Abstract
Polyphosphoinositides (PPIn) are essential signaling phospholipids that make remarkable contributions to the identity of all cellular membranes and signaling cascades in mammalian cells. They exert regulatory control over membrane homeostasis via selective interactions with cellular proteins at the membrane–cytoplasm interface. This review article briefly summarizes our current understanding of the key roles that PPIn play in orchestrating and regulating crucial electrical and chemical signaling events in mammalian neurons and the significant neuro-pathophysiological conditions that arise following alterations in their metabolism.
Keywords: Endoplasmic reticulum, Voltage gated Ca2+ channel, Voltage gated K+ channel, Membrane contact site, Phosphatidylinositol, Phospholipase C, Polyphosphoinositide, Phospholipids, Plasma membrane, Phosphoinositide, Neuron, Ion channel
Introduction
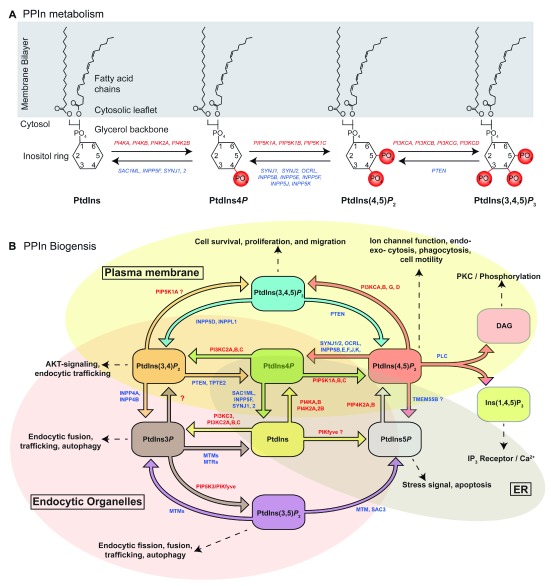
Polyphosphoinositides (PPIn) are a family of minor (low-abundance), negatively charged phospholipid molecules found on the cytoplasmic leaflet of all cellular membranes that play critical roles in membrane homeostasis and cellular signaling 1. Structurally, they consist of two fatty acid chains (that insert into the cytosolic leaflet of cellular membranes), a glycerol moiety, and an inositol headgroup ( Figure 1A).
Figure 1. Phosphoinositide metabolism and biogenesis.
( A) Phosphoinositide metabolism. Hypothetical equilibrium reaction involving four polyphosphoinositide (PPIn) species at the membrane–cytosol interface. The basic structure of the parent PPIn, phosphatidylinositol (PtdIns), forms the substrate for subsequent PPIn species. Red labels represent gene names of lipid kinases that catalyze the addition of phosphate groups (phosphorylate) at specific positions of the inositol ring. Blue labels represent gene names of lipid phosphatases that remove phosphate groups (dephosphorylate) at specific positions of the inositol ring. ( B) Phosphoinositide biogenesis. Diagram summarizing the major PPIn lipid kinase and phosphatase reaction pathways. Red and blue labels are the gene names of enzymes capable of catalyzing each reaction. Gene names with question marks (?) represent enzymes with some uncertainty surrounding their ability to catalyze a specific reaction. Dashed arrows represent the major cellular roles for each individual PPIn. Colored circles represent the approximate cellular locations of each PPIn species. ER, endoplasmic reticulum; PKC, protein kinase C; PTEN, phosphatase and tensin homolog.
In primary mammalian cells, about 80% of the phosphoinositide (PI) molecules have stearoyl/arachidonyl as their fatty acid chains 2– 4 ( Figure 1A, “Fatty acid chains”). Typically, this is designated C18:0/C20:4 (the number of carbons:number of double bonds in each fatty acid) or 38:4 for the whole molecule. A small but increasing body of evidence suggests that the fatty acid chains of a given PPIn themselves could represent a signaling code. For example, it has been suggested that different fatty acid chains may confer substrate preferences at the level of one or more lipid kinases and lipid phosphatases 5, 6; however, this is an area of work that requires further investigation.
The vast majority of work detailing the ability of PPIn to act as signaling moieties involves the inositol headgroup. Indeed, it is the inositol headgroup that can be selectively phosphorylated by specific lipid kinases ( Figure 1A) at one of three positions (D-3, D-4, or D-5) to generate seven PPIn species from the parent, phosphatidylinositol (Ptdlns). Each of the seven PPIn species—three monophosphorylated phosphoinositides (PtdIns3 P, PtdIns4 P, and PtdIns5 P), three bisphosphorylated phosphoinositides (PtdIns(3,5) P 2 [Phosphatidylinositol 3,5-bisphosphate], PtdIns(4,5) P 2, and PtdIns(3,5) P 2), and a single trisphosphorylated phosphoinositide (PtdIns(3,4,5) P 3 [Phosphatidylinositol 3,4,5 trisphosphate]) ( Figure 1B)—has signature cellular locations ( Figure 2). For example, PtdIns4 P within the cell can be found at the plasma membrane (PM), endosomes, and trans-Golgi network, whereas the majority of PtdIns(4,5) P 2 or PtdIns(3,4,5) P 3 within cells are found mostly at the PM. Precise spatial regulation of PPIn distribution is critical for regulated cellular function and is carefully controlled through the catalytic actions of around 50 (34 phosphatases and 20 kinases) 7 differentially localized PPIn-metabolizing enzymes, each with highly specific preferences for a given PPIn species headgroup.
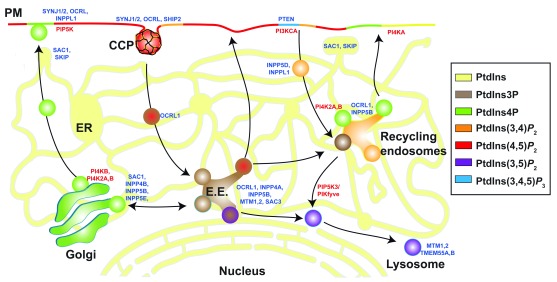
Figure 2. Phosphoinositide zip code.
Cellular distribution of polyphosphoinositide (PPIn) species and metabolizing enzymes. Diagram depicting the signature distribution of each PPIn species and approximate location of enzymes regulating each species. Blue and red labels represent PPIn phosphatases and PPIn kinases, respectively. E.E., early endosome; ER, endoplasmic reticulum; PM, plasma membrane; PtdIns, phosphatidylinositol.
Despite contributing a small fraction to the bulk of cellular phospholipids, PPIn make striking contributions to practically every aspect of cell biology/physiology. They do so by recruiting and interacting with proteins at the membrane–cytoplasm interface to organize and shape organelle identity. There are many excellent reviews 1, 8– 10 that discuss PPIn distribution, metabolism, and function across many cell types; these articles are wonderful starting points to inform readers of the general principles and importance of these essential signaling lipids. This review article briefly summarizes our current understanding of the essential role(s) of PPIn in orchestrating and regulating crucial signaling events in the mammalian nervous system and puts particular emphasis on recent work. Further highlighting the roles of these lipids, we discuss the implications for human health and devastating disorders that arise when phosphoinositide metabolism goes awry.
Biogenesis, distribution, and roles of polyphosphoinositides in the nervous system
To begin, we focus on the biogenesis of each individual PPIn species and their membrane distribution and define their cellular roles in healthy cells of the nervous system.
Phosphatidylinositol
PtdIns, the precursor of all PPIn ( Figure 1A and B), is the most abundant PPIn species, contributing about 10 to 20 mol % of total membrane phospholipid content. The most abundant isoform is PtdIns 38:4, which (it should be noted) is different from the most abundant isoform in heterologous expression cell lines (PtdIns 36:1) 11, 12. The difference in fatty acid composition in cultured cells compared with primary cells remains to be fully determined. We know very little about its subcellular distribution despite being several orders of magnitude more concentrated in cellular membranes than other PPIn species. PtdIns is synthesized following the simple conjugation reaction of myo-inositol and CDP-DAG, catalyzed by a PtdIns synthase (PIS) enzyme in endoplasmic reticulum (ER) membranes. Following its biogenesis, PtdIns is transported out of the ER through one of the following three routes: (1) vesicular transport, (2) non-vesicular lipid transfer protein mechanisms at membrane contact sites 13– 17, or (3) via highly mobile PIS-containing vesicles 18. The use of lipid-binding domains for PtdIns4 P and PtdIns3 P has revealed that target membranes for PtdIns delivery include the plasma and Golgi membranes 19– 21 as well as a pool of endo-membranes ( Figure 1B). It remains to be seen whether significant amounts of PtdIns concentrate at these specific endo-membranes or it is rapidly transferred de novo to generate mono-phosphorylated species. Most investigations have focused on PtdIns as an essential precursor lipid for the generation of PtdIns4 P or PtdIns(4,5) P 2 18; this is almost certainly due to the lack of a faithful biosensor to rigorously investigate its distribution and metabolism.
For the nervous system, alterations in the concentration of one of the essential substrates for PtdIns synthesis, myo-inositol, or expressional change in a myo-inositol transporter, SMIT1 ( SLC5A3 gene), modify neuronal excitability through downstream alterations in PtdIns(4,5) P 2 metabolism 22 and direct interactions with KCNQ1/KCNE2 complexes 23, respectively. This information pairs well with older literature demonstrating that lithium, administered at therapeutically relevant doses, reduces myo-inositol and subsequently PtdIns to aid in the recovery of mood disorders, including bipolar affective disorder 24, 25. For PtdIns transfer proteins (PITPs), such as the Sec14-like or START-like proteins, there are strong links to human disease, such as the progressive neurodegenerative disorder vitamin E status ataxia with vitamin E deficiency (AVED) and a rare autosomal recessive disorder called Cayman-type cerebellar ataxia, to name a few (reviewed in 26). Further underscoring the importance of PITPs, a murine knockout model of PITPα presents striking neurological defects 27. Together, these data underscore the importance of PtdIns transport and metabolism for regulated nervous system function. Despite this knowledge, there are significant questions that remain unanswered in neurons, including the steady-state cellular distribution/metabolism of PtdIns and how this may be affected during signaling reactions or disease, and the role of membrane contact site proteins that transport PtdIns, such as TMEM24 13. Hopefully, the development of tools to visualize PtdIns will offer helpful insights into some of these unanswered questions.
Phosphatidylinositol 3-monophosphate
Phosphatidylinositol 3-monophosphate (PtdIns3 P) is the signature PPIn of endosomes and autophagosomes. Despite its relatively low abundance (20%–30% of PtdIns4 P), it is a key regulator of endocytic trafficking, fusion, and autophagy (for review, see 28) via PtdIns3 P-dependent interactions with PX or FYVE domains on proteins involved in cargo sorting, positioning, and maturation. PtdIns3 P is derived mainly from phosphorylation of PI by PI3K-II or PI3K-III 29– 31, and additional contributions are made from dephosphorylation of phosphatidylinositol 3,4-bisphosphate (PtdIns[3,4] P 2) by PtdIns(3,4) P 2 4-phosphatases and PtdIns(3,5) P 2 by PtdIns(3,5) P 2 5-phosphatases ( Figure 1B). For the nervous system, it has been reported that PI(3)P is involved (through WDR91–Rab7 interactions) in the regulation of dendritic arborization and post-natal development of the mouse brain 32, control of axonal transport and growth 33, and GABAergic neurotransmission at inhibitory post-synapses 34. Finally, underscoring a major role for PtdIns3 P in the nervous system, deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration 35.
Phosphatidylinositol 4-monophosphate
Phosphatidylinositol 4-monophosphate (PtdIns4 P) can be directly synthesized from PtdIns at the plasma and Golgi membranes via the actions of PtdIns 4-kinases, with neuronal PM PtdIns4 P also potentially augmented via the actions of synaptojanins 36 and oculocerebrorenal syndrome of Lowe (OCRL) proteins 37, 38, which dephosphorylate PtdIns(4,5) P 2 into PtdIns4 P ( Figure 1B). These two biosynthetic pathways, supplemented by PtdIns4 P generated by dephosphorylation of PtdIns(3,4) P 2 by PtdIns 3-phosphatase enzymes, ensure that PtdIns4 P is found across several different organelle compartments, including the PM, Golgi, and endosomes ( Figure 2). All of the PtdIns 4-kinases ( Figure 1B) are expressed in the brain, and PI4KA (PI4KIIIα) and PI4KB (PI4KIIIβ) isoforms are localized throughout the nervous system. PI4KIIIα appears to be more highly expressed in spinal cord and cerebral cortex neurons, whereas PI4KIIIβ has enhanced distribution in the cerebellar cortex 39, 40. Localization studies from the Human Protein Atlas have revealed that PI4K2A (PI4KIIα) is expressed across different neuronal and astrocyte populations, and there are high levels in Purkinje cells, hippocampus, and dentate gyrus; PI4K2B (PI4KIIβ) is expressed in the cerebellum, and the highest expression is reported for the hippocampus. Taken together, there is a large body of evidence that each of these enzymes is localized throughout the brain, including in many classes of neuron.
In the peripheral nervous system, PI4KIIIα was recently reported to play an essential role in myelin formation as Schwann cell–specific inactivation of the gene caused myelination defects and gross alterations in actin architecture 41. Currently, there is little direct information visualizing the distribution of PtdIns4 P in central nervous system neurons. Information gained from sympathetic superior cervical ganglia (SCG) neurons 11, expressing a biosensor for PtdIns4 P (P4M) 42, suggests that a significant portion of the lipid resides at the PM at rest and that other pools are in intracellular organelles (likely the trans Golgi and endosomes). Such a distribution is consistent with other reports from mammalian expression system cells 42, 43, suggesting a conserved localization of PtdIns4 P-metabolizing enzymes. Interestingly, the same authors [11] revealed a threefold accelerated synthesis of PM PtdIns4 P in SCG neurons, suggesting higher enzymatic activity of the lipid 4-kinase. Thus, there may be subtle differences in enzyme abundance, activity, and localization in primary neuronal cells. Refined experimental designs/tools will be necessary to analyze the molecular mechanisms underlying the accelerated synthesis of PM PtdIns4 P in neurons. For the other main cellular source of PtdIns4 P, the trans Golgi, information from non-neuronal cells reveals that PI4KB and PI4K2A and -2B all contribute to its synthesis. PI4KB is recruited to the Golgi by Arf1 44– 46, whereas PI4K2A and -2B contribute to Golgi PtdIns4 P via lipid modifications and perhaps cholesterol-rich domains 47– 51.
Both PM and Golgi PtdIns4 P pools appear under further regulatory control by the lipid transfer proteins ORP5/8 (oxysterol-binding protein-related proteins5/8) 52– 55 and OSBP (oxysterol-binding protein) 56, 57, respectively. At the PM, ORP5/8 are localized to regions of close proximity (15–20 nm) between the ER and PM, termed ER-PM contact sites. These membrane contacts visualized in excitable cells 58– 60, including neurons 61, are sites of close organelle membrane apposition that facilitate information transfer (lipids and ions), independent of vesicular transport. Such membrane fusion-independent lipid transport is likely to be essential in complex cells, like neurons, where organelle compartments are often separated by large distances. Through binding of their N-terminal pleckstrin homology (PH) domains with PtdIns4 P 55 or PtdIns(4,5) P 2 53 or both 52, the ER-localized ORP5/8 dock with the PM. Despite not being functionally characterized in neurons, the ubiquitously expressed ORP5/8, similar to other mammalian cells, are likely to facilitate the counter-transport of phosphatidylserine (to the PM) for PtdIns4 P (to the ER). Transported PtdIns4 P is then likely to be dephosphorylated to PtdIns by the ER PtdIns4 P-4-phosphatase, Sac1. Thus, ORP5/8 may serve not only to tune PM PtdIns4 P but also to aid in the maintenance of ER PtdIns levels. At ER–Golgi membrane contact sites, OSBP1 also serves to regulate PtdIns4 P abundance. Once positioned at ER–Golgi membrane contact sites, OSBP exchanges cholesterol (on ER membrane) for PtdIns4 P (on trans-Golgi membrane) 56, 57. Compelling evidence for the importance of PtdIns4 P in the nervous system is demonstrated by PI4K2A gene-trapped mice developing late-onset spinocerebellar axonal degeneration and the presence of PI4K2A on synaptic vesicles 62.
Phosphatidylinositol 5-monophosphate
Phosphatidylinositol 5-monophosphate (PtdIns5 P) remains the most enigmatic of the PPIs because of its low abundance (similar to that of PtdIns3 P) and the current lack of a faithful biosensor. It is for these reasons that the effectors controlled by PtdIns5 P and the pathways it regulates are poorly understood relative to the other PPIn family members. How PtdIns5 P is biosynthesized remains controversial. Work on non-neuronal mammalian cells suggests two pathways for its generation: (1) directly by phosphorylation of PtdIns by a PI 5-kinase (such as PIKfyve or type I PI5K enzymes) 63– 65 or (2) indirectly via dephosphorylation of PtdIns(3,5) P 2 by the myotubularin phosphatases 66. PtdIns5 P was initially discovered as having a signaling role in the nucleus 67, 68 since reports of PtdIns5 P being involved in Akt/mammalian target of rapamycin (Akt/mTOR) signaling 69 and apoptosis 70 have been documented (for review see 71, 72. For the nervous system, there is little direct information regarding PtdIns5 P.
Phosphatidylinositol 4,5-bisphosphate
Phosphatidylinositol 4,5-bisphosphate (PtdIns[4,5] P 2) is the signature PPIn of the PM ( Figure 2) and undoubtedly the best-characterized PPIn of the nervous system. It is produced primarily through the phosphorylation of PtdIns4 P by type I PtdIns4 P 5-kinases (α, β, and γ), although there may be minor contributions from PtdIns(3,4,5) P 3 5-phosphatases (PTEN) or PtdIns5 P 4-kinases ( Figure 1B). PtdIns(4,5) P 2 is under a further layer of regulation from PtdIns(4,5) P 2 5-phosphatases, like synaptojanin 1 and 2 and OCRL (Figures 1B and 2). Underscoring the importance of these enzymes for human health, mutations in the genes that encode the PtdIns(4,5) P 2 5-phosphatases result in a host of human disorders of the nervous system, including seizures 73, Alzheimer’s 74, Down syndrome 75, Parkinson’s 76, and Lowe syndrome 37.
PtdIns(4,5) P 2 plays an essential role in regulating many essential PM events, including electrical signaling ( Figure 3Ai), synaptic plasticity 77, endocytosis, and exocytosis ( Figure 3Aiii). It also acts as a substrate for phospholipase C (PLC) following G protein–coupled receptor (GPCR) activation ( Figure 3Aii). To date, around 100 ion channels and transporters have been shown to be directly regulated by this lipid (for review, see [10]); many of these PtdIns(4,5) P 2-sensitive channels, including voltage-gated potassium channels 78– 80 and voltage-gated calcium channels 81, are found in cells of the nervous system ( Figure 3Ai). Thus, alterations in abundance or distribution (or both) of this minor lipid can significantly alter electrical activity in neurons 11, 82. One such mechanism that dynamically modulates PM PtdIns(4,5) P 2 abundance is binding of modulatory neurotransmitters to receptors coupled to PLC ( Figure 3ii). The consequence of PLC activation is rapid hydrolysis of PtdIns(4,5) P 2 into soluble IP 3 and membrane-bound diacylglycerol (DAG). Consequently, modulatory neurotransmitters of the nervous system that couple to G q have the potential to nearly synchronously switch off specific ion channels, initiate Ca 2+ from IP 3R on ER membranes, and recruit protein kinase C (PKC) to the PM. Termination of these signaling reactions, following removal of neurotransmitter from the synaptic cleft, allows PtdIns(4,5) P 2 to be rapidly resynthesized 11. The source or sources of PtdIns4 P that serve as precursor sources for the PM PtdIns(4,5) P 2 pool that supports ion channel activity appear to originate from the PM 11, 83 and trans-Golgi membranes 84.
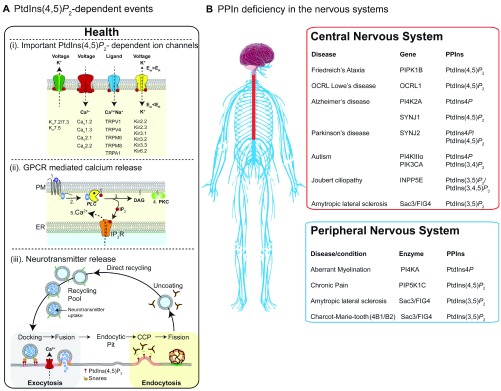
Figure 3. Roles for polyphosphoinositides (PPIn) in the nervous system in health and disease.
( A) PtdIns(4,5) P 2-dependent events. Critical events regulated by plasma membrane (PM) PtdIns(4,5) P 2 within the nervous system. (i) Four families of ion channels that require PtdIns(4,5) P 2 as a co-factor for full function. (ii) PtdIns(4,5) P 2 is the critical precursor for generation of IP 3-mediated Ca 2+ release and protein kinase C (PKC)-mediated phosphorylation. Binding of ligand (1) releases the heterotrimeric G-protein G q (2) to activate phospholipase C (PLC), which subsequently hydrolyses PM PtdIns(4,5) P 2 into membrane-bound DAG and soluble IP 3 (3). DAG then can recruit PKC to phosphorylate protein targets (4) while IP 3 binds to the IP 3R on endoplasmic reticulum (ER) membranes to initiate Ca 2+ releases into the cytoplasm (5). (iii) Critical involvement of PtdIns(4,5) P 2 in neurotransmitter release. During calcium-regulated synaptic vesicle release, PtdIns(4,5) P 2 is required to attract many proteins to the PM active zone for docking and fusion. After fusion, the vesicle membrane is recovered via the clathrin adapter protein AP2 to form clathrin-coated pits (CCP), before dynamin-dependent membrane scission occurs during the final stages of endocytosis. ( B) PPIn deficiency in the nervous system. Diseases and cellular consequences for altered PPIn metabolism in the central (red box) and peripheral (blue box) nervous systems. CCP, clathrin coated pit; GPCR, G protein–coupled receptor; PtdIns, phosphatidylinositol.
Phosphatidylinositol 3,4-bisphosphate
PtdIns(3,4) P 2 is localized mainly to the PM and endocytic compartments ( Figure 2), where it typically interacts with TAPP domain-containing proteins to orchestrate signaling cascades. Current evidence suggests that the majority of PtdIns(3,4) P 2 is formed through the actions of INPP5D (SHIP1) and INPPL1 (SHIP2) phosphatases following class I PI3K-mediated generation of PtdIns(3,4,5) P 3 at the PM 85– 87 ( Figure 1B and 2) or by phosphorylation of PtdIns4 P via class II PI3K lipid kinases 88. PtdIns(3,4) P 2 is under a further layer of regulation through the metabolic phosphatase actions of INPP4A/B 89 and PTEN 87, which act on their substrates to generate PtdIns3 P and PtdIns4 P, respectively. PtdIns(3,4) P 2 is involved in maturation of late-stage clathrin-coated pits [89] and in fast endophilin-mediated endocytosis 90, 91. Loss of INPP4A function leads to neurodegeneration through a mechanism thought to involve enhanced neuronal susceptibility to glutamate-induced excitotoxicity 89, underscoring a potential neuroprotective role for INPP4A by tuning PtdIns(3,4) P 2 signaling pathways. Finally, clustering of PtdIns(3,4) P 2 appears necessary and sufficient for actin-mediated neurite initiation and dendrite morphogenesis 92. Thus, PtdIns(3,4) P 2 is thought to play a role in the maintenance of nervous system function via its role at the PM, endocytotic membranes, and more distal membrane compartments.
Phosphatidylinositol 3,5-bisphosphate
PtdIns(3,5) P 2 is the low-abundance (<0.1% of total PPIs) signature PPIn of late endosomal/lysosomal membranes ( Figure 2). Current knowledge indicates that the synthesis and turnover of PtdIns(3,5) P 2 are tightly controlled by a large protein complex that includes Vac14, PIKfyve, and FIG4. Through direct interactions, Vac14 nucleates the complex between the PtdIns3 P 5-kinase (PIKfyve) and PtdIns(3,5) P 2 5-phosphatase (Sac3/FIG4) to ensure tight coordination between synthesis and degradation of PtdIns(3,5) P 2 66, 93 ( Figure 1B). At steady state, the relatively low abundance of PtdIns(3,5) P 2 is important for membrane trafficking, endocytic vesicle fission/fusion, organelle pH, and intracellular ion channel function 94– 98. Mouse models of Fig4 and Vac14 deletions and a mutation within PIKfyve exhibit embryonic lethality or severe neurodegenerative phenotypes 66, 99, 100. PtdIns(3,5) P 2 abundance has also been correlated with long-term depression, and the activity of PIKfyve is seemingly involved in modifying synaptic strength 100, 101. Together, these observations suggest that PtdIns(3,5) P 2 is essential for proper development in the nervous system.
Phosphatidylinositol 3,4,5 trisphosphate
PtdIns(3,4,5) P 3 is generated following binding of extracellular stimuli—for example, growth factors epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and insulin-like growth factor-I (IGF-I)—to receptors that activate class I PI3K to phosphorylate PtdIns(4,5) P 2 into PtdIns(3,4,5) P 3 ( Figure 1B). Receptor-mediated elevations in PtdIns(3,4,5) P 3 lead to recruitment of protein kinases (for example, AKT, BKT, and PDK1) to the PM to shape downstream cellular signaling cascades. PtdIns(3,4,5) P 3/PI3K activity has been implicated in many facets of nervous system function; for example, PtdIns(3,4,5) P 3 appears to be involved in clustering Syntaxin1A to regulate neurotransmitter release 102, while levels of Akt regulate axon branching, formation of dendritic spines, cell hypertrophy, growth cone expansion, and axon regeneration in neurons 103– 105. PtdIns(3,4,5) P 3 levels are under tight regulatory control by the catalytic activity of the protein and lipid phosphatase, PTEN. PTEN is widely expressed in mouse brain, and there is some preferential distribution in Purkinje neurons and some pyramidal neurons 106, where it is thought to be involved in neuronal migration, size, and survival 106, 107. Interestingly, PTEN has been reported as a potential target for neuroprotection and neuroregeneration following insult or injury (for review, see 108). In these studies, upregulation of mTOR activity in corticospinal neurons via conditional deletion of PTEN, a negative regulator of mTOR, enables successful regeneration of a group of injured axons 109. Along similar lines, conditional inactivation 110 or inhibition 111 of PTEN function in oligodendrocytes is required to regulate myelin thickness and preserve axon integrity.
Polyphosphoinositides in diseases of the nervous system
In health, the PPIn zip code ( Figure 2) is established through the combined spatial and temporal activities of over 50 PPI-metabolizing enzymes [7]. Through the actions of each of the 34 PPIn phosphatases and 20 PPIn kinases, each of the seven PPIn species is generated and interacts with over 400 different proteins at the membrane–cytosol interface 112. Given the sheer coverage of the intracellular PPIn interactome, it is perhaps not surprising that mutations in phosphoinositide kinases and phosphatases have been implicated in many human diseases of the nervous system ( Figure 3B). To date, over 20 monogenetic disorders have been reported to be caused by mutations in PPIn enzymes. Indeed, the role of PPIn phosphatases and kinases in health and disease has been covered comprehensively in several reviews 7, 113– 116. Here, we focus our attention on a few of the more commonly occurring neurological disorders that have been suggested to arise through defects in PPIn metabolism.
Several studies have suggested that mutations in genes coding for PPIn-metabolizing enzymes are associated with autism spectrum disorders. Interestingly, the majority of the PPIn enzymes associated with autism are PPIn kinases, and isoforms of the class 1 PI3K family (for review, see 117), PI4K 118, and PIP5K 119 are all reported to play prominent roles. For the PI4Ks, mutations in the peripheral membrane adaptor protein of the PI4KIIIα signaling complex, EFR3, are significantly more common among autism spectrum cases than controls 118. Thus, considerable evidence suggests that involvement in PPIn signaling in autism; however, how each of these PPI-metabolizing proteins contributes to the pathophysiology awaits further delineation.
Amyotrophic lateral sclerosis (ALS), commonly known as Lou Gehrig’s disease, is a progressive neurodegenerative disease characterized by selective motor neuron death leading to muscle atrophy, paralysis, and motor impairment. Currently, two proteins related to PPIn metabolism have been determined to be disease-causing ALS mutations. The first is a substitution of proline with serine at residue 56 on the vesicle-associated membrane protein (VAMP)-associated protein (VAP) VAPB gene (P56S; designated ALS8). VAPB is a conserved integral membrane protein of the ER found in all eukaryotic cells and regulates PPIn transport and homeostasis at ER-membrane contact sites 14, 15, 120– 122. At present, the molecular mechanism or mechanisms underlying ALS8 pathogenesis remain poorly understood; however, in transgenic mice, expression of human VAPB with the ALS8 mutation causes various motor behavioral abnormalities, including progressive hyperactivity 123. Thus, future investigations appear warranted to determine the downstream neuropathology associated with this mutation. The second PPIn protein associated with ALS involves the PtdIns(3,5) P 2 5-phosphatase, Sac3/FIG4. Mutations in Sac3/Fig4 result in a significant loss of protein function, resulting in this autosomal dominant form of ALS, designated ALS11 [99]. Further implicating alterations in PtdIns(3,5) P 2 metabolism as a potential risk factor for disease progression, mutations in PIKfyve production have been linked to neurological disorders such as ALS and Charcot–Marie–Tooth disease 66, 124. Indeed, mutations in myotubularin-related 2 (MTMR2), which preferentially dephosphorylates PtdIns3 P and PtdIns(3,5) P 2 into PtdIns and PtdIns5 P, respectively, cause autosomal recessive Charcot–Marie–Tooth disease type 4B (CMT4B1) 125– 127. This disorder manifests as childhood onset of progressive muscle weakness of the distal muscles and sensory loss that is characterized by decreased nerve conduction velocity and demyelination in the nerve 125. A less severe MTMR2 −/− mouse model develops azoospermia and abnormal peripheral nerve myelination with marked myelin sheath focal outfoldings in Schwann cells rather than peripheral motor neurons.
A growing body of evidence suggests that intracellular levels of PPIn are significantly altered in the two most prevalent neurodegenerative disorders: Alzheimer’s 74, 128, 129 and Parkinson’s 73, 76, 130. For Alzheimer’s disease, genetic polymorphisms or mutations in genes such as INPP5D 131 and SYNJ1 132 are risk factors for late-onset Alzheimer’s disease (LOAD), and there are several reports of amyloid beta–dependent alterations in the catalytic activity of synaptojanin 133 and PI4K2A 134. For Parkinson’s disease, an autosomal recessive R258Q mutation within the Sac domain of synaptojanin 1 was recently designated PARK20 76. At the cellular level, this mutation alters synaptic development and this is accompanied by endocytic defects and accumulation of clathrin-coated intermediates. At the behavioral level, mice harboring this mutation develop neurological symptoms similar to those of human patients. Further emphasizing the link between dysfunction in early endocytic traffic and Parkinson’s disease, loss-of-function mutations in the ER-lysosome tethering protein VPS13C result in a distinct form of early-onset parkinsonism characterized by rapid and severe disease progression and early cognitive decline 135, 136.
These highlighted examples fully underscore the importance of regulated PPIn metabolism for human health. Given the ubiquitous distribution of PPIn across all mammalian cells, the scale of the PPIn interactome, and their essential role in choreographing critical signaling events, it is perhaps inevitable that every human disease will exhibit some form of PPIn dysfunction.
Conclusions and future directions
In the past 20 years, there has been an explosion of research on PPIn signaling. The overarching narrative of this work is that PPIn are indispensable and universal signaling entities that initialize, organize, and contribute to nearly all aspects of cellular life. Despite these heroic efforts, there is a lack of information that translates and integrates what we understand in expression systems to crucial primary cells like neurons. This author is especially excited to better understand the neuronal localization and function of each of the enzymes listed in Figure 1B. For membrane contact sites, very little is known in neurons apart from beautiful characterizations of their morphology. Simple questions remain unanswered, such as their primary roles, the consequence(s) of their absence, and heterogeneity/redundancy of proteins within defined membrane contact sites. For disease, we need to determine at the molecular level how alterations in PPIn at specific organelle membranes translate to progressive changes in human behavior, ultimately leading to neuropathies and frequently death. Finally, with continued development of pharmacological tools, investigators can begin leveraging what we know about PPIn metabolism (and their broad control of cellular reactions across multiple membranes) to potentially relieve symptoms of disease without actually addressing the underlying genetic or idiopathic factors initiating the disease.
In conclusion, PPIn play a central role in coordinating virtually all aspects of a cell’s life and death. Such fundamental involvement demands continued research into the biology of PPIn, specifically primary cells (like neurons), with the goal to develop diagnostics and novel therapeutic strategies to expedite treatment of human disorders.
Abbreviations
ALS, amyotrophic lateral sclerosis; ER, endoplasmic reticulum; mTOR, mammalian target of rapamycin; OCRL, oculocerebrorenal syndrome of Lowe; ORP, oxysterol-binding protein-related protein; OSBP, oxysterol-binding protein; PtdIns, phosphatidylinositol; PI, phosphoinositide; PIS, phosphatidylinositol synthase; PITP, polyphosphoinositide transfer protein; PLC, phospholipase C; PM, plasma membrane; PPIn, polyphosphoinositides; PTEN, phosphatase and tensin homolog; SCG, superior cervical ganglia; VAP, vesicle-associated membrane protein-associated protein
Acknowledgments
The author apologizes to friends and colleagues whose work was omitted because of space limitations and a focus on newer literature.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Vytas A Bankaitis, Department of Molecular & Cellular Medicine, Texas A&M Health Science Center, College Station, TX 77843-1114, USA
Tamas Balla, Section on Molecular Signal Transduction, Program for Developmental Neuroscience, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA
Bernard Payrastre, Université Toulouse 3, Institut des Maladies Métaboliques et Cardiovasculaires, Toulouse, France
Funding Statement
This work was supported by National Institutes of Health grant R01 GM127513 and University of California Funds.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Balla T: Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev. 2013;93(3):1019–137. 10.1152/physrev.00028.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Traynor-Kaplan A, Kruse M, Dickson EJ, et al. : Fatty-acyl chain profiles of cellular phosphoinositides. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(5):513–522. 10.1016/j.bbalip.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mujalli A, Chicanne G, Bertrand-Michel J, et al. : Profiling of phosphoinositide molecular species in human and mouse platelets identifies new species increasing following stimulation. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(9):1121–1131. 10.1016/j.bbalip.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 4. Wang C, Palavicini JP, Wang M, et al. : Comprehensive and Quantitative Analysis of Polyphosphoinositide Species by Shotgun Lipidomics Revealed Their Alterations in db/db Mouse Brain. Anal Chem. 2016;88(24):12137–12144. 10.1021/acs.analchem.6b02947 [DOI] [PubMed] [Google Scholar]
- 5. Shulga YV, Anderson RA, Topham MK, et al. : Phosphatidylinositol-4-phosphate 5-kinase isoforms exhibit acyl chain selectivity for both substrate and lipid activator. J Biol Chem. 2012;287(43):35953–63. 10.1074/jbc.M112.370155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shulga YV, Topham MK, Epand RM: Study of arachidonoyl specificity in two enzymes of the PI cycle. J Mol Biol. 2011;409(2):101–12. 10.1016/j.jmb.2011.03.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Staiano L, De Leo MG, Persico M, et al. : Mendelian disorders of PI metabolizing enzymes. Biochim Biophys Acta. 2015;1851(6):867–81. 10.1016/j.bbalip.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 8. Di Paolo G, De Camilli P: Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443(7112):651–7. 10.1038/nature05185 [DOI] [PubMed] [Google Scholar]
- 9. Hammond GR, Balla T: Polyphosphoinositide binding domains: Key to inositol lipid biology. Biochim Biophys Acta. 2015;1851(6):746–58. 10.1016/j.bbalip.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hille B, Dickson EJ, Kruse M, et al. : Phosphoinositides regulate ion channels. Biochim Biophys Acta. 2015;1851(6):844–56. 10.1016/j.bbalip.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kruse M, Vivas O, Traynor-Kaplan A, et al. : Dynamics of Phosphoinositide-Dependent Signaling in Sympathetic Neurons. J Neurosci. 2016;36(4):1386–400. 10.1523/JNEUROSCI.3535-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Hicks AM, DeLong CJ, Thomas MJ, et al. : Unique molecular signatures of glycerophospholipid species in different rat tissues analyzed by tandem mass spectrometry. Biochim Biophys Acta. 2006;1761(9):1022–9. 10.1016/j.bbalip.2006.05.010 [DOI] [PubMed] [Google Scholar]
- 13. Lees JA, Messa M, Sun EW, et al. : Lipid transport by TMEM24 at ER-plasma membrane contacts regulates pulsatile insulin secretion. Science. 2017;355(6326): pii: eaah6171. 10.1126/science.aah6171 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Kim YJ, Guzman-Hernandez ML, Wisniewski E, et al. : Phosphatidylinositol-Phosphatidic Acid Exchange by Nir2 at ER-PM Contact Sites Maintains Phosphoinositide Signaling Competence. Dev Cell. 2015;33(5):549–61. 10.1016/j.devcel.2015.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Chang CL, Liou J: Phosphatidylinositol 4,5-Bisphosphate Homeostasis Regulated by Nir2 and Nir3 Proteins at Endoplasmic Reticulum-Plasma Membrane Junctions. J Biol Chem. 2015;290(23):14289–301. 10.1074/jbc.M114.621375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim S, Kedan A, Marom M, et al. : The phosphatidylinositol-transfer protein Nir2 binds phosphatidic acid and positively regulates phosphoinositide signalling. EMBO Rep. 2013;14(10):891–9. 10.1038/embor.2013.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nile AH, Bankaitis VA, Grabon A: Mammalian diseases of phosphatidylinositol transfer proteins and their homologs. Clin Lipidol. 2010;5(6):867–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim YJ, Guzman-Hernandez ML, Balla T: A highly dynamic ER-derived phosphatidylinositol-synthesizing organelle supplies phosphoinositides to cellular membranes. Dev Cell. 2011;21(5):813–24. 10.1016/j.devcel.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Nakatsu F, Baskin JM, Chung J, et al. : PtdIns4P synthesis by PI4KIIIα at the plasma membrane and its impact on plasma membrane identity. J Cell Biol. 2012;199(6):1003–16. 10.1083/jcb.201206095 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Balla A, Kim YJ, Varnai P, et al. : Maintenance of hormone-sensitive phosphoinositide pools in the plasma membrane requires phosphatidylinositol 4-kinase IIIα. Mol Biol Cell. 2008;19(2):711–21. 10.1091/mbc.e07-07-0713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balla A, Tuymetova G, Tsiomenko A, et al. : A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1. Mol Biol Cell. 2005;16(3):1282–95. 10.1091/mbc.e04-07-0578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dai G, Yu H, Kruse M, et al. : Osmoregulatory inositol transporter SMIT1 modulates electrical activity by adjusting PI(4,5)P 2 levels. Proc Natl Acad Sci U S A. 2016;113(23):E3290–9. 10.1073/pnas.1606348113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abbott GW, Tai KK, Neverisky DL, et al. : KCNQ1, KCNE2, and Na +-coupled solute transporters form reciprocally regulating complexes that affect neuronal excitability. Sci Signal. 2014;7(315):ra22. 10.1126/scisignal.2005025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berridge MJ, Downes CP, Hanley MR: Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59(3):411–9. 10.1016/0092-8674(89)90026-3 [DOI] [PubMed] [Google Scholar]
- 25. Brown KM, Tracy DK: Lithium: the pharmacodynamic actions of the amazing ion. Ther Adv Psychopharmacol. 2013;3(3):163–76. 10.1177/2045125312471963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nile AH, Tripathi A, Yuan P, et al. : PITPs as targets for selectively interfering with phosphoinositide signaling in cells. Nat Chem Biol. 2014;10(1):76–84. 10.1038/nchembio.1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alb JG, Jr, Cortese JD, Phillips SE, et al. : Mice lacking phosphatidylinositol transfer protein-alpha exhibit spinocerebellar degeneration, intestinal and hepatic steatosis, and hypoglycemia. J Biol Chem. 2003;278(35):33501–18. 10.1074/jbc.M303591200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schink KO, Tan KW, Stenmark H: Phosphoinositides in Control of Membrane Dynamics. Annu Rev Cell Dev Biol. 2016;32:143–71. 10.1146/annurev-cellbio-111315-125349 [DOI] [PubMed] [Google Scholar]
- 29. Franco I, Gulluni F, Campa CC, et al. : PI3K class II α controls spatially restricted endosomal PtdIns3 P and Rab11 activation to promote primary cilium function. Dev Cell. 2014;28(6):647–58. 10.1016/j.devcel.2014.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marat AL, Haucke V: Phosphatidylinositol 3-phosphates-at the interface between cell signalling and membrane traffic. EMBO J. 2016;35(6):561–79. 10.15252/embj.201593564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Devereaux K, Dall'Armi C, Alcazar-Roman A, et al. : Regulation of mammalian autophagy by class II and III PI 3-kinases through PI3P synthesis. PLoS One. 2013;8(10):e76405. 10.1371/journal.pone.0076405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu K, Xing R, Jian Y, et al. : WDR91 is a Rab7 effector required for neuronal development. J Cell Biol. 2017;216(10):3307–21. 10.1083/jcb.201705151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lorenzo DN, Badea A, Davis J, et al. : A PIK3C3-ankyrin-B-dynactin pathway promotes axonal growth and multiorganelle transport. J Cell Biol. 2014;207(6):735–52. 10.1083/jcb.201407063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Papadopoulos T, Rhee HJ, Subramanian D, et al. : Endosomal Phosphatidylinositol 3-Phosphate Promotes Gephyrin Clustering and GABAergic Neurotransmission at Inhibitory Postsynapses. J Biol Chem. 2017;292(4):1160–77. 10.1074/jbc.M116.771592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou X, Wang L, Hasegawa H, et al. : Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proc Natl Acad Sci U S A. 2010;107(20):9424–9. 10.1073/pnas.0914725107 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Cremona O, Di Paolo G, Wenk MR, et al. : Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99(2):179–88. 10.1016/S0092-8674(00)81649-9 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Nández R, Balkin DM, Messa M, et al. : A role of OCRL in clathrin-coated pit dynamics and uncoating revealed by studies of Lowe syndrome cells. eLife. 2014;3:e02975. 10.7554/eLife.02975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Attree O, Olivos IM, Okabe I, et al. : The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature. 1992;358(6383):239–42. 10.1038/358239a0 [DOI] [PubMed] [Google Scholar]
- 39. Zólyomi A, Zhao X, Downing GJ, et al. : Localization of two distinct type III phosphatidylinositol 4-kinase enzyme mRNAs in the rat. Am J Physiol Cell Physiol. 2000;278(5):C914–20. 10.1152/ajpcell.2000.278.5.C914 [DOI] [PubMed] [Google Scholar]
- 40. Balla A, Vereb G, Gülkan H, et al. : Immunohistochemical localisation of two phosphatidylinositol 4-kinase isoforms, PI4K230 and PI4K92, in the central nervous system of rats. Exp Brain Res. 2000;134(3):279–88. 10.1007/s002210000469 [DOI] [PubMed] [Google Scholar]
- 41. Alvarez-Prats A, Bjelobaba I, Aldworth Z, et al. : Schwann-Cell-Specific Deletion of Phosphatidylinositol 4-Kinase Alpha Causes Aberrant Myelination. Cell Rep. 2018;23(10):2881–90. 10.1016/j.celrep.2018.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Hammond GR, Machner MP, Balla T: A novel probe for phosphatidylinositol 4-phosphate reveals multiple pools beyond the Golgi. J Cell Biol. 2014;205(1):113–26. 10.1083/jcb.201312072 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Dickson EJ, Jensen JB, Vivas O, et al. : Dynamic formation of ER-PM junctions presents a lipid phosphatase to regulate phosphoinositides. J Cell Biol. 2016;213(1):33–48. 10.1083/jcb.201508106 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. De Matteis M, Godi A, Corda D: Phosphoinositides and the golgi complex. Curr Opin Cell Biol. 2002;14(4):434–47. 10.1016/S0955-0674(02)00357-5 [DOI] [PubMed] [Google Scholar]
- 45. Godi A, Pertile P, Meyers R, et al. : ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P 2 on the Golgi complex. Nat Cell Biol. 1999;1(5):280–7. 10.1038/12993 [DOI] [PubMed] [Google Scholar]
- 46. Wong K, Meyers ddR, Cantley LC: Subcellular locations of phosphatidylinositol 4-kinase isoforms. J Biol Chem. 1997;272(20):13236–41. 10.1074/jbc.272.20.13236 [DOI] [PubMed] [Google Scholar]
- 47. Minogue S, Chu KM, Westover EJ, et al. : Relationship between phosphatidylinositol 4-phosphate synthesis, membrane organization, and lateral diffusion of PI4KIIalpha at the trans-Golgi network. J Lipid Res. 2010;51(8):2314–24. 10.1194/jlr.M005751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Waugh MG, Minogue S, Chotai D, et al. : Lipid and peptide control of phosphatidylinositol 4-kinase IIalpha activity on Golgi-endosomal Rafts. J Biol Chem. 2006;281(7):3757–63. 10.1074/jbc.M506527200 [DOI] [PubMed] [Google Scholar]
- 49. Waugh MG, Minogue S, Anderson JS, et al. : Localization of a highly active pool of type II phosphatidylinositol 4-kinase in a p97/valosin-containing-protein-rich fraction of the endoplasmic reticulum. Biochem J. 2003;373(Pt 1):57–63. 10.1042/BJ20030089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barylko B, Mao YS, Wlodarski P, et al. : Palmitoylation controls the catalytic activity and subcellular distribution of phosphatidylinositol 4-kinase II{alpha}. J Biol Chem. 2009;284(15):9994–10003. 10.1074/jbc.M900724200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barylko B, Gerber SH, Binns DD, et al. : A novel family of phosphatidylinositol 4-kinases conserved from yeast to humans. J Biol Chem. 2001;276(11):7705–8. 10.1074/jbc.C000861200 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Sohn M, Korzeniowski M, Zewe JP, et al. : PI(4,5)P 2 controls plasma membrane PI4P and PS levels via ORP5/8 recruitment to ER-PM contact sites. J Cell Biol. 2018;217(5):1797–813. 10.1083/jcb.201710095 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Ghai R, Du X, Wang H, et al. : ORP5 and ORP8 bind phosphatidylinositol-4, 5-biphosphate (PtdIns(4,5) P 2) and regulate its level at the plasma membrane. Nat Commun. 2017;8(1):757. 10.1038/s41467-017-00861-5 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Moser von Filseck J, Vanni S, Mesmin B, et al. : A phosphatidylinositol-4-phosphate powered exchange mechanism to create a lipid gradient between membranes. Nat Commun. 2015;6: 6671. 10.1038/ncomms7671 [DOI] [PubMed] [Google Scholar]
- 55. Chung J, Torta F, Masai K, et al. : INTRACELLULAR TRANSPORT. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science. 2015;349(6246):428–32. 10.1126/science.aab1370 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Mesmin B, Bigay J, Polidori J, et al. : Sterol transfer, PI4P consumption, and control of membrane lipid order by endogenous OSBP. EMBO J. 2017;36(21):3156–74. 10.15252/embj.201796687 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Mesmin B, Bigay J, Moser von Filseck J, et al. : A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell. 2013;155(4):830–43. 10.1016/j.cell.2013.09.056 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Porter KR, Palade GE: Studies on the endoplasmic reticulum. III. Its form and distribution in striated muscle cells. J Biophys Biochem Cytol. 1957;3(2):269–300. 10.1083/jcb.3.2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Block BA, Imagawa T, Campbell KP, et al. : Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988;107(6 Pt 2):2587–600. 10.1083/jcb.107.6.2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Franzini-Armstrong C: STUDIES OF THE TRIAD : I. Structure of the Junction in Frog Twitch Fibers. J Cell Biol. 1970;47(2):488–99. 10.1083/jcb.47.2.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wu Y, Whiteus C, Xu CS, et al. : Contacts between the endoplasmic reticulum and other membranes in neurons. Proc Natl Acad Sci U S A. 2017;114(24):E4859–E4867. 10.1073/pnas.1701078114 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Guo J, Wenk MR, Pellegrini L, et al. : Phosphatidylinositol 4-kinase type IIα is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles. Proc Natl Acad Sci U S A. 2003;100(7):3995–4000. 10.1073/pnas.0230488100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sbrissa D, Ikonomov OC, Deeb R, et al. : Phosphatidylinositol 5-phosphate biosynthesis is linked to PIKfyve and is involved in osmotic response pathway in mammalian cells. J Biol Chem. 2002;277(49):47276–84. 10.1074/jbc.M207576200 [DOI] [PubMed] [Google Scholar]
- 64. Sbrissa D, Ikonomov OC, Shisheva A: PIKfyve, a mammalian ortholog of yeast Fab1p lipid kinase, synthesizes 5-phosphoinositides. Effect of insulin. J Biol Chem. 1999;274(31):21589–97. 10.1074/jbc.274.31.21589 [DOI] [PubMed] [Google Scholar]
- 65. Tronchère H, Laporte J, Pendaries C, et al. : Production of phosphatidylinositol 5-phosphate by the phosphoinositide 3-phosphatase myotubularin in mammalian cells. J Biol Chem. 2004;279(8):7304–12. 10.1074/jbc.M311071200 [DOI] [PubMed] [Google Scholar]
- 66. Zolov SN, Bridges D, Zhang Y, et al. : In vivo, Pikfyve generates PI(3,5)P 2, which serves as both a signaling lipid and the major precursor for PI5P. Proc Natl Acad Sci U S A. 2012;109(43):17472–7. 10.1073/pnas.1203106109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gozani O, Karuman P, Jones DR, et al. : The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell. 2003;114(1):99–111. 10.1016/S0092-8674(03)00480-X [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Clarke JH, Letcher AJ, D'santos CS, et al. : Inositol lipids are regulated during cell cycle progression in the nuclei of murine erythroleukaemia cells. Biochem J. 2001;357(Pt 3):905–10. 10.1042/bj3570905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gupta A, Toscano S, Trivedi D, et al. : Phosphatidylinositol 5-phosphate 4-kinase (PIP4K) regulates TOR signaling and cell growth during Drosophila development. Proc Natl Acad Sci U S A. 2013;110(15):5963–8. 10.1073/pnas.1219333110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Emerling BM, Hurov JB, Poulogiannis G, et al. : Depletion of a putatively druggable class of phosphatidylinositol kinases inhibits growth of p53-null tumors. Cell. 2013;155(4):844–57. 10.1016/j.cell.2013.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Marat AL, Wallroth A, Lo WT, et al. : mTORC1 activity repression by late endosomal phosphatidylinositol 3,4-bisphosphate. Science. 2017;356(6341):968–72. 10.1126/science.aaf8310 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Rostislavleva K, Soler N, Ohashi Y, et al. : Structure and flexibility of the endosomal Vps34 complex reveals the basis of its function on membranes. Science. 2015;350(6257):aac7365. 10.1126/science.aac7365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hardies K, Cai Y, Jardel C, et al. : Loss of SYNJ1 dual phosphatase activity leads to early onset refractory seizures and progressive neurological decline. Brain. 2016;139(Pt 9):2420–30. 10.1093/brain/aww180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhu L, Zhong M, Elder GA, et al. : Phospholipid dysregulation contributes to ApoE4-associated cognitive deficits in Alzheimer's disease pathogenesis. Proc Natl Acad Sci U S A. 2015;112(38):11965–70. 10.1073/pnas.1510011112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Voronov SV, Frere SG, Giovedi S, et al. : Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down's syndrome. Proc Natl Acad Sci U S A. 2008;105(27):9415–20. 10.1073/pnas.0803756105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cao M, Wu Y, Ashrafi G, et al. : Parkinson Sac Domain Mutation in Synaptojanin 1 Impairs Clathrin Uncoating at Synapses and Triggers Dystrophic Changes in Dopaminergic Axons. Neuron. 2017;93(4):882–896.e5. 10.1016/j.neuron.2017.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Kim SJ, Jeong MJ, Jo HJ, et al. : Identification of postsynaptic phosphatidylinositol-4,5-bisphosphate (PIP 2) roles for synaptic plasticity using chemically induced dimerization. Sci Rep. 2017;7(3351): 3351. 10.1038/s41598-017-03520-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Suh BC, Horowitz LF, Hirdes W, et al. : Regulation of KCNQ2/KCNQ3 current by G protein cycling: the kinetics of receptor-mediated signaling by G q. J Gen Physiol. 2004;123(6):663–83. 10.1085/jgp.200409029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Suh BC, Hille B: Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35(3):507–20. 10.1016/S0896-6273(02)00790-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Wang HS, Pan Z, Shi W, et al. : KCNQ2 and KCNQ3 potassium channel subunits: Molecular correlates of the M-channel. Science. 1998;282(5395):1890–3. 10.1126/science.282.5395.1890 [DOI] [PubMed] [Google Scholar]
- 81. Suh BC, Leal K, Hille B: Modulation of high-voltage activated Ca 2+ channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron. 2010;67(2):224–38. 10.1016/j.neuron.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Vivas O, Kruse M, Hille B: Nerve growth factor sensitizes adult sympathetic neurons to the proinflammatory peptide bradykinin. J Neurosci. 2014;34(36):11959–71. 10.1523/JNEUROSCI.1536-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dickson EJ, Jensen JB, Hille B: Golgi and plasma membrane pools of PI(4)P contribute to plasma membrane PI(4,5)P 2 and maintenance of KCNQ2/3 ion channel current. Proc Natl Acad Sci U S A. 2014;111(22):E2281–90. 10.1073/pnas.1407133111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Stephens LR, Hughes KT, Irvine RF: Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991;351(6321):33–9. 10.1038/351033a0 [DOI] [PubMed] [Google Scholar]
- 85. Hawkins PT, Jackson TR, Stephens LR: Platelet-derived growth factor stimulates synthesis of PtdIns(3,4,5)P 3 by activating a PtdIns(4,5)P 2 3-OH kinase. Nature. 1992;358(6382):157–9. 10.1038/358157a0 [DOI] [PubMed] [Google Scholar]
- 86. Misawa H, Ohtsubo M, Copeland NG, et al. : Cloning and characterization of a novel class II phosphoinositide 3-kinase containing C2 domain. Biochem Biophys Res Commun. 1998;244(2):531–9. 10.1006/bbrc.1998.8294 [DOI] [PubMed] [Google Scholar]
- 87. Sasaki J, Kofuji S, Itoh R, et al. : The PtdIns(3,4)P 2 phosphatase INPP4A is a suppressor of excitotoxic neuronal death. Nature. 2010;465(7297):497–501. 10.1038/nature09023 [DOI] [PubMed] [Google Scholar]
- 88. Malek M, Kielkowska A, Chessa T, et al. : PTEN Regulates PI(3,4)P 2 Signaling Downstream of Class I PI3K. Mol Cell. 2017;68(3):566–580.e10. 10.1016/j.molcel.2017.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Posor Y, Eichhorn-Grünig M, Haucke V: Phosphoinositides in endocytosis. Biochim Biophys Acta. 2015;1851(6):794–804. 10.1016/j.bbalip.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 90. Boucrot E, Ferreira AP, Almeida-Souza L, et al. : Endophilin marks and controls a clathrin-independent endocytic pathway. Nature. 2015;517(7535):460–5. 10.1038/nature14067 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Renard HF, Simunovic M, Lemière J, et al. : Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature. 2015;517(7535):493–6. 10.1038/nature14064 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 92. Zhang SX, Duan LH, He SJ, et al. : Phosphatidylinositol 3,4-bisphosphate regulates neurite initiation and dendrite morphogenesis via actin aggregation. Cell Res. 2017;27(2):253–273. 10.1038/cr.2017.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jin N, Chow CY, Liu L, et al. : VAC14 nucleates a protein complex essential for the acute interconversion of PI3P and PI(3,5)P 2 in yeast and mouse. EMBO J. 2008;27(24):3221–34. 10.1038/emboj.2008.248 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Hirschi M, Herzik MA, Wie J, et al. : Cryo-electron microscopy structure of the lysosomal calcium-permeable channel TRPML3. Nature. 2017;550(7676):411–414. 10.1038/nature24055 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 95. Dong XP, Shen D, Wang X, et al. : PI(3,5)P 2 controls membrane trafficking by direct activation of mucolipin Ca 2+ release channels in the endolysosome. Nat Commun. 2010;1:38. 10.1038/ncomms1037 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 96. She J, Guo J, Chen Q, et al. : Structural insights into the voltage and phospholipid activation of the mammalian TPC1 channel. Nature. 2018;556(7699):130–134. 10.1038/nature26139 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 97. Jha A, Ahuja M, Patel S, et al. : Convergent regulation of the lysosomal two-pore channel-2 by Mg 2+, NAADP, PI(3,5)P 2 and multiple protein kinases. EMBO J. 2014;33(5):501–11. 10.1002/embj.201387035 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Wang X, Zhang X, Dong XP, et al. : TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151(2):372–83. 10.1016/j.cell.2012.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 99. Chow CY, Zhang Y, Dowling JJ, et al. : Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448(7149):68–72. 10.1038/nature05876 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 100. Zhang Y, McCartney AJ, Zolov SN, et al. : Modulation of synaptic function by VAC14, a protein that regulates the phosphoinositides PI(3,5)P 2 and PI(5)P. EMBO J. 2012;31(16):3442–56. 10.1038/emboj.2012.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. McCartney AJ, Zolov SN, Kauffman EJ, et al. : Activity-dependent PI(3,5)P 2 synthesis controls AMPA receptor trafficking during synaptic depression. Proc Natl Acad Sci U S A. 2014;111(45):E4896–905. 10.1073/pnas.1411117111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Khuong TM, Habets RL, Kuenen S, et al. : Synaptic PI(3,4,5)P 3 is required for Syntaxin1A clustering and neurotransmitter release. Neuron. 2013;77(6):1097–108. 10.1016/j.neuron.2013.01.025 [DOI] [PubMed] [Google Scholar]
- 103. Grider MH, Park D, Spencer DM, et al. : Lipid raft-targeted Akt promotes axonal branching and growth cone expansion via mTOR and Rac1, respectively. J Neurosci Res. 2009;87(14):3033–42. 10.1002/jnr.22140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Song Y, Ori-McKenney KM, Zheng Y, et al. : Regeneration of Drosophila sensory neuron axons and dendrites is regulated by the Akt pathway involving Pten and microRNA bantam. Genes Dev. 2012;26(14):1612–25. 10.1101/gad.193243.112 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 105. Ueda Y, Hayashi Y: PIP 3 regulates spinule formation in dendritic spines during structural long-term potentiation. J Neurosci. 2013;33(27):11040–7. 10.1523/JNEUROSCI.3122-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lachyankar MB, Sultana N, Schonhoff CM, et al. : A role for nuclear PTEN in neuronal differentiation. J Neurosci. 2000;20(4):1404–13. 10.1523/JNEUROSCI.20-04-01404.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. van Diepen MT, Eickholt BJ: Function of PTEN during the formation and maintenance of neuronal circuits in the brain. Dev Neurosci. 2008;30(1–3):59–64. 10.1159/000109852 [DOI] [PubMed] [Google Scholar]
- 108. Chang N, El-Hayek YH, Gomez E, et al. : Phosphatase PTEN in neuronal injury and brain disorders. Trends Neurosci. 2007;30(11):581–6. 10.1016/j.tins.2007.08.006 [DOI] [PubMed] [Google Scholar]
- 109. Liu K, Lu Y, Lee JK, et al. : PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13(9):1075–81. 10.1038/nn.2603 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 110. Harrington EP, Zhao C, Fancy SP, et al. : Oligodendrocyte PTEN is required for myelin and axonal integrity, not remyelination. Ann Neurol. 2010;68(5):703–16. 10.1002/ana.22090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. De Paula ML, Cui QL, Hossain S, et al. : The PTEN inhibitor bisperoxovanadium enhances myelination by amplifying IGF-1 signaling in rat and human oligodendrocyte progenitors. Glia. 2014;62(1):64–77. 10.1002/glia.22584 [DOI] [PubMed] [Google Scholar]
- 112. Jungmichel S, Sylvestersen KB, Choudhary C, et al. : Specificity and commonality of the phosphoinositide-binding proteome analyzed by quantitative mass spectrometry. Cell Rep. 2014;6(3):578–91. 10.1016/j.celrep.2013.12.038 [DOI] [PubMed] [Google Scholar]
- 113. Burke JE: Structural Basis for Regulation of Phosphoinositide Kinases and Their Involvement in Human Disease. Mol Cell. 2018;71(5):653–73. 10.1016/j.molcel.2018.08.005 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 114. Billcliff PG, Lowe M: Inositol lipid phosphatases in membrane trafficking and human disease. Biochem J. 2014;461(2):159–75. 10.1042/BJ20140361 [DOI] [PubMed] [Google Scholar]
- 115. Wen PJ, Osborne SL, Meunier FA: Phosphoinositides in neuroexocytosis and neuronal diseases. Curr Top Microbiol Immunol. 2012;362:87–98. 10.1007/978-94-007-5025-8_4 [DOI] [PubMed] [Google Scholar]
- 116. Liu Y, Bankaitis VA: Phosphoinositide phosphatases in cell biology and disease. Prog Lipid Res. 2010;49(3):201–17. 10.1016/j.plipres.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gross C: Defective phosphoinositide metabolism in autism. J Neurosci Res. 2017;95(5):1161–73. 10.1002/jnr.23797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gupta AR, Pirruccello M, Cheng F, et al. : Rare deleterious mutations of the gene EFR3A in autism spectrum disorders. Mol Autism. 2014;5:31. 10.1186/2040-2392-5-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cuscó I, Medrano A, Gener B, et al. : Autism-specific copy number variants further implicate the phosphatidylinositol signaling pathway and the glutamatergic synapse in the etiology of the disorder. Hum Mol Genet. 2009;18(10):1795–804. 10.1093/hmg/ddp092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Dong R, Saheki Y, Swarup S, et al. : Endosome-ER Contacts Control Actin Nucleation and Retromer Function through VAP-Dependent Regulation of PI4P. Cell. 2016;166(2):408–23. 10.1016/j.cell.2016.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 121. Chang CL, Liou J: Homeostatic regulation of the PI(4,5)P 2-Ca 2+ signaling system at ER-PM junctions. Biochim Biophys Acta. 2016;1861(8 Pt B):862–73. 10.1016/j.bbalip.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chang CL, Hsieh TS, Yang TT, et al. : Feedback regulation of receptor-induced Ca 2+ signaling mediated by E-Syt1 and Nir2 at endoplasmic reticulum-plasma membrane junctions. Cell Rep. 2013;5(3):813–25. 10.1016/j.celrep.2013.09.038 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 123. Aliaga L, Lai C, Yu J, et al. : Amyotrophic lateral sclerosis-related VAPB P56S mutation differentially affects the function and survival of corticospinal and spinal motor neurons. Hum Mol Genet. 2013;22(21):4293–305. 10.1093/hmg/ddt279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. McCartney AJ, Zhang Y, Weisman LS: Phosphatidylinositol 3,5-bisphosphate: low abundance, high significance. Bioessays. 2014;36(1):52–64. 10.1002/bies.201300012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Quattrone A, Gambardella A, Bono F, et al. : Autosomal recessive hereditary motor and sensory neuropathy with focally folded myelin sheaths: Clinical, electrophysiologic, and genetic aspects of a large family. Neurology. 1996;46(5):1318–24. 10.1212/WNL.46.5.1318 [DOI] [PubMed] [Google Scholar]
- 126. Laporte J, Bedez F, Bolino A, et al. : Myotubularins, a large disease-associated family of cooperating catalytically active and inactive phosphoinositides phosphatases. Hum Mol Genet. 2003;12(Spec No 2):R285–92. 10.1093/hmg/ddg273 [DOI] [PubMed] [Google Scholar]
- 127. Laporte J, Biancalana V, Tanner SM, et al. : MTM1 mutations in X-linked myotubular myopathy. Hum Mutat. 2000;15(5):393–409. [DOI] [PubMed] [Google Scholar]
- 128. Kam TI, Park H, Gwon Y, et al. : FcγRIIb-SHIP2 axis links Aβ to tau pathology by disrupting phosphoinositide metabolism in Alzheimer's disease model. eLife. 2016;5: pii: e18691. 10.7554/eLife.18691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhu L, Zhong M, Zhao J, et al. : Reduction of synaptojanin 1 accelerates Aβ clearance and attenuates cognitive deterioration in an Alzheimer mouse model. J Biol Chem. 2013;288(44):32050–63. 10.1074/jbc.M113.504365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Drouet V, Lesage S: Synaptojanin 1 mutation in Parkinson's disease brings further insight into the neuropathological mechanisms. Biomed Res Int. 2014;2014:289728. 10.1155/2014/289728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. : Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45(12):1452–8. 10.1038/ng.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Miranda AM, Herman M, Cheng R, et al. : Excess Synaptojanin 1 Contributes to Place Cell Dysfunction and Memory Deficits in the Aging Hippocampus in Three Types of Alzheimer's Disease. Cell Rep. 2018;23(10):2967–2975. 10.1016/j.celrep.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 133. Berman DE, Dall'Armi C, Voronov SV, et al. : Oligomeric amyloid-beta peptide disrupts phosphatidylinositol-4,5-bisphosphate metabolism. Nat Neurosci. 2008;11(5):547–54. 10.1038/nn.2100 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 134. Wu B, Kitagawa K, Liu B, et al. : Attenuation of amyloid beta (Abeta)-induced inhibition of phosphatidylinositol 4-kinase activity by Abeta fragments, Abeta20-29 and Abeta31-35. Neurosci Lett. 2006;396(2):148–52. 10.1016/j.neulet.2005.11.026 [DOI] [PubMed] [Google Scholar]
- 135. Lesage S, Drouet V, Majounie E, et al. : Loss of VPS13C Function in Autosomal-Recessive Parkinsonism Causes Mitochondrial Dysfunction and Increases PINK1/Parkin-Dependent Mitophagy. Am J Hum Genet. 2016;98(3):500–513. 10.1016/j.ajhg.2016.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kumar N, Leonzino M, Hancock-Cerutti W, et al. : VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J Cell Biol. 2018;217(10):3625–3639. 10.1083/jcb.201807019 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation