Abstract
The first example of an environmentally-benign chitosan supported copper catalyzed conjugate silylation of α,β-unsaturated acceptors was accomplished in water under mild conditions. This protocol provides an efficient pathway to achieve an important class of β-silyl carbonyl compounds and the desired products were obtained in good to excellent yields. Gram-scale synthesis and easy transformation of obtained β-silyl products were also been demonstrated. Remarkably, this chitosan supported copper catalyst can be easily recycled and reused six times without any significant decrease of catalytic activity. The advantages of this newly developed method include operational simplicity, good functional group tolerance, scale-up ability, ready availability, and easy recyclability of catalyst.
Keywords: chitosan supported copper, heterogeneous catalyst, organosilicon compound, easily recyclable
1. Introduction
The development of methods for the synthesis of organosilicon compounds has attracted intense attention because of their myriad uses in organic synthesis [1,2] as well as their potential applications in materials science [3] and biological chemistry [4,5]. Traditionally, most β-silyl carbonyl compounds are prepared via conjugate addition process by using stoichiometric amounts of silyl metal reagents [6,7,8]. Besides, the methods that utilized a catalytic amount of metal in the presence of silylzincates [9,10,11] or silylcuprates [12] were also developed for further improvement of efficiency. In recent years, the utilization of Suginome’s silylboron reagent [13], dimethylphenylsilyl pinacolatoboronate (1), provided an alternative and effective strategy to directly install the dimethylphenylsilyl moiety to α,β-unsaturated acceptors. Although, Rh(I) [14,15,16,17], Cu(I) [18,19,20], and metal-free NHC [21] catalysts were successfully applied for activating the Si–B bond of Suginome’s reagent, Cu(II)-catalysis is still in high demand because its protocol is much more convenient with lower cost. Until now, only a few methods based on Cu(II) catalysis have been disclosed for this transformation. For example, our group [22] and Santos [23] independently reported that Cu2(OH)2CO3 and CuSO4 respectively could catalyze the silylation of α,β-unsaturated compounds in the presence of a base. Kobayashi and co-workers [24] carried out this reaction in an enantioselective manner by using a chiral complex obtained from Cu(acac)2 and a chiral 2,2’-bipyridine ligand. In consideration of limited examples of Cu(II) catalysis and environmental impact, it is necessary to develop a green, efficient, and much milder strategy using Cu(II) catalysis to obtain functionalized organosilicon compounds bearing a carbonyl moiety. Furthermore, the recycling and reuse of Cu(II) catalyst still remains a challenge due to the deactivation or decomposition of non-immobilized Cu(II) salts.
Transition metal catalysts immobilized on a heterogeneous support played an important role in discovering unique reactivities and selectivities different from homogeneous systems [25,26]. The observed heterogeneous catalyst displays several advantages such as easy isolation, operational simplicity, environmental compatibility and remarkable reusability. Various supports—including magnetic materials [27], zeolites [28,29], silica [30], and polymers [31]—have been adopted for the immobilization of transition metals. Recently, chitosan (CS) has received extensive interest due to its non-toxicity, biodegradability, and reasonable ability of chelation [32,33]. It was proven that chitosan supported metal complexes were efficient to catalyze C–C [34,35,36,37,38,39], C–N [40], C–O [41], and C–S [42] bonds forming reactions. Meanwhile, we reported that chitosan supported copper is capable of catalyzing the transfer of pinacolatoboron moiety to α,β-unsaturated carbonyls, leading to the formation of C–B bond [43]. However, to the best of our knowledge, very few examples describing the C–Si formation in chitosan supported heterogeneous system were reported previously and the topic still represents a challenge.
With our continuous efforts in exploring applications of chitosan supported metal catalysts [22,43], we were interested in developing a green and mild protocol for synthesis of useful organosilicon compounds. Hence, we herein report a simple and easily available chitosan supported Cu(II) material as a highly reactive and recyclable catalyst for the desired β-silyl conjugate additions of α,β-unsaturated acceptors.
2. Materials and Methods
2.1. Materials
Chitosan (degree of acetylation = 5%; MW: 10,000–50,000, determined by GPC) was purchased from Aladdin (Shanghai, China), dimethylphenylsilyl pinacolatoboronate (CAS: 185990-03-8) was purchased from Energy Chemical (Shanghai, China) and Cu(II) salts were purchased from J&K (Beijing, China). All α,β-unsaturated acceptors, bases were obtained commercially from Energy Chemical (Shanghai, China) and used without further purification. Chitosan supported copper catalysts CS@CuSO4, CS@Cu(OAc)2, and CS@Cu(acac)2 were prepared according to the procedures reported [42].
2.2. Analytical Methods
Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance III 600 MHz spectrometer (Karlsruhe, Germany), operating at 600 for 1H and 150 MHz for 13C NMR in CDCl3 unless otherwise noted. CDCl3 is served as the internal standard (δ = 7.26 ppm) for 1H NMR and (δ = 77.0 ppm) for 13C NMR. Data for 1H-NMR is reported as follows: chemical shift (ppm, scale), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet and/or multiplet resonances, br = broad), coupling constant (Hz), and integration. Data for 13C NMR is reported in terms of chemical shift (ppm, scale), multiplicity, and coupling constant (Hz). Flash column chromatographic purification of products was accomplished using forced-flow chromatography on silica gel (200–300 mesh). The weight percentage and metal leaching of copper were determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES) (PerkinElmer, Waltham, MA, USA) analysis. The copper loading of CS@CuSO4, CS@Cu(OAc)2, and CS@Cu(acac)2 were found to be 1.85, 1.42, and 1.50 mmol/g, respectively.
2.3. General Procedure for Preparation of Chitosan Supported Copper Catalyst
Chitosan (5 g) was suspended in 100 mL of water. Copper salts (1 g) was added to this suspension and the mixture was stirred for 3 h. The catalyst was separated using a centrifuge (5000 rpm, 10 min), and dried under vacuum at 50 °C.
2.4. General Procedure for the Sample Preparation for ICP Analysis to Determine Catalyst Loading
Chitosan supported copper catalyst (~20 mg) was placed in a clean test tube and heated with H2SO4 (1 mL) at 200 °C. After 30 min, several drops of concentrated HNO3 were added carefully and the tube was shaken occasionally. HNO3 was continuously added until a clear solution was obtained and excess amount of HNO3 was allowed to evaporate under heating. After the solution was cooled to room temperature, 1 mL of aqua regia was added carefully. Effervescence of gas was observed and the solution become clearer. The solution was then transferred to a volumetric flask and made up to 50 mL with water which was submitted for ICP analysis.
2.5. General Procedure for the Sample Preparation for ICP Analysis to Determine Metal Leaching
After the reaction was finished, the reaction mixture was filtered. The filtrate obtained was concentrated and diluted with 10 mL of THF. Then 50% v/v of the crude THF solution (5 mL) was then passed through a membrane filter (0.25 or 0.45 μm) into a clean test tube. After evaporation of solvent, the solid obtained in the test tube was heated to 200 °C and 1.0 mL of concentrated H2SO4 was added. Following similar procedure described above, concentrated HNO3 were added at regular intervals until the resulting solution was clear. After the solution was cooled to room temperature, 1 mL of aqua regia was added carefully. Effervescence of gas was observed and the solution become clearer. The solution was then transferred to a volumetric flask and made up to 50 mL with water which was submitted for ICP analysis.
2.6. General Procedure for CS@Cu-Catalyzed Silylation of α,β-Unsaturated Acceptors in Water
CS@Cu(acac)2 (10.0 mg, 5 mol % Cu loading) and 4-picoline (3.3 mg, 6 mol %) were mixed in water (2 mL). The mixture was stirred for 1 h at room temperature, followed by successive addition of substrate (2) (0.3 mmol) and PhMe2Si-B(pin) (1) (94.4 mg, 0.36 mmol). After stirring for 12 h at room temperature, the reaction mixture was filtered and the filtrate was extracted with EtOAc (20 mL × 3). Combined organic layers were dried over anhydrous Na2SO4. After being concentrated under reduced pressure, the crude mixture was purified by flash column chromatography (200–300 mesh silica gel, petroleum ether/EtOAc = 10:1~20:1) to afford the desired product 3.
2.7. General Procedure for Gram-Scale Synthesis of 3a
CS@Cu(acac)2 (150 mg, 5 mol % Cu loading) and 4-picoline (49.5 mg, 6 mol %) were mixed in water (30 mL). The mixture was stirred for 1 h at room temperature, followed by successive addition of chalcone (2a) (0.94 g, 4.5 mmol) and PhMe2Si-B(pin) (1) (1.42 g, 5.4 mmol). After stirring for 12 h at room temperature, the reaction mixture was filtered and the filtrate was extracted with EtOAc (30 mL × 3). Combined organic layers were dried over anhydrous Na2SO4. After being concentrated under reduced pressure, the crude mixture was purified by flash column chromatography (200–300 mesh silica gel, petroleum ether/EtOAc = 10:1) to afford the desired product 3a.
2.8. Recycling and Reuse of CS@Cu Catalyst
In order to demonstrate the recyclability of CS@Cu(acac)2 catalyst, the silyl conjugate addition was repeated six times with the same sample. The initial amount of catalyst was 50.0 mg (5 mol % Cu loading). Reactions were carried out for 12 h. After the reaction, catalyst was filtered off, washed by EtOAc and water, and then dried for 4 h at 60 °C before next run. The recovery rate of the catalyst at the time of reusing is about 92%.
3. Results and Discussion
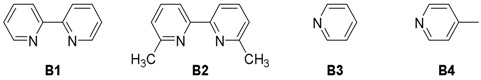
For the initial investigation, we tested the silyl conjugate addition of PhMe2SiB(pin) (1) to chalcone (2a) catalyzed by an array of chitosan supported copper catalysts in water (Table 1, entries 1–3). To our delight, CS@CuSO4, CS@Cu(OAc)2, and CS@Cu(acac)2 were all able to catalyze this transformation to obtain desired β-silyl product in 30%, 22%, and 41% yield respectively. Encouraged by this observation, we turned to screen different bases, including inorganic bases and a series of pyridine derivatives to further improve the conversion (Table 1, entries 4–9). It was found that the reactivity obviously increased in a more basic aqueous solution when sodium carbonate or potassium carbonate were used as base (Table 1, entries 4 and 5). However, 2,2’-bipyridine (B1) and its analog (B2) resulted in no effect to the reaction system, probably due to their poor dispersability in water (Table 1, entries 6 and 7). Furthermore, we had previously disclosed that 2,2’-bipyridine was effective to activate Si-B bond in the presence of a non-ionic surfactant [22]. Gratifyingly, significant improvements were achieved when pyridine (B3) and 4-picoline (B4) were used as additives (Table 1, entries 8 and 9). 4-Picoline is favorable for this reaction because it may perform the roles of the ligand for copper and Brønsted base for activating water molecules [23]. In the absence of catalyst, the reaction did not proceed at all (Table 1, entry 10). Subsequently, several aprotic solvents were examined (Table 1, entries 11–14), upon using dichloromethane, tetrahydrofuran, diethylether and toluene as solvents, product 3a was not observed due to the lack of proton source. Protic solvents such as methanol and ethanol only resulted in very low conversion because of their poor ability to provide proton (Table 1, entries 15 and 16). Notably, it was feasible to reduce the catalyst loading to half without compromising on yield (Table 1, entry 17). In addition, the reaction atmosphere did not affect the reactivity as illustrated (Table 1, entry 18). Therefore, the optimized reaction conditions were determined to run the reaction at room temperature in H2O with 6 mol % 4-picoline, using CS@Cu(acac)2 as catalyst (Table 1, entry 9).
Table 1.
Optimization of reaction conditions a.
 | ||||
| Entry | Catalyst | Base | Solvent | Yield (%) b |
| 1 | CS@CuSO4 | none | H2O | 30 |
| 2 | CS@Cu(OAc)2 | none | H2O | 22 |
| 3 | CS@Cu(acac)2 | none | H2O | 41 |
| 4 | CS@Cu(acac)2 | Na2CO3 | H2O | 50 |
| 5 | CS@Cu(acac)2 | K2CO3 | H2O | 65 |
| 6 | CS@Cu(acac)2 | B1 | H2O | 40 |
| 7 | CS@Cu(acac)2 | B2 | H2O | 38 |
| 8 | CS@Cu(acac)2 | B3 | H2O | 86 |
| 9 | CS@Cu(acac)2 | B4 | H2O | 92 |
| 10 | - | B4 | H2O | NR |
| 11 | CS@Cu(acac)2 | B4 | DCM | NR |
| 12 | CS@Cu(acac)2 | B4 | THF | NR |
| 13 | CS@Cu(acac)2 | B4 | Et2O | NR |
| 14 | CS@Cu(acac)2 | B4 | Toluene | NR |
| 15 | CS@Cu(acac)2 | B4 | MeOH | 25 |
| 16 | CS@Cu(acac)2 | B4 | EtOH | 8 |
| 17 c | CS@Cu(acac)2 | B4 | H2O | 89 |
| 18 d | CS@Cu(acac)2 | B4 | H2O | 91 |
 | ||||
a Reaction conditions: substrate 2 (0.3 mmol), Me2PhSi-B(pin) 1 (1.2 equiv.), catalyst (5 mol % Cu loading), base (6 mol %), H2O (2 mL), room temperature, air, 12 h; b Isolated yield of product; c 2.5 mol % Cu loading was used; d Performed under Ar atomosphere.
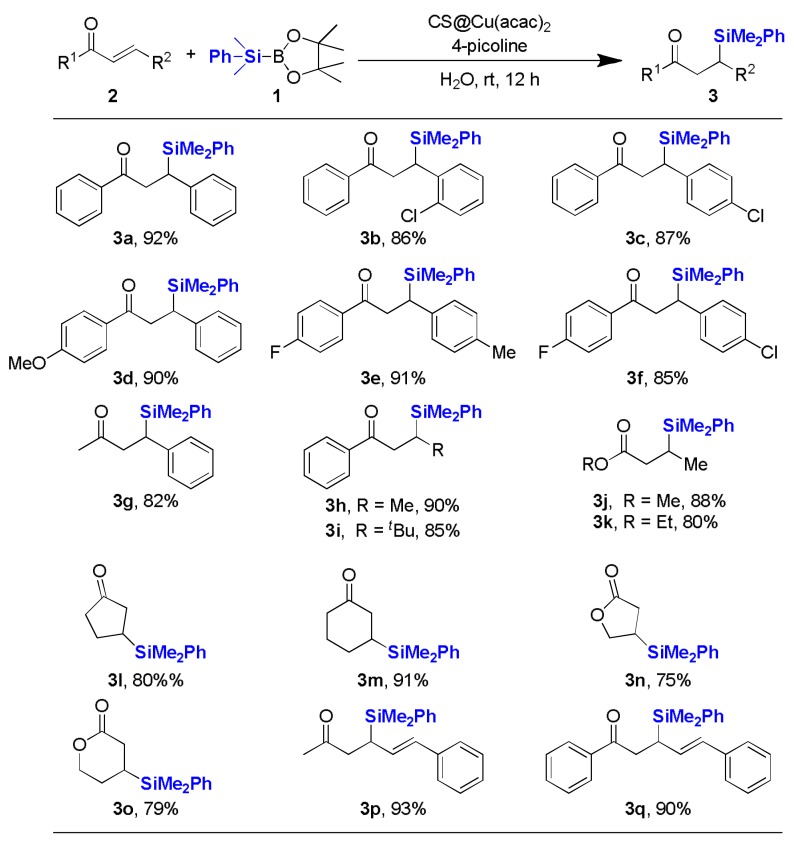
With the optimized conditions in hand, the substrate scope of α,β-unsaturated acceptors was surveyed and the results were summarized in Figure 1. Chalcone derivatives bearing a chloro-substituent at ortho- or para-position both proceeded smoothly to give corresponding products in good yields (3b,3c) (3b–3q are in Supplementary Materials). Electron-donating group as well as the electron-withdrawing one was tolerated in this kind of silylation process (3d). Next, di-substituted chalcones were also found to be suitable substrates besides mono-substituted derivatives (3e,3f). Silylation of α,β-unsaturated ketones having aromatic moieties on one side while aliphatic ones on the other, all performed well under the optimized conditions (3g,3h), even with sterically congested substrate (3i). It is worthy to note that great improvements were achieved for methyl- and ethyl-esters by using our newly developed strategy, compared to the previous report (3j,3k) [23]. Not only acyclic α,β-unsaturated carbonyls, but also cyclic ketones were applicable and exhibited comparable reactivities (3l,3m). Remarkably, lactones which are common motifs of many natural products could also be reacted with 1 to yield β-silyl products in 75% and 79%, respectively (3n,3o). Notably, 1,4-addition products were obtained exclusively without the formation of 1,6-addition byproducts when α,β,γ,δ-unsaturated dienones were used as substrates (3p,3q). The allylsilane products 3p and 3q could be further employed as a useful synthon in the Hosomi–Sakurai reaction [44]. Therefore, it was demonstrated that a wide range of α,β-unsaturated acceptors could be silylated effectively which were catalyzed by chitosan supported copper catalyst with 4-picoline as base.
Figure 1.
Substrate scope of α,β-unsaturated acceptors a,b. a Reaction conditions: substrate 2 (0.3 mmol), Me2PhSi-B(pin) 1 (1.2 equiv.), CS@Cu(acac)2 (5 mol % Cu loading), 4-picoline (6 mol %), H2O (2 mL), room temperature, air, 12 h; b Isolated yields were listed.
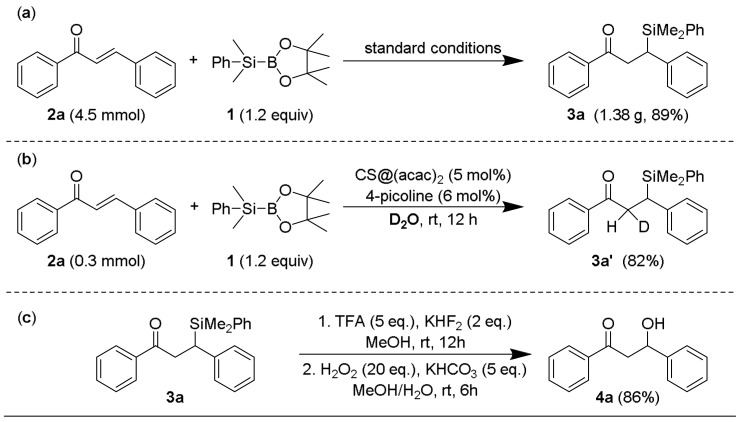
For the purpose of practical application, we further carried out this reaction in a gram scale. As shown in Scheme 1a, β-dimethylphenylsilyl substituted chalcone (3a) (in Supplementary Materials) was successfully synthesized in 89% yield under the standard conditions. Next, when this reaction was performed in deuterium oxide instead of water, about 50% deuterium was observed in α-methylene, as revealed by 1H NMR spectrum (Scheme 1b). This result indicated that water performs not only as a solvent but also as an important proton source for the protonation step of the whole catalytic cycle. To be mentioned, using water in this silyl conjugate addition made our method green and environmental friendly, because water is non-toxic, cheap, and non-flammable unlike organic solvents. In order to confirm the utility of obtained β-silyl products, further conversion of 3a by Fleming–Tamao oxidation [45] easily produced corresponding β-hydroxyl compound 4a (Scheme 1c). This process supplied a simple and efficient protocol to introduce hydroxyl group into complicated structure of natural products and biological active compounds.
Scheme 1.
(a) Gram scale synthesis β-silyl compound; (b) reaction in deuterium oxide; (c) further conversion of β-silyl products to β-hydroxyl compounds.
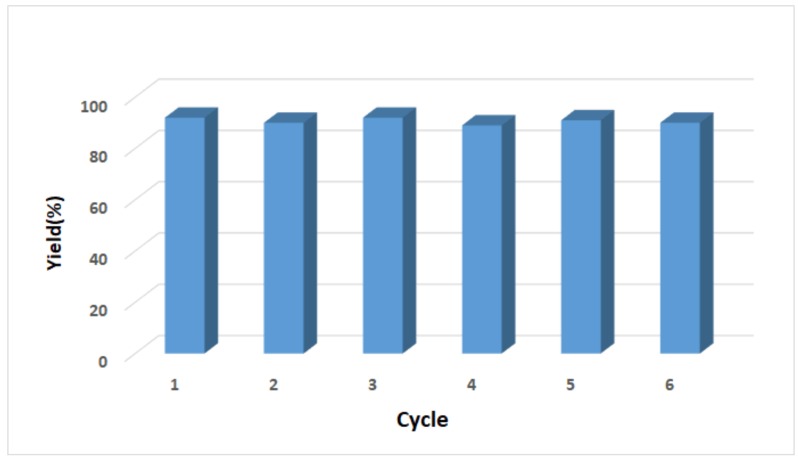
Recovery and recycling of heterogeneous catalyst are usually crucial considerations for transition metal-catalyzed reactions, from the aspects of economy and sustainability. Thus, the recyclability of chitosan supported copper catalyst was evaluated in the reaction of chalcone (2a) with 1 as shown in Figure 2. In each cycle, recovered CS@Cu(acac)2 was treated with another new substrate for the next run under the optimized conditions. Remarkably, the catalyst still remained catalytically active after being reused six times. To date, there has only been one report about the recycling of catalyst for just two runs in similar silylation of cyclopentenone, along with apparent decline of reactivity [24]. In addition, we halted the reaction after six hours when about 60% conversion of starting material was received. The catalyst was then removed by filtration at the reaction temperature and the residue reacted for the rest time. It was found that n additional product was formed in the absence of catalyst. Finally, ICP analysis of the filtered aqueous solution after reaction showed no detectable leaching of copper. Both the filtration experiment and metal leaching test strongly suggested chitosan supported copper catalysis is heterogeneous in nature.
Figure 2.
Recycling of chitosan supported copper catalyst.
4. Conclusions
In summary, we have prepared a heterogeneous chitosan supported copper catalyst which is highly active to catalyze the silyl conjugate addition of α,β-unsaturated acceptors with Suginome’s silylboron reagent. The catalytic reaction was performed in good to excellent yields in water under mild conditions. Various substituted acyclic and cyclic α,β-unsaturated ketones, esters proceeded well under the optimized conditions. Besides, α,β,γ,δ-unsaturated acyclic dienones could also be silylated regioselectively to give corresponding 1,4-addition products. Water possibly plays a predominant role in accelerating the protonation step to achieve high yields. Gram scale synthesis and conversion to β-hydroxy compound made this method more versatile. Remarkably, the catalyst could be easily recovered and recycled six cycles without any significant decrease of reactivity. As a development towards the application of chitosan, our method stands a chance over the previously reported methods in terms of substrate scope, operational simplicity, and recyclability of catalyst.
Acknowledgments
The authors acknowledge the financial support from the National Natural Science Foundation of China (Nos. 21774029, 21774107, 31371750), the Natural Science Foundation of Hubei Province of China (No. 2016CFB104) and Hubei Engineering University.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4360/10/4/385/s1, Characterization data, and spectra for the 1H and 13C NMR of compounds 3a–3q.
Author Contributions
Lei Zhu and Caiqin Qin conceived and designed the experiments; Bojie Li, Shan Wang, and Wei Wang performed the experiments; Liang Ding analyzed the structure data and prepared the draft manuscript; Liansheng Wang contributed reagents/materials/analysis tools; Lei Zhu and Caiqing Qin reviewed and modified the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Colvin E.W. Silicon in Organic Synthesis. 1st ed. Butterworths; London, UK: 1981. [Google Scholar]
- 2.Weber W.P. Silicon Reagents for Organic Synthesis. 1st ed. Springer; Berlin, Germany: 1983. [Google Scholar]
- 3.Allen R.B., Kochs P., Chandra G. Organosilicon Materials. 1st ed. Springer; Berlin, Germany: 1997. [Google Scholar]
- 4.Mutahi M.W., Nittoli T., Guo L., Sieburth S.M. Silicon-based metalloprotease inhibitors: Synthesis and evaluation of silanol and silanediol peptide analogues as inhibitors of angiotensin-converting enzyme. J. Am. Chem. Soc. 2002;124:7363–7375. doi: 10.1021/ja026158w. [DOI] [PubMed] [Google Scholar]
- 5.Sieburth S.M., Chen C.A. Silanediol protease inhibitors: From conception to validation. Eur. J. Org. Chem. 2006;2006:311–322. doi: 10.1002/ejoc.200500508. [DOI] [Google Scholar]
- 6.Still W.C. Conjugate addition of trimethysilyllithium, a preparation of 3-silyl ketones. J. Org. Chem. 1976;41:3063–3064. doi: 10.1021/jo00880a044. [DOI] [Google Scholar]
- 7.Fleming I., Lee D. Conjugate addition of silyl groups to β-unsubstituted enones, & Si-to-OH conversion: A synthesis of (±)-lavandulol. Tetrahedron Lett. 1996;37:6929–6930. doi: 10.1016/0040-4039(96)01519-5. [DOI] [Google Scholar]
- 8.Dambacher J., Bergdahl M. Empolying the simple monosilylcopper reagent, Li[PhMe2SiCuI], in 1,4-addition reactions. Chem. Commun. 2003:144–145. doi: 10.1039/B210792A. [DOI] [PubMed] [Google Scholar]
- 9.Lipshutz B.H., Sclafani J.A., Takanami T. Silyl cuprate couplings: Less silicon, accelerated yet catalytic in copper. J. Am. Chem. Soc. 1998;120:4021–4022. doi: 10.1021/ja980152i. [DOI] [Google Scholar]
- 10.Oestreich M., Weiner B. Copper-catalyzed conjugate addition of a bis(triorganosilyl) zinc and a methyl(triorganosilyl) magnesium. Synlett. 2004:2139–2142. doi: 10.1055/s-2004-831331. [DOI] [Google Scholar]
- 11.Auer G., Weiner B., Oestreich M. Copper-free and copper-promoted conjugate addition reactions of bis(triorganosilyl) zincs and tris(triorganosilyl) zincates. Synthesis. 2006:2113–2116. doi: 10.1002/chin.200645173. [DOI] [Google Scholar]
- 12.Weickgenannt A., Oestreich M. Silicon- and tin-based cuprates: Now catalytic in copper! Chem. Eur. J. 2010;16:402–412. doi: 10.1002/chem.200902222. [DOI] [PubMed] [Google Scholar]
- 13.Suginome M., Matsuda T., Ito Y. Convenient preparation of silylboranes. Organometallics. 2000;19:4647–4649. doi: 10.1021/om000254t. [DOI] [Google Scholar]
- 14.Walter C., Auer G., Oestreich M. Rhodium-catalyzed enantioselective conjugate silyl transfer: 1,4-addition of silyl boronic esters to cyclic enones and lactones. Angew. Chem. Int. Ed. 2006;45:5675–5677. doi: 10.1002/anie.200601747. [DOI] [PubMed] [Google Scholar]
- 15.Walter C., Oestreich M. Catalytic asymmetric C-Si bond formation to acyclic α,β-unsaturated acceptors by RhI-catalyzed conjugate silyl transfer using a Si–B linkage. Angew. Chem. Int. Ed. 2008;47:3818–3820. doi: 10.1002/anie.200800361. [DOI] [PubMed] [Google Scholar]
- 16.Walter C., Fröhlich R., Oestreich M. Rhodium(I)-catalyzed enantioselective 1,4-addition of nucleophilic silicon. Tetrahedron. 2009;65:5513–5520. doi: 10.1016/j.tet.2009.01.111. [DOI] [Google Scholar]
- 17.Hartmann E., Oestreich M. Asymmetric conjugate silyl transfer in iterative catalytic sequences: Synthesis of the C7–C16 fragment of (+)-neopeltolide. Angew. Chem. Int. Ed. 2010;49:6195–6198. doi: 10.1002/anie.201002916. [DOI] [PubMed] [Google Scholar]
- 18.Lee K., Hoveyda A.H. Enantioselective conjugate silyl additions to cyclic and acyclic unsaturated carbonyls catalyzed by Cu complexes of chiral N-heterocyclic carbenes. J. Am. Chem. Soc. 2010;132:2898–2900. doi: 10.1021/ja910989n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welle A., Petrignet J., Tinant B., Wouters J., Riant O. Copper-catalyzed domino silylative aldol reaction leading to stereocontrolled chiral quaternary carbons. Chem. Eur. J. 2010;16:10980–10983. doi: 10.1002/chem.201000907. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahem I., Santoro S., Himo F., Córdova A. Enantioselective conjugate silyl additions to α,β-unsaturated aldehydes catalyzed by combination of transition metal and chiral amine catalysts. Adv. Synth. Catal. 2011;353:245–252. doi: 10.1002/adsc.201000908. [DOI] [Google Scholar]
- 21.O’Brien J.M., Hoveyda A.H. Metal-free catalytic C-Si bond formation in an aqueous medium. Enantioselective NHC-catalyzed silyl conjugate additions to cyclic and acyclic α,β-unsaturated carbonyls. J. Am. Chem. Soc. 2011;133:7712–7715. doi: 10.1021/ja203031a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W., Li B., Xiao Z., Yan F., Wei P., Wang L., Zhu L. Basic copper carbonate-catalyzed silyl conjugate additions to α,β-unsaturated carbonyls in water. J. Chin. Chem. Soc. 2018;65:81–86. doi: 10.1002/jccs.201700186. [DOI] [Google Scholar]
- 23.Calderone J.A., Santos W.L. Copper(II)-catalyzed silyl conjugate addition to α,β-unsaturated conjugated compounds: Brønsted base-assisted activation of Si-B bond in water. Org. Lett. 2012;14:2090–2093. doi: 10.1021/ol300618j. [DOI] [PubMed] [Google Scholar]
- 24.Kitanosono T., Zhu L., Liu C., Xu P., Kobayashi S. An insoluble copper(II) acetylacetonate-chiral bipyridine complex that catalyzes asymmetric silyl conjugate addition in water. J. Am. Chem. Soc. 2015;137:15422–15425. doi: 10.1021/jacs.5b11418. [DOI] [PubMed] [Google Scholar]
- 25.Mizuno N., Misono M. Heterogeneous catalysis. Chem. Rev. 1998;98:199–218. doi: 10.1021/cr960401q. [DOI] [PubMed] [Google Scholar]
- 26.Yin L., Liebscher J. Carbon-carbon coupling reactions catalyzed by heterogeneous palladium catalysts. Chem. Rev. 2007;107:133–173. doi: 10.1021/cr0505674. [DOI] [PubMed] [Google Scholar]
- 27.Schultz A.M., Salvador P.A., Rohrer G.S. Enhanced photochemical activity of α-Fe2O3 films supported on SrTiO3 substrates under visible light illumination. Chem. Commun. 2012;48:2012–2014. doi: 10.1039/c2cc16715h. [DOI] [PubMed] [Google Scholar]
- 28.Dey R., Screedhar B., Ranu B.C. Molecular sieves-supported palladium(II) catalyst: Suzuki coupling of chloroarenes and an easy access to useful intermediates for the synthesis of irbesartan, iosartan and boscalid. Tetrahedron. 2010;66:2301–2305. doi: 10.1016/j.tet.2010.02.011. [DOI] [Google Scholar]
- 29.Zhu Y., Hua Z., Zhou X., Song Y., Gong Y., Zhou J., Zhao J., Shi J. CTAB-templated mesoporous TS-1 zeolites as active catalysts in a desulfurization process: The decreased hydrophobicity is more favourable in thiophene oxidation. RSC Adv. 2013;3:4193–4198. doi: 10.1039/c3ra23276j. [DOI] [Google Scholar]
- 30.Opanasenko M., Štěpnička P., Čejka J. Heterogeneous Pd catalysts supported on silica matrices. RSC Adv. 2014;4:65137–65162. doi: 10.1039/C4RA11963K. [DOI] [Google Scholar]
- 31.Zhang J., Han D., Zhang H., Chaker M., Zhao Y., Ma D. In Situ recyclable gold nanoparticles using CO2-switchable polymers for catalytic reduction of 4-nitrophenol. Chem. Commun. 2012;48:11510–11512. doi: 10.1039/c2cc35784d. [DOI] [PubMed] [Google Scholar]
- 32.Kumar M.N.V.R., Muzzarelli R.A.A., Muzzarelli C., Sashiwa H., Domb A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004;104:6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- 33.Kadib A.E. Chitosan as a sustainable organocatalyst: A concise overview. ChemSusChem. 2015;8:217–244. doi: 10.1002/cssc.201402718. [DOI] [PubMed] [Google Scholar]
- 34.Hardy J.J.E., Hubert S., Macquarrie D.J., Wilson A.J. Chitosan-based heterogeneous catalysts for Suzuki and Heck reactions. Green Chem. 2004;6:53–56. doi: 10.1039/b312145n. [DOI] [Google Scholar]
- 35.Kadib A.E., Molvinger K., Bousmina M., Brunel D. Improving catalytic activity by synergic effect between base and acid pairs in hierarchically porous chitosan@titania nanoreactors. Org. Lett. 2010;12:948–951. doi: 10.1021/ol9029319. [DOI] [PubMed] [Google Scholar]
- 36.Primo A., Quignard F. Chitosan as efficient porous support for dispersion of highly active gold nanoparticles: Design of hydrid catalyst for carbon-carbon bond formation. Chem. Commun. 2010;46:5593–5595. doi: 10.1039/c0cc01137a. [DOI] [PubMed] [Google Scholar]
- 37.Makhubela B.C.E., Jardine A., Smith G.S. Rh(I) complexes supported on a biopolymer as recyclable and selective hydroformylation catalysts. Green Chem. 2012;14:338–347. doi: 10.1039/C1GC15979H. [DOI] [Google Scholar]
- 38.Sk M.P., Jana C.K., Chattopadhyay A. A gold-carbon nanoparticle composite as an efficient catalyst for homocoupling reaction. Chem. Commun. 2013;49:8235–8237. doi: 10.1039/c3cc43726d. [DOI] [PubMed] [Google Scholar]
- 39.Shen C., Xu J., Ying B., Zhang P. Heterogeneous chitosan@copper(II)-catalyzed remote trifluoromethylation of aminoquinolines with Langlois reagent by radical cross-coupling. ChemCatChem. 2016;8:3560–3564. doi: 10.1002/cctc.201601068. [DOI] [Google Scholar]
- 40.Baig R.B.N., Varma R.S. Copper on chitosan: A recyclable heterogeneous catalyst for azide-alkyne cycloaddition reactions in water. Green Chem. 2013;15:1839–1843. doi: 10.1039/c3gc40401c. [DOI] [Google Scholar]
- 41.Ying B., Xu J., Zhu X., Shen C., Zhang P. Catalyst-controlled selectivity in the synthesis of C2- and C3-sulfonate esters from quinoline N-oxides and arylsulfonyl chlorides. ChemCatChem. 2016;8:2604–2608. doi: 10.1002/cctc.201600555. [DOI] [Google Scholar]
- 42.Shen C., Xu J., Yu W., Zhang P. A highly active and easily recoverable chitosan@copper catalyst for the C-S coupling and its application in the synthesis of zolimidine. Green Chem. 2014;16:3007–3012. doi: 10.1039/C4GC00161C. [DOI] [Google Scholar]
- 43.Xu P., Li B., Wang L., Qin C., Zhu L. A green and recyclable chitosan supported catalyst for the borylation of α,β-unsaturated acceptors in water. Catal. Commun. 2016;86:23–26. doi: 10.1016/j.catcom.2016.08.002. [DOI] [Google Scholar]
- 44.Hosomi A., Sakurai H.J. Chemistry of organosilicon compounds. 99. Conjugate addition of allylsilanes to .alpha,beta-enones. A New method of stereoselective introduction of the angular allyl group in fused cyclic alpha, beta-enones. J. Am. Chem. Soc. 1977;99:1673–1675. doi: 10.1021/ja00447a080. [DOI] [Google Scholar]
- 45.Fleming I., Barbero A., Walter D. Stereochemical control in organic synthesis using silicon-containing compounds. Chem. Rev. 1997;97:2063–2192. doi: 10.1021/cr941074u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.