Abstract
Self-referential processing is critical to understanding social anxiety disorder (SAD). This study examined neural differences in self-referential processing in healthy controls (HC) and participants with SAD at pre- and post-treatment. Participants (n = 64) underwent fMRI scanning while viewing a video of themselves (“Self”) or another person (“Other”). SAD participants were randomized to cognitive behavior therapy (CBT), acceptance and commitment therapy (ACT), or waitlist, and were re-scanned at post-treatment. In SAD vs. HC, the fusiform face area (FFA) showed significantly more activation during Self vs. Other, and greater SAD severity was associated with significantly more activation during Self vs. Other in the right FFA and the left extrastriate body area (EBA). Greater reduction in SAD severity was associated with stronger connectivity between the amygdala and FFA during Self vs. Other at post-treatment, whereas the strength of connectivity during Self and Other was comparable at post-treatment for those with less SAD reduction. Thus, there were significant differences in activation and functional connectivity of brain regions implicated in self-referential processing in SAD. Change in connectivity between the amygdala and FFA were observed as a function of change in SAD severity, suggesting that improvements in SAD severity may correct this altered functional connectivity.
Keywords: Social Phobia, Anxiety, Video-Feedback, fMRI, self-observation, social anxiety disorder, self-referential processing, cognitive behavior therapy, acceptance and commitment therapy
1. Introduction
Social anxiety disorder (SAD) is characterized by excessive concern with negative evaluation (American Psychiatric Association, 2013). While much is known about the clinical presentation of SAD and its treatment, less is known about neural mechanisms underlying core processes of SAD. The threat-detection neural circuitry underlying fears of negative evaluation involves the amygdala, precuneus, insula, anterior cingulate, and prefrontal cortices (Brühl et al, 2014; Etkin and Wager, 2007). However, beyond fears of negative evaluation, SAD is also characterized by altered self-referential processing, and this is likely reflected in unique areas of neural activation.
Rapee and Heimberg (1997) proposed that SAD involves negatively-biased mental self-representations. Self-representation is based on memories of social successes and failures. This self-representation is compared with an expected performance to predict the probability of an aversive outcome, such that the representation itself becomes aversive. Video-feedback may combat distorted mental representations by providing corrective learning experiences (Harvey et al., 2000). While several investigations have explored self-referential processing in response to language cues (i.e., criticism and praise; Blair et al., 2008; Abraham et al., 2013; Yoon et al., 2016) or in subclinical samples (Abraham et al., 2013), the neural mechanisms of self-referential processing in regard to videos of the self in SAD are not well understood. Given the importance of self-referential video feedback for treatment, it is important to further understand the neural mechanisms underlying this task.
1.1. Neural Underpinnings of Self-Referential Processing in Healthy Controls
Meta-analytic findings suggest that three key steps are required for self-referential processing for videos of the self (Platek et al., 2008): (1) sensory processing in the fusiform, particularly in terms of stimulus detection for faces, which (2) relays information to the precuneus for processing of facial information, and then (3) to cortical areas, especially the middle frontal gyrus which is involved in the discrimination between self- and other-content. The fusiform gyrus, an area involved in face processing more broadly (which is related to the sensory processing of the first step in the model described above, Kanwisher et al., 1997), has previously been shown to be more active in responses to images of the self- versus other-face (Sugiura et al., 2005). In addition, the extrastriate body area (EBA), a brain region involved in processing of body stimuli, is likely involved in processing self- vs. other-referential body stimuli (Vocks et al., 2010). Cortical areas implicated in self-referential processing more broadly, particularly in stimuli other than videos of the self, have been shown to involve activation of the cortical midline structures (CMS), including the medial orbitofrontal cortex, ventromedial prefrontal cortex (vmPFC), dorsomedial PFC, dorsal anterior cingulate cortex, and precuneus (Northoff et al., 2006).
1.2. Neural Underpinnings of Self-Referential Processing in SAD
The fusiform face area (FFA), a key region in self-referential processing, is differentially activated in SAD compared to HC. When viewing images of fearful faces, SAD participants demonstrated greater reactivity in bilateral fusiform compared to HC (Frick et al., 2013). This suggests that the individuals with SAD might demonstrate altered sensory processing, specifically facial processing, compared to HC. When individuals with SAD and HC observed a video recording of their own working memory task performance, compared to others’ performance, differential activation in the fusiform was observed for those with higher SAD severity (Pujol et al., 2013). To our knowledge, this is the only study comparing self- to other-referential processing of videos in SAD and HC, and this study did not include observation of a speech performance task, a common therapeutic tool in SAD treatment.
Altered processing of bodily stimuli may also be critical in understanding disrupted self-referential processing in SAD. Individuals with greater levels of social inhibition, or the tendency to inhibit emotional expression in social relationships which often occurs in SAD, demonstrate greater activation of the EBA during observation of threatening versus neutral body movements (Kret et al., 2011). However, the importance of altered EBA activation in self-referential processing has not been extended to SAD. Differential activation in the EBA has been demonstrated during self-referential body processing in anorexia, bulimia, and unspecified eating disorders. Specifically, individuals with eating disorders who received treatment demonstrated increased activation in the EBA during self- versus other-body processing relative to individuals who did not receive treatment (Vocks et al., 2011). Thus, treatment for eating disorders may be associated with altered sensory processing, reflected in more activation of brain regions typically associated with processing of bodily stimuli. However, it is unclear whether self-referential bodily processing is altered in SAD or whether these alterations are corrected in response to treatment.
1.3. Neural Mechanisms of SAD Treatment
A few prior studies have described neural changes from pre- to post-SAD treatment. Young and colleagues (2017) found that in a sample of patients with SAD who were assigned to cognitive behavior therapy (CBT) or acceptance and commitment therapy (ACT), degree of SAD reduction in treatment correlated with functional connectivity between the amygdala and ventromedial prefrontal cortex at post-treatment. Three additional studies have explored other implicit (Burklund et al., 2015) or explicit emotion regulation strategies in response to treatment for SAD, including internet delivered CBT (Månsson et al., 2013), and CBT (Goldin et al., 2013, 2014). However, none of the studies that have explored neural mechanisms of SAD treatment have reported on changes in neural activation during self-referential processing of a video observation task.
1.4. Current Study
In the current study, fMRI BOLD signal was recorded as participants with and without SAD observed video-recordings of their own (“self-observation”) and another person’s (“other-observation”) speech performance. We hypothesized that exposure to self-observation (vs. other-observation) would be associated with enhanced activation in regions associated with self-referential processing, namely the FFA, EBA and medial PFC, and regions associated with anxiety, namely the amygdala, in SAD compared to HC. We also hypothesized that severity of social anxiety across the entire sample would be associated with differential patterns of activation in the FFA and EBA, with greater social anxiety severity associated with greater activation during self- vs. other-observation. We explored functional connectivity between the right amygdala, given its role in fear conditioning (Marek & Sah, 2018), and all other brain regions during self- vs. other-observation for SAD vs. HC. We did not have a priori hypotheses about functional connectivity given the lack of prior research on this topic in self-referential processing for SAD vs. HC. Therefore, this was an exploratory aim. In addition, we examined changes in self-referential processing from pre- to post-treatment. We hypothesized that individuals who received an evidence-based treatment for SAD (namely cognitive behavior therapy, CBT, and acceptance and commitment therapy, ACT; Hayes et al., 1999) would show a correction in disrupted neural activation during self-referential processing compared to individuals on a waitlist. As the parent trial from which these data were collected did not reveal any major treatment outcome differences between CBT and ACT (Craske et al., 2014), we did not anticipate any differences between the two active treatment comparisons in terms of neural activation. Finally, we compared functional connectivity at post-treatment, as well as the correlation between functional connectivity at post-treatment and SAD severity reduction from pre- to post-treatment. As with the baseline functional connectivity aim, these were exploratory aims.
2. Methods
2.1. Participants
Demographic and full methodological details are reported in Table 1 and in Craske and colleagues (2014). Participants were medication-free or stabilized (n = 8) on psychotropic medications and could not meet criteria for severe major depression, a history of bipolar disorder, psychosis, active suicide ideation, current substance abuse or dependence, or major medical problems. Individuals could not participate if they were pregnant, left-handed, had metal implants or claustrophobia.
Table 1.
Demographic Details
| HC | SAD | Total Sample | p | |
|---|---|---|---|---|
| Age (Mean, SD) | 29.1 (7.4) | 27.3 (6.0) | 27.7 (6.3) | .37 |
| Race n (%) | Fisher’s exact = .75 | |||
| Caucasian | 9 (69.2) | 25 (49.0) | 34 (53.1) | |
| Latino | 1 (7.7) | 6 (11.8) | 7 (10.9) | |
| Asian American | 3 (23.1) | 11 (21.6) | 14 (21.9) | |
| African American | 0 (0.0) | 2 (3.9) | 3 (3.1) | |
| Other | 0 (0.0) | 6 (11.8) | 6 (9.4) | |
| Missing | 0 (0.0) | 1 (2.0) | 1 (1.6) | |
| Gender (% Female) | 8 (61.5) | 24 (47.1) | 32 (50.0) | .35 |
| Medications | ||||
| Current meds for emotional problem n (% yes) | 0 (0.0) | 8 (16.0) | 8 (12.5) | .12 |
| Previous meds for emotional problem n (% yes) | 0 (0.0) | 23 (46.0) | 23 (35.9) | .02 |
| Missing med information | 0 (0.0) | 1 (2.0) | 1 (1.6) | |
| Marital Status | .81 | |||
| Married | 1 (7.7) | 4 (7.8) | 5 (7.8) | |
| Single | 11 (84.6) | 44 (86.3) | 55 (85.9) | |
| Divorced | 1 (7.7) | 1 (2.0) | 2 (3.1) | |
| Cohabitating | 0 (0.0) | 1 (2.0) | 1 (1.6) | |
| Separated | 0 (0.0) | 1 (2.0) | 1 (1.6) | |
| Education | .23 | |||
| Less than high school | 0 (0.0) | 1 (2.0) | 1 (1.6) | |
| High school | 0 (0.0) | 10 (19.6) | 10 (15.6) | |
| Some college | 7 (53.8) | 13 (25.5) | 20 (31.2) | |
| College Degree | 5 (38.5) | 22 (43.1) | 27 (42.19) | |
| Post-college training | 1 (7.7) | 5 (9.8) | 6 (9.3) | |
| Occupational Status | .04 | |||
| Professional/White Collar | 9 (69.2) | 17 (33.3) | 26 (40.6) | |
| Blue Collar | 0 (0.0) | 9 (17.6) | 9 (14.1) | |
| Unemployed/Other | 4 (30.8) | 25 (49.0) | 29 (45.3) |
2.1.1. Social Anxiety Participants.
Individuals who met criteria for SAD, generalized type according to the Anxiety Disorders Interview Schedule (ADIS; Di Nardo et al., 1994), as indicated by a clinician severity rating greater than 4, were invited to participate (n = 100). The current study included 51 participants who completed the self-referential processing task.
2.1.2. Healthy control participants.
HC (n = 13) were similar to all SAD participants on age and gender and did not meet criteria for any DSM-IV mood or anxiety disorders.
2.2. Measures
2.3.1. Liebowitz Social Anxiety Scale-Self Report Version (LSAS; Liebowitz, 1987).
The LSAS is a 27-item measure of social anxiety severity that assesses both fear and avoidance of social and performance situations on a 0–3 point Likert scale. The LSAS has high internal consistency, as well as good convergent and discriminant validity (Fresco et al., 2001). As social anxiety disorder status was determined by a clinical interview and not self-report, the LSAS measure has sufficient variability, even within the HC condition (range 0 – 27 within HC) and can be used as a dimensional measure of social anxiety severity.
2.3. Procedure
The study took place at the Anxiety and Depression Research Center at the University of California, Los Angeles campus and was approved by the UCLA Human Subjects Protection Committee. Following completion of informed consent and the baseline assessment, participants completed a laboratory session in which they completed a variety of emotional reactivity and regulation tasks regarding public speaking. Participants were asked to give a three-minute video-recorded speech on two topics (global warming and corporal punishment). Two researchers observed the speech delivery. The researchers told participants that the recorded tape would be prepared so that other “impartial observers” could watch and evaluate its content and delivery. Researchers instructed participants to discuss one or both of the topics during the speech, and to prepare for 5-minutes prior to the speech. After preparation, participants were given a 2-minute break to enhance speech anticipation.
Participants returned to the laboratory for a separate visit to complete the fMRI scan. The task for the current study included viewing two blocked presentations of separate 30 s videos of the participant’s speech (“Self”) interspersed with two presentations of 30 s news videos (“Other”) with a 10s interstimulus interval. Researchers instructed participants as follows: “During the next scan, you’ll simply be watching video clips of yourself and others speaking. You will not need to make any button presses for this scan.”
“Other” stimuli consisted of two 30s videos depicting standardized news reports of a well-known female news anchor, which was included to control for familiarity, movement and speech of the self-referential video observation task. The content of the videos included segments on invisibility technology and school yearbooks. “Self” stimuli included two idiographic 30s videos depicting each participant delivering a previously recorded speech in the laboratory on a prior session, recorded from the waist up.
2.4. Therapy
Participants randomized to receive either CBT (n = 17) or ACT (n = 20) received 12 weekly, 1-hour therapy sessions, whereas participants on the waitlist (n = 14) were invited to complete either treatment at the end of their final assessment. Full details on the therapy are available elsewhere (Craske et al., 2014). However, most participants completed therapy between 12 – 16 weeks from baseline (range 11 – 18 weeks). In the sample of participants who completed the task for the current study, mean LSAS severity scores from pre- to post-treatment were as follows: CBT Pre-treatment: 81.55 (SD = 15.75), Post-treatment: 61.82; (SD = 20.47); ACT Pre-treatment: 90.92 (SD = 21.25), Post-treatment: 66.75 (SD = 19.98), Waitlist Pre-treatment: 69.70 (SD = 22.43), Post-treatment: 69.70 (SD = 15.35). In each active condition, 4 participants dropped out of therapy.
2.5. Image acquisition and analysis
Data were collected on a Trio 3.0 Tesla MRI scanner at the UCLA Ahmanson-Lovelace Brain Mapping Center. High-resolution structural T2-weighted echo-planar images were acquired for each participant (spin-echo; TR=5000 ms; TE=34 ms, matrix size= 128 × 128, resolution 1.6 mm × 1.66 mm × 3 mm; FOV= 200 mm; 36 axial slices, 3 mm thick, flip angle=90°, bandwidth =1302 Hz/Px). Functional imaging used T2*-weighted echo planar image sequences (gradient-echo, TR=3000 ms, TE=25ms, flip angle=90°, matrix size=64 × 64, resolution 3.1 mm × 3.1 mm × 3.0 mm, FOV=200 mm, 36 axial slices, bandwidth=2604 Hz/Px, duration: 2 min 51s, 1 functional run/participant, third task per run).
Data were analyzed using SPM8 (Wellcome Department of Cognitive Neurology, Institute for Neurology, London, UK). Functional images were realigned to the mean to correct for head motion, co-registered with high resolution structural images and warped into MNI space using the segmentation procedure in SPM8 (resampled at 3×3×3 mm; Mazziotta et al., 2001). Images were then smoothed using an 8-mm Gaussian kernel, full-width half-max and high pass filtered at 128 seconds to account for low-frequency drift.
Statistical analysis of functional data used general linear models using a block design to model box car functions for the duration of video clips (30s), convolved with the canonical double-gamma hemodynamic response function (HRF) and including six motion parameters as covariates of no interest. Additionally, regressors identifying individual volumes with large global signal intensity change (outliers thresholded at 2.5 standard deviations from average global signal intensity within the run) or volumes with motion spikes (1.5mm or greater) were included to remove variance in signal due to noise. Runs with more than 10% of volumes with large global signal intensity changes or motion spikes were removed from analyses. Contrast images were computed for the mean activation of ‘Self > Other’ and ‘Other > Self’.
For hypothesis 1, contrasts of interest in the whole-brain analyses included Self < or > Other, first for each group separately and then in a between-group, two-sample, one-sided t-test. Of note, SPM corrects for differences in sample size and heteroscedasticity by assuming unequal variance between samples and using restricted maximum likelihood (REML) and weighted least squares to produce maximum likelihood estimators using non-sphericity.
For hypothesis 2, a secondary whole-brain analysis included LSAS as a regressor for the same contrast. Specifically, we entered LSAS as a regressor of activation in SAD only, HC only, and in the two-sample SAD vs. HC comparison.
To address hypothesis 3, differential functional connectivity between SAD and HC with the amygdala as a seed region, given its role in threat-responding for social anxiety disorder (Pape and Pare, 2010), we conducted psychophysiological interaction (PPI). A whole brain PPI analyzes anatomically defined regions of interest (ROI, using the AAL) of the right and left amygdala in each group separately and in a two-sample between group comparison.
To address hypothesis 4, differential activation from pre- to post-treatment for Treatment versus Waitlist and for CBT vs. ACT, we ran a two-sample t-test on the change in activation from pre- to post-treatment.
Finally, to address hypothesis 5,differential functional connectivity from pre- to post-treatment with the right amygdala as the seed region was calculated using PPI analyses in a two-sample between group comparison. To address hypothesis 6, this PPI analysis was repeated with change in LSAS entered as a covariate.
For all analyses, we determined cluster-size thresholds using Monte Carlo simulations in AFNI’s 3DClustSim (http://afni.nimh.nih.gov/afni/) which allowed for improved assessment of functional activation and correction for multiple comparisons (Forman et al., 1995) based on a grey matter mask. Given our a priori interest in the FFA, an anatomical mask of the left and right FFA were also entered into 3DClustSim. These analyses resulted in a recommended resampled cluster size of 30.5 voxels for the left FFA, 32.9 voxels for the right FFA, and 39.3 voxels for the rest of the brain at an alpha level of 0.05 and a voxelwise threshold of p < 0.005 (two-tailed tests).
3. Results
3.1. Baseline
3.1.1. Hypothesis 1: Whole-brain group differences at pre-treatment.
In a two-sample t-test, SAD had a greater difference in activation for the Self > Other contrast compared to HC in right middle occipital gyrus, extending into the right fusiform gyrus (see Table 2). For differences for Self > Other with HC and SAD separately, see supplemental material.
Table 2.
Whole-brain effects by group using a p-value of .005 combined with an extent threshold of 40 contiguous voxels at pre-treatment
| x | y | z | t | Voxels | |
|---|---|---|---|---|---|
| Whole Brain: SAD vs. HC (Self vs. Other) | |||||
| SAD > HC | |||||
| Middle Occipital Gyrus/Fusiform | 24 | −85 | 10 | 3.5358 | 86 |
| HC > SAD | |||||
| No suprathreshold clusters | |||||
| Whole Brain: LSAS Covaried (All Ps) | |||||
| Self > Other | |||||
| Fusiform | 27 | −67 | −2 | 4.6601 | 238 |
| Middle Occipital Gyrus | −21 | −85 | 10 | 3.8227 | 61 |
| Middle Occipital Gyrus/EBA | −45 | −79 | 13 | 4.401 | 65 |
| Other>Self | |||||
| No suprathreshold clusters | |||||
| PPI: Right Amygdala Seed, HC Only | |||||
| No suprathreshold clusters | |||||
| PPI: Right Amygdala Seed, HC Only | |||||
| No suprathreshold clusters | |||||
| PPI: Right Amygdala Seed, SAD Only | |||||
| Self > Other | |||||
| No suprathreshold clusters detected | |||||
| Other > Self | |||||
| Fusiform | 24 | −40 | −14 | −3.8172 | 38 |
| Fusiform | −27 | −37 | −17 | −3.8469 | 44 |
| Middle frontal gyrus | −33 | 17 | −55 | −4.9236 | 59 |
| Medial Orbitofrontal gyrus | −3 | 50 | −17 | −4.4868 | 59 |
| Cerebellum Crus I | −21 | −79 | −26 | −3.6506 | 53 |
| Cerebellum Crus I | −30 | −73 | −35 | −3.6642 | 54 |
| Cerebellum Crus I | 15 | −76 | −26 | −3.97 | 92 |
| Whole Brain: Right Amygdala seed for Self>Other | |||||
| SAD > HC | |||||
| Insula | 39 | 7 | −17 | 3.9497 | 41 |
| HC > SAD | |||||
| Middle Temporal Gyrus | −54 | −49 | 7 | −3.5223 | 51 |
3.1.2. Hypothesis 2. Relationship of social anxiety severity to brain activation during Self-vs. Other-observation at pre-treatment
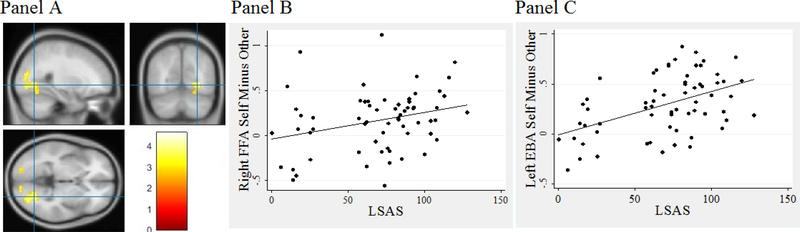
When LSAS was included as a regressor in the model, one region of activation included the right fusiform gyrus which was significant in the Self > Other contrast (see Table 2) and was extracted as an ROI to allow for graphing. LSAS was a significant predictor of the difference in activation for Self > Other in the right fusiform gyrus, and greater LSAS severity was associated with a greater activity in response to Self relative to Other (see Figure 1). Other areas with significant activation in this Self > Other comparison in the same direction included the bilateral middle occipital gyrus. Activation in the left middle occipital gyrus included an area in the visual cortex that extended into the EBA.
Figure 1.
Pre-treatment correlation between social anxiety severity (LSAS) and fusiform face area (FFA) and extrastriate body area (EBA) activation
Note. Panel A depicts neural activation when the LSAS was entered as a regressor. Panel B depicts the association between the right FFA for Self – Other correlated with LSAS. Panel C depicts the association between the left EBA for Self – Other correlated with LSAS.
3.1.3. Hypothesis 3. Differential functional connectivity with right amygdala seed in SAD vs. HC
3.1.3.1. Within-group functional connectivity.
In HC, no suprathreshold clusters were detected in right amygdala-seeded functional connectivity analyses for the Self vs. Other contrast. In SAD, greater functional connectivity was detected to Other compared to Self in bilateral fusiform, left middle frontal gyrus, and right frontal medial orbitofrontal gyrus, among other regions.
3.1.3.2. Between-group functional connectivity.
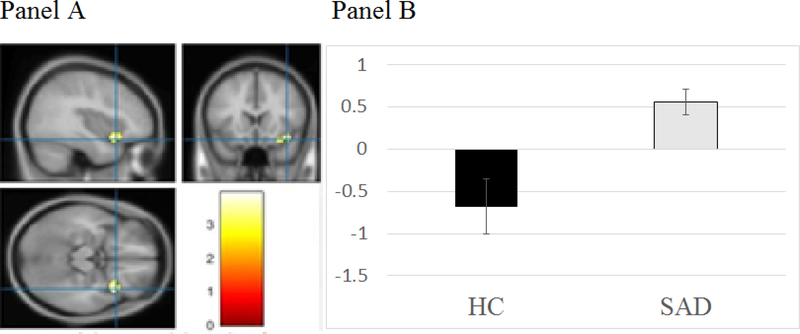
A right amygdala seeded PPI analysis found that, compared to HC, SAD participants showed greater functional connectivity for Self > Other in the right insula (see Table 2 and Figure 2a-b). There was greater functional connectivity for Self > Other between the insula and amygdala in SAD compared to HC.
Figure 2.
Pre-treatment functional connectivity with the right amygdala as a seed region for the socially anxious (SAD) and healthy control (HC) groups
Note: Panel A depicts the functional connectivity between right amygdala and right insula differences for SAD vs. HC. Panel B depicts the right amygdala and right insula connectivity strength for Self – Other for SAD and HC.
3.2. Pre- to post-treatment
3.2.1. Hypothesis 4. Differences from pre- to post-treatment.
3.2.1.1. Treatment vs. waitlist.
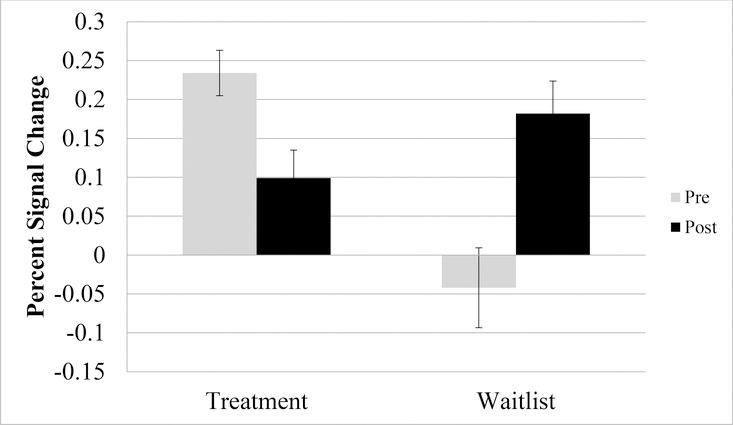
A two-sample t-test comparing Treatment versus Waitlist from pre- to post-treatment on differences in Self > Other resulted in 6 significant clusters of activation, including in the left insula, anterior cingulate cortex, and parietal/occipital regions. Across all regions, the Treatment group demonstrated greater differential activation to Self > Other Contrast at pre-treatment compared to post-treatment (this pattern was reversed for Waitlist; see Table 3 and Figure 3, as an example region in the left insula). Visual inspection of the direction of these effects demonstrated that activation to Self and Other decreased from pre- to post-treatment in the Treatment group, whereas activation to Self and Other increased from pre- to post-treatment in the Waitlist group.
Table 3.
Whole-brain effects by group using a p-value of .005 combined with an extent threshold of 40 contiguous voxels at from pre- to post-treatment
| x | y | z | Peak intensity | # voxels | |
|---|---|---|---|---|---|
| Treatment vs. Waitlist | |||||
| Self > Other | |||||
| Left Insula | −42 | 11 | 10 | 4.52 | 189 |
| Anterior Cingulate Cortex | −3 | 35 | 37 | 3.69 | 90 |
| Left Inferior Parietal | −36 | −49 | 37 | 4.14 | 83 |
| Right Inferior Parietal | 33 | −52 | 49 | 3.77 | 56 |
| Right Middle Frontal | 45 | −1 | 43 | 3.83 | 48 |
| Visual Cortex | 15 | −46 | 52 | 3.32 | 46 |
| Other > Self | |||||
| No suprathreshold clusters | |||||
| CBT vs. ACT | |||||
| Putamen | 27 | −13 | −5 | 4.24 | 63 |
| PPI: Right Amygdala Seed, SAD Only (Pre vs. Post) | |||||
| Medial prefrontal cortex | −3 | 50 | −14 | 4.86 | 74 |
| PPI: Right amygdala seed, LSAS Change | |||||
| FFA | 45 | −37 | −14 | 4.50 | 44 |
| Cingulate | −15 | 8 | 43 | 5.00 | 75 |
Figure 3.
Pre- to post-treatment left insula activation during self- versus other-referential processing in the treatment and waitlist groups
3.2.1.2. CBT vs. ACT.
A two-sample t-test comparing CBT vs. ACT revealed only one significant region of differential activation in the putamen for Self > Other. This difference was driven by stronger activation in the putamen Self > Other in ACT relative to CBT.
3.2.2. Hypothesis 5: Functional connectivity
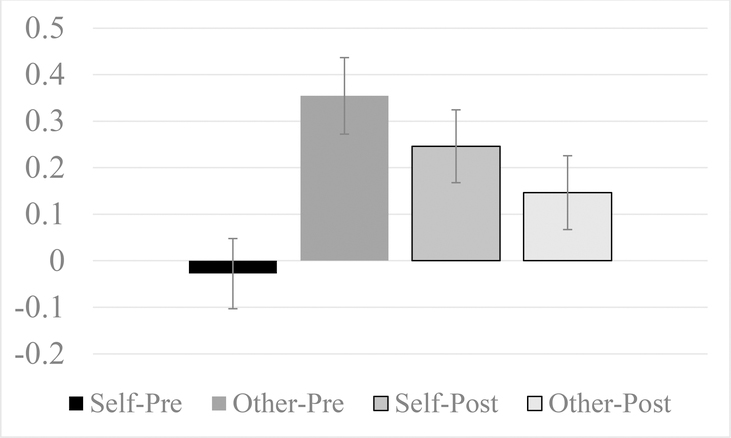
Across all groups at post-treatment, there was less of a difference in functional connectivity between the right amygdala and vmPFC for Self > Other relative to the difference at pre-treatment, where connectivity was stronger during Other compared to Self (see Figure 4). When only the treatment group was analyzed, this effect was only marginally significant, possibly due to power.
Figure 4.
Right amygdala to ventromedial prefrontal cortex (VMPFC) connectivity
3.2.3. Hypothesis 6: Functional connectivity by LSAS reduction.
LSAS reduction was negatively correlated with change in functional connectivity between the right amygdala and right FFA. Specifically, those with greater reduction in LSAS demonstrated stronger connectivity during Self and weaker connectivity during Other at post-treatment, whereas the strength of connectivity during Self and Other was comparable at post-treatment for those with less LSAS change.
4. Discussion
Compared to healthy controls, individuals with social anxiety disorder demonstrated greater pre-treatment activation in the fusiform face area, a region implicated in self-referential processing (Kanwisher et al., 1997), during self-observation compared to other-observation. This finding is consistent with our first hypothesis, and with prior research in which emotionally evocative stimuli were associated with enhanced activation in the fusiform face area in individuals with social anxiety disorder (Frick et al., 2013). The current study extends this work by demonstrating altered fusiform face activation during self-versus other-referential processing.
Furthermore, greater severity of social anxiety at baseline was associated with greater activation the fusiform face area during self-referential processing compared to other-referential processing. This finding is consistent with prior research in which differential activation of the fusiform face area was observed during self-observation of a working memory task based on severity of social anxiety symptoms (Pujol et al., 2013). Similar findings were observed when social anxiety severity was regressed on activation in the extrastriate body area, a region implicated in self-referential processing of body stimuli in clinical samples (Kret et al., 2011). Therefore, the second hypothesis was also confirmed.
Altered functional connectivity between the amygdala and the fusiform face area at pre-treatment was also observed in individuals with social anxiety disorder. In other words, the correlation in activation between the amygdala and the fusiform face area was more positive in individuals with social anxiety disorder during other-referential processing compared to self-referential processing. At post-treatment, those who experienced a greater reduction in social anxiety severity had a correction in this pattern of neural activation. Specifically, greater improvement in social anxiety severity from pre- to post-treatment was associated with more positive functional connectivity between the amygdala and fusiform face area in self-referential processing versus other-referential processing compared to individuals who experienced less change in social anxiety severity. This suggests that improvements in social anxiety symptoms may be associated with a correction of dysfunctional connectivity, from negative connectivity at baseline to positive connectivity at post-treatment, between the amygdala and fusiform face area during self-referential processing. One possibility is that at baseline, visual processing is more independent of amygdala activation, such that rather than reacting to the visual stimulus, participants with social anxiety detect threat and react strongly. Perhaps with a reduction in social anxiety severity, participants demonstrate greater stimulus-driven reactivity, reflected in stronger connectivity between affective circuitry (amygdala) and visual processing regions (FFA).
At pre-treatment, the social anxiety group had more positive functional connectivity between the amygdala and the insula during self-referential processing versus other-referential processing compared to healthy controls. The insula is involved interoception and internal awareness of the body (Paulus and Stein, 2006). Heightened awareness of bodily cues and sensations is both common in individuals predisposed toward anxiety, and acts as a trigger for anxious responding, which is associated with heightened amygdala activation (Paulus and Stein, 2006). In the group that received treatment, the difference in insula activation during self- vs other-referential processing decreased from pre- to post-treatment. The opposite finding emerged for the waitlist group. This finding suggests the possibility that treatment may have led to reduced interoceptive sensitivity, which was reflected in less activation of the insula during observation of an aversive stimulus. Alternatively, the self-referential stimulus may have been less distressing for those who received treatment, and perhaps more distressing for those on the waitlist. In this scenario, self-referential processing may have elicited a stronger bodily reaction in the waitlist group, which was reflected in greater insula activation during self versus other.
There were no major differences in neural activation during self-referential processing between the group randomized to CBT versus ACT. The only difference that emerged was in activation of the putamen, which was greater for self-referential processing relative to other-referential processing at post-treatment for those in ACT relative to CBT. Overactivation of the putamen is associated with anxiety-related distress (Carlisi et al., 2017), though other studies have reported opposite effects (Etkin and Wager, 2007). For these reasons, it is difficult to interpret this finding, especially in light of the comparable treatment response between CBT and ACT on self-report and clinician-rated anxiety in the parent trial (Craske et al., 2014). However, the putamen should be explored as an area for future investigation in the context of self-referential processing in social anxiety disorder.
Contrary to expectations, pre-treatment alterations in prefrontal cortex (PFC) and amygdala activation were not observed. This was somewhat surprising given that the amygdala and PFC regions are implicated in threat encoding, threat detection and fear expression across species and tasks (LeDoux, 2014). One possibility is that the task may have evoked a negative self-comparison response rather than a fear response. Unfortunately, we did not assess self-report ratings of negative social comparisons in response to the task. However, at post-treatment across all participants (treatment and waitlist), there was enhanced positive connectivity between the right amygdala and the ventromedial prefrontal cortex during self- relative to other-referential processing. A robust body of literature suggests that positive connectivity between the amygdala and prefrontal cortex is important for emotion regulation (Brühl et al., 2014; Etkin and Wager, 2007). However, when the analyses were reduced to include only the treatment condition, this effect was only marginally significant, potentially due to a smaller sample size. Therefore, this finding should be interpreted with caution.
Several key limitations of the current study are noteworthy. The first is the small sample size for the healthy control group, due to the focus in the larger study on social anxiety disorder. Second, there were only two opportunities for self-observation as well as for other-observation, precluding an opportunity to determine habituation or other time-course effects over multiple stimulus presentations. Third, the other-observation including the presentation of a well-known national news anchor, which might have resulted in differential patterns of activation compared to the observation of a novel stimulus. However, given that self-referential processing was the key construct under scrutiny in this study, familiarity with the other stimuli does not appear to be a critical confounding variable. In fact, a familiar news anchor was included to control for effects of differential familiarity as novel faces may elicit greater amygdala activity. Finally, as participants did not provide any behavioral responses during the task, we are unable to confirm that participants were focused on the task.
In conclusion, this is the first study to demonstrate differential neural activation during self-referential processing in social anxiety disorder compared to healthy controls using a video observation of a speech performance, a task common in the treatment of social anxiety disorder. Key areas of differences during self-referential processing included visual processing regions including the fusiform face area and extrastriate body area, and insula. The amygdala demonstrated differences in functional connectivity to these and other regions based on social anxiety severity, with more positive functional connectivity between the amygdala and insula during self-referential processing for those with social anxiety disorder. In individuals who received treatment for social anxiety, improvements in sensory processing regions were observed. Finally, greater improvement in social anxiety from pre- to post-treatment was associated with more positive functional connectivity between the amygdala and fusiform face area during self-referential processing at post-treatment. These findings suggest that self-referential processing is disrupted in social anxiety disorder, which may play a key role in maintaining anxiety and related impairments, and that corrections in social anxiety severity are reflected in neural changes from pre- to post-treatment in the activation and connectivity with sensory processing regions during self-referential processing.
Supplementary Material
Highlights.
Alterations in facial (FFA) processing brain regions correlated with social anxiety (SAD)
Change in connectivity between the FFA and amygdala were observed at post-treatment
Improvements in SAD severity correlated with change in FFA-amygdala connectivity at post-treatment
Acknowledgments
Funding Information: This project was funded by the National Institutes of Mental Health 1 R21 MH081299 (PIs: Craske, Lieberman and Taylor). The sponsor was not involved in the interpretation or reporting of study results.
Footnotes
Conflicts of Interest: Drs. Craske, Brown, Lieberman, Young and Goldin reported receiving funding from the National Institute of Health. Dr. Brown reported receiving funding from the Department of Defense.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham A, Kaufmann C, Redlich R, Hermann A, Stark R, Stevens S, et al. , (2013). Self-referential and anxiety-relevant information processing in subclinical social anxiety: an fMRI study. Brain Imaging Behav, 7, 35–48, DOI: 10.1007/s11682-012-9188-x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders (5th ed.). American Psychiatric Publishing, Arlington, VA. [Google Scholar]
- Blair K, Geraci M, Devido J, McCaffrey D, Chen G, Vythilingam M, et al. , (2008). Neural response to self- and other referential praise and criticism in generalized social phobia. JAMA Psychiatry. 65, 1178–1184. doi: 10.1001/archpsyc.65.10.1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl AB, Delsignore A, Komossa K, Weidt S, 2014. Neuroimaging in social anxiety disorder – A meta-analytic review resulting in a new neurofunctional model. Neurosci. Biobehav. Rev, 47, 260–280. doi: 10.1016/j.neubiorev.2014.08.003 [DOI] [PubMed] [Google Scholar]
- Burklund LJ, Craske MG, Taylor SE, Lieberman MD, 2015. Altered emotion regulation capacity in social phobia as a function of comorbidity. Soc Cogn Affect Neurosci, 10:199–208. doi: 10.1093/scan/nsu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisi CO, Norman LJ, Lukito SS, Radua J, Mataix-Cols D, Rubia K, 2017. Comparative multimodal meta-analysis of structural and functional brain abnormalities in autism spectrum disorder and obsessive-compulsive disorder. Biol Psychiatry, 82(2), 83–102. doi: 10.1016/j.biopsych.2016.10.006 [DOI] [PubMed] [Google Scholar]
- Craske MG, Niles AN, Burklund LJ, Wolitzky-Taylor KB, Vilardaga JCP, Arch J et al. , 2014. Randomized controlled trial of cognitive behavioral therapy and acceptance and commitment therapy for social phobia: Outcomes and moderators. J Consult Clin Psychol, 82(6), 1034–1048. doi: 10.1037/a0037212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nardo PA, Brown TA, Barlow DH, 1994. Anxiety Disorders Interview Schedule for DSM-IV: Lifetime Version. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Etkin A, Wager TD, 2007. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The Am J Psychiatry, 164, 1476–1488. doi: 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC, 1995. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine, 33, 636–647. 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fresco DM, Coles ME, Heimberg RG, Liebowitz MR, Hami S, Stein MB, et al. , 2001. The Liebowitz Social Anxiety Scale: A comparison of the psychometric properties of self-report and clinician-administered formats. Psychol Med, 31, 1025–1035. 10.1017/S0033291701004056 [DOI] [PubMed] [Google Scholar]
- Frick A, Howner K, Fischer H, Kristiansson M, Furmark T, 2013. Altered fusiform connectivity during processing of fearful faces in social anxiety disorder. Transl Psychiatry, 3, e312. doi: 10.1038/tp.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ, 2013. Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA psychiatry, 70:1048–1056. doi: 10.1001/jamapsychiatry.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Weeks J, Heimberg RG, Gross JJ, 2014. Impact of cognitive-behavioral therapy for social anxiety disorder on the neural bases of emotional reactivity to and regulation of social evaluation. Behav res ther, 62:97–106. doi: 10.1016/j.brat.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Clark DM, Ehlers A, Rapee RM, 2000. Social anxiety and self-impression: Cognitive preparation enhances the beneficial effects of video feedback following a stressful social task. Behav Res Ther, 38, 1183–1192. 10.1016/S0005-7967(99)00148-5. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM, 1997. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci, 17, 4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kret ME, Denollet J, Grèzes J, de Gelder B, 2011. The role of negative affectivity and social inhibitionin perceiving social threat: An fMRI study. Neuropsychologia, 49, 1187–1193. doi: 10.1016/j.neuropsychologia.2011.02.007 [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, 1987. Social phobia. Modern problems in pharmacopsychiatry, 22, 141–173. [DOI] [PubMed] [Google Scholar]
- Månsson KN, Carlbring P, Frick A, Engman J, Olsson C-J, Bodlund O, Furmark T, Andersson G, 2013. Altered neural correlates of affective processing after internet-delivered cognitive behavior therapy for social anxiety disorder. Psychiatry Res: Neuroimaging, 214:229–237. doi: 10.1016/j.pscychresns.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Marek R, Sah P, 2018. Neural Circuits Mediating Fear Learning and Extinction. Adv Neurobiol, 21, 35–48. doi: 10.1007/978-3-319-94593-4_2. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, De Greck M, Bermpohl F, Dobrowolny H, Panksepp J, 2006. Self-referential processing in our brain-a meta-analysis of imaging studies on the self. Neuroimage, 31, 440–457. DOI: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D, 2010. Plastic synaptic networks of the amygdala for the acquisition, expression and extinction of conditioned fear. Physiol Rev, 90, 419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek SM, Wathne K, Tierney NG, Thomson JW, 2008. Neural correlates of self-face recognition: an effect-location meta-analysis. Brain research, 1232, 173–184. 10.1016/j.brainres.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Pujol J, Giménez M, Ortiz H, Sorian-Mas C, López-Solà M, Farré M, et al. , 2013. Neural response to the observable self in social anxiety disorder. Psychol Med, 43, 721–731. doi: 10.1017/S0033291712001857 [DOI] [PubMed] [Google Scholar]
- Rapee RM, Heimberg RG, 1997. A cognitive-behavioral model of anxiety in social phobia. Behav Res Ther, 35, 741–756. 10.1016/S0005-7967(97)00022-3 [DOI] [PubMed] [Google Scholar]
- Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R, 2005. Cortical mechanisms of visual self-recognition. Neuroimage, 24, 143–149. 10.1016/j.neuroimage.2004.07.063 [DOI] [PubMed] [Google Scholar]
- Vocks S, Busch M, Grönemeyer D, Schulte D, Herpertz S, Suchan B, 2010. Differential neuronal responses to the self and others in the extrastriate body area and the fusiform body area. CABN, 10, 422–429. [DOI] [PubMed] [Google Scholar]
- Vocks S, Schulte D, Busch M, Grönemeyer D, Herpertz S, Suchan B, 2011. Changes in neuronal correlates of body image processing by means of cognitive-behavioural body image therapy for eating disorders: A randomized controlled fMRI study. Psychol Med, 41, 1651–1663. doi: 10.1017/S0033291710002382. [DOI] [PubMed] [Google Scholar]
- Yoon H, Kim. J, Shin Y, Choi S, Lee S, Kim J (2016). Neural activity during self-referential working memory and the underlying role of the amygdala in social anxiety disorder. Neuroscience Letters, 627, 139–147. doi: 10.1016/j.neulet.2016.05.068. [DOI] [PubMed] [Google Scholar]
- Young KS, Burklund LJ, Torre J, Saxbe D, Lieberman MD, Craske MG, 2017. Treatment for social anxiety disorder alters functional connectivity in emotion regulation neural circuitry. Psychiatry Res, 261, 44–51. doi: 10.1016/j.pscychresns.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.