Abstract
We describe detection in the United Kingdom (UK) of the drug-resistant Neisseria gonorrhoeae FC428 clone, with ceftriaxone resistance and intermediate azithromycin resistance. Two female patients developed infection following contact with UK-resident men from the same sexual network linked to travel to Ibiza, Spain. One case failed treatment with ceftriaxone, and azithromycin and gentamicin, before successful treatment with ertapenem. Both isolates had indistinguishable whole-genome sequences. Urgent action is essential to contain this drug-resistant strain.
Keywords: gonorrhoeae, treatment failure, antimicrobial resistance, antimicrobial treatment, whole-genome sequencing
Antimicrobial resistance in Neisseria gonorrhoeae is a major public health concern, in particular resistance to ceftriaxone which is the last available drug for empirical monotherapy for gonorrhoea. Here, we report further international spread of the N. gonorrhoeae FC428 clone associated with ceftriaxone resistance and intermediate resistance to azithromycin.
The FC428 clone was first isolated in Japan in January 2015 [1], with a second isolate, FC460, obtained from the same patient [2]. This clone was subsequently reported in 2017 in Australia (A7536, A7846) [3], Canada (47707) [4], Denmark (GK124) [5] and France (F90) [6], and in 2018 in Ireland (IR72) [7]. However despite this global dissemination, sustained local transmission has not been reported to date. We describe a transmission cluster that probably involved at least four people resident in the United Kingdom (UK). We report the clinical management, microbiology and whole-genome sequencing findings.
Case descriptions
Case 1, a female patient, presented to a sexual health clinic in the UK in October 2018 with urinary symptoms. She had had unprotected vaginal intercourse with more than one male partner, 2 months before presentation, while on holiday in Ibiza, Spain. These partners were normally resident in the UK. The patient had a positive N. gonorrhoeae nucleic acid amplification test (NAAT) and endocervical culture. Before the results of susceptibility testing were available, she was treated empirically, in line with national treatment guidelines at the time, with a single dose of intramuscular ceftriaxone 500 mg and oral azithromycin 1 g. A NAAT test of cure (TOC) 2 weeks after treatment was negative. We were unable to trace her partners.
Case 2, a female patient, presented to another sexual health clinic elsewhere in the UK in November 2018 for an asymptomatic sexual health screen. Two weeks previously, she had had unprotected vaginal, oral and anal sex with an asymptomatic UK-resident man who had been in Ibiza and had links with the same sexual network as Case 1. She had positive vaginal and rectal N. gonorrhoeae NAAT results, a negative throat N. gonorrhoeae NAAT and was culture-negative at all three sites. She was treated empirically 1 week later with a single dose of intramuscular ceftriaxone 1 g and at this visit reported rectal symptoms. She initially responded clinically but her symptoms relapsed, and she was culture-positive on a vaginal sample taken 10 days later by her general practitioner, and culture and NAAT-positive at both the rectum and urogenital tract but not the throat when she attended the sexual health clinic a further 4 days later for treatment. She failed subsequent treatment with single dose intramuscular gentamicin 240 mg plus oral azithromycin 2 g but cleared the infection with intravenous ertapenem 1 g once daily for 3 days. A NAAT TOC taken 2 weeks later was negative at both sites.
The male sexual contact of Case 2 tested negative for gonorrhoea on urine NAAT testing in November 2018 without treatment and he did not report symptoms before or after sexual intercourse with Case 2. However, given his link to the same sexual network as Case 1, he is likely to have been the source of Case 2’s infection, and to have spontaneously cleared his infection [8]. Further information on other partners of Case 2’s male contact was not available. Case 2 also had unprotected oral sex and protected vaginal sex with a new male partner 8 days after receiving the first treatment (ceftriaxone), before her symptoms relapsed. This partner subsequently tested N. gonorrhoeae NAAT-positive at the pharynx but had a negative pharyngeal culture and negative urine NAAT. Although this case may have had pre-existing asymptomatic pharyngeal carriage, we considered that his positive test was possibly explained by acquisition of N. gonorrhoeae from Case 2. He was therefore also treated with intravenous ertapenem 1 g once daily for 3 days and had a negative TOC 2 weeks later.
Phenotypic characterisation
One N. gonorrhoeae isolate from each culture-positive case (Case 1: H18-209 and Case 2: H18-502) was sent to Public Health England’s national reference laboratory for characterisation. Antimicrobial susceptibility testing was undertaken using minimum inhibitory concentration (MIC) gradient diffusion strips. Results were interpreted using European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints [9].
Both isolates showed resistance to ceftriaxone (MIC 1.0 mg/L), cefixime (MIC 2.0 mg/L), penicillin (MIC 2.0 mg/L), tetracycline (MIC 2.0 mg/L) and ciprofloxacin (MIC > 32 mg/L), and intermediate resistance to azithromycin (MIC 0.5 mg/L). The isolates were susceptible to spectinomycin (MIC 8.0 mg/L) and did not produce a β-lactamase. MICs for gentamicin and ertapenem were 4.0 mg/L and 0.032 mg/L, respectively, which represent low values likely to be susceptible although no formal breakpoints are defined.
Genomic characterisation
Whole genome sequencing (WGS) was undertaken using Illumina MiSeq and Oxford Nanopore Technologies (ONT) sequencing as previously described [10]. Raw sequence reads are available under NCBI BioProject PRJNA523794. Maximum likelihood phylogenies [11], based on mapping to the NCCP11945 reference genome, and corrected for recombination [12], were used to compare the sequences of the cases’ isolates to previously described ceftriaxone-resistant isolates [1-7,10,13-15].
Antimicrobial resistance determinants were identified from ONT and Illumina data as described previously, using a combination of de novo assembly and mapping-based approaches [16]. BEAST v1.10.4 [17] was used to generate time-dated phylogenies using a recombination-corrected whole genome alignment and a Hasegawa-Kishino-Yano (HKY) substitution model. A strict molecular clock was fitted. As there were a limited number of genomes, the prior distribution for the clock rate was chosen to match the credibility interval for the N. gonorrhoeae mutation rate determined previously [18]. Outputs from three independent chains were merged after checking for convergence and a sufficient effective sample size.
The two isolates’ genomes were indistinguishable and were from multilocus sequence typing (https://pubmlst.org/neisseria) ST-1903, N. gonorrhoeae multi-antigen sequence typing (NG-MAST; http://www.ng-mast.net) ST-1614 (porB 1053, tbpB 33) and N. gonorrhoeae sequence typing for antimicrobial resistance (NG-STAR; https://ngstar.canada.ca) ST233. The penA allele was identical to that seen in FC428 (penA-60.001), containing two key ceftriaxone resistance mutations, A311V and T483S, as well as G545S, I312M and V316T. There were no 23S rRNA mutations; azithromycin intermediate resistance was conferred by an mtrR promoter deletion (−35A) and ciprofloxacin resistance by parC S87R and gyrA S91F and D95A substitutions.
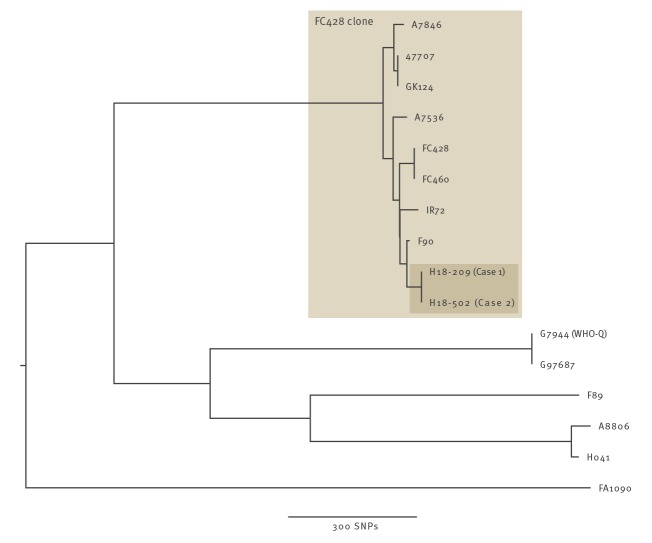
Comparison with previously sequenced ceftriaxone-resistant isolates demonstrated the cases’ isolates were from the FC428 clone (Figure 1).
Figure 1.
Ceftriaxone-resistant Neisseria gonorrhoeae isolates (n=15), including from Case 1 and Case 2, United Kingdom, 2018
SNP: single nucleotide polymorphism.
Maximum likelihood phylogeny, based on mapping to the NCCP11945 reference genome, corrected for recombination. Previously described ceftriaxone-resistant isolates were used for comparison [1-7,10,13-15]. All isolates shown were resistant to ceftriaxone, except FA1090, which was included to root the tree.
A time-dated Bayesian phylogeny of all available sequences from the FC428 clone is shown in Figure 2, with published details of the location of each case’s country of residence and sexual contacts. The current cases’ genomes are most closely related to F90; both the present culture-positive patients and the F90 patient had not travelled outside of Europe; their sexual contacts were, respectively, in Spain (with men from the UK) and in France. The remainder of the cases were diagnosed globally (Ireland, Japan, Canada, Denmark, Australia), however, all had had sexual contacts in Asia.
Figure 2.
Time-dated Bayesian phylogeny of Neisseria gonorrhoeae FC428 clone cases and epidemiological details, 2015–2019 (n = 10)
The right-hand panel indicates the country of residence and any links to sexual contacts in other countries. Cases with a sexual contact in their country of residence are shown with that country coloured on the basis of the sexual contact. The box indicating Spain for H18-502 is shown partially shaded to reflect Case 2’s partner’s sexual contact in Spain.

Within the FC428 clone, after correction for recombination, 267 unique single nucleotide polymorphisms (SNPs) were identified among the genomes. Such diversity makes direct transmission between any of the previously described cases unlikely, with the exception of GK124 and 47707 which were genetically indistinguishable. To our knowledge, this link has not been previously reported. These isolates from Denmark and Canada were from a man and a woman who both reported sexual contact in China with a member of the opposite sex.
Based on previously determined rates of N. gonorrhoeae evolution, the date of the most recent common ancestor of the FC428 clone was estimated as October 2001 (95% highest posterior density interval: May 1999 to March 2004). This is considerably before the first reported case from the clone, FC428, in January 2015. This suggests the clone emerged and persisted in a setting where antimicrobial susceptibility testing for N. gonorrhoeae is not routinely undertaken. This allowed it to remain undetected for probably more than 10 years and enabled the emergence of diversity in the infection reservoir with which each of the reported cases has separately come into contact.
Discussion
There is growing evidence that the FC428 clone has the potential to spread globally, which is of concern given it is resistant and intermediate resistant to the only two remaining empirical treatment options for N. gonorrhoeae, ceftriaxone and azithromycin. Although reported new cases are uncommon and mostly described following sexual contact in South East Asia, our cases, with the previously reported F90 case, provide evidence of transmission occurring within Europe. As the transmission between our cases is likely to have occurred between UK residents visiting Ibiza, a well-known European party destination, there is a risk that further undetected transmission has occurred.
The location of the sexual contacts of the remaining cases described within the clone, suggests South East Asia or China, is the likely reservoir for this clone. There is therefore an urgent need for improved access in the region to N. gonorrhoeae diagnostics and antimicrobial resistance detection [19]. This is required for surveillance so that the burden of antimicrobial resistance can be better quantified and rational antibiotic treatment guidelines made. At the level of the individual patient, it is needed to prevent onward transmission of drug-resistant gonorrhoea.
We report a N. gonorrhoeae transmission cluster involving the FC428 clone with two confirmed female cases and at least two probable additional infections in the male partners of the cases. Given that the partners of Case 1 could not be contacted and that it is unclear how long the asymptomatic male contact of Case 2 was infected [8,20] and unclear if this N. gonorrhoeae strain was initially acquired by UK residents before or during travel to Ibiza, it is likely that there has been onward transmission from one or more undetected cases. As a result, Public Health England have introduced enhanced monitoring of data submitted by local laboratories on N. gonorrhoeae antimicrobial resistance to ensure all ceftriaxone-resistant isolates identified are sent to Public Health England for confirmation and investigation promptly to help reduce further spread.
Case 2 required treatment with 3 days of intravenous ertapenem after failed treatment with ceftriaxone, and with azithromycin and gentamicin. However, widespread use of ertapenem may pose logistical difficulties where sexual health services are not set up to administer intravenous therapy on consecutive days. In addition, use of carbapenems may promote drug resistance in other organisms, including Enterobacteriaceae, where carbapenems are usually reserved for multidrug-resistant infections. Therefore, new treatments for N. gonorrhoeae infection are urgently required. Zoliflodacin is a novel antibiotic active against urogenital infections, but with only limited efficacy in pharyngeal infections [21]. Several other potential agents are under development [22].
European public health agencies and sexual health clinicians should be aware that the FC428 clone has potential to spread in Europe. This threatens the effectiveness of gonorrhoea treatment. New treatments are required that are effective against all infected sites and can be delivered by existing sexual health infrastructure. Ongoing surveillance of antimicrobial resistance with culture or emerging molecular methods [23], test of cure, extra-genital sampling and effective partner notification are vital to maintain control of N. gonorrhoeae.
Acknowledgements
This work was supported by the National Institute for Health Research Oxford Biomedical Research Centre and the Health Protection Research Unit (NIHR HPRU) in Healthcare Associated Infections and Antimicrobial Resistance at the University of Oxford in partnership with Public Health England (PHE) [HPRU-2012-10041]. DWE is a Big Data Institute Robertson Fellow.
We acknowledge the contribution of several unlisted authors, whose names have been withheld to preserve patient confidentially. We would also like to acknowledge Magnus Unemo, Daniel Golparian and Béatrice Berçot for providing sequencing data from previously published studies.
Conflict of interest: None declared.
Authors’ contributions: DWE wrote the first draft of the manuscript and analysed the sequencing data. TS and LB performed the sequencing. NS undertook bioinformatic analysis. KT, HM, MG, CI, DG, JS, CB, JS, DT, CC, NP, BN, PH, and HF formed part of the Public Health England national incident management team overseeing the clinical care of the patients and the public health response. MC and RP were responsible for the microbiology at PHE. BN, PH and HF revised the manuscript. All authors reviewed the final version of the manuscript.
References
- 1. Nakayama S, Shimuta K, Furubayashi K, Kawahata T, Unemo M, Ohnishi M. New ceftriaxone- and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan. Antimicrob Agents Chemother. 2016;60(7):4339-41. 10.1128/AAC.00504-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yahara K, Nakayama S-I, Shimuta K, Lee KI, Morita M, Kawahata T, et al. Genomic surveillance of Neisseria gonorrhoeae to investigate the distribution and evolution of antimicrobial-resistance determinants and lineages. Microb Genom. 2018;4(8): Epub ahead of print. 10.1099/mgen.0.000205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lahra MM, Martin I, Demczuk W, Jennison AV, Lee KI, Nakayama SI, et al. Cooperative Recognition of Internationally Disseminated Ceftriaxone-Resistant Neisseria gonorrhoeae Strain. Emerg Infect Dis. 2018;24(4). 10.3201/eid2404.171873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lefebvre B, Martin I, Demczuk W, Deshaies L, Michaud S, Labbé AC, et al. Ceftriaxone-Resistant Neisseria gonorrhoeae, Canada, 2017. Emerg Infect Dis. 2018;24(2):381-3. 10.3201/eid2402.171756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Terkelsen D, Tolstrup J, Johnsen CH, Lund O, Larsen HK, Worning P, et al. Multidrug-resistant Neisseria gonorrhoeae infection with ceftriaxone resistance and intermediate resistance to azithromycin, Denmark, 2017. Euro Surveill. 2017;22(42):1273. 10.2807/1560-7917.ES.2017.22.42.17-00659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poncin T, Fouere S, Braille A, Camelena F, Agsous M, Bebear C, et al. Multidrug-resistant Neisseria gonorrhoeae failing treatment with ceftriaxone and doxycycline in France, November 2017. Euro Surveill. 2018;23(21):e1002344. 10.2807/1560-7917.ES.2018.23.21.1800264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Golparian D, Rose L, Lynam A, Mohamed A, Bercot B, Ohnishi M, et al. Multidrug-resistant Neisseria gonorrhoeae isolate, belonging to the internationally spreading Japanese FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, Ireland, August 2018. Euro Surveill. 2018;23(47):4339. 10.2807/1560-7917.ES.2018.23.47.1800617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrazzo JM, Apicella MA. 214 - Neisseria gonorrhoeae (Gonorrhea). In: Blaser JEBDJ, ed. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases (8th Edition). Philadelphia: Churchill Livingstone 2015: pp 2446–62. [Google Scholar]
- 9.European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. Version 8.1. EUCAST; 2018. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.1_Breakpoint_Tables.pdf
- 10. Eyre DW, Sanderson ND, Lord E, Regisford-Reimmer N, Chau K, Barker L, et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill. 2018;23(27):364. 10.2807/1560-7917.ES.2018.23.27.1800323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307-21. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 12. Didelot X, Wilson DJ. ClonalFrameML: efficient inference of recombination in whole bacterial genomes. PLOS Comput Biol. 2015;11(2):e1004041. 10.1371/journal.pcbi.1004041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother. 2012;56(3):1273-80. 10.1128/AAC.05760-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lahra MM, Ryder N, Whiley DM. A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia. N Engl J Med. 2014;371(19):1850-1. 10.1056/NEJMc1408109 [DOI] [PubMed] [Google Scholar]
- 15. Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, et al. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother. 2011;55(7):3538-45. 10.1128/AAC.00325-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eyre DW, De Silva D, Cole K, Peters J, Cole MJ, Grad YH, et al. WGS to predict antibiotic MICs for Neisseria gonorrhoeae. J Antimicrob Chemother. 2017;72(7):1937-47. 10.1093/jac/dkx067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ, Rambaut A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018;4(1):vey016. 10.1093/ve/vey016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Silva D, Peters J, Cole K, Cole MJ, Cresswell F, Dean G, et al. Whole-genome sequencing to determine transmission of Neisseria gonorrhoeae: an observational study. Lancet Infect Dis. 2016;16(11):1295-303. 10.1016/S1473-3099(16)30157-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weston EJ, Wi T, Papp J. Strengthening global surveillance for antimicrobial drug–resistant Neisseria gonorrhoeae through the enhanced gonococcal antimicrobial surveillance program. Emerg Infect Dis. 2017;23(13). 10.3201/eid2313.170443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK. Asymptomatic gonorrhea in men. Diagnosis, natural course, prevalence and significance. N Engl J Med. 1974;290(3):117-23. 10.1056/NEJM197401172900301 [DOI] [PubMed] [Google Scholar]
- 21. Taylor SN, Marrazzo J, Batteiger BE, Hook EW, 3rd, Seña AC, Long J, et al. Single-dose zoliflodacin (etx0914) for treatment of urogenital gonorrhea. N Engl J Med. 2018;379(19):1835-45. 10.1056/NEJMoa1706988 [DOI] [PubMed] [Google Scholar]
- 22. Suay-García B, Pérez-Gracia MT. Future prospects for Neisseria gonorrhoeae treatment. Antibiotics (Basel). 2018;7(2):E49. 10.3390/antibiotics7020049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whiley DM, Mhango L, Jennison AV, Nimmo G, Lahra MM. Direct detection of penA gene associated with ceftriaxone-resistant Neisseria gonorrhoeae FC428 strain by using PCR. Emerg Infect Dis. 2018;24(8):1573-5. 10.3201/eid2408.180295 [DOI] [PMC free article] [PubMed] [Google Scholar]