Abstract
A five-microRNA signature for HPV negative head and neck squamous cell carcinoma (HNSCC) identifies patients at risk for inferior outcomes. Combining established clinical variables with this novel microRNA signature affords an opportunity to personalize and intensify treatment strategies in this high-risk patient population.
In this issue of Clinical Cancer Research, Hess and colleagues (1) report on a prognostication strategy for patients with HPV-negative head and neck squamous cell carcinoma (HNSCC), where a five-miRNA signature was associated with disease recurrence and inferior survival. In this study, pre-treatment specimens were obtained from patients with HPV negative HNSCC treated with surgery and post-operative radiation therapy (+/− cytotoxic chemotherapy). In an initial training set of 162 tumors, slightly over 1,000 miRNAs were identified and examined for association with recurrence following treatment, leading to the identification of an optimized 5 miRNA expression signature (hsa-let-7g-3p, hsa-miR-6508–5p, hsa-miR-210–5p, hsa-miR-4306 and hsa-miR-7161–3p) associated with recurrence. This 5-miRNA signature was then refined in a second cohort of patients using a threshold defined from the training cohort. In both cohorts of patients, the signature could be used to define high and low-risk groups, with dramatically different rates of recurrence, disease-specific survival, and most importantly overall survival. Specifically, in both patient cohorts, the low risk group demonstrated a survival of approximately 70% while that in the high-risk group was 30%. Combining this signature with clinical parameters including tumor stage, nodal stage and extranodal extension facilitated the stratification of patients into four risk groups based upon freedom from recurrence. Although this latter analysis is limited by numbers per risk group, the separation between groups is quite impressive.
MicroRNAs are small noncoding RNAs of approximately 22 nucleotides that are generated in the nucleus and transported into the cytoplasm for processing. A series of processing steps generates a sequence capable of negatively regulating gene expression and disrupting oncologic signaling pathways. Since their initial discovery, a variety of miRNAs have been linked to tumorigenesis and outcome in multiple disease sites. Indeed, as pointed out by the authors, two of the miRNAs identified in the signature (hsa-mir-210–5p and hsa-let-7g-3p), have been previously linked to outcome in HNSCC (2,3). However, despite a wealth of literature examining miRNAs associated with outcome in a variety of different malignancies there are no miRNAs currently used for prognostic of predictive purposes in clinical practice.
At present the clinical and translational landscape of HNSCC is dominated by the HPV positive versus negative distinction. Indeed, these two types of HNSCC can really be thought of as two distinct malignancies. HPV-positive HNSCC presents in younger patients, has an indirect association with smoking and alcohol and a better prognosis. Conversely, HPV negative HNSCC presents in older patients, is usually directly linked to smoking and alcohol exposure and has comparatively worse outcomes. Based upon the increasing incidence of HPV positive HNSCC (specifically in the oropharynx), there has been a seismic shift in the study of HNSCC toward identifying the mechanisms of this virally mediated disease and away from the more classic form of HNSCC. While the responsiveness of the community to the HPV epidemic is laudatory, the fact remains that the outcomes for locally advanced HPV negative HNSCC (the kind of patient represented in the current study) remain far, far poorer than their HPV positive counterparts. Indeed, patients with HPV positive HNSCC can generally expect a survival rate of approximately 85%, compared to 50%−60% in a similarly staged HPV negative patient (4). Moreover, in HPV-negative patients, we lack validated biomarkers to either identify groups of patients who will do worse with the current standard of care or identify targetable drivers of resistance to current therapy
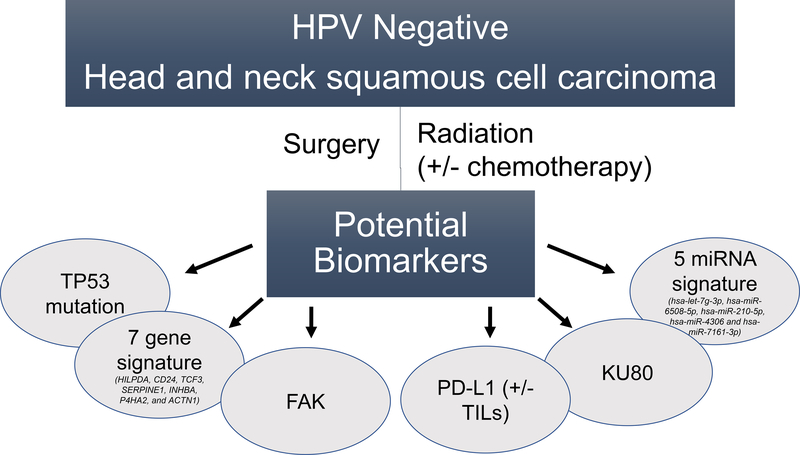
Thus, the current study is a welcome addition to several previously identified biomarkers in HPV negative HNSCC (Fig. 1). These biomarkers have been identified in the context of HPV negative HNSCC treated with radiation, usually following surgery and sometimes with concurrent chemotherapy. As HPV-negative HNSCC leads to death largely via local and regional recurrence of disease, these biomarkers have, by and large, been associated specifically with resistance to radiation. Indeed, several of these biomarkers have been directly targeted to increase sensitivity to radiation in pre-clinical models and may provide mechanistic insight to pathways of innate radioresistance in HPV negative HNSCC (5,6). In examining the current 5-miRNA signature a broad variety of pathways may be affected, including several important for response to radiation such as DNA damage repair, apoptosis, hypoxia response, and even immune signaling (Hess et al., Supplementary Figure 12). However, one of the challenges of identifying pathways associated with any miRNA is the sheer number of canonical pathways modulated by each individual miRNA, let alone a signature of five. Moreover, the 5-miRNA signature in the current study appears to be more associated with distant than loco-regional failure (Hess et al., Supplementary Figure 4 A & B). Thus, the biological effect associated with one or several miRNAs in the signature may be related more to invasion and motility than intrinsic resistance to radiation. Additional pre-clinical studies are necessary to integrate this clinical finding into a framework of pathways that may be driving each type of recurrence (distant and loco-regional) as opposed to those just along for the ride.
Figure 1.
Graphical representation of biomarkers of outcome and potential radioresistance in HPV-negative head and neck cancer (HNSCC).
Perhaps the more important question, however, is how best to move forward clinically with this signature as well as other biomarkers of outcome in HPV-negative HNSCC. The answer to this question is far from clear. Quite rightly the authors point out that prospective validation is necessary. However, this is often easier said than done. Proceeding from an interesting novel prognostic or predictive biomarker to broad clinical applicability is exceedingly challenging. This process presents multiple hurdles, not the least of which are prospective validation in a uniformly treated group of patients, a highly reproducible assay, ability to perform the assay beyond a centralized laboratory, and the logistics of integrating a given assay into routine clinical use (7). Although these difficulties are certainly surmountable, they require both patience as well as collaboration between multiple groups of stakeholders to reach fruition. Means of streamlining and consolidating this process must be identified, if novel biomarkers such as those identified here and others, are to be fully utilized to improve patient care.
Acknowledgments:
H.D. Skinner is supported by the National Cancer Institute of the National Institutes of Health (R01CA16848507).
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Hess J, Unger K, Maihoefer C, Schüttrumpf L, Wintergerst L, Heider T, et al. A Five-MicroRNA Signature Predicts Survival and Disease Control of Patients with Head and Neck Cancer Negative for HPV-infection. 2018. doi 10.1158/1078-0432.CCR-18-0776 J Clinical Cancer Research. [DOI] [PubMed] [Google Scholar]
- 2.Peng SC, Liao CT, Peng CH, Cheng AJ, Chen SJ, Huang CG, et al. MicroRNAs MiR-218, MiR-125b, and Let-7g predict prognosis in patients with oral cavity squamous cell carcinoma. PLoS One 2014;9(7):e102403 doi 10.1371/journal.pone.0102403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gee HE, Camps C, Buffa FM, Patiar S, Winter SC, Betts G, et al. hsa-mir-210 is a marker of tumor hypoxia and a prognostic factor in head and neck cancer. Cancer 2010;116(9):2148–58 doi 10.1002/cncr.25009. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363(1):24–35 doi 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skinner HD, Giri U, Yang L, Woo SH, Story MD, Pickering CR, et al. Proteomic Profiling Identifies PTK2/FAK as a Driver of Radioresistance in HPV-negative Head and Neck Cancer. Clinical Cancer Research 2016;22(18):4643–50 doi 10.1158/1078-0432.Ccr-15-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skinner HD, Giri U, Yang LP, Kumar M, Liu Y, Story MD, et al. Integrative Analysis Identifies a Novel AXL-PI3 Kinase-PD-L1 Signaling Axis Associated with Radiation Resistance in Head and Neck Cancer. Clinical Cancer Research 2017;23(11):2713–22 doi 10.1158/1078-0432.Ccr-16-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall JA, Salgado R, Lively T, Sweep F, Schuh A. A risk-management approach for effective integration of biomarkers in clinical trials: perspectives of an NCI, NCRI, and EORTC working group. Lancet Oncol 2014;15(4):e184–93 doi 10.1016/S1470-2045(13)70607-7. [DOI] [PubMed] [Google Scholar]