Abstract
Background
Skeletal muscle loss, commonly known as sarcopenia, is highly prevalent and prognostic of adverse outcomes in oncology. However, there is limited information on adults with early breast cancer and examination of other skeletal muscle indices, despite the potential prognostic importance. This study characterizes and examines age-related changes in body composition of adults with early breast cancer and describes the creation of a novel integrated muscle measure.
Methods
Female patients diagnosed with stage I-III breast cancer with abdominal computerized tomography (CT) scans within 12 weeks from diagnosis were identified from local tumor registry (N=241). Skeletal muscle index (muscle area per height [cm2/m2]), skeletal muscle density, and subcutaneous and visceral adipose tissue areas, were determined from CT L3 lumbar segments. We calculated a novel integrated skeletal measure, skeletal muscle gauge, which combines skeletal muscle index and density (SMI × SMD).
Results
241 patients were identified with available CT imaging. Median age 52 years and range of 23-87. Skeletal muscle index and density significantly decreased with age. Using literature based cut-points, older adults (≥65 years) had significantly higher proportions of sarcopenia (63 vs 28%) and myosteatosis (90 vs 11%) compared to younger adults (<50 years). Body mass index was positively correlated with skeletal muscle index and negatively correlated with muscle density. Skeletal muscle gauge correlated better with increasing age (rho = 0.52) than either skeletal muscle index (rho = 0.20) or density (rho = 0.46).
Conclusions
Wide variations and age-related changes in body composition metrics were found using routinely obtained abdominal CT imaging. Skeletal muscle index and density provide independent, complementary information, and the product of the two metrics, skeletal muscle gauge, requires further research to explore its impact on outcomes in women with curable breast cancer.
Keywords: Sarcopenia, Aging, Breast Cancer, Geriatric Oncology, Skeletal Muscle Index
Introduction
With an estimated 246,000 new cases in 2016, breast cancer is the most common new cancer diagnosis in the United States and one of the leading causes of cancer mortality in women worldwide (1). Nearly one third of U.S. patients with breast cancer are over age 65 and in other developed countries this percentage may rise to 40% (2). Increasing our awareness of considerations for treating the older breast cancer population will be important in caring for a large and growing population of adults with breast cancer.
Decreased skeletal muscle mass, commonly known as sarcopenia in oncology, is a common finding in adults with cancer and has been associated with increased treatment related toxicities and poorer survival in a myriad of cancer treatment settings (3). However, less is known about the body composition of adults with early stage breast cancer. A recent study of patients with metastatic disease found a correlation between pre-existing sarcopenia and treatment toxicity (4). Another study of breast cancer survivors found that sarcopenia was associated with overall mortality and may increase breast-cancer related mortality (5).
Routine computed tomography (CT) imaging can be utilized to assess muscle mass, but it is also able to assess muscle radiodensity and other non-muscular body composition measures. Radiodensity is a qualitative measure of muscle composition, conveying the degree of myosteatosis or fatty infiltration of muscle tissue (6). This metric, similar to skeletal muscle mass, can confer prognostic value for cancer outcomes (7–10). One study of metastatic breast cancer showed that low CT-derived muscle radiodensity associated with reduced overall survival and time to next treatment (11). Adipose tissue content, including subcutaneous (SAT) and visceral adipose tissue (VAT), can also be determined from CT imaging, and can also be associated with treatment outcome. For instance, CT-derived visceral adiposity has been linked to doxorubicin toxicity in a curable breast cancer population (12).
The goal of this study was to characterize the body composition of adults with early stage breast cancer (stage I-III) across a wide spectrum of age ranges and examine age-related changes in body composition. In addition, we describe the development of an integrated measure of skeletal muscle – Skeletal Muscle Gauge – that combines skeletal muscle mass and radiodensity into a novel composite measure.
Methods
Patient Data Collection
Eligible patients were treated at the North Carolina Cancer Hospital (NCCH) and identified through a review of patients in the North Carolina tumor registry between years 2008-2013. The eligibility criteria included female patients with Stage I- III breast cancer (American Joint Committee on Cancer, version 7) of any histological type, grade, hormone receptor, or HER-2 status and required a stored CT scan of the patient’s abdomen within 12 weeks from the date of breast cancer diagnosis. Medical records were reviewed for clinical characteristics, tumor histology, and staging for eligible patients. Institutional Review Board at the University of North Carolina (UNC) at Chapel Hill approved the study (IRB# 15-0579).
CT-based body composition analysis
Skeletal muscle area was measured using established methodology (13). In brief, abdominal CT images were acquired from the UNC Picture Archiving and Communication System office. CT images were examined on Impac radiological software (Mountain View, CA), and transverse sections at the level of L3 were extracted for analysis. L3 lumbar segments were processed using the ABACS automatic CT image segmentation software (14, 15). The software recognizes muscle tissue based on density threshold between −29 and +150 HU, while using a priori information about the L3 muscle shape to avoid mislabeling parts of neighboring organs. Images were than reviewed and corrected as necessary under the guidance of a diagnostic radiologist (HY, co-author). Using the formula for calculating skeletal muscle index (SMI) [(lean tissue area-cm2)/(patient height-m2)], we generated SMIs for each patient. Mean skeletal muscle density (SMD) was derived by averaging HU radiodensity for the total sectional skeletal muscle. Literature based cut-points were utilized to define sarcopenia (low muscle mass) and myosteatosis (low muscle radiodensity) (7, 8). SAT area was calculated from extramuscular tissue with density between −190 and −30 HU, and VAT from non-subcutaneous tissue with density between −150 and −50 HU. To integrate both the skeletal muscle quantity (SMI) and the skeletal muscle density (SMD), we generated the skeletal muscle gauge (SMG) from multiplying SMI × SMD, with arbitrary units (AU).
Statistics
Differences between age groups were analyzed using Pearson’s chi-square tests for categorical variables, and ANOVA, with a two-sided post-hoc analysis (Tukey’s multiple comparisons test), for continuous variables. Correlations were assessed using Pearson correlation coefficients (rho). Linear regression was used to quantify the relationship between continuous variables by estimating the slope of the best-fit line. In all instances, the phrase “significantly different” denotes p < 0.05. Analyses were conducting using GraphPad Prism and SAS statistical software (Cary, NC).
Results
Using the NC tumor registry, 1217 non-repeating entries and 1080 records were accessible in the electronic medical record. 480 of these patients had received abdominal CT imaging for staging purposes of which 241 patients had received the abdominal CT scan within 12 weeks of breast cancer diagnosis.
Patient Characteristics
Patient characteristics by age group are presented in Table 1. The 65+ age group had the fewest patients (n=30). One BMI value (72.7, in the 50-64 age group) was eliminated as an outlier (Grubbs test, α = 0.01) from analyses. BMI and the proportion of obese patients were similar across age groups, as were the proportion of patients categorized as stage II versus III.
Table 1.
Patient characteristics and body composition measures by age group
| ALL | <50 | 50-64 | ≥65 | |
|---|---|---|---|---|
| N | 241 | 105 | 106 | 30 |
| Age at Diagnosis (years) | ||||
| Mean (range) | 52 (23-87) | 42 (23-50) | 57 (50-64) | 71 (65-88) |
| Body Mass Index (BMI) | ||||
| Mean (range) | 29 (17-73) | 28 (17-45) | 29 (19-73) | 28 (20-42) |
| % Obese | 38 | 36 | 42 | 33 |
| Stage at Diagnosis | ||||
| 2 | 144 (60%) | 61 (58 %) | 66 (62%) | 17 (57%) |
| 3 | 97 (40%) | 44 (42%) | 40 (38%) | 13 (43%) |
| Tumor Classification | ||||
| HR+/Her2− | 139 (58%) | 59 (56%) | 59 (55%) | 21 (70%) |
| Her2+ | 49 (20%) | 24 (23%) | 22 (21%) | 3 (10%) |
| Triple Negative | 53 (22%) | 22 (21%) | 25 (24%) | 6 (20%) |
| Race | ||||
| White | 168 (70%) | 73 (70%) | 72 (68%) | 23 (77%) |
| Black | 65 (27%) | 25 (24%) | 34 (32%) | 6 (20%) |
| Other | 8 (3%) | 7 (7%) | 0 (0%) | 1 (3%) |
| Body Composition Measures (Means with 95% confidence intervals, or percentages) | ||||
| Muscle Area (cm2) | 119 [116, 121] | 122 [119, 126] | 119 [115, 122] | 104 [97.7, 111] |
| Skeletal Muscle Index (cm2/m2) | 44.5 [43.5, 45.4] | 45.9 [44.5,47.3] | 44.3 [42.8, 45.8] | 40.2 [37.7, 42.6] |
| Skeletal Muscle Density (HU) | 35.6 [34.5, 36.8] | 40.0 [38.5, 41.4] | 33.1 [31.4, 34.7] | 29.7 [26.7, 32.7] |
| Skeletal Muscle Gauge (AU) | 1576 [1519, 1632] | 1818 [1744, 1891] | 1447 [1370, 1524] | 1184 [1061, 1308] |
| VAT (cm2) | 90.8 [81.6. 100] | 68.8 [57.5, 80.1] | 104 [88.9, 120] | 120 [95.3, 145] |
| SAT (cm2) | 267 [248, 286] | 263 [237, 290] | 279 [248, 310] | 238 [189, 286] |
| % Sarcopenic | 34 | 28 | 32 | 63 |
| % Myosteatic | 34 | 44 | 81 | 90 |
| % Sarcopenic & Myosteatic | 22 | 8 | 26 | 57 |
Abbreviations: HR, Hormone Receptor. HU, Hounsfield units; SAT, subcutaneous adipose tissue area. VAT, visceral adipose tissue area.
Body Composition Measures
Table 1 also presents age-stratified body composition metrics. Overall, many skeletal muscle measures were significantly lower in the 65+ age bracket versus the <50 age group, including skeletal muscle area, SMI, SMD, and SMG (p < 0.001). The 65+ group was significantly different from the aged 50-64 group for SMI and SMD as well (p < 0.001, p < 0.01, respectively). SMG showed sharply decreasing values between the <50, 50-64, and 65+ groups. VAT showed an opposite pattern as the skeletal muscle metrics, with mean values increasing across aged groups (65+ versus <50, p < 0.01, 50-64 versus <50, p < 0.001).
The overall proportion of sarcopenic patients in our study was 34%. A significantly greater number of older patients (≥65) were sarcopenic (63%) than patients in the other age groups (28% for <50, p < 0.001 and 32% for 50-64, p < 0.01). Significantly more 50-64 and 65+ years old patients were considered myosteatic than patients <50 years old (p < 0.001). Very few (8%) of patients aged <50 were both sarcopenic and myosteatic, whereas 26% of patients aged 50-64 and 57% of patients aged 65+ (65+ versus both other age groups, p < 0.001) met this criteria. We examined the proportion of combined sarcopenic and obese patients per group as well, and found no patients meeting these criteria in the <50 age group, but 8% and 10% of the 50-64 and 65+ age groups met criteria, respectively.
Skeletal Muscle Index (SMI) and Skeletal Muscle Density (SMD) correlations
Overall, SMI decreased as age increased, but had a very weak correlation (rho = 0.20, Fig 1A). For every one-year increase in age, SMI decreased approximately 0.13 units. SMI was strongly positively correlated with BMI (slope = 0.68 rho = 0.60, Fig 1C). SMI increased slightly as visceral fat increased (slope = 0.03, Fig 1E) and was more strongly correlated with BMI (rho = 0.59, Fig 1C) than age (rho = 0.20, Fig 1A). SMD decreased as age increased (rho=0.46, Fig 1B). For every one-year increase in age, SMD decreased by 0.36 units. SMD decreased dramatically as BMI increased (slope = −0.72, rho = 0.52, Fig 1D), and decreased as VAT increased (slope = −0.08, rho = 0.68, Fig 1F). Variability in SMD was more strongly correlated with VAT (rho = 0.68, Fig 1F), than BMI (rho = 0.52) or age (rho = 0.46). A comparison of SMI to SMD found a very minimal negative correlation between the two indices (rho = 0.14) (figure 2).
Figure 1. Scatter plots of SMI and SMD.
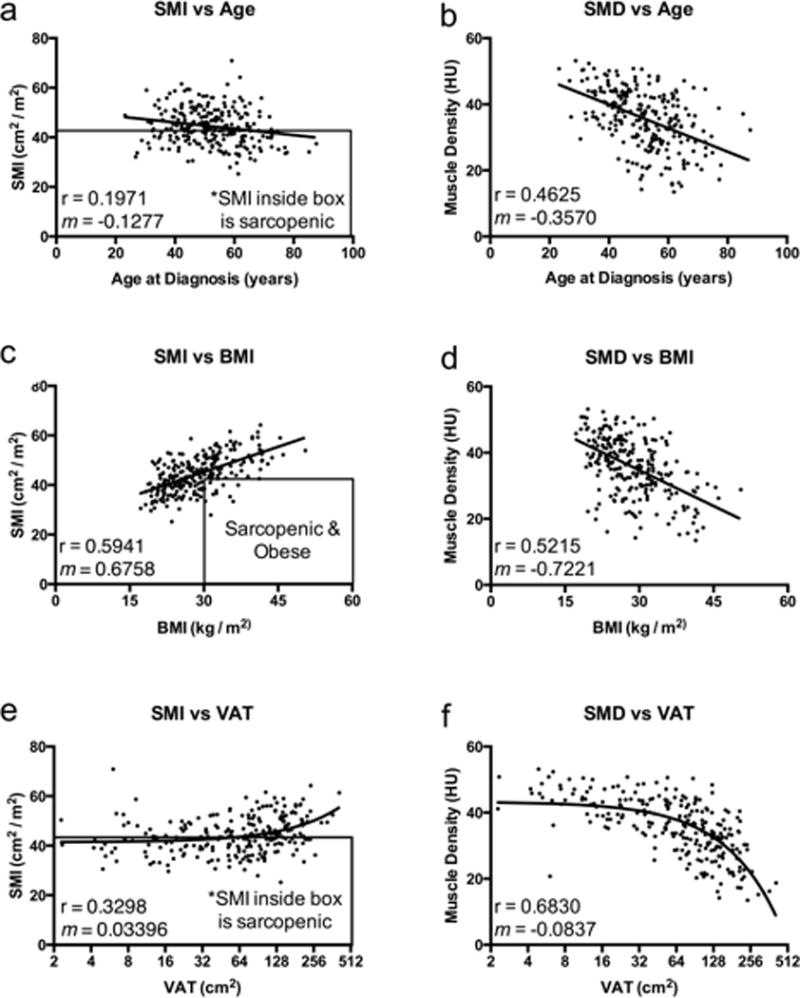
(A) Skeletal Muscle Index (SMI) versus Age of Diagnosis. (B) Skeletal Muscle Density (SMD) versus Age of Diagnosis. (C) SMI versus Body Mass Index (BMI). (D) SMD versus BMI. (E) SMI versus Visceral Adipose Tissue (VAT). (F) SMD versus VAT. rho and slopes (m) for the correlations are indicated on the individual graphs. VAT values were drawn on a log2 scale based on the wide ranging data stratification, and the best fit line represents a straight line fit.
Figure 2. Scatter plot of SMI versus SMD.
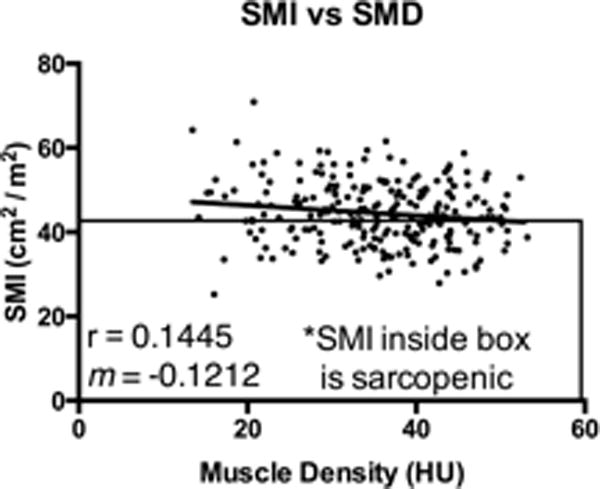
Skeletal Muscle Index (SMI) is plotted against Skeletal Muscle Density (SMD). R2 value and slope (m) for the correlation is indicated on the graph.
Integration of Skeletal Muscle Index and Density= Skeletal Muscle Gauge
We found minimal correlation between the two principle skeletal muscle indices (SMI and SMD), and diametrically opposite relationships of these variables with other body composition measures (including BMI). Given this, and the existing literature supporting the independent prognostic ability of SMI and SMD, we proposed a mathematical combination of the skeletal measures. We chose to multiply the skeletal measures (SMI × SMD) in order to derive a new measure that was equally weighted of the respective individual measures. SMG was negatively correlated with age and held a stronger correlation to age (rho = 0.52) than either SMI (rho = 0.20) or SMD (rho = 0.46) (figure 3).
Figure 3. Scatter plot of SMG versus Age.
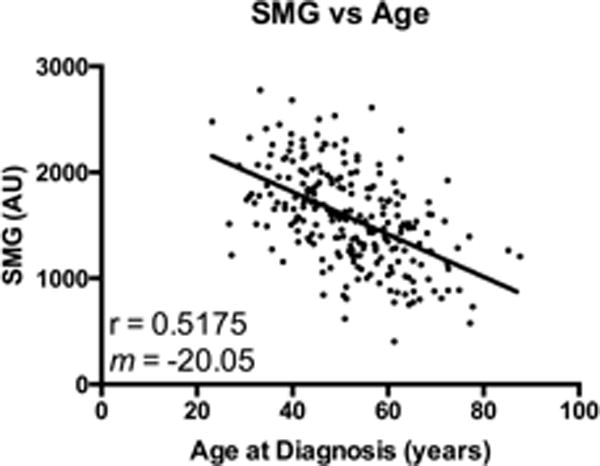
Skeletal Muscle Gauge (SMG) is plotted against Age of Diagnosis. AU, arbitrary units (Skeletal Muscle Index [SMI] × Skeletal Muscle Density [SMD]). R2 value and slope (m) for the linear regression is indicated on the graph.
Conclusions
In this study we examined the body composition of women diagnosed with early stage breast cancer using abdominal CT images obtained as part of routine oncologic care and examined age-related differences in body mass composition. We found wide variation in body composition variables across the age spectrum and a high prevalence of sarcopenia and myosteatosis that was most prevalent in the ≥65 population. This is consistent with other studies of sarcopenia in adults with cancer (16), and the overall proportion of sarcopenic patients in our population was somewhat higher than that expected in a healthy population of women (17).
While sarcopenia, defined by SMI alone, has offered substantial prognostic value in a number of studies (18), it is by no means the only valuable CT-based body composition metric available to researchers. SMD conveys the composition of muscle tissue, independent of muscle quantity, and is inversely related to fatty infiltration of skeletal muscle, known as myosteatosis. SMD is prognostic of survival in gastrointestinal (7, 8), pancreatic (9), and lung cancers (8), with independent prognostic value from SMI (8). Indeed, at least two studies have found prognostic relationship of SMD but not SMI on overall survival (10, 19). One study, of unresectable pancreatic cancer patients, found that higher myosteatosis but not sarcopenia was correlated with increased systemic inflammation and reduced survival (10). Another study of patients with metastatic renal cell carcinoma, found that SMD but not SMI has positive prognostic relationship with overall survival and progression-free survival (19). These findings strongly support the independent value of myosteatosis from sarcopenia. In comparing SMI to SMD in our study, several salient similarities and differences can be drawn. For example, both SMI and SMD decreased with age, but SMD was more age-sensitive than SMI. The relationship of BMI to SMI and SMD is diametrically opposite: SMI and BMI are positively correlated, whereas SMD and BMI are negatively correlated. Similarly, SMI positively correlates with VAT levels, whereas SMD negatively correlates with the same. The clear contrast between SMI and SMD supports the independent prognostic value of the two metrics. To this end, correlation of SMI to SMD yielded a very poor relationship between the two indices (rho = 0.14).
SMI and SMD are defined independently of one another and both are demonstrated prognostic indicators for cancer outcomes, however there are mathematical means to combine these measures that both could lead to more unified body composition and outcomes reporting, and that may confer increased sensitivity for prognostic purposes. To better understand SMI and SMD as measures of skeletal muscle, a diamond metaphor can be used. The value of a diamond consists of its size (carats) and quality (color and clarity). This is akin to SMI – a measure of skeletal muscle quantity, and SMD – a surrogate measure of muscle ‘quality’. Both size and quality are necessary for determining the overall diamond value, and a mathematical means of gauging a total value is to multiply individual components together. The two variables contain different units of measurement, disallowing addition. Multiplication retains the equal influence of each variable on the combined number regardless of absolute size variability. Multiplying SMI × SMD provides an integrated measure of quality and quantity of an individual’s skeletal muscle. Linear regression models comparing all our variables showed that the combination of SMI with SMD into SMG resulted in a superior coefficient of determination of age (R2 value of 0.27, versus 0.04 and 0.21, respectively), demonstrating its potential value over its constituent measures.
Given the implicit significance of age to the definition of sarcopenia, the fact that SMG has a stronger correlation to age in a curable breast cancer population is exciting, and warrants further study. Several recent studies from our group have demonstrated the predictive strength of the SMG variable. Metastatic breast cancer patients with lower SMG values had significantly increased high-grade toxicities and hospitalizations due to toxicity, reduced time to treatment failure, and reduced overall survival when undergoing taxane-based chemotherapy. For most of these key outcomes, SMG was the most sensitive body composition correlate to toxicity (20). SMG was further shown to be the best body composition metric for predicting toxicity in early stage breast cancer. Here similarly to patients with metastatic disease, SMG was significantly associated with high-grade hematological and gastrointestinal toxicities, as well as chemotherapy-related hospitalizations (21). In colorectal cancer patients, SMG was more highly correlated with 5-fluorouracil toxicity than either SMI or SMD (22). Finally, low SMG values have also correlated with impaired physical functioning in older adults with cancer, including prolonged Timed Up and Go and impairments in Instrumental Activities of Daily Living, ability to walk a block, climb steps, and bend over. Here, SMG values more closely mirrored SMD than SMI values (23). Thus in various oncological body composition studies, SMG has demonstrated frank superiority or similar correlation with outcomes as its constituent measures, marking it as a promising unified body composition metric.
Our study has some limitations. First, it was limited by the number of patients older than 65 in our dataset. However, given the large age-related group differences we observed and more importantly the focus on age as a continuous rather than grouped value, we expect that these results will replicate in larger older patient populations. One challenge to studying patients with curable breast cancer is the overall lack of CT imaging available as staging CT scans are recommended only for patients with Stage III breast cancer (24). Here, only 22% of all patients in the patient registry database received abdominal CTs within our pre-determined cutoff of 12 weeks surrounding the date of diagnosis, and therefore our findings may not be representative of all patients with early breast cancer. Also, the declared cutoff ranges for sarcopenic and myosteatic patients, though based on previous literature, may not prove appropriate for the non-metastatic breast cancer population, nor be predictive of outcomes for these patients. Finally, it is challenging to compare body composition values to outcomes in this population of patients with curable breast cancer as the absolute recurrence and mortality rate in this population to date remains extremely low. However it will be valuable to continue following these individuals over time to assess these long-term measures.
In conclusion, this is the largest study to date systemically characterizing CT-based body composition measures in adults with potentially curable breast cancer. We present a new metric, skeletal muscle gauge, which correlates better with aging than either SMD or SMI and has already shown great value in predicting oncological outcomes. Due to the ease of generating these body composition measures from routine CT imaging, we foresee oncologists incorporating skeletal muscle metrics to improve prognostication and identify patients at-risk for adverse outcomes. These patients could benefit from supportive interventions, such as resistance exercise therapy, which has been shown to reverse sarcopenia and associated with improved quality of life (25).
Acknowledgments
We thank Dr. Vickie Baracos, University of Alberta, for generously providing image analysis software. We thank Ms. Debra Witzler (NC Tumor Registry program) for database support. Finally we thank Ms. Ruck and Dr. Roberts of the UNC Center for Aging and Health for their assistance throughout the Medical Student Training in Aging Research summer program. Research supported by the Medical Student Training in Aging Research (MSTAR) program (NIA 2-T35-AG038047-06), through the American Federation for Aging Research, and by the UNC Oncology Clinical Translational Research Training Program (NCI 5K12CA120780-07). A. M. Deal was funded in part by the Breast Cancer Research Foundation (New York, NY). Dr. Shachar gratefully was supported by the Friends of Rambam Medical Center and The J & G Zukier Medical Fund donation.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Wildiers H, Kunkler I, Biganzoli L, Fracheboud J, Vlastos G, Bernard-Marty C, et al. Management of breast cancer in elderly individuals: recommendations of the International Society of Geriatric Oncology. Lancet Oncol. 2007;8(12):1101–15. doi: 10.1016/S1470-2045(07)70378-9. [DOI] [PubMed] [Google Scholar]
- 3.Rier HN, Jager A, Sleijfer S, Maier AB, Levin MD. The Prevalence and Prognostic Value of Low Muscle Mass in Cancer Patients: A Review of the Literature. Oncologist. 2016 doi: 10.1634/theoncologist.2016-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15(8):2920–6. doi: 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- 5.Villasenor A, Ballard-Barbash R, Baumgartner K, Baumgartner R, Bernstein L, McTiernan A, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012;6(4):398–406. doi: 10.1007/s11764-012-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging. 2010;14(5):362–6. doi: 10.1007/s12603-010-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamandl D, Paireder M, Asari R, Baltzer PA, Schoppmann SF, Ba-Ssalamah A. Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol. 2015 doi: 10.1007/s00330-015-3963-1. [DOI] [PubMed] [Google Scholar]
- 8.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–47. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 9.Okumura S, Kaido T, Hamaguchi Y, Fujimoto Y, Masui T, Mizumoto M, et al. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015;157(6):1088–98. doi: 10.1016/j.surg.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Rollins KE, Tewari N, Ackner A, Awwad A, Madhusudan S, Macdonald IA, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr. 2015 doi: 10.1016/j.clnu.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Rier HN, Jager A, Sleijfer S, van Rosmalen J, Kock MC, Levin MD. Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast. 2017;31:9–15. doi: 10.1016/j.breast.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Wong AL, Seng KY, Ong EM, Wang LZ, Oscar H, Cordero MT, et al. Body fat composition impacts the hematologic toxicities and pharmacokinetics of doxorubicin in Asian breast cancer patients. Breast Cancer Res Treat. 2014;144(1):143–52. doi: 10.1007/s10549-014-2843-8. [DOI] [PubMed] [Google Scholar]
- 13.Chung H, Cobzas D, Birdsell L, Lieffers J, Baracos V, editors. SPIE Medical Imaging. International Society for Optics and Photonics; 2009. Automated segmentation of muscle and adipose tissue on CT images for human body composition analysis. [Google Scholar]
- 14.ABACS automated CT image segmentation software. [Available from: https://sourceforge.net/projects/ctsegtool.
- 15.Popuri K, Cobzas D, Esfandiari N, Baracos V, Jagersand M. Body Composition Assessment in Axial CT Images Using FEM-Based Automatic Segmentation of Skeletal Muscle. IEEE transactions on medical imaging. 2016;35(2):512–20. doi: 10.1109/TMI.2015.2479252. [DOI] [PubMed] [Google Scholar]
- 16.Kazemi-Bajestani SM, Mazurak VC, Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Seminars in cell & developmental biology. 2015 doi: 10.1016/j.semcdb.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Tanko LB, Movsesyan L, Mouritzen U, Christiansen C, Svendsen OL. Appendicular lean tissue mass and the prevalence of sarcopenia among healthy women. Metabolism. 2002;51(1):69–74. doi: 10.1053/meta.2002.28960. [DOI] [PubMed] [Google Scholar]
- 18.Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. doi: 10.1016/j.ejca.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Antoun S, Lanoy E, Iacovelli R, Albiges-Sauvin L, Loriot Y, Merad-Taoufik M, et al. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer. 2013;119(18):3377–84. doi: 10.1002/cncr.28218. [DOI] [PubMed] [Google Scholar]
- 20.Shachar SS, Deal AM, Weinberg M, Nyrop KA, Williams GR, Nishijima TF, et al. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane-Based Chemotherapy. Clin Cancer Res. 2017;23(3):658–65. doi: 10.1158/1078-0432.CCR-16-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shachar SS, Deal AM, Weinberg M, Williams GR, Nyrop KA, Popuri K, et al. Body Composition as a Predictor of Toxicity in Patients Receiving Anthracycline and Taxane-Based Chemotherapy for Early-Stage Breast Cancer. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams GR, Deal AM, Shachar SS, Walko C, Patel J, O’Neil B, et al., editors. The impact of sarcopenia on toxicity and pharmacokinetics of 5-fluorouracil (5FU) in colorectal cancer. American Society of Clinical Oncology - Gastrointentional Cancers Symposium; 2017. Journal of Clinical Oncology. [Google Scholar]
- 23.Williams GR, Deal AM, Muss HB, Weinberg MS, Sanoff HK, Nyrop KA, et al. Skeletal muscle measures and physical function in older adults with cancer: sarcopenia or myopenia? Oncotarget. 2017 doi: 10.18632/oncotarget.16866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Network NCC. The NCCN Breast Cancer Clinical Practice Guidelines in Oncology (V.3.2015) 2015 [Available from: nccn.org.
- 25.Adams SC, Segal RJ, McKenzie DC, Vallerand JR, Morielli AR, Mackey JR, et al. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Breast Cancer Res Treat. 2016;158(3):497–507. doi: 10.1007/s10549-016-3900-2. [DOI] [PubMed] [Google Scholar]


