Abstract
The pleiotropic effects of statins have been evaluated to assess their potential benefit in the treatment of various inflammatory and immune-mediated diseases including periodontitis. Herein, the adjunctive use of statins in periodontal therapy in vitro, in vivo, and in clinical trials was reviewed. Statins act through several pathways to modulate inflammation, immune response, bone metabolism, and bacterial clearance. They control periodontal inflammation through inhibition of proinflammatory cytokines and promotion of anti-inflammatory and/or proresolution molecule release, mainly, through the ERK, MAPK, PI3-Akt, and NF-κB pathways. Moreover, they are able to modulate the host response activated by bacterial challenge, to prevent inflammation-mediated bone resorption and to promote bone formation. Furthermore, they reduce bacterial growth, disrupt bacterial membrane stability, and increase bacterial clearance, thus averting the exacerbation of infection. Local statin delivery as adjunct to both nonsurgical and surgical periodontal therapies results in better periodontal treatment outcomes compared to systemic delivery. Moreover, combination of statin therapy with other regenerative agents improves periodontal healing response. Therefore, statins could be proposed as a potential adjuvant to periodontal therapy. However, optimization of the combination of their dose, type, and carrier could be instrumental in achieving the best treatment response.
1. Introduction
Periodontitis is an inflammatory disease of infectious origin characterized by progressive destruction of periodontal soft and hard tissues leading to tooth loss. The main symptoms comprise gingival inflammation, formation of periodontal pocket, alveolar bone loss, abscess, or tooth mobility [1]. The pathogenesis of periodontitis involves a complex interaction of immune and inflammatory cascades initiated by bacteria of the oral biofilm [2]. Persistent inflammation and dysbiosis worsen periodontal tissue damage, and the host response plays a vital role in this phenomenon contributing to tissue destruction [3].
The conventional treatment comprising scaling and root planing (SRP) presents limitations in certain cases involving deep periodontal pockets, inaccessible areas, or severe periodontitis [4]. Therefore, several adjunctive pharmacological therapeutics have been tested to improve its outcomes. In this context, systemic and local deliveries of drugs such as antibiotics, bisphosphonates, anti-inflammatory drugs, anticytokines, probiotics, and prebiotics have been tested so far to reduce bacterial load and to control inflammation [5–9]. Likewise, the use of statins in periodontal treatment has been explored recently [10]. Statins, or inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase), are a group of drugs, used primarily to treat hyperlipidemia and to prevent cardiovascular diseases [11]. After their discovery in the 70s, they have been widely prescribed worldwide [12]. They differ mainly in their ring structure, and these structural differences modify their pharmacological properties including hydrophilicity and lipophilicity. The lactone ring is present in an active form (already hydrolyzed) in all statins except for simvastatin, lovastatin, and mevastatin, in which the lactone ring is activated (hydrolyzed) in the liver. The lactone form of the statins enables their transport, metabolism, and clearance [13] (Table 1).
Table 1.
Physical properties of different types of statins.
| Drug | Source | Solubility | Molecular mass (Da) |
|---|---|---|---|
| Atorvastatin | Synthetic | Lipophilic | 1209.42 |
| Simvastatin | Natural | Lipophilic | 418.6 |
| Lovastatin | Natural | Lipophilic | 404.5 |
| Mevastatin | Natural | Lipophilic | 390.52 |
| Pravastatin | Natural | Hydrophilic | 446.52 |
| Fluvastatin | Synthetic | Lipophilic | 411.47 |
| Cerivastatin | Synthetic | Lipophilic | 459.56 |
| Pitavastatin | Synthetic | Lipophilic | 421.46 |
| Rosuvastatin | Synthetic | Hydrophilic | 481.54 |
Apart from their lipid-lowering properties, statins possess pleiotropic effects due to their anti-inflammatory, antioxidative, antibacterial, and immunomodulatory properties [14–17]. Statins have also been reported to have anabolic effects on the bone by augmenting bone morphogenetic protein-2 (BMP-2) expression, thus contributing towards the differentiation and activity of osteoblasts (OBs) [18]. In view of their beneficial properties, statins have been presented as new potential candidates for improving periodontal therapy outcomes [19, 20].
In several preclinical and clinical studies, statins have exhibited contradictory results [21–23] depending on the mode of delivery (local vs systemic), anatomy and severity of the lesions, type of disease, and treatment approach (nonsurgical vs surgical). Therefore, the aim of this literature review was to establish a better understanding of the prophylactic and therapeutic effects of all statin types administered locally or systemically as adjuvant to nonsurgical/surgical periodontal treatment in existing preclinical models and clinical settings and to explore the biological mechanisms underlying these healing and proregenerative effects in the management of periodontitis.
2. Methods
2.1. Literature Search
Studies published in English language only were included, and the last search was carried out in September 2018. Regarding studies performed on animal models and clinical trials, a systematic literature search was performed in the PubMed/MEDLINE and ScienceDirect databases. A hand search has also been performed after checking references of the identified articles. Concerning in vivo studies, the following keywords were used for the search: periodontitis OR periodontal disease OR alveolar bone loss OR periodontal attachment loss OR periodontal pocket AND simvastatin OR statin OR rosuvastatin OR atorvastatin OR cerivastatin OR mevastatin OR lovastatin OR pravastatin OR Fluvastatin OR pitavastatin OR Hydroxymethylglutaryl-CoA Reductase Inhibitors AND mouse OR dog OR pig OR rat OR rodent OR rabbit OR monkey OR in vivo. A study was considered eligible if it met the following criteria: (1) experimentally induced periodontitis (EIP) and/or acute/chronic periodontal defects (ACP), (2) treatment of EIP and/or ACP with statins (local or systemic or combination) with or without SRP or other periodontal treatment modalities, and (3) at least one periodontal parameter assessed as outcome. Exclusion criteria for in vivo studies were the following: (1) periapical lesions, (2) tooth extraction models, (3) orthodontic movements, (4) calvarial models, (5) long bone defects, and (6) drug-induced gingival enlargement.
Concerning clinical studies, the following keywords were used for the search: periodontitis OR periodontal disease OR alveolar bone loss OR periodontal attachment loss OR periodontal pocket AND simvastatin OR statin OR rosuvastatin OR atorvastatin OR cerivastatin OR mevastatin OR lovastatin OR pravastatin OR Fluvastatin OR pitavastatin OR Hydroxymethylglutaryl-CoA Reductase Inhibitors. A study was considered eligible if it met the following criteria: (1) randomized and controlled clinical trials, (2) cohort clinical studies, (3) longitudinal studies, (4) patients with diagnosis of chronic or aggressive periodontitis, (5) systemic or local administration of statins with nonsurgical or surgical periodontal treatment, and (6) at least one periodontal parameter: pocket depth (PD), clinical attachment level (CAL), bone loss (BL), or tooth loss (TL) assessed as outcome. Exclusion criteria for clinical studies were the following: (1) no follow-up, (2) no periodontal treatment, and (3) reviews, letters, and case reports.
2.2. Study Selection
Titles and abstracts of the studies were screened independently by two reviewers (CP and FB) and categorized as suitable or not for inclusion. Full reports were reviewed independently for studies appearing to meet the inclusion criteria or for which there was insufficient information in the title and abstract to allow a clear decision. Disagreements between the authors were resolved after discussion with a third reviewer (OH).
2.3. Risk of Bias Assessment
Risk of bias was assessed using the Cochrane Collaboration's tool for assessing risk of bias which provided guidelines for the following parameters: sequence generation, allocation concealment method, blinding of the examiner, address of incomplete outcome data, and free of selective outcome reporting. The degree of bias was categorized as follows: low risk if all the criteria were met, moderate risk when only one criterion was missing, and high risk if two or more criteria were missing. Two reviewers (FB and CP) independently performed the quality assessment, and any disagreement was resolved by a third investigator (OH) (Supplemental Table 1).
3. Results
3.1. Effect of Statins on the Inflammatory-Immune Crosstalk
Localization of periodontium at the interface between the teeth and jaws exposes periodontal tissues to continuous bacterial challenge which could contribute to exacerbation of the immune response during periodontal wound healing. Recruitment of inflammatory cells at the periodontal site, including polymorphonuclear (PMN) leukocytes, macrophages, and lymphocytes, is associated to the release of a complex nexus of cytokines. When the inflammatory front migrates toward the alveolar bone, it stimulates osteoclastogenesis and subsequent alveolar bone destruction [24]. Therefore, the importance of inflammation control at the soft tissue level cannot be undermined.
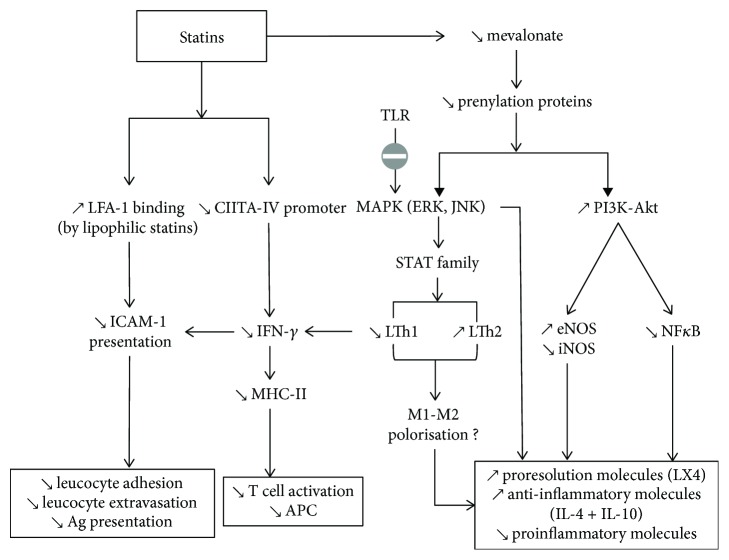
The effects of statins on the inflammatory-immune crosstalk involved in the periodontal wound healing have been evaluated. Statins decrease the levels of proinflammatory cytokines (interleukin-1 beta (IL-1β), interleukin-8 (IL-8), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α)) and increase the release of anti-inflammatory mediators (IL-10) and chemokines [25, 26]. There are several pathways implicated in the action of statins, notably suppression of HMG-CoA reductase, thereby inhibiting Rac and p21Ras phosphorylation. As Rac and p21Ras are coupled to the transcription of proinflammatory molecules via MAP kinase (MAPK) pathways, therefore, statins also suppress nuclear factor kappa B (NF-κB) activation, thus reducing the expression of proinflammatory molecules [27] (Figure 1).
Figure 1.
Effect of statins on the inflammatory-immune crosstalk. Direct LFA1 site binding by lipophilic statins decreases ICAM-1 presentation leading to reduced leukocyte chemotaxis and antigen presentation. Statins inhibit MHC-II induction by IFN-γ leading to decreased T-cell activation. Statins lower mevalonate release, leading to resolution of inflammation via the ERK, MAPK, and PI3K-Akt pathways.
3.1.1. Effect of Statins on Inflammatory Molecules
In vitro, the effect of statins on inflammatory mediators' secretion was demonstrated to be cell specific. For instance, in human oral epithelial cells [15] and OBs [28], statins reduced IL-6, IL-8 release, whereas, in T-cells [29, 30], statins increased the expression of IL-4, IL-5, IL-10 and IL-13. In vivo, statins confirmed the reduction of cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), IL-1β, IL-6, IL-8, TNF-α, interferon-gamma (IFN-γ), C-reactive protein (CRP), colony-stimulating factors (CSF2, CSF3), recruitment of mononuclear inflammatory cells, and several Toll-like receptors (TLRs) in various EIP or ACP models [26, 31–35]. Clinical trials also corroborated the downregulation of inflammation by the use of statins, as demonstrated by increased IL-10 level in gingival crevicular fluid (GCF) from hyperlipidemic patients treated with statins [19].
3.1.2. Effect of Statins on Proresolution Molecules
Periodontal wound healing and regeneration involve a constant “tug-of-war” between the proinflammatory and anti-inflammatory/proresolution mediators [36, 37]. Anti-inflammatory effects of statins enhancing resolution of periodontal inflammation, that is, initiated by several endogenous chemical and lipid mediators, such as the lipoxins (LXs), resolvins (RVs), protectins, and maresins, could possibly explain the positive treatment outcomes [38, 39]. However, further studies need to explore the exact effect of statins on the proresolution mediators.
3.1.3. Effect of Statins on Host Modulation
Literature reports contradictory results regarding the effect of statins on different types of immune cells. For instance, in an ACP model, simvastatin did not change circulating white blood cell (WBC) counts in a study [33], whereas leukocyte infiltration was decreased by atorvastatin gavage in an EIP model [40]. Similarly, regulatory T (Treg) cells that control adaptive immunity against pathogens and activate other effector immune cells were reported to be regulated by statins. In this regard, atorvastatin and simvastatin demonstrated an increase in the number of human Treg cells and differentiation of CD4 into Treg in vitro [41, 42].
Furthermore, TLRs have an important role in the immune-inflammatory crosstalk with a consequent impact on periodontal wound healing response. In the context of periodontal treatment, targeting TLRs has been proposed as it could enhance antimicrobial properties, suppress adverse inflammation, or activate tissue repair [43]. Interestingly, simvastatin inhibited the stimulation of several TLRs (1, 2, 3, 4, 6, 7, and 9) by Aggregatibacter actinomycetemcomitans (A.a) LPS in vivo, reducing its capability to escape innate immune response [33]. Hence, statins play an instrumental role in the modulation of inflammatory and immune responses.
3.1.4. Inhibition of Major Histocompatibility Complex Class II (MHC-II) by Statins
In case of nonresolving periodontal lesions, bacterial antigens are processed and presented by antigen-presenting cells and macrophages. Such process is associated to massive immune cell recruitment implicated in tissular destruction [2]. In this regard, statins are able to inhibit MHC-II expression due to inhibition of the inducible promoter IV of the class II transactivator (CIITA) as observed in several cell types, including monocytes and macrophages [44]. This effect renders statins to have a potential host-modulating impact on periodontal treatment.
3.1.5. Lymphocyte Function-Associated Antigen-1 LFA1 Site Binding by Statins
Lymphocyte function-associated antigen-1 (LFA-1), an integrin with its main ligand intercellular adhesion molecule-1 (ICAM-1), is activated on the surface of fibroblasts (FBs) by IFN-γ and represents a critical phase in the early stage of inflammation. ICAM-1 regulates LFA-1-dependent neutrophil transmigration and recruitment to the inflammation site [45]. Several studies have demonstrated the inhibition of LFA-1 by statins in many inflammatory and immune diseases other than periodontitis. Statins inhibit ICAM-1 upregulation and chemotaxis of monocytes [46]. Lovastatin, simvastatin, and mevastatin, but not pravastatin, were able to inhibit the LFA-1/ICAM-1 interaction in vitro by binding to the L-site of LFA-1 [47]. In this way, statins limit the exacerbation of immune-mediated inflammatory response at the lesion site. However, the impact of statins on LFA-1 binding in the context of periodontal wound healing remains unexplored.
3.1.6. Effect of Statins on Nitric Oxide Synthase (NOS)
NOS plays an important role in host defence and homeostasis and has been implicated in the pathogenesis of periodontitis, where it is expressed in FBs, epithelial cells, rests of Malassez, macrophages, osteoclasts (OC), and vascular endothelial cells [48, 49]. In chronic periodontitis, bacterial challenge induces proinflammatory cytokine release and a higher expression of inducible NOS (iNOS) and NOS derived from FBs and WBCs that migrate to the periodontal lesion [50–52] leading to inflammation-mediated bone resorption [53]. Various studies demonstrated a NOS-inhibiting effect by the use of statins. For instance, in vivo, rosuvastatin significantly reduced inflammation-mediated tissue destruction and gingival iNOS expression [54].
Concerning the underlying mechanism of action, statins attenuate the production of reactive oxygen species (ROS) induced by NADPH oxidase by suppressing Rac's geranylation. Phosphatidylinositol-3 active kinase (PI3-Akt) is a kinase that phosphorylates and stimulates eNOS. Mevalonate is able to inhibit PI3-Akt; therefore, by reducing the concentration of mevalonate, statins upregulate eNOS-derived NO production resulting in vasorelaxation that leads to improved angiogenesis and wound healing response [27].
3.1.7. Effect of Statins on Matrix Metalloproteinases (MMPs)
MMPs degrade extracellular matrix proteins, especially collagen, contributing to the degradation of periodontal tissue including alveolar bone [55]. Most statins have been reported to potently inhibit the expression of MMP-1, MMP-8, and MMP-9 upregulated by LPS as demonstrated for simvastatin in mononuclear cells in vitro [56]. Moreover, in vivo, a decrease of MMP-1, MMP-2, MMP-8, and MMP-9 was observed by the use of statins [31, 57–59]. Thus, statins prevent periodontal tissue and alveolar bone destruction by inhibiting the release of MMPs.
3.2. Effect of Statins on Bone Metabolism
Statins have an impact on bone metabolism through increase of osteogenesis, decrease of OB apoptosis, and osteoclastogenesis [60]. Statins allow periodontal regeneration via the Ras/Smad/extracellular signal-regulated kinase (Erk)/BMP-2 pathway that enhances bone formation [61] and by antagonizing TNF-α through Ras/Rho/mitogen-activated protein kinase (MAPK) that causes osteoclastic differentiation [62]. Moreover, they significantly increase OB differentiation factors such as alkaline phosphatase (ALP), osteocalcin (OCN), bone sialoprotein (BSP), BMP-2 [63], osteopontin (OPN), and vascular endothelial growth factor (VEGF) [64] (Figure 2).
Figure 2.
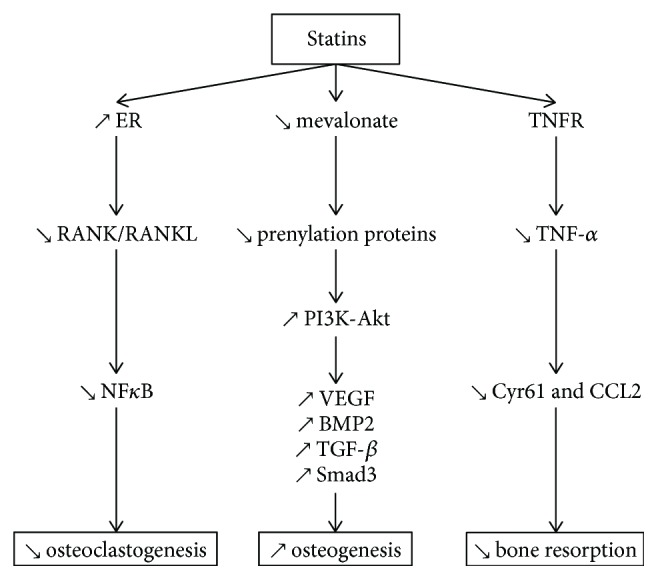
Effects of statins on several pathways involved in bone metabolism. Statins decrease osteoclastogenesis via RANK/RANKL and NF-κB signaling. Statins promote osteogenesis by increasing VEGF, BMP2, and TGF-β expression through the PI3-Akt pathway. Statins prevent inflammation-mediated bone resorption by decreasing TNF-α, via TNFR.
3.2.1. Role of Statins in the Promotion of Osteogenesis
Inhibition of HMG-CoA by statins decreases prenylation of farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GPP) leading to increased levels of BMP-2 and VEGF through the PI3-Akt pathway. Interestingly, both VEGF and BMP-2 regulate OB differentiation and bone formation during bone repair and regeneration [65, 66]. Concerning BMP, simvastatin and lovastatin increased the levels of BMP-2, consequently, increasing OB activity in vitro [58, 63]. Statins present a cost-effective option when compared with growth factors such as BMP-2 [67, 68].
Hydrophobic statins (simvastatin, atorvastatin, and cerivastatin) also increased mRNA expression of VEGF in OBs [69]. Likewise, simvastatin increased osteoprotegerin (OPG) expression in periodontal tissue [58] and enhanced matrix calcification in human bone marrow stem cells by diminishing the mean size of the fibroblastic colony-forming units (CFU-Fs) [70]. In vivo, statins stimulated bone growth and repair by increasing angiogenesis [71]. In particular, the lactone-form statins (lovastatin and simvastatin) stimulated OB differentiation of mouse periodontal ligament cells (PDLs) via the ERK1/2 pathway (phosphorylation) and enhanced intercellular matrix mineralization [63].
3.2.2. Role of Statins in the Inhibition of Bone Destruction
Statins act through certain pathways that avert bone degradation. Several clinical trials confirm the reduction of alveolar bone loss by statins, as an adjunct to SRP [72]. Many studies reported significantly decreased bone resorption by the use of simvastatin, rosuvastatin, and atorvastatin [26, 28, 32, 73]. Interestingly, simvastatin reduced TNF-α-induced synthesis of Cysteine-rich 61 (Cyr61) and chemokine ligand 2 (CCL2) [74] that are potential osteolytic mediators in inflammatory bone diseases, in human OB, thereby decreasing bone loss. Besides, statins increase bone formation by inhibiting OB apoptosis, augmenting TGF-β against the Smad3 signaling pathway. As an evidence, pitavastatin, mevastatin, and simvastatin induced the expression of Smad3 in nontransformed OBs (MC3T3-E1) [75]. Consequently, statins prevent bone destruction and also promote bone healing and regeneration.
3.2.3. Role of Statins in the Inhibition of Osteoclastogenesis
Statins suppress osteoclastogenesis through the OPG/receptor activator of the nuclear factor kappa-B ligand (RANKL)/RANK signaling pathway. Statins (simvastatin, atorvastatin, and fluvastatin) inhibited, in vitro and in vivo, the expression of the receptor activator of RANK which along with RANKL is required for the differentiation of OC precursors [26, 31, 33, 58, 76]. Nevertheless, IL-10 is also implicated in inhibiting bone resorption by preventing the RANK/RANKL pathway ([77]); hence, statins could potentially reduce the inflammation-mediated bone resorption [25]. Another mechanism for osteoclastogenesis involving unprenylated Rap GTP-binding protein 1A (Rap-1A), a RAS super family of small GTP-binding protein member, has been studied in the context of statins. Rosuvastatin, pravastatin, cerivastatin, and simvastatin caused accumulation of unprenylated Rap-1A in rabbit osteoclast-like cells and macrophages, inhibiting osteoclast-mediated resorption. Interestingly, hydrophilic statin (cerivastatin) was more effective than hydrophobic statin (rosuvastatin) to inhibit OC prenylation [78]. Additionally, the mRNA expression of cathepsin K, a key marker of OC differentiation, is reduced by simvastatin through inhibition of Src signaling and modulation of MAPK including ERK1/ERK2. Moreover, upregulation of AKT leads to a decrease of OC activity via RANKL and BMP-2 [79].
3.3. Antibacterial Effect of Statins
Periodontitis is a polymicrobial disease involving keystone pathogen such as Porphyromonas gingivalis (P.g) that is able to hijack the adaptive immune response. Therefore, elimination of the periodontal pathogens is the cornerstone of periodontal treatment. Uncontrolled infection hinders periodontal wound healing and may worsen the therapeutic outcome by reducing the clinical attachment gain. Statins exhibit antimicrobial effects attributed to an increased bacterial clearance from the infection site as demonstrated in a model of sepsis (Figure 3) ([80]). Hence, statins could provide an additional benefit during periodontal wound healing (Table 2).
Figure 3.
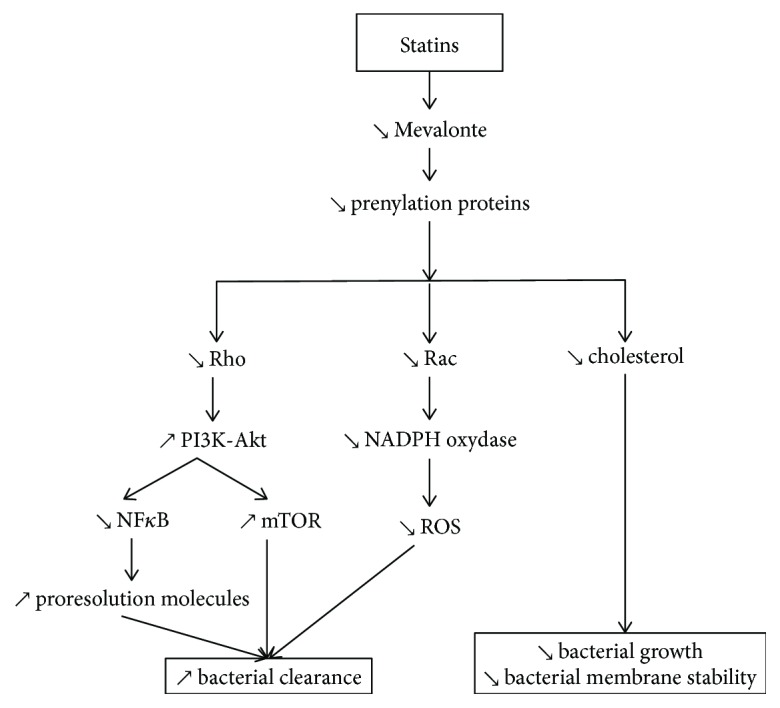
Antibacterial effect of statins. Statins arrest bacterial growth and disrupt their membrane stability by decreasing cholesterol. Statins increase bacterial clearance by decreasing NF-κB and ROS signaling (via the PI3K-Akt and NADPH oxidase pathways, respectively) and by enhancing proresolution molecule release.
Table 2.
Representative in vitro studies evaluating the impact of statins on periodontal pathogens.
| Local drug delivery | ||||
|---|---|---|---|---|
| Reference | Experimental design | Type of statin dose | Results | Periodontal consideration |
| [82] | MIC was determined against P.g (ATCC 33277) and A.a (ATCC 25586) using serial dilution method | Simvastatin, 1 μg/mL to 500 μg/mL | ↘ P.g
↘ A.a |
Simvastatin had an antibacterial effect against the keystone pathogens involved in periodontal disease |
|
| ||||
| [138] | A.a (ATCC 43719), P. nigrescens (ATCC 33563), or P.g (ATCC 33277) were cultured on a trilayer functional CS membrane with EGCG and lovastatin | Lovastatin 0.1, 0.5, 1, and 2 mg | ↘ P.g
↘ A.a |
Lovastatin had an antibacterial effect against periodontopathogenic bacteria |
Cholesterol is an integral component needed by bacteria for maintaining their membrane integrity. Statins can counter bacteria by inhibiting the intermediate in the isoprenoid biosynthesis pathway necessary for membrane stability, which is substituted by cholesterol and protects bacteria from the toxic effect of statins. Statins, therefore, kill bacteria directly and by lowering accessible host cholesterol content for bacterial growth and protection. Such effects may be due to the disruption of teichoic acid structures reducing biofilm formation ([81]). Statins display antibacterial activity towards anaerobic bacteria, including periodontal pathogens such as A.a and P.g. For instance, low concentration of simvastatin was proven to be effective against A.a and P.g even if A.a was more sensitive (MIC < 1 μg/mL) than P.g (MIC until 2 μg/mL dilution) [82]. The hydrophobic nature of simvastatin may explain its antibacterial activity against periodontal pathogens where it disrupts the bacterial membrane in a “soap-like” manner causing its death [83]. Nevertheless, not all statins exhibit antibacterial activity. The degree of HMG-CoA reductase inhibition corresponds directly to the cholesterol-lowering capabilities of statins [84] but it does not seem commensurate with their antibacterial potency [85].
Some other mechanisms are modulated by the action of statins on lipoxin A4 (LXA4) production, a proresolving lipid mediator that enhances bacterial clearance, consequently reducing the severity of periodontal disease [86, 87]. Furthermore, the mechanistic target of rapamycin (mTOR) signaling, regulated principally by TLRs via two major pathways (NF-κB-dependent pathway and a PI3-Akt-dependent pathway), is also involved in bacterial clearance [88]. It is known that statins inhibit isoprenoid synthesis, impeding intracellular signaling molecules like Rho or Rac [89].
Therefore, it is plausible that statins possess certain antibacterial properties that could facilitate periodontal treatment. However, since periodontitis is a polymicrobial disease, the susceptibility of various other periodontal pathogens to statins must also be evaluated.
3.4. Effects of Statins in Induced Periodontitis Models
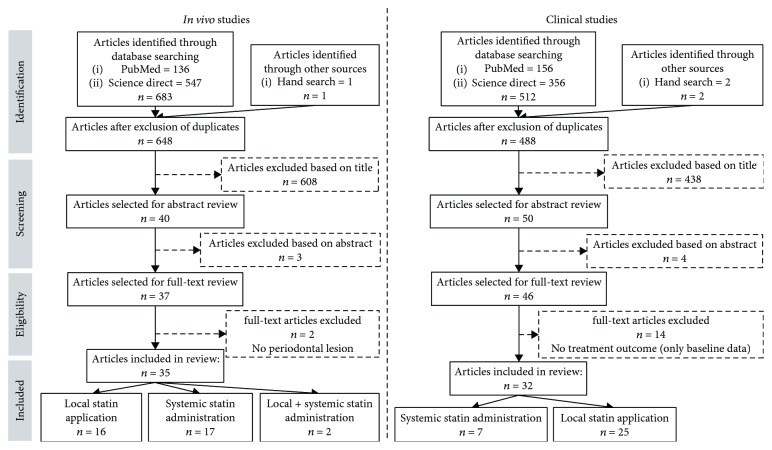
Statins have been tested in several induced periodontitis models to evaluate improvement in periodontal parameters and their underlying biological mechanisms. In vivo, 35 studies were identified based on the inclusion criteria (Figure 4), out of which 16 involved local statin delivery (Table 3), 17 used systemic route (Table 4), and 2 employed a combination of both modes (Table 5). In the studies evaluating local statin application, 8 studies involved the treatment of EIPs while the remaining 8 investigated the treatment of ACP models, one of which was induced by LPS injection of Escherichia coli (E. coli) [90]. Concerning the systemic administration of statins (Table 4), 14 out of the total 17 studies treated EIPs, whereas the 3 remaining studies involved ACP models by LPS injections of A.a [32, 33] and P.g into the gingiva [76].
Figure 4.
Selection of the studies.
Table 3.
In vivo studies evaluating the impact of local statin administration on periodontal wound healing.
| Local drug delivery | ||||
|---|---|---|---|---|
| Reference | Experimental periodontitis induction model (i) Animal (ii) Method (iii) Site |
Periodontitis treatment (i) Type of treatment (ii) Type and dose of statin (iii) Mode and time of statin delivery |
Results | Periodontal considerations |
| [139] | Rats (retired female breeder) EIP by ligatures Maxillary right M2 |
Nonsurgical treatment (therapeutic) Simvastatin prodrug 0.5 mg, 1.0 mg, and 1.5 mg Local injections of the drug/SIM/SIM-mPEG carrier 10 μL into the palatal gingiva between maxillary M1 and M2 Three weekly injections until euthanasia |
↗ amount of uninflamed connective tissue in the M1-M2 interproximal area ↘ bone loss, especially with 1.5 mg SIM/SIM-mPEG ↘ percentage of neutrophils |
Simvastatin limited periodontal breakdown by reducing bone loss and the extent of gingival inflammation |
|
| ||||
| [73] | Rats (male) ACP (maxillary bone defect) Maxillary M1 extraction followed by socket healing, preparation of a critical-sized periodontal defect (2.0 mm diameter and 1.0 mm depth) on the mesial aspect of the M2, and manual removal of the residual bone and cementum on mesial aspect of M2 |
Surgical treatment (therapeutic) Simvastatin 1 mg Encapsulated in double-walled PDLLA-PLGA microspheres Combinations: simvastatin-BSA, simvastatin-PDGF, simvastatin |
↗ neo-osteogenesis ↗ bone mineral density ↗ bone volume fraction ↗ number and thickness of trabeculae ↘ trabecular separation ↗ cementogenesis of the periodontal apparatus ↘ inflammatory cell infiltration |
Simvastatin promoted osteogenic differentiation, reduced inflammation, and facilitated osteogenesis. Sequential PDGF-simvastatin delivery was able to accelerate osteogenesis, bone maturation, fiber realignment, and cementogenesis of the periodontal apparatus, thus accelerating periodontal regeneration |
|
| ||||
| [94] | Rats (male) ACP (tooth-associated alveolar bone defect model) extraction of M1 followed by 4 weeks of socket healing, preparation of a critical-sized intrabony periodontal defect in the M1 edentulous ridge next to the mesial aspect of the M2 finished by a 2.6 mm diameter and 1.0 mm deep osteotomy (completely removing the mesial wall of the osteotomy), and cementum removal (to expose the mesial aspect of M2) |
Surgical treatment (therapeutic) Simvastatin 1 mg PDLLA-PLGA hybrid microspheres encapsulating simvastatin/PDGF/BSA to fill the defects |
↗ neo-osteogenesis (histologically) PDL fibers not inserted on the root surface (mainly parallel) ↗ bone volume fraction % (not significant) |
Simvastatin histologically improved bone healing but better healing response was observed in the group receiving PDGF |
|
| ||||
| [95] | Rats (female) ACP (fenestration defects) Defects 2 mm high, 4 mm wide, and 1.5 mm deep over mandibular molar roots |
Nonsurgical treatment (therapeutic) Simvastatin 0.5 mg Local injection of 0.5 mg SIM per site dissolved in 70% ethanol or as SIM-ALN-CD Three weekly injections Treatment started 15 days after the defect preparation |
↗ insignificant improvement of bone fill compared to other groups New cementum formation (not significant) But better bone healing response after systemic ALN administration followed by simvastatin injections |
Simvastatin had a local bone healing effect which can be augmented by addition of certain other regenerative molecules like ALN |
|
| ||||
| [138] | Dogs (male) ACP (maxillary bone defect) Extraction of all maxillary PM2 followed by healing and preparation of one-walled intrabony defects (4 × 5 × 4 mm: buccolingual, mesiodistal, and depth, respectively) on the mesial and distal sides of maxillary bilateral PM1 Removal of residual cementum by SRP |
Surgical treatment (therapeutic) Lovastatin 0.1, 0.5, 1, or 2 mg per trilayer functional CS with the EGCG membrane area (cm2) |
↗ new bone formation in the EGCG14-CS-lovastatin 1 group (62.03%) > BioMend® group (46.07%) > control group (42.32%) Evidence of new cementum deposition observed on the root surface No inflammatory cell infiltrate was noted in the EGCG14-CS-lovastatin 1 group Fibrous connective tissue approximated to the surgical defect |
The trilayer functional CS membrane with EGCG and lovastatin enhanced periodontal regeneration and bone formation rate |
|
| ||||
| [140] | Dogs (male) ACP (maxillary bone defect) Extraction of maxillary 2nd and 3rd incisors followed by 8 weeks of socket healing and, later, preparation of three-walled intrabony defects (4 × 4 × 5 mm: buccolingual, mesiodistal, and depth, respectively) on the mesial side of maxillary bilateral canines Removal of residual cementum by SRP |
Nonsurgical treatment (therapeutic) Lovastatin 4 mg dissolved in chloroform to form a 3 wt % PLGA solution Local injections of PLGA-lovastatin-CS-tetracycline 0.3% nanoparticles prepared as a hydrogel by mixing with gelatin (10 mg/100 mm3) to fill the defects |
↗ new deposits of cementum on the root surface ↗ active plasmacytoid osteoblastic rimming along the trabecular surface of the bone adjacent to the defect ↗ percentage of new bone formation (41.32%) No evident inflammation |
PLGA-lovastatin-chitosan-tetracycline nanoparticles showed a good osteogenic potential. They promoted new bone and cementum formation |
|
| ||||
| [96] | Rats (male) ACP (mandibular bone defect) Preparation of surgical defects 0.8 mm in diameter through the alveolar bone over the mesiobuccal root of the mandibular M1 bilaterally |
Surgical treatment (therapeutic) Simvastatin 2.5% gel Defect was filled with 2.5% simvastatin gel Single topical application |
↘ marrow spaces in simvastatin-treated defects ↗ collagen fibril organization ↗ OPN in bone matrix ↗ alveolar bone regeneration |
Simvastatin gel improved the quality of the new bone and decreased bone resorption |
|
| ||||
| [99] | Dogs (males and females) ACP (mandibular bone defect) Preparation of bilateral 3-walled intrabony defects (4 × 4 × 4 mm) distal of the mandibular PM2 and mesial of the PM4 and class II furcation defects at the buccal furcation of the mandibular M1 measuring 4 mm occlusal apically and 4 mm buccolingually followed by healing and SRP of defect sites |
Nonsurgical treatment (therapeutic) Simvastatin 0.5 mg or 2.0 mg in 30 μL methylcellulose gel Three weekly injections |
↗ edentulous ridge thickness (29% greater with simvastatin) ↗ bone loss in class II furcation defects ↗ length of new cementum in the interproximal intrabony defect ↗ bone height with simvastatin (2 mg) No new cementum was observed in furcations |
Simvastatin was not appropriate for the treatment of class II furcation defects. However, it improved bone healing in intrabony defects and edentulous ridges significantly |
|
| ||||
| [22] | Rats (male) EIP by ligatures Maxillary M2 bilaterally |
Nonsurgical treatment (therapeutic) Atorvastatin 2% w/v containing CS gel Local 100 μL volume application every other day until euthanasia |
↘ IL-1β, IL-6, and IL-8 ↗ IL-10 (time dependent) ↘ alveolar bone resorption (significantly with ATV + CS application and insignificantly with ATV alone) ↘ attachment loss Improvement of inflammatory and osteoclastic activity score over time |
Atorvastatin with chitosan downregulated inflammation-mediated bone resorption |
|
| ||||
| [90] | Rats (female) EIP by injection of E. coli LPS 10 μL of endotoxin injection (1 mg/mL of LPS in PBS) between M1 and M2 |
Nonsurgical treatment (preventative) Simvastatin 0.5 mg of simvastatin and 3.75 mg of SIM-ALN-CD in H2O Three weekly 12 μL injection bilaterally into the palatal/interproximal gingiva of M1 and M2 Treatment started one week before induction |
↗ bone preservation during experimental periodontitis by prophylactic SIM-ALN-CD injection ↘ subsulcular inflammation ↘ alveolar bone loss ↘ OC number |
Simvastatin protected against alveolar bone loss and soft tissue inflammation |
|
| ||||
| [98] | Dogs (female) ACP (mandibular bone defect) Preparation of dehiscence defects (5 × 3 mm) bilaterally on the lateral aspect of the mandibular PM2 mesial roots and removal of root cementum Split-mouth design |
Surgical treatment (therapeutic) Simvastatin Graft surgery with HA grafts bilaterally covered with resorbable bilayer collagen membranes hydrated with 10 mg simvastatin (graft surgery performed at the time of defect preparation) Local injection 10 mg SIM (0.5 mg/kg) in ethanol (100 μL) Three weekly injections (one week after the graft surgery and defect preparation) |
↗ width of new bone in edentulous ridge Distance between CEJ and the alveolar crest was more coronal in dehiscence defects treated with simvastatin (insignificant) Three weeks post-op after simvastatin injection (firm swelling about 1 × 1 cm to 3.5 × 3.5 cm in size), disappeared in 2 months |
Simvastatin improved new bone formation where periosteum existed and did not induce severe side effects except for moderate swelling that, eventually, subsided |
|
| ||||
| [59] | Rats (male) EIP by ligatures Left mandibular M1 |
Nonsurgical treatment (therapeutic) Simvastatin 1 mg/mL (Natrosol + simvastatin gel solution) into the periodontal pocket SRP and irrigation with simvastatin Single injection |
↘ MMP-8 expression ↘ bone loss |
Simvastatin reduced periodontal bone loss |
|
| ||||
| [141] | Rats (male) EIP by ligatures Maxillary M2 |
Nonsurgical treatment (therapeutic) Simvastatin 0.2 mg in 50 μL PBS topically injected into the buccal gingivae Twice a week for 70 days |
↗ ALP activity ↗ bone nodule formation No inflammatory cells around the new bone ↘ bone loss Simvastatin recovered the ligature-induced alveolar bone resorption (46% reversal of bone height) |
Simvastatin increased bone regeneration and reduced inflammation |
|
| ||||
| [142] | Rats (male) EIP by ligatures Mandibular left M1 |
Nonsurgical treatment (preventative) Simvastatin 0.5 mg/kg body weight orally Followed by laser therapy Treatment started 1 day before induction and daily until euthanasia |
↘ bone loss ↘ carbonylated proteins in gingiva |
Simvastatin reduced bone loss |
|
| ||||
| [91] | Rats (female ovarectomized) EIP by ligatures Mandibular right M1 |
Nonsurgical treatment (protective) Simvastatin 10−6 M, 3 × 10−7 M, 10−7 M subperiosteal injections (0.05 mL) Twice a week since the first day of ligature insertion to the 25th day |
↘ periodontal breakdown ↘ bone loss in alveolar bone crest zone in a dose-dependent manner (10−7 > 10−6 > 3 × 10−7) |
Simvastatin reduced bone loss in a dose-dependent manner |
|
| ||||
| [143] | Rat (female) EIP (ligature) Maxillary M2 bilaterally |
Nonsurgical treatment (therapeutic) Simvastatin SIM-PPi conjugate Different treatments including SIM-PPi (dissolved in 25%, 2.56 mg, equivalent to 1.5 mg SIM) and SIM acid (dissolved in PBS, 1.56 mg, equivalent to 1.5 mg of SIM) locally injected (10 μL) into the palatal gingiva between the maxillary M1 and M2 On the first day of weeks 1, 2 and 3 after ligature placement |
↗ alveolar bone crest preservation with SIM-PPi ↗ bone volume ↗ trabecular thickness ↗ trabecular number ↘ trabecular separation ↘ neutrophil and lymphocyte score ↘ OC score |
Simvastatin improved periodontal bone regeneration and decreased periodontal inflammation |
Table 4.
In vivo studies evaluating the impact of systemic statin administration on periodontal wound healing.
| Systemic drug delivery | ||||
|---|---|---|---|---|
| Reference | Experimental periodontitis induction model (i) Animal (ii) Method (iii) Site |
Periodontitis treatment (i) Type of treatment (ii) Type and dose of statin (iii) Mode and time of statin delivery |
Results | Periodontal considerations |
| [31] | Rats (male) EIP by ligatures Maxillary left M2 |
Nonsurgical treatment (protective) Atorvastatin 1 mg/kg, 5 mg/kg, and 10 mg/kg 1 hour before induction and thereafter once daily |
↘ MMP-2, MMP-9 ↘ RANK-L, RANK ↗ OPG ↗ GSH levels ↘ IL-1β, TNF-α, and MPO (dose dependent) ↘ COX-2 level ↘ MDA activity ↘ alveolar bone loss is dose dependent |
Atorvastatin protected against alveolar bone loss in a dose-dependent manner |
|
| ||||
| [58] | Rats (female) EIP by ligatures Maxillary left M2 |
Nonsurgical treatment (protective) Simvastatin 3, 10, and 30 mg/kg/day 1 hour before induction and thereafter once daily |
↗ BMP-2 and OPG levels ↗ TRAP activity ↘ MPO activity (dose dependent) ↘ IL-1β and TNF-α ↗ IL-10 ↘ gingival GSH ↗ gingival MDA and NOX ↘ iNOS, MMP-1, MMP-8, RANK, and RANKL expression No differences in AST and ALT levels Inhibition of alveolar bone loss |
Simvastatin prevented inflammatory bone resorption and possessed antioxidant properties |
|
| ||||
| [144] | Rats (male) EIP by ligatures Maxillary left M2 |
Nonsurgical treatment (protective) Atorvastatin 1, 3, and 9 mg/kg Atorvastatin mixed in sterile saline by gavage 30 min before ligature placement and then daily until euthanasia |
↘ alveolar bone loss in the furcation area as well as in proximal faces of upper M2 (47% reduction with 9 mg dose compared to that with the control) Insignificant bone loss protection with 1 and 3 mg doses |
Atorvastatin had protective effect against alveolar bone loss |
|
| ||||
| [40] | Rats (male) EIP by ligatures Maxillary left M2 |
Nonsurgical treatment (protective + therapeutic) Atorvastatin 0.3 mg/kg or 27 mg/kg by gavage In combination with ALN 30 min before ligature placement and thereafter once daily until euthanasia or 5 days after the start of periodontitis induction and then daily until euthanasia |
↘ TRAP and MPO activity ↘ cementum resorption ↘ neutrophilia and lymphomonocytosis ↘ alveolar bone loss both prophylactically (39%) and therapeutically (53.4%) with lower dose of ALN + ATV (0.01 mg/kg+0.3 mg/kg, respectively) Prevented BALP reduction with lower dose of ALN + ATV No effect on serum transaminases |
Atorvastatin reduced alveolar bone loss, cemental resorption, and inflammatory cell infiltration both prophylactically and therapeutically |
|
| ||||
| [145] | Rats (male) EIP by ligatures Maxillary left M2 |
Nonsurgical treatment (protective) Atorvastatin 0.3, 3, and 27 mg/kg by gavage 30 min before ligature placement and thereafter once daily until euthanasia |
↘ alveolar bone in a dose-dependent manner (39% for 3 mg/kg and 56% for 27 mg/kg doses) Prevented the reduction of BALP serum levels (27 mg/kg) Prevented leukocytosis (27 mg/kg) |
Atorvastatin prevented alveolar bone loss with both prophylactic and therapeutic doses |
|
| ||||
| [32] | Rats (female with metabolic syndrome) ACP (injection of 20 μg of A.a LPS in PBS) into the palatal gingiva between the maxillary M1 and M2, thrice per week for 4 weeks |
Nonsurgical treatment (protective) Simvastatin 20 mg/kg/day Daily via gavage for 4 weeks Treatment started on the same day as injection of LPS |
↘ LPS induced alveolar bone loss in both lean and fat rats (significantly) ↘ infiltration of mononuclear cells ↘ inflammatory score ↘ LPS stimulated RANKL and CSF2 expression in both lean and fat rats ↘ bone resorption |
Simvastatin downregulated inflammation-mediated bone resorption |
|
| ||||
| [33] | Rats (female) ACP injection of 20 μg/rat of A.a LPS through the palatal gingiva between the maxillary M1 and M2 thrice per week for 8 weeks |
Nonsurgical treatment (protective) Simvastatin (20 mg/kg/day) daily via oral gavage for 8 weeks |
↘ LPS induced alveolar bone loss (31%) ↘ LPS induced osteoclastogenesis ↘ TNF-α, IL-1α, IL-1β, IL-6, CSF-2, CSF-3, MCP-1, and MMP-9 ↘ LPS induced TLR family members' expression |
Simvastatin downregulated inflammation-mediated bone resorption |
|
| ||||
| [25] | Rats (male) EIP by ligatures Maxillary M2 |
Nonsurgical treatment (protective) Rosuvastatin 20 mg/kg in water by gavage 1 h before ligation and then once daily until euthanasia |
↗ IL-10 ↘ IL-1β ↗ MDA ↗ GSH ↘ inflammatory infiltrate ↘ OC number ↗ OB number ↘ alveolar bone loss (significantly) |
Rosuvastatin protected against alveolar bone loss |
|
| ||||
| [54] | Rats (male) EIP by ligatures Hyperlipidemia induction through diet Maxillary M2 |
Nonsurgical treatment (protective) Rosuvastatin 20 mg/kg in water by gavage 1 h before ligation and then once daily until euthanasia |
↘ gingival iNOS (significantly) ↘ inflammation and hyperemia ↘ alveolar bone loss |
Rosuvastatin protected against inflammation-induced bone degradation |
|
| ||||
| [34] | Rats (male) EIP by ligatures Mandibular M1 and maxillary M2 bilaterally |
Nonsurgical treatment (therapeutic) Simvastatin 10 mg/kg in water once daily orally until euthanasia Treatment started 8 days after periodontitis induction |
↘ alveolar bone loss ↘ IL-6 ↘ CRP |
Simvastatin decreased inflammation and alveolar bone loss |
|
| ||||
| [93] | Rats (male hypertensive) EIP by ligatures Mandibular M1 bilaterally |
Nonsurgical treatment (protective) Rosuvastatin 2 mg/kg oral gavage Treatment started since the day of induction daily until euthanasia |
↘ bone loss in furcation area ↘ attachment loss ↘ TRAP-positive multinucleated cells |
Rosuvastatin reduced alveolar bone loss and osteoclastogenesis |
|
| ||||
| [97] | Rats EIP by ligatures Mandibular M1 |
Nonsurgical treatment (protective + therapeutic) Simvastatin Different treatments: simvastatin-simvastatin: aqueous suspension of simvastatin by gavage (35 mg/kg/day) administration before and after periodontitis induction; simvastatin-water: simvastatin administration before and filtered water after periodontitis induction; and water-simvastatin: water administration before and simvastatin after periodontitis induction |
No significant differences between groups receiving simvastatin before the induction of periodontitis and those that received water No protective effect of simvastatin against the development of periodontitis |
Simvastatin did not possess protective or therapeutic effects against periodontitis development |
|
| ||||
| [146] | Rats (male) EIP by ligatures Mandibular left M1 |
Nonsurgical treatment (therapeutic) Simvastatin 25 mg/kg Dissolved in saline Treatment started 14 days after the initiation of periodontitis induction |
↗ TG levels ↘ MDA level ↗ IL-10 ↘ MMP-9 ↘ bone loss No difference on TNF-α levels |
Simvastatin promoted the anti-inflammatory mediators to counter alveolar bone loss |
|
| ||||
| [35] | Rats (male, cyclosporine A-induced alveolar bone loss) EIP by ligatures Mandibular right M1 |
Nonsurgical treatment (protective) Simvastatin 20 mg/kg orally daily for 30 days The treatment and induction started on the same day |
↗ Ca2+ concentrations (significantly) No effect of simvastatin treatment in the presence of periodontal disease on serum ALP levels but it blocked the cyclosporine A-mediated decrease of ALP No significant effect on alveolar bone turnover but with concomitant cyclosporine A and simvastatin delivery Simvastatin completely inhibited cyclosporine A-induced bone loss |
Simvastatin did not prevent alveolar bone loss in periodontitis but it completely countered the cyclosporine A-induced bone loss |
|
| ||||
| [147] | Rats (male) EIP by ligatures Mandibular right M1 |
Nonsurgical treatment (protective) Simvastatin 20 mg/kg The treatment and induction started on the same day |
↗ ALP activity in periodontal inflammation ↘ alveolar bone loss |
Simvastatin protected against alveolar bone loss |
|
| ||||
| [76] | Mice (male) ACP (P.g LPS injection) 1 mg/kg P.g LPS injection at the gingiva of left mandibular M2 on days 4 and 7 |
Nonsurgical treatment (protective) Fluvastatin 3 mg/kg IP injections on days 1, 4, and 7 |
↘ LPS induced OC (by >50%) ↘ LPS-induced bone erosion ↘ RANKL |
Fluvastatin prevented inflammation-induced bone erosion |
|
| ||||
| [26] | Rats (male, GIOP) EIP by ligatures Maxillary left M2 |
Nonsurgical treatment (protective) Atorvastatin 27 mg/kg ATV orally 30 min before induction and once daily afterwards |
↘ bone loss ↘ MPO, TNF-α, IL-1β, IL-6, and IL-8 ↗ IL-10, GSH, SOD, and CAT levels ↘ RANKL and DKK-1 ↗ OPG, WNT10 β, and β-catenin expressions and BALP activity |
Atorvastatin prevented alveolar bone loss in periodontitis and reduced inflammation |
Table 5.
In vivo studies evaluating the impact of a combination of local and systemic statin administration on periodontal wound healing.
| Local + systemic drug delivery | ||||
|---|---|---|---|---|
| Reference |
Experimental periodontitis induction model
(i) Animal (ii) Method (iii) Site |
Periodontitis treatment
(i) Type of treatment (ii) Type and dose of statin (iii) Mode and time of statin delivery |
Results | Periodontal considerations |
| [57] | Rats (male) EIP by ligature mandibular M1 |
Nonsurgical treatment (therapeutic) Atorvastatin Systemically (5 mg/kg in a volume of 0.5 mL) and locally (0.1 mg/kg in a volume of 0.05 mL) at a dose of 0.1 mg/kg in a volume of 0.05 mL |
↗ alveolar bone area % ↗ VEGF ↘ MMP-9 ↘ alveolar bone and attachment loss Local application showed better results on periodontium healing |
Atorvastatin increased the alveolar bone regeneration while decreasing the periodontal inflammation and attachment loss |
|
| ||||
| [92] | Rats (female ovarectomized) EIP by ligatures Maxillary M1 and M2 bilaterally |
Nonsurgical treatment (therapeutic) Simvastatin Local injection (0.8 mg/0.05 mL) Oral (25 mg/kg) For two months until euthanasia |
↗ alveolar crest height (28% with local & oral and 27% with local) ↗ BV/TV ↗ trabecular thickness ↘ trabecular separation |
Simvastatin reduced bone degradation when administered locally, systemically, or both locally and systemically together |
The animals included in the studies are healthy unless stated otherwise. Treatment was considered (1) “preventative” when it started at least one day before the start of EIP/ACP induction, (ii) “protective” when it started the same day as that of EIP/ACP induction, and (iii) “therapeutic” when it started at least one day after the start of EIP/ACP induction.
Regarding the mode of periodontitis induction, in total, 24 out of 35 studies had EIP with ligatures (cotton, nylon, or silk), whereas 11 used ACP including the 4 studies where periodontitis was induced by bacterial LPS. Studies were mostly performed in rodents (Tables 3, 4, and 5). In ACP models, the surgically created lesions were mainly intrabony defects, fenestration defects, dehiscence defects, furcation class II defects, and 3-walled intrabony defects.
In 6 studies, animals with systemic diseases (i.e, osteoporosis [26, 91, 92], metabolic syndrome [32], cyclosporine A-associated alveolar bone loss [35], hyperlipidemia [54], or hypertension [93] were used to evaluate the effect of statins treatment. Overall, 22 studies involved treatment with simvastatin, 7 with atorvastatin, 3 with rosuvastatin, 2 with lovastatin, and only one with fluvastatin. Some studies investigated more than one type of statin. In vivo, the systemic dosage used ranged from 0.3 to 30 mg/kg with 20 mg/kg as the most commonly tested dose. The dose of locally delivered statins varied with the type of carrier/scaffold used (Table 3). Five studies demonstrated insignificant improvements [94–98]. Interestingly, 3 of them involved surgical treatment of ACP models by local statin application [94, 96, 98] and one study employed nonsurgical local statin therapy [95], whereas only one EIP was treated with systemic statin delivery [97]. One study even demonstrated a negative impact of statin use [99].
3.5. Clinical Outcomes
The selected studies evaluating the effect of statins in the context of periodontal treatment included 23 controlled and randomized clinical trials, 8 cohort studies, and 1 longitudinal study (Figure 4). Primary outcomes varied between improvement of clinical attachment level (CAL), reduction of pocket depth (PD), tooth loss, radiographic bone defect depth, periodontal inflamed surface area (PISA), and serum and/or GCF proinflammatory cytokines level. Most of the studies focused on the local administration (n = 25) of statins (Table 6), while 7 investigated the impact of systemic route (Table 7). Essentially, effects of statins have been evaluated as an adjunct to both nonsurgical and surgical treatments, mainly in the context of chronic periodontitis in healthy patients.
Table 6.
Clinical studies evaluating the impact of local statin administration on periodontal wound healing.
| Local drug delivery | |||||
|---|---|---|---|---|---|
| Reference Study area Type of study |
Drug Mode of delivery Dose |
Number of patients Periodontal status Type of patients |
Type of treatment Study design (groups) Follow-up |
Results | Periodontal considerations |
| [130] (India) RCT with split-mouth design |
Simvastatin in methylcellulose gel 1.2 g of SIM |
30 Periodontitis (Armitage 1999) Healthy patients (nonsmokers) Sites with periodontal pocket measuring ≥ 5 mm and vertical bone loss ≥ 2 mm in different quadrants of the mouth |
Nonsurgical treatment Group I: SRP + placebo gel Group II: SRP + SIM gel 6 months follow-up |
All subjects tolerated the drug ↗ periodontal parameters with or without SIM ↗ CAL (p = 0.02) ↗ INFRA 2 (p < 0.01) ↘ PD significantly (p = 0.04) ↘ INFRA 1 (p < 0.01) |
Simvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [23] (India) RCT |
Rosuvastatin 1.2% rosuvastatin (RSV) gel |
90 Chronic periodontitis Healthy patients (nonsmokers) |
Nonsurgical treatment Groups I: SRP + placebo gel Group II: SRP + 1.2% RSV gel Group III: SRP + 1% MF gel 12 months follow-up |
↗ CAL ↘ PD significant ↗ bone fill ↘ PI ↘ mSBI ↘ DDR |
Rosuvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [102] (India) RCT |
Atorvastatin and rosuvastatin 1.2% atorvastatin or 1.2% rosuvastatin gel local drug delivery (1.2 mg/0.1 mL) |
90 No data Healthy patients (nonsmokers) Mandibular class II furcation defects with PD ≥ 5 mm and horizontal PD ≥ 3 mm |
Nonsurgical treatment Group I: SRP + placebo Group II: SRP + 1.2% RSV gel Group III: SRP + 1.2% ATV gel 9 month follow-up |
↘ PI and mSBI in all groups The 2 statins lead to the following: ↘ PD ↗ mean gain in CAL ↗ mean percentage of DDR Statistically greater results for RSV than for ATV |
Statins increased periodontal regeneration and CAL gain |
|
| |||||
| [103] (India) Cohort study |
Simvastatin SIM gel (1.2 mg/0.1 mL) |
50 Chronic periodontitis Healthy patients (nonsmokers) |
Nonsurgical treatment Group I: SRP alone Group II: SRP + SIM gel 3 months follow-up |
↘ IL-6 and IL-8 ↗ IL-10 significantly ↘ PI, mSBI, and PD No effect on CAL |
Simvastatin gel decreased periodontal inflammation and promote periodontal regeneration |
|
| |||||
| [21] (India) RCT |
Simvastatin 1.2% simvastatin gel |
46 Chronic periodontitis Healthy patients (nonsmokers) |
Nonsurgical treatment Group I: SRP Group II: SRP + SIM gel 45 days follow-up |
↘ PI, GI, and SBI No significant difference for PD and CAL ↘ mean IL-6 levels No significant difference for IL-8 levels |
Simvastatin gel decreased periodontal inflammation |
|
| |||||
| [104] (India) Cohort study with split-mouth design |
Simvastatin Combination of DFDBA and a 10−8 M solution of the drug simvastatin |
15 No data Healthy patients (nonsmokers) Identical bilateral infrabony defect |
Surgical treatment (Kirkland flap) Group A: DFDBA alone Group B: DFDBA + SIM 24 weeks follow-up |
↘ PD ↗ mean gain in CAL (better with DFDBA + SIM) ↘ infrabony defect depth (greater reduction with DFDBA + SIM) ↗ linear defect fill (better with DFDBA + SIM) |
Simvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [148] (India) RCT |
Atorvastatin 1.2% atorvastatin gel (ATV gel (1.2 mg/0.1 mL) |
75 Well-controlled type 2 diabetic patients (nonsmokers) Chronic periodontitis |
Nonsurgical treatment Group 1: SRP + ATV Group 2: SRP + placebo 9 months follow-up |
↗ mSBI ↘ PD ↗ CAL gain ↘ IBD depth and DDR No significant difference for PI at all time intervals evaluated |
Atorvastatin increased periodontal regeneration |
|
| |||||
| [125] (India) RCT |
Atorvastatin 1.2% atorvastatin gel (ATV gel (1.2 mg/0.1 mL)) |
71 Smokers Chronic periodontitis |
Nonsurgical treatment Group 1: SRP + ATV Group 2: SRP + placebo 9 months follow-up |
↘ PD ↗ mean CAL gain ↘ mean percentage of DDR ↘ mSBI ↘ IBD depth No statistically significant difference in the site-specific PI score and full-mouth PI score between the groups at any visit |
Atorvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [105] (India) Cohort |
Atorvastatin 1.2% ATV gel |
96 Healthy patients (nonsmokers) Chronic periodontitis |
Surgical treatment Group I: OFD + PRF Group II: OFD + PRF + 1.2% ATV Group III: OFD alone 9 months follow-up |
ATV gel and PRF alone showed significantly the following: ↘ PD ↗ mean CAL gain ↘ IBD depth No statistically significant difference in PI and mSBI scores between the groups at 9 months |
Atorvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [101] (India) RCT |
Atorvastatin and simvastatin 10 mL of 1.2% ATV gel (1.2 mg/0.1 mL) and 10 mL of 1.2% SIM gel (1.2 mg/0.1 mL) |
96 Healthy patients (nonsmokers) Chronic periodontitis |
Nonsurgical treatment Group I: SRP + 1.2% ATV Group II: SRP + 1.2% SIM Group III: SRP + placebo 9 months follow-up |
The 2 statins lead to the following: ↘ PD ↘ mSBI ↘ IBD depth ↗ mean CAL gain Statistically greater results for ATV than for SIM for PD reduction, CAL gain and percentage of IBD reduction |
Atorvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [149] (India) RCT |
Simvastatin Single topical transmucosal injection 1.2 mg SIM |
60 Chronic periodontitis Healthy patients (nonsmokers) |
Nonsurgical treatment Group I: SRP + placebo Group II: SRP + SIM 6 months follow-up |
↘ mSBI ↘ mean PD ↗ mean CAL ↗ IBD fill ↘ GI |
Simvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [126] (India) RCT |
Simvastatin SIM 1.2 μg/inj. (0.12 μg/mm3) Methylcellulose gel |
72 Chronic periodontitis Healthy patients (nonsmokers) Mandibular buccal class II furcation defects |
Nonsurgical treatment Group I: SRP + placebo Group II: SRP + 1.2 mg SIM 6 months follow-up |
↘ SBI and PB ↗ CAL ↗ IBD fill |
Simvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [150] (India) RCT |
Atorvastatin 1.2% ATV methyl cellulose gel |
60 patients Chronic periodontitis Healthy patients (nonsmokers) |
Nonsurgical treatment Group I: SRP + 1.2% ATV Groups II: SRP + placebo gel 9 months follow-up |
↘ PD ↘ mSBI ↗ mean CAL gain ↗ IBD fill |
Simvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [151] (India) RCT |
Simvastatin 1.2% SIM gel |
38 Chronic periodontitis Well-controlled type II diabetes Nonsmokers |
Nonsurgical treatment Group I: SRP + SIM Group II: SRP + placebo 9 months follow-up |
↘ PD ↗ mean CAL gain ↗ mean radiographic bone fill ↘ mSBI |
Simvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [152] (India) RCT |
Rosuvastatin 1.2% rosuvastatin (RSV) gel |
65 Chronic periodontitis Healthy (nonsmokers) |
Nonsurgical treatment Group I: SRP + RSV Group II: SRP + placebo 6 months follow-up |
↘ mSBI ↘ PD ↗ mean CAL gain ↗ IBD fill |
Rosuvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [20] (India) RCT |
Atorvastatin + rosuvastatin 1.2% RSV and 1.2% ATV gel |
90 Chronic periodontitis Healthy (nonsmokers) |
Nonsurgical treatment Group I: SRP + placebo Group II: SRP + 1.2% RSV gel Group III: SRP + 1.2% ATV gel 9 months follow-up |
The 2 statins lead to the following: ↘ mSBI ↘ PD ↗ mean CAL gain ↗ IBD fill Statistically greater results for RSV than for ATV for PD reduction, CAL gain, IBD reduction, and msSBI reduction |
Atorvastatin and rosuvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [106] (India) RCT |
Rosuvastatin 1.2% RSV gel |
90 Chronic periodontitis Healthy (nonsmokers) |
Surgical treatment 2/3-walled intrabony defects Group I: OFD alone Group II: OFD + PRF Group III: OFD + PRF + 1.2% RSV gel 9 months follow-up |
↘ PD ↗ mean CAL gain ↗ IBD fill |
Rosuvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [107] (India) RCT |
Rosuvastatin 1.2% RSV gel |
110 Chronic periodontitis Healthy (nonsmokers) Mandibular degree II furcation defects |
Surgical treatment Group 1: OFD + placebo gel Group II: OFD + PRF + HA Group III: OFD + RSV 1.2 mg gel + PRF + HA 9 months follow-up |
↘ PD ↗ mean CAL gain ↗ IBD fill ↘ PI and mSBI |
Rosuvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [153] (India) RCT |
Atorvastatin 1.2% atorvastatin gel |
90 Chronic periodontitis Healthy patients (nonsmokers) Intrabony defect |
Nonsurgical treatment Group I: SRP + ALN Group II: SRP + 1.2% ATV Group III: SRP + placebo group 9 months follow-up |
↘ PD ↗ mean CAL gain ↗ IBD fill ↘ mSBI |
Local delivery of atorvastatin increased periodontal regeneration |
|
| |||||
| [154] (India) RCT |
Simvastatin 0.1 mL SIM gel (1.2 mg/0.1 mL) |
24 Aggressive periodontitis Healthy patients (nonsmokers) Intrabony defect |
Nonsurgical treatment Group I: SRP + placebo gel Group II: SRP + SIM gel 6 months follow-up |
↘ PD ↗ mean CAL gain ↗ IBD fill ↘ mSBI All patients tolerated the drug with no postapplication complications No statistically significant difference between groups I and II regarding PI |
Simvastatin increased periodontal regeneration |
|
| |||||
| [108] (India) RCT |
Simvastatin 1.2 mg Simvastatin gel |
20 Chronic periodontitis Healthy patients (nonsmokers) |
Surgical treatment PD ≥ 5 mm in the mandibular molar region bilaterally Group I: OFD + SIM Group II: OFD + placebo gel 9 months follow-up |
↗ IBD fill for group I Significant results at 9 months in both groups: ↘ GI, PD ↗ mean CAL gain |
Simvastatin increased periodontal regeneration |
|
| |||||
| [155] (India) RCT |
Simvastatin 10 μL prepared SIM gel (1.2 mg/0.1 mL) |
40 Chronic periodontitis Healthy patients Smokers only |
Nonsurgical treatment Group I: SRP + SIM 1.2% Group II: SRP + placebo 9 months follow-up |
↘ mSBI ↘ PD ↗ mean CAL gain ↗ IBD fill |
Simvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [156] (India) RCT |
Simvastatin 1.2% simvastatin gel |
60 Chronic periodontitis Healthy (nonsmokers) |
Nonsurgical treatment Group A: SRP + placebo Group B: SRP + SIM gel 6 months follow-up |
↘ mSBI and PD ↗ mean CAL gain ↗ IBD fill ↘ IL-6 levels |
This study showed the efficacy of SIM as a local drug delivery system in the treatment of chronic periodontitis not only in clinical but also in molecular levels |
|
| |||||
| [137] (Chile) RCT |
Atorvastatin 2% atorvastatin dentifrice |
36 Chronic periodontitis Controlled diabetic only All types of smoking status |
Nonsurgical treatment Group I: SRP + ATV dentifrice Group II: SRP + placebo dentifrice 1 month follow-up |
↘ PISA ↘ mean PD ↘ % of sites with PD ≥ 5 mm ↗ mean CAL gain ↘ % of sites with CAL ≥ 5 mm ↘ BOP ↘ GI |
Simvastatin increased periodontal regeneration and CAL gain |
|
| |||||
| [100] (India) Cohort study |
Atorvastatin + simvastatin Drug in sodium alginate suspension administered with calcium chloride solution, subgingival delivery 1.2% simvastatin, or 1.2% atorvastatin |
45 Moderate to severe chronic periodontitis Healthy (nonsmokers) |
Nonsurgical treatment Group I: SRP alone Group II: SRP + 1.2% SIM Group III: SRP + 1.2% ATV 6 months follow-up |
The test groups did not show any statistically significant difference when compared with the control group | No significant benefit for periodontal regeneration with the use of statin |
Table 7.
Clinical studies evaluating impact of systemic statin administration on periodontal wound healing.
| Systemic drug delivery | |||||
|---|---|---|---|---|---|
| Reference Study area Type of study |
Drug Mode of delivery Dose |
Number of patients Periodontal status Type of patients |
Type of treatment Study design (groups) Follow-up |
Results | Periodontal considerations |
| [109] (USA) Retrospective cohort study |
Not reported | 1021 Chronic periodontal disease All types of patients (diabetic, smokers, antibiotic users, anti-inflammatory users…) |
Nonsurgical treatment Hyperlipidemic vs healthy Mean follow-up = 7.1 years |
Any statin use during the first 3 years after the initial periodontal exam was associated with a 48% decreased tooth loss rate in year 4 and subsequent years | Statins reduced tooth loss in chronic periodontitis |
|
| |||||
| [112] (Mexico) RCT |
Atorvastatin 20 mg/day |
38 Chronic periodontitis Healthy (all types of smoking status) |
Nonsurgical treatment Group I: SRP + ATV Group II: SRP + placebo 3 months follow-up |
↘ dental mobility ↘ distance from the crestal alveolar bone to the cementoenamel junction |
Atorvastatin reduced tooth mobility and bone loss |
|
| |||||
| [110] (Turkey) No control group Longitudinal |
Atorvastatin 10 or 20 mg |
20 Chronic periodontitis Hyperlipidemic patients (nonsmokers) |
Nonsurgical treatment SRP 6 months follow-up |
↘ median values for the PI, GI, PD, and BOP (%) ↗ median value of CAL gain All lipid parameters decreased after the periodontal treatment No comparison with the control group |
Atorvastatin reduced periodontal breakdown Improved periodontal health may influence metabolic control of hyperlipidemia |
|
| |||||
| [113] (Turkey) Cohort study |
Atorvastatin 10 or 20 mg |
80 Chronic periodontitis Healthy or hyperlipidemic patients (nonsmokers) |
Nonsurgical treatment Group I: healthy patient + SRP Group II: hyperlipidemic patients + prescribed diet (HD) Group III: hyperlipidemic patients + atorvastatin (HS) 3 months follow-up |
↗ BOP ↘ IL-6 (serum and GCF) ↘ TNF-α (GCF) levels |
Systemic atorvastatin had beneficial effects on periodontal inflammation |
|
| |||||
| [111] (Germany) Cohort study |
Simvastatin (n = 87), lovastatin (n = 27), pravastatin (n = 53), fluvastatin (n = 37), atorvastatin (n = 34), and cerivastatin (n = 42) | 2689 All types of periodontal disease Hyperlipidemic vs normolipidemic All types of smoking status |
All types of periodontal treatment Group I: participants undergoing statin treatment Group II: patients without statins 5.3 years mean follow-up |
No effect on PD and CAL ↘ tooth loss |
Statins had the beneficial effect of protecting against tooth loss |
|
| |||||
| [56] (USA) Cohort study |
Simvastatin Not reported |
117 Chronic periodontitis Diabetic vs healthy All types of smoking status |
Nonsurgical treatment Group I: nondiabetic patients not taking statin Group II: nondiabetic patients taking statin Group III: diabetic patients not taking statin Group IV: diabetic patients taking statin 6 weeks follow-up |
↘ PD in diabetic patients ↗ CAL in nondiabetic patients ↘ MMP-1 level in GCF of nondiabetic and diabetic patients No difference was found for MMP-8 and MMP-9 levels in GCF |
Statin intake was associated with reduced PD in diabetic patients and MMP-1 level in GCF in either nondiabetic or diabetic patients |
|
| |||||
| [114] (India) Cohort study |
Atorvastatin 20 mg/day |
107 Chronic periodontitis Hyperlipidemic vs normolipidemic Nonsmokers |
Nonsurgical periodontal treatment Group 1: hyperlipidemic + SIM Group 2: hyperlipidemic + diet Group 3: normolipidemic patients 3 months follow-up |
↘ GI Mean change in PD is negatively associated with LDL-C Mean change in GI is positively associated with HDL-C |
Patients with hyperlipidemia were more prone to periodontal disease Statin intake had beneficial effects on periodontal inflammation |
3.6. Statins as a Local Adjunct to Nonsurgical Periodontal Treatment
The effect of local delivery of statins as an adjunct to nonsurgical periodontal therapy (SRP) was studied in 20 clinical trials (Table 6). Atorvastatin and simvastatin have been the most commonly studied statins. Amongst the identified studies, 13 demonstrated a significant PD reduction, CAL gain, and IBD fill in healthy patients, 2 in well-controlled type II diabetes patients, and 3 in smokers. At contrary, in 2 studies, the test groups using atorvastatin or simvastatin did not show any significant differences when compared with the control [21, 100]. For instance, with simvastatin, the mean PD gain was 1.23 ± 0.57 mm for the control group versus 1.83 ± 0.07 mm for the test group (p = 0,112) and the mean CAL gain was 2.09 ± 0.08 mm for the control group versus 2.43 ± 0.01 mm for the test group (p = 0.889) after 45 days. Nevertheless, authors found a statistically significant reduction of PI, BOP, IL-6, and IL-8 levels [21].
Only 4 studies compared the outcomes obtained with more than one statin; however, contradictory results were observed. For instance, one study did not show any significant difference between atorvastatin and simvastatin [100], whereas better results were obtained with atorvastatin in another study [101]. Nevertheless, two studies highlighted greater efficacy with rosuvastatin in comparison with atorvastatin [20, 102].
Interestingly, studies that have investigated the effects of statin treatment on the biological markers from GCF showed that simvastatin administration reduced significantly IL-6, IL-8 and increased the anti-inflammatory IL-10 [21, 100, 103].
3.7. Statins as a Local Adjunct to Surgical Periodontal Treatment
Statins have also been inspected for their role in the surgical treatment outcomes. In all identified studies where statins (simvastatin, atorvastatin, and rosuvastatin) were locally administered concomitant to surgical approach (including the use of biomaterials or PRF), a significant reduction of PD, improvement of CAL, and bone defect fill was achieved in the test group in comparison to the control group [104–108] (Table 6). Amongst these studies, the mean difference of PD between the test and control groups ranged from 1.3 ± 0.21 mm to 2.51 ± 0.22 mm (p < 0.001). Thus, the mean difference of CAL between the test and control groups ranged from 1.16 ± 0.09 mm to 2.35 ± 0.08 (p < 0.001). Moreover, the mean difference of bone defect fill between the test and control groups ranged from 1.336 ± 0.714 to 3.08 ± 0.07 (p < 0.001).
3.8. Impact of Systemic Administration of Statins on Nonsurgical Periodontal Treatment Outcomes
The impact of systemic administration of statins on nonsurgical periodontal treatment outcomes was evaluated in a few studies (Table 7). From the 7 studies identified, 4 demonstrated significant improvements regarding reduction of PD, CAL gain, and/or tooth loss in comparison to the control group [56, 109–111]. At contrary, 3 other studies did not show any significant differences in periodontal outcomes between the statin-treated and control groups [112–114]. These discrepancies could be due to the very short follow-up of the abovementioned 3 studies (3 months) compared to the other ones (from 3 months to 7 years follow-up). Moreover, one of the studies did not compare the treatment group with a control group [110].
4. Discussion
Statins exhibit multiple effects, including modulation of inflammatory-immune crosstalk, bone regeneration, and antibacterial activity, to promote periodontal wound healing and regeneration (Figure 5). They act through several closely interrelated pathways highlighting potential therapeutic targets. The hydrophobic or hydrophilic nature of statins determines their efficacy, action on periodontal pathogens, and treatment response and appears to be largely cell and tissue dependent [69, 78]. Further insight into this may help selecting the best statin.
Figure 5.
Pleiotropic effects of statins in the context of periodontitis management. Statin biological properties might be of interest for the management of periodontitis as they act on each tissular compartment and mechanisms including inflammatory-immune crosstalk, bone metabolism and bacterial clearance.
Moreover, the mode of statin delivery also affects the treatment outcomes. Oral systemic administration of statins reduces periodontal inflammation and consequent tooth loss [111] but the low resultant dose available to the tissues after hepatic bypass renders them relatively less efficacious [60]. On the other hand, a higher dose to enhance efficacy can manifest systemic side effects such as statin-induced myopathy, hepatotoxicity, nephrotoxicity, pulmonary manifestations, ophthalmological manifestations, gastrointestinal hemorrhage risk, and oral manifestations (dryness, itch, bitterness, and cough) [115, 116]. Therefore, to avoid these side effects, various local application strategies have been tested that allow site-specific delivery reducing the required dose, frequency of application, and bioavailability in the blood [60, 117, 118], concomitantly improving patient compliance [119].
The development and selection of an optimal statin delivery carrier are crucial as it enhances the statin retention on the lesion and acts as a scaffold for cell growth and differentiation [120]; therefore, it should be capable to withstand the oral environment, continuous fluid exchange inside the pocket, and salivary influx.
Several studies demonstrate that anti-inflammatory properties of statins vary according to the type and dose of statin used [121]. On a cellular level, modulation of macrophage polarization from a proinflammatory M1 to a proresolution M2 phenotype by systemic delivery of immune modulatory drugs resolved persistent inflammation associated with chronic periodontitis [122]. In this context, statins' ability to switch M1 to M2 to promote periodontal wound healing and regeneration needs to be explored. Furthermore, it is yet to be established if statin-induced reduction in plasma total cholesterol and LDL cholesterol levels in the periodontal space could decrease macrophage recruitment to improve the treatment outcome.
Despite the documented anti-inflammatory properties of statins, a local high-dose statin application causes considerable soft tissue inflammation [123]. Accordingly, studies determined that reducing the simvastatin dose from 2.2 mg to 0.5 mg reduced inflammation without compromising its bone growth potential [67]. A 10 mg/kg/day dose in rats is equivalent to 70 mg/day for humans, so it is a high systemic dose compared to that commonly used in clinical practice (20-40 mg/day) [124].
Concerning locally applied statins, most clinical studies investigated the 1.2% dose (mainly atorvastatin, simvastatin, and rosuvastatin) [20, 23, 125, 126]. Therefore, other doses should be tested to compare efficacy.
Most of the review articles have focused on the use of statins as adjunct to the nonsurgical SRP in clinical settings [127–129]. Here, this review encompasses the use of statins (local, systemic, or combination), alone or in addition to other drugs or scaffolds, in nonsurgical or surgical periodontal treatment in vitro, in vivo, and in clinical trials. However, the potential of statins in surgical periodontal therapy remains relatively less explored except for a few studies where treatment outcomes were improved, primarily, with the combination of some other regenerative agents such as allograft or PRF [105, 106]. Cognizant of the numerous studies involving statins, not all statin types have been studied so far; thus, exploring all natural and synthetic statins to compare their efficacy and safety could be instrumental.
Notably, 17 out of 32 clinical studies were carried out by the same group of researchers on similar population; therefore, generalizations should be drawn with caution. Additionally, in most studies involving statins, the follow-up period was no longer than 9 months [103, 130]. Hence, it is imperative to follow clinical studies for periods longer than those commonly investigated so as to achieve a deeper and more genuine insight into their long-term benefits. Discrepancies amongst outcomes between time points are of importance to clearly conclude. For instance, the meta-analysis performed by Sinjab et al. [131] declared the outcomes of the control group of a study [20] to be better by considering the data up to 6 months follow-up, whereas the meta-analysis performed by Ambrósio et al. regarded the treatment group of the same study to have better outcomes as the follow-up data until 9 months was taken into account [132].
Moreover, the studies carried out so far mainly involved hyperlipidemic patients, diabetic patients, or smokers. Systemic diseases, such as obesity or metabolic syndrome, have been linked with periodontitis [133]. It has been demonstrated that such conditions modify significantly the host response to periodontal pathogens [134] but also could impaired treatment response. For instance, in a rat model of metabolic syndrome, the effects induced by statins in rats with metabolic syndrome were different in comparison with rats without [32] highlighting the potential modulation of pharmacologic effect due to the systemic condition. Even if clinical trials performed in diabetes patients or exhibiting hyperlipidemia showed promising results when statins were administered concomitantly to nonsurgical periodontal treatment [56, 110, 113, 114], more studies are required to better understand the differential biological mechanisms modulated by statin's administration. It would also be of importance to assess statins' tolerance and efficacy in subjects with different systemic conditions where periodontal treatment response is impaired (e.g., liver diseases, kidney dysfunction, and immunocompromised states).
In clinical trials, the local application of statins with surgical periodontal treatment always showed significant improvements in periodontal parameters [105, 106]. However, in vivo, statin application in ACP models showed contradictory results [99] which could be explained by the limitations of animal models to simulate conditions identical to human periodontal disease. Nevertheless, as a direct optimization of treatment protocols in humans is not ethically permissible, the utility of preclinical models to get directions and overall assessment of the expected treatment outcomes in clinical scenarios cannot be undermined.
Concerning the systemic administration of statins, a study reported that using a combination of two pharmacokinetically different statins (20 mg/day of atorvastatin plus 40 mg/day of pravastatin) in hyperlipidemic patients for one year improved their lipid profiles compared to those on monotherapies [135]. Besides, a case of a hyperlipidemic patient experiencing certain side effects with a high dose of systemic simvastatin who could well tolerate a combination of reduced doses of simvastatin and rosuvastatin instead has also been reported [136]. To the best of our knowledge, no two statins have been combined for periodontal treatment so far; nonetheless, combination of two statins could be tested for its impact on periodontal treatment response.
Likewise, the impact of incorporating statins with antimicrobial agents, growth factors, or other proregenerative molecules within a local application system could be studied as adjunct to SRP. Statin integration into gels [21] or dentifrice [137] could enhance ease of application and patient's compliance and could be potentially beneficial in the maintenance phase to counter periodontal breakdown that persists after conventional periodontal treatment. The literature does not report the impact of statins on patients with extremely poor oral hygiene; nonetheless, it could be interesting to explore the impact of statins on oral hygiene indicators.
5. Conclusion
Statins have been studied in depth in the context of bone regeneration, but soft tissue healing remains relatively less explored. Further research into it could present statins as a potential adjunctive therapeutic strategy with a positive impact on both hard and soft periodontal tissue healing. Furthermore, the impact of statins on proresolution molecules has not been investigated in the context of periodontal wound healing and regeneration. This could unveil new vistas for statins as regenerative therapeutics. Since all available statins have not been tested yet, new studies need to evaluate the impact of other statins on antibacterial, inflammatory, immune, and osteoprogenitor responses. To conclude, choosing an optimum dose of statins, based on the mode of drug delivery and the carrier employed, may enhance the positive impact of statins on the periodontal treatment outcomes. Moreover, combining statins with growth factors or other drugs in an efficient carrier system may be beneficial to promote periodontal regeneration.
Acknowledgments
The authors are very grateful to the Agence Nationale de la Recherche (The French National Research Agency) (nos. ANR-14-CE16-0025-04 and ANR-17-CE17-0024-01) for their valuable support.
Abbreviations
- M1:
First molar
- M2:
Second molar
- M3:
Third molar
- mPEG:
Polyethylene glycol monomethyl ether
- PDLLA-PLGA:
Poly-(d,l-lactide) and poly-(d,l-lactide-co-glycolide
- BSA:
Bovine serum albumin
- PDGF:
Platelet-derived growth factor
- PM:
Premolar
- PDL:
Periodontal ligament cells
- EGCG:
Epigallocatechin-3-gallate
- CS:
Chitosan
- BALP:
Bone alkaline phosphatase
- LPS:
Lipopolysaccharide
- PBS:
Phosphate buffered saline
- ALN-CD:
Alendronate-β-cyclodextrin
- SIM:
Simvastatin
- CEJ:
Cementoenamel junction
- HA:
Hydroxyapatite
- TGF-β:
Transforming growth factor beta
- E. coli:
Escherichia coli
- PPi:
Isopropyl alcohol
- TRAP:
Tartrate-resistant acid phosphatase
- GSH:
Glutathione
- MDA:
Malondialdehyde
- MPO:
Myeloperoxidase
- GIOP:
Glucocorticoid-induced osteoporosis
- DKK1:
Dickkopf-related protein
- CAT:
Enzyme catalase
- SOD:
Enzyme superoxide dismutase
- MMPs:
Matrix metalloproteinases
- MCP:
Monocyte chemotactic protein
- CSF:
Colony-stimulating factor
- A.a:
Aggregator actinomycetemcomitans
- P.g:
Porphyromonas gingivalis
- COX:
Cyclooxygenase
- ALP:
Alkaline phosphatase
- AST:
Aspartate aminotransferase
- ALT:
Alanine aminotransferase
- IP:
Intraperitoneal
- TG:
Triglyceride
- ATV:
Atorvastatin
- PD:
Pocket depth
- RANKL:
Receptor activator of the NF-κB ligand
- RANK:
Receptor activator of NF-κB
- OPG:
Osteoprotegerin
- OPN:
Osteopontin
- BV/TV:
Bone volume/tissue volume
- CAL:
Clinical attachment level
- SRP:
Scaling and root planing
- INFRA:
Radiographic infrabony defect fill
- MF:
Metformin
- DDR:
Defect depth reduction
- DFDBA:
Demineralized freeze-dried bone allograft
- OFD:
Open flap debridement
- BOP:
Bleeding on probing
- GI:
Gingival index
- PI:
Plaque index
- mSBI:
Modified sulcus bleeding index
- IBD:
Intrabony defect
- PRF:
Platelet-rich fibrin
- PISA:
Periodontal inflamed surface area
- LDL-C:
Low-density lipoprotein cholesterol
- HDL-C:
High-density lipoprotein cholesterol
- OB:
Osteoblasts
- OC:
Osteoclasts
- EIP:
Experimentally induced periodontitis
- ACP:
Acute/chronic periodontal defect
- NOX:
Nitrate/nitrite levels
- VEGF:
Vascular endothelial growth factor.
Conflicts of Interest
The authors declare no conflicts of interest related to this study.
Authors' Contributions
CP and FB performed the electronic search and drafted the manuscript. OH drafted and critically revised the manuscript. IB, PS, and NB-J critically revised the manuscript. All authors reviewed the final version of the manuscript. Catherine Petit and Fareeha Batool contributed equally to this work.
Supplementary Materials
Risk of bias assessment of included clinical studies.
References
- 1.Kinane D. F., Stathopoulou P. G., Papapanou P. N. Periodontal diseases. Nature Reviews Disease Primers. 2017;3, article 17038 doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 2.Cekici A., Kantarci A., Hasturk H., van Dyke T. E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000. 2014;64(1):57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen I., Lambris J. D., Hajishengallis G. Porphyromonas gingivalis disturbs host–commensal homeostasis by changing complement function. Journal of Oral Microbiology. 2017;9(1, article 1340085) doi: 10.1080/20002297.2017.1340085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobb C. M. Clinical significance of non-surgical periodontal therapy: an evidence-based perspective of scaling and root planing. Journal of Clinical Periodontology. 2002;29(Supplement 2):22–32. doi: 10.1034/j.1600-051X.29.s2.4.x. [DOI] [PubMed] [Google Scholar]
- 5.Agossa K., Morand D. N., Tenenbaum H., Davideau J. L., Huck O. Systemic application of anti-inflammatory agents in periodontal treatment. Clinical Anti-Inflammatory & Anti-Allergy Drugs. 2016;2(1):3–13. doi: 10.2174/221270380201160517185229. [DOI] [Google Scholar]
- 6.Akram Z., Abduljabbar T., Kellesarian S. V., Abu Hassan M. I., Javed F., Vohra F. Efficacy of bisphosphonate as an adjunct to nonsurgical periodontal therapy in the management of periodontal disease: a systematic review. British Journal of Clinical Pharmacology. 2017;83(3):444–454. doi: 10.1111/bcp.13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alshammari A., Patel J., al-Hashemi J., et al. Kava-241 reduced periodontal destruction in a collagen antibody primed Porphyromonas gingivalis model of periodontitis. Journal of Clinical Periodontology. 2017;44(11):1123–1132. doi: 10.1111/jcpe.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gokhale S. R., Padhye A. M. Future prospects of systemic host modulatory agents in periodontal therapy. British Dental Journal. 2013;214(9):467–471. doi: 10.1038/sj.bdj.2013.432. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Cabezas R., Davideau J. L., Tenenbaum H., Huck O. Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: a systematic review and meta-analysis. Journal of Clinical Periodontology. 2016;43(6):520–530. doi: 10.1111/jcpe.12545. [DOI] [PubMed] [Google Scholar]
- 10.Estanislau I. M. G., Terceiro I. R. C., Lisboa M. R. P., et al. Pleiotropic effects of statins on the treatment of chronic periodontitis - a systematic review. British Journal of Clinical Pharmacology. 2015;79(6):877–885. doi: 10.1111/bcp.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin-Ruiz E., Olry-de-Labry-Lima A., Ocaña-Riola R., Epstein D. Systematic review of the effect of adherence to statin treatment on critical cardiovascular events and mortality in primary prevention. Journal of Cardiovascular Pharmacology and Therapeutics. 2018;23(3):200–215. doi: 10.1177/1074248417745357. [DOI] [PubMed] [Google Scholar]
- 12.Pasin L., Landoni G., Castro M. L., et al. The effect of statins on mortality in septic patients: a meta-analysis of randomized controlled trials. PLoS One. 2013;8(12, article e82775) doi: 10.1371/journal.pone.0082775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fong C. W. Statins in therapy: understanding their hydrophilicity, lipophilicity, binding to 3-hydroxy-3-methylglutaryl-CoA reductase, ability to cross the blood brain barrier and metabolic stability based on electrostatic molecular orbital studies. European Journal of Medicinal Chemistry. 2014;85:661–674. doi: 10.1016/j.ejmech.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 14.Margaritis M., Sanna F., Antoniades C. Statins and oxidative stress in the cardiovascular system. Current Pharmaceutical Design. 2017;23(46):7040–7047. doi: 10.2174/1381612823666170926130338. [DOI] [PubMed] [Google Scholar]
- 15.Sakoda K., Yamamoto M., Negishi Y., Liao J. K., Node K., Izumi Y. Simvastatin decreases IL-6 and IL-8 production in epithelial cells. Journal of Dental Research. 2006;85(6):520–523. doi: 10.1177/154405910608500608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitaker E. J., Alshammari A. Bacteriostatic effect of simvastatin on selected oral streptococci in vitro. Contemporary Clinical Dentistry. 2017;8(1):59–63. doi: 10.4103/ccd.ccd_848_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeiser R. Immune modulatory effects of statins. Immunology. 2018;154(1):69–75. doi: 10.1111/imm.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou H., Xie Y., baloch Z., Shi Q., Huo Q., Ma T. The effect of atorvastatin, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor (HMG-CoA), on the prevention of osteoporosis in ovariectomized rabbits. Journal of Bone and Mineral Metabolism. 2017;35(3):245–254. doi: 10.1007/s00774-016-0750-2. [DOI] [PubMed] [Google Scholar]
- 19.Cicek Ari V., Ilarslan Y. D., Erman B., et al. Statins and IL-1β, IL-10, and MPO levels in gingival crevicular fluid: preliminary results. Inflammation. 2016;39(4):1547–1557. doi: 10.1007/s10753-016-0390-7. [DOI] [PubMed] [Google Scholar]
- 20.Pradeep A. R., Garg V., Kanoriya D., Singhal S. 1.2% rosuvastatin versus 1.2% atorvastatin gel local drug delivery and redelivery in treatment of intrabony defects in chronic periodontitis: a randomized placebo-controlled clinical trial. Journal of Periodontology. 2016;87(7):756–762. doi: 10.1902/jop.2016.150706. [DOI] [PubMed] [Google Scholar]
- 21.Gunjiganur Vemanaradhya G., Emani S., Mehta D. S., Bhandari S. Effect of 1.2% of simvastatin gel as a local drug delivery system on gingival crevicular fluid interleukin-6 & interleukin-8 levels in non surgical treatment of chronic periodontitis patients. Archives of Oral Biology. 2017;82:55–61. doi: 10.1016/j.archoralbio.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Özdoğan A. I., İlarslan Y. D., Kösemehmetoğlu K., et al. In vivo evaluation of chitosan based local delivery systems for atorvastatin in treatment of periodontitis. International Journal of Pharmaceutics. 2018;550(1-2):470–476. doi: 10.1016/j.ijpharm.2018.08.058. [DOI] [PubMed] [Google Scholar]
- 23.Pankaj D., Sahu I., Kurian I. G., Pradeep A. R. Comparative evaluation of subgingivally delivered 1.2% rosuvastatin and 1% metformin gel in treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. Journal of Periodontology. 2018;89(11, article 16):1318–1325. doi: 10.1002/JPER.17-0434. [DOI] [PubMed] [Google Scholar]
- 24.Graves D. T., Li J., Cochran D. L. Inflammation and uncoupling as mechanisms of periodontal bone loss. Journal of Dental Research. 2011;90(2):143–153. doi: 10.1177/0022034510385236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kırzıoğlu F. Y., Tözüm Bulut M., Doğan B., et al. Anti-inflammatory effect of rosuvastatin decreases alveolar bone loss in experimental periodontitis. Journal of Oral Science. 2017;59(2):247–255. doi: 10.2334/josnusd.16-0398. [DOI] [PubMed] [Google Scholar]
- 26.Sousa L. H., Linhares E. V. M., Alexandre J. T., et al. Effects of atorvastatin on periodontitis of rats subjected to glucocorticoid-induced osteoporosis. Journal of Periodontology. 2016;87(10):1206–1216. doi: 10.1902/jop.2016.160075. [DOI] [PubMed] [Google Scholar]
- 27.Pahan K. Lipid-lowering drugs. Cellular and Molecular Life Sciences. 2006;63(10):1165–1178. doi: 10.1007/s00018-005-5406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazzerini P. E., Capperucci C., Spreafico A., et al. Rosuvastatin inhibits spontaneous and IL-1β-induced interleukin-6 production from human cultured osteoblastic cells. Joint, Bone, Spine. 2013;80(2):195–200. doi: 10.1016/j.jbspin.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Arora M., Chen L., Paglia M., et al. Simvastatin promotes Th2-type responses through the induction of the chitinase family member Ym1 in dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(20):7777–7782. doi: 10.1073/pnas.0508492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mira E., Leon B., Barber D. F., et al. Statins induce regulatory T cell recruitment via a CCL1 dependent pathway. The Journal of Immunology. 2008;181(5):3524–3534. doi: 10.4049/jimmunol.181.5.3524. [DOI] [PubMed] [Google Scholar]
- 31.Fernandes de Araújo R., Oliveira Souza T., Moreno de Moura L., et al. Atorvastatin decreases bone loss, inflammation and oxidative stress in experimental periodontitis. PLoS One. 2013;8(10, article e75322) doi: 10.1371/journal.pone.0075322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin J., Machado E. R., Yu H., et al. Simvastatin inhibits LPS-induced alveolar bone loss during metabolic syndrome. Journal of Dental Research. 2014;93(3):294–299. doi: 10.1177/0022034513516980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin J., Zhang X., Lu Z., et al. Simvastatin inhibits lipopolysaccharide-induced osteoclastogenesis and reduces alveolar bone loss in experimental periodontal disease. Journal of Periodontal Research. 2014;49(4):518–526. doi: 10.1111/jre.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machado W. M., Prestes A. P., Costa T. P., et al. The effect of simvastatin on systemic inflammation and endothelial dysfunction induced by periodontitis. Journal of Periodontal Research. 2014;49(5):634–641. doi: 10.1111/jre.12145. [DOI] [PubMed] [Google Scholar]
- 35.Nassar P. O., Nassar C. A., Guimarães M. R., et al. Simvastatin therapy in cyclosporine A-induced alveolar bone loss in rats. Journal of Periodontal Research. 2009;44(4):479–488. doi: 10.1111/j.1600-0765.2008.01143.x. [DOI] [PubMed] [Google Scholar]
- 36.Morand D.-N., Davideau J. L., Clauss F., Jessel N., Tenenbaum H., Huck O. Cytokines during periodontal wound healing: potential application for new therapeutic approach. Oral Diseases. 2017;23(3):300–311. doi: 10.1111/odi.12469. [DOI] [PubMed] [Google Scholar]
- 37.Van Dyke T. E., Hasturk H., Kantarci A., et al. Proresolving nanomedicines activate bone regeneration in periodontitis. Journal of Dental Research. 2015;94(1):148–156. doi: 10.1177/0022034514557331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasturk H., Kantarci A., Ohira T., et al. RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. The FASEB Journal. 2006;20(2):401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 39.Spite M., Serhan C. N. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circulation Research. 2010;107(10):1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goes P., Melo I. M., Silva L. M. C. M., et al. Low-dose combination of alendronate and atorvastatin reduces ligature-induced alveolar bone loss in rats. Journal of Periodontal Research. 2014;49(1):45–54. doi: 10.1111/jre.12077. [DOI] [PubMed] [Google Scholar]
- 41.Kagami S.-I., Owada T., Kanari H., et al. Protein geranylgeranylation regulates the balance between Th17 cells and Foxp3+ regulatory T cells. International Immunology. 2009;21(6):679–689. doi: 10.1093/intimm/dxp037. [DOI] [PubMed] [Google Scholar]
- 42.Mausner-Fainberg K., Luboshits G., Mor A., et al. The effect of HMG-CoA reductase inhibitors on naturally occurring CD4+CD25+ T cells. Atherosclerosis. 2008;197(2):829–839. doi: 10.1016/j.atherosclerosis.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 43.Hajishengallis G. Toll gates to periodontal host modulation and vaccine therapy. Periodontology 2000. 2009;51(1):181–207. doi: 10.1111/j.1600-0757.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S. . J., Qin H., Benveniste E. . N. The IFN-γ-induced transcriptional program of the CIITA gene is inhibited by statins. European Journal of Immunology. 2008;38(8):2325–2336. doi: 10.1002/eji.200838189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hajishengallis G., Sahingur S. E. Novel inflammatory pathways in periodontitis. Advances in Dental Research. 2014;26(1):23–29. doi: 10.1177/0022034514526240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montecucco F., Burger F., Pelli G., et al. Statins inhibit C-reactive protein-induced chemokine secretion, ICAM-1 upregulation and chemotaxis in adherent human monocytes. Rheumatology. 2009;48(3):233–242. doi: 10.1093/rheumatology/ken466. [DOI] [PubMed] [Google Scholar]
- 47.Weitz-Schmidt G., Welzenbach K., Brinkmann V., et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nature Medicine. 2001;7(6):687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 48.Korkmaz Y., Bloch W., Behrends S., Schroder H., Addicks K., Baumann M. A. NO-cGMP signaling molecules in the rat epithelial rests of Malassez. European Journal of Oral Sciences. 2004;112(1):55–60. doi: 10.1111/j.0909-8836.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 49.Tóthová L.'u., Celec P. Oxidative stress and antioxidants in the diagnosis and therapy of periodontitis. Frontiers in Physiology. 2017;8:p. 1055. doi: 10.3389/fphys.2017.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batista A. C., Silva T. A., Chun J. H., Lara V. S. Nitric oxide synthesis and severity of human periodontal disease. Oral Diseases. 2002;8(5):254–260. doi: 10.1034/j.1601-0825.2002.02852.x. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y.-S., Pi S. H., Lee Y. M., Lee S. I., Kim E. C. The anti-inflammatory role of heme oxygenase-1 in lipopolysaccharide and cytokine-stimulated inducible nitric oxide synthase and nitric oxide production in human periodontal ligament cells. Journal of Periodontology. 2009;80(12):2045–2055. doi: 10.1902/jop.2009.090145. [DOI] [PubMed] [Google Scholar]
- 52.Popkov V. L., Fil'chukova I. A., Lapina N. V., Galenko-Yaroshevskii V. P., Dukhanin A. S. Activity of nitric oxide synthase and concentration of nitric oxide end metabolites in the gingiva under experimental pathological conditions. Bulletin of Experimental Biology and Medicine. 2005;140(4):391–393. doi: 10.1007/s10517-005-0499-4. [DOI] [PubMed] [Google Scholar]
- 53.Herrera B. S., Martins-Porto R., Maia-Dantas A., et al. iNOS-derived nitric oxide stimulates osteoclast activity and alveolar bone loss in ligature-induced periodontitis in rats. Journal of Periodontology. 2011;82(11):1608–1615. doi: 10.1902/jop.2011.100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kırzıoğlu F. Y., Özmen Ö., Doğan B., Bulut M. T., Fentoğlu Ö., Özdem M. Effects of rosuvastatin on inducible nitric oxide synthase in rats with hyperlipidaemia and periodontitis. Journal of Periodontal Research. 2018;53(2):258–266. doi: 10.1111/jre.12513. [DOI] [PubMed] [Google Scholar]
- 55.Franco C., Patricia H.-R., Timo S., Claudia B., Marcela H. Matrix metalloproteinases as regulators of periodontal inflammation. International Journal of Molecular Sciences. 2017;18(2):p. 440. doi: 10.3390/ijms18020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poston C. J., Pierce T. C., Li Y., et al. Statin intake is associated with MMP-1 level in gingival crevicular fluid of patients with periodontitis. Oral Diseases. 2016;22(5):438–444. doi: 10.1111/odi.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balli U., Keles G. C., Cetinkaya B. O., Mercan U., Ayas B., Erdogan D. Assessment of vascular endothelial growth factor and matrix metalloproteinase-9 in the periodontium of rats treated with atorvastatin. Journal of Periodontology. 2014;85(1):178–187. doi: 10.1902/jop.2013.130018. [DOI] [PubMed] [Google Scholar]
- 58.Dalcico R., de Menezes A. M. A., Deocleciano O. B., et al. Protective mechanisms of simvastatin in experimental periodontal disease. Journal of Periodontology. 2013;84(8):1145–1157. doi: 10.1902/jop.2012.120114. [DOI] [PubMed] [Google Scholar]
- 59.Santos B. F. E., Souza E. Q. M., Brigagão M. R. P. L., Lima D. C. d., Fernandes L. A. Local application of statins in the treatment of experimental periodontal disease in rats. Journal of Applied Oral Science. 2017;25(2):168–176. doi: 10.1590/1678-77572016-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y., Bradley A. D., Wang D., Reinhardt R. A. Statins, bone metabolism and treatment of bone catabolic diseases. Pharmacological Research. 2014;88:53–61. doi: 10.1016/j.phrs.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 61.Chen P.-Y., Sun J.-S., Tsuang Y.-H., Chen M.-H., Weng P.-W., Lin F.-H. Simvastatin promotes osteoblast viability and differentiation via Ras/Smad/Erk/BMP-2 signaling pathway. Nutrition Research. 2010;30(3):191–199. doi: 10.1016/j.nutres.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 62.Yamashita M., Otsuka F., Mukai T., et al. Simvastatin antagonizes tumor necrosis factor-α inhibition of bone morphogenetic proteins-2-induced osteoblast differentiation by regulating Smad signaling and Ras/Rho-mitogen-activated protein kinase pathway. Journal of Endocrinology. 2008;196(3):601–613. doi: 10.1677/JOE-07-0532. [DOI] [PubMed] [Google Scholar]
- 63.Kim I. S., Jeong B. C., Kim O. S., et al. Lactone form 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) stimulate the osteoblastic differentiation of mouse periodontal ligament cells via the ERK pathway. Journal of Periodontal Research. 2011;46(2):204–213. doi: 10.1111/j.1600-0765.2010.01329.x. [DOI] [PubMed] [Google Scholar]
- 64.Maeda T., Matsunuma A., Kurahashi I., Yanagawa T., Yoshida H., Horiuchi N. Induction of osteoblast differentiation indices by statins in MC3T3-E1 cells. Journal of Cellular Biochemistry. 2004;92(3):458–471. doi: 10.1002/jcb.20074. [DOI] [PubMed] [Google Scholar]
- 65.Hu K., Olsen B. R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. Journal of Clinical Investigation. 2016;126(2):509–526. doi: 10.1172/JCI82585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reyes R., Rodríguez J. A., Orbe J., Arnau M. R., Évora C., Delgado A. Combined sustained release of BMP2 and MMP10 accelerates bone formation and mineralization of calvaria critical size defect in mice. Drug Delivery. 2018;25(1):750–756. doi: 10.1080/10717544.2018.1446473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stein D., Lee Y., Schmid M. J., et al. Local simvastatin effects on mandibular bone growth and inflammation. Journal of Periodontology. 2005;76(11):1861–1870. doi: 10.1902/jop.2005.76.11.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Z., Li Y., Zhou F., Piao Z., Hao J. Effects of statins on bone mineral density and fracture risk: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2016;95(22, article e3042) doi: 10.1097/MD.0000000000003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maeda T., Kawane T., Horiuchi N. Statins augment vascular endothelial growth factor expression in osteoblastic cells via inhibition of protein prenylation. Endocrinology. 2003;144(2):681–692. doi: 10.1210/en.2002-220682. [DOI] [PubMed] [Google Scholar]
- 70.Baek K. H., Lee W. Y., Oh K. W., et al. The effect of simvastatin on the proliferation and differentiation of human bone marrow stromal cells. Journal of Korean Medical Science. 2005;20(3):438–444. doi: 10.3346/jkms.2005.20.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y.-S., Ou M. E., Liu H., et al. The effect of simvastatin on chemotactic capability of SDF-1α and the promotion of bone regeneration. Biomaterials. 2014;35(15):4489–4498. doi: 10.1016/j.biomaterials.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 72.de Monès E., Schlaubitz S., Catros S., Fricain J. C. Statins and alveolar bone resorption: a narrative review of preclinical and clinical studies. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2015;119(1):65–73. doi: 10.1016/j.oooo.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 73.Chang P.-C., Dovban A. S., Lim L. P., Chong L. Y., Kuo M. Y., Wang C. H. Dual delivery of PDGF and simvastatin to accelerate periodontal regeneration in vivo . Biomaterials. 2013;34(38):9990–9997. doi: 10.1016/j.biomaterials.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 74.Lin L.-D., Lin S. K., Chao Y. L., et al. Simvastatin suppresses osteoblastic expression of Cyr61 and progression of apical periodontitis through enhancement of the transcription factor Forkhead/winged helix box protein O3a. Journal of Endodontics. 2013;39(5):619–625. doi: 10.1016/j.joen.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 75.Kaji H., Naito J., Inoue Y., Sowa H., Sugimoto T., Chihara K. Statin suppresses apoptosis in osteoblastic cells: role of transforming growth factor-β-Smad3 pathway. Hormone and Metabolic Research. 2008;40(11):746–751. doi: 10.1055/s-0028-1082051. [DOI] [PubMed] [Google Scholar]
- 76.Pokhrel N. K., Kim Y. G., Kim J. Y., Kim H. H., Lee Y. Fluvastatin inhibits osteoclast differentiation and Porphyromonas gingivalis lipopolysaccharide-induced alveolar bone erosion in mice. Journal of Periodontology. 2017;88(4):390–398. doi: 10.1902/jop.2016.160536. [DOI] [PubMed] [Google Scholar]
- 77.Evans K. E., Fox S. W. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biology. 2007;8(1):p. 4. doi: 10.1186/1471-2121-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hughes A., Rogers M. J., Idris A. I., Crockett J. C. A comparison between the effects of hydrophobic and hydrophilic statins on osteoclast function in vitro and ovariectomy-induced bone loss in vivo. Calcified Tissue International. 2007;81(5):403–413. doi: 10.1007/s00223-007-9078-1. [DOI] [PubMed] [Google Scholar]
- 79.Yamashita M., Otsuka F., Mukai T., et al. Simvastatin inhibits osteoclast differentiation induced by bone morphogenetic protein-2 and RANKL through regulating MAPK, AKT and Src signaling. Regulatory Peptides. 2010;162(1-3):99–108. doi: 10.1016/j.regpep.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 80.Chaudhry M. Z., Wang J. H., Blankson S., Redmond H. P. Statin (cerivastatin) protects mice against sepsis-related death via reduced proinflammatory cytokines and enhanced bacterial clearance. Surgical Infections. 2008;9(2):183–194. doi: 10.1089/sur.2006.077. [DOI] [PubMed] [Google Scholar]
- 81.Ko H. H. T., Lareu R. R., Dix B. R., Hughes J. D. In vitro antibacterial effects of statins against bacterial pathogens causing skin infections. European Journal of Clinical Microbiology & Infectious Diseases. 2018;37(6):1125–1135. doi: 10.1007/s10096-018-3227-5. [DOI] [PubMed] [Google Scholar]
- 82.Emani S., Gunjiganur G. V., Mehta D. S. Determination of the antibacterial activity of simvastatin against periodontal pathogens, Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans: an in vitro study. Contemporary Clinical Dentistry. 2014;5(3):377–382. doi: 10.4103/0976-237X.137959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bergman P., Linde C., Pütsep K., et al. Studies on the antibacterial effects of statins - in vitro and in vivo . PLoS One. 2011;6(8, article e24394) doi: 10.1371/journal.pone.0024394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liao J. K., Laufs U. Pleiotropic effects of statins. Annual Review of Pharmacology and Toxicology. 2005;45(1):89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ko H. H. T., Lareu R. R., Dix B. R., Hughes J. D. Statins: antimicrobial resistance breakers or makers? PeerJ. 2017;5, article e3952 doi: 10.7717/peerj.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Russell C. D., Schwarze J. The role of pro-resolution lipid mediators in infectious disease. Immunology. 2014;141(2):166–173. doi: 10.1111/imm.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Serhan C. N., Jain A., Marleau S., et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. The Journal of Immunology. 2003;171(12):6856–6865. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 88.Abdel-Nour M., Tsalikis J., Kleinman D., Girardin S. E. The emerging role of mTOR signalling in antibacterial immunity. Immunology and Cell Biology. 2014;92(4):346–353. doi: 10.1038/icb.2014.3. [DOI] [PubMed] [Google Scholar]
- 89.Laufs U., Kilter H., Konkol C., Wassmann S., Böhm M., Nickenig G. Impact of HMG CoA reductase inhibition on small GTPases in the heart. Cardiovascular Research. 2002;53(4):911–920. doi: 10.1016/S0008-6363(01)00540-5. [DOI] [PubMed] [Google Scholar]
- 90.Price U., le H. . O. T., Powell S. E., et al. Effects of local simvastatin-alendronate conjugate in preventing periodontitis bone loss. Journal of Periodontal Research. 2013;48(5):541–548. doi: 10.1111/jre.12036. [DOI] [PubMed] [Google Scholar]
- 91.Vaziri H., Naserhojjati-Roodsari R., Tahsili-Fahadan N., et al. Effect of simvastatin administration on periodontitis-associated bone loss in ovariectomized rats. Journal of Periodontology. 2007;78(8):1561–1567. doi: 10.1902/jop.2007.060480. [DOI] [PubMed] [Google Scholar]
- 92.Xu X.-C., Chen H., Zhang X., et al. Simvastatin prevents alveolar bone loss in an experimental rat model of periodontitis after ovariectomy. Journal of Translational Medicine. 2014;12(1):p. 284. doi: 10.1186/s12967-014-0284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Messora M. R., Apolinário Vieira G. H., Vanderlei J. M. T. M. M., et al. Rosuvastatin promotes benefits on induced periodontitis in hypertensive rats. Journal of Periodontal Research. 2017;52(4):734–744. doi: 10.1111/jre.12442. [DOI] [PubMed] [Google Scholar]
- 94.Chong L. Y., Chien L. Y., Chung M. C., et al. Controlling the proliferation and differentiation stages to initiate periodontal regeneration. Connective Tissue Research. 2013;54(2):101–107. doi: 10.3109/03008207.2012.751985. [DOI] [PubMed] [Google Scholar]
- 95.Killeen A. C., Rakes P. A., Schmid M. J., et al. Impact of local and systemic alendronate on simvastatin-induced new bone around periodontal defects. Journal of Periodontology. 2012;83(12):1463–1471. doi: 10.1902/jop.2012.110683. [DOI] [PubMed] [Google Scholar]
- 96.Maciel-Oliveira N., Bradaschia-Correa V., Arana-Chavez V. E. Early alveolar bone regeneration in rats after topical administration of simvastatin. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 2011;112(2):170–179. doi: 10.1016/j.tripleo.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 97.Moraes R. M. Effects of simvastatin on prevention and progression of induced periodontitis in rats. Journal of Dental Health, Oral Disorders & Therapy. 2017;8(1) doi: 10.15406/jdhodt.2017.08.00270. [DOI] [Google Scholar]
- 98.Rutledge J., Schieber M. D., Chamberlain J. M., et al. Simvastatin application to augment facial jaw bone in a dog model: pilot study. Journal of Periodontology. 2011;82(4):597–605. doi: 10.1902/jop.2010.100214. [DOI] [PubMed] [Google Scholar]
- 99.Morris M. S., Lee Y., Lavin M. T., et al. Injectable simvastatin in periodontal defects and alveolar ridges: pilot studies. Journal of Periodontology. 2008;79(8):1465–1473. doi: 10.1902/jop.2008.070659. [DOI] [PubMed] [Google Scholar]
- 100.Surve S. M., Acharya A. B., Thakur S. L. Efficacy of subgingivally delivered atorvastatin and simvastatin as an adjunct to scaling and root planing. Drug Metabolism and Personalized Therapy. 2015;30(4):263–269. doi: 10.1515/dmpt-2015-0024. [DOI] [PubMed] [Google Scholar]
- 101.Martande S. S., Kumari M., Pradeep A. R., Singh S. P., Suke D. K. Comparative evaluation of efficacy of subgingivally delivered 1.2% atorvastatin and 1.2% simvastatin in the treatment of intrabony defects in chronic periodontitis: a randomized controlled trial. Journal of Dental Research, Dental Clinics, Dental Prospects. 2017;11(1):18–25. doi: 10.15171/joddd.2017.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Garg S., Pradeep A. R. 1.2% rosuvastatin and 1.2% atorvastatin gel local drug delivery and redelivery in the treatment of class II furcation defects: a randomized controlled clinical trial. Journal of Periodontology. 2017;88(3):259–265. doi: 10.1902/jop.2016.160399. [DOI] [PubMed] [Google Scholar]
- 103.Grover H. S., Kapoor S., Singh A. Effect of topical simvastatin (1.2 mg) on gingival crevicular fluid interleukin-6, interleukin-8 and interleukin-10 levels in chronic periodontitis – a clinicobiochemical study. Journal of Oral Biology and Craniofacial Research. 2016;6(2):85–92. doi: 10.1016/j.jobcr.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kinra P., Gupta H., Khan S., Ahmad M. S. Evaluation of the relative efficacy of an allograft used alone and that in combination with simvastatin in the treatment of human periodontal Infrabony defects – a clinical and radiological study. Journal of Taibah University Medical Sciences. 2010;5(2):75–88. doi: 10.1016/S1658-3612(10)70136-0. [DOI] [Google Scholar]
- 105.Martande S. S., Kumari M., Pradeep A. R., Singh S. P., Suke D. K., Guruprasad C. N. Platelet-rich fibrin combined with 1.2% atorvastatin for treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. Journal of Periodontology. 2016;87(9):1039–1046. doi: 10.1902/jop.2016.150306. [DOI] [PubMed] [Google Scholar]
- 106.Pradeep A. R., Garg V., Kanoriya D., Singhal S. Platelet-rich fibrin with 1.2% rosuvastatin for treatment of Intrabony defects in chronic periodontitis: a randomized controlled clinical trial. Journal of Periodontology. 2016;87(12):1468–1473. doi: 10.1902/jop.2016.160015. [DOI] [PubMed] [Google Scholar]
- 107.Pradeep A. R., Karvekar S., Nagpal K., Patnaik K., Raju A., Singh P. Rosuvastatin 1.2 mg in situ gel combined with 1:1 mixture of autologous platelet-rich fibrin and porous hydroxyapatite bone graft in surgical treatment of mandibular class II furcation defects: a randomized clinical control trial. Journal of Periodontology. 2016;87(1):5–13. doi: 10.1902/jop.2015.150131. [DOI] [PubMed] [Google Scholar]
- 108.Ranjan R., Patil S. R., Veena H. R. Effect of in-situ application of simvastatin gel in surgical management of osseous defects in chronic periodontitis–a randomized clinical trial. Journal of Oral Biology and Craniofacial Research. 2017;7(2):113–118. doi: 10.1016/j.jobcr.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cunha-Cruz J., Saver B., Maupome G., Hujoel P. P. Statin use and tooth loss in chronic periodontitis patients. Journal of Periodontology. 2006;77(6):1061–1066. doi: 10.1902/jop.2006.050280. [DOI] [PubMed] [Google Scholar]
- 110.Fentoğlu Ö., Sözen T., Öz S. G., et al. Short-term effects of periodontal therapy as an adjunct to anti-lipemic treatment. Oral Diseases. 2010;16(7):648–654. doi: 10.1111/j.1601-0825.2010.01668.x. [DOI] [PubMed] [Google Scholar]
- 111.Meisel P., Kroemer H. K., Nauck M., Holtfreter B., Kocher T. Tooth loss, periodontitis, and statins in a population-based follow-up study. Journal of Periodontology. 2014;85(6):e160–e168. doi: 10.1902/jop.2013.130456. [DOI] [PubMed] [Google Scholar]
- 112.Fajardo M. E., Rocha M. L., Sánchez-Marin F. J., Espinosa-Chávez E. J. Effect of atorvastatin on chronic periodontitis: a randomized pilot study. Journal of Clinical Periodontology. 2010;37(11):1016–1022. doi: 10.1111/j.1600-051X.2010.01619.x. [DOI] [PubMed] [Google Scholar]
- 113.Fentoğlu Ö., Kırzıoğlu F. Y., Özdem M., Koçak H., Sütçü R., Sert T. Proinflammatory cytokine levels in hyperlipidemic patients with periodontitis after periodontal treatment. Oral Diseases. 2012;18(3):299–306. doi: 10.1111/j.1601-0825.2011.01880.x. [DOI] [PubMed] [Google Scholar]
- 114.Sangwan A., Tewari S., Singh H., Sharma R. K., Narula S. C. Effect of hyperlipidemia on response to nonsurgical periodontal therapy: statin users versus nonusers. European Journal of Dentistry. 2016;10(1):69–76. doi: 10.4103/1305-7456.175685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grover H. S., Luthra S., Maroo S. Are statins really wonder drugs? Journal of the Formosan Medical Association. 2014;113(12):892–898. doi: 10.1016/j.jfma.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 116.Martinez A. I., Freeman P. R., Moga D. C. Statin use and gastrointestinal hemorrhage: a large retrospective cohort study. American Journal of Cardiovascular Drugs. 2018;46:107–110. doi: 10.1007/s40256-018-0301-4. [DOI] [PubMed] [Google Scholar]
- 117.Da Rocha H. A., Silva C. F., Santiago F. L., Martins L. G., Dias P. C., De Magalhães D. Local drug delivery systems in the treatment of periodontitis: a literature review. Journal of the International Academy of Periodontology. 2015;17(3):82–90. [PubMed] [Google Scholar]
- 118.Joshi D., Garg T., Goyal A. K., Rath G. Advanced drug delivery approaches against periodontitis. Drug Delivery. 2016;23(2):363–377. doi: 10.3109/10717544.2014.935531. [DOI] [PubMed] [Google Scholar]
- 119.Mombelli A., Samaranayake L. P. Topical and systemic antibiotics in the management of periodontal diseases. International Dental Journal. 2004;54(1):3–14. doi: 10.1111/j.1875-595x.2004.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 120.Park J.-B. The use of simvastatin in bone regeneration. Medicina Oral, Patología Oral y Cirugía Bucal. 2009;14(9):e485–e488. [PubMed] [Google Scholar]
- 121.Schwinté P., Mariotte A., Anand P., et al. Anti-inflammatory effect of active nanofibrous polymeric membrane bearing nanocontainers of atorvastatin complexes. Nanomedicine. 2017;12(23):2651–2674. doi: 10.2217/nnm-2017-0198. [DOI] [PubMed] [Google Scholar]
- 122.Sima C., Glogauer M. Macrophage subsets and osteoimmunology: tuning of the immunological recognition and effector systems that maintain alveolar bone. Periodontology 2000. 2013;63(1):80–101. doi: 10.1111/prd.12032. [DOI] [PubMed] [Google Scholar]
- 123.Thylin M. R., McConnell J. C., Schmid M. J., et al. Effects of simvastatin gels on murine calvarial bone. Journal of Periodontology. 2002;73(10):1141–1148. doi: 10.1902/jop.2002.73.10.1141. [DOI] [PubMed] [Google Scholar]
- 124.Wang J. W., Xu S. W., Yang D. S., Lv R. K. Locally applied simvastatin promotes fracture healing in ovariectomized rat. Osteoporosis International. 2007;18(12):1641–1650. doi: 10.1007/s00198-007-0412-2. [DOI] [PubMed] [Google Scholar]
- 125.Kumari M., Martande S. S., Pradeep A. R. Subgingivally delivered 1.2% atorvastatin in the treatment of chronic periodontitis among smokers: a randomized, controlled clinical trial. Journal of Investigative and Clinical Dentistry. 2017;8(2, article e12213) doi: 10.1111/jicd.12213. [DOI] [PubMed] [Google Scholar]
- 126.Pradeep A. R., Priyanka N., Kalra N., Naik S. B., Singh S. P., Martande S. Clinical efficacy of subgingivally delivered 1.2-mg simvastatin in the treatment of individuals with class II furcation defects: a randomized controlled clinical trial. Journal of Periodontology. 2012;83(12):1472–1479. doi: 10.1902/jop.2012.110716. [DOI] [PubMed] [Google Scholar]
- 127.Akram Z., Vohra F., Javed F. Efficacy of statin delivery as an adjunct to scaling and root planing in the treatment of chronic periodontitis: a meta-analysis. Journal of Investigative and Clinical Dentistry. 2018;9(2, article e12304) doi: 10.1111/jicd.12304. [DOI] [PubMed] [Google Scholar]
- 128.Meza-Mauricio J., Soto-Peñaloza D., Peñarrocha-Oltra D., Montiel-Company J. M., Peruzzo D. C. Locally applied statins as adjuvants to non-surgical periodontal treatment for chronic periodontitis: a systematic review and meta-analysis. Clinical Oral Investigations. 2018;22(7):2413–2430. doi: 10.1007/s00784-018-2507-x. [DOI] [PubMed] [Google Scholar]
- 129.Muniz F. W. M. G., Taminski K., Cavagni J., Celeste R. K., Weidlich P., Rösing C. K. The effect of statins on periodontal treatment—a systematic review with meta-analyses and meta-regression. Clinical Oral Investigations. 2018;22(2):671–687. doi: 10.1007/s00784-018-2354-9. [DOI] [PubMed] [Google Scholar]
- 130.Agarwal S., Chaubey K. K., Chaubey A., Agarwal V., Madan E., Agarwal M. C. Clinical efficacy of subgingivally delivered simvastatin gel in chronic periodontitis patients. Journal of Indian Society of Periodontology. 2016;20(4):409–416. doi: 10.4103/0972-124X.194270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sinjab K., Zimmo N., Lin G. H., Chung M. P., Shaikh L., Wang H. L. The effect of locally delivered statins on treating periodontal intrabony defects: a systematic review and meta-analysis. Journal of Periodontology. 2017;88(4):357–367. doi: 10.1902/jop.2016.160384. [DOI] [PubMed] [Google Scholar]
- 132.Ambrósio L. M. B., Rovai E. S., Sendyk D. I., Holzhausen M., Pannuti C. M. Does the adjunctive use of statins provide additional benefits to nonsurgical periodontal treatment? A systematic review and meta-analysis. Journal of Periodontal Research. 2018;53(1):12–21. doi: 10.1111/jre.12480. [DOI] [PubMed] [Google Scholar]
- 133.Linden G. J., Lyons A., Scannapieco F. A. Periodontal systemic associations: review of the evidence. Journal of Periodontology. 2013;84(4-s):S8–19. doi: 10.1902/jop.2013.1340010. [DOI] [PubMed] [Google Scholar]
- 134.Zhou Q., Leeman S. E., Amar S. Signaling mechanisms in the restoration of impaired immune function due to diet-induced obesity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):2867–2872. doi: 10.1073/pnas.1019270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Athyros V. G., Papageorgiou A. A., Demitriadis D. S., Kontopoulos A. G. Atorvastatin plus pravastatin for the treatment of heterozygous familial hypercholesterolaemia-a pilot study. Current Medical Research and Opinion. 2001;17(4):267–272. doi: 10.1185/030079901753403162. [DOI] [PubMed] [Google Scholar]
- 136.Ogai Y. A., Banks T. A., Gada S. M. Statin allergy: clinical experience and structural relation as a framework for evaluation. The Journal of Allergy and Clinical Immunology: In Practice. 2015;3(6):993–995. doi: 10.1016/j.jaip.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 137.Rosenberg D. R., Andrade C. X., Chaparro A. P., et al. Short-term effects of 2% atorvastatin dentifrice as an adjunct to periodontal therapy: a randomized double-masked clinical trial. Journal of Periodontology. 2015;86(5):623–630. doi: 10.1902/jop.2015.140503. [DOI] [PubMed] [Google Scholar]
- 138.Lee B.-S., Lee C. C., Lin H. P., et al. A functional chitosan membrane with grafted epigallocatechin-3-gallate and lovastatin enhances periodontal tissue regeneration in dogs. Carbohydrate Polymers. 2016;151:790–802. doi: 10.1016/j.carbpol.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 139.Bradley A. D., Zhang Y., Jia Z., et al. Effect of simvastatin prodrug on experimental periodontitis. Journal of Periodontology. 2016;87(5):577–582. doi: 10.1902/jop.2016.150599. [DOI] [PubMed] [Google Scholar]
- 140.Lee B. S., Lee C. C., Wang Y. P., et al. Controlled-release of tetracycline and lovastatin by poly(D,L-lactide-co-glycolide acid)-chitosan nanoparticles enhances periodontal regeneration in dogs. International Journal of Nanomedicine. 2016;11:285–297. doi: 10.2147/IJN.S94270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Seto H., Ohba H., Tokunaga K., Hama H., Horibe M., Nagata T. Topical administration of simvastatin recovers alveolar bone loss in rats. Journal of Periodontal Research. 2008;43(3):261–267. doi: 10.1111/j.1600-0765.2007.01024.x. [DOI] [PubMed] [Google Scholar]
- 142.Swerts A. A., Santos B. F. E., Bruzadelli S. R., Brigagão M. R. P. L., Lima D. C. ., Fernandes L. A. Treatment of experimental periodontal disease by laser therapy in simvastatin-modified rats. Journal of Applied Oral Science. 2017;25(4):387–395. doi: 10.1590/1678-7757-2016-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang X., Jia Z., Almoshari Y., Lele S. M., Reinhardt R. A., Wang D. Local application of pyrophosphorylated simvastatin prevents experimental periodontitis. Pharmaceutical Research. 2018;35(8):p. 164. doi: 10.1007/s11095-018-2444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goes P., Lima A. P. S., Melo I. M., Rêgo R. O. C. C., Lima V. Effect of atorvastatin in radiographic density on alveolar bone loss in Wistar rats. Brazilian Dental Journal. 2010;21(3):193–198. doi: 10.1590/S0103-64402010000300003. [DOI] [PubMed] [Google Scholar]
- 145.Goes P., Lima N. A., Rodrigues J. A. G., Benevides N. M. B., Brito G. A. C., Lima V. Anti-inflammatory and anti-resorptive effects of atorvastatin on alveolar bone loss in Wistar rats. Brazilian Dental Journal. 2016;27(3):267–272. doi: 10.1590/0103-6440201600600. [DOI] [PubMed] [Google Scholar]
- 146.Mouchrek Júnior J. C. E., Macedo C. G., Abdalla H. B., et al. Simvastatin modulates gingival cytokine and MMP production in a rat model of ligature-induced periodontitis. Clinical, Cosmetic and Investigational Dentistry. 2017;9:33–38. doi: 10.2147/CCIDE.S134125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nassar C. A., Battistetti G. D., Nahsan F. P., et al. Evaluation of the effect of simvastatin on the progression of alveolar bone loss in experimental periodontitis--an animal study. Journal of the International Academy of Periodontology. 2014;16(1):2–7. [PubMed] [Google Scholar]
- 148.Kumari M., Martande S. S., Pradeep A. R., Naik S. B. Efficacy of subgingivally delivered 1.2% atorvastatin in the treatment of chronic periodontitis in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Journal of Periodontology. 2016;87(11):1278–1285. doi: 10.1902/jop.2016.130227. [DOI] [PubMed] [Google Scholar]
- 149.Pradeep A. R., Thorat M. S. Clinical effect of subgingivally delivered simvastatin in the treatment of patients with chronic periodontitis: a randomized clinical trial. Journal of Periodontology. 2010;81(2):214–222. doi: 10.1902/jop.2009.090429. [DOI] [PubMed] [Google Scholar]
- 150.Pradeep A. R., Kumari M., Rao N. S., Martande S. S., Naik S. B. Clinical efficacy of subgingivally delivered 1.2% atorvastatin in chronic periodontitis: a randomized controlled clinical trial. Journal of Periodontology. 2013;84(7):871–879. doi: 10.1902/jop.2012.120393. [DOI] [PubMed] [Google Scholar]
- 151.Pradeep A. R., Rao N. S., Bajaj P., Kumari M. Efficacy of subgingivally delivered simvastatin in the treatment of patients with type 2 diabetes and chronic periodontitis: a randomized double-masked controlled clinical trial. Journal of Periodontology. 2013;84(1):24–31. doi: 10.1902/jop.2012.110721. [DOI] [PubMed] [Google Scholar]
- 152.Pradeep A. R., Karvekar S., Nagpal K., Patnaik K., Guruprasad C. N., Kumaraswamy K. M. Efficacy of locally delivered 1.2% rosuvastatin gel to non-surgical treatment of patients with chronic periodontitis: a randomized, placebo-controlled clinical trial. Journal of Periodontology. 2015;86(6):738–745. doi: 10.1902/jop.2015.140631. [DOI] [PubMed] [Google Scholar]
- 153.Pradeep A. R., Kanoriya D., Singhal S., Garg V., Manohar B., Chatterjee A. Comparative evaluation of subgingivally delivered 1% alendronate versus 1.2% atorvastatin gel in treatment of chronic periodontitis: a randomized placebo-controlled clinical trial. Journal of Investigative and Clinical Dentistry. 2017;8(3, article e12215) doi: 10.1111/jicd.12215. [DOI] [PubMed] [Google Scholar]
- 154.Priyanka N., Abhilash A., Saquib S., et al. Clinical efficacy of subgingivally delivered 1.2 mg simvastatin in the treatment of patients with aggressive periodontitis: a randomized controlled clinical trial. The International Journal of Periodontics & Restorative Dentistry. 2017;37(2):e135–e141. doi: 10.11607/prd.2936. [DOI] [PubMed] [Google Scholar]
- 155.Rao N. S., Pradeep A. R., Bajaj P., Kumari M., Naik S. B. Simvastatin local drug delivery in smokers with chronic periodontitis: a randomized controlled clinical trial. Australian Dental Journal. 2013;58(2):156–162. doi: 10.1111/adj.12042. [DOI] [PubMed] [Google Scholar]
- 156.Rath A., Mahenra J., Thomas L., Sandhu M., Namasi A., Ramakrishna T. A clinical, radiological and il-6 evaluation of subgingivally delivered simvastatin in the treatment of chronic periodontitis. International Journal of Drug Delivery. 2012;4:70–81. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk of bias assessment of included clinical studies.