Abstract
Tris(pentafluorophenyl)borane was used as Lewis acid catalyst to lower the ring opening polymerization temperature (ROP) of 1,3-benzoxazines. Dynamic scanning calorimeter studies revealed that on-set ROP temperatures were decreased as much as 98 °C for model benzoxazine compounds. Catalytic polymerization was traced by both FTIR and 1H NMR, and revealed that tris(pentafluorophenyl)borane acted rapidly and fast curing achieved. Moreover, thermal properties of resulting polybenzoxazines were investigated by thermogravimetric analysis (TGA) and found out that the catalyst has high impact on char yield and even 3 mol % catalyst augmented char yields up to 13%.
Keywords: ring-opening polymerization, benzoxazines, polybenzoxazines, curing temperature, tris(pentafluorophenyl)borane
1. Introduction
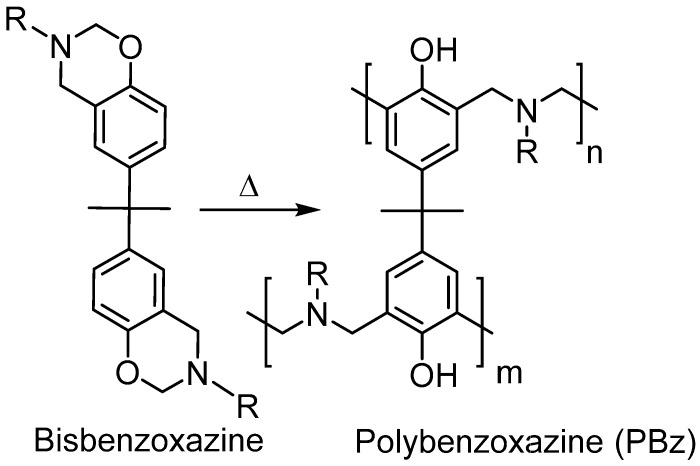
Polybenzoxazines have attracted interest in the last decade due to superior properties in the field of high performance materials. These polymers can be considered as a class of phenolic thermosets resembling to novolac and resole type systems. Thus, they bear in many properties of traditional phenolics, such as excellent mechanical and thermal properties. Besides, polybenzoxazines exhibit unique features that makes them a contender to many high performance polymers. They have low water absorption, molecular design flexibility, and high char yield, and generally high glass transition temperatures [1,2,3,4,5,6]. Most of these features are mainly caused by the aminoalkyl repeat units (–CH2–N(R)–CH2–) and hydrogen bonds between amine and phenolic –OH groups [7,8,9]. Moreover, the cyclic structural nature of corresponding 1,3-benzoxazine monomers limit shrinkage during curing and also stabilize the monomer at a wide temperature range from room temperature to ca. 150 °C, depending on the functionalities that are present on the monomer. [10] Thus, these monomers can be stored for prolonged times even under humid and relatively hot environment. The stability of oxazine ring, on the other hand, makes the polymerization be conducted at high temperatures that can be considered as a drawback [11]. Generally, polybenzoxazines are synthesized by thermally induced ring opening polymerization (ROP) of 1,3-benzoxazines over 220 °C (Scheme 1). These values are higher than usual curing temperatures (ca. 177 °C) that are used to produce composites especially for aero-structures. Therefore, reduction in the cure temperature is highly desirable for industrial applications.
Scheme 1.
Ring opening polymerization (ROP) of a bisbenzoxazine monomer.
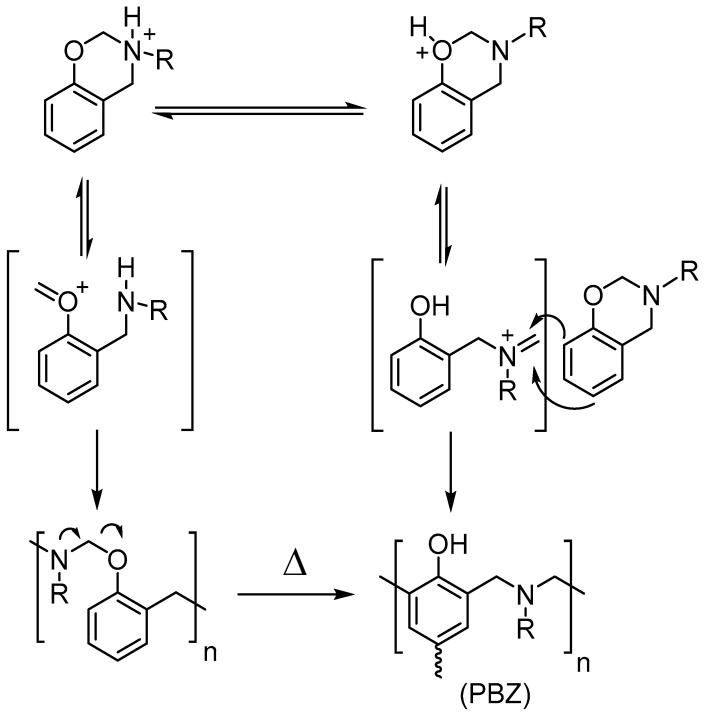
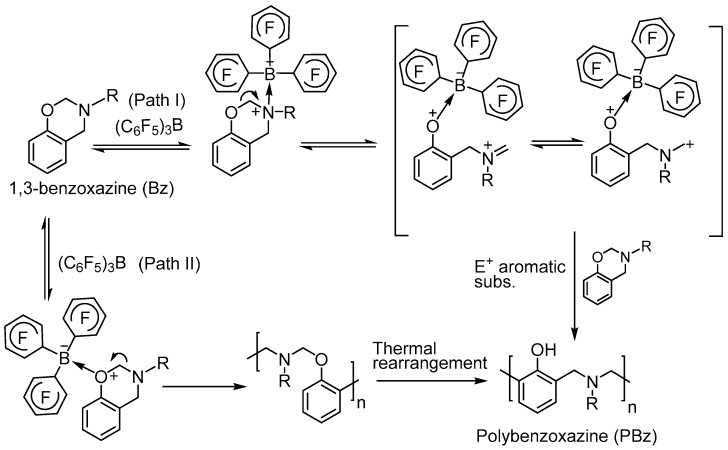
The polymerization of benzoxazines proceeds through a cationic ring opening pathway where the oxygen and nitrogen on the heterocycle easily stabilizes the formed cations during the process [12,13,14]. This way, the distorted semi-chair oxazine ring is opened at high temperatures leading to Friedel-Crafts reaction at ortho or para position of aromatics. Actually, two major mechanistic paths take place according to the cation formation either on nitrogen or oxygen atoms that are on the oxazine ring. If the cation is formed on the oxygen atom of oxazine, then polybenzoxazine is thoroughly produced over ring-opening and Friedel-Crafts reactions. However, cation formation on the nitrogen atom causes ring-opening and subsequent etherification to give –OH blocked polybenzoxazine that eventually rearranges to form phenolic polybenzoxazine at high temperatures (Scheme 2) [15,16].
Scheme 2.
Proposed mechanism for ROP of 1,3-benzoxazines.
The nature of polymerization of 1,3-benzoxazines is suitable to be catalysed by acids or other species that are able to produce cation on the oxazine ring. Designing self-catalytic monomers and admixing catalysts with benzoxazines are the usually applied strategies to reduce the curing temperature. Both approach has superiorities and drawbacks, thus selection may vary according to the specific application. For the first strategy, several different benzoxazine monomers containing –COOH and –OH groups were reported [17,18,19,20,21]. In this connection, it should be pointed out that although acidic groups readily reduce the ROP temperatures, decarboxylation observed during the polymerization causes voids in the final polymers due to released CO2 is the major deterrent of this strategy [22]. The materials formed this way may exhibit drastic mechanical failures in most applications. Therefore, carboxylic acid containing benzoxazines were best suited to produce sponge like networks, typically for chromatographic column applications. Alternative approach involving the use of –OH functional benzoxazines as self-catalysts appears to be more beneficial since problems associated with gas release is prevented [23]. The ROP temperature reductions were around 40 °C as the interaction of –OH groups with the cationic intermediates formed during ring opening process accelerates the polymerization. Notably, monomers with unoccupied phenolic –OH groups act as built-in catalysts and effectively reduce ROP temperatures which can be considered as an alternative solution to harsh curing conditions [24]. Actually, the described self-catalyst strategy is designed for the systems that contain only benzoxazines and derivatives. On the other hand, this approach needs synthesis of structurally related monomers and for some monomers multi step synthetic routes may be required. As stated, the second strategy, based on addition of catalysts into benzoxazine formulations, appears to be easier and more flexible. Several compounds, namely amines, amine salts, thiols, phenols, toluene sulfonates, and colloidal sulfur were used to promote ROP of benzoxazines [25,26,27,28,29,30,31,32,33]. Moreover, admixing Lewis acids with benzoxazines profoundly affected the curing and ROP temperatures were drastically reduced down to 120–160 °C. Typically, PCl5, POCl3, TiCl4, AlCl3, and FeCl3 acted as efficient catalysts, particularly when with solvents and ring opening of oxazine took place even at room temperature [34,35,36]. Although these catalysts are economical and potent, their miscibility in neat benzoxazines is limited. Therefore, Lewis acids in complex structures would obviously increase both their solubility and stabilities. Acetylacetonato complexes of 4th period transition metals can be given as a striking example in this context. These complexes with nucleophiles promote the polymerization efficiently and 120 °C as onset of ROP temperatures were obtained [37]. Highly active catalytic nature of Lewis acid often initiate ROP at low temperatures and viscosity increases rapidly, providing a practical advantage for fast curing requirements. Thus, miscibility of the Lewis catalyst come into prominence for such purposes and tris(pentafluorophenyl)borane (B(C6F5)3) has a potential in this manner. It was generally used to catalyse several organic reactions and it also played role in borylation chemistry [38]. For example, substituted quinolones were synthesized by B(C6F5)3 initiated aldehyde-aniline-alkyne reaction [39]. Moreover, B(C6F5)3 was shown to be an excellent activator component in homogeneous Ziegler-Natta processes [40]. Besides its catalytic activity, this acid was used to prepare carbonyl-borane adducts showing solid state luminescence [41] and also as receptor to detect NH3 on an organic field-effect transistor [42]. The multirole of B(C6F5)3 prompted us to further extend its usage in other areas particularly in benzoxazine based thermosets. Herein, we present our investigations for the impact of the B(C6F5)3 on ROP of benzoxazines.
2. Materials and Methods
2.1. Materials
4,4′-Isopropylidenediphenol (bisphenol A) (Aldrich, 97%, St. Louis, MO, USA), aniline (Aldrich, ≥99.5%, St. Louis, MO, USA) paraformaldehyde (Acros, 96%, Geel, Belgium), benzylamine (Aldrich, 99%), 4-tert-butylphenol (Aldrich, 99%, St. Louis, MO, USA), butylamine (Aldrich, ≥99%, St. Louis, MO, USA), xylenes (Aldrich, ≥96%, St. Louis, MO, USA), ethanol (Aldrich, ≥99.5%, St. Louis, MO, USA), toluene (Carlo Erba, 99.5%, Barcelona, Spain), tetrahydrofuran (Sigma-Aldrich, ≥99%, St. Louis, MO, USA), hexane (Aldrich, 95%, St. Louis, MO, USA), and diethyl ether (Aldrich, ≥98%, St. Louis, MO, USA), sodium hydroxide (Sigma-Aldrich, ≥98%, St. Louis, MO, USA) were used as received.
2.2. Characterization
All 1H NMR spectra were recorded on an Agilent NMR System VNMRS 500 spectrometer (Agilent, Santa Clara, CA, USA) at room temperature in CDCl3 with Si(CH3)4 as an internal standard. FT-IR spectra were recorded on a Perkin-Elmer FT-IR Spectrum (Boston, MA, USA) One spectrometer. Differential Scanning Calorimetry (DSC) was performed on Perkin–Elmer Diamond DSC from 0 to 320 °C, with a heating rate of 10 °C/min. under nitrogen flow. Thermal gravimetric analysis (TGA) was performed on Perkin-Elmer Diamond TA/TGA with a heating rate of 10 °C/min under nitrogen flow. Gel permeation chromatography (GPC) measurements were performed on a Viscotek GPC max auto sampler system (Malvern, UK) consisting of a pump, a Viscotek UV detector, and Viscotek a differential refractive index (RI) detector with three ViscoGEL GPC columns (G2000H HR, G3000H HR, and G4000H HR, 7.8 mm internal diameter, 300 mm length) in series. Tetrahydrofuran (THF) was used as an eluent at flow rate of 1.0 mL·min−1 at 30 °C. Both of the detectors were calibrated with PS standards having narrow-molecular-weight distribution. Data were analysed using ViscotekOmniSEC Omni-01 software (Malvern, UK).
2.3. Synthesis of Aniline Based Bisbenzoxazine (B-a)
In a 100 mL round bottomed flask, paraformaldehyde (34.9 mmol, 1.05 g), bisphenol A (8.76 mmol, 2.0 g), and aniline (17.5 mmol, 1.6 mL) were dissolved with 20 mL of xylenes. The reaction mixture was heated to 120 °C for 12 h. The solvent was evaporated under vacuum. The resulting oily product was dissolved in diethyl ether (200 mL) and extracted with 3 M sodium hydroxide for three times to remove unreacted bisphenol A. Then, to neutralize the solution diethyl ether solution washed with distilled water (150 mL) for three times. The solution was dried with anhydrous Na2SO4 and filtered. Diethyl ether was evaporated under vacuum. The product was dried under vacuum at room temperature for 24 h.
2.4. Synthesis of Benzylamine Based Monobenzoxazine (P-Bn)
In a 100 mL round bottomed flask, paraformaldehyde (106 mmol, 3.19 g), phenol (53.1 mmol, 5.0 g), and benzylamine (53.1 mmol, 5.80 mL) were dissolved with 50 mL of toluene and 25 mL of ethanol mixture. The reaction mixture was refluxed for 12 h. The solvent was evaporated under vacuum. The resulting oily product was dissolved in diethyl ether (200 mL) and extracted with 3 M sodium hydroxide for various times to remove unreacted bisphenol A. Then, to neutralize the solution diethyl ether solution washed with distilled water (150 mL) for three times. The solution was dried with anhydrous Na2SO4 and filtered. Diethyl ether was evaporated under vacuum. The product was dried under vacuum at room temperature for 24 h.
2.5. Synthesis of 4-t-butylphenol Based Monobenzoxazine (t-P-Bt)
4-t-Butylphenol (5.0 g, 33.3 mmol) was added to a 100 mL round bottomed flask and cooled to 0 °C in an ice-bath. Butylamine (2.43 g, 33.3 mmol) was added dropwise to 4-tert-butylphenol for 15 min. and stirred for 5 min. Paraformaldehyde (1.99 g, 66.4 mmol) was added partly to the mixture over 20 min with stirring at 0 °C. After the mixture was stirred over 15 min. at room temperature, temperature was raised to 105 °C and stirred for 2 h. The reaction medium cooled to room temperature, dissolved in diethyl ether and extracted with aqueous sodium hydroxide (10 wt %, 250 mL), two times with distilled water and dried with magnesium sulfate. The product was dried under vacuum at room temperature for 24 h.
3. Results and Discussion
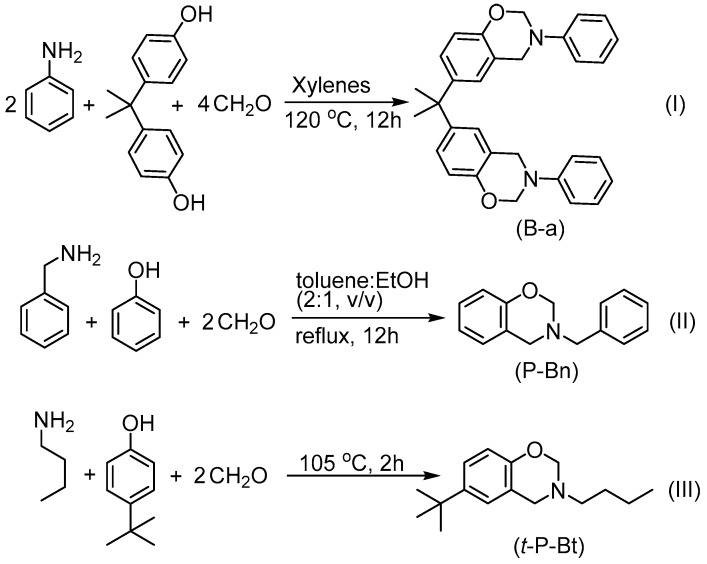
As stated, previous studies on the ROP mechanism of benzoxazines disclosed that the polymerization is initiated by thermally induced formation of cationic intermediates on N– and O– atoms, followed by the concurrent cleavage of methylene bridge on the oxazine ring. The formed cationic species immediately attack N–, O–, or aryl group and the rearrangement of labile bonds finally produce polybenzoxazines. In such a polymerization mechanism, Lewis acids are appropriate to consider as effective catalyst system since Lewis acids can strongly bind and generate cationic species on nitrogen and oxygen atoms of oxazine ring. Thus, the energy requirement for cleaving the oxazine ring reduces drastically and the ROP temperature decrease becomes imminent. It should be noted that the solubility of many Lewis acids are limited in organic compounds, and thus, in a benzoxazine medium, the solubility of the acid and polarity of benzoxazine monomer would affect the catalyst performance. Therefore, preparation of benzoxazine based formulations containing Lewis acids, such as FeCl3, TiCl4, AlCl3, ZnCl2 can be problematic due to their low solubility in organic solvents. Alternatively, ligated Lewis acids may provide better solubility and catalytic activity. Apart from solubility issues, boron compounds are known to show high complexation affinity against amines and oxazine ring bearing tertiary amine in its structure, which would presumably strongly bind central boron atom of a boron based Lewis acid [43]. It seemed, therefore, appropriate to investigate the catalytic activity of B(C6F5)3 on the ROP of benzoxazines. Initially, mono and bisbenzoxazines were synthesized as model monomers by using conventional method, according to the literature (Scheme 3) [44,45,46]. Spectral characterization of these monomers was performed using 1H NMR analysis (Figures S1–S3).
Scheme 3.
Synthesis of B-a, P-Bn, t-P-Bt monomers.
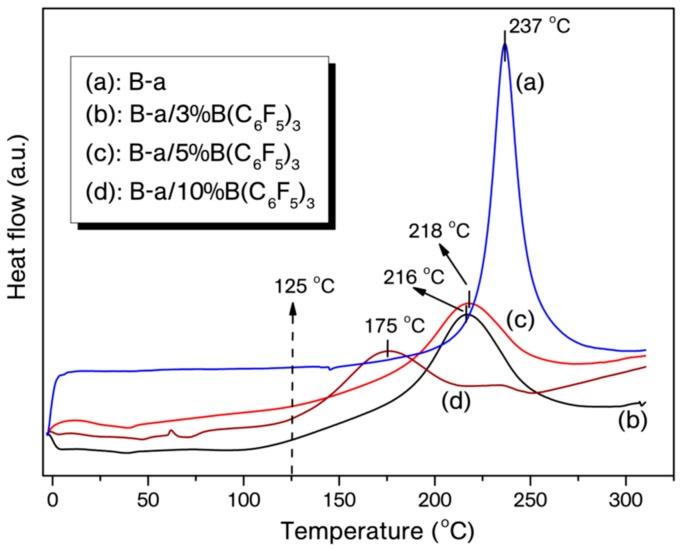
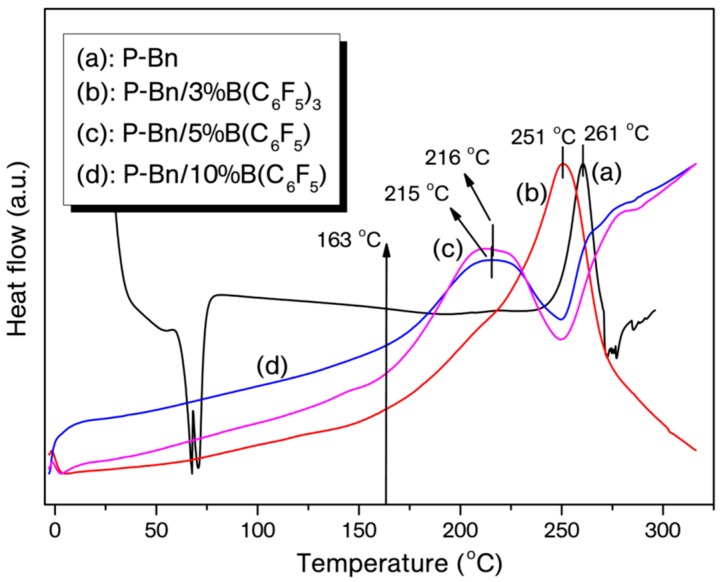
Typically, the catalyst was admixed with benzoxazine monomers in either 3 or 5 mol % by dissolving the components in tetrahydrofuran (1 mL) to obtain homogeneous mixtures. The solvent in the mixtures were removed by blowing nitrogen gas and then subjected to vacuum (ca. 10 mm-Hg) for 5 min. at room temperature. As is known, most of the benzoxazines have the ROP temperatures (curing maximum, Tmax) generally over 220 °C, which can be monitored as a distinct exotherm. Thus, ROP of B-a and P-Bn with B(C6F5)3 were traced under N2 environment with a heating rate of 10 °C·min−1 using a DSC device. The overlaid DSC thermograms for benzoxazine/catalyst mixtures presented in Figure 1 and Figure 2 and DSC data tabulated in Table 1 clearly show the ROP temperature reduction of these monomers with added B(C6F5)3. Moreover, the impact of catalyst amount on the ROP was also examined by increasing the B(C6F5)3 content from 3 to 10 mol % and the on-set of curing temperatures dropped to 125 from 212 °C for B-a and 163 from 242 °C for P-Bn (Figure 1 and Figure 2), corresponding to 98 and 81 °C reduction on on-set curing temperature, respectively.
Figure 1.
Differential Scanning Calorimetry (DSC) thermograms of pristine B-a (a), B-a/3%B(C6F5)3 (b), B-a/5%B(C6F5)3 (c), B-a/10%B(C6F5)3 (d).
Figure 2.
DSC thermograms of pristine P-Bn (a), P-Bn/3%B(C6F5)3 (b), P-Bn/5%B(C6F5)3 (c), P-Bn/10%B(C6F5)3 (d).
Table 1.
DSC a characteristics of B-a, P-Bn and their mixtures with various amounts of B(C6F5)3.
| Entry | On-Set of Curing (°C) | End-Set of Curing (°C) | Maximum Curing (°C) | ΔH (J/g) |
|---|---|---|---|---|
| B-a | 212 | 266 | 237 | −201 |
| B-a/3% B(C6F5)3 | 180 | 251 | 216 | −156 |
| B-a/5% B(C6F5)3 | 180 | 252 | 217 | −117 |
| B-a/10% B(C6F5)3 | 125 | 216 | 174 | −103 |
| P-Bn | 242 | 272 | 261 | −35 |
| P-Bn/3% B(C6F5)3 | 217 | 270 | 251 | −130 |
| P-Bn/5% B(C6F5)3 | 174 | 248 | 216 | −45 |
| P-Bn/10% B(C6F5)3 | 163 | 247 | 215 | −74 |
a DSC thermograms were collected under 20 mL·min−1 N2 flow and 10 °C·min−1 heating rate.
Previous mechanistic studies concerning Lewis acid catalysed ROP of benzoxazines disclosed the characteristic and two major polymerization mechanism was proposed. Correspondingly, a plausible mechanism for the B(C6F5)3 promoted ROP is proposed in Scheme 4. In the case of boron compounds, path I appears to be dominant process when compared to path II, due to the high complexation affinity of N and B atoms thus favours the electron transfer from N to B in the first step.
Scheme 4.
Tris(pentafluorophenyl)borane catalysed ROP of benzoxazine.
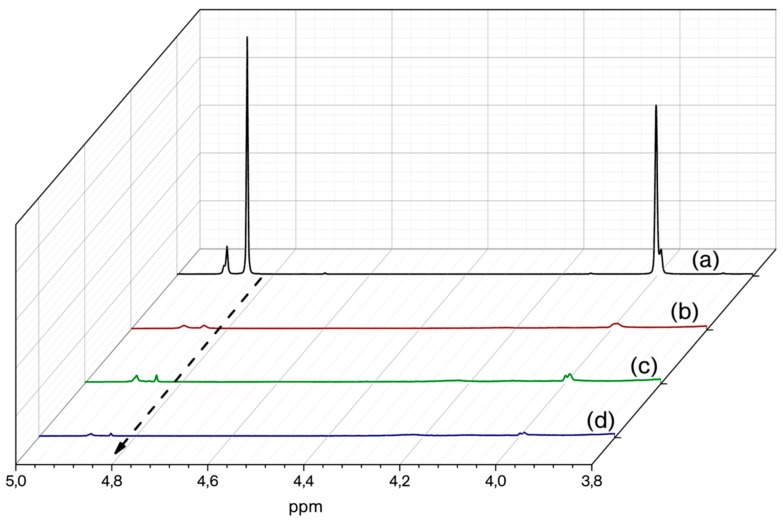
It was shown that linear and soluble polybenzoxazines can be obtained by using mono functional benzoxazines with blocked para position on the phenol moiety. For example, blocking the para position with tertiary butyl groups prevents the attack of carbocation to this position thus avoids crosslinking. Hence, t-P-Bt was deliberately selected so as to follow ring-opening reaction by 1H NMR. Initially, the solubility of the cured t-P-Bt was tested after curing at 220 °C for 1 h and the obtained polymer was analysed by GPC using THF as eluent to determine the molecular weight. The GPC chromatogram (Figure S4) showed a molecular weight of Mn: 980 Da with 3.01 polydispersity index. The relatively high dispersity observed is the consequence of the nature of the condensation polymerizations. As stated, the solubility of B(C6F5)3 in the reaction medium is a crucial factor for the ring-opening of benzoxazines. It is even likely that ring-opening can be achieved at r.t. in certain solvents. However, it is important to point out that it does not necessarily mean that the overall polymerization can take place at r.t. To test this probability, we have conducted a reaction followed by 1H NMR spectroscopy. 1H NMR spectra of the aliquot samples of the solution of t-P-Bt and B(C6F5)3 (5 mol %) in CDCl3 at the beginning and after 30, 60, and 120 min were recorded to observe the changes of the signals corresponding to N–CH2–O protons of the oxazine ring. As can be seen from Figure 3, the intensity of the peak at 4.85 ppm decreases drastically within 30 min., indicating that B(C6F5)3 catalyses ring-opening reaction at r.t. in solution medium. These results reveal that the catalyst acts rapidly and starts the ring-opening upon admixing, thus provide clear evidence for the fast curing at higher temperatures.
Figure 3.
Overlaid 1H NMR spectra of t-P-Bt/5% B(C6F5)3 mixture as prepared (a); t-P-Bt/5% B(C6F5)3 mixture after 30 min (b); t-P-Bt/5% B(C6F5)3 mixture after 60 min (c); t-P-Bt/5% B(C6F5)3 mixture after 120 min (d).
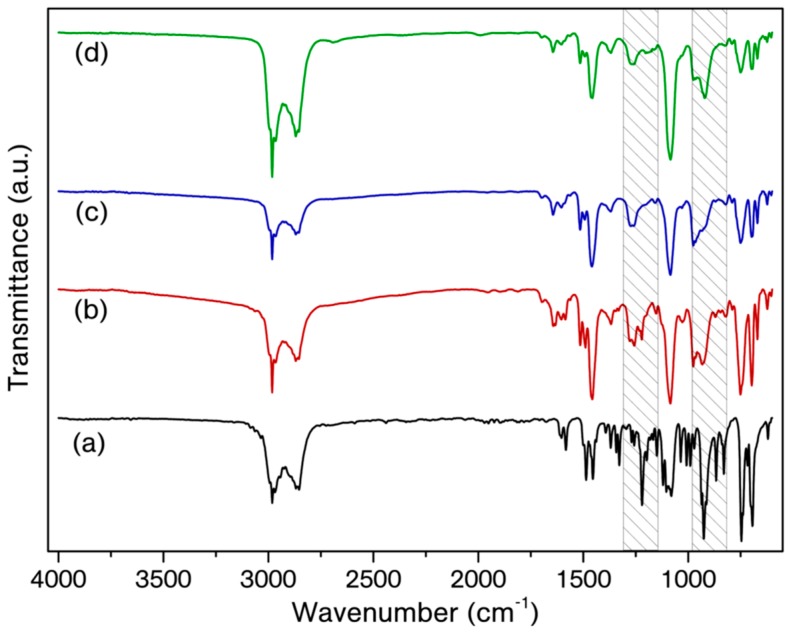
On the other hand, a similar curing study was also performed by using P-Bn monomer, which forms crosslinked polybenzoxazine. Therefore, FTIR spectroscopy was used to track the ring-opening of oxazine ring for certain time intervals (Figure 4). The overlaid FTIR spectra of the resulting products clearly reveals the change on the oxazine ring bands. The intensity of the C–O–C band at 1218 cm−1 is reduced after 30 min and the reduction becomes more pronounced after 60 min. Moreover, polymerization of benzoxazine resins is generally proved by the disappearance of the band at 960–900 cm−1, corresponding to the C–H out-of-plane bending of the benzene and the O–C stretching of the oxazine ring [47]. The intensity of the related band at 926 cm−1 is reduced by time and a new band at 967 cm−1 emerges, evidencing the ring-opening of oxazine by B(C6F5)3 catalyst.
Figure 4.
Overlaid FTIR spectra of P-Bn/5% B(C6F5)3 mixture as prepared (a), P-Bn/5% B(C6F5)3 mixture after 30 min. (b), P-Bn/5% B(C6F5)3 mixture after 60 min. (c), P-Bn/5% B(C6F5)3 mixture after 120 min. (d).
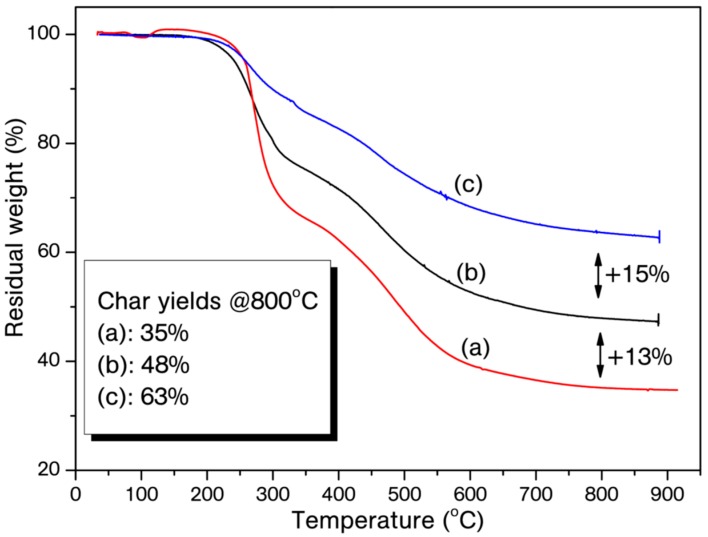
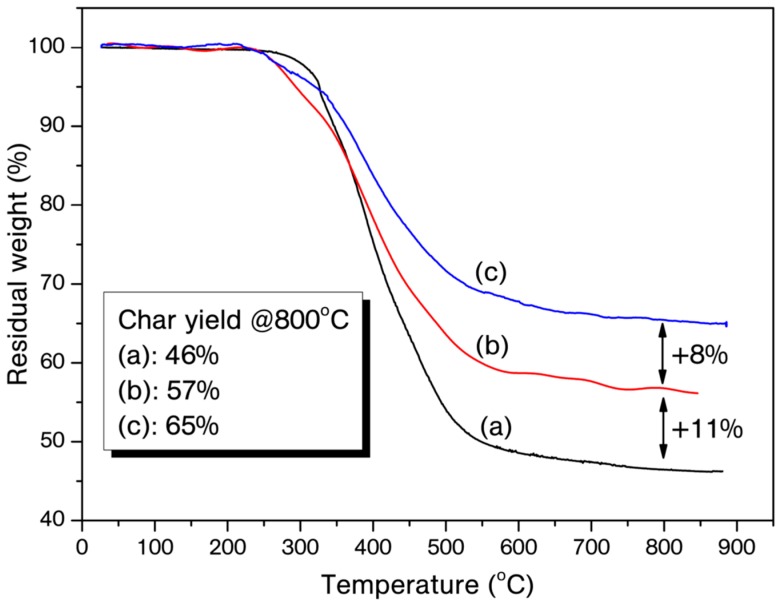
The thermal stability of the polybenzoxazines for each catalyst ratio was studied by using thermo-gravimetric analysis (TGA). TGA traces and related thermo-gravimetric results are presented in Figure 5 and Figure 6, and Table 2, respectively. Although cured P-Bn/B(C6F5)3 have slight differences for T5%, a pronounced effect of the catalyst on the thermal stability for T10%, Tc and Tmax values is visible. On the other hand, the initial degradation values for cured B-a/B(C6F5)3 are lower than that of the cured pristine B-a. Polybenzoxazines formed from monofunctional benzoxazines generally have low crosslinking degree and the catalyst presumably increases the crosslinking density of polybenzoxazine of P-Bn. In contrast, B-a already produces stiff network due to the difunctional structure, thus any positive effect on initial thermal degradation is not observed. Notably, the addition of B(C6F5)3 showed drastic increment in the char yield at 800 °C for both of the systems. Similar positive effect was also observed for polybenzoxazines obtained by transition metal based catalytic ROP. Although the B atom is not a transition metal, char yield improvement is significant with B(C6F5)3 catalyst and only 3 mol % addition of B(C6F5)3 augmented the char yields 11–13%. Three reasons can be discussed to explain this phenomenon. The high degree of crosslinking due to the catalyst, the coordination between boron and nitrogen atoms that may delay the amine degradation in polybenzoxaine, and apart from central boron atom in the catalyst, fluorine atoms may also contribute to this observed effect due to the well-known flame retardation property of aromatic fluorine compounds [48,49].
Figure 5.
Thermo-gravimetric analysis (TGA) traces of cured P-Bn (a), P-Bn/3% B(C6F5)3 (b), P-Bn/5% B(C6F5)3 (c).
Figure 6.
TGA traces of cured B-a (a), B-a/3% B(C6F5)3 (b), B-a/5% B(C6F5)3 (c).
Table 2.
Thermal properties of the cured P-Bn, B-a and B(C6F5)3 (3 and 5 mol %) mixtures.
| Sample | T5% (°C) | T10% (°C) | Tmax (°C) a | Tc (%) |
|---|---|---|---|---|
| P-Bn | 257 | 267 | 270 *, 466 | 35 |
| P-Bn/3% B(C6F5)3 | 258 | 263 | 272 *, 492 | 48 |
| P-Bn/5% B(C6F5)3 | 244 | 297 | 268 *, 557 | 63 |
| B-a | 326 | 364 | 390 | 46 |
| B-a/3% B(C6F5)3 | 294 | 347 | 284 *, 392 | 57 |
| B-a/5% B(C6F5)3 | 323 | 340 | 271 *, 391 | 65 |
a These values extracted from derivative of TGA (Figures S5 and S6 in Supplementary Materials); *Major Tmax; T5%: The temperature for which the weight loss is 5%; T10%: The temperature for which the weight loss is 10%; Tmax: The temperature for maximum weight loss; Tc: The char yield at 800 °C.
4. Conclusions
We have established the catalytic potential of tris(pentafluorophenyl)borane in the polymerization of benzoxazines. The benzoxazine/catalyst mixtures were easily prepared and the catalyst solubility was high due to the its ligated nature and the strong binding affinity between N and B atom. As expected, borane catalyst acted rapidly and initiated the ROP of model benzoxazine compounds, which disclosed that the catalyst is suitable for fast curing demands. The strong catalytic properties of the catalyst exhibited an unusual impact on thermal properties of the resulting polybenzoxazines. Especially, char yield improvement is significant with the catalyst and only 3 mol% addition of B(C6F5)3 augmented the char yields 11–13%. Therefore, this catalyst may find applications where high char yields are required and metal based catalyst impurities would be a drawback.
Acknowledgments
Authors thank to Istanbul Technical University Research Fund.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4360/10/3/239/s1.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Conflicts of Interest
The authors declare no competing financial interest.
References
- 1.Ghosh N.N., Kiskan B., Yagci Y. Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007;32:1344–1391. doi: 10.1016/j.progpolymsci.2007.07.002. [DOI] [Google Scholar]
- 2.Wirasate S., Dhumrongvaraporn S., Allen D.J., Ishida H. Molecular origin of unusual physical and mechanical properties in novel phenolic materials based on benzoxazine chemistry. J. Appl. Polym. Sci. 1998;70:1299–1306. doi: 10.1002/(SICI)1097-4628(19981114)70:7<1299::AID-APP6>3.0.CO;2-H. [DOI] [Google Scholar]
- 3.Kiskan B. Adapting benzoxazine chemistry for unconventional applications. React. Funct. Polym. 2017 doi: 10.1016/j.reactfunctpolym.2017.06.009. [DOI] [Google Scholar]
- 4.Kukut M., Kiskan B., Yagci Y. Self-curable benzoxazine functional polybutadienes synthesized by click chemistry. Des. Monomers Polym. 2009;12:167–176. doi: 10.1163/156855509X412108. [DOI] [Google Scholar]
- 5.Lin R.-C., Mohamed M., Chen T., Kuo S.-W. Coumarin- and carboxyl-functionalized supramolecular polybenzoxazines form miscible blends with polyvinylpyrrolidone. Polymers. 2017;9:146. doi: 10.3390/polym9040146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu W.-H., Huang K.-W., Kuo S.-W. Heteronucleobase-functionalized benzoxazine: Synthesis, thermal properties, and self-assembled structure formed through multiple hydrogen bonding interactions. Polym. Chem. 2012;3:1546–1554. doi: 10.1039/c2py20090b. [DOI] [Google Scholar]
- 7.Kim H.D., Ishida H. A study on hydrogen-bonded network structure of polybenzoxazines. J. Phys. Chem. A. 2002;106:3271–3280. doi: 10.1021/jp010606p. [DOI] [Google Scholar]
- 8.Dong H., Xin Z., Lu X., Lv Y. Effect of n-substituents on the surface characteristics and hydrogen bonding network of polybenzoxazines. Polymer. 2011;52:1092–1101. doi: 10.1016/j.polymer.2011.01.009. [DOI] [Google Scholar]
- 9.Hu W.H., Huang K.W., Chiou C.W., Kuo S.W. Complementary multiple hydrogen bonding interactions induce the self-assembly of supramolecular structures from heteronucleobase-functionalized benzoxazine and polyhedral oligomeric silsesquioxane nanoparticles. Macromolecules. 2012;45:9020–9028. doi: 10.1021/ma302077x. [DOI] [Google Scholar]
- 10.Ishida H., Allen D.J. Physical and mechanical characterization of near-zero shrinkage polybenzoxazines. J. Polym. Sci. B. 1996;34:1019–1030. doi: 10.1002/(SICI)1099-0488(19960430)34:6<1019::AID-POLB1>3.0.CO;2-T. [DOI] [Google Scholar]
- 11.Andronescu C., Gârea S.A., Deleanu C., Iovu H. Characterization and curing kinetics of new benzoxazine monomer based on aromatic diamines. Thermochim. Acta. 2012;530:42–51. doi: 10.1016/j.tca.2011.11.035. [DOI] [Google Scholar]
- 12.Hamerton I., McNamara L.T., Howlin B.J., Smith P.A., Cross P., Ward S. Examining the initiation of the polymerization mechanism and network development in aromatic polybenzoxazines. Macromolecules. 2013;46:5117–5132. doi: 10.1021/ma401014h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baqar M., Agag T., Huang R., Maia J., Qutubuddin S., Ishida H. Mechanistic pathways for the polymerization of methylol-functional benzoxazine monomers. Macromolecules. 2012;45:8119–8125. doi: 10.1021/ma301963d. [DOI] [Google Scholar]
- 14.Chutayothin P., Ishida H. Cationic ring-opening polymerization of 1,3-benzoxazines: Mechanistic study using model compounds. Macromolecules. 2010;43:4562–4572. doi: 10.1021/ma901743h. [DOI] [Google Scholar]
- 15.Sudo A., Kudoh R., Nakayama H., Arima K., Endo T. Selective formation of poly(n,o-acetal) by polymerization of 1,3-benzoxazine and its main chain rearrangement. Macromolecules. 2008;41:9030–9034. doi: 10.1021/ma8013178. [DOI] [Google Scholar]
- 16.Endo T. Toward elucidating the role of number of oxazine rings and intermediates in the benzoxazine backbone on their thermal characteristics. Macromolecules. 2016;49:8466–8478. [Google Scholar]
- 17.Andreu R., Reina J.A., Ronda J.C. Carboxylic acid-containing benzoxazines as efficient catalysts in the thermal polymerization of benzoxazines. J. Polym. Sci. A. 2008;46:6091–6101. doi: 10.1002/pola.22921. [DOI] [Google Scholar]
- 18.Zuniga C., Larrechi M.S., Lligadas G., Ronda J.C., Galia M., Cadiz V. Polybenzoxazines from renewable diphenolic acid. J. Polym. Sci. A. 2011;49:1219–1227. doi: 10.1002/pola.24541. [DOI] [Google Scholar]
- 19.Kiskan B., Demirel A.L., Kamer O., Yagci Y. Synthesis and characterization of nanomagnetite thermosets based on benzoxazines. J. Polym. A. 2008;46:6780–6788. doi: 10.1002/pola.23023. [DOI] [Google Scholar]
- 20.Andreu R., Reina J.A., Ronda J.C. Studies on the thermal polymerization of substituted benzoxazine monomers: Electronic effects. J. Polym. Sci. A Chem. 2008;46:3353–3366. doi: 10.1002/pola.22677. [DOI] [Google Scholar]
- 21.Soto M., Hiller M., Oschkinat H., Koschek K. Multifunctional benzoxazines feature low polymerization temperature and diverse polymer structures. Polymers. 2016;8:278. doi: 10.3390/polym8080278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuniga C., Lligadas G., Carlos Ronda J., Galia M., Cadiz V. Self-foaming diphenolic acid benzoxazine. Polymer. 2012;53:3089–3095. doi: 10.1016/j.polymer.2012.05.044. [DOI] [Google Scholar]
- 23.Lin C.H., Feng Y.R., Dai K.H., Chang H.C., Juang T.Y. Synthesis of a benzoxazine with precisely two phenolic oh linkages and the properties of its high-performance copolymers. J. Polym. Sci. A. 2013;51:2686–2694. [Google Scholar]
- 24.Zhang W., Froimowicz P., Arza C.R., Ohashi S., Xin Z., Ishida H. Latent catalyst-containing naphthoxazine: Synthesis and effects on ring-opening polymerization. Macromolecules. 2016;49:7129–7140. doi: 10.1021/acs.macromol.6b01177. [DOI] [Google Scholar]
- 25.Kocaarslan A., Kiskan B., Yagci Y. Ammonium salt catalyzed ring-opening polymerization of 1,3-benzoxazines. Polymer. 2017;122:340–346. doi: 10.1016/j.polymer.2017.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arslan M., Kiskan B., Yagci Y. Combining elemental sulfur with polybenzoxazines via inverse vulcanization. Macromolecules. 2016;49:767–773. doi: 10.1021/acs.macromol.5b02791. [DOI] [Google Scholar]
- 27.Semerci E., Kiskan B., Yagci Y. Thiol reactive polybenzoxazine precursors: A novel route to functional polymers by thiol-oxazine chemistry. Eur. Polym. J. 2015;69:636–641. doi: 10.1016/j.eurpolymj.2015.02.030. [DOI] [Google Scholar]
- 28.Bektas S., Kiskan B., Orakdogen N., Yagci Y. Synthesis and properties of organo-gels by thiol-benzoxazine chemistry. Polymer. 2015;75:44–50. doi: 10.1016/j.polymer.2015.08.026. [DOI] [Google Scholar]
- 29.Sudo A., Yamashita H., Endo T. Ring-opening polymerization of 1,3-benzoxazines by p-toluenesulfonates as thermally latent initiators. J. Polym. Sci. A. 2011;49:3631–3636. doi: 10.1002/pola.24801. [DOI] [Google Scholar]
- 30.Wang J., Xu Y.Z., Fu Y.F., Liu X.D. Latent curing systems stabilized by reaction equilibrium in homogeneous mixtures of benzoxazine and amine. Sci. Rep. 2016;6:38584. doi: 10.1038/srep38584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun J., Wei W., Xu Y., Qu J., Liu X., Endo T. A curing system of benzoxazine with amine: Reactivity, reaction mechanism and material properties. RSC Adv. 2015;5:19048–19057. doi: 10.1039/C4RA16582A. [DOI] [Google Scholar]
- 32.Gorodisher I., DeVoe R.J., Webb R.J. Catalytic opening of lateral benzoxazine rings by thiols. Handb. Benzoxazine Resins. 2011:211–234. doi: 10.1016/B978-0-444-53790-4.00056-4. [DOI] [Google Scholar]
- 33.Dunkers J., Ishida H. Reaction of benzoxazine-based phenolic resins with strong and weak carboxylic acids and phenols as catalysts. J. Polym. Sci. A. 1999;37:1913–1921. doi: 10.1002/(SICI)1099-0518(19990701)37:13<1913::AID-POLA4>3.0.CO;2-E. [DOI] [Google Scholar]
- 34.Kiskan B., Yagci Y., Sahmethogilu E., Toppare L. Preparation of conductive polybenzoxazines by oxidative polymerization. J. Polym. Sci. A Chem. 2007;45:999–1006. doi: 10.1002/pola.21848. [DOI] [Google Scholar]
- 35.Wang Y.X., Ishida H. Cationic ring-opening polymerization of benzoxazines. Polymer. 1999;40:4563–4570. doi: 10.1016/S0032-3861(99)00074-9. [DOI] [Google Scholar]
- 36.Cid J.A., Wang Y.X., Ishida H. Cationic polymerization of benzoxazine monomers by boron trifluoride complex. Polym. Polym. Compos. 1999;7:409–420. [Google Scholar]
- 37.Sudo A., Hirayama S., Endo T. Highly efficient catalysts-acetylacetonato complexes of transition metals in the 4th period for ring-opening polymerization of 1,3-benzoxazine. J. Polym. Sci. A. 2010;48:479–484. doi: 10.1002/pola.23810. [DOI] [Google Scholar]
- 38.Lawson J.R., Melen R.L. Tris(pentafluorophenyl)borane and beyond: Modern advances in borylation chemistry. Inorg. Chem. 2017;56:8627–8643. doi: 10.1021/acs.inorgchem.6b02911. [DOI] [PubMed] [Google Scholar]
- 39.Fasano V., Radcliffe J.E., Ingleson M.J. Mechanistic insights into the b(c6f5)3-initiated aldehyde–aniline–alkyne reaction to form substituted quinolines. Organometallics. 2017;36:1623–1629. doi: 10.1021/acs.organomet.7b00174. [DOI] [Google Scholar]
- 40.Erker G. Tris(pentafluorophenyl)borane: A special boron lewis acid for special reactions. Dalton Trans. 2005:1883–1890. doi: 10.1039/b503688g. [DOI] [PubMed] [Google Scholar]
- 41.Hansmann M.M., López-Andarias A., Rettenmeier E., Egler-Lucas C., Rominger F., Hashmi A.S.K., Romero-Nieto C. B(c6f5)3: A lewis acid that brings the light to the solid state. Angew. Chem. Int. Edit. 2016;55:1196–1199. doi: 10.1002/anie.201508461. [DOI] [PubMed] [Google Scholar]
- 42.Huang W., Besar K., LeCover R., Rule A.M., Breysse P.N., Katz H.E. Highly sensitive nh3 detection based on organic field-effect transistors with tris(pentafluorophenyl)borane as receptor. J. Am. Chem. Soc. 2012;134:14650–14653. doi: 10.1021/ja305287p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamilton C.W., Baker R.T., Staubitz A., Manners I. B-n compounds for chemical hydrogen storage. Chem. Soc. Rev. 2009;38:279–293. doi: 10.1039/B800312M. [DOI] [PubMed] [Google Scholar]
- 44.Imran M., Kiskan B., Yagci Y. Concise synthesis and characterization of unsymmetric 1,3-benzoxazines by tandem reactions. Tetrahedron Lett. 2013;54:4966–4969. doi: 10.1016/j.tetlet.2013.07.041. [DOI] [Google Scholar]
- 45.Wang X., Chen F., Gu Y. Influence of electronic effects from bridging groups on synthetic reaction and thermally activated polymerization of bisphenol-based benzoxazines. J. Polym. Sci. A Chem. 2011;49:1443–1452. doi: 10.1002/pola.24566. [DOI] [Google Scholar]
- 46.Demir K.D., Kiskan B., Yagci Y. Thermally curable acetylene-containing main-chain benzoxazine polymers via sonogashira coupling reaction. Macromolecules. 2011;44:1801–1807. doi: 10.1021/ma1029746. [DOI] [Google Scholar]
- 47.Han L., Iguchi D., Gil P., Heyl T.R., Sedwick V.M., Arza C.R., Ohashi S., Lacks D.J., Ishida H. Oxazine ring-related vibrational modes of benzoxazine monomers using fully aromatically substituted, deuterated, 15n isotope exchanged, and oxazine-ring-substituted compounds and theoretical calculations. J. Phys. Chem. A. 2017;121:6269–6282. doi: 10.1021/acs.jpca.7b05249. [DOI] [PubMed] [Google Scholar]
- 48.Achiha T., Nakajima T., Ohzawa Y., Koh M., Yamauchi A., Kagawa M., Aoyama H. Thermal stability and electrochemical properties of fluorine compounds as nonflammable solvents for lithium-ion batteries. J. Electrochem. Soc. 2010;157:A707–A712. doi: 10.1149/1.3377084. [DOI] [Google Scholar]
- 49.Dhara M.G., Banerjee S. Fluorinated high-performance polymers: Poly(arylene ether)s and aromatic polyimides containing trifluoromethyl groups. Prog. Polym. Sci. 2010;35:1022–1077. doi: 10.1016/j.progpolymsci.2010.04.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.