Abstract
Clostridium septicum is a Gram-positive, anaerobic, spore-forming bacillus found in the intestine. It is linked to colon cancer and immunosuppression. Infection with C septicum may vary in manifestation and is associated with more than 60% mortality rate. In this article, we present a case of incidental isolation of C septicum in a patient who presented with fever and later on colonoscopy was found to have colon carcinoma. Bacteremia may be the unexpected initial presentation of undiagnosed colon carcinoma.
Keywords: Clostridium septicum, colon carcinoma, bacteremia
Introduction
Few bacterial infections have been associated with malignancy; one of them is Clostridium septicum, which is known to be associated with gastrointestinal (GI) and solid organ malignancy, immunosuppression, neutropenia, and diabetes mellitus.1 It has a variable clinical presentation, and if not treated early, it is associated with a high mortality with the majority of deaths occurring within the first 24 hours.2 We report a case of C septicum bacteremia associated with colon carcinoma. We understand that it is important for clinicians to be aware of clinicians of this rare but known association between colon cancer and C septicum.
Case Presentation
A 71-year-old female with comorbidities such as hypertension and end-stage renal disease (on hemodialysis) presented to our emergency department with complain of back pain of 1 day duration. Only significant finding noted on physical examination was decreased range of motion in lumbar spine associated with moderate to severe pain. Review of systems was otherwise reported negative. The patient had low-grade fever 100.4°F, tachycardia 115 beats per minute, respiratory rate 22 breaths per minute, and blood pressure 160/78 mm Hg on admission. Complete blood count on admission was significant for macrocytic anemia with hemoglobin 10.1 mg/dL and mean corpuscular volume 101.3. Significant left shift was noted on differential white blood cell (WBC) count with 96.2% neutrophils (total WBC count on admission was 9500/dL). Comprehensive metabolic panel showed deranged renal function blood urea nitrogen 40 mg/dL (8-20 mg/dL) and creatinine 9.79 mg/dL (0.4-1.3 mg/dL). The patient met systemic inflammatory response syndrome criteria; therefore, 2 sets of blood cultures were sent for further sepsis workup and patient was started on broad-spectrum antibiotics (vancomycin and meropenem) empirically. Computed tomography chest/abdomen/pelvis was done, which was negative for any pulmonary or intra-abdominal focus of infection. However, computed tomography abdomen/pelvis showed presence of spinal canal stenosis in the lumbar area. Blood cultures sent on day 1 and day 3 (total 3 sets) grew C septicum. Repeat blood culture sent on day 5 was reported negative. Based on sensitivity report, antibiotics were switched to piperacillin/tazobactam.
Due to patient’s new-onset symptoms of worsening back pain, magnetic resonance imaging lumbar spine was done, which ruled out acute process/any mass lesion in the lumbar spine. Presence of multilevel degenerative disease and spinal canal stenosis was confirmed and any other pathology was ruled out.
The patient improved symptomatically and WBC count also improved over the next few days. However, due to known but rare association of C septicum with GI malignancy, upper GI endoscopy and colonoscopy (Figure 1) was performed on day 6, which showed nonobstructive mass in ascending colon suspicious for malignancy. Biopsy was taken and sent for histopathology examination, which was reported positive for well-differentiated adenocarcinoma (Figure 2).
Figure 1.
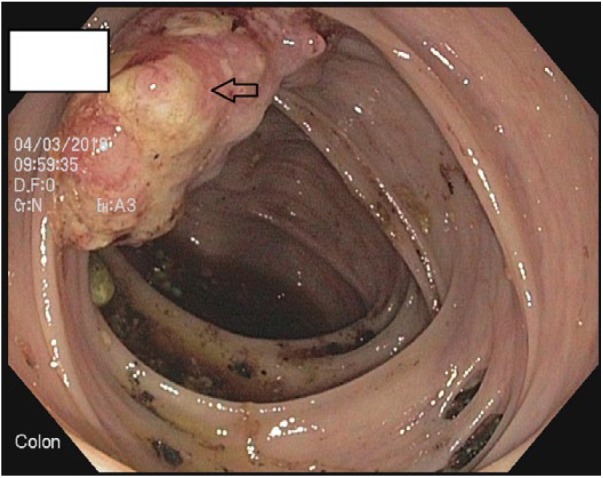
Colonoscopy showing nonobstructive mass in ascending colon suspicious for malignancy.
Figure 2.
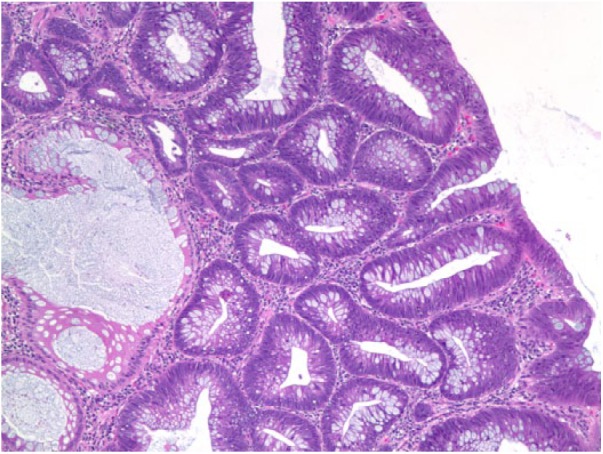
Histopathological section from the colonic mass showing adenocarcinoma.
As there was no metastasis, the patient underwent hemicolectomy for the aforementioned lesion in the ascending colon. She was followed-up in 3 months and underwent surveillance colonoscopy, which showed no local recurrence of the malignancy.
Discussion
Clostridium septicum is a rare infection and is known to be associated with colorectal malignancy.3 Other similar association with colon cancer is Streptococcus bovis; however, as compared with the latter, C septicum is a lethal infection if not treated early. In one case review, among infection with Clostridium species, malignancy was found to be associated in 50% of patients with C septicum, whereas malignancy was seen in only 11% of patients with other clostridial infections and the remaining patients with spontaneous clostridial species infection all had evidence of immunosuppression.4
The clinical spectrum of C septicum varies and can present as cellulitis, fasciitis, myonecrosis, abscess, aortitis, or septic shock.5 There should be a high suspicion of colonic malignancy in the patients with C septicum bacteremia with or without underlying signs of sepsis. C septicum can present with nonspecific features such as fever, and the infection can be easily missed in asymptomatic patients with colon carcinoma.6 In the present case, we sent a blood culture as a part of sepsis workup without other clinical manifestations of colon cancer. Incidentally, C septicum infection was found leading to evaluation for the possible cause. The early initiation of treatment with antibiotics is also very important as sepsis-related mortality varies between 45% and 70%.1,5 This association between C septicum and colon cancer could be due to over proliferation of tumor tissue leading to relatively less blood supply with the development of tissue necrosis, which creates a favorable environment for anaerobic bacterial proliferation.
Clostridium septicum is more aerotolerant than C perfringens and therefore has predilection for normal tissue.7 There may be multiple virulence factors involved including production of several toxins and potential to aggressively invade the tissue.8 Tumor-induced mucosal ulceration results in disruption of the intact barrier, owing to translocation of the bacteria hematogenously from the bowel leading to sepsis. Once the malignancy outgrows its blood supply, the anaerobic environment created is ideal for bacterial growth.5 The infection spreads and tends to affect areas supplied by a single artery.9 Impaired host immunity as in our case from end-stage renal disease is also believed to facilitate translocation.
Cecal cancers are the most common locations linked with this infection likely due to characteristic pH, osmotic changes, and electrolyte changes conducive for multiplication of C septicum; however, tumor may be located at the other area such as ascending colon in our case.7,10 Mao et al observed that 4 patients who grew of C septicum in their blood cultures were also found to have colon cancer.7
In the present case, there was early hematogenous dissemination of C septicum from the colon carcinoma that led to early workup of malignancy and eventually colon carcinoma was diagnosed on colonoscopy. The patient was treated with antibiotics in a timely fashion and she underwent curative resection of colon carcinoma. Therefore, evidence of C septicum infection can be useful tool to detect any occult malignancy.
Conclusions
The vigorous search for colorectal carcinoma should be done in any patient growing C septicum on blood culture with or without any symptoms.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting case report.
Informed Consent: Written informed consent was obtained from the patient.
ORCID iDs: Amrendra Mandal  https://orcid.org/0000-0002-5873-5443
https://orcid.org/0000-0002-5873-5443
Vijay Gayam  https://orcid.org/0000-0001-5194-9134
https://orcid.org/0000-0001-5194-9134
References
- 1. Mirza NN, McCloud JM, Cheetham MJ. Clostridium septicum sepsis and colorectal cancer—a reminder. World J Surg Oncol. 2009;7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nanjappa S, Shah S, Pabbathi S. Clostridium septicum gas gangrene in colon cancer: importance of early diagnosis. Case Rep Infect Dis. 2015;2015:694247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hermsen JL, Schurr MJ, Kudsk KA, Faucher LD. Phenotyping Clostridium septicum infection: a surgeon’s infectious disease. J Surg Res. 2008;148:67-76. [DOI] [PubMed] [Google Scholar]
- 4. Larson CM, Bubrick MP, Jacobs DM, West MA. Malignancy, mortality, and medicosurgical management of Clostridium septicum infection. Surgery. 1995;118:592-598. [DOI] [PubMed] [Google Scholar]
- 5. Khan AA, Davenport K. A reminder of the association between Clostridium septicum and colonic adenocarcinoma. Int Semin Surg Oncol. 2006;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chew SS, Lubowski DZ. Clostridium septicum and malignancy. ANZ J Surg. 2001;71:647-649. [DOI] [PubMed] [Google Scholar]
- 7. Mao E, Clements A, Feller E. Clostridium septicum sepsis and colon carcinoma: report of 4 cases. Case Rep Med. 2011;2011:248453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pelletier JPR, Plumbley JA, Rouse EA, Cina SJ. The role of Clostridium septicum in paraneoplastic sepsis. Arch Pathol Lab Med. 2000;124:353-356. [DOI] [PubMed] [Google Scholar]
- 9. Griffin AS, Crawford MD, Gupta RT. Massive gas gangrene secondary to occult colon carcinoma. Radiol Case Rep. 2016;11:67-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seder CW, Kramer M, Long G, Uzieblo MR, Shanley CJ, Bove P. Clostridium septicum aortitis: report of two cases and review of the literature. J Vasc Surg. 2009;49:1304-1309. [DOI] [PubMed] [Google Scholar]


