. 2013;28(1):26–37. doi: 10.1177/0748730412469353. [DOI] [PubMed] [Google Scholar]
- 73.Pfister S., Weber T., Härtig W. Novel role of cystic fibrosis transmembrane conductance regulator in maintaining adult mouse olfactory neuronal homeostasis. J Comp Neurol. 2015;523(3):406–430. doi: 10.1002/cne.23686. [DOI] [PubMed] [Google Scholar]
- 74.Blaug S., Quinn R., Quong J., Jalickee S., Miller S.S. Retinal pigment epithelial function: a role for CFTR? Doc Ophthalmol Adv Ophthalmol. 2003;106(1):43–50. doi: 10.1023/a:1022514031645. [DOI] [PubMed] [Google Scholar]
- 75.Constable P.A., Lawrenson J.G., Arden G.B. Light and alcohol evoked electro-oculograms in cystic fibrosis. Doc Ophthalmol Adv Ophthalmol. 2006;113(2):133–143. doi: 10.1007/s10633-006-9023-z. [DOI] [PubMed] [Google Scholar]
- 76.Grubb B.R., Rogers T.D., Kulaga H.M. Olfactory epithelia exhibit progressive functional and morphological defects in CF mice. Am J Physiol Cell Physiol. 2007;293(2):C574–C583. doi: 10.1152/ajpcell.00106.2007. [DOI] [PubMed] [Google Scholar]
- 77.Miwa T., Furukawa M., Tsukatani T., Costanzo R.M., DiNardo L.J., Reiter E.R. Impact of olfactory impairment on quality of life and disability. Arch Otolaryngol Head Neck Surg. 2001;127(5):497–503. doi: 10.1001/archotol.127.5.497. [DOI] [PubMed] [Google Scholar]
- 78.Reppert S.M., Weaver D.R. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 79.Panda S., Antoch M.P., Miller B.H. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 80.Murray J.M., Sletten T.L., Magee M. Prevalence of circadian misalignment and its association with depressive symptoms in delayed sleep phase disorder. Sleep. 2017;40(1) doi: 10.1093/sleep/zsw002. [DOI] [PubMed] [Google Scholar]
- 81.Saini C., Petrenko V., Pulimeno P. A functional circadian clock is required for proper insulin secretion by human pancreatic islet cells. Diabetes Obes Metab. 2016;18(4):355–365. doi: 10.1111/dom.12616. [DOI] [PubMed] [Google Scholar]
- 82.Reutrakul S., Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci. 2014;1311:151–173. doi: 10.1111/nyas.12355. [DOI] [PubMed] [Google Scholar]
- 83.Zhu J.L., Hjollund N.H., Andersen A.M., Olsen J. Shift work, job stress, and late fetal loss: the National Birth Cohort in Denmark. J Occup Environ Med. 2004;46(11):1144–1149. doi: 10.1097/01.jom.0000145168.21614.21. [DOI] [PubMed] [Google Scholar]
- 84.Weyler R.T., Yurko-Mauro K.A., Rubenstein R. CFTR is functionally active in GnRH-expressing GT1-7 hypothalamic neurons. Am J Physiol. 1999;277(3 pt 1):C563–C571. doi: 10.1152/ajpcell.1999.277.3.C563. [DOI] [PubMed] [Google Scholar]
- 85.Kim Y.S., Kim Y.B., Kim W.B. Histamine 1 receptor-Gβγ-cAMP/PKA-CFTR pathway mediates the histamine-induced resetting of the suprachiasmatic circadian clock. Mol Brain. 2016;9(1):49. doi: 10.1186/s13041-016-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nelson C.S., Marino J.L., Allen C.N. Melatonin receptor potentiation of cyclic AMP and the cystic fibrosis transmembrane conductance regulator ion channel. J Pineal Res. 1999;26(2):113–121. doi: 10.1111/j.1600-079x.1999.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 87.Jensen J.L., Jones C.R., Kartsonaki C., Packer K.A., Adler F.R., Liou T.G. Sleep phase delay in cystic fibrosis: a potential new manifestation of cystic fibrosis transmembrane regulator dysfunction. Chest. 2017;152(2):386–393. doi: 10.1016/j.chest.2017.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Belcher R., Gumenyuk V., Roth T. Insomnia in shift work disorder relates to occupational and neurophysiological impairment. J Clin Sleep Med. 2015;11(4):457–465. doi: 10.5664/jcsm.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sack R.L., Auckley D., Auger R.R. Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review. Sleep. 2007;30(11):1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bahammam A.S., Alaseem A.M., Alzakri A.A., Almeneessier A.S., Sharif M.M. The relationship between sleep and wake habits and academic performance in medical students: a cross-sectional study. BMC Med Educ. 2012;12:61. doi: 10.1186/1472-6920-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hershner S.D., Chervin R.D. Causes and consequences of sleepiness among college students. Nat Sci Sleep. 2014;6:73–84. doi: 10.2147/NSS.S62907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Magee M., Sletten T.L., Ferguson S.A. Associations between number of consecutive night shifts and impairment of neurobehavioral performance during a subsequent simulated night shift. Scand J Work Environ Health. 2016;42(3):217–227. doi: 10.5271/sjweh.3560. [DOI] [PubMed] [Google Scholar]
- 93.Benedetti F., Riccaboni R., Dallaspezia S., Locatelli C., Smeraldi E., Colombo C. Effects of CLOCK gene variants and early stress on hopelessness and suicide in bipolar depression. Chronobiol Int. 2015;32(8):1156–1161. doi: 10.3109/07420528.2015.1060603. [DOI] [PubMed] [Google Scholar]
- 94.Scheer F.A., Hilton M.F., Mantzoros C.S., Shea S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yaggi H.K., Araujo A.B., McKinlay J.B. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29(3):657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 96.Wong P.M., Hasler B.P., Kamarck T.W., Muldoon M.F., Manuck S.B. Social jetlag, chronotype, and cardiometabolic risk. J Clin Endocrinol Metab. 2015;100(12):4612–4620. doi: 10.1210/jc.2015-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.St-Onge M.P., Grandner M.A., Brown D. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–e386. doi: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carter S.J., Durrington H.J., Gibbs J.E. A matter of time: study of circadian clocks and their role in inflammation. J Leukoc Biol. 2016;99(4):549–560. doi: 10.1189/jlb.3RU1015-451R. [DOI] [PubMed] [Google Scholar]
- 99.Fu L., Lee C.C. The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer. 2003;3(5):350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 100.Milross M.A., Piper A.J., Norman M. Subjective sleep quality in cystic fibrosis. Sleep Med. 2002;3(3):205–212. doi: 10.1016/s1389-9457(01)00157-5. [DOI] [PubMed] [Google Scholar]
- 101.Dancey D.R., Tullis E.D., Heslegrave R., Thornley K., Hanly P.J. Sleep quality and daytime function in adults with cystic fibrosis and severe lung disease. Eur Respir J. 2002;19(3):504–510. doi: 10.1183/09031936.02.00088702. [DOI] [PubMed] [Google Scholar]
- 102.Jankelowitz L., Reid K.J., Wolfe L., Cullina J., Zee P.C., Jain M. Cystic fibrosis patients have poor sleep quality despite normal sleep latency and efficiency. Chest. 2005;127(5):1593–1599. doi: 10.1378/chest.127.5.1593. [DOI] [PubMed] [Google Scholar]
- 103.Naqvi S.K., Sotelo C., Murry L., Simakajornboon N. Sleep architecture in children and adolescents with cystic fibrosis and the association with severity of lung disease. Sleep Breath Schlaf Atm. 2008;12(1):77–83. doi: 10.1007/s11325-007-0123-0. [DOI] [PubMed] [Google Scholar]
- 104.Maisonneuve P., Marshall B.C., Knapp E.A., Lowenfels A.B. Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. J Natl Cancer Inst. 2013;105(2):122–129. doi: 10.1093/jnci/djs481. [DOI] [PubMed] [Google Scholar]
- 105.Than B.L.N., Linnekamp J.F., Starr T.K. CFTR is a tumor suppressor gene in murine and human intestinal cancer. Oncogene. 2016;35(32):4179–4187. doi: 10.1038/onc.2015.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang J.T., Jiang X.H., Xie C. Downregulation of CFTR promotes epithelial-to-mesenchymal transition and is associated with poor prognosis of breast cancer. Biochim Biophys Acta. 2013;1833(12):2961–2969. doi: 10.1016/j.bbamcr.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 107.Xie C., Jiang X.H., Zhang J.T. CFTR suppresses tumor progression through miR-193b targeting urokinase plasminogen activator (uPA) in prostate cancer. Oncogene. 2013;32(18):2282–2291.e1-7. doi: 10.1038/onc.2012.251. [DOI] [PubMed] [Google Scholar]
- 108.Son J.W., Kim Y.J., Cho H.M. Promoter hypermethylation of the CFTR gene and clinical/pathological features associated with non-small cell lung cancer. Respirol Carlton Vic. 2011;16(8):1203–1209. doi: 10.1111/j.1440-1843.2011.01994.x. [DOI] [PubMed] [Google Scholar]
- 109.Govindan R., Ding L., Griffith M. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150(6):1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bombieri C., Benetazzo M., Saccomani A. Complete mutational screening of the CFTR gene in 120 patients with pulmonary disease. Hum Genet. 1998;103(6):718–722. doi: 10.1007/s004390050897. [DOI] [PubMed] [Google Scholar]
- 111.Li J., Zhang J.T., Jiang X. The cystic fibrosis transmembrane conductance regulator as a biomarker in non-small cell lung cancer. Int J Oncol. 2015;46(5):2107–2115. doi: 10.3892/ijo.2015.2921. [DOI] [PubMed] [Google Scholar]
- 112.Ashour N., Angulo J.C., Andrés G. A DNA hypermethylation profile reveals new potential biomarkers for prostate cancer diagnosis and prognosis. Prostate. 2014;74(12):1171–1182. doi: 10.1002/pros.22833. [DOI] [PubMed] [Google Scholar]
- 113.Wilson T.J., Zamler D.B., Doherty R., Castro M.G., Lowenstein P.R. Reversibility of glioma stem cells’ phenotypes explains their complex in vitro and in vivo behavior: discovery of a novel neurosphere-specific enzyme, cGMP-dependent protein kinase 1, using the genomic landscape of human glioma stem cells as a discovery tool. Oncotarget. 2016;7(39):63020–63041. doi: 10.18632/oncotarget.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu J., Yong M., Li J. High level of CFTR expression is associated with tumor aggression and knockdown of CFTR suppresses proliferation of ovarian cancer in vitro and in vivo. Oncol Rep. 2015;33(5):2227–2234. doi: 10.3892/or.2015.3829. [DOI] [PubMed] [Google Scholar]
- 115.Kim S., Beyer B.A., Lewis C., Nadel J.A. Normal CFTR inhibits epidermal growth factor receptor-dependent pro-inflammatory chemokine production in human airway epithelial cells. PloS One. 2013;8(8):e72981. doi: 10.1371/journal.pone.0072981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hung M.S., Chen I.C., Lin P.Y. Epidermal growth factor receptor mutation enhances expression of vascular endothelial growth factor in lung cancer. Oncol Lett. 2016;12(6):4598–4604. doi: 10.3892/ol.2016.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Herbst R.S. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59(suppl 2):21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 118.Chen J., Chen Y., Chen Y. Epidermal CFTR suppresses MAPK/NF-κB to promote cutaneous wound healing. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2016;39(6):2262–2274. doi: 10.1159/000447919. [DOI] [PubMed] [Google Scholar]
- 119.Mattoscio D., Evangelista V., De Cristofaro R. Cystic fibrosis transmembrane conductance regulator (CFTR) expression in human platelets: impact on mediators and mechanisms of the inflammatory response. FASEB J. 2010 doi: 10.1096/fj.10-159921. fj.10-159921. [DOI] [PubMed] [Google Scholar]
- 120.Crites K.S., Morin G., Orlando V. CFTR knockdown induces proinflammatory changes in intestinal epithelial cells. J Inflamm Lond Engl. 2015;12:62. doi: 10.1186/s12950-015-0107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tomlinson D.C., Baxter E.W., Loadman P.M., Hull M.A., Knowles M.A. FGFR1-induced epithelial to mesenchymal transition through MAPK/PLCγ/COX-2-mediated mechanisms. PloS One. 2012;7(6):e38972. doi: 10.1371/journal.pone.0038972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shakibaei M., Harikumar K.B., Aggarwal B.B. Resveratrol addiction: to die or not to die. Mol Nutr Food Res. 2009;53(1):115–128. doi: 10.1002/mnfr.200800148. [DOI] [PubMed] [Google Scholar]
- 123.Gray R.D., Imrie M., Boyd A.C., Porteous D., Innes J.A., Greening A.P. Sputum and serum calprotectin are useful biomarkers during CF exacerbation. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2010;9(3):193–198. doi: 10.1016/j.jcf.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 124.Martin C., Coolen N., Wu Y. CFTR dysfunction induces vascular endothelial growth factor synthesis in airway epithelium. Eur Respir J. 2013;42(6):1553–1562. doi: 10.1183/09031936.00164212. [DOI] [PubMed] [Google Scholar]
- 125.Wojewodka G., De Sanctis J.B., Bernier J. Candidate markers associated with the probability of future pulmonary exacerbations in cystic fibrosis patients. PLoS One. 2014;9(2):e88567. doi: 10.1371/journal.pone.0088567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bellin M.D., Laguna T., Leschyshyn J. Insulin secretion improves in cystic fibrosis following ivacaftor correction of CFTR: a small pilot study. Pediatr Diabetes. 2013;14(6):417–421. doi: 10.1111/pedi.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fontés G., Ghislain J., Benterki I. The ΔF508 mutation in the cystic fibrosis transmembrane conductance regulator is associated with progressive insulin resistance and decreased functional β-cell mass in mice. Diabetes. 2015;64(12):4112–4122. doi: 10.2337/db14-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Philpott C.M., Boak D. The impact of olfactory disorders in the United Kingdom. Chem Senses. 2014;39(8):711–718. doi: 10.1093/chemse/bju043. [DOI] [PubMed] [Google Scholar]