Short abstract
Background
Morning urine formaldehyde concentrations could predict the severe degree of dementia in patients with post-stroke dementia and Alzheimer’s disease. However, the routinely available technique of high-performance liquid chromatography (HPLC) for detecting urine formaldehyde requires expensive and sophisticated equipment.
Methods
We established a fluorescence spectrophotometric method by using a formaldehyde-specific fluorescent probe-NaFA (λex/em = 430/543 nm). As a standard reference method, the same batch of urine samples was analysed by HPLC with a fluorescence detector (λex/em = 346/422 nm). Then we compared the limits of detection and the limits of quantization detected by these two methods and addressed the relationship between urine formaldehyde and human cognitive ability. The Mini-Mental State Examination (MMSE), Clinical Dementia Rating and Activities of Daily Living scale were used to evaluate cognition function in 30 Alzheimer’s disease patients and 52 healthy age-matched controls.
Results
Limits of detection and limits of quantization (1.27 and 2.48 μM) of the NaFA probe method were more accurate than Fluo-HPLC (1.52 and 2.91 μM). There was no difference in the detected formaldehyde values within day and day-to-day. Notably, only 3/82 urine formaldehyde concentrations detected by NaFA probe were below zero, while 12/82 of the values analysed by Fluo-HPLC were abnormal. More importantly, there were negatively correlated between urine formaldehyde concentrations detected by NaFA probe and MMSE scores, but positively correlated with Clinical Dementia Rating scores in Alzheimer’s disease patients.
Conclusions
This detecting urine formaldehyde method by NaFA probe was more rapid, sensitive and accurate than Fluo-HPLC.
Keywords: Formaldehyde, NaFA, spectrophotometry, high-performance liquid chromatography, dementia, Alzheimer’s disease
Introduction
Exogenous gaseous formaldehyde (FA), notoriously known as an indoor air pollutant, induces animal memory loss1 and human cognitive impairments.2 Surprisingly, endogenous FA has been found to be existing in all invertebrate and vertebrate cells.3 Normal brain FA concentration in healthy mice, rats and human is about 300 μM, which can be detected by gas chromatography/mass spectrometry (GC/MS),4 or high-performance liquid chromatography with a fluorescent probe (Fluo-HPLC).5 Notably, an abnormal high concentration of hippocampal FA (∼500 μM) has been found in the patients with Alzheimer’s disease (AD), APP/PSI transgenic AD-like model mice and the senescence-accelerated mouse SAMP.6 Injection of 500 μM FA indeed impairs memory in mice and rats.7 Unsurprisingly, excess FA has strong neurotoxicity8 because it can accelerate Aβ aggregation and Tau hyperphosphorylation in normal adult mice and monkeys9, 10 and lead to cognitive decline.11 In clinic, morning urine FA concentrations are negatively correlated with cognitive ability in elder people,12 especially in patients with Alzheimer’s disease (AD).6 A cross-sectional survey in 577 participants has found that urine FA with cut-off (41.8 μM) could predict the severe degree of dementia in patients with poststroke dementia (PSD) or AD.13
In recent decades, different kinds of methods have been established to detect urine FA concentrations. For example, the average concentration of human FA urine analysed by headspace gas chromatography (GC) is ∼33 μM.14 This result is similar to the value (∼29 μM) detected by Fluo-HPLC, which is based on the fact that ampicillin has chemical reaction with FA.13 Similarly, urine FA concentration in rats analysed by HPLC with ultraviolet (UV) detector is approximately 32 μM.15 A more sensitive radiometric method by using 14C-dimedone reagent has been established, and the range of FA values are about 10–20 μM.16 These data indicate that urine FA concentrations in healthy adult humans are less than 40 μM (the pathological concentration in AD patients). Although these existing approaches provide accurate and sensitive detection of FA, several disadvantages, such as sophisticated experimental procedures and noxious analytical reagents, have hindered their clinical and practical applications. Therefore, a simple, sensitive and efficient method for determining trace amounts of urine FA is urgently needed.
Here, we established a highly sensitive and selective spectrophotometric method for detecting urine FA at room temperature by using NaFA probe because it can specifically react with FA to produce a fluorescent derivant (λex/em = 430/543 nm).
Materials and methods
Ethics
This clinical investigation (2014SY39) was approved by the Ethics Committee at the Capital Medical University, China.
Participants
The study was registered at the Chinese Clinical Trial Registry (http://www.chictr.org/cn, Unique Identifier: ChiCTR-OOC-14005576), and conducted between March 2008 and December 2014. We recruited participants from representative regions in Beijing, China. The mean age of all individuals in this cross-sectional survey was 78.69 ± 2.58 years (n = 82). Participants who refused to provide urine samples, or had a life-threatening illness, or were unable to participate in the assessment, were excluded from the entire survey. Informed consent was obtained from each participant directly or indirectly from his or her guardian.
Clinical evaluation
The cognitive status of patients/participants was assessed by neurologists using the Activities of Daily Living (ADL),17 Clinical Dementia Rating (CDR)18 and Mini Mental State Examination (MMSE).19 A MMSE score ≤20 (adjusted for education level of the participants from rural regions) was defined to be cognitive impairment. The MMSE is widely applied to assess the cognitive ability of patients suffering from memory decline.19 PSD and AD were distinguished and diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders (fourth edition) revised (DSM-IV-R) criteria, as described previously.20 Information on medical history and medications for each participant were obtained from primary health-care records, or provided by each participant or his or her guardian.
Morning urine samples
The morning urine samples from participants were collected and immediately placed on ice, before being stored at –70°C until analysis. After centrifugation (8000 × g, 4°C, 10 min), supernatant fractions of urine were subjected to urine FA analysis.
Chemical reagents
FA-specific fluorescent probe-NaFA was provided by Prof. Weiying Lin (Institute of Fluorescent Probes for Biological Imaging, University of Jinan, China). Ampicillin (D-(2)-a-aminobenzylpenicillin anhydrous), dimethyl sulfoxide (DMSO), 37% FA solution and trichloroacetic acid (TCA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other reagents were of analytical grade. All solvents were of HPLC grade (Tedia, Fairfield, OH, USA) and deionized water was obtained from the Milli-Q system.
Detection of urine FA by fluorescent spectrophotometer
To determine the optimal time of derivative reaction between FA and FA probe-NaFA at room temperature, seven 100-μL aliquots of different concentrations of FA standard solutions (1, 5, 10, 50, 100, 500 and 1000 μM, pH at 7.4, respectively) were added to seven vials in order to prepare a series of calibration standards. In addition, 800 μL of PBS with pH at 7.4 was pipetted into a separate vial after the aliquots of 100 μL 20 μM NaFA solution with pH at 7.4 were added to each of these seven vials. These mixtures were pipetted into 96-well plates and incubated at room temperature for 0, 30, 60 and 120 min, respectively. Then the fluorescent intensities of the derivant between standard FA and NaFA, between urine FA and NaFA were quantified by a fluorescent spectrophotometer at λex/em = 430/543 nm (Multi-Mode Microplate Reader, SpectraMax i3, Molecular Devices, California, USA), respectively (Supplementary Figure 1).
The calibration curves which covered the FA concentration range of 1–1000 μmM were prepared. For routine analysis, a one-point calibration in duplicate was prepared daily and used for quantitative calculation of urine samples.
Detection of urine FA by Fluo-HPLC
As a standard method reference, the same batch of urine samples was routinely analysed by using Fluo-HPLC (λex/em = 346/422 nm) as described previously.13, 21 Briefly, 200 μL human urine was pipetted into a 2-mL glass vial, to which 800 μL water, 100 μL ampicillin solution (2.5 mg/mL, in water) and 250 μL TCA (20%, w/v, in water) were added. The vial was heated in a 90°C water bath for 1 h. After cooling to room temperature, the content of the vial was transferred to a 10-mL glass centrifuge tube. The vial was rinsed with 1 mL diethyl ether twice and also transferred to the centrifuge tube. About 0.5 g sodium chloride (NaCl) was added to precipitation urine proteins. After centrifugation, the upper layer was extracted with another 1 mL diethyl ether. The diethyl ether was evaporated to dryness with a gentle stream of nitrogen. The residue was redissolved in 500 μL acetonitrile–water (50:50), and was ready for Fluo-HPLC analysis (Agilent HP1100, USA) (Supplementary Figure 1).
Variations of within-day and day-to-day
Urine samples (n = 10) were analysed for fluorescent intensity on day 1 to evaluate the variation in within-day FA concentrations at 0, 30, 60 and 120 min, respectively. Another group was used to evaluate the variation in within day and day-to-day of FA concentrations, respectively.
Data analysis
Statistical analyses were performed using IBM SPSS software for Windows (version 19.0; SPSS Inc., Chicago, IL, USA). Graphs were generated using GraphPad Prism version 5.01 (GraphPad Software Inc., San Diego, CA, USA). The clinical characteristics and urine formaldehyde concentrations were compared using the χ2 statistic for categorical variables and analysis of variance for continuous variables. The concentrations of formaldehyde in urine samples from AD patients and age-matched controls were compared using Student’s t-test. Differences between the groups were considered statistically significant when the P value was less than 0.05.
Results
Optimization of the reaction conditions
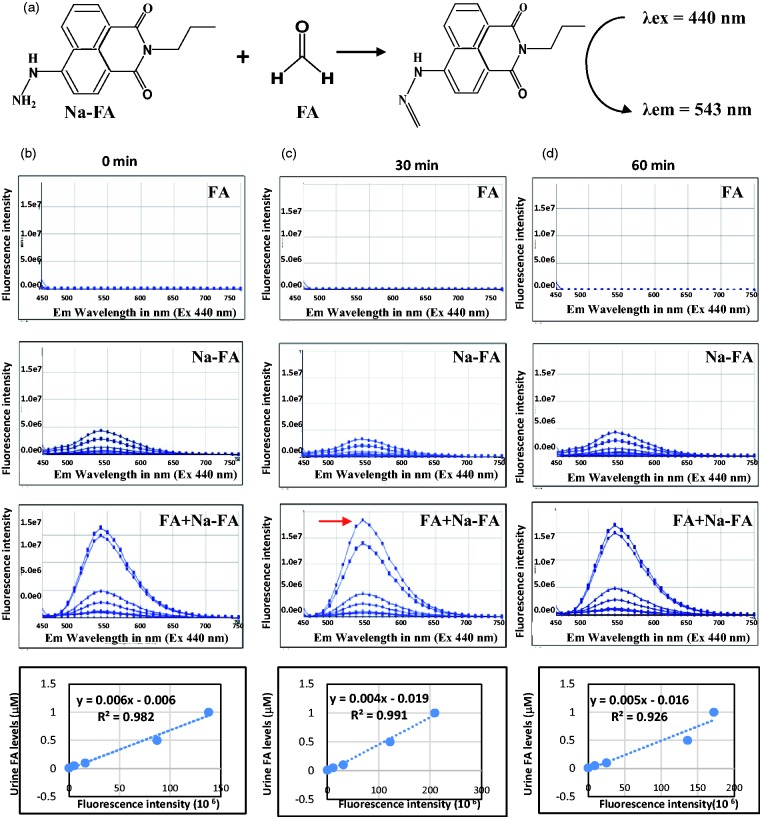
To examine the sensitivity of the NaFA probe method, we explored the experimental parameters’ reaction time at room temperature (25 ± 1°C). We found that the highest fluorescent intensity was at 30 min and then gradually declined at 1 h after the standard FA was mixed with NaFA probe at room temperature (Figure 1(a) to (d)). The derivant between NaFA and FA can be detected by fluorescent spectrophotometry at λex/em = 430/543 nm.22 Therefore, reaction times at 30 min and room temperature at 25°C were the optimal conditions for the derivative reaction.
Figure 1.
The standard curves of FA derivation with NaFA (a FA-specific fluorescent probe) analysed by fluorescence spectrophotometry. (a) The derivant between FA and NaFA detected at λex/em = 430/543 nm and (b) to (d) The chemical reaction time and standard curves between FA and NaFA at 0, 30, 60 and 120 min, respectively.
FA: formaldehyde.
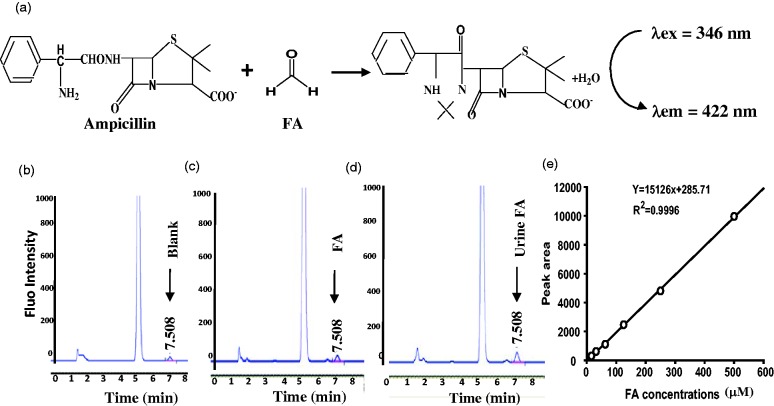
As a standard reference method,5,13 the available approach of Fluo-HPLC for detecting urine FA was carried out in this study. We found that ampicillin had chemical reaction with FA and formed a derivant, which can be detected by HPLC with a fluorescence detector (λex/em = 346/422 nm). There was a hypothetical standard curve between FA and area of fluorescent peaks (R2 = 0.996) (Figure 2(a) to (e)).
Figure 2.
The standard curves of FA derivation with ampicillin analysed by Fluo-HPLC. (a) The derivant between FA and ampicillin detected at λex/em = 346/422 nm. (b) to (d). The HPLC peak of blank control, FA and urine FA, respectively. Retention time: 7.508 min and (e) Standard curves between FA and peak area of fluorescence intensity.
FA: formaldehyde.
Limits of detection and limits of quantization of methods
Next we tested the limits of detection (LOD) and the limits of quantization (LOQ) of urine FA, which were analysed by these two methods. In routine analysis, a one-point calibration in duplicate could be used instead of a calibration curve, but a new calibration curve was prepared to check the instrumental system in case of any deviation. The LOD and LOQ were calculated from the confidence intervals of the regression line of the calibration line following the method as previously described.23–25 Our results showed that the LOD and LOQ of using FA probe method were 1.27 and 2.48 μM, while 1.52 and 2.91 μM for Fluo-HPLC method (Table 1). The average recoveries were 97.3% and 98.1% with relative standard deviations (RSDs, <8%). These data indicate that this NaFA probe method is more accurate than Fluo-HPLC.
Table 1.
LOD, LOQ, RSD and urine FA concentrations detected by using FA probe and Fluo-HPLC methods within a day at different time points, respectively.
| Test items | 0 min | 30 min | 1 h | 2 h | Mean ± S.D. | Recovery (%) | RSD (%) | LOD (μM) | LQD (μM) |
|---|---|---|---|---|---|---|---|---|---|
| FA probe-urine FA | 94.52 | 96.85 | 92.17 | 91.03 | 93.64 ± 6.26 | 97.3 | 6.68 | 1.27 | 2.48 |
| HPLC-urine FA | 52.49 | 53.33 | 53.58 | 54.06 | 53.36 ± 3.85 | 98.1 | 7.22 | 1.52 | 2.91 |
Note: Data in means (n = 10). No significant difference among the values detected by these two methods within a day (P > 0.05), respectively.
FA: formaldehyde; LOD: limits of detection; LOQ: limits of quantization; RSD: relative standard deviations; HPLC: high-performance liquid chromatography.
Repeatability and stability of comparative methods
Ten replicates of urine samples were analysed at various time points in a day to evaluate within-day variations. The same samples of urines were also analysed on different days to evaluate day-to-day variations. The data of variations of within-day are summarized in Table 1. These data indicate that there is no difference in the concentrations of FA in urine samples within a day (P > 0.05). The results of variation of day-to-day showed that the average relative standard deviations were 7.07 for FA probe method and 3.73 for Fluo-HPLC method (Table 2). The average recoveries were 97.6% and 96.2% with RSDs (<8%). These data indicate that these two methods can effectively and steadily determine urine FA concentrations. However, the method of NaFA probe was more sensitive than Fluo-HPLC because the average concentrations of urine FA detected by prior approach (93.64 ± 10.29 μM) were higher than the latter method (53.36 ± 8.32 μM) (Table 2).
Table 2.
Urine FA concentrations detected by using FA probe and HPLC methods at different days, respectively.
| Test items | One day | Two days | Mean | S.D. | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|
| FA probe-Urine FA (μM) | 96.85 | 103.87 | 100.36 | 7.07 | 97.6 | 7.04 |
| HPLC-Urine FA (μM) | 53.42 | 56.79 | 55.10 | 3.73 | 96.2 | 6.76 |
Note: Data in means (n = 10). No significant difference among the values detected by these two methods day-to-day (P > 0.05), respectively.
FA: formaldehyde; RSD: relative standard deviations; HPLC: high-performance liquid chromatography.
Relationship between urine FA and human cognitive ability
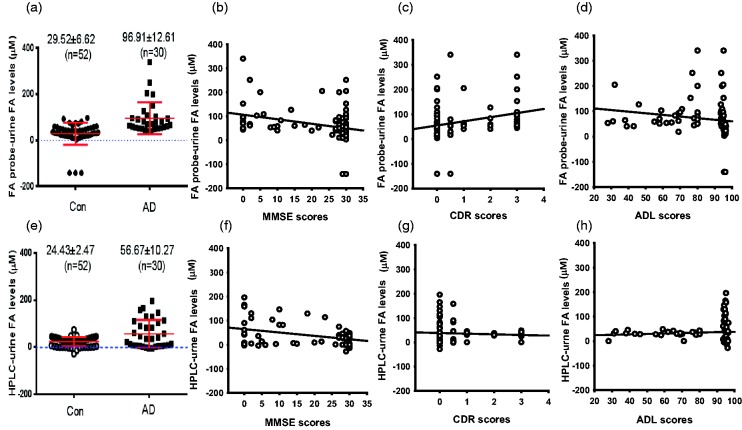
To address whether urine FA predicts the degree of dementia, we evaluated human cognitive ability by using the Mini-Mental State Examination (MMSE), CDR and ADL in 30 AD patients and 52 age-matched controls, and analysed urine FA by these two methods (Table 3). The results showed that urine FA concentrations in AD patients (96.91 ± 12.61 μM) analysed by using FA probe were obviously higher than age-matched controls (29.52 ± 6.62 μM, P < 0.01) (Figure 3(a)). More importantly, using the NaFA method, urine FA concentrations were negatively correlated with MMSE scores but positively correlated with CDR scores, and not relative with ADL in 82 participants (Figure 3(b) to (c)). As a standard reference method, urine FA concentrations detected by Fluo-HPLC were 56.67 ± 10.27 μM in AD patients and 24.43 ± 2.47 μM in healthy controls (Figure 3(e)). However, the correlation coefficient between MMSE/CDR and urine FA detected by Fluo-HPLC was lesser than that analysed by NaFA probe (Figure 3(f) to (h)). These data indicate that the method of NaFA probe is more suitable to analyse urine FA.
Table 3.
Comparative urine FA concentrations detected by using FA probe and Fluo-HPLC methods in patients with Alzheimer’s disease and age-matched controls, respectively.
| Test items/groups | Controls (n = 52) | AD (n = 30) | P |
|---|---|---|---|
| Age (years old) | 77.01 ± 3.45 | 80.02 ± 2.36 | >0.05 |
| Sex (male/female) | 21/31 | 12/18 | >0.05 |
| Education (years) | 8.65 ± 2.76 | 8.38 ± 2.82 | >0.05 |
| MMSE scores | 29.19 ± 2.15 | 6.16 ± 1.37 | <0.01 |
| CDR scores | 0.08 ± 0.03 | 2.50 ± 0.14 | <0.01 |
| ADL scores | 94.84 ± 2.37 | 63.63 ± 3.13 | <0.01 |
| FA probe-urine FA (μM) | 29.52 ± 6.62 | 96.91 ± 12.61 | <0.01 |
| HPLC-urine FA (μM) | 24.43 ± 2.47 | 56.67 ± 10.27 | <0.01 |
FA: formaldehyde; AD: Alzheimer’s disease; MMSE: Mini-Mental State Examination; CDR: Clinical Dementia Rating; ADL: Activities of Daily Living.
Figure 3.
The relationship between the score of MMSE/CDR/ADL and urine FA concentration analysed by these two methods. (a to d) Urine FA detected by using FA probe (a), the relationship between FA and MMSE (b), CDR (c), ADL (d), respectively. (e) to (h) Urine FA detected by using Fluo-HPLC, (e) the relationship between FA and MMSE (f), CDR (g), ADL (h), respectively.
FA: formaldehyde; MMSE: mini-mental state examination; CDR: Clinical Dementia Rating; ADL: Activities of Daily Living scale.
Discussion
Compared with the methods of GC/MS, Fluo-HPLC and UV-HPLC, the technique of NaFA probe for detecting urine FA was relatively simple and rapid. It is a promising approach for clinical diagnosis and medicine monitoring in AD patients.
The accuracy, repeatability and stability of the new method are critical for clinical biochemical investigations. In this study, LOD and LOQ (1.27 and 2.48 μM) of NaFA probe method were more accurate than Fluo-HPLC (1.52 and 2.91 μM). There was no difference in urine FA concentrations within day and day-to-day. Only 3/82 urine FA concentrations detected by NaFA probe were below zero, while 12/82 of the values analysed by Fluo-HPLC were abnormal. Each urine FA value in AD patients (30/30) detected by NaFA probe was higher than the average concentrations in healthy controls (29.52 ± 6.62 μM) (Figure 3(a)). However, only 19/30 of urine FA concentrations analysed by Fluo-HPLC were higher than the average concentrations in healthy controls (24.43 ± 2.47 μM) (Figure 3(e)). More importantly, the correlation coefficient between MMSE/CDR and urine FA detected by NaFA probe was higher than Fluo-HPLC. These data indicate that the method of NaFA probe is more accurate than Fluo-HPLC.
The specificity of the derivant reagent determines the accuracy of these two methods of NaFA probe and Fluo-HPLC. The fluorescent probe of NaFA has been developed recently,26 and it has a substantial high specificity and sensitivity to FA, because NaFA did not have chemical reaction with a lot of relevant analytes, such as PBS, glyoxal, methylglyoxal, sodium pyruvate, p-hydroxybenzaldehyde, trichloroacetaldehyde, acetaldehyde, 4-nitro-benzaldehyde, acetone, NaClO, H2O2, tert-butyl hydroperoxide, TBHP, nitric oxide (NO); CaCl2, MgCl2, Na2SO3, NaNO2, NaHSO3, NaHS, L-Arg, GSH, L-Cys, DL-Hcy, D-Phe, N-acetylglycine and N-acetyl-cysteine. In this study, we found that the selectivity and sensitivity of Na-FA probe to urine FA were similar to previous report.26 For example, these unsaturated aldehydes at 50 μM, including glyoxal, methylglyoxal, acetaldehyde, trichloroacetaldehyde, 4-nitro-benzaldehyde, p-hydroxybenzaldehyde, had extremely lower sensitivity than FA (Supplementary Figure 2(a) and (b)). The average FA concentration of these aldehydes detected by NaFA probe was about 1.5 μM, which was much less than urine FA (about 30 μM, P < 0.01). Meanwhile, we found that these FA scavengers at 10 μM, including L-Cys,27 N-acetyl-cysteine28 and NaHSO3,26 could reduce urine FA concentrations (Supplementary Figure 2(b)). However, urine components contain only ∼0.16 μM L-cys and almost nothing of N-acetyl-cysteine and NaHSO3. These data indicate that the abovementioned compounds do not disturb the sensitivity of urine FA. More importantly, the derivant between FA and NaFA can be specifically detected by fluorescent spectrophotometry at λex/em: 440/543 nm.22 Although the method of Fluo-HPLC at λex/em: 346/422 nm is based on that the derivant between ampicillin and FA, the specificity of ampicillin via above relevant analytes was not examined.5 In this study, LOD and LOQ of the NaFA probe method were more sensitive than Fluo-HPLC, suggesting that this method of NaFA probe has relatively good accuracy and sensitivity.
Another critical question was whether the pH values of urine affect the detected FA concentrations by using this NaFA-probe method. First, there was no statistical difference in the average pH values of urine samples of six AD patients and six age-matched controls selected from sample bank randomly, were 6.76 ± 0.24 and 6.84 ± 0.39, respectively (Supplementary Figure 3(a)). Second, to rule out the effects of pH on urine FA concentrations, we artificially adjusted the pH values to 6.2, 6.8 and 7 of these randomly selected 12 samples, respectively, and then analysed urine FA concentration. Notably, under the same condition of these urine samples at the same pH, there was a marked difference in urine FA concentrations between AD patients and healthy controls (Supplementary Figure 3(b) to (d)). These data confirm that FA concentrations in urine from AD patients are higher than healthy controls, which is consistent with previous results detected by HPLC13,21 or other methods.29 Third, we observed that a very few pH values of urine samples were lower 6.0; therefore, the effects of the same sample at different pH on urine FA concentrations need to be investigated. We found that there was a negative relationship between pH value and urine FA concentration (Supplementary Figure 3(e)). Moreover, FA concentration of urine sample at pH 6.2 was higher than the same sample at pH 6.8 or 7.2 (Supplementary Figure 3(f)). This result indicates that the more acidic environment can enhance the fluorescence urine FA values. This unexpected phenomenon seemly did not support that application of this method of NaFA probe to specifically detect urine FA. However, urine acidification from AD patients was a possible clinical biochemical character because FA can be oxidized to form formic acid, which results in urine acidification with or without enzyme-dependent pathways30 (Supplementary Figure 4(a) and (b)). Thus, urine acidification of AD patients contributes to the improvement of sensitivity of this method. The pH value in the urine of a healthy human is about 6.5. We found that the slight acidifications ranging from 6.2 to 7.2 did not affect the detected FA concentrations in urine of healthy controls (Supplementary Figure 3(f)). However, if by any chance low pH of urine from healthy controls leads to an abnormally high concentration of FA, the doctors can easily distinguish between AD and healthy controls according to the clinical scores of MMSE by cognitive examination. Therefore, this Na-FA probe can sensitively and specifically detect urine FA of AD patients.
Conclusion
Using the NaFA fluorescent probe, an abnormally high concentration of urine FA was observed in AD patients than age-matched controls. The present fluorescent spectrophotometric approach is a simple, rapid, sensitive and practical alternative for diagnosing dementia or monitoring medicine metabolism.
Supplemental Material
Supplemental Material for A rapid and sensitive fluorescence method for detecting urine formaldehyde in patients with Alzheimer’s disease by Li Ai, Jun Wang, Tingting Li, Chang Zhao, Yonghe Tang, Weishan Wang, Shengjie Zhao, Wenjing Jiang, Yalan Di, Xuechao Fei, Hongjun Luo, Hui Li, Wenhong Luo, Yan Yu, Weiying Lin, Rongqiao He and Zhiqian Tong in Annals of Clinical Biochemistry
Acknowledgements
We thank HJ Luo, H Li and WH Luo (Central Laboratory, Shantou University Medical College, China) for HPLC analysis of FA; and C Zhao, YH Tang and WY Lin (Institute of Fluorescent Probes for Biological Imaging, University of Jinan, China) for fluorescent analysis of FA. A special thanks to RQ He (Institute of Biophysics, University of Chinese Academy of Sciences, China) for reviewing this article.
Contributorship
All authors declare that they have intellectually participated in this work and are solely responsible for its results.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Natural Science Foundation of China (21472067, 21672083) and Beijing Municipal Natural Science Foundation (7172022).
Ethical approval
This clinical investigation (2014SY39) was approved by the Ethics Committee at the Capital Medical University, China.
Guarantor
ZQT.
References
- 1.Lu Z, Li C, Qiao Y, et al. Effect of inhaled formaldehyde on learning and memory of mice. Indoor Air 2008; 18: 77–83. [DOI] [PubMed] [Google Scholar]
- 2.Kilburn KH, Wohsa R, Thornton JC. Formaldehyde impairs memory, equilibrium, and dexterity in histology technicians: effects which persist for days after exposure. Arch Environ Health 1987; 42: 117–120. [DOI] [PubMed] [Google Scholar]
- 3.Kalasz H. Biological role of formaldehyde, and cycles related to methylation, demethylation, and formaldehyde production. Mini Rev Med Chem 2003; 3: 175–192. [DOI] [PubMed] [Google Scholar]
- 4.Heck HD, White E, Casanova-Schmitz M. Determination of formaldehyde in biological tissues by gas chromatography/mass spectrometry. Biomed Mass Spectrom 1982; 9: 347–353. [DOI] [PubMed] [Google Scholar]
- 5.Tong Z, Han C, Luo W, et al. Aging-associated excess formaldehyde leads to spatial memory deficits. Sci Rep 2013; 3: 1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong ZQ, Zhang J, Luo WH, et al. Urine formaldehyde level is inversely correlated to mini mental state examination scores in senile dementia. Neurobiol Aging 2011; 32: 31–42. [DOI] [PubMed] [Google Scholar]
- 7.Mei Y, Jiang C, Wan Y, et al. Aging-associated formaldehyde-induced norepinephrine deficiency contributes to age-related memory decline. Aging Cell 2015; 14: 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Songur A, Ozen OA, Sarsilmaz M. The toxic effects of formaldehyde on the nervous system. Rev Environ Contam Toxicol 2010; 203: 105–118. [DOI] [PubMed] [Google Scholar]
- 9.Yang M, Lu J, Miao J, et al. Alzheimer's disease and methanol toxicity (part 1): chronic methanol feeding led to memory impairments and tau hyperphosphorylation in mice. J Alzheimers Dis 2014; 41: 1117–1129. [DOI] [PubMed] [Google Scholar]
- 10.Yang M, Miao J, Rizak J, et al. Alzheimer's disease and methanol toxicity (part 2): lessons from four Rhesus Macaques (Macaca mulatta) chronically fed methanol. J Alzheimers Dis 2014; 41: 1131–1147. [DOI] [PubMed] [Google Scholar]
- 11.Tulpule K, Dringen R. Formaldehyde in brain: an overlooked player in neurodegeneration? J Neurochem 2013; 127: 7–21. [DOI] [PubMed] [Google Scholar]
- 12. Yu J, Su T, Zhou T, et al. Uric formaldehyde levels are negatively correlated with cognitive abilities in healthy older adults. Neurosci Bull 2014; 30: 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong Z, Wang W, Luo W, et al. Urine formaldehyde predicts cognitive impairment in post-stroke dementia and Alzheimer's disease. J Alzheimers Dis 2017; 55: 1031–1038. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi A, Takigawa T, Abe M, et al. Determination of formaldehyde in urine by headspace gas chromatography. Bull Environ Contam Toxicol 2007; 79: 1–4. [DOI] [PubMed] [Google Scholar]
- 15.Deng Y, Yu PH. Simultaneous determination of formaldehyde and methylglyoxal in urine: involvement of semicarbazide-sensitive amine oxidase-mediated deamination in diabetic complications. J Chromatogr Sci 1999; 37: 317–322. [DOI] [PubMed] [Google Scholar]
- 16.Szarvas T, Szatlóczky E, Volford J, et al. Determination of endogenous formaldehyde level in human blood and urine by dimedone-14C radiometric method. J Radioanal Nuclear Chem 1986; 106: 357–367. [Google Scholar]
- 17.Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med 2003; 348: 1333–1341. [DOI] [PubMed] [Google Scholar]
- 18.Fillenbaum GG, Peterson B, Morris JC. Estimating the validity of the clinical Dementia Rating Scale: the CERAD experience. Consortium to establish a registry for Alzheimer's Disease. Aging (Milano) 1996; 8: 379–385. [DOI] [PubMed] [Google Scholar]
- 19.Woodford HJ, George J. Cognitive assessment in the elderly: a review of clinical methods. QJM 2007; 100: 469–484. [DOI] [PubMed] [Google Scholar]
- 20.Bleich S, Wiltfang J, Kornhuber J. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med 2003; 349: 609–610. [DOI] [PubMed] [Google Scholar]
- 21.Luo W, Li H, Zhang Y, et al. Determination of formaldehyde in blood plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl 2001; 753: 253–257. [DOI] [PubMed] [Google Scholar]
- 22.Tang Y, Kong X, Liu ZR, et al. Lysosome-targeted turn-on fluorescent probe for endogenous formaldehyde in living cells. Anal Chem 2016; 88: 9359–9363. [DOI] [PubMed] [Google Scholar]
- 23.Peter C. Meier aRFZ. Statistical methods in analytical chemistry. 2nd ed New York: Wiley-Interscience, 2000, p.424. [Google Scholar]
- 24.Alankar Shrivastava VBG. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron Young Sci 2011; 2: 21–25. [Google Scholar]
- 25.Uhrovcik J. Strategy for determination of LOD and LOQ values – some basic aspects. Talanta 2014; 119: 178–180. [DOI] [PubMed] [Google Scholar]
- 26.Tang Y, Kong X, Xu A, et al. Development of a two-photon fluorescent probe for imaging of endogenous formaldehyde in living tissues. Angew Chem Int Ed Engl 2016; 55: 3356–3359. [DOI] [PubMed] [Google Scholar]
- 27.Ashby MT, Nagy P. On the kinetics and mechanism of the reaction of cysteine and hydrogen peroxide in aqueous solution. J Pharm Sci 2006; 95: 15–18. [DOI] [PubMed] [Google Scholar]
- 28.Visseren F, Verkerk MS, van der Bruggen T, et al. Iron chelation and hydroxyl radical scavenging reduce the inflammatory response of endothelial cells after infection with Chlamydia pneumoniae or influenza A. Eur J Clin Invest 2002; 32(Suppl 1): 84–90. [DOI] [PubMed] [Google Scholar]
- 29.Yue X Zhang Y, Xing W, et al. A sensitive and rapid method for detecting formaldehyde in brain tissues. Anal Cell Pathol 2017; 2017: 9043134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conaway CC, Whysner J, Verna LK, et al. Formaldehyde mechanistic data and risk assessment: endogenous protection from DNA adduct formation. Pharmacol Ther 1996; 71: 29–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for A rapid and sensitive fluorescence method for detecting urine formaldehyde in patients with Alzheimer’s disease by Li Ai, Jun Wang, Tingting Li, Chang Zhao, Yonghe Tang, Weishan Wang, Shengjie Zhao, Wenjing Jiang, Yalan Di, Xuechao Fei, Hongjun Luo, Hui Li, Wenhong Luo, Yan Yu, Weiying Lin, Rongqiao He and Zhiqian Tong in Annals of Clinical Biochemistry