Abstract
Irritable bowel syndrome (IBS) is a functional gut disorder that typically manifests in early adult years. IBS patients report that pain is the most distressing symptom with the greatest impact on quality of life. Pain-sensitivity genes and the gut microbiome may influence severity of symptoms as well as response to self-management (SM) interventions. Based on current understanding of the science of SM, pain neurophysiology, and the gut-brain axis, our team developed a pain SM intervention to be added to evidence-based self-management instruction to increase the individual's SM knowledge and skills (self-efficacy, self-regulation, and goal-setting). The purpose of this randomized controlled longitudinal pilot study is to examine the feasibility, acceptability, and preliminary effectiveness of the IBS-pain SM intervention on IBS-pain SM behaviors and related health outcomes. A sample of 80 young adults (age 18–29 years old) will be recruited and randomly assigned to the experimental or control group. Both groups will receive 10 electronic video modules focused on IBS-pain SM knowledge and skills. The experimental group also will receive nurse-led one-on-one phone consultations to facilitate monitoring and problem-solving. All participants will be followed over 12 weeks. Primary outcomes will be measured at baseline, 6 weeks, and 12 weeks, including IBS-pain SM behaviors, quality of life, and well-being. The influence of pain-sensitivity genes and the gut microbiome on IBS-pain SM behaviors and health outcomes also will be assessed.
Keywords: genetics, gut microbiome, irritable bowel syndrome, pain, self-management
1 ∣. INTRODUCTION
Intense, recurrent abdominal (visceral) pain is a predominant symptom of irritable bowel syndrome (IBS), a functional gut disorder that typically manifests in the early adult years (Lacy, Chey, & Lembo, 2015). IBS is common, with prevalence reaching over 20% in some regions of the world, and affects more women than men (Canavan, West, & Card, 2014; Longstreth et al., 2003, 2006).
Individuals with IBS report that pain is the most distressing symptom and has the greatest impact on quality of life (Lacy et al., 2015). While women report more severe IBS-related pain, both younger men and women report more severe pain than do older adults (Tang, Yang, Liang, et al., 2012; Tang, Yang, Wang, & Lin, 2012). Current approaches to improve self-management (SM) of IBS-related pain do not target individual, context-specific factors of pain. Therefore, individuals with IBS-related pain often endure a long and frustrating course of learning how to manage pain on their own. The proposed pilot project was developed based on this common situation and will provide preliminary data about feasibility and outcomes of an intervention with personalized pain SM with nurse-led support for individuals with IBS-related pain.
1.1 ∣. IBS pain
IBS-related pain is associated with sensitization of the central nervous system, peripheral nervous system, and/or mechanisms engaged within the bowel (Camilleri, Lasch, & Zhou, 2012). While it is unclear how abnormal pain signaling along the pain processing pathway contributes to IBS pain burden, approximately half of all patients with IBS have visceral hypersensitivity and report distress as a result (Frissora & Koch, 2005; Kanazawa, Hongo, & Fukudo, 2011; Whitehead, Palsson, & Jones, 2002). Pain susceptibility is influenced by genetic variation, and IBS-related pain also is influenced by the gut microbiome (Hughes et al., 2013; Kerckhoffs et al., 2009; Rajilic-Stojanovic et al., 2011; Simren et al., 2013). The brain-gut-microbiota axis has been recognized recently as an important mechanism in the regulation of health and the pain/stress response (Dinan & Cryan, 2012). This bidirectional communication network enables top-down signaling from the brain to influence the motor, sensory, and secretory modalities of the GI tract, and conversely, bottom-up signaling from the gut to affect brain function, especially in the hypothalamus and amygdala, which are devoted to emotion and pain/stress (Cong, Xu, Romisher, et al., 2016).
Self-management of IBS symptoms is the crux of therapy, and strategies to support the daily integration of health behaviors to reduce the negative impact of this condition are needed (Fukudo et al., 2015). Several groups have developed and tested IBS-SM programs and have found that IBS knowledge increases after web-based, individual or group, and telephone interventions (AHRQ, 2014; Chang, Lembo, & Sultan, 2014; Trinkley & Nahata, 2014). Heitkemper's team reported that an 8-week nurse-led IBS-SM intervention signicantly improved symptoms at 3 and 6 months when compared to usual care (Jarrett et al., 2009, 2016). In addition, they found that a polymorphism of the COMT gene moderated the effect of the intervention on symptom improvement (Han et al., 2017).
Adolescents and young adults, who are most often affected by IBS, reported a high-level of satisfaction with self-guided SM resources including symptom self-monitoring, goal setting, problem-solving, and pain coping skills training (Stinson et al., 2014). Pain neuroscience education has also been found to reduce fear of pain, pain catastrophizing, and pain intensity (Robins, Perron, Heathcote, & Simons, 2016).
This pilot study was guided by the Individual and Family Self-Management Theory (IFSMT), in which SM takes place in the context of risk and protective factors specific to the condition, physical and social environment, and individual and family (Ryan & Sawin, 2009). Because it has already been shown that IBS-SM interventions are equal or superior to usual care (Cong, Perry, Bernier, Young, & Starkweather, 2017), the intervention tested the previous model against the addition of nurse-led social support component designed to personalize the use of SM skills and facilitate activation of skills into SM behaviors. Adolescents and young adults will be randomized to receive either the IBS pain SM intervention alone (control) or the IBS pain SM intervention plus the nurse-led support for monitoring and problem-solving (experimental group).
The aims of this study are to evaluate: (1) feasibility and acceptability of the intervention; (2) preliminary effectiveness of the IBS-pain SM intervention plus nurse-led support on pain responses, IBS-pain SM behaviors, and related health outcomes. A secondary aim is to explore the influence of contextual risk factors of IBS, including peripheral and central pain sensitivity, single-nucleotide polymorphisms (SNPs) of candidate pain-sensitivity genes, and the gut microbiome, on pain and SM behaviors.
2 ∣. METHODS
2.1 ∣. Study design
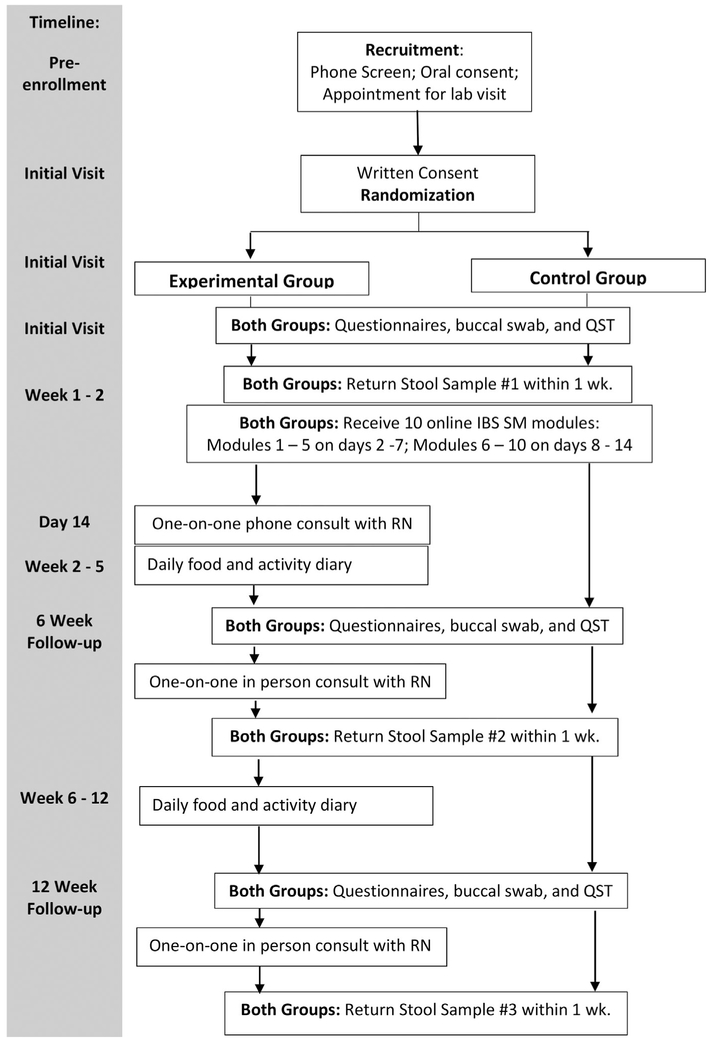
A randomized controlled longitudinal design is being used. Both the experimental and control groups will receive 10 electronic video modules focused on knowledge and skills of IBS SM. The experimental group also will receive one-on-one consultation with a nurse for personalized self-monitoring and goal setting. All participants will be followed for 12 weeks after enrollment, and primary outcomes will be measured at baseline, 6, and 12 weeks (see Figure 1). The study protocol has been approved by the University institutional review board and affiliated study settings.
FIGURE 1.
Data collection flow chart. Experimental group, IBS pain SM intervention plus the nurse-led support; Control, IBS pain SM intervention alone; QST, quantitative sensory testing; IBS, irritable bowel syndrome; SM, self-management; RN, registered nurse
2.1.1 ∣. Randomization
After giving written consent, participants are randomized into either the control or experimental group using a stratified and blocked randomization scheme (Figure 1). In this scheme, gender is the stratification factor and the block size is four. Together, these components result in approximately equal ratios of male:female participants in the two study groups throughout the study period. Given the relatively small sample sizes in this study, achieving a balance of gender in the groups reduces the possibility that this covariate could operate as a confounding factor.
2.1.2 ∣. Blinding
All efforts are made to maintain blinding of study team members to the group assignment of individual participants. This is done by limiting the number of team members who perform the randomization (1–2 members). The study nurses are only contacting participants in the experimental group. All other team members remain blinded to group assignment, including laboratory personnel who are processing the biological samples. Dummy codes will be applied to the dataset so that it is not possible to determine the randomization scheme.
2.2 ∣. Study setting
The study participants are being recruited from the general community as well as targeted advertising at two large public university campuses and two gastrointestinal (GI) clinics in the Northeastern US. Recruitment is taking place through advertisements placed in local newspapers and transportation vehicles, general flyers in the community, public places, major local university campuses and hospitals, and email invitation at major campuses, which instruct potential participants to call a research-designated phone line. The intervention and data collection are conducted in the biobehavioral laboratories on the two university campuses.
2.3 ∣. Participants
2.3.1 ∣. Inclusion criteria
Those included will be: (1) men and women 18–29 years of age; (2) with a diagnosis of IBS by a healthcare provider with current report of pain (volunteers asked to bring provider-verification of IBS diagnosis based on Rome-III criteria to initial study appointment); (3) able to read and speak English; and (4) have daily access to a computer connected to the internet.
2.3.2 ∣. Exclusion criteria
(1) Other chronic painful conditions including but not limited to fibromyalgia, chronic pelvic pain, or chronic interstitial cystitis; (2) infectious diseases (hepatitis, HIV, MRSA); (3) celiac disease or inflammatory bowel disease; (4) diabetes mellitus; (5) serious mental health conditions (e.g., bipolar disorder, schizophrenia, and mania); (6)women during pregnancy or within 3 months postpartum; (7)regular use of opioids, iron supplements, prebiotics/probiotics or antibiotics, or substance abuse; and (8) injury to non-dominant hand or presence of open skin lesions, disturbed sensation, carpal tunnel syndrome, or rash. The rationale for these exclusion criteria is to control for correlates of pain sensitivity, genetic and genomic attributes, and IBS-related health outcomes.
2.3.3 ∣. Sample size
Eighty male and female young adults will be recruited to the study and randomized into the intervention or control group, with 40 subjects in each group. We estimated the sample size by reference to previous studies that evaluated effects of SM interventions for individuals with IBS (Cong, Perry, et al., 2017).
The most common outcome variable shared by these studies is the IBS-Quality of Life scale (Dorn et al., 2015; Hahn, Kirchdoerfer, Fullerton, & Mayer, 1997; Jarrett et al., 2009; Stinson et al., 2014). Across studies, standardized effects on this variable are typically either “medium” (Cohen’s d ≈ 0.5) or “very large” (d ≥ 1.0). As the current study focuses on feasibility, acceptability, and preliminary assessments of effectiveness, our sample size objective reflects an expected intervention effect of d = 0.75, Types I and II error probabilities of 0.05 and 0.2, respectively, and possible attrition up to 30%.
A power analysis to detect differences in the gut microbiome in response to the IBS SM intervention was not possible because no related previous study was found. A sample of 40 participants in each group will provide preliminary data to investigate the effects of the IBS-pain SM intervention on the gut microbiome, while accounting for potential attrition.
2.4 ∣. Intervention
2.4.1 ∣. Online education and training modules (both groups)
The research team has developed 10 online modules, which span pathophysiology and symptoms management of IBS (see Table 1). The modules include: IBS-related pain neurophysiology and the brain-gut axis mechanism, triggers of IBS-related pain, medication use, IBS pain management strategies, such as medication use, progressive muscle relaxation, guided imagery, mindfulness, belly breathing, and pain problem-solving, as well as managing daily activities. Each module is approximately 5–10 min in length and contains animated contents with interactive learning strategies. Language and concepts are presented at an 8th grade level.
TABLE 1.
IBS module topics and descriptions
| Module topic | Description |
|---|---|
| IBS persistent abdominal pain neurophysiology and brain-gut axis mechanism | The pathophysiology of pain, visceral pain sensitivity and the brain gut axis will be covered in this module using simple terms, definitions of medical terminology and basic principles of neurophysiology and the microbiome. |
| Triggers of IBS | Common triggers of IBS, particularly psychological stress and certain foods, will be reviewed. Strategies to decrease psychological stress, food logging, and dietary modifications will be discussed. |
| IBS symptom management strategies | Basic principles of symptom management will be discussed such as what symptoms mean, monitoring symptoms, tracking symptoms, and knowing your body (self-scanning). Pharmacological and non-pharmacological strategies for symptom management as well as recommendations for healthy living will be reviewed (e.g., physical activity, diet, avoiding/decreasing exposures to toxins and substances). |
| Pharmacological therapies | General categories of IBS medications will be explained, including the mechanisms of action, indications and contraindications. Over the counter and herbal remedies will be discussed so that participants are aware of how they are used and side-effects. (e.g., Use of certain probiotics and their long-term effects). |
| Pain problem solving | This module will discuss strategies to use for decreasing the impact of pain on daily life. Food logging, physical activity, socialization, and strengthening coping skills will be discussed. |
| Progressive muscle relaxation | Methods to use for progressive muscle relaxation will be discussed, specifically related to the abdominal region. Step-by-step instructions and demonstration of progressive muscle relaxation will be included. |
| Belly breathing | Methods of belly breathing will be shown. Step-by-step instructions on how to use belly breathing for anxiety, stress reduction and pain will be highlighted. |
| Guided imagery | Methods of using guided imagery will be reviewed. Step-by-step instructions and demonstration will be given. |
| Mindfulness | Concepts of mindfulness will be reviewed. Application of mindfulness to daily life will be discussed. |
| Integration into daily life | How to instructions on applying all the various concepts into daily life will be discussed with instructions on how to set weekly goals. Examples include incorporating breathing exercises, dietary changes, and coping strategies into weekly goals. |
The development of these modules utilized university e-learning principles as well as the online learning standards provided by Quality Matters (Available online at: https://www.qualitymatters.org). The content of these modules was assessed by several healthcare providers for accuracy and relevance, and the usability was tested prior to the start of the study. The modules are distributed through the university's Research Electronic Data Capture (REDCap) system (Harris et al., 2009), which is a web-based, secure, research-driven platform that supports data capture, validated data entry and export, and audit trails for tracking data manipulation. Upon completion of the consent process and the baseline data collection, participants in both the experimental and control groups are provided with a unique link to the REDCap to access these electronic video modules and are instructed to complete one module each day for the next 2 weeks.
2.4.2 ∣. One-on-one consultation with a registered nurse (experimental group)
Two research nurses with active registered nurse (RN) licenses provide one-on-one consultation to the participants in the experimental group, either by phone or in person. Both of the RNs have more than 2 years’ experience providing care in the pediatric and young adult population.
The first phone consultation is scheduled for the experimental group (IBS pain SM intervention plus the nurse-led support) after the completion of the video modules. Prior to the consultation, the RN assesses the participant's central and peripheral pain sensitivity as well as the dietary and symptom history by reviewing the QST and questionnaire results from the baseline visit.
During the initial consultation, the RN inquiries about the participant's most recent (within the previous 2 weeks) experience with symptom episodes, stress, diet, activity/exercise, medication changes, and quality of life. During this conversation, the RN integrates findings from the participant's baseline visit and provides recommendations for improved SM. At the end of the consultation, the RN asks the participant to set a structured goal to work toward prior to the subsequent follow-up visit. This process includes choosing and describing a SM activity, detailing a schedule for the activity, and identifying barriers. Consultations occur at 2, 6, and 12 weeks.
At the 6-week and 12-week follow-up visits, a research RN meets the participant in person and reviews findings from the online self-monitoring diary and accomplishments from the previously selected goal. The participants are given a choice in continuing to work toward their established goal, adding an additional goal, or choosing a new goal. The RN assists in problem-identification and solving throughout the consultation. Each consultation is estimated to take between 15–30 min.
2.4.3 ∣. Online symptom self-monitoring diary (experimental group)
To facilitate self-monitoring, participants in the experimental group are asked to fill out a daily log of IBS symptom ratings during the 12-week study period. The online diary is distributed and completed via the REDCap and covers self-monitoring of IBS-pain, diet, sleep, stress, and stool quality. The diary takes a few minutes to complete each day and is introduced to the participant after the first RN consultation.
2.4.4 ∣. Monitoring of physical activity (experimental group)
The participants are asked to record pedometer readings in the online diary. Physical activity has been shown to decrease symptoms of IBS (Johannesson, Simren, Strid, Bajor, & Sadik, 2011) as well as pain sensitivity (Dishman et al., 2006), although the precise level (frequency and intensity) remains unclear. At the end of the first visit, an electronic pedometer (Pro-Form—SP-100 Pedometer) is given to participants to measure steps taken, distance traveled, and calories burned.
2.4.5 ∣. Measurements and data collection
After obtaining informed consent and randomization, initial data collection as well as follow-up visits are scheduled (Figure 1). Table 2 outlines the self-report measurements to be used at baseline, 6, and 12 weeks. All study measurements are collected and managed using the university REDCap system.
TABLE 2.
Instruments administered at baseline, 6-weeks, and 12-weeks of the study
| Instruments | Purpose | Items |
|---|---|---|
| National Institute of Nursing Research Common Data Elements (CDEs) | Demographics. | 12 Items |
| Patient-Reported Outcomes Measurement Information System (PROMIS) | Anxiety, fatigue, depression, applied cognition, and sleep. | 39 Items |
| Quality of Life in Neurological Disorders (Neuro-QOL) | Physical, mental and social health. | 6 Items |
| Short Form (SF) Health Survey | Quality of life measures. | 36 Items |
| Index of Self-Regulation | Self-regulation for physical activity. | 9 Items |
| Self-efficacy for Managing Chronic Disease | Individual’s self-efficacy and confidence of managing their chronic disease. | 6 Items |
| PROMIS Global Health Form | General domains of health and function, such as physical, mental social health, pain and fatigue, and QOL. | 10 Items |
| Food Frequency Questionnaire (FFQ) | Measure the type and quantity of food intake. | 34 items |
| Brief Pain Inventory (BPI) | Pain severity and interferes/impact of pain on function. | 9 Items |
| Coping Strategies Questionnaire-Revised (CSQ-R) | Cognitive coping responses to pain. | 27 items |
| IBS Quality of Life (IBS-QOL) Questionnaire | Assess QOL in IBS population. | 34 items |
| Use of recommended pharmacological therapies and healthcare utilization | Use of pharmacological therapies and number of visits in the past 6 weeks to healthcare providers for their IBS pain. | Various |
2.4.6 ∣. Demographics and general questionnaires
These include questionnaires from the recommended NINR common data elements, such as a Demographics Form, Patient-Reported Outcomes Measurement Information System (PROMIS®), Quality of Life in Neurological Disorders (Neuro-QOL), and the Short Form Health Survey. SM questionnaires include the Self-Efficacy for Managing Chronic Disease, Index of Self-Regulation, and the PROMIS Global health form (Available online at: https://cde.nlm.nih.gov/cde/search?selectedOrg=NINR).
2.4.7 ∣. Food frequency questionnaire (FFQ)
Food intake affects the gut microbiome (Ohman, Stridsberg, Isaksson, Jerlstad, & Simren, 2012; Voreades, Kozil, & Weir, 2014), and specific foods can influence IBS pain (Bohn, Storsrud, Tornblom, Bengtsson, & Simren, 2013). The self-reported FFQ will be used to measure the type and quantity of food intake (Kristal, Feng, Coates, Oberman, & George, 1997).
2.4.8 ∣. Brief pain inventory (BPI)
The BPI measures two domains of pain, the severity of pain and the degree to which pain interferes with feeling and function, using 0–10 rating scales (Keller et al., 2004). The BPI process variable measures pain at the time of data collection, and the distal outcome measures pain at each data point.
2.4.9 ∣. Coping strategies questionnaire-revised (CSQ-R)
The CSQ-R (Robinson et al., 1997) is designed to assess six cognitive coping responses to pain. Subjects rate the frequency of using each coping strategy and its perceived control over their pain on a 7-point Likert-type scale, from “never do that” to “always do that” (Robinson et al., 1997).
2.4.10 ∣. IBS-quality of life (IBS-QOL) questionnaire
The IBS-QOL is designed to assess QOL specific to IBS populations (Hahn etal., 1997). A 5-point Likert scale is used to measure responses. All item scores are summed, and higher scores show better QOL.
2.4.11 ∣. Use of recommended pharmacological therapies and healthcare utilization
Subjects are asked to document the use of pharmacological therapies and number of visits to healthcare providers for their IBS pain in the past 6 weeks. Number of visits is summed within categories: primary care providers (e.g., internist, family medicine practitioner), specialists (e.g., gastroenterologist), mental health visits (counselor, psychiatrist, or psychologist), and complementary alternative therapists (e.g., acupuncturist, massage therapist).
2.4.12 ∣. Pain sensitivity
Quantitative sensory testing (QST) is used to measure pain sensitivity using standardized stimuli to test both nociceptive and non-nociceptive systems in the periphery and central nervous systems (Rolke et al., 2006; Verdugo & Ochoa, 1992). A standardized protocol of administration based on previous studies (Starkweather et al., 2016), including examination room conditions and instructions provided for the patient, are strictly followed by all research team members. A total of seven tests are used to quantify 13 functional sensory pathways.
The QST protocol will be run on the subject's non-dominant forearm. A practice run on the dominant forearm is performed in order to verify the participant's understanding of the protocol. The medial side of the non-dominant forearm is utilized for testing purposes. QST is conducted in the following order: mechanical cutaneous pain, thermal pain, and pressure pain. The protocol is described in detail in a previous manuscript (Starkweather et al., 2016).
2.4.13 ∣. Pain-susceptibility SNPs genotyping
Buccal cell samples are collected at the time of initial visit. Participants are instructed to rinse their mouth twice with water and then roll the sterile buccal brush firmly on the inside of the check. The buccal cell sample is stored at −80 °C in the biobehavioral laboratary until processing. Genomic DNA will then be extracted from buccal brushes using standard kits and protocols (Qiagen, Germantown, MD, Gentra® Puregene® Buccal Cell Kit, #158845) and stored at −80 °C for subsequent processing in batches. Specific SNPs of interest as pain-susceptibility genes include oxytocin receptor gene (OXTR), glucocorticoid receptor gene (NR3C1), OPRM1, COMT, and CYP2D6 (Hall et al., 2012; Karling et al., 2011; Makker, Chilimuri, & Bella, 2015; Song et al., 2012). Taqman SNP genotyping assays (VIC/FAM) are performed according to manufacturer protocol using an Applied Biosystems Step One Plus PCR machine and ABI allelic discrimination software (Thermo Fisher Scientific, Waltham, MA).
2.4.14 ∣. Gut microbiome sequencing
Participants are instructed to collect stool samples using the OMNIgene. GUT (OMR-200) collection kit (DNA Genotek Inc., Ottawa, Canada) and send back the samples within 1 week after the initial visit and at 6- and 12-week follow-up visits. Samples are then frozen and maintained at −80 °C at the Center's biobehavioral laboratary until processing. 16S rRNA gene sequencing analysis is conducted at the university-run Microbial Analysis, Resource, and Service facility.
Bacterial DNA is then isolated from stool samples by a protocol tested in preliminary experiments (Cong, Judge et al., 2017; Cong, Xu, Janton et al., 2016). The V4 region of the 16S rRNA genes of the microbial community has been shown to work well for human fecal samples. Using 16S rRNA sequencing of time series from different individuals, one can compare changes in the composition and diversity of the microbiome over time and between subjects.
The physiology of the gut microbiome is determined using RNA-seq. The cDNA libraries are prepared using the TruSeq kit (Illumina, San Diego, CA) and sequenced on an Illumina HiSeq. The readings are then mapped against a curated set of reference genomes using CLC Genomics Workbench (CLC Bioinformatics [Qiagen], Redwood City, CA). If microbial species are abundant for which no genomes are available, additional sequencing of the metagenome is carried out based on nucleotide usage patterns. The sequences are annotated using a MG-RAST metagenomic server.
2.5 ∣. Data analysis
Study data are analyzed using the SPSS, SAS, and R statistical packages, as appropriate. Self-report clinical data is exported from the REDCap database to the SPSS v.23 database for management and analysis. QST and SNPs data are also entered into SPSS for analysis. Gut microbial data are analyzed using the Mothur software and imported into R 3.3.1. The final statistical analysis, including hypothesis testing, will be conducted using the R 3.3.1 package and SAS version 9.4 (Cary, NC). The plans for data analysis are described according to each aim.
For Aim 1, to document the feasibility of the study design and its interventions in the population of young adults with IBS, the analysis will include a description of the number of individuals invited to participate, the proportion of those individuals who meet inclusion criteria, and the proportion who eventually provide informed consent. Among those who are randomized to the two study groups, the summaries of completion of video modules and activity logs will be tallied. In the experimental group, daily IBS symptom ratings and completion of follow-up consultations with the study nurse will be examined. A rate of 75% for the completion of all modules, activity logs, and IBS symptom ratings will be deemed as adequate to demonstrate feasibility of the study protocol (Jarrett et al., 2016).
For Aim 2, the initial stage of data analysis will concentrate on comparing the effectiveness of the IBS-pain SM intervention (IBS pain SM knowledge and skills ± support for monitoring and problemsolving) on IBS-pain SM behaviors and related health outcomes (BPI severity and interference, anxiety, depression, fatigue, CSQ-R scales, IBS-QOL, pharmacological, and nonpharmacological therapy use, physical activity and healthcare visits). Because the study involves three repeated measurements (initial visit, 6-, and 12-week follow-up) in two independent groups, repeated measures analysis of covariance will be used to estimate and contrast means between the experimental and control conditions at the 6- and 12-week time points, with the initial visit value of the dependent variable as a covariate. Differences in means are evaluated as main effects of treatment, time effects, and treatment-by-time interactions.
The secondary aim is to explore the influence of genetic polymorphisms and gut microbiome characteristics on pain and other symptoms and on change in symptoms and well-being in the groups at 6 and 12 weeks post-intervention. Mixed-effects linear modeling will be used to evaluate treatment effects while simultaneously investigating effects of pain sensitivity genotypes and pain sensitivity QST measures on outcome variables, to explore whether pain sensitivity traits are associated with IBS symptoms and management strategies and/or mediate or moderate intervention effectiveness. For microbiome data, descriptive statistical methods are used to document temporal changes in microbiome characteristics relative to: (1) week of study participation; (2) initiation and completion of RN consultations and food/activity diaries in the experimental group; and (3) changes in dietary composition in both groups. In turn, changes in IBS outcome measures for individual participants over time will be graphed relative to changes in their microbiome characteristics, to assess any indication that changes in outcome variables to be preceded by changes in microbiome.
3 ∣. DISCUSSION
3.1 ∣. Progress to date
Participant recruitment began on October 12, 2016. As of September 30, 2017, 79 subjects had been screened and 60 had been enrolled in the study. Of these enrolled participants, 27 have completed the study, 17 dropped out, and 16 are still enrolled. All 27 completed participants completed questionnaires, somatosensory testing, DNA samples for genotyping, and stool samples at all three time points. Data management and analysis, including genetic and genomic data sequencing, clustering and analysis, and IBS related clinical data analysis, are ongoing.
Of the 27 individuals who have completed the study, 14 were enrolled into the experimental group. One participant was randomized to the experimental arm but declined and opted to continue in the control arm. Of the participants in the experimental group, only 8 completed all entries in the daily log, although 11 of 14 completed at least half of the entries.
3.2 ∣. Challenges encountered
At this time, the most challenging aspect of our study implementation has been the high dropout rate, currently at 28%. This dropout rate is similar in both the treatment and the control groups, and the majority of these dropouts occurred prior to 6 weeks (the second study visit). Of note, 30% of these dropouts were participants recruited within 1 month prior to the academic winter break. We speculate that timing of mid-term and final exams may have affected study participation as well as subject burden.
In order to address this issue of study retention, the research team initiated multiple strategies. The participants were contacted up to three times via email with reminders about future appointments. These reminders are built into the daily workings of the research laboratory and did not increase experimental burden. Possibly the most significant strategy was the modification in the consent process to stress the time commitment required for study participation. This included a detailed explanation of the time needed to watch the videos and complete the daily log. The study module video schedule was changed so that participants could watch them back-to-back as their schedule allowed, instead of having to watch them on specific days. The policy on stool sample delivery was also changed to allow for individuals to mail in samples at their convenience and receive an e-gift card. Future statistical analyses will determine the effectiveness of the modifications on study retention.
Another issue that required attention was the slow recruitment rate within the first year of the study. To address this, the study team expanded clinical recruitment sites to two more campuses in the local region and increased the age limit to 29 years old. Also, noting the slow recruitment and high drop-out rate around exam and holiday weeks, the team made a conscious effort to monitor the scheduling accordingly.
Although challenges exist, it is hoped that this pilot study will demonstrate whether adding nurse consultations to existing instructional material can enhance symptom management in patients with IBS and whether pain-sensitivity genes and the gut microbiome can influence severity of symptoms as well as response to self-management (SM) interventions. The present study will inform larger tests of interventions to promote symptom management in this population.
ACKNOWLEDGMENT
This publication was supported by the National Institute of Nursing Research of the National Institutes of Health (NIH-NINR) under award number: NIH-NINR P20NR016605 (PI Starkweather)—Pilot 1 sub-award (PI: Cong).
Funding information
National Institute of Nursing Research of the National Institutes of Health (NIH-NINR), Grant number: NIH-NINR P20NR016605
REFERENCES
- AHRQ. (2014). Patient self-management support programs: An evaluation. Retrieved from http://www.ahrq.gov/research/findings/final-reports/ptmgmt/index.html
- Bohn L, Storsrud S, Tornblom H, Bengtsson U, & Simren M (2013). Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. American Journal of Gastroenterology, 108, 634–641. 10.1038/ajg.2013.105 [DOI] [PubMed] [Google Scholar]
- Camilleri M, Lasch K, & Zhou W (2012). Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. American Journal of Physiology: Gastrointestinal and Liver Physiology, 303, G775–G785. [DOI] [PubMed] [Google Scholar]
- Canavan C, West J, & Card T (2014). The epidemiology of irritable bowel syndrome. Clinical Epidemiology, 6, 71–80. 10.2147/CLEP.S40245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Lembo A, & Sultan S (2014). American Gastroenterological Association Institute Technical review on the pharmacological management of irritable bowel syndrome. Gastroenterology, 147, 1149–1172, e1142. 10.1053/j.gastro.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Cong X, Judge M, Xu W, Diallo A, Janton S, Brownell EA, … Graf J (2017). Influence of feeding type on gut microbiome development in hospitalized preterm infants. Nursing Research, 66, 123–133. 10.1097/NNR.0000000000000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong X, Perry M, Bernier KM, Young EE, & Starkweather A (2017). Effects of self-management interventions in patients with irritable bowel syndrome: Systematic review. Western Journal of Nursing Research, 10.1177/0193945917727705. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong X, Xu W, Janton S, Henderson WA, Matson A, McGrath JM, … Graf J (2016). Gut microbiome developmental patterns in early life of preterm infants: Impacts of feeding and gender. PLoS ONE, 11(4), e0152751 10.1371/journal.pone.0152751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong X, Xu W, Romisher R, Poveda S, Forte S, Starkweather A, & Henderson WA (2016). Gut microbiome and infant health: Brain-gut-microbiota axis and host genetic factors. Yale Journal of Biology and Medicine, 89, 299–308. [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, & Cryan JF (2012). Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology, 37, 1369–1378. 10.1016/j.psyneuen.2012.03.007 [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, … Zigmond MJ (2006). Neurobiology of exercise. Obesity (Silver Spring), 14, 345–356. 10.1038/oby.2006.46 [DOI] [PubMed] [Google Scholar]
- Dorn SD, Palsson OS, Woldeghebriel M, Fowler B, McCoy R, Weinberger M, & Drossman DA (2015). Development and pilot testing of an integrated, web-based self-management program for irritable bowel syndrome (IBS). Neurogastroenterology and Motility, 27(1), 128–134. 10.1111/nmo.12487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frissora CL, & Koch KL (2005). Symptom overlap and comorbidity of irritable bowel syndrome with other conditions. Current Gastroenterology Report, 7(4), 264–271. [DOI] [PubMed] [Google Scholar]
- Fukudo S, Kaneko H, Akiho H, Inamori M, Endo Y, Okumura T, … Shimosegawa T (2015). Evidence-based clinical practice guidelines for irritable bowel syndrome. Journal of Gastroenterology, 50, 11–30. 10.1007/s00535-014-1017-0 [DOI] [PubMed] [Google Scholar]
- Hahn BA, Kirchdoerfer LJ, Fullerton S, & Mayer E (1997). Evaluation of a new quality of life questionnaire for patients with irritable bowel syndrome. Alimentary Pharmacology and Therapeutics, 11, 547–552. [DOI] [PubMed] [Google Scholar]
- Hall KT, Lembo AJ, Kirsch I, Ziogas DC, Douaiher J, Jensen KB, … Kaptchuk TJ (2012). Catechol-O-methyltransferase val158met polymorphism predicts placebo effect in irritable bowel syndrome. PLoS ONE, 7(10), e48135 10.1371/journal.pone.0048135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CJ, Kohen R, Jun S, Jarrett ME, Cain KC, Burr R, & Heitkemper MM (2017). COMT Val158Met polymorphism and symptom improvement following a cognitively focused intervention for irritable bowel syndrome. Nursing Research, 66, 75–84. 10.1097/NNR.0000000000000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PA, Zola H, Penttila IA, Blackshaw LA, Andrews JM, & Krumbiegel D (2013). Immune activation in irritable bowel syndrome: can neuroimmune interactions explain symptoms? American Journal of Gastroenterology, 108, 1066–1074. 10.1038/ajg.2013.120 [DOI] [PubMed] [Google Scholar]
- Jarrett ME, Cain KC, Burr RL, Hertig VL, Rosen SN, & Heitkemper MM (2009). Comprehensive self-management for irritable bowel syndrome: Randomized trial of in-person versus combined in-person and telephone sessions. American Journal of Gastroenterology, 104, 3004–3014. 10.1038/ajg.2009.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett ME, Han CJ, Cain KC, Burr RL, Shulman RJ, Barney PG, … Heitkemper MM (2016). Relationships of abdominal pain, reports to visceral and temperature pain sensitivity, conditioned pain modulation, and heart rate variability in irritable bowel syndrome. Neurogastroenterology and Motility, 28, 1094–1103. 10.1111/nmo.12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesson E, Simren M, Strid H, Bajor A, & Sadik R (2011). Physical activity improves symptoms in irritable bowel syndrome: A randomized controlled trial. American Journal of Gastroenterology, 106, 915–922. 10.1038/ajg.2010.480 [DOI] [PubMed] [Google Scholar]
- Kanazawa M, Hongo M, & Fukudo S (2011). Visceral hypersensitivity in irritable bowel syndrome. Journal of Gastroenterology and Hepatology, 26(Suppl 3), 119–121. 10.1111/j.1440-1746.2011.06640.x [DOI] [PubMed] [Google Scholar]
- Karling P, Danielsson A, Wikgren M, Soderstrom I, Del-Favero J, Adolfsson R, & Norrback KF (2011). The relationship between the val158met catechol-O-methyltransferase (COMT) polymorphism and irritable bowel syndrome. PLoS ONE, 6(3), e18035 10.1371/journal.pone.0018035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, & Cleeland CS (2004). Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clinical Journal of Pain, 20, 309–318. [DOI] [PubMed] [Google Scholar]
- Kerckhoffs AP, Samsom M, van der Rest ME, de Vogel J, Knol J, Ben-Amor K, & Akkermans LM (2009). Lower Bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World Journal of Gastroenterology, 15, 2887–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristal AR, Feng Z, Coates RJ, Oberman A, & George V (1997). Associations of race/ethnicity, education, and dietary intervention with the validity and reliability of a food frequency questionnaire: The Women’s Health Trial feasibility study in minority populations. American Journal of Epidemiology, 146, 856–869. [DOI] [PubMed] [Google Scholar]
- Lacy BE, Chey WD, & Lembo AJ (2015). New and emerging treatment options for irritable bowel syndrome. Gastroenterology and Hepatology (NY), 11(4 Suppl 2), 1–19. [PMC free article] [PubMed] [Google Scholar]
- Longstreth GF, Wilson A, Knight K, Wong J, Chiou CF, Barghout V, … Ofman JJ (2003). Irritable bowel syndrome, health care use, and costs: A U.S. managed care perspective. American Journal of Gastroenterology, 98, 600–607. [DOI] [PubMed] [Google Scholar]
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, & Spiller RC (2006). Functional bowel disorders. Gastroenterology, 130, 1480–1491. 10.1053/j.gastro.2005.11.061 [DOI] [PubMed] [Google Scholar]
- Makker J, Chilimuri S, & Bella JN (2015). Genetic epidemiology of irritable bowel syndrome. World Journal of Gastroenterology, 21, 11353–11361. 10.3748/wjg.v21.i40.11353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman L, Stridsberg M, Isaksson S, Jerlstad P, & Simren M (2012). Altered levels of fecal chromogranins and secretogranins in IBS: Relevance for pathophysiology and symptoms? American Journal of Gastroenterology, 107, 440–447. 10.1038/ajg.2011.458 [DOI] [PubMed] [Google Scholar]
- Rajilic-Stojanovic M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, & de Vos WM (2011). Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology, 141, 1792–1801. 10.1053/j.gastro.2011.07.043 [DOI] [PubMed] [Google Scholar]
- Robins H, Perron V, Heathcote LC, & Simons LE (2016). Pain neuroscience education: State of the art and application in pediatrics. Children (Basel), 3(4), 43 10.3390/children3040043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ME, Riley JL 3rd, Myers CD, Sadler IJ, Kvaal SA, Geisser ME, & Keefe FJ (1997). The Coping Strategies Questionnaire: A large sample, item level factor analysis. Clinical Journal of Pain, 13(1), 43–49. [DOI] [PubMed] [Google Scholar]
- Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, & Treede RD (2006). Quantitative sensory testing: A comprehensive protocol for clinical trials. European Journal of Pain, 10(1), 77–88. 10.1016/j.ejpain.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Ryan P, & Sawin KJ (2009). The individual and family self-management theory: Background and perspectives on context, process, and outcomes. Nursing Outlook, 57, 217–225, e216. 10.1016/j.outlook.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simren M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, … Vanner S (2013). Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut, 62, 159–176. 10.1136/gutjnl-2012-302167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YA, Park SY, Park YL, Chung CY, Lee GH, Cho DH, … Joo YE (2012). Association between single nucleotide polymorphisms of the transient receptor potential vanilloid 1 (TRPV-1) gene and patients with irritable bowel syndrome in Korean populations. Acta Gastroenterologica Belgica, 75, 222–227. [PubMed] [Google Scholar]
- Starkweather AR, Heineman A, Storey S, Rubia G, Lyon DE, Greenspan J, & Dorsey SG (2016). Methods to measure peripheral and central sensitization using quantitative sensory testing: A focus on individuals with low back pain. Applied Nursing Research, 29, 237–241. 10.1016/j.apnr.2015.03.013 [DOI] [PubMed] [Google Scholar]
- Stinson JN, Lalloo C, Harris L, Isaac L, Campbell F, Brown S, & Karim A (2014). ICanCope with Pain: User-centred design of a web- and mobile-based self-management program for youth with chronic pain based on identified health care needs. Pain Research and Management, 19, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YR, Yang WW, Liang ML, Xu XY, Wang MF, & Lin L (2012). Age-related symptom and life quality changes in women with irritable bowel syndrome. World Journal of Gastroenterology, 18, 7175–7183. 10.3748/wjg.v18.i48.7175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YR, Yang WW, Wang YL, & Lin L (2012). Sex differences in the symptoms and psychological factors that influence quality of life in patients with irritable bowel syndrome. European Journal of Gastroenterology and Hepatology, 24, 702–707. 10.1097/MEG.0b013e328351b2c2 [DOI] [PubMed] [Google Scholar]
- Trinkley KE, & Nahata MC (2014). Medication management of irritable bowel syndrome. Digestion, 89, 253–267. 10.1159/000362405 [DOI] [PubMed] [Google Scholar]
- Verdugo R, & Ochoa JL (1992). Quantitative somatosensory thermotest. A key method for functional evaluation of small calibre afferent channels. Brain, 115(Pt 3), 893–913. [DOI] [PubMed] [Google Scholar]
- Voreades N, Kozil A, & Weir TL (2014). Diet and the development of the human intestinal microbiome. Frontiers in Microbiology, 5, 494 10.3389/fmicb.2014.00494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead WE, Palsson O, & Jones KR (2002). Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology, 122, 1140–1156. [DOI] [PubMed] [Google Scholar]