Abstract
Objectives:
To test the hypothesis that maternal plasma alpha-tocopherol levels are associated with protection from childhood wheeze, and that this protection is modified by gamma-tocopherol.
Study eesign:
We conducted a prospective nested study in the INSPIRE birth cohort of 652 children with postpartum maternal plasma vitamin E isoforms used as a surrogate for pregnancy concentrations. Our outcomes were wheezing and recurrent wheezing over a 2-year period, ascertained using validated questionnaires. We assessed the association of alpha- and gamma-tocopherol with wheezing outcomes using multivariable adjusted logistic regression, and tested for interaction between the isoforms with respect to the risk for wheezing outcomes.
Results:
Children with wheezing (N=547, n=167; 31%) and recurrent wheezing (N=545, n=55; 10.1%) over a 2-year period, were born to mothers with significantly lower postpartum maternal plasma concentrations of alpha-tocopherol, P = .016 and p=0.007, respectively. In analyses of interquartile range increases, alpha-tocopherol was associated with decreased risk of wheezing (adjusted odds ratio [aOR] 0.70 [95% CI:0.53,0.92]); and recurrent wheezing (aOR 0.63 [95% CI:0.42,0.95]). For gamma-tocopherol, the aOR for wheezing was 0.79 (95% CI:0.56-1.10) and the aOR for recurrent wheezing was 0.56 (95% CI:0.33-0.94, with non-monotonic association). The association of alpha-tocopherol with wheezing was modified by gamma-tocopherol (p-interaction=0.05).
Conclusions:
Increases in postpartum maternal plasma alpha-tocopherol isoform concentrations were associated with decreased likelihood of wheezing over a 2-year period. Gamma-tocopherol modified this association.
Keywords: alpha tocopherol, gamma tocopherol, wheezing, vitamin E, infancy, maternal
Asthma, characterized by airway inflammation, oxidative stress and airway hyperreactivity, is one of the most common chronic childhood diseases worldwide, with approximately 80% of disease onset during childhood.(1-5) Marked variation in asthma rates around the world,(6) rapid increases in population-specific rates of asthma over time as countries develop in a westernized fashion,(7) and shifts in risk of asthma for migrating populations towards that of their adoptive country(8) suggests that the environment, not genes alone, is likely a significant contributor to disease development. There are currently no effective interventions to prevent childhood asthma, but these observations suggest that the ability to modify diet, environmental exposures and lifestyle are likely to prevent disease if causal early life and modifiable risk factors can be identified.
Dietary interventions for asthma are appealing given the need for safe and cost efficient prevention efforts. Vitamin E is known to be a potent antioxidant as well as a factor affecting cell-mediated immunity, likely important in asthma development (9-11) and lung growth.(12, 13) Vitamin E is a fat soluble vitamin requiring dietary intake in humans. Ninety-nine percent of body vitamin E is estimated to reside in the tissues, especially in slowly turned over body stores (likely in the adipose tissue) that maintain steady state plasma concentrations.(14, 15) Plasma concentrations do not fluctuate much due to this reservoir.(14, 16) Vitamin E concentrations in the plasma can be increased by absorption of dietary vitamin E, but are mostly maintained by recirculation from the tissues.(15) Short courses of exogenous supplementation of vitamin E produce short-lived increases in plasma concentrations;(17) whereas, increases in tissue concentrations, where vitamin E is exerting its effects, require long-term modification of vitamin E intake.(18) It is estimated that >90% of American adults do not meet the estimated average requirement of 12 mg/day of α-tocopherol.(19) During pregnancy, maternal vitamin E plasma concentrations in women who are not taking vitamin E supplements gradually increase until delivery and decrease during the postpartum period.(16, 20)
We have previously demonstrated a protective effect of the vitamin E isoform, alpha-tocopherol, on incident adult-onset asthma, as well as an opposing effect of the vitamin E isoform, gamma-tocopherol, at higher concentrations.(9) Although all isoforms of vitamin E are known to scavenge free radicals,(21) we have demonstrated in in vivo mouse models of allergic inflammation that individual isoforms of vitamin E have different effects. (10, 22-24) Alpha-tocopherol, the most abundant form of vitamin E in the body, reduced allergic airway inflammation to allergen challenge for the offspring of female allergic mice in a dose-dependent fashion.(23) In contrast, gamma-tocopherol supplementation in the same mouse model increased allergic airway responses, but has been demonstrated to reduce neutrophilic airway inflammation in other models.(24-29) As the ratio of these isoforms varies by oil source consumed, dietary modification favoring one isoform over another is both possible and inexpensive.(22)
In a recent systematic review and meta-analysis, maternal vitamin E was associated with reduced subsequent wheezing in childhood.(30) To date, the independent associations and interaction of both major vitamin E isoforms, alpha-tocopherol and gamma-tocopherol, on the development of pediatric wheezing illnesses have not been studied. The objective of this study was to assess the relationships of maternal vitamin E isoforms on childhood wheezing.(31, 32)
Methods:
The Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure (INSPIRE) study is a prospective, ongoing birth cohort of 1,951 healthy term infants enrolled during the first few months of infancy designed to investigate the association of early life environmental exposures with the development of allergic and respiratory outcomes. Methods for the INSPIRE birth cohort have previously been published, (overview in the Appendix; available at www.jpeds.com).(33) In brief, baseline interviews, sample collections, and standardized questionnaires were conducted at enrollment within the first few months of the infant’s birth. Follow-up for the outcomes of wheezing, asthma and allergic diseases, is ongoing annually using validated instruments including the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire.(34)
The current study includes 652 mother-child dyads with available postpartum maternal blood samples from the subset of 1,090 participants who were approached to provide a maternal blood sample (Figure 1; available at www.jpeds.com). Written informed consent was obtained from the parent at enrollment. The study was approved by the Institutional Review Board at Vanderbilt University Medical Center. Data were collected and managed using the REDCap tool hosted at Vanderbilt University.(35)
Figure 1:
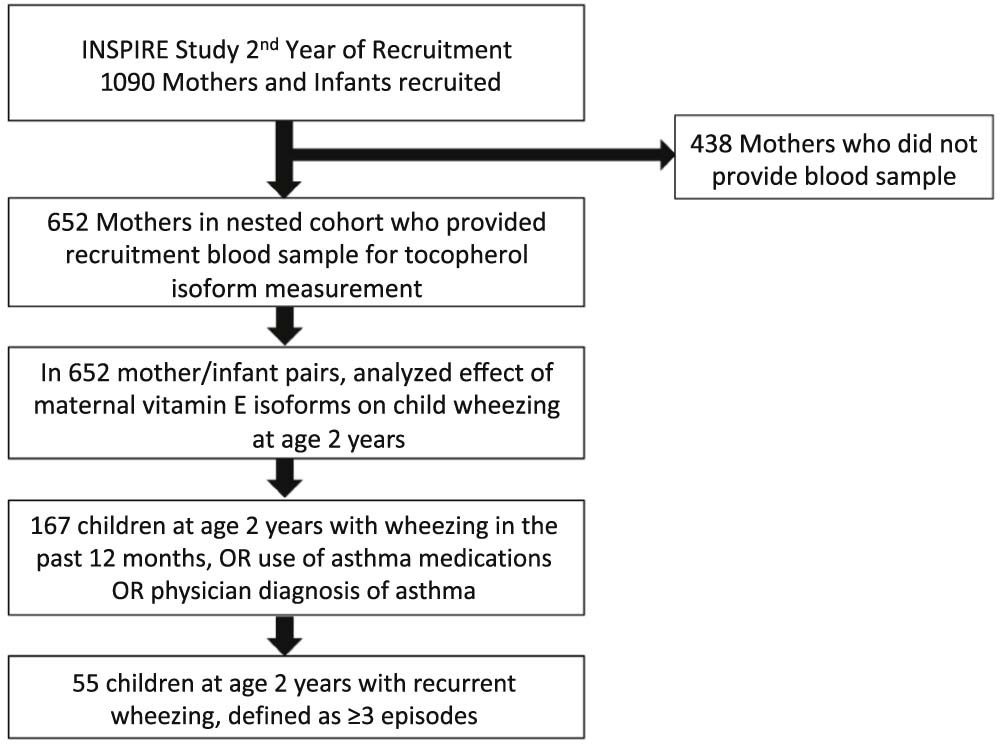
Flow diagram of this nested cohort study of maternal alpha- and gamma-tocopherol isoforms and the outcomes of 2-year wheezing and 2-year recurrent wheezing.
Maternal alpha- and gamma-tocopherol concentrations:
During the second recruitment phase of INSPIRE postpartum period enrollment encounters, we obtained a finger-stick blood sample on a subset of 652 mothers (59.8% of second recruitment cohort). Postpartum levels of vitamin E have been shown to slowly decrease after pregnancy, and the stability of vitamin E plasma concentrations over time supports the acceptability of a single time point measure as a surrogate for pregnancy concentrations.(14, 16) Plasma levels of alpha- and gamma-tocopherol were assessed using high performance liquid chromatography per a previously published protocol.(36, 37)
Childhood respiratory outcomes:
Our primary childhood respiratory outcome, which we will refer to as wheezing over a 2-year period, throughout this report, was defined at age 2 years as wheezing in the past 12 months, or receipt of asthma medications in the past 12 months or a parent reported diagnosis of asthma. To capture more severe wheezing, our second childhood respiratory outcome was recurrent wheezing, defined as ≥3 episodes of wheezing between the child’s first birthday and the 2 year visit. A detailed overview of the outcome definitions can be found in the Appendix.
Statistical Analyses:
Demographic and other baseline characteristics were described for the nested cohort (N=652) using median and interquartile range (IQR) for continuous variables; frequencies and proportions were used for categorical variables and were compared using Kruskal-Wallis non-parametric test and Chi-square test for overall difference in distribution across the three alpha- and gamma-tocopherol tertiles.(9) (Table 3 and Table 4; available at www.jpeds.com)
Table 3:
Study Participants’ Demographic Characteristics Stratified by Alpha Tocopherol in Tertiles (N=652)
| Variable | Alpha Tocopherol 4.18- 54.9 μmol/L n=218 |
Alpha Tocopherol 54.9- 92.0 μmol/L n=217 |
Alpha Tocopherol 92.0- 217.8 μmol/L n=217 |
|
|---|---|---|---|---|
| Infants | Median [IQR: 25th, 75th] or n (%) | Median [IQR: 25th, 75th] or n (%) | Median [IQR: 25th, 75th] or n (%) | p-value |
| Gestational Age (weeks) | 39 [39.0, 40.0] | 39.0 [39.0,40.0] | 39.0 [38.1,40.0] | 0.7 |
| Infant Age at Enrollment (days) | 63 [21, 97] | 59 [18, 82] | 24 [14, 65] | 0.001 |
| Infant Race or Ethnicity | 0.035 | |||
| Black | 55 (25) | 47 (22) | 32 (15) | |
| White | 123(56) | 134 (62) | 143 (66) | |
| Hispanic | 21 (10) | 25 (12) | 18 (8) | |
| Other | 19 (9) | 11 (5) | 24 (11) | |
| Birth Weight (grams) | 3405 [3126, 3717] | 3405 [3121, 3717] | 3518 [3206, 3859] | 0.028 |
| Sex | 0.59 | |||
| Female | 109 (50) | 98 (45) | 102 (47) | |
| Male | 109 (50) | 119 (55) | 115 (53) | |
| Parents | ||||
| Maternal Age (years) | 25.0 [22.0, 30.0] | 26.0 [23.0, 31.0] | 28.0 [24.0, 32.0] | <0.001 |
| Postpregnancy BMI* | 26.9 (24.0, 31.9) | 28.1 (24.4, 33.1) | 28.0 (24.2, 32.5) | 0.63 |
| Mother ever smoked | 82 (38) | 85 (39) | 82 (38) | 0.52 |
| Smoked during pregnancy | 46 (21) | 41 (19) | 28 (13) | 0.18 |
| Prenatal vitamin use during pregnancy | 202 (93) | 197 (91) | 210 (97) | 0.04 |
| Breastfeeding at enrollment | 101 (46) | 108 (50) | 122 (56) | 0.34 |
| Maternal history of asthma | 50 (23) | 49 (23) | 42 (19) | 0.61 |
| Maternal history of allergies | 59 (27) | 59 (27) | 53 (24) | 0.76 |
| Paternal history of asthma | 37 (17) | 30 (14) | 33 (15) | 0.86 |
| Paternal history of allergies | 58 (27) | 58 (27) | 50 (23) | 0.58 |
BMI, body mass index (kg/m2) was available in N=517 participants
Table 4:
Study Participants’ Demographic Characteristics Stratified by Gamma Tocopherol in Tertiles (N=652)
| Variable | Gamma Tocopherol 0- 5.9 μmol/L n=220 |
Gamma Tocopherol 5.9- 11.2 μmol/L n=215 |
Gamma Tocopherol 11.2- 51.3 μmol/L n=217 |
|
|---|---|---|---|---|
| Infants | Median [IQR: 25th, 75th] or n (%) | Median [IQR: 25th, 75th] or n (%) | Median [IQR: 25th, 75th] or n (%) | p-value |
| Gestational Age (weeks) | 39.0 [39.0,40.0] | 39.0 [39.0,40.0] | 39.0 [38.0,40.0] | <0.001 |
| Infant Age at Enrollment (days) | 54 [16,77] | 48 [17,86] | 44 [16,85] | 0.98 |
| Infant Race or Ethnicity | 0.46 | |||
| Black | 48 (22) | 46 (21) | 40 (18) | |
| White | 136 (62) | 129 (60) | 135 (62) | |
| Hispanic | 24 (11) | 17 (8) | 23 (11) | |
| Other | 12 (5) | 29 (11) | 19 (9) | |
| Birth Weight (grams) | 3462 [3202,3775] | 3405 [3107,3717] | 3433 [3150,3802] | 0.38 |
| Sex | 0.88 | |||
| Female | 105(48) | 99 (46) | 105 (48) | |
| Male | 115 (52) | 116 (54) | 112 (52) | |
| Parents | ||||
| Maternal Age (years) | 27.0 [23.0, 32.0] | 26.0 [23.0, 31.0] | 26.0 [22.0, 30.0] | 0.094 |
| Postpregnancy BMI* | 26.4 [23.6, 30.6] | 27.4 [24.5, 32.4] | 29.2 [25.4, 34.4] | <0.001 |
| Mother ever smoked | 64 (29) | 85 (40) | 91 (42) | 0.012 |
| Smoked during pregnancy | 26 (12) | 51 (24) | 38 (18) | 0.002 |
| Prenatal vitamin use during pregnancy | 207 (94) | 202 (94) | 200 (92) | 0.67 |
| Breastfeeding at enrollment | 129 (59) | 106 (49) | 96 (44) | 0.032 |
| Maternal history of asthma | 51 (23) | 48 (22) | 42 (19) | 0.6 |
| Maternal history of allergies | 68 (31) | 61 (28) | 42 (19) | 0.016 |
| Paternal history of asthma | 38 (17) | 23 (11) | 39 (18) | 0.14 |
| Paternal history of allergies | 64 (29) | 57 (27) | 45 (21) | 0.11 |
BMI, body mass index (kg/m2) was available in N=517 participants
We assessed the relationship of alpha-tocopherol and gamma-tocopherol on wheezing and recurrent wheezing over a 2-year period, using univariate analyses and multivariable logistic regression. We also tested for interaction between the two vitamin isoforms to examine whether the relationship of alpha-tocopherol with wheezing risk was modified by gamma-tocopherol, as we have shown in adults with asthma.(9) Vitamin E isoforms were analyzed with log transformation or restricted cubic splines with 3 knots when non-linear associations were detected (as for gamma-tocopherol). To depict interaction association, tertiles of maternal gamma-tocopherol isoforms were used for ease of interpretation in plotting the continuous relationship of alpha-tocopherol and child wheezing outcome. Maternal gamma-tocopherol levels were imputed to 0.01 (n=4) when log transformation was necessary.
Covariates were selected a priori for their known relationship with wheezing, recurrent wheezing, or impact on host antioxidant defense and included infant sex, race, birth weight (grams), breast feeding, maternal smoking during pregnancy, maternal asthma, and maternal insurance status. Post-pregnancy maternal BMI (kg/m2) was missing in ~20% of the sub-cohort, thus to assess the impact of casewise deletions when it was included as a covariate for adjustment, we performed sensitivity analysis with multiple imputations. We controlled for potential differences in the timing of postpartum maternal vitamin E isoform measurement by performing sensitivity analyses with infant age at enrollment using restricted cubic splines to account for non-linear associations. Although we have a term birth cohort, to control for potential residual confounding, we performed a separate analysis with wheezing over a 2-year period and infant gestational age as an additional covariate in multivariable regression. Separate analyses were conducted with adjustment for RSV positive or HRV positive status during acute upper respiratory infection in infancy (both assessed by PCR) along with the main a priori covariates listed above.
A 2-sided 5% significance level was used for all statistical inferences. Statistical analyses were performed using R version 3.4.0(38)
Results:
Our study population is comprised of 652 mother-child dyads from the INSPIRE cohort with available maternal tocopherol isoform concentrations. The subset cohort reflects the demographics of the local population, with a proportion of race and ethnicities reflective of urban Davidson County and suburban and rural Williamson, Sumner Counties in Tennessee, from which the study population is derived.(39) (Table I and Table 2 [available at www.jpeds.com]). Children who had mothers with available serum tocopherol isoform concentrations for study were not significantly different from those in the rest of the INSPIRE cohort in regard to infant sex, birth weight, gestational age, prenatal vitamin use, breast feeding, type of insurance, or maternal BMI (data not shown). Cohort characteristics were compared by tertiles of both alpha and gamma-tocopherol (Table 3 and Table 4).
Table I:
Study Participants’ Demographic Characteristics
| Maternal Vitamin E Subcohort (N=652) | |
|---|---|
| Infants | Median [IQR: 25th, 75th] or n (%) |
| Gestational age (weeks) | 39 [39, 40] |
| Infant age at enrollment (days) | 50 [16, 80] |
| Infant race or ethnicity | |
| Black | 134 (21%) |
| White | 400 (61%) |
| Hispanic | 64 (10%) |
| Other | 54 (8%) |
| Birth weight (grams) | 3433 [3150, 3774] |
| Sex | |
| Female | 343 (53%) |
| Male | 309 (47%) |
| Parents | |
| Maternal age at enrollment (years) | 26 [23,31] |
| Post pregnancy BMI* | 27.5 [24.2, 32.4] |
| Mother ever smoked | 240 (37%) |
| Smoked during pregnancy | 115 (18%) |
| Prenatal vitamin use during pregnancy | 609 (93%) |
| Breastfeeding at enrollment | 331 (51%) |
| Maternal history of asthma | 141 (22%) |
| Maternal history of allergies | 171 (26%) |
| Paternal history of asthma | 100 (15%) |
| Paternal history of allergies | 166 (25%) |
BMI, body mass index (kg/m2) was available in N=517 of the vitamin E participants
Table 2:
Study Participants’ Demographic Characteristics, Compared to the INSPIRE 2nd Year Recruitment Cohort
| Maternal Vitamin E Subcohort (N=652) |
INSPIRE 2nd Year Cohort (n=1,090) |
|
|---|---|---|
| Infants | Median [IQR: 25th, 75th] or n (%) | Median [IQR: 25th, 75th] or n (%) |
| Gestational age (weeks) | 39 [39, 40] | 39 [39, 40] |
| Infant age at enrollment (days) | 50 [16, 80] | 51 [16, 85] |
| Infant race or ethnicity | ||
| Black | 134 (21%) | 204 (19%) |
| White | 400 (61%) | 702 (64%) |
| Hispanic | 64 (10%) | 97 (9%) |
| Other | 54 (8%) | 87 (8%) |
| Birth weight (grams) | 3433 [3150, 3774] | 3405 [3121, 3717] |
| Sex | ||
| Female | 343 (53%) | 576 (53%) |
| Male | 309 (47%) | 514 (47%) |
| Parents | ||
| Maternal age at enrollment (years) | 26 [23,31] | 26 [22,31] |
| Post pregnancy BMI* | 27.5 [24.2, 32.4] | 27.4 [23.9, 32.4] |
| Mother ever smoked | 240 (37%) | 406 (37%) |
| Smoked during pregnancy | 115 (18%) | 211 (19%) |
| Prenatal vitamin use during pregnancy | 609 (93%) | 1015 (93%) |
| Breastfeeding at enrollment | 331 (51%) | 541 (50%) |
| Maternal history of asthma | 141 (22%) | 227 (21%) |
| Maternal history of allergies | 171 (26%) | 228 (21%) |
| Paternal history of asthma | 100 (15%) | 160 (15%) |
| Paternal history of allergies | 166 (25%) | 253 (23%) |
BMI, body mass index (kg/m2) was available in N=517 of the vitamin E participants
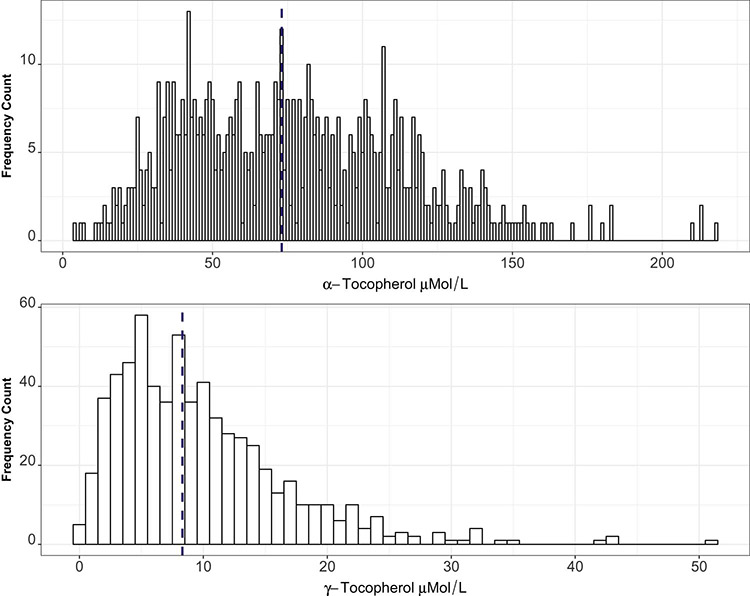
Alpha-tocopherol concentrations in our population ranged from a minimum level of 4.2 μmol/L to a maximum of 217.8 μmol/L, with a median of 73.1 μmol/L (IQR: 46.1,102.3) (Figure 2; available at www.jpeds.com). Only 4 mothers were deficient in alpha-tocopherol using currently suggested cutoffs for deficiency at 11.6 μmol/L.(21) There is no currently established serum limit to define excess of alpha-tocopherol.
Figure 2:
Distribution of maternal vitamin E isoforms in the study population (N=652). Dashed vertical line denotes median value of each isoform for the population.
Gamma-tocopherol concentrations in our population ranged from a minimum of 0 μmol/L to a maximum of 51.3 μmol/L, with a median of 8.3 μmol/L (IQR 4.7, 13.2) (Figure 2). There are no established serum cutoffs for deficiency or excess of gamma-tocopherol.(21)
Association of Alpha- and Gamma-tocopherol with Wheezing over a 2-year period:
In univariate analysis, maternal vitamin E alpha-tocopherol isoform concentrations were significantly lower among children with wheezing over a 2-year period (n=167 [31%]), median (IQR) of 69 μmol/L (42, 96) compared with children who did not wheeze (n=380 [69%]) (75 μmol/L [50,106]), p=0.016. There was no significant difference in maternal gamma-tocopherol isoform concentrations among children who had wheezing over a 2-year period (8.0 μmol/L [4.4, 14.3]) versus children who did not wheeze (8.2 μmol/L [4.7, 12.9]), p=0.92.
We then assessed the association of maternal tocopherol concentrations with risk of wheezing over a 2-year period using logistic regression. Increasing alpha-tocopherol levels were associated with decreased odds of wheezing over a 2-year period, unadjusted univariate odds ratio 0.70, (95% CI: [0.54-0.91]), for interquartile range difference (46, 102 μmol/L) and adjusted odds ratio (aOR) 0.70 (95% CI: 0.53,0.92). There was no significant association with gamma-tocopherol on wheeze over a 2-year period; unadjusted OR for gamma-tocopherol, 0.83 (95% CI: 0.60-1.15) and aOR 0.79 (95% CI: 0.56-1.10) for IQR difference (4.7, 13 μmol/L). The relationship of the isoforms with predicted probability of wheezing over a 2-year period was plotted to visualize the relationship between increasing alpha-tocopherol and decreased likelihood of wheezing across the whole range of values. Gamma-tocopherol had a nonmonotonic relationship with risk of wheezing, whereby lower concentrations trended with decreased likelihood of wheezing and higher concentrations with increased likelihood of wheezing. (Figure 3).
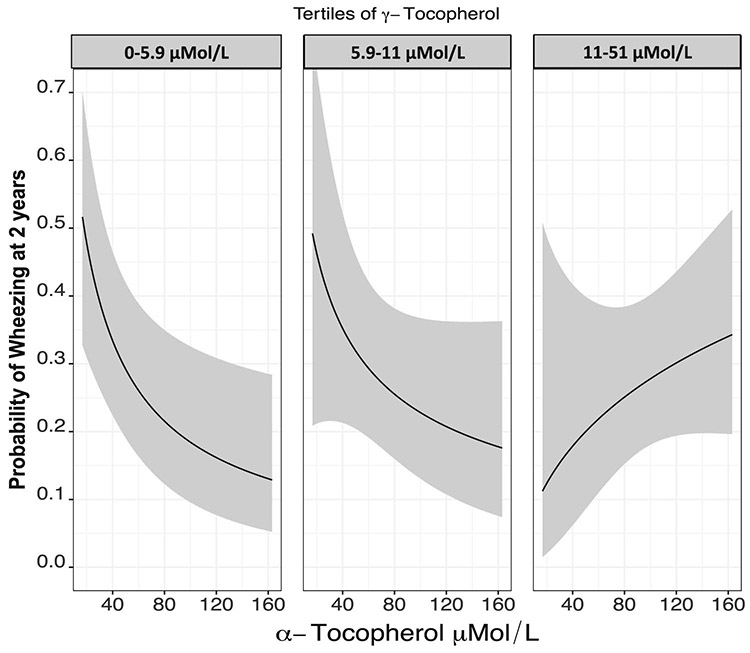
Figure 3:
Predicted probability of 2-year wheezing and 2 year recurrent wheezing by maternal alpha- and gamma- tocopherol concentrations. Multiple logistic regression was used to assess the association of alpha- or gamma-tocopherol separately with 2-year wheezing. Alpha-tocopherol was fit with log transformation whereas gamma-tocopherol showed statistically significant non-linearity and was fit with restricted cubic splines. Model covariates included infant sex, race, birth weight, breast feeding status, maternal smoking during pregnancy, maternal asthma and insurance status. The Y axis in Panel A represents the adjusted predicted probability of 2-year wheezing and the Y axis in Panel B represents the adjusted predicted probability of 2-year recurrent wheezing. The X axis in both panels presents maternal concentrations of alpha-tocopherol (log transformed, P=0.011 for 2-year wheezing and P=0.027 for 2-year recurrent wheezing) or gamma-tocopherol(P=0.065, P non-linearity =0.022 for 2-year recurrent wheezing and P=0.037, P non-linearity=0.010).
We assessed for the interaction between maternal alpha- and gamma-tocopherol concentrations on the primary outcome of wheezing over a 2-year period with adjustment for potential confounders using multivariable logistic regression. Although increasing maternal alpha-tocopherol concentrations were inversely associated with child wheezing at age 2 years, increasing maternal gamma-tocopherol concentrations appear to attenuate this protective association (p-interaction via likelihood ratio test= 0.05) (Table 5 and Figure 4).
Table 5.
Interaction between alpha- and gamma-tocopherol on 2-year wheezing
| Univariate analysis: Distribution of alpha-tocopherol concentrations by 2-year wheezing status in subjects with low, medium, and high gamma-tocopherol concentrations (tertiles) | ||||
|---|---|---|---|---|
| Gamma-tocopherol concentration (tertiles, ng/mg creatinine) |
||||
| Low | Medium | High | ||
| Alpha-tocopherol concentration (ng/mg creatinine) [IQR] | No wheezing | 49 [36,74] | 73 [55,98] | 106 [83,123] |
| Alpha-tocopherol concentration (ng/mg creatinine) [IQR] | Wheezing | 42 [26,53] | 67 [45,82] | 107 [87,121] |
| P value | 0.004 | 0.059 | 0.42 | |
| Multivariable regression analysis: The protective association of alpha-tocopherol with 2-year wheezing outcome differs by gamma-tocopherol level | ||||
| Gamma-tocopherol concentration (tertiles, ng/mg creatinine) |
||||
| Low | Medium | High | ||
| Unadjusted ORs* | 0.51 (0.32,0.80) | 0.54 (0.28,1.05) | 1.64 (0.71,3.81) | |
| Alpha-tocopherol concentration (ng/mg creatinine) IQR difference | aORs* | 0.49 (0.30,0.80) | 0.59 (0.29,1.18) | 1.65 (0.67,4.01) |
ORs were estimated for the association of alpha tocopherol with 2-year wheezing in low, medium, and high tertiles of gamma-tocopherol. Multivariable logistic regression was used for the adjusted association including infant sex, race, birth weight, breastfeeding status, maternal smoking during pregnancy, maternal asthma, and insurance status. P interaction (likelihood ratio test) cross-product alpha (continuous)—and gamma-tocopherol tertiles = 0.05. Alpha-tocopherol was natural log transformed.
Figure 4.
Effect modification of gamma-tocopherol concentrations on the association of alpha tocopherol with the outcome of 2-year wheezing assessed using multivariable logistic regression modeling. Model covariates included infant sex, race, birth weight, breast feeding status, maternal smoking during pregnancy, maternal asthma and insurance status. Alpha-tocopherol concentration is on the x-axis, and tertiles of gamma-tocopherol are represented in each panel. P-interaction (Likelihood ratio test) cross-product alpha- and gamma-tocopherol=0.054.
When additionally adjusting for maternal post-partum BMI, we performed multiple imputations as 20% of values were missing. In separate models, the association of alpha-tocopherol and gamma-tocopherol remained similarly associated with wheezing over a 2-year period (alpha-tocopherol, p=0.03; gamma-tocopherol, p=0.06). In modeling that examined interaction between the two isoforms with BMI multiply imputed, gamma tocopherol in tertiles and alpha tocopherol had similar effect modification (p=0.06).
To control for potential differences due to timing of postpartum maternal gamma and alpha tocopherol measurement and residual confounding we also performed additional adjustment for infant age at enrollment and gestational age. In a model containing infant age at enrollment, the association of alpha-tocopherol and gamma-tocopherol remained similarly associated with wheezing over a 2-year period (alpha-tocopherol, p=0.008; gamma-tocopherol, p=0.08, p-value for interaction=0.02). In a model containing both infant age at enrollment and gestational age the association of alpha-tocopherol and gamma-tocopherol also remained similarly associated with wheezing over a 2-year period was unchanged. Neither of these age and timing of exposure measurement-related factors altered the association between maternal alpha- and gamma-tocopherol and wheezing over a 2-year period.
We explored whether the relationship of alpha- and gamma- tocopherol with the subsequent development of childhood wheezing was influenced by infant upper respiratory viral infections by including RSV or HRV detection (PCR confirmed) during ARI in infancy as an additional covariate in multivariable logistic regressions. Alpha-tocopherol remained associated with wheezing over a 2-year period (OR: 0.71, 95%CI: 0.54, 0.93, p =0.014). Adjustment for infant RSV ARI did not attenuate the association of gamma-tocopherol with wheezing over a 2-year period (OR: 0.81, 95%CI 0.58, 1.14, p=0.08). The interaction of alpha-tocopherol with gamma-tocopherol on wheezing over a 2-year period (p=0.05) remained unchanged with adjustment for RSV ARI in infancy. Similarly, adjustment for HRV detection during an infant ARI did not affect the association between alpha-tocopherol (OR: 0.71, 95%CI: 0.54, 0.94, p =0.016) or gamma-tocopherol (OR: 0.80, 95%CI 0.57, 1.12, p=0.065) and wheezing over a 2-year period, nor the interaction between the 2 isoforms (p-interaction=0.06).
Association of Alpha- and Gamma-tocopherol with Recurrent Wheezing over a 2-year period:
In univariate analysis, maternal vitamin E alpha-tocopherol isoform concentrations were also significantly lower among mothers of children with recurrent wheezing over a 2-year period (n=55 [10.1%]), median (IQR) of 53 μmol/L (40, 89) compared with concentrations in mothers of children with less frequent or no wheezing (n=490 [74.8%]) (75 μmol/L [49,104]), p=0.007. There was no significant difference in gamma-tocopherol isoform concentrations in mothers of children who had recurrent wheezing over a 2-year period (7.6 μmol/L [4.2, 10.9]) versus mothers of children who did not wheeze (8.3 μmol/L [4.7, 10.9)]), p=0.35.
We then assessed the association of tocopherol concentrations with risk of recurrent wheezing over a 2-year period using logistic regression analysis. Increasing alpha-tocopherol concentrations had a monotonic association with decreased odds of recurrent wheezing over a 2-year period, unadjusted OR 0.60 (95% CI: 0.41-0.88), IQR range difference (46, 102 μmol/L), and aOR 0.63 (95% CI: 0.42,0.95). Gamma-tocopherol again had a nonmonotonic relationship with risk of recurrent wheezing characterized by protective relationship at lower concentrations and increasing likelihood for recurrent wheezing at higher concentrations, and is best depicted graphically (Figure 3). Using difference estimates for interquartile ranges (4.7, 13 μmol/L), the gamma-tocopherol unadjusted OR for recurrent wheezing over a 2-year period was 0.67 (95% CI: 0.42-1.07) and aOR 0.56 (95% CI 0.33-0.94).
Discussion:
This study demonstrates the important differential relationship of the 2 most prevalent isoforms of vitamin E, alpha- and gamma-tocopherol, on the risk of childhood wheezing. Increasing maternal vitamin E alpha-tocopherol concentrations were associated with decreased odds of childhood wheezing, and increasing gamma-tocopherol concentrations had a nonmonotonic relationship with risk of wheezing and recurrent wheezing. As dietary oils and supplements of vitamin E can contain widely different ratios of both alpha- and gamma-tocopherol, dietary changes or supplementation make vitamin E isoforms a readily modifiable exposure.(22)
Our observations build upon what we and others have reported in studies assessing the relationship of tocopherol isoforms with the development of adult-onset asthma,(9) rate of lung function decline,(40) and adult lung function.(13) An intrauterine effect of alpha-tocopherol on infant and child respiratory morbidity is supported by studies demonstrating associations of increasing maternal alpha-tocopherol concentrations with increased intrauterine crown-rump length in the first trimester, and higher FEV1, and FVC at age 5 years.(41) Biological plausibility is supported by our animal studies demonstrating that selective alpha-tocopherol supplementation of pregnant mice decreases allergic airway inflammation in their offspring, and gamma-tocopherol supplementation potentiates it.(23, 24, 42) Other animal and human studies have demonstrated potential benefits for gamma-tocopherol on neutrophilic airway inflammation after endotoxin challenge.(25-29) Our study demonstrates the different effects of alpha- and gamma-tocopherol, and potential effect modification of the two most common vitamin E isoforms on the outcome of childhood wheezing.
These findings are of significance. First, although previous human studies of self-reported intake of total vitamin E by food frequency questionnaire have sometimes demonstrated a protective effect of vitamin E intake on asthma development, the alpha- and gamma-tocopherol content can vary widely.(43, 44) The differential effect of these isoforms may explain why these findings have been inconsistent, especially in comparing studies done in regions where dietary sources of vitamin E, and thus individual isoform concentrations, may vary greatly.(45–48) Second, we demonstrate a protective effect on respiratory outcomes at normal physiologic levels of alpha-tocopherol, suggesting that high dose supplementation is not necessary. Because our observed associations were found within normal population ranges of tocopherols, any future intervention could likely be delivered primarily with dietary modification and avoid the known adverse effects of high dose supplementation.(48-50) Third, our data suggest that gamma-tocopherol, while protective at lower concentrations, is associated with increasing risk of wheeze with increasing concentrations.
One maternal vitamin E supplementation study on prevention of wheezing has been done, utilizing a daily 400 IU of RRR alpha-tocopherol co-administered with 1000mg vitamin C during pregnancy, in a secondary analysis of data originally collected for the primary outcome of preeclampsia. This study, powered to detect a 30% decrease in wheezing, detected no difference for wheezing at age 2, aOR 0.97 (0.62-1.52), or for wheezing frequency of more than once per week at age 2, aOR 0.83 (0.26-2.59); there were non-significant reductions in use of respiratory medications, aOR 0.75 (0.50-1.13), p=0.068.(51) Gamma-tocopherol effects were not accounted for in this study. Infants in the study were not typical of the usual population (multiple births, many admitted to neonatal special care.)
Strengths of our study include maternal tocopherol isoform ascertainment in over 600 INSPIRE participants, a diverse population-based study cohort, an analysis evaluating multiple confounders that would contribute to risk for developing wheezing over a 2-year period, and the consistency of our findings between children who wheezed both intermittently and recurrently. Despite the strengths, there are limitations that must be considered. We did not have maternal blood available during pregnancy to measure maternal vitamin E isoforms. However, we believe that our measurement following birth is a valid assessment of maternal status during pregnancy due to the slow changes in vitamin E equilibrium, as tocopherols are fat-soluble vitamins with predominantly extra-plasma stores. There was a trend with proximity to birth at enrollment and increased maternal tocopherol concentrations. This is likely because during pregnancy, maternal vitamin E plasma concentrations in women who are not taking vitamin E supplements gradually increase until delivery.(16, 20) During the postpartum period, plasma concentrations gradually decrease, resembling 3rd trimester concentrations immediately after delivery, and 2nd trimester concentrations by 6 weeks postpartum.(16, 20) In sensitivity analyses that adjusted for the timing of postpartum sample collection in relation to infant age at enrollment our results were not affected. Future studies will need to further explore the timing of isoform measurement in pregnancy and post-partum in relation to longitudinal risk of wheezing outcomes.(52) Another limitation is that we did not correct vitamin E isoform concentrations with lipid profiles which are important for placing our measures in context of the overall dietary status of the mother. Due to the small volumes of blood from maternal finger stick, we were not able to correct for lipid profile and it is possible that a small number of our vitamin E isoform measurements have been altered by extremes of maternal lipid status,(53-55) though such adjustments may not be necessary.(56)
Our observations suggests that future preventive studies for asthma and allergic diseases should select formulations of vitamin E that quantify the content of both isoforms and contain predominantly alpha-tocopherol. These findings provide important new insights into the future design of interventions utilizing dietary modification for prevention of early life respiratory morbidity and recurrent wheezing, common and significant diseases of early childhood. Further study will be done as the INSPIRE cohort ages to evaluate the effects of maternal alpha- and gamma-tocopherol on the development of childhood asthma and allergy outcomes, and pathways through which they may exert a protective effect.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health: NIH/NHLBI (T32 HL87738) and NIH/NIGMS (5T32 GM007569-41 [to C.S.]), NIH/NIAID (U19 AI 095227 [to T.H.]), NIH (K24 AI 077930 [to T.H.]). The project described was also supported by the National Center for Advancing Translational Sciences (CTSA award No. UL1TR000445). Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. The authors declare no conflicts of interest.
Abbreviations:
- INSPIRE
Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure
- ISAAC
International Study of Asthma and Allergies in Childhood
- RSS
respiratory severity score
- PCR
polymerase chain reaction
- ARI
acute respiratory illness
- FEV1
Forced expiratory volume at 1 second
- aOR
adjusted odds ratio
- CI
confidence interval
- IQR
interquartile range
- RSV
Respiratory Syncytial Virus
Footnotes
Portions of this study were presented as an abstract at the American Academy of Allergy Asthma & Immunology (AAAAI) annual meeting, March 3-6, 2017, Atlanta, Georgia, and at the Respiratory Disease Young Investigators’ Forum, October 13-16, 2016, Chicago, IL.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Riedl M, Nel A. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr Opin Allergy Clin Immunol. 2008. February;8:49–56. [DOI] [PubMed] [Google Scholar]
- 2.Wood L, Gibson P, Garg M. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur Respir J. 2003. January;21:177–86. [DOI] [PubMed] [Google Scholar]
- 3.Blackwell D, Lucas J, Clarke T. Summary Health Statistics for U.S. adults: National Health Interview Survey, 2012. National Center for Health Statistics Vital Health Stat. 2014;10. [PubMed] [Google Scholar]
- 4.Bloom B, Jones L, Freeman G. Summary Health Statistics for U.S. children: National Health Interview Survey, 2012. National Center for Health Statistics Vital Health Stat. 2013;10. [PubMed] [Google Scholar]
- 5.Yunginger J, Reed C, O'Connell E, Melton L 3rd, O'Fallon W, Silverstein M. A community-based study of the epidemiology of asthma. Incidence rates, 1964-1983. Am Rev Respir Dis. 1992. October;146:888–94. [DOI] [PubMed] [Google Scholar]
- 6.Lai C, Beasley R, Crane J, Foliaki S, Shah J, Weiland S, et al. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2009. June;64:476–83. [DOI] [PubMed] [Google Scholar]
- 7.Platts-Mills T The allergy epidemics: 1870-2010. J Allergy Clin Immunol. 2015. July;136:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tobias A, Soriano J, Chinn S, Anto J, Sunyer J, Burney P, et al. Symptoms of asthma, bronchial responsiveness and atopy in immigrants and emigrants in Europe. European Community Respiratory Health Survey. Eur Respir J. 2001. September;18:459–65. [DOI] [PubMed] [Google Scholar]
- 9.Larkin E, Gao Y, Gebretsadik T, Hartman T, Wu P, Wen W, et al. New risk factors for adult-onset incident asthma. A nested case-control study of host antioxidant defense. Am J Respir Crit Care Med. 2015;191:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook-Mills J, Gebretsadik T, Abdala-Valencia H, Green J, Larkin E, Dupont W, et al. Interaction of vitamin E isoforms on asthma and allergic airway disease. Thorax. 2016;71:954–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook-Mills J, McCary C. Isoforms of vitamin E differentially regulate inflammation. Endocr Metab Immune Disord Drug Targets. 2010. December;10:348–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam S, Narra V, Cote GM, Manganaro TF, Donahoe PK, Schnitzer JJ. Prenatal vitamin E treatment improves lung growth in fetal rats with congenital diaphragmatic hernia. J Pediatr Surg. 1999. January;34:172–6. [DOI] [PubMed] [Google Scholar]
- 13.Marchese M, Kumar R, Colangelo L, Avila P, Jacobs D, Gross M, et al. The vitamin E isoforms α-tocopherol and γ-tocopherol have opposite associations with spirometric parameters: the CARDIA study. Respir Res. 2014;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novotny J, Fadel J, Holstege D, Furr H, Clifford A. This kinetic, bioavailability, and metabolism study of RRR-α-tocopherol in healthy adults suggests lower intake requirements than previous estimates. J Nutr. 2012;142:2105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton G, Traber M. Vitamin E: antioxidant activity, biokinetics, and bioavailability. Annu Rev Nutr. 1990;10:357–82. [DOI] [PubMed] [Google Scholar]
- 16.Cikot R, Steegers-Theunissen R, Thomas C, de Boo T, Merkus H, Steegers E. Longitudinal vitamin and homocysteine levels in normal pregnancy. Br J Nutr. 2001;85:49–58. [DOI] [PubMed] [Google Scholar]
- 17.Eichhorn J, Lee R, Dunster C, Basu S, Kelly F. Alpha- and gamma-tocopherol plasma and urinary biokinetics following alpha-tocopherol supplementation. Ann NY Acad Sci. 2004;1031:339–40. [DOI] [PubMed] [Google Scholar]
- 18.Baxter L, Marugan J, Xiao J, Incao A, McKew J, Zheng W, et al. Plasma and tissue concentrations of alpha-tocopherol and delta-tocopherol following high dose dietary supplementation in mice. Nutrients. 2012. June;4:467–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McBurney M, Yu E, Ciappio E, Bird J, Eggersdorfer M, Mehta S. Suboptimal Serum alpha-Tocopherol Concentrations Observed among Younger Adults and Those Depending Exclusively upon Food Sources, NHANES 2003-2006. PloS one. 2015; 10:e0135510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oostenbrug G, Mensink R, Al M, van Houwelingen A, Hornstra G. Maternal and neonatal plasma antioxidant levels in normal pregnancy, and the relationship with fatty acid unsaturation. Br J Nutr. 1998;80:67–73. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine Report: Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academies Press; 2000. p. 186–283. [PubMed] [Google Scholar]
- 22.Cook-Mills JM, Abdala-Valencia H, Hartert T. Two Faces of Vitamin E in the Lung. Am J Respir Crit Care Med. 2013. August;188:279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdala-Valencia H, Berdnikovs S, Soveg F, Cook-Mills J. α-Tocopherol supplementation of allergic female mice inhibits development of CD11c+CD11b+ dendritic cells in utero and allergic inflammation in neonates. Am J Physiol Lung Cell Mol Physiol. 2014;307:L482–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdala-Valencia H, Soveg F, Cook-Mills J. γ-Tocopherol supplementation of allergic female mice augments development of CD11c+CD11b+ dendritic cells in utero and allergic inflammation in neonates. Am J Physiol Lung Cell Mol Physiol. 2016;310:L759–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burbank A, Duran C, Almond M, Wells H, Jenkins S, Jiang Q, et al. A short course of gamma-tocopherol mitigates LPS-induced inflammatory responses in humans ex vivo. J Allergy Clin Immunol. 2017;140:1179–81.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez M, Wagner J, Kala A, Mills K, Wells H, Alexis N, et al. Vitamin E, γ-tocopherol, reduces airway neutrophil recruitment after inhaled endotoxin challenge in rats and in healthy volunteers. Free Radic Biol Med. 2013;60:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills K, Lay J, Wu W, Robinette C, Kesic M, Dreskin S, et al. Vitamin E, gamma-tocopherol, diminishes ex vivo basophil response to dust mite allergen. Allergy. 2014. April;69:541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner J, Harkema J, Jiang Q, Illek B, Ames B, Peden D. Gamma-tocopherol attenuates ozone-induced exacerbation of allergic rhinosinusitis in rats. Toxicol Pathol. 2009. June;37:481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner J, Jiang Q, Harkema J, Ames B, Illek B, Roubey R, et al. Gamma-tocopherol prevents airway eosinophilia and mucous cell hyperplasia in experimentally induced allergic rhinitis and asthma. Clin Exp Allergy. 2008. March;38:501–11. [DOI] [PubMed] [Google Scholar]
- 30.Wu H, Zhang C, Wang Y, Li Y. Does vitamin E prevent asthma or wheeze in children: A systematic review and meta-analysis. Paediatr Respir Rev. 2017:ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Wu P, Dupont W, Griffin M, Carroll K, Mitchel E, Gebretsadik T, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu P, Hartert T. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011. September;9:731–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larkin E, Gebretsadik T, Moore M, Anderson L, Dupont W, Chapell J, et al. Objectives, design and enrollment results from the Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure Study. BMC Pulm Med. 2015;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asher M, Keil U, Anderson H, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–91. [DOI] [PubMed] [Google Scholar]
- 35.Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. April;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bieri J, Brown E, Smith J. Determination of individual carotenoids in human plasma by high performance liquid chromatography. J Liq Chromatogr. 1985;8:473–84. [Google Scholar]
- 37.Craft N, Brown E, Smith J. Effects of storage and handling conditions on concentrations of individual carotenoids, retinol, and tocopherol in plasma. Clin Chem. 1988;1988:44–8. [PubMed] [Google Scholar]
- 38.Computing RFfS. A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: [October-17-2016]; Available from: http://www.R-project.org. [Google Scholar]
- 39.Bureau USC. Quick Facts Nashville-Davidson, Williamson County, Sumner County (balance) Tennessee. https://www.census.gov/quickfacts/table/PST045215/47165,47187,4752006: United States Cenus Bureau; 2017. [March-10-2017]. [Google Scholar]
- 40.Hanson C, Lyden E, Furtado J, Campos H, Sparrow D, Vokonas P, et al. Serum tocopherol levels and vitamin E intake are associated with lung function in the normative aging study. Clin Nutr. 2016;35:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner SW, Campbell D, Smith N, Craig LCA, McNeill G, Forbes SH, et al. Associations between fetal size, maternal alpha-tocopherol and childhood asthma. Thorax. 2010. May;65:391–7. [DOI] [PubMed] [Google Scholar]
- 42.Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Vitamin E Isoforms as Modulators of Lung Inflammation. Nutrients. 2013. November;5:4347–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Litonjua A, Rifas-Shiman S, Ly N, Tantisira K, Rich-Edwards J, Camargo CJ, et al. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am J Clin Nutr. 2006;84:903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allan K, Prabhu N, Craig L, McNeill G, Kirby B, McLay J, et al. Maternal vitamin D and E intakes during pregnancy are associated with asthma in children. Eur Respir J. 2015;45:1027–36. [DOI] [PubMed] [Google Scholar]
- 45.Goszcz K, Deakin S, Duthie G, Stewart D, Leslie S, Megson I. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Front Cardiovasc Med. 2015;2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farina N, Llewellyn D, Isaac M, Tabet N. Vitamin E for Alzheimer's dementia and mild cognitive impairment. Cochrane Database Syst Rev. 2017. January 27;1:CD002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwingshackl L, Boeing H, Stelmach-Mardas M, Gottschald M, Dietrich S, Hoffmann G, et al. Dietary Supplements and Risk of Cause-Specific Death, Cardiovascular Disease, and Cancer: A Systematic Review and Meta-Analysis of Primary Prevention Trials. Adv Nutr. 2017. January;8:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vitamin E Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/ - en10: National Institutes of Health; 2016. [cited 2017 March-10-2017]. [Google Scholar]
- 49.Alpha Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994. April 14;330:1029–35. [DOI] [PubMed] [Google Scholar]
- 50.Miller E 3rd, Pastor-Barriuso R, Dalal D, Riemersma R, Appel L, Guallar E Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005. January 04;142:37–46. [DOI] [PubMed] [Google Scholar]
- 51.Greenough A, Shaheen S, Shennan A, Seed P, Poston L. Respiratory outcomes in early childhood following antenatal vitamin C and E supplementation. Thorax. 2010;65:998–1003. [DOI] [PubMed] [Google Scholar]
- 52.Nwaru B, Virtanen S, Alfthan G, Karvonen A, Genuneit J, Lauener R, et al. Serum vitamin E concentrations at 1 year and risk of atopy, atopic dermatitis, wheezing, and asthma in childhood: the PASTURE study. Allergy. 2014. January;69:87–94. [DOI] [PubMed] [Google Scholar]
- 53.Rubinstein H, Dietz A, Srinavasan R. Relation of Vitamin E and Serum Lipids. Clinica Chimica Acta. 1969;23:1–6. [DOI] [PubMed] [Google Scholar]
- 54.Horwitt M, Harvey C, Dahm C Jr., Searcy M. Relationship between tocopherol and serum lipid levels for determination of nutritional adequacy. Ann N Y Acad Sci. 1972. December 18;203:223–36. [DOI] [PubMed] [Google Scholar]
- 55.Thurnham D, Davies J, Crump B, Situnayake R, Davis M. The use of different lipids to express serum tocopherol: lipid ratios for the measurement of vitamin E status. Ann Clin Biochem. 1986. September;23:514–20. [DOI] [PubMed] [Google Scholar]
- 56.Ford E, Schleicher R, Mokdad A, Ajani U, Liu S. Distribution of serum concentrations of alpha-tocopherol and gamma-tocopherol in the US population. Am J Clin Nutr. 2006. August;84:375–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.