Abstract
Background
Aspirin use in COPD has been associated with reduced all-cause mortality in meta-regression analysis with few equivocal studies. However, the effect of aspirin on COPD morbidity is unknown.
Methods
Self-reported daily aspirin use was obtained at baseline from SPIROMICS participants with COPD (FEV1/FVC < 70%). Acute exacerbations of COPD (AECOPD) were prospectively ascertained through quarterly structured telephone questionnaires up to 3 years and categorized as moderate (symptoms treated with antibiotics or oral corticosteroids) or severe (requiring ED visit or hospitalization). Aspirin users were matched one-to-one with nonusers, based on propensity score. The association of aspirin use with total, moderate, and severe AECOPD was investigated using zero-inflated negative binomial models. Linear or logistic regression was used to investigate the association with baseline respiratory symptoms, quality of life, and exercise tolerance.
Results
Among 1,698 participants, 45% reported daily aspirin use at baseline. Propensity score matching resulted in 503 participant pairs. Aspirin users had a lower incidence rate of total AECOPD (adjusted incidence rate ratio [IRR], 0.78; 95% CI, 0.65-0.94), with similar effect for moderate but not severe AECOPD (IRR, 0.86; 95% CI, 0.63-1.18). Aspirin use was associated with lower total St. George’s Respiratory Questionnaire score (β, –2.2; 95% CI, –4.1 to –0.4), reduced odds of moderate-severe dyspnea (modified Medical Research Council questionnaire score ≥ 2; adjusted odds ratio, 0.69; 95% CI, 0.51-0.93), and COPD Assessment Test score (β, –1.1; 95% CI, –1.9 to –0.2) but not 6-min walk distance (β, 0.7 m; 95% CI, –14.3 to 15.6).
Conclusions
Daily aspirin use is associated with reduced rate of COPD exacerbations, less dyspnea, and better quality of life. Randomized clinical trials of aspirin use in COPD are warranted to account for unmeasured and residual confounding.
Trial Registry
ClinicalTrials.gov; No.: NCT01969344; URL: www.clinicaltrials.gov
Key Words: acute exacerbation of chronic bronchitis, antiplatelet drugs, COPD, dyspnea, quality of life
Abbreviations: 6MWD, 6-min walk distance; AECOPD, acute exacerbation of COPD; CAT, COPD Assessment Test; IRR, incidence rate ratio; MDCT, multidetector row CT; mMRC, modified Medical Research Council; SGRQ, St. George’s Respiratory Questionnaire; SPIROMICS, Subpopulations and Intermediate Outcome Measures in COPD Study
Pharmacologic therapy for COPD consists of treatment using combination inhaler therapy focused on bronchodilation and decreasing pulmonary inflammation. However, the systemic manifestations of COPD, including systemic inflammation, are inadequately addressed by current therapies. Inhaled corticosteroids have not been shown to reduce circulating inflammatory biomarkers.1 Chronic macrolide antibiotics, which have antiinflammatory and immunomodulatory properties in addition to antibacterial effects, have been associated with fewer acute exacerbations of COPD (AECOPD) among patients with severe COPD and a history of exacerbations; however, use has been limited by concern for antibiotic resistance and significant hearing loss.2, 3 Likewise, the phosphodiesterase-4 inhibitor roflumilast reduced the rate of COPD exacerbations only in patients with chronic bronchitis and severe disease, and its use has been limited by gastrointestinal side effects.4, 5 Statins, which are thought to exert antiinflammatory effects and reduce C-reactive protein (CRP),6 were ineffective in reducing the exacerbation rate among patients with moderate-to-severe COPD in a clinical trial.7
Aspirin, which inhibits platelet activity by blocking conversion of arachidonic acid and has both direct and indirect antiinflammatory properties, has been associated with reduced all-cause mortality among patients with COPD in most,8, 9, 10, 11, 12 but not all,13, 14 previous studies. Aspirin has also been associated with reduced odds of mechanical ventilation and shorter hospital stay during AECOPD and reduced emphysema progression in the general population.9, 15 To our knowledge, whether aspirin use is associated with COPD morbidity has not previously been explored. This study investigates the association of aspirin use with AECOPD, respiratory symptoms, and quality of life, using a large, well-characterized observational cohort.
Materials and Methods
The Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) is an observational cohort study that enrolled current and former smokers (≥ 20 pack-years) and nonsmokers (≤ 1 pack-year) aged 40 to 80 years from 12 clinical sites in the United States, recruited from 2010 to 2015 and followed up to 36 months with the goal of identifying intermediate outcome measures that predict long-term clinical end points of morbidity.16 Participants with COPD (postbronchodilator ratio of FEV1 to FVC < 70%) were selected for this analysis. Participants were specifically asked at baseline whether they were currently taking a daily aspirin (yes/no). Information about dose, adherence, and duration of therapy was not available and therefore not accounted for in the analysis.
Outcomes
Respiratory events, ED visits, and hospitalizations were ascertained through structured telephone questionnaires administered quarterly throughout follow-up. The primary outcome of interest is total number of moderate or severe AECOPD, which was subdivided into moderate AECOPD (defined as respiratory symptoms treated with antibiotics or oral corticosteroids or requiring physician’s office or urgent care visit) and severe AECOPD (defined as respiratory symptoms requiring an ED visit or hospitalization). Secondary outcomes of interest included baseline COPD health status based on total COPD Assessment Test (CAT) score,17 moderate-severe dyspnea defined as modified Medical Research Council (mMRC) questionnaire18 score ≥ 2, exercise capacity based on 6-min walk distance (6MWD) in meters,19 and respiratory-specific quality of life based on the St. George’s Respiratory Questionnaire (SGRQ) score.20
Propensity Score and Matching
A propensity score—the predicted probability that a participant reports taking daily aspirin at baseline—was obtained for each participant, using a nonparsimonious multivariable generalized mixed logistic regression model with random intercepts for study site. The model included age (continuous), sex, race (African American vs other), educational achievement (< high school vs ≥ high school), percent predicted postbronchodilator FEV1 (continuous),21 smoking status (current smoker based on self-report or, if unavailable, exhaled carbon monoxide > 6 parts per million vs former smoker),22 presence of hypertension, coronary heart disease (history of coronary artery disease, heart attack, or angina; coronary heart disease), congestive heart failure, diabetes, stroke, body mass index (categorical: underweight [< 18.5 kg/m2] vs normal/overweight [18.5 to < 30 kg/m2] vs obese [≥ 30 kg/m2]), anemia23 (dichotomous, using sex-specific cutoffs of hemoglobin), oxygen use, statin use, β-blocker use, and inhaler therapy (long-acting antimuscarinic, long-acting β-agonist, or inhaled corticosteroid) use during the preceding 3 months.
Aspirin users were then matched one-to-one without replacement with nonusers based on the propensity score, using a caliper width equal to 0.2 times the standard deviation of the logit of the propensity score.24, 25 For longitudinal analysis of the primary outcome of interest, only participants who had accrued follow-up time and had nonmissing exacerbation data were eligible to be matched. For cross-sectional analysis of secondary outcomes, participants with data for any outcome regardless of subsequent follow-up were rematched on the basis of propensity score, using the above method. Analysis of each secondary outcome included only participant pairs where both participants had nonmissing data for that outcome.
Statistical Analysis
Baseline characteristics and health-related variables were compared between aspirin users and nonusers in the unmatched and propensity score-matched samples, using the χ2 test for proportions and the Student t test or Kruskal-Wallis test for continuous variables to evaluate means and medians respectively. Subsequent analyses (except sensitivity analyses) were performed in the matched sample.
Crude exacerbation rates were calculated as number of AECOPD per person-year of follow-up and compared between aspirin users and nonusers with a Student t test. Median follow-up time was compared between the groups, using the Kruskal-Wallis test. Unconditional zero-inflated negative binomial models accounting for total follow-up time as an offset variable with FEV1 as the zero inflation variable were constructed to investigate the association of aspirin use with total AECOPD, moderate AECOPD, and severe AECOPD separately controlling for all covariates that were included in the propensity score model to account for residual confounding.26, 27 Several sensitivity analyses were performed to determine whether results were robust to different modeling strategies in the unmatched sample: (1) fully adjusted zero-inflated negative binomial regression, (2) bivariate zero-inflated negative binomial model adjusted for propensity score as a continuous variable, and (3) inverse probability of the treatment weights.28, 29 Furthermore, to minimize the effect of possible changes in aspirin use over time, death, or loss to follow-up, the association of aspirin use with number of AECOPD within the first year of follow-up was investigated using a similar model (without the offset variable) among propensity score-matched participants who had both accrued at least 365 days of follow-up.
Since previous systemic therapies in COPD demonstrated clinical benefit among patients with more severe obstruction or chronic bronchitis,2, 3, 4 effect modification of the association between aspirin use and AECOPD by disease severity (mild/moderate vs severe) and presence of chronic bronchitis as defined by either the classic or alternative (SGRQ) definition30 was explored by multiplicative interaction and by calculating stratum-specific estimates. Furthermore, effect modification by emphysema burden at baseline was explored in light of recent evidence that aspirin use reduced emphysema progression in a general population cohort.15 Whole-lung multidetector row CT (MDCT) scans were performed at baseline for both coached total lung capacity and coached residual volume. Emphysema-like lung was calculated on the basis of the percentage of total lung voxels < –950 Hounsfield units at total lung capacity.31 Effect modification based on the presence of emphysema (< 5% vs ≥ 5%) and emphysema severity (continuous percent emphysema) was evaluated, including MDCT scanner model as an additional covariate. Finally, in order to investigate the possibility of differential misclassification of cardiovascular events as AECOPD, effect modification by the presence of cardiometabolic phenotype (≥ 2 cardiac/metabolic comorbidities: hypertension, coronary heart disease, congestive heart failure, stroke, diabetes, and obesity)23 was also explored.
In the cross-sectional analysis of secondary outcomes, unconditional multivariable linear regression was used to investigate the association of aspirin use with baseline 6MWD, total CAT and SGRQ scores while unconditional multivariable logistic regression was used to investigate the association of aspirin use with moderate-severe dyspnea (mMRC score ≥ 2). Participants who were missing data on an outcome were removed along with their matched pair in order to maintain a balanced sample.
Analyses were conducted with SAS 9.4 (SAS Institute). The threshold of significance for main effects was P < .05, while interaction terms were considered significant at P < .1.32 SPIROMICS was approved by the institutional review board at each center (e-Table 1), and all participants provided written informed consent (ClinicalTrials.gov: NCT01969344).
Results
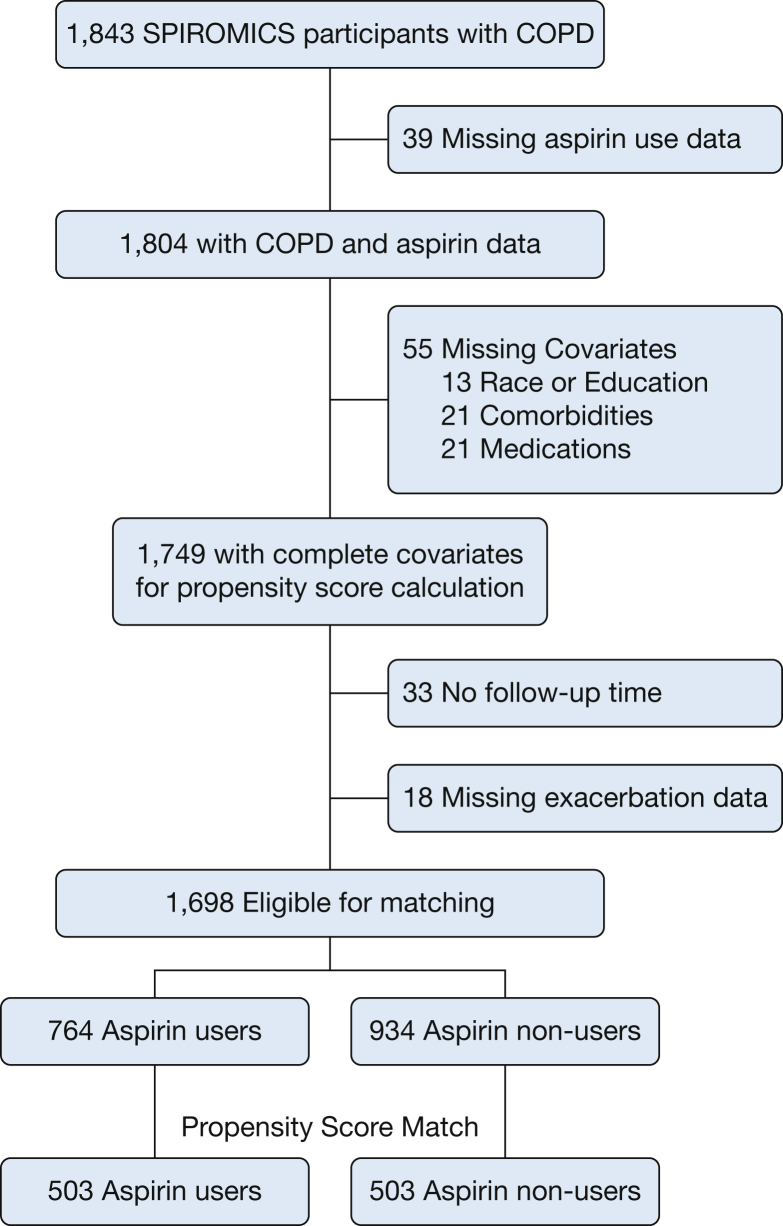
Among 1,843 SPIROMICS participants with COPD, 1,749 (95%) had complete data on aspirin use and all relevant covariables for propensity score calculation (Fig 1). Of the 1,698 participants (92%) who accrued follow-up time and had nonmissing exacerbation data, 764 (45%) reported using aspirin daily at baseline. Compared with nonusers, aspirin users were significantly older; more likely to be male, white, and obese; less likely to be smokers; had better lung function but more cardiovascular comorbidities; and were more likely to use cardiovascular medications (Table 1). Matching resulted in 503 pairs of participants with all baseline characteristics balanced between aspirin users and nonusers (Table 1). Overall, the matched sample participants were on average 66.5 years old, had an average postbronchodilator FEV1 of 62% predicted, were male more often than female (58%) and white more often than black (88%), and with fewer than two cardiovascular comorbidities (62%). At baseline, the sample consisted of 30% current smokers and 23% home oxygen users, with 43% reporting statin use, 48% reporting inhaled corticosteroid use, and 58% reporting long-acting bronchodilator use during the previous 3 months.
Figure 1.
CONSORT diagram of SPIROMICS participants. CONSORT = Consolidated Standards of Reporting Trials; SPIROMICS = Subpopulations and Intermediate Outcome Measures in COPD Study.
Table 1.
Baseline Characteristics
| Characteristic | SPIROMICS Participants With COPD and Complete Data |
Matched Participants:Longitudinal Analysis, Whole Follow-up Period |
||||
|---|---|---|---|---|---|---|
| Daily Aspirin Users (n = 764) | Aspirin Nonusers (n = 934) | P Value | Daily Aspirin Users (n = 503) | Aspirin Nonusers (n = 503) | P Value | |
| Age, mean ± SD | 67.5 ± 6.8 | 63.5 ± 8.4 | < .0001 | 66.5 ± 6.8 | 66.5 ± 7.6 | 1 |
| Female | 270 (35.3) | 450 (48.2) | < .0001 | 210 (41.8) | 208 (41.4) | .9 |
| Race: black | 80 (10.5) | 167 (17.9) | < .0001 | 63 (12.5) | 60 (11.9) | .8 |
| Less than high school education | 267 (35) | 386 (41.3) | .007 | 189 (37.6) | 196 (39) | .6 |
| FEV1 % predicted (postbronchodilator) | 63.1 ± 22.6 | 59.7 ± 23.2 | .003 | 62.4 ± 23.2 | 61.7 ± 22.9 | .7 |
| Current smoker | 206 (27) | 363 (38.9) | < .0001 | 155 (30.8) | 150 (29.8) | .7 |
| Cigarettes/d among current smokers, median (Q1-Q3) | 12 (6-20) | 15 (8-20) | .05 | 10.5 (6-20) | 15 (7-20) | .2 |
| Supplemental home oxygen | 182 (23.8) | 212 (22.7) | .6 | 115 (22.9) | 118 (23.5) | .8 |
| Percent emphysema on MDCT scan, mean ± SD | 10.5 ± 10.7 | 11.8 ± 11.8 | .02 | 11.0 ± 11.4 | 10.6 ± 10.7 | .5 |
| Emphysema ≥ 5% on MDCT scan | 423 (55.9) | 549 (59) | .2 | 282 (56.3) | 280 (55.9) | .9 |
| Anemia: hemoglobin < 13 g/dL (males) or < 12 g/dL (females) | 84 (11) | 69 (7.4) | .01 | 41 (8.2) | 52 (10.3) | .2 |
| Hypertension | 477 (62.4) | 382 (40.9) | < .0001 | 280 (55.7) | 274 (54.5) | .7 |
| Coronary arterial disease | 239 (31.3) | 88 (9.4) | < .0001 | 79 (15.7) | 83 (16.5) | .7 |
| Congestive heart failure | 38 (5) | 15 (1.6) | < .0001 | 13 (2.6) | 13 (2.6) | 1 |
| Stroke | 39 (5.1) | 35 (3.8) | .2 | 25 (5) | 22 (4.4) | .7 |
| Diabetes | 153 (20) | 80 (8.6) | < .0001 | 75 (14.9) | 71 (14.1) | .7 |
| Statin use (prior 3 mo) | 433 (56.7) | 246 (26.3) | < .0001 | 216 (42.9) | 213 (42.4) | .8 |
| β-Blocker use (prior 3 mo) | 231 (30.2) | 106 (11.4) | < .0001 | 83 (16.5) | 96 (19.1) | .3 |
| Inhaled corticosteroid use (prior 3 mo) | 338 (44.2) | 452 (48.4) | .09 | 235 (46.7) | 247 (49.1) | .4 |
| Long-acting bronchodilator use (prior 3 mo) | .5 | .9 | ||||
| Long-acting muscarinic antagonist (LAMA) | 95 (12.4) | 108 (11.6) | 59 (11.7) | 55 (10.9) | ||
| Long-acting β-agonist (LABA) | 138 (18.1) | 194 20.8) | 97 (19.3) | 106 (21.1) | ||
| Both LAMA and LABA | 199 (26.1) | 249 (26.7) | 132 (26.2) | 134 (26.6) | ||
| Neither LAMA nor LABA | 332 (43.5) | 383 (41) | 215 (42.7) | 208 (41.4) | ||
| Body mass index, mean ± SD | 28.1 ± 5.2 | 26.7 ± 5.2 | < .0001 | 27.8 ± 5.2 | 27.6 ± 5.2 | .5 |
| Underweight (< 18.5 kg/m2) | 18 (2.4) | 42 (4.5) | < .0001 | 13 (2.6) | 12 (2.4) | .9 |
| Normal or overweight (18.5 to < 30 kg/m2) | 474 (62) | 651 (69.7) | 326 (64.8) | 332 (66) | ||
| Obese (≥ 30 kg/m2) | 272 (35.6) | 241 (25.8) | 164 (32.6) | 159 (31.6) | ||
| Propensity score, mean ± SD | 0.57 ± 0.22 | 0.35 ± 0.19 | < .0001 | 0.47 ± 0.18 | 0.47 ± 0.18 | .8 |
MDCT = multidetector row CT; Q1, Q3 = quartiles 1 and 3; SPIROMICS = Subpopulations and Intermediate Outcome Measures in COPD Study.
Boldface entries indicate a statistically significant difference, with a P value less than .05.
Association of Aspirin Use With COPD Exacerbation Rate
In the propensity score-matched sample participants taking aspirin were monitored for a median of 2.7 years (Q1-Q3, 1.8-3.0 years) compared with 2.6 years (Q1-Q3, 1.8-3.0 years) for aspirin nonusers (Kruskal-Wallis P = .15). One-half of the sample did not report any exacerbations during follow-up, with an overall crude exacerbation rate of 0.58 ± 1.0 (0.40 ± 0.73 [moderate AECOPD] and 0.19 ± 0.56 [severe AECOPD]) per person-year. Comparison of crude AECOPD rates by aspirin use is displayed in Table 2.
Table 2.
Crude COPD Exacerbation Rates by Aspirin Use in Propensity Score-Matched Sample
| COPD Exacerbation Rate | Aspirin Users (Mean ± SD) | Aspirin Nonusers (Mean ± SD) | P Value |
|---|---|---|---|
| Total exacerbations | 0.51 ± 0.86 | 0.65 ± 1.1 | .03 |
| Moderate exacerbations | 0.34 ± 0.63 | 0.44 ± 0.82 | .02 |
| Severe exacerbations | 0.17 ± 0.47 | 0.20 ± 0.63 | .4 |
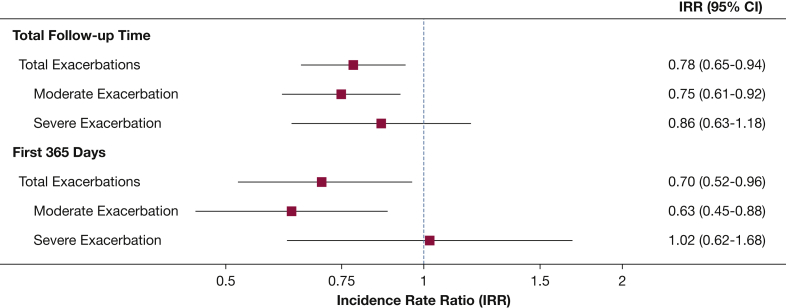
In adjusted analysis of the matched sample, aspirin users had an estimated 22% lower incidence rate of total AECOPD (incidence rate ratio [IRR], 0.78; 95% CI, 0.65-0.94), which was similar to the reduction in moderate AECOPD (IRR, 0.75; 95% CI, 0.61-0.92) with no statistically significant difference in severe AECOPD (IRR, 0.86; 95% CI, 0.63-1.18) (Fig 2). Sensitivity analyses produced similar results (Table 3). When considering only exacerbations during the first year of follow-up among the sample of matched participants who had accrued at least 1 year of follow-up (n = 788), aspirin users had a 30% lower incidence rate of total AECOPD (IRR, 0.70; 95% CI, 0.52-0.96), 37% lower incidence of moderate AECOPD (IRR, 0.63; 95% CI, 0.45-0.88) but no association with severe AECOPD (IRR, 1.02; 95% CI, 0.62-1.68 (Fig 2).
Figure 2.
Association of aspirin use with incidence rate of acute exacerbations of COPD.
Table 3.
Sensitivity Analyses
| Analysis Method | No. | Total AECOPD | Moderate AECOPD | Severe AECOPD |
|---|---|---|---|---|
| Primary propensity-matched analysis (main analysis) | 1,008 | 0.78 (0.65-0.94) | 0.75 (0.61-0.92) | 0.86 (0.63-1.18) |
| Fully adjusted unmatched sample | 1,698 | 0.81 (0.70-0.95) | 0.78 (0.65-0.94) | 0.91 (0.71-1.18) |
| Bivariate analysis adjusting for propensity score | 1,698 | 0.78 (0.67-0.92) | 0.77 (0.63-0.93) | 0.86 (0.66-1.11) |
| Inverse probability of the treatment weights | 1,698 | 0.71 (0.62-0.82) | 0.66 (0.56-0.78) | 0.88 (0.71-1.10) |
AECOPD = acute exacerbation of COPD.
Subgroup Analyses
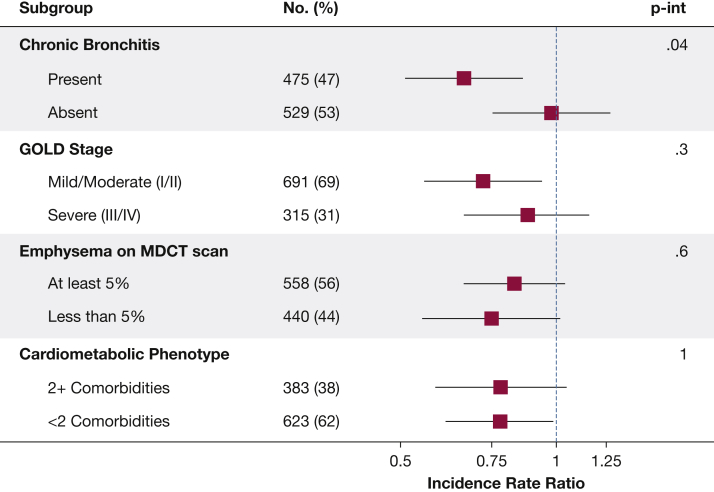
In the propensity score-matched sample, comparing aspirin users and nonusers, the prevalence of baseline chronic bronchitis (47% vs 47%), severe COPD (32% vs 31%), total emphysema ≥ 5% (56% vs 56%), and cardiometabolic phenotype (39% vs 37%) was similar. In stratified analysis aspirin use was more strongly associated with total AECOPD among participants with chronic bronchitis (IRRCB+, 0.66; 95% CI, 0.51-0.86; Pinteraction = .04) (Fig 3). This finding was reflected in moderate AECOPD (IRRCB+, 0.62; 95% CI, 0.47-0.83; IRRCB–, 0.96; 95% CI, 0.72-1.29; Pinteraction = .04) but not severe AECOPD (IRRCB+, 0.73; 95% CI, 0.48-1.13; IRRCB–, 1.1; 95% CI, 0.69-1.74; Pinteraction = .2). There was no effect modification by COPD severity (Pinteraction = .3), presence of emphysema (Pinteraction = .6), emphysema severity (Pinteraction = .4), or cardiometabolic phenotype (Pinteraction = 1) (Fig 3).
Figure 3.
Association of aspirin use with incidence of COPD exacerbations, based on presence of chronic bronchitis at baseline, COPD severity, presence of emphysema (≥ 5%), and presence of cardiometabolic phenotype at baseline. GOLD = Global Initiative for Chronic Obstructive Lung Disease; MDCT = multidetector row CT; P-int = P value for interaction with aspirin use.
Association of Aspirin Use With Respiratory Symptoms and Quality of Life
A total of 1,044 participants were matched for the cross-sectional analysis at baseline. Because of missing outcome data, 66 participant pairs were excluded from the SGRQ analysis, as were 9 pairs from the mMRC analysis, 55 pairs from the CAT analysis, and 62 pairs from the 6MWD analysis. Aspirin users had SGRQ scores that were on average 3 points lower than those of nonusers (95% CI, –4.9 to –1.1), had 34% lower odds of reporting moderate-severe dyspnea based on mMRC score ≥ 2 (adjusted OR, 0.66; 95% CI, 0.49-0.90), and lower total CAT score (β, –1.1; 95% CI, –1.9 to –0.2). There was no association between aspirin use and 6MWD (β, 0.7 m; 95% CI, –14.3 to 15.6).
Discussion
Daily aspirin use in a large stable COPD cohort was associated with reduced incidence rate of AECOPD during 3 years of follow-up, as well as better quality of life and less dyspnea in cross-sectional analysis at baseline. In subgroup analysis aspirin use was more strongly associated with reduced incidence of AECOPD among participants reporting symptoms of chronic bronchitis at baseline. Although aspirin use has previously been linked with reduced mortality risk in patients with COPD, to our knowledge this is the first study to investigate the association of daily aspirin use with respiratory morbidity in COPD. These findings imply that aspirin may reduce the risk of respiratory morbidity and improve symptoms in COPD.
In the absence of data from a randomized controlled trial, this study allows evaluation of aspirin use in COPD while attempting to minimize the influence of confounding by indication. The reduced incidence of total and moderate AECOPD among aspirin users was independent of concurrent respiratory or cardiovascular medication use and robust when analyzing the entire follow-up period, limiting the analysis to the first year of follow-up, and across all sensitivity analyses. There was no association between aspirin use and incidence of severe AECOPD requiring ED visit or hospitalization. This may be a consequence of few severe AECOPD during the study, which limits the power to detect a difference, or the higher prevalence of respiratory viral infections, which are more likely to lead to hospitalization for AECOPD33 and would not be augmented by aspirin therapy.
Four previous studies have reported reduced all-cause mortality among aspirin users with stable COPD8, 12 and following AECOPD,9, 10 while two found no such association.13, 14 Meta-regression analysis by Pavasini et al11 demonstrated a significant reduction in all-cause mortality without modification by smoking status, sex, cardiovascular comorbidities, or use of other cardiovascular medications. Findings of this study add to the existing literature by highlighting that aspirin use is also associated with reduced respiratory morbidity across several domains—including exacerbation risk, quality of life, and dyspnea—factors related to patient well-being and health-care utilization. Notably, however, the difference in SGRQ and CAT scores between aspirin users and nonusers at baseline (3 and 1.1, respectively), while statistically significant, did not reach the minimal clinically important difference of these surveys (4 and 2, respectively)34, 35; although similar effect sizes have been reported in most studies evaluating mean SGRQ difference with long-acting muscarinic antagonist36 and long-acting β-agonist.37, 38
COPD phenotyping in this study allows for the identification of subgroup effects that inform future studies. It appears that participants who reported cough and phlegm at baseline, consistent with the chronic bronchitis phenotype that has been associated with greater risk for morbidity and mortality,39 had a significantly stronger association between aspirin use and reduced AECOPD. On the basis of the findings of this study, future studies investigating aspirin therapy in COPD may benefit from oversampling participants with the chronic bronchitis phenotype. There was a trend toward stronger association between aspirin use and AECOPD among participants with mild or moderate COPD compared with those with severe COPD. While this may be due to a higher proportion of severe rather than moderate AECOPD in the severe COPD group (36% vs 28% of total exacerbations, respectively), it may alternatively reflect imprecision of the effect estimate in the severe COPD group due to inadequate power. There was no difference in the association of aspirin use with AECOPD, based on the presence or burden of emphysema at baseline. Emphysema progression, which was attenuated by aspirin use in a recent study,15 could not be evaluated due to lack of follow-up MDCT scans.
Aspirin has both systemic and local pulmonary mechanisms of action that may explain these study findings including inactivation of platelets and reduced inflammation. A urinary metabolite of thromboxane A2, which is secreted by activated platelets, has been shown to be elevated among patients with COPD and represents the pathway irreversibly blocked by aspirin.40, 41 Circulating inflammatory markers IL-6 and C-reactive protein, when persistently elevated, may represent a systemic inflammatory phenotype of COPD and are attenuated by aspirin in other patient populations.42, 43 Furthermore, treatment with aspirin reduced proinflammatory cytokines in bronchoalveolar lavage samples from 33 healthy volunteers.44
This study has several limitations. Aspirin use was ascertained through self-report, a potential source of measurement error. Aspirin dose was unavailable and therefore a dose effect could not be investigated. In addition, duration of aspirin use and adherence to therapy before and during the study were unavailable, which limits inferences regarding baseline symptoms and may lead to uncaptured therapeutic crossover during follow-up. AECOPD were not routinely adjudicated using medical records in this study, which may lead to misclassification of events. Mild AECOPD, defined as respiratory symptoms requiring increased use of inhaler medications without health-care utilization, oral corticosteroid, or antibiotic initiation, were not systematically ascertained in this study and therefore the association of aspirin use with mild AECOPD was not investigated. SPIROMICS was not specifically designed to address aspirin use in COPD, and despite the use of statistical methods such as propensity score matching to minimize confounding by indication and residual confounding, observational studies remain vulnerable to bias due to inability to completely control for both measured and unmeasured factors relating to individuals, their providers, and the health-care system. For example, aspirin users may represent participants with higher health literacy who take measures to protect against exacerbations that were not ascertained in this study such as increased physical activity, avoidance of environmental exposures or sick contacts, and adherence to vaccinations or medications. A randomized controlled study is necessary to establish whether a direct causal effect exists between aspirin use and AECOPD. Finally, reported AECOPD may represent cardiovascular events that are clinically misclassified; however, the presence of cardiovascular risk factors did not modify the association between aspirin use and AECOPD.
Conclusions
In conclusion, on the basis of findings from a large multicenter observational cohort, daily aspirin use at baseline was associated with lower incidence rate of AECOPD, as well as less severe respiratory symptoms and better quality of life at baseline. Prospective randomized clinical trials of aspirin use are warranted to explore its potential effectiveness in reducing COPD morbidity.
Acknowledgments
Author contributions: A. F. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, and is the guarantor of this study. A. F., N. P., and N. N. H. contributed substantially to the design of the study, interpretation of results, drafting and revising the manuscript. R. P. B., A. P. C., C. B. C., M. T. D., M. K. H., E. A. H., R. E. K., J. A. K., S. P. P., R. W., R. G. B., and N. N. H. contributed to the acquisition of the data, interpretation of the results, and revisions of the manuscript for critically important intellectual content. C. P. A., W. W. L., R. P., and L. M. P. contributed to interpretation of the results and revisions of the manuscript for critically important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: A. F., N. P., C. P. A., A. P. C., R. E. K., J. A. K., W. W. L., R. P., L. M. P., and R. G. B. report no relationships with industry sponsors who supported SPIROMICS through the Foundation for the NIH and the COPD Foundation. R. P. B. serves on the advisory board of GlaxoSmithKline, Boehringer-Ingelheim Pharmaceuticals, Inc, and Mylan Specialty LP and received research grant support from GlaxoSmithKline. C. B. C. has consulted with PulmonX, has received research funding from Equinox Fitness Clubs, and is employed part-time by the GlaxoSmithKline Global Respiratory Franchise. M. T. D. has consulted with AstraZeneca/MedImmune and GlaxoSmithKline and has contracted clinical trials with AstraZeneca/MedImmune, Boehringer-Ingelheim Pharmaceuticals, Inc, GlaxoSmithKline, and Novartis Pharmaceuticals Corporation. M. K. H. has consulted for AstraZeneca, Boehringer-Ingelheim Pharmaceuticals, Inc, and GlaxoSmithKline and received research support from Sunovion and Novartis. E. A. H. is a founder and shareholder of VIDA Diagnostics, a company commercializing lung image analysis software developed, in part, at the University of Iowa. S. P. P. has consulted with AstraZeneca/MedImmune, GlaxoSmithKline, Novartis Pharmaceuticals Corporation, Regeneron Pharmaceuticals, Inc, Sanofi, and Sunovion. R. W. has consulted with AstraZeneca/MedImmune, Boehringer-Ingelheim Pharmaceuticals, Inc, GlaxoSmithKline, Sanofi, and Sunovion and has received grant support from AstraZeneca, Boehringer-Ingelheim, and GlaxoSmithKline. N. N. H. serves on the advisory board of AstraZeneca, GlaxoSmithKline, and Mylan and has received research grants from AstraZeneca, Boehringer-Ingelheim Pharmaceuticals, Inc, Forest Research Institute, Inc, and GlaxoSmithKline.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
*SPIROMICS Investigators: The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. More information about the study and how to access SPIROMICS data may be found at www.spiromics.org. The authors acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E. Alexis, PhD; Wayne H. Anderson, PhD; Igor Barjaktarevic, MD, PhD; R. Graham Barr, MD, DrPH; Eugene R. Bleecker, MD; Richard C. Boucher, MD; Russell P. Bowler, MD, PhD; Elizabeth E. Carretta, MPH; Stephanie A. Christenson, MD; Alejandro P. Comellas, MD; Christopher B. Cooper, MD, PhD; David J. Couper, PhD; Gerard J. Criner, MD; Ronald G. Crystal, MD; Jeffrey L. Curtis, MD; Claire M. Doerschuk, MD; Mark T. Dransfield, MD; Christine M. Freeman, PhD; MeiLan K. Han, MD; Nadia N. Hansel, MD, MPH; Annette T. Hastie, PhD; Eric A. Hoffman, PhD; Robert J. Kaner, MD; Richard E. Kanner, MD; Eric C. Kleerup, MD; Jerry A. Krishnan, MD, PhD; Lisa M. LaVange, PhD; Stephen C. Lazarus, MD; Fernando J. Martinez, MD; Deborah A. Meyers, PhD; Wendy C. Moore, MD; John D. Newell Jr, MD; Laura Paulin, MD, MHS; Stephen Peters, MD, PhD; Cheryl Pirozzi, MD; Elizabeth C. Oelsner, MD, MPH; Wanda K. O’Neal, PhD; Victor E. Ortega, MD, PhD; Robert Paine III, MD; Nirupama Putcha, MD, MHS; Sanjeev Raman, MBBS, MD; Stephen I. Rennard, MD; Donald P. Tashkin, MD; J. Michael Wells, MD; Robert A. Wise, MD; and Prescott G. Woodruff, MD, MPH.
Additional information: The e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: Dr Fawzy is supported by the National Institutes of Health (NIH)/National Institute of Environmental Health Sciences (NIEHS) [Grant F32ES28576]. SPIROMICS was supported by contracts from the NIH/National Heart, Lung, and Blood Institute (NHLBI) [Grants HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C], and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc; Chiesi Farmaceutici SpA; Forest Research Institute, Inc; GlaxoSmithKline; Grifols Therapeutics, Inc; Ikaria, Inc; Nycomed GmbH; Takeda Pharmaceutical Company; Novartis Pharmaceuticals Corporation; ProterixBio; Regeneron Pharmaceuticals, Inc; Sanofi; and Sunovion.
Contributor Information
Nadia N. Hansel, Email: nhansel1@jhmi.edu.
SPIROMICS Investigators:
Neil E. Alexis, Wayne H. Anderson, Igor Barjaktarevic, R. Graham Barr, Eugene R. Bleecker, Richard C. Boucher, Russell P. Bowler, Elizabeth E. Carretta, Stephanie A. Christenson, Alejandro P. Comellas, Christopher B. Cooper, David J. Couper, Gerard J. Criner, Ronald G. Crystal, Jeffrey L. Curtis, Claire M. Doerschuk, Mark T. Dransfield, Christine M. Freeman, MeiLan K. Han, Nadia N. Hansel, Annette T. Hastie, Eric A. Hoffman, Robert J. Kaner, Richard E. Kanner, Eric C. Kleerup, Jerry A. Krishnan, Lisa M. LaVange, Stephen C. Lazarus, Fernando J. Martinez, Deborah A. Meyers, Wendy C. Moore, John D. Newell, Jr., Laura Paulin, Stephen Peters, Cheryl Pirozzi, Elizabeth C. Oelsner, Wanda K. O’Neal, Victor E. Ortega, Robert Paine, III, Nirupama Putcha, Sanjeev Raman, Stephen I. Rennard, Donald P. Tashkin, J. Michael Wells, Robert A. Wise, and Prescott G. Woodruff
Supplementary Data
References
- 1.Sin D.D., Man S.P., Marciniuk D.D. The effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(11):1207–1214. doi: 10.1164/rccm.200709-1356OC. [DOI] [PubMed] [Google Scholar]
- 2.Albert R.K., Connett J., Bailey W.C. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herath S.C., Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD) Cochrane Database Syst Rev. 2013;11:CD009764. doi: 10.1002/14651858.CD009764.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Calverley P.M., Sanchez-Toril F., McIvor A., Teichmann P., Bredenbroeker D., Fabbri L.M. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(2):154–161. doi: 10.1164/rccm.200610-1563OC. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S. Side-effects of roflumilast. Lancet. 2012;379(9817):710–711. doi: 10.1016/S0140-6736(12)60304-3. [DOI] [PubMed] [Google Scholar]
- 6.Albert M.A., Danielson E., Rifai N., Ridker P.M., PRINCE Investigators Effect of statin therapy on C-reactive protein levels: the Pravastatin Inflammation/CRP Evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286(1):64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 7.Criner G.J., Connett J.E., Aaron S.D. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med. 2014;370(23):2201–2210. doi: 10.1056/NEJMoa1403086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekstrom M.P., Hermansson A.B., Strom K.E. Effects of cardiovascular drugs on mortality in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):715–720. doi: 10.1164/rccm.201208-1565OC. [DOI] [PubMed] [Google Scholar]
- 9.Goto T., Faridi M.K., Camargo C.A., Hasegawa K. The association of aspirin use with severity of acute exacerbation of chronic obstructive pulmonary disease: a retrospective cohort study. NPJ Prim Care Respir Med. 2018;28(1):7. doi: 10.1038/s41533-018-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison M.T., Short P., Williamson P.A., Singanayagam A., Chalmers J.D., Schembri S. Thrombocytosis is associated with increased short and long term mortality after exacerbation of chronic obstructive pulmonary disease: a role for antiplatelet therapy? Thorax. 2014;69(7):609–615. doi: 10.1136/thoraxjnl-2013-203996. [DOI] [PubMed] [Google Scholar]
- 11.Pavasini R., Biscaglia S., d’Ascenzo F. Antiplatelet treatment reduces all-cause mortality in COPD patients: a systematic review and meta-analysis. COPD. 2016;13(4):509–514. doi: 10.3109/15412555.2015.1099620. [DOI] [PubMed] [Google Scholar]
- 12.Short P.M., Lipworth S.I., Elder D.H., Schembri S., Lipworth B.J. Effect of β blockers in treatment of chronic obstructive pulmonary disease: a retrospective cohort study. BMJ. 2011;342:d2549. doi: 10.1136/bmj.d2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campo G., Pavasini R., Malagù M. Relationship between troponin elevation, cardiovascular history and adverse events in patients with acute exacerbation of COPD. COPD. 2015;12(5):560–567. doi: 10.3109/15412555.2014.995293. [DOI] [PubMed] [Google Scholar]
- 14.Søyseth V., Brekke P.H., Smith P., Omland T. Statin use is associated with reduced mortality in chronic obstructive pulmonary disease. Eur Respir J. 2007;29(2):279–283. doi: 10.1183/09031936.00106406. [DOI] [PubMed] [Google Scholar]
- 15.Aaron C.P., Schwartz J.E., Hoffman E.A. A longitudinal cohort study of aspirin use and progression of emphysema-like lung characteristics on CT imaging: the MESA Lung Study. Chest. 2018;154(1):41–50. doi: 10.1016/j.chest.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Couper D., LaVange L.M., Han M. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69(5):491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones P.W., Harding G., Berry P., Wiklund I., Chen W.H., Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 18.Bestall J.C., Paul E.A., Garrod R., Garnham R., Jones P.W., Wedzicha J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holland A.E., Spruit M.A., Troosters T. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 20.Jones P.W., Quirk F.H., Baveystock C.M., Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 21.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 22.Middleton E.T., Morice A.H. Breath carbon monoxide as an indication of smoking habit. Chest. 2000;117(3):758–763. doi: 10.1378/chest.117.3.758. [DOI] [PubMed] [Google Scholar]
- 23.Putcha N., Fawzy A., Paul G.G. Anemia and adverse outcomes in a chronic obstructive pulmonary disease population with a high burden of comorbidities: an analysis from SPIROMICS. Ann Am Thorac Soc. 2018;15(6):710–717. doi: 10.1513/AnnalsATS.201708-687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coca-Perraillon M. Matching with propensity scores to reduce bias in observational studies. Paper presented at: Proceedings of NorthEast SAS Users Group Conference (NESUG); September 17-20, 2006; Philadelphia, PA.
- 26.Stuart E.A. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeSantis S.M., Lazaridis C., Ji S., Spinale F.G. Analyzing propensity matched zero-inflated count outcomes in observational studies. J Appl Stat. 2014;41(1):127–141. doi: 10.1080/02664763.2013.834296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.d’Agostino R.B. Tutorial in biostatistics: propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 29.Curtis L.H., Hammill B.G., Eisenstein E.L., Kramer J.M., Anstrom K.J. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45(10):S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 30.Kim V., Crapo J., Zhao H. Comparison between an alternative and the classic definition of chronic bronchitis in COPDgene. Ann Am Thorac Soc. 2015;12(3):332–339. doi: 10.1513/AnnalsATS.201411-518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sieren J.P., Newell J.D., Jr., Barr R.G. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194(7):794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selvin S. 3rd ed. Oxford University Press; New York: 2004. Monographs in Epipemiology and Biostatistics, Vol. 35: Statistical Analysis of Epidemiologic Data. [Google Scholar]
- 33.Wedzicha J.A. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1(2):115–120. doi: 10.1513/pats.2306030. [DOI] [PubMed] [Google Scholar]
- 34.Jones P.W. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 35.Kon S.S., Canavan J.L., Jones S.E. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203. doi: 10.1016/S2213-2600(14)70001-3. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan A. Effect of tiotropium on quality of life in COPD: a systematic review. Prim Care Respir J. 2010;19(4):315–325. doi: 10.4104/pcrj.2010.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tashkin D.P., Fabbri L.M. Long-acting β-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res. 2010;11(1):149. doi: 10.1186/1465-9921-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigo G.J., Nannini L.J., Rodríguez-Roisin R. Safety of long-acting β-agonists in stable COPD. Chest. 2008;133(5):1079–1087. doi: 10.1378/chest.07-1167. [DOI] [PubMed] [Google Scholar]
- 39.Kim V., Criner G.J. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(3):228–237. doi: 10.1164/rccm.201210-1843CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davì G., Basili S., Vieri M. Enhanced thromboxane biosynthesis in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156(6):1794–1799. doi: 10.1164/ajrccm.156.6.9706026. [DOI] [PubMed] [Google Scholar]
- 41.Patrignani P., Filabozzi P., Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest. 1982;69(6):1366–1372. doi: 10.1172/JCI110576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikonomidis I., Andreotti F., Economou E., Stefanadis C., Toutouzas P., Nihoyannopoulos P. Increased proinflammatory cytokines in patients with chronic stable angina and their reduction by aspirin. Circulation. 1999;100(8):793–798. doi: 10.1161/01.cir.100.8.793. [DOI] [PubMed] [Google Scholar]
- 43.Agustí A., Edwards L.D., Rennard S.I. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7(5):e37483. doi: 10.1371/journal.pone.0037483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamid U., Krasnodembskaya A., Fitzgerald M. Aspirin reduces lipopolysaccharide-induced pulmonary inflammation in human models of ARDS. Thorax. 2017;72(11):971–980. doi: 10.1136/thoraxjnl-2016-208571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.