Abstract
Interventions to extend lifespan and improve health with increasing age would have significant impact on a growing aged population. There are now several pharmaceutical interventions that extend lifespan in laboratory rodent models with rapamycin, an inhibitor of mechanistic target of rapamycin (mTOR) being the most well studied. In this study, we report on the hematological effects on a cohort of middle-aged common marmosets (Callithrix jacchus) that were enrolled in a study to test the effects of daily rapamycin treatment on aging in this species. In addition, we assessed whether sex was a significant factor in either baseline assessment or as an interaction with rapamycin treatment. Among our cohort at baseline, we found few differences in either basic morphology or hematological markers of blood cell counts, metabolism or inflammation between male and female marmosets. After dosing with rapamycin, surprisingly we found trough blood concentrations of rapamycin were significantly lower in female compared to male marmosets. Despite this pharmacological difference, both sexes had only minor changes in cellular blood counts after 9 months of rapamycin. These data then suggest that the potential clinical hematological side effects of rapamycin are not likely outcomes of long-term rapamycin in relatively healthy, middle-aged marmosets.
Keywords: mTOR, leukocytes, erythrocytes, longevity, healthy aging
INTRODUCTION
There is reasonable evidence that pharmaceutical interventions can extend lifespan and improve healthy aging in laboratory organisms. Among these pro-longevity interventions, the most well-studied compounds is rapamycin, an inhibitor of mechanistic target of rapamycin (mTOR) signaling. Rapamycin, initially reported as an anti-fungal and anti-proliferative in mammalian cells, complexes with 12-kDa FK506-binding protein (FKBP12) which as a complex specifically binds and inhibits mTOR complex 1 (mTORC1). In mouse models, intervention with rapamycin has been shown to significantly extend lifespan; in particular, this has been true whether rapamycin has been given as a chronic treatment (beginning either early or late in life) or either intermittently or as a short-term treatment late in life (Apelo, Pumper, Baar, Cummings, & Lamming, 2016; Bitto et al., 2016; Harrison et al., 2009; Miller et al., 2014). Moreover, rapamycin in mouse models has been shown to delay, or even reverse, the progression of multiple age-related pathologies including cardiac dysfunction, neurodegeneration, kidney disease and periodontitis (An et al., 2017; Dai et al., 2014; Shavlakadze et al., 2018; Tang et al., 2013). While there is evidence that rapamycin might improve some off-target age-related conditions in humans when provided as a short-term therapeutic (Kraig et al., 2018; Mannick et al., 2014; Mannick et al., 2018), it is unclear whether this may similarly delay aging in humans as in rodent subjects.
The challenge facing clinical adaption of laboratory findings in aging research, as well as other areas of medical research, is practical translation to clinical populations. This process is generally slow and often fails to replicate findings in human studies that are readily apparent when tested in laboratory animals, primarily rodents (Woolf, 2008). The potential reasons for these lacks of success are multitude, but a significant issue is in the fundamental biological and physiological differences that exist between humans and rodents. Testing long-term efficacy of pro-longevity treatments in non-human primate models as an additional pre-clinical model may benefit translation efforts because of the relative biological similarity of these animals to humans. For example, the cause of death of most mouse strains is some form of cancer whereas human and non-human primate mortality is typically caused by an array of different age-related pathologies. In this regard, the common marmoset (Callithrix jacchus) has emerged as a practical non-human primate model for longevity studies due in part to its short lifespan for a primate, small size, and amenability to long-term study that are discussed in more detail elsewhere in this special issue [cites to other REF this special issue].
In this regard, our group has reported on basic pharmacological and physiological effects of chronic, long-term rapamycin treatment in the marmoset. In these pilot studies, we determined a dosing regimen of rapamycin that effectively inhibited mTOR signaling in vivo and was practical for long-term dosing (Tardif et al., 2015). Moreover, we reported that this dose of rapamycin was sufficient to stimulate autophagy, a component of protein homeostasis that acts as one of the downstream effectors of mTOR signaling (Lelegren, Liu, Ross, Tardif, & Salmon, 2016). We also reported that this dose of rapamycin does not generally promote hyperlipidemia or glucose metabolic dysfunction, two significant concerns of this drug in current clinical application of this drug as a therapeutic as well as common outcomes in mouse studies (Lamming et al., 2012; Liu et al., 2014; Ross et al., 2015). Thus, in a relatively small-scale study we found evidence that the physiological outcomes of long-term rapamycin treatment may differ to some degree between rodent models and non-human primates.
Based on the findings, we have now built a large-scale study to test whether rapamycin intervention can extend longevity and slow the development of age-related diseases in the marmoset. As this is an on-going long-term study, it is important to develop easily addressed markers of both safety and efficacy of this cohort of animals. In clinical practice, basic bloodwork represents one of the main sources of such information with pharmacological treatment. There are reported effects on basic clinical hematological markers, including thrombocytopenia, leucopenia and anaemia, among patients receiving rapamycin (Hong & Kahan, 2000; Kreis et al., 2000). Because these subjects were kidney transplant patients receiving a cocktail of immunosuppressant drugs, it is unclear whether these would be likely outcomes of rapamycin treatment in relatively healthy patients. A recent study in older, healthy subjects receiving rapamycin for 6–8 weeks showed small, but significant changes in several blood cell parameters including reduced red blood cells (RBC), hematocrit, and hemoglobin as well as changes in several cell volume markers (Kraig et al., 2018). While none of these were reported to have clinically significant effects, there is still concern in long-term, even life-long, administration of drugs to populations. Here we report on hematological and clinical markers of metabolism and inflammation associated with long-term administration of rapamycin in middle-aged marmosets. In addition, we have begun to address the potential sex-differences on biochemical and physiological outcomes of chronic rapamycin treatment based on some reports of increased rapamycin benefit in mice due in part to sex-specific rapamycin metabolism (Harrison et al., 2009; Miller et al., 2014).
METHODS
Animals:
All marmosets used in this research were housed at the Barshop Institute for Longevity and Aging Studies at UT Health San Antonio (UTHSA). The Institutional Animal Care and Use Committee (IACUC) of UTHSA is responsible for monitoring housing and animal condition regularly to ensure all guidelines are met for the safety and health of the animals. This research was reviewed and approved by the UTHSA IACUC and experiments were conducted in compliance with the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals and adhered to the American Society of Primatologists (ASP) principles for the ethical treatment of non-human primates.
Animals chosen for enrollment in this study were selected based on age as well as a record of relatively good health as assessed by veterinary examination. Animals in this study were either born within our animal facility at UTHSA or transferred from the Southwest National Primate Research Center (SNPRC) in San Antonio TX. At UTHSA, animals were maintained in our vivarium under a modified specific pathogen-free barrier facility described in greater detail elsewhere (Ross et al., 2017). In our first cohort of animals, 23 marmosets were enrolled with 11 males and 12 females. The average age of all animals at the beginning of treatment was ~8 years overall with males averaging ~9 years and females ~7 years (Table 1). The range of ages among all animals at this time was 4.2–10.1 years. Based on their reported lifespan, the average age of this group is approximately mid-life for this species (Nishijima et al., 2012; Ross et al., 2017).
Table 1.
Baseline parameters (pre-dosing).
| Male | Female | Sex effect (p) | |
|---|---|---|---|
| N | 11 | 12 | |
| Age (y) | 9.2 (0.2) | 7.2 (0.8) | - |
| Body weight (g) | 440 (12) | 407 (17) | 0.15 |
| RBC (Thou/mm3) | 7.5 (0.3) | 6.6 (0.7) | 0.10 |
| Hemoglobin (g/dL) | 16.4 (0.5) | 14.4 (0.8) | 0.054 |
| Hematocrit (%) | 54.3 (2.0) | 47.8 (2.8) | 0.09 |
| MCV (fL) | 72.8 (1.3) | 72.0 (1.5) | 0.72 |
| MCH (pg) | 22.0 (0.4) | 21.8 (0.4) | 0.75 |
| MCHC (g/dL) | 30.3 (0.3) | 30.3 (0.4) | 0.84 |
| RDW (%) | 16.3 (0.6) | 15.6 (0.4) | 0.30 |
| Platelet (Thou/mm3) | 561.0 (37.4) | 574.1 (52.1) | 0.85 |
| WBC (Thou/mm3) | 6.0 (0.6) | 5.3 (0.5) | 0.35 |
| Gran (Thou/mm3) | 2.4 (0.3) | 2.3 (0.3) | 0.79 |
| Lymph (Thou/mm3) | 3.3 (0.3) | 2.7 (0.5) | 0.30 |
| Mono (Thou/mm3) | 0.5 (0.3) | 0.2 (0.1) | 0.18 |
| Eos (Thou/mm3) | 0.08 (0.02) | 0.03 (0.01) | 0.11 |
| Baso (Thou/mm3) | 0.01 (0.01) | 0.03 (0.01) | 0.21 |
RBC – red blood cell, MCV – mean corpuscular volume, MCH – mean corpuscular hemoglobin, MCHC mean corpuscular hemoglobin concentration, RDW – red cell distribution width, WBC – white blood cells, Gran – granulocytes, Lymph – lymphocytes, Mono – monocytes, Eos – eosinophils, Baso – basophils. For each, data are average (standard error of mean; SEM) for group. P value italicized to indicate p = 0.05.
While enrolled in study, each animal received three diet choices daily provided ad libitum: Harlan Teklad purified marmoset diet (TD99468), Mazuri Callitrichid gel diet (5MI5) and ZuPreem. Each diet was provided in an irradiated or sterilized form in accordance with our barrier standard operating procedures. All animals were maintained as female – vasectomized male pairs for the duration of study in one over one marmoset caging. Treatments and data collections for this study occurred between February 2016 and November 2016.
Rapamycin:
Rapamycin encapsulated in Eudragit (and Eudragit alone) were purchased from Rapamycin Holdings (San Antonio TX). Encapsulated rapamycin was homogenized into yogurt by hand as previously described (Tardif et al., 2015). Marmoset training to receive oral dosing by syringe has also been described previously (Tardif et al., 2015). Once trained, marmosets were treated once daily with a dose of rapamycin roughly equivalent to 1 mg rapamycin/kg body weight. The final dose for each animal was based on binning animals within a 50 g interval (i.e., all animals between 400–450 g received equivalent dose, etc.). Animals were dosed Monday-Friday with dose received between 08:00 and 10:00. Animals were not dosed on Saturday or Sunday. Concentrations of rapamycin mixed in yogurt or in marmoset blood were assessed by tandem mass spectrometry by the Bioanalytical Pharmacology Core of the San Antonio Nathan Shock Center for Excellence in Biology of Aging (Harrison et al., 2009; Miller et al., 2014; Tardif et al., 2015)
Blood draws and clinical blood counts/chemistry:
Blood draws were performed at baseline (before beginning treatment) and then again after 9 months of daily treatment. Animals were fasted overnight and a 2.0 ml femoral blood collection was placed into SST and EDTA tubes. Plasma was separated from 0.5 ml of whole blood and stored at −80˚ C for analysis of rapamycin concentration at the UTHSA Bioanalytical Pharmacology Core. The remaining whole blood was transported to the SNPRC pathology lab for analysis of blood cell count, metabolic markers including glucose, cholesterol and triglycerides and C-reactive protein (CRP) concentrations.
Statistical analysis:
To test for sex-differences data for baseline characteristics and rapamycin concentrations in blood were analyzed by Student’s t test. Two way ANOVA were used to test for effect of sex or rapamycin treatment in samples from 9 month treatment group. For these data, change from baseline was first calculated for each individual animal. Post-hoc analysis when applicable was performed using the method of Holm-Sidak. For all, statistical significance was determined at a p < 0.05.
RESULTS
In our previous pilot studies, we demonstrated that delivery of encapsulated, slow-release rapamycin by oral dosing in the marmoset can result in clinically effective trough concentrations of rapamycin in the blood and inhibition of mTOR peripherally (Tardif et al., 2015). As a means to test the effect of such dosing on aging in this species, we have recruited middle-aged marmosets into a study designed to test both longevity and changes in aging physiology with chronic oral treatment of rapamycin for the remainder of life. Here, we begin to report on basic health outcomes from an initial cohort of animals treated for a period of approximately 9 months.
Due to the relative lack of information on basic hematology and markers of metabolism and inflammation, etc. of marmosets at this age, we first addressed for sex-specific differences among our cohorts of animals. In large part, males and females of these ages were surprisingly similar regarding their complete blood count with only hematocrit differing between the two (Table 1). Similarly, we found no difference in fasting concentrations of glucose or triglycerides in the blood, though cholesterol concentrations were significantly higher in male marmosets compared to females (Figure 1). We also found no significant sex difference in the concentrations of C-reactive protein, a general marker of inflammations (Figure 1).
Figure 1.
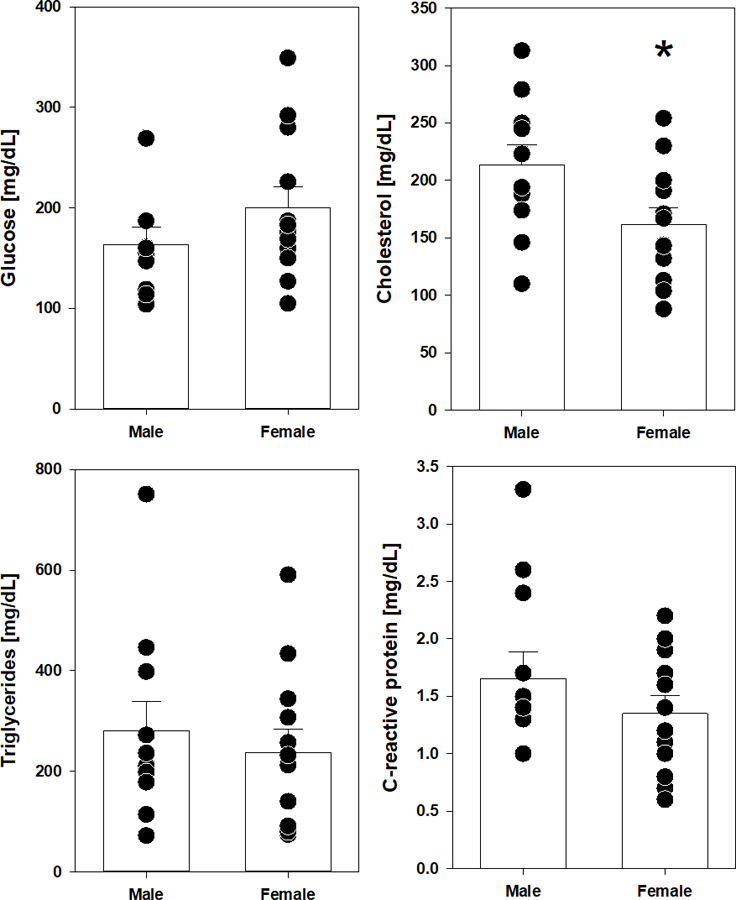
Baseline assessments of fasting glucose, cholesterol, triglycerides and C-reactive protein in middle-aged marmosets prior to rapamycin dosing. Filled circles indicate value for individual animal of indicated sex. Bars represent average value for group with error bars of standard error of mean (SEM). Asterisks indicate p <0.05 by t test.
Dosing was based upon body weight range of the individual animals (adjusted every two months) and final dosage was approximately 1 mg rapamycin/kg body weight. In our previous pilot study, we showed that the delivery of a dose similar to this produced trough concentrations (24 hours since last treatment) of rapamycin of ~5 ng/mL (Tardif et al., 2015). This previous report also details the pharmacokinetics of orally-delivered encapsulated rapamycin as we continue to use in this study. Here, with approximately double the number of animals, the average trough concentration of rapamycin for all animals 24 hours after dosing was 6.4 ± 1.0 ng/mL (Figure 2). Interestingly, when these concentrations were separated by sex we found that rapamycin concentrations in male marmosets were significantly higher than those in females (Figure 2). Rapamycin concentrations in the blood averaged 8.4 ± 1.7 ng/mL for male marmosets and 4.4 ± 0.6 ng/mL for female marmosets.
Figure 2.
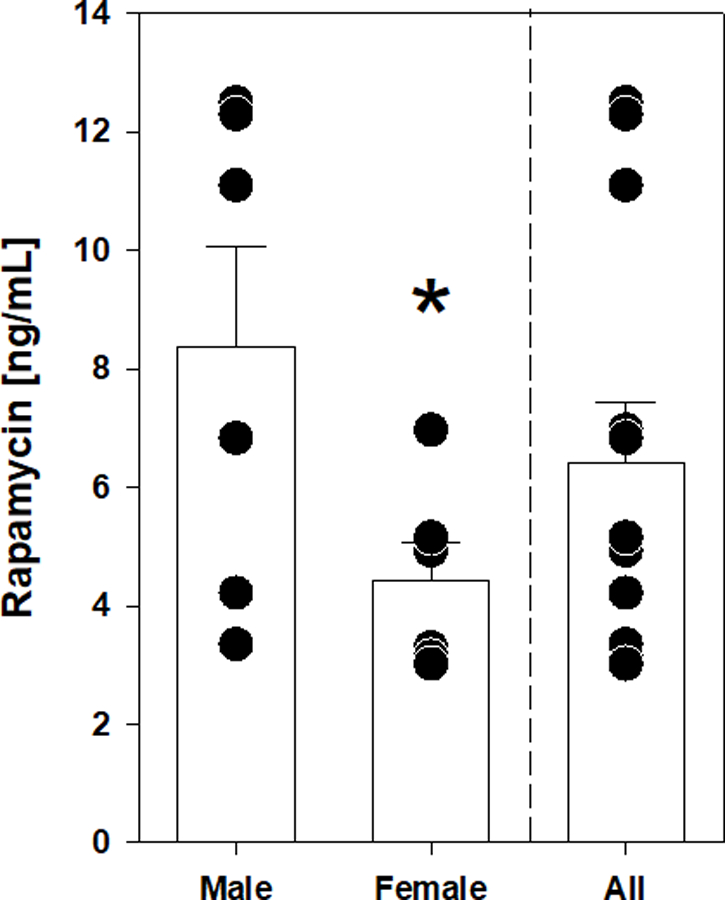
Concentration of rapamycin in blood for entire cohort and separated by sex. Filled circles indicate value for individual animal of indicated sex. Bars represent average value for group with error bars of standard error of mean (SEM). Asterisks indicate p <0.05 by t test comparing male and female groups. Dashed line separates data for all animals in cohort (All) with that binned by sex.
Follow-up assessment was performed after 9 months of treatment in this cohort of animals. A total of 6 males and 6 females received rapamycin for this period while 5 males and 5 females were included in the control cohort; one control female died prior to this time point and was not included in further data analysis. While there were minor differences in body weight between treatments at this time point, particularly for males, these differences did not reach statistical difference (Table 2). Complete blood cell counts at this time point show largely relatively minor changes compared to values assessed prior to treatment. Interestingly, we found no significant effect of sex among these changes and a significant effect of rapamycin on only two outcomes: change in red blood cell (RBC) count and change in basophil count (Table 2). In both cases, rapamycin resulted in significantly higher values for these outcomes compared to samples from control animals. We also found no significant interaction effects between sex and rapamycin treatment. Importantly, rapamycin had no effect on total white blood cell (WBC) count or on any particular leukocytes other than basophils. In general, this would suggest rapamycin is not driving a general decline in immune cell count at this point in treatment.
Table 2.
Change in parameters after 9 months treatment.
| Male Control | Male Rapa | Female Control | Female Rapa | Sex Effect | Treat Effect | Sex x treatment Interaction | |
|---|---|---|---|---|---|---|---|
| N | 5 | 6 | 5 | 6 | |||
| Δ Body weight (g) | 35 (18) | −16 (16) | 5 (20) | 11 (18) | 0.90 | 0.23 | 0.13 |
| Δ RBC (Thou/mm3) | 0.0 (0.9) | 1.4 (0.8) | −2.3 (0.9) | 0.8 (0.8) | 0.10 | 0.02 | 0.33 |
| Δ Hemoglobin (g/dL) | 0.6 (2.0) | 1.7 (1.8) | −4.5 (2.0) | 0.6 (1.8) | 0.12 | 0.12 | 0.32 |
| Δ Hematocrit (%) | −1.2 (6.5) | 3.9 (5.9) | −17.2 (6.5) | −1.3 (5.9) | 0.11 | 0.11 | 0.40 |
| Δ MCV (fL) | −2.0 (9.9) | 4.1 (9.0) | −16.1 (9.9) | −9.1 (9.0) | 0.17 | 0.50 | 0.96 |
| Δ MCH (pg) | 0.7 (3.1) | 2.0 (2.8) | −3.7 (3.1) | −1.5 (2.8) | 0.20 | 0.57 | 0.89 |
| Δ MCHC (g/dL) | 1.8 (4.3) | 6.2 (3.9) | −4.3 (4.3) | 1.8 (3.9) | 0.21 | 0.32 | 0.84 |
| Δ RDW (%) | −0.2 (1.9) | 2.8 (1.8) | −3.3 (1.9) | −1.3 (1.8) | 0.07 | 0.20 | 0.79 |
| Δ Platelet (Thou/mm3) | −82 (84) | 50 (77) | −76 (84) | −54 (77) | 0.55 | 0.36 | 0.51 |
| Δ WBC (Thou/mm3) | 0.0 (1.0) | 0.5 (1.0) | −1.5 (1.0) | −1.5 (1.0) | 0.35 | 0.30 | 0.57 |
| Δ Gran (Thou/mm3) | −0.6 (0.7) | 0.3 (0.6) | −1.1 (0.7) | −0.1 (0.6) | 0.53 | 0.16 | 0.92 |
| Δ Lymph (Thou/mm3) | 0.5 (0.5) | 0.0 (0.5) | −0.3 (0.5) | −0.6 (0.5) | 0.16 | 0.42 | 0.81 |
| Δ Mono (Thou/mm3) | −0.5 (0.3) | 0.1 (0.3) | −0.1 (0.3) | 0.1 (0.3) | 0.37 | 0.19 | 0.47 |
| Δ Eos (Thou/mm3) | 0.08 (0.14) | 0.02 (0.13) | 0.12 (0.14) | 0.23 (0.13) | 0.36 | 0.86 | 0.53 |
| Δ Baso (Thou/mm3) | 0.06 (0.03) | 0.12 (0.03) | −0.02 (0.03) | 0.08 (0.03) | 0.09 | 0.02 | 0.47 |
RBC – red blood cell, MCV – mean corpuscular volume, MCH – mean corpuscular hemoglobin, MCHC mean corpuscular hemoglobin concentration, RDW – red cell distribution width, WBC – white blood cells, Gran – granulocytes, Lymph – lymphocytes, Mono – monocytes, Eos – eosinophils, Baso – basophils. For each, data are average (standard error of mean; SEM) for group. P value bolded indicate p < 0.05.
Rapamycin also had no significant effect on changes in fasting blood glucose, cholesterol or triglycerides compared to pre-treatment values in either male (Figure 3) of female marmosets (Figure 4). While there is high variation among groups, there was also no effect of sex on these outcomes. C-reactive protein levels were also unaffected by sex or rapamycin treatment suggesting limited effects on general inflammation among these animals. For all assessments, there was no significant interaction effect between sex and rapamycin treatment as measured by two way ANOVA.
Figure 3.
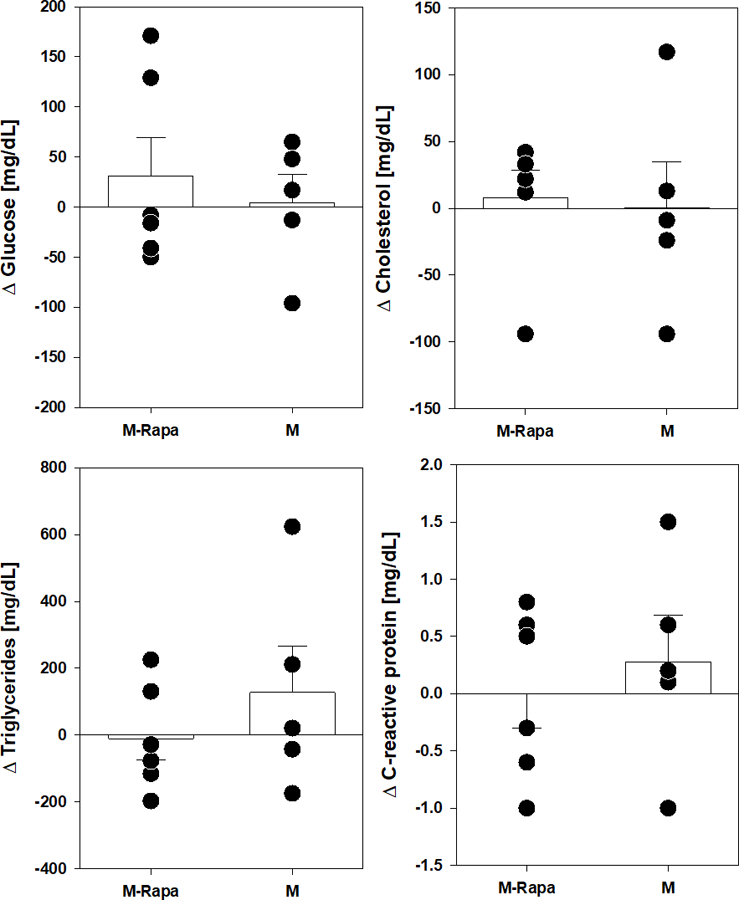
Metabolic and inflammatory marker changes after 9 months rapamycin in male marmosets. Filled circles indicate value for individual animal of indicated group. Rapa-M are males treated with rapamycin, M are control males. Bars represent average value for group with error bars of standard error of mean (SEM).
Figure 4.
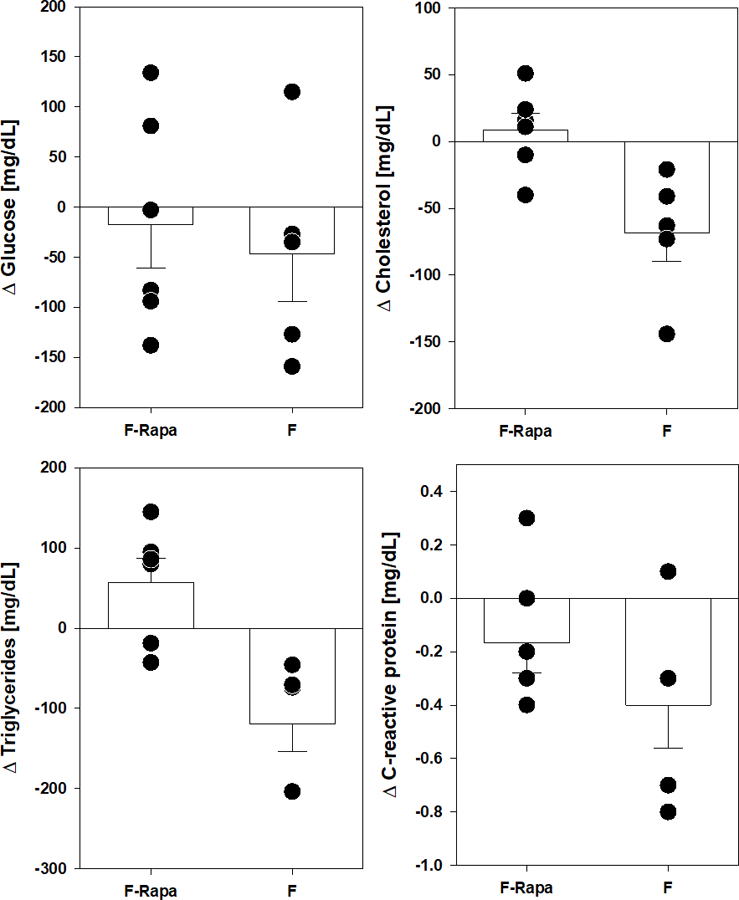
Metabolic and inflammatory marker changes after 9 months rapamycin in female marmosets. Filled circles indicate value for individual animal of indicated group. Rapa-F are females treated with rapamycin, F are control females. Bars represent average value for group with error bars of standard error of mean (SEM).
DISCUSSION
In this study, we report at least two important findings from a novel cohort of marmosets enrolled in a study to test the effect of rapamycin treatment on longevity in this species. First, we report that among middle-aged animals in our unique specific-pathogen free marmoset vivarium that there are few hematological differences between male and female animals prior to treatment. Second, the effects of chronic (9 month) treatment with rapamycin has relatively minor effects on hematological markers and, importantly, no marked clinical manifestations of significant potential side effects noted for this drug, namely thrombocytopenia, leucopenia and anaemia (Hong & Kahan, 2000; Kreis et al., 2000).
It is interesting that we noted very little difference between male and female middle-aged marmosets in their basic characterization prior to treatment. We noted marginally significant higher concentrations of hemoglobin in males and significantly lower cholesterol in females but all other markers were not different between the sexes. Others have reported a lack of difference in body weight at least in young animals and (Araujo et al., 2000) and marmosets generally described as monomorphic (Tardif, Power, Ross, & Rutherford, 2013). However, there have also been numerous reports of sex-specific phenotypes including behavior (Yamamoto, Domeniconi, & Box, 2004), endocrine beyond the basic sex hormones (de Sousa, Galvao, Sales, de Castro, & Galvao-Coelho, 2015), and even at the molecular level in terms of gene expression differences (Reinius et al., 2008). Hematological differences have been reported between male and female marmosets though in relatively young animals (Silva et al., 2014). It is of note that our animals originated from at least two sources (UTHSA and SNPRC) but were housed within our animal facility for a period of at least 2 months prior to sampling. It has been noted that housing conditions can have a significant impact on hematological and blood chemistry markers (Ross et al., 2017).
One intriguing sex-specific effect we noted was a significant difference between male and female marmosets in their trough concentrations of rapamycin. While sex differences in the physiological effects of rapamycin are well reported, sex differences in pharmacology of this drug are a bit more muddled. One report suggests that among genetically heterogeneous HET3 mice fed encapsulated rapamycin, female mice have significantly higher concentrations of rapamycin in the blood than male mice (Miller et al., 2014). However, others suggest no difference between sexes in either HET3 mice or inbred C57BL/6 mice fed encapsulated rapamycin or C57BL/6 mice injected with rapamycin (Bitto et al., 2016; Harrison et al., 2009; Weiss, Fernandez, Liu, Strong, & Salmon, 2018; Zhang et al., 2014). At least one human study using relatively healthy subjects also reported no clear sex difference (Kraig et al., 2018). It is unclear whether our results here suggest a species-specific pharmacological outcome in marmosets or lack of significant power to actually test this idea. Follow up tests as we include more subjects will certainly help clarify this issue.
In general, our results suggest little effect of rapamycin on these clinical laboratory markers through at least 9 months of treatment. Overall, this suggests that it is largely safe and, potentially, that detrimental effects on hematological markers previously noted for rapamycin may be limited at this particular dose, time-frame and species. In a previous study we also mentioned no significant effect of rapamycin on some hematological markers in marmosets (Tardif et al., 2015) and a similar lack of effect was noted on clinical hematological parameters in dogs treated with rapamycin, albeit at a much lower dose than in this study and for a shorter period of time (Urfer et al., 2017). The approximate dose used here has been used in both rodents and marmosets for relatively long-periods of time and is known to have species-specific effects on physiological markers. For example, rodents, particularly mice, develop transient dysfunctions in glucose metabolism when treated with rapamycin (Lamming et al., 2012; Liu et al., 2014; Weiss et al., 2018). On the other hand, we previously showed that a similar dose of rapamycin in marmosets does not significantly alter fasting glucose or glucose tolerance in marmosets (Ross et al., 2015). Here again, we find no significant effect of rapamycin on fasting glucose or on ancillary markers of metabolism like cholesterol or triglycerides. Rapamycin has been associated with hyperlipidemia, but again generally in health-compromised populations (Morrisett et al., 2002). Here, and before (Ross et al., 2015), we report limited effects on lipid panels in marmosets treated with rapamycin. It will be of interest to clarify whether such discrepancies between populations are species-specific differences or are related more to the health and poly-pharmaceutical therapeutics of the clinical populations studied.
As mentioned, the animals tested in this study are part of a larger cohort of animals testing the effect of rapamycin on longevity in the common marmoset. The dose of rapamycin is in line with those previously reported for lifespan extending effects in mice as well as reported studies using human subjects and clinical dosing. One important outcome from this study overall will be to determine potential predictive markers of the likelihood of longevity outcomes of interventions; i.e., biomarkers. At this point, it is still unclear what standard clinical laboratory testing might tell us in this case; however, longitudinal assessment of these same animals, as is planned, will help clarify whether age-related markers exist(Kaeberlein, 2018). In addition, expansion of potential markers such as epigenetic clock (Horvath & Raj, 2018), changes in the metabolome (Hoffman et al., 2016), frailty assessments (Kane, Gregson, Theou, Rockwood, & Howlett, 2017; Kim, Myers, Wyckoff, Cherry, & Jazwinski, 2017) or tests addressing cellular physiology (Salmon, Dorigatti, Huber, Li, & Nathanielsz, 2018) or others will be important to address during the course of this study.
ACKNOWLEDGEMENTS
The animal care provided by the Department of Lab Animal Research (LAR) at UTHSA is acknowledged. Research was supported by NIH R01 AG050797. Effort was also supported in part by the San Antonio Nathan Shock Center for Excellence in the Biology of Aging (P30 AG013319) and the San Antonio Claude A. Pepper Older Americans Independence Center ((P30 AG044271). ABS was also supported Geriatric Research, Education and Clinical Center of the South Texas Veterans Health Care System. This material is the result of work supported with resources and the use of facilities at South Texas Veterans Health Care System, San Antonio, Texas. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
REFERENCES
- An JY, Quarles EK, Mekvanich S, Kang A, Liu A, Santos D, . . . Kaeberlein M (2017). Rapamycin treatment attenuates age-associated periodontitis in mice. GeroScience, 39(4), 457–463. doi: 10.1007/s11357-017-9994-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelo SIA, Pumper CP, Baar EL, Cummings NE, & Lamming DW (2016). Intermittent Administration of Rapamycin Extends the Life Span of Female C57BL/6J Mice. Journals of Gerontology Series a-Biological Sciences and Medical Sciences, 71(7), 876–881. doi: 10.1093/gerona/glw064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo A, Arruda MF, Alencar AI, Albuquerque F, Nascimento MC, & Yamamoto ME (2000). Body weight of wild and captive common marmosets (Callithrix jacchus). International Journal of Primatology, 21(2), 317–324. doi: Doi 10.1023/A:1005433722475 [DOI] [Google Scholar]
- Bitto A, Ito TK, Pineda VV, LeTexier NJ, Huang HZ, Sutlief E, . . . Kaeberlein M (2016). Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. Elife, 5, ARTN e16351. doi: 10.7554/eLife.16351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D-F, Karunadharma PP, Chiao YA, Basisty N, Crispin D, Hsieh EJ, . . . Rabinovitch PS (2014). Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell, 13(3), 529–539. doi: 10.1111/acel.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa MBC, Galvao ACD, Sales CJR, de Castro DC, & Galvao-Coelho NL (2015). Endocrine and Cognitive Adaptations to Cope with Stress in Immature Common Marmosets (Callithrix jacchus): Sex and Age Matter. Frontiers in Psychiatry, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, . . . Miller RA (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460(7253), 392–395. doi: 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JM, Tran V, Wachtman LM, Green CL, Jones DP, & Promislow DE (2016). A longitudinal analysis of the effects of age on the blood plasma metabolome in the common marmoset, Callithrix jacchus. Exp Gerontol, 76, 17–24. doi: 10.1016/j.exger.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JC, & Kahan BD (2000). Sirolimus-induced thrombocytopenia and leukopenia in renal transplant recipients: risk factors, incidence, progression, and management. Transplantation, 69(10), 2085–2090. [DOI] [PubMed] [Google Scholar]
- Horvath S, & Raj K (2018). DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics, 19(6), 371–384. doi: 10.1038/s41576-018-0004-3 [DOI] [PubMed] [Google Scholar]
- Kaeberlein M (2018). How healthy is the healthspan concept? Geroscience doi: 10.1007/s11357-018-0036-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane AE, Gregson E, Theou O, Rockwood K, & Howlett SE (2017). The association between frailty, the metabolic syndrome, and mortality over the lifespan. GeroScience, 39(2), 221–229. doi: 10.1007/s11357-017-9967-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Myers L, Wyckoff J, Cherry KE, & Jazwinski SM (2017). The frailty index outperforms DNA methylation age and its derivatives as an indicator of biological age. GeroScience, 39(1), 83–92. doi: 10.1007/s11357-017-9960-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig E, Linehan LA, Liang H, Romo TQ, Liu Q, Wu Y, . . . Kellogg DL Jr. (2018). A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Exp Gerontol, 105, 53–69. doi: 10.1016/j.exger.2017.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis H, Cisterne JM, Land W, Wramner L, Squifflet JP, Abramowicz D, . . . Vialtel P (2000). Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation, 69(7), 1252–1260. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, . . . Baur JA (2012). Rapamycin-Induced Insulin Resistance Is Mediated by mTORC2 Loss and Uncoupled from Longevity. Science, 335(6076), 1638–1643. doi: 10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelegren M, Liu Y, Ross C, Tardif S, & Salmon AB (2016). Pharmaceutical inhibition of mTOR in the common marmoset: effect of rapamycin on regulators of proteostasis in a non-human primate. Pathobiol Aging Age Relat Dis, 6, 31793. doi: 10.3402/pba.v6.31793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Diaz V, Fernandez E, Strong R, Ye L, Baur JA, . . . Salmon AB (2014). Rapamycin-induced metabolic defects are reversible in both lean and obese mice. Aging (Albany NY), 6(9), 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, . . . Klickstein LB (2014). mTOR inhibition improves immune function in the elderly. Sci Transl Med, 6(268), 268ra179. doi: 10.1126/scitranslmed.3009892 [DOI] [PubMed] [Google Scholar]
- Mannick JB, Morris M, Hockey HP, Roma G, Beibel M, Kulmatycki K, . . . Klickstein LB (2018). TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci Transl Med, 10(449). doi: 10.1126/scitranslmed.aaq1564 [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, . . . Strong R (2014). Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell, 13(3), 468–477. doi: 10.1111/acel.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisett JD, Abdel-Fattah G, Hoogeveen R, Mitchell E, Ballantyne CM, Pownall HJ, . . . Kahan BD (2002). Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. Journal of Lipid Research, 43(8), 1170–1180. doi: 10.1194/jlr.M100392-JLR200 [DOI] [PubMed] [Google Scholar]
- Nishijima K, Saitoh R, Tanaka S, Ohsato-Suzuki M, Ohno T, & Kitajima S (2012). Life span of common marmoset (Callithrix jacchus) at CLEA Japan breeding colony. Biogerontology, 13(4), 439–443. doi: 10.1007/s10522-012-9388-1 [DOI] [PubMed] [Google Scholar]
- Reinius B, Saetre P, Leonard JA, Blekhman R, Merino-Martinez R, Gilad Y, & Jazin E (2008). An evolutionarily conserved sexual signature in the primate brain. PLoS Genet, 4(6), e1000100. doi: 10.1371/journal.pgen.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C, Salmon A, Strong R, Fernandez E, Javors M, Richardson A, & Tardif S (2015). Metabolic consequences of long-term rapamycin exposure on common marmoset monkeys (Callithrix jacchus). Aging (Albany NY), 7(11), 964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CN, Austad S, Brasky K, Brown CJ, Forney LJ, Gelfond JA, . . . Tardif SD (2017). The development of a specific pathogen free (SPF) barrier colony of marmosets (Callithrix jacchus) for aging research. Aging (Albany NY), 9(12), 2544–2558. doi: 10.18632/aging.101340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon AB, Dorigatti J, Huber HF, Li C, & Nathanielsz PW (2018). Maternal nutrient restriction in baboon programs later-life cellular growth and respiration of cultured skin fibroblasts: a potential model for the study of aging-programming interactions. GeroScience, 40(3), 269–278. doi: 10.1007/s11357-018-0024-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavlakadze T, Zhu J, Wang S, Zhou W, Morin B, Egerman MA, . . . Glass DJ (2018). Short-term Low-Dose mTORC1 Inhibition in Aged Rats Counter-Regulates Age-Related Gene Changes and Blocks Age-Related Kidney Pathology. J Gerontol A Biol Sci Med Sci, 73(7), 845–852. doi: 10.1093/gerona/glx249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva ID, da Silva FDR, Fuzessy LF, Tavela AD, Carretta M, Silva VHD, . . . Boere V (2014). Hematology and blood biochemistry in wild hybrid marmosets from the Atlantic Forest, Brazil. Ciencia Rural, 44(9), 1596–1602. doi: 10.1590/0103-8478cr20120822 [DOI] [Google Scholar]
- Tang Z, Bereczki E, Zhang H, Wang S, Li C, Ji X, . . . Pei JJ (2013). Mammalian target of rapamycin (mTor) mediates tau protein dyshomeostasis: implication for Alzheimer disease. J Biol Chem, 288(22), 15556–15570. doi: 10.1074/jbc.M112.435123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif S, Ross C, Bergman P, Fernandez E, Javors M, Salmon A, . . . Richardson A (2015). Testing efficacy of administration of the antiaging drug rapamycin in a nonhuman primate, the common marmoset. J Gerontol A Biol Sci Med Sci, 70(5), 577–587. doi: 10.1093/gerona/glu101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif SD, Power ML, Ross CN, & Rutherford JN (2013). Body Mass Growth in Common Marmosets: Toward a Model of Pediatric Obesity. American Journal of Physical Anthropology, 150(1), 21–28. doi: 10.1002/ajpa.22110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DEL, & Kaeberlein M (2017). A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. GeroScience, 39(2), 117–127. doi: 10.1007/s11357-017-9972-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R, Fernandez E, Liu Y, Strong R, & Salmon AB (2018). Metformin reduces glucose intolerance caused by rapamycin treatment in genetically heterogeneous female mice. Aging (Albany NY) doi: 10.18632/aging.101401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf SH (2008). The meaning of translational research and why it matters. JAMA, 299(2), 211–213. doi: 10.1001/jama.2007.26 [DOI] [PubMed] [Google Scholar]
- Yamamoto ME, Domeniconi C, & Box H (2004). Sex differences in common Marmosets (Callithrix jacchus) in response to an unfamiliar food task. Primates, 45(4), 249–254. doi: 10.1007/s10329-004-0088-6 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, . . . Fischer K (2014). Rapamycin Extends Life and Health in C57BL/6 Mice. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 69A(2), 119–130. doi: 10.1093/gerona/glt056 [DOI] [PMC free article] [PubMed] [Google Scholar]


