Abstract
Mitral valve surgery has evolved over 4 decades from one based on the principles of prosthetic replacement to a subspecialty with a foundation based on the principles of repair. This review will attempt to enumerate the contemporary techniques of mitral valve repair and a pathoanatomically directed approach with which to apply them by focusing on degenerative disease and associated complexities.
Keywords: cardiac surgery, mitral valve, transesophageal echocardiography, intraoperative assessment, cardiac anesthesia
Mitral valve (MV) surgery has evolved over 4 decades from one based on the principles of prosthetic replacement to a subspecialty based on the principles of repair. MV repair has further evolved from the sound principles of quadrangular resection as a one-size-fits-all approach to degenerative disease, to a more tailored approach based on specific anatomic features.1-3 This review will attempt to enumerate the contemporary techniques of MV repair by focusing on a pathoanatomically directed approach to degenerative disease and its associated complexities.
Classification and Management
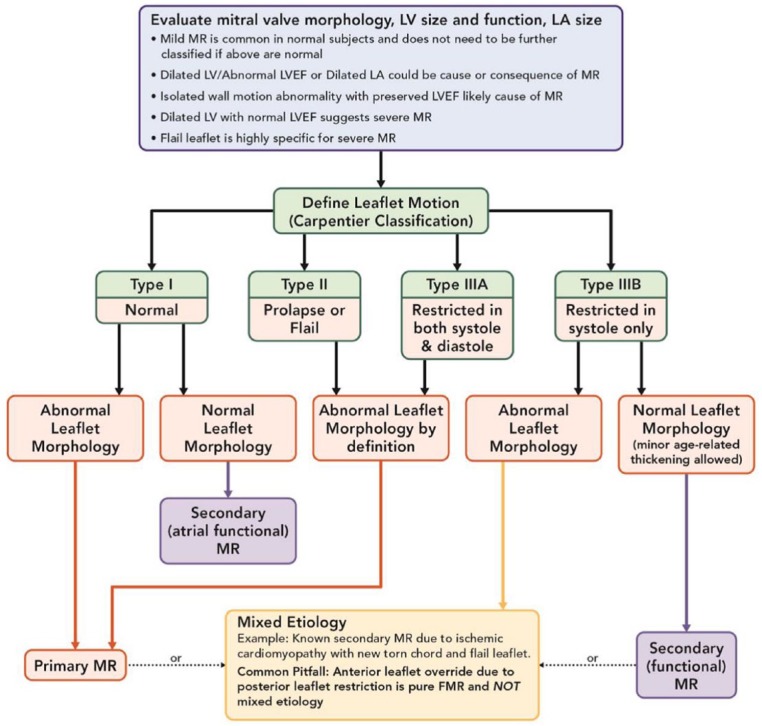
Mitral regurgitation (MR) is the most common mitral pathology affecting adults in the United States over 55 years of age, and it is pathologically classified as either primary, secondary, or mixed.3,4 Primary MR accounts for cases of myxomatous degenerative disease and annular dilatation, secondary MR accounts for functional or restrictive diseases, and MR is considered mixed when both pathology categories exist together.3 Degenerative disease has a 3% overall prevalence in the United States, and it is the most common global MR etiology (60.7%), while rheumatic degeneration is the next most common (22.5%), followed by functional (ischemic or nonischemic) as the third most common etiology.4,5 The most common definition of MV leaflet motion used is the Carpentier classification.1-3 Type I is normal leaflet motion such as central MR due to annular dilation or leaflet perforation, Type II includes excess leaflet motion such as prolapse or flail associated with degenerative primary MR, Type IIIA occurs when the leaflet is restricted in both systole and diastole such as in rheumatic disease, and Type IIIB exists when the leaflet is restricted in systole only such as with functional secondary MR (Figure 1).
Figure 1.
Classification of mitral regurgitation based on leaflet motion.
Abbreviations: FMR, functional mitral regurgitation; MR, mitral regurgitation; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction.
Adapted from O’Gara et al.3
As experience has been gained with regard to the natural history of severe primary MR, the practice of watchful waiting is being replaced by earlier referral for MV repair, even in asymptomatic patients.6-8 Of course, this is based on the ability of the center and surgeon to guarantee repair rates above 90% to 95% for primary MR, regardless of whether the patient is symptomatic.8,9 Enhancements in imaging quality and improved knowledge of the evidence should facilitate earlier diagnosis and referral for surgical correction. However, a knowledge and clinical referral gap currently persists as the majority of patients presenting for surgical correction today have advanced heart failure symptoms, depressed ejection fraction, or atrial fibrillation.10-12 This is despite a current overall repair rate for primary MR now being over 80% in the United States, with nearly 20% of isolated MV repair operations being performed through nonsternotomy minimally invasive techniques, nearly 10% robotically.11-13 In several highly experienced centers where consistent pathoanatomically directed MV repair techniques are applied, repair rates for primary degenerative disease may now be achieved in essentially 100% of patients, a growing number of these consistently being achieved robotically.13-16
Pathoanatomic Assessment
Contemporary MV surgery is safe with overall excellent 30-day outcomes.11,12 Nevertheless, to achieve superior repair rates with long-term durability, the importance of programmatic attention to pathoanatomically directed surgery cannot be overstated. The imaging cardiologist, mitral surgeon, and cardiac anesthesiologist each play an integral role in mitral heart teams to ensure that image-guided operative strategies are implemented for optimal results. Once the diagnosis is correctly made and the type and severity of MR identified by the referral process, it is really up to the intraoperative team to clearly identify the detailed mechanism of the MR along with the potential pitfalls in order to finally craft the operative approach before skin incision is made. It is this final step of a mutual understanding on MR mechanism and strategic approach that defines excellence in outcome-driven MV repair centers.
Intraoperative transesophageal interrogation is not the time to make the diagnosis of MR severity. Instead, it is the essential moment to carefully assess the pathoanatomy of the lesion(s) and to identify anatomic risk factors that should be avoided in tailoring the correct repair solution for each individual patient by using all means necessary.2,3,13,17-19 This exercise, most commonly performed by the echo-boarded cardiac anesthesiologist, should not focus on the obvious lesion such as a P2 flail, but on identifying the potential secondary mechanisms that could be masked by an obvious lesion. After a thorough 2-dimensional assessment where the majority of these features can be identified, 3-dimensional (3D) reconstruction with/without color flow may clarify or confirm potential subtle surgical pitfalls. The specific areas of focus for the imaging anesthesiologist or cardiologist to greatly assist the mitral surgeon may include the identification of the following: (a) the existence of anterior mitral leaflet (AML) prolapse—either masked by an obvious posterior mitral leaflet (PML) lesion or an AML pseudo-prolapse lesion if the PML is restricted; (b) locating the existence of commissural prolapse at the medial or lateral location as a separate lesion—this is a commonly missed diagnosis perhaps best identified in the bicommisural view or with 3D; (c) PML clefts adjacent to the primary lesion—this is best identified with 3D and very important to avoid a technical error of over resecting the primary PML lesion and exposing clefts for residual MR; (d) mitral annular calcification with secondary and tertiary PML chordal impingement/tethering—perhaps best identified in the long-axis views of the PML or with the ventricular view on 3D; (e) AML secondary chordal tethering resulting in lack of coaptation or primary AML prolapse—this is best identified in long-axis views and they can be most useful for the surgeon to divide if felt to be contributing to the lack of coaptation; and (f) predictors of systolic anterior motion (SAM) to include an acute aorto-mitral angle, reduced coaptation-septal distance, hyperdynamic small left ventricular cavity, and excess AML or PML height—all relevant to the surgeon to focus on PML height reduction techniques.
When identifying the lesion(s) in need of repair, it is important to also identify the tissue features of the leaflets involved in the pathology. Type II lesions can occur in a spectrum of myxomatous degeneration. Repair techniques may vary depending on how focal or diffuse the leaflet involvement may be. Fibroelastic deficiency, most common in older patients, is a focal phenomenon usually involving the P2 segment of the PML without much thickening or myxoid changes to other areas of the valve. When the majority of the PML is diffusely thickened with prominent clefts and increased PML height but with minimal AML involvement, this is known as forme fruste. Bileaflet involvement with diffuse myxoid thickening and excess tissue is commonly known as Barlow’s disease.2,3,15,20 On initial valve assessment by intraoperative echocardiography, it is imperative to recognize that as the degree of myxomatous degenerative involvement increases, so does the potential existence of secondary mechanisms enumerated earlier. In fact, special vigilance is required if the patient has forme fruste or bileaflet/Barlow’s pathology and is of advanced age as the existence of secondary mechanisms are all but assured, and these should be identified and a pathoanatomic surgical solution formulated prior to commencing the operation.
Pathoanatomically Directed MV Repair
The principle tenets of MV repair include 4 main components: (a) restoration of the depth of AML-PML coaptation to greater than 5 mm; (b) stabilization and remodeling of the mitral annulus; (c) restoration of normal leaflet motion without tension to the leaflets; and (d) elimination of MR to trace or less on the immediate postoperative transesophageal echocardiogram.2,3 If the surgical MV repair adheres to all of these principles, operative success can be consistently achieved and long-term durability most often assured.
Traditional quadrangular resection techniques, involving resecting all of P2 then anastomosing P1 to P2 after performing annular compression sutures in order to minimize leaflet tension, have enjoyed durable outcomes in young patients with focal disease.21 However, due to its limited application to advancing pathologies, most surgeons have largely abandoned this technique in favor of limited resection or nonresection techniques using polytetrafluoroethylene (PTFE) neochordal support.22 Durable outcomes and equivalent hemodynamic results have been achieved with both limited resection and nonresection techniques.23-26 Though some advocate for one approach over the other, given the broad array of pathologic findings now amenable for durable MV repair and the mainstream application of minimally invasive and robotic techniques, the contemporary MV surgeon must be versatile in all available reconstructive methods.
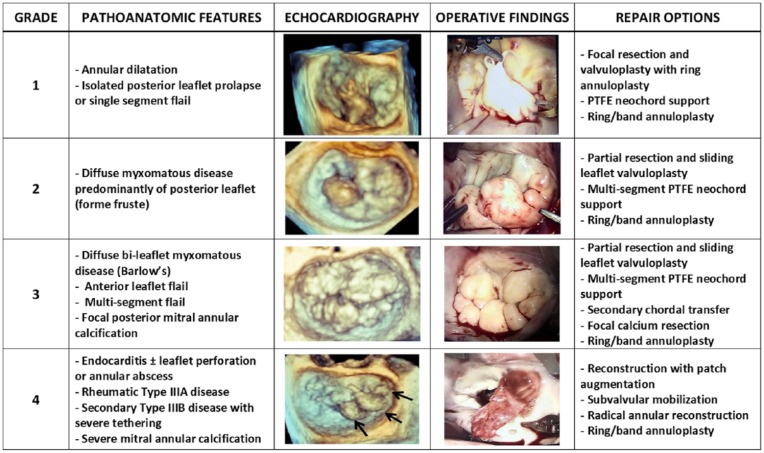
Only following a detailed understanding of the precise mechanism of the MR by imaging, including the identification of potential secondary mechanisms, should the MV operation commence. It remains quite common that even with what may appear to preoperatively be quite clear Type II disease, patients may have combined pathologies such as focal restrictive Type IIIA or IIIB disease. One should be particularly suspicious of this when patients with primary MR due to a flail leaflet present in a decompensated state with advanced symptoms and left ventricular dilatation. Patients with true mixed etiologies deserve special attention as this may increase the technical complexity to achieve a durable repair. To assist in identifying pathoanatomic features and tailoring which surgical strategies may be most effective for successful MV repair, use of a preoperative 4-grade complexity score may be helpful (Figure 2).
Figure 2.
The West Virginia University Pathoanatomic Complexity Score.
Abbreviation: PTFE, polytetrafluoroethylene.
Grade 1 disease includes straightforward annular dilatation alone or focal single-segment prolapse/flail that is often readily treated by focal triangular resection or PTFE neochords and annuloplasty. This is the common category of annular dilatation associated with left atrial dilatation and for fibroelastic deficiency or Type II primary MR with P2 prolapse in which MV repair should be essentially 100% achievable in all experienced centers. Should a nonresection strategy be considered, important technical points for PTFE chord placement include the following: (a) placement of the PTFE chords in the mid-papillary muscle body and not too close to the head to avoid entanglement with native chords, (b) assure that the sutures are directed into the central orifice between the papillary muscles to avoid subvalvular impingement in diastole, and (c) placement of the PTFE chords aligned with the ipsilateral papillary muscle matched to the appropriate length of adjacent normal chords to avoid excess tension during systole.
Grade 2 disease involves more diffuse PML disease with minimal to no involvement of the AML. This is often accompanied by multi-scallop involvement, often with clefts and more diffuse myxoid leaflet changes consistent with forme fruste. Attention to be directed at identifying potential echocardiographic SAM predictors in these cases as well as other secondary mechanisms. These patients may often require some form of PML height reduction as part of a successful repair. This may be achieved by multi-segment PTFE neochordal reconstruction or focal partial resection and sliding leaflet valvuloplasty (Video 1; all supplementary videos are available in the online version of the article). In some cases, where the clefts are not prominent but diffuse P2 disease exists and PML height reduction is desired, a triangular resection in conjunction with PTFE supported leaflet re-approximation, or a “respectful resection,” may be utilized.22 These techniques require careful preoperative imaging and intraoperative inspection to avoid SAM. When performing variations on PML resection methods, it is important to divide secondary and tertiary chords felt to contribution to the diffuse nature of the disease. This step can be most helpful to both debulk the excess PML tissue while preserving primary chordal connection and leaflet motion during diastole.
Grade 3 disease involves bileaflet myxomatous pathology such as Barlow’s or when the flail involves the AML or several segments of both leaflets. This category may also be utilized when focal posterior MAC is involved in the diffuse PML or bileaflet presentation. Surgical techniques for MV repair would be cumulative to those applied for Grade 2 disease but may include multi-segment PTFE support, focal resection of the MAC involved in secondary/tertiary tethering of the PML, and/or the use of secondary chordal transfer for primary support (Video 2). Steps outlined for Grade 2 become even more important when faced with secondary and tertiary PML chordal involvement of MAC or in states of mixed restriction.
Grade 4 disease may involve more invasive and complex MAC that may necessitate some degree of resection and annular reconstruction.27 This category also applies to cases of rheumatic Type IIIA leaflet tethering that may require leaflet augmentation with autologous pericardium,28 cases of Type IIIB restriction without inferobasal aneurysms that may require adjunctive leaflet or subvalvular remodeling techniques, or cases of acute bacterial endocarditis with associated leaflet destruction and annular involvement that may require radical debridement and patch reconstruction in order to restore leaflet coaptation (Video 3). These cases comprise the most complex cases for repair and require the surgeon to call on the entire armamentarium of techniques used for Grades 1 to 3 disease. Autologous patch use can be fresh or fixed with glutaraldehyde based on institutional preference, though there is an increasing trend toward minimal fixation or fresh pericardial use to avoid late leaflet calcification. Should the surgeon or center not be prepared or experienced in these techniques, total chord-sparing MV replacement may be a very reasonable and safe alternative.
Future Directions
As more centers become versatile with the application of advanced MV repair techniques to increased image-guided pathoanatomic complexity, so should their pursuit of close longitudinal patient follow-up. Technical advancements must be matched with quality assessment and patient safety. Postoperative transthoracic echocardiography either pre-dismissal or within 30 days should become minimum standard of care in all advanced mitral programs, and 1-year echocardiographic follow-up should be encouraged. Surgeons and centers accomplished in MV repair commonly embark on development of minimally invasive and robotic programs. For fully developed pathoanatomically directed MV programs, robotic technology may become a tool not just reserved for the Grade 1 patients with straightforward pathology, but a standard approach for all Grades and all-comers including the elderly or infirm as a manner in which to perhaps actually decrease the morbidity of a sternotomy approach.16,29,30
Conclusions
Repair of MR in the setting of advancing pathologic complexity can be successful using a variety of proven techniques. Understanding the pathoanatomic mechanism of MR with thorough preoperative imaging is essential to navigate predictable pitfalls and tailor pathology-specific repair solutions to achieve durable long-term outcomes.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD: Vinay Badhwar  https://orcid.org/0000-0002-7916-3544
https://orcid.org/0000-0002-7916-3544
References
- 1. Carpentier A. Cardiac valve surgery—the “French correction.” J Thorac Cardiovasc Surg. 1983;86:323-337. [PubMed] [Google Scholar]
- 2. Badhwar V, Smith A, Cavalcante JL. A pathoanatomic approach to the management of mitral regurgitation. Trends Cardiovasc Med. 2016;26:126-134. [DOI] [PubMed] [Google Scholar]
- 3. O’Gara PT, Grayburn PA, Badhwar V, et al. 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70:2421-2449. [DOI] [PubMed] [Google Scholar]
- 4. Nkomo VT, Gardin JM, Skelton TN, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005-1011. [DOI] [PubMed] [Google Scholar]
- 5. Jones EC, Devereux RB, Roman MJ, et al. Prevalence and correlates of mitral regurgitation in a population-based sample (the Strong Heart Study). Am J Cardiol. 2001;87:298-304. [DOI] [PubMed] [Google Scholar]
- 6. Suri RM, Vanoverschelde JL, Grigioni F, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA. 2013;310:609-616. [DOI] [PubMed] [Google Scholar]
- 7. Kang DH, Park SJ, Sun BJ, et al. Early surgery versus conventional treatment for asymptomatic severe mitral regurgitation: a propensity analysis. J Am Coll Cardiol. 2014;63:2398-2407. [DOI] [PubMed] [Google Scholar]
- 8. Enriquez-Sarano M, Suri RM, Clavel MA, et al. Is there an outcome penalty linked to guideline-based indications for valvular surgery? Early and long-term analysis of patients with organic mitral regurgitation. J Thorac Cardiovasc Surg. 2015;150:50-58. [DOI] [PubMed] [Google Scholar]
- 9. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252-289. [DOI] [PubMed] [Google Scholar]
- 10. Wang A, Grayburn P, Foster JA, et al. Practice gaps in the care of mitral valve regurgitation: Insights from the American College of Cardiology mitral regurgitation gap analysis and advisory panel. Am Heart J. 2016;172:70-79. [DOI] [PubMed] [Google Scholar]
- 11. Gammie JS, Chikwe J, Badhwar V, et al. Isolated mitral valve surgery: the Society of Thoracic Surgeons Adult Cardiac Surgery Database analysis. Ann Thorac Surg. 2018;106:716-727. [DOI] [PubMed] [Google Scholar]
- 12. Badhwar V, Rankin JS, He X, et al. The Society of Thoracic Surgeons Mitral Repair/Replacement Composite Score: a report of the Society of Thoracic Surgeons Quality Measurement Task Force. Ann Thorac Surg. 2016;101:2265-2271. [DOI] [PubMed] [Google Scholar]
- 13. Wei LM, Roberts HG, Badhwar V. Mitral surgery on the precipice of transformation. J Thorac Cardiovasc Surg. 2018;155:945-946. [DOI] [PubMed] [Google Scholar]
- 14. Tatum JM, Bowdish ME, Mack WJ, et al. Outcomes after mitral valve repair: a single-center 16-year experience. J Thorac Cardiovasc Surg. 2017;154:822-830.e2. [DOI] [PubMed] [Google Scholar]
- 15. Adams DH, Rosenhek R, Falk V. Degenerative mitral valve regurgitation: best practice revolution. Eur Heart J. 2010;31:1958-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coyan G, Wei LM, Althouse A, et al. Robotic mitral valve operations by experienced surgeons are cost-neutral and durable at 1 year. J Thorac Cardiovasc Surg. 2018;156:1040-1047. [DOI] [PubMed] [Google Scholar]
- 17. Cobey FC, Ferreira R, Ursprung WW, Karhausen J, Swaminathan M, Mackensen GB. A novel approach to assess the three-dimensional anatomy of a mitral valve regurgitant jet orifice. J Cardiothorac Vasc Anesth. 2017;31:169-173. [DOI] [PubMed] [Google Scholar]
- 18. Cobey FC, Shernan SK. Emerging from two-dimensional shadows, the value of added dimensions in the accurate assessment of mitral regurgitation. J Am Soc Echocardiogr. 2016;29:A26-A27. [DOI] [PubMed] [Google Scholar]
- 19. Bhatia M, Kumar P, Martinelli SM. Surgical Echocardiography of the MV: Focus on 3D. Semin Cardiothorac Vasc Anesth. 2018; 23:26-36. doi: 10.1177/1089253218789409 [DOI] [PubMed] [Google Scholar]
- 20. Barlow JB, Pocock WA. Billowing, floppy, prolapsed or flail mitral valves? Am J Cardiol. 1985;55:501-502. [DOI] [PubMed] [Google Scholar]
- 21. Carpentier A, Chauvaud S, Fabiani JN, et al. Reconstructive surgery of mitral valve incompetence: ten year appraisal. J Thorac Cardiovasc Surg. 1980;79:338-348. [PubMed] [Google Scholar]
- 22. Roberts HG, Rankin JS, Wei LM, Cook CC, Salman M, Badhwar V. Respectful resection to enhance the armamentarium of mitral valve repair: is less really more? J Thorac Cardiovasc Surg. 2018;156:1854-1855. doi: 10.1016/j.jtcvs.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 23. Lawrie GM, Earle EA, Earle N. Intermediate-term results of a nonresectional dynamic repair technique in 662 patients with mitral valve prolapse and mitral regurgitation. J Thorac Cardiovasc Surg. 2011;141:368-376. [DOI] [PubMed] [Google Scholar]
- 24. Seeburger J, Falk V, Borger MA, et al. Chordae replacement versus resection for repair of isolated posterior mitral leaflet prolapse: à ègalité. Ann Thorac Surg. 2009;87:1715-1720. [DOI] [PubMed] [Google Scholar]
- 25. Padala M, Powell SN, Croft LR, Thourani VH, Yoganathan AP, Adams DH. Mitral valve hemodynamics after repair of acute posterior leaflet prolapse: quadrangular resection versus triangular resection versus neochordoplasty. J Thorac Cardiovasc Surg. 2009;138:309-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mazine A, Friedrich JO, Nedadur R, et al. Systematic review and meta-analysis of chordal replacement versus leaflet resection for posterior mitral leaflet prolapse. J Thorac Cardiovasc Surg. 2018;155:120-128.e10. [DOI] [PubMed] [Google Scholar]
- 27. Carpentier AF, Pellerin M, Fuzellier JF, Relland JY. Extensive calcification of the mitral valve annulus: pathology and surgical management. J Thorac Cardiovasc Surg. 1996;111:718-729. [DOI] [PubMed] [Google Scholar]
- 28. Thomas MP, Badhwar V. A three-step technique for repair of rheumatic disease of the mitral valve. Cardiol Young. 2014;24:1104-1107. [DOI] [PubMed] [Google Scholar]
- 29. Badhwar V, Peterson ED, Jacobs JP, et al. Longitudinal outcome of isolated mitral repair in older patients: results from 14 604 procedures performed from 1991 to 2007. Ann Thorac Surg. 2012;94:1870-1877. [DOI] [PubMed] [Google Scholar]
- 30. Wang A, Brennan JM, Zhang S, et al. Robotic mitral valve repair in older individuals: an analysis of the Society of Thoracic Surgeons Database. Ann Thorac Surg. 2018;106:1388-1393. doi: 10.1016/j.athoracsur.2018.05.074 [DOI] [PubMed] [Google Scholar]