Abstract
Importance
Ibrutinib (Imbruvica®), a Bruton tyrosine kinase (BTK) inhibitor, is a new targeted agent approved by the US Food and Drug Administration for the treatment of chronic lymphocytic leukemia (CLL), mantle cell lymphoma, and Waldenström macroglobulinemia. Ibrutinib is overall well-tolerated but requires long-term treatment until disease progression or intolerable toxicity occurs. Little is known regarding its cutaneous adverse effects.
Objective
To describe the hair and nail manifestations associated with the long-term use of ibrutinib for the treatment of CLL.
Design
Prospective study of patients enrolled in a single-arm phase II clinical trial of ibrutinib for CLL between March 2014 and October 2015
Setting
A single institution study at the National Institutes of Health.
Participants
The study evaluated 66 patients with CLL.
Main outcomes and measures
The primary outcome, nail and hair changes associated with ibrutinib therapy, was assessed by an 11question survey. In addition, the severity of nail changes was determined from a 0–3 rating scale for both onychoschizia and onychorrhexis.
Results
Among 66 patients, 44 (66.6%) reported brittle fingernails at a median of 6.5 months after starting ibrutinib (95% CI 6–12 months). 15 patients (22.7%) developed brittle toenails after a median of 9 months of ibrutinib therapy (95% CI 6–15). Textural hair changes were reported in 17 patients (25.7%), at a median of 9 months of ibrutinib treatment (95% CI 6–12).
Conclusion and relevance
Hair and nail abnormalities are commonly associated with ibrutinib and appear several months after initiating therapy. Ibrutinib inhibits BTK by covalently binding to cysteine 481.Whether ibrutinib affects the hair and nails by binding and altering cysteine rich proteins of hair and nails or due to other off-target remains unknown.
INTRODUCTION
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib is an orally bioavailable molecule that was FDA approved for mantle cell lymphoma (MCL) in 2013 (560mg once daily) and for chronic lymphocytic leukemia (CLL) in 2014 (420mg once daily)1–4. Ibrutinib inhibits BTK, a key kinase for B-cell signaling, by covalently binding to Cys481, resulting in decreased proliferation and survival of malignant B cells.5 A limited number of kinases possess a homologous cysteine residue, making ibrutinib highly selective for BTK.6 Adverse events include fatigue, diarrhea, upper respiratory tract infections, skin rash7, and ecchymosis.2–4 Ibrutinib is administered until disease progression or intolerable/severe toxicity occurs; however, as with other tyrosine kinase inhibitors, cutaneous toxicity represents a potential barrier to long-term tolerability.8 Ibrutinib is generally well tolerated but we noted that patients frequently described nail and hair changes with long-term use.9 In this paper, we provide specific details on hair and nail manifestations and introduce a potential therapeutic intervention.
METHODS
86 CLL patients enrolled to treatment with ibrutinib at the NIH (clinicaltrials.gov NCT01500733). Two patients died and two patients withdrew from the study prior to data collection. 82 patients remained of which 66 patients were available for detailed review of hair and nail abnormalities (March 2014-October 2015). 15 patients withdrew from the study and in 1 patient the survey was not completed.
Study results, in part, have been reported previously.9 A survey was completed by a member of the study team at the time of each follow-up visit. The survey was composed of 11 questions: 1) time of onset of nail changes after ibrutinib treatment, 2) onychoschizia and onychorrhexis of fingernails, 3) severity of fingernail changes, 4) brittle toenails, 5) hair changes, 6) other skin or mucosal involvement, 7) pain, 8) bleeding, 9) treatment of hair and nail changes, 10) progression of hair and nails abnormalities over the course of therapy with ibrutinib and 11) impact on quality of life. The degree of severity was based on the grading score by Van de Kerkhof et al.10 Onychoschizia was defined as lamellar splitting and graded from 0–3: 0-none, 1-mild: splitting not involving the whole free edge of the nail plate, 2-moderate: splitting involving the whole free edge of the nail plate, 3-severe: splitting involving the whole free edge of the nail plate and covering 1/3 of the nail plate. Onychorrhexis was defined as longitudinal ridging and also graded from 0–3: 0-none, 1-mild: few superficial longitudinal ridges and grooves, 2-moderate: few deep ridges and grooves and 3-severe: more than 70% of the nail plate showing deep ridges and corresponding grooves. All patients provided informed consent.
RESULTS
The age range of the 66 study participants was 55 to 85 years old, with 43 males and 23 females. 44/66 (67%) participants described new onset fingernail changes after a median time of 6.5 months (95% CI 6–12 months) .15/66 (22.7%) developed brittle toenails after a median of 9 months (95% CI 6–15 months) of ibrutinib therapy (Table 1). Nail changes manifested as mild to moderate onychoschizia and onychorrhexis, corresponding to grade 1 and 2 CTCAE v3.0 adverse events, respectively (Figure 1, Figure 2).10 17/66 (25.7%) patients developed hair changes at a median of 9 months (95% CI 6–12 months) after starting ibrutinib. Most hair changes manifested as straightening and softening of the hair, although 4/17 patients reported increased curliness of hair. Among 5 patients who used oral biotin at 2.5 mg daily, 3 reported significant improvement in their nails. 55% of affected individuals reported a negative impact of hair and nail changes on their quality of life.
Table 1.
Summary of Results
| Outcomes | Number of patients (%) | Number of Males affected (%) |
Number of females affected (%) |
Severity | ||
|---|---|---|---|---|---|---|
|
Fingernail involvement |
44 (66.6) | 29 (67) | 15 (65) | Grade 1 (45%) Grade 2 (55%) |
||
| Toenail involvement | 15 (22.7) | 13 (30) | 2 (8) | Grade 1–2 | ||
|
Discomfort due to nail appearance and pain |
33 (55) | 17 (40) | 16 (70) | |||
| Hair changes | 17 (25.7) | 7 (16) | 10 (43) | Grade 1–2 | ||
Figure 1. Ibrutinib-associated onychorrhexis of the fingernails.
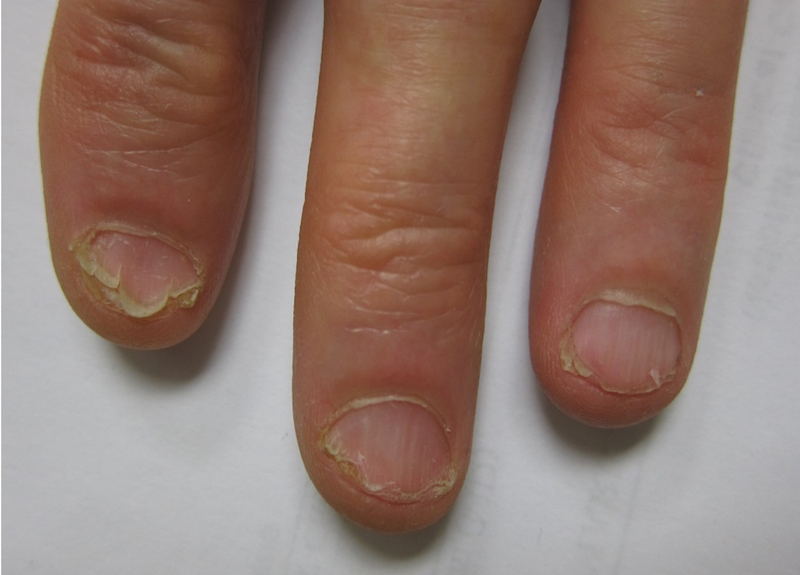
Moderate onychorrhexis of the fingernails in a CLL patient after 6 months of ibrutinib therapy.
Figure 2: Ibrutinib-associated onychoschizia of the toenails.
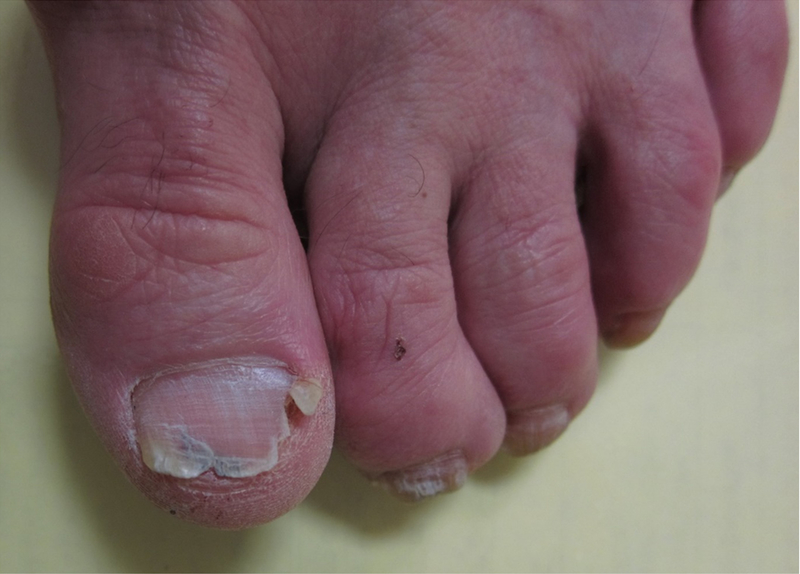
Onychoschizia of the toenails 11 months after starting ibrutinib.
DISCUSSION
Nail brittleness affects approximately 20% of the population, most frequently the elderly and women, and is associated with onychoschizia, onychorrhexis, or both.10 The pathogenesis of brittle nails is multifactorial, and may result from disruption of intercellular adhesion between corneocytes as well as factors affecting the nail matrix.10 Nail texture is influenced by corneocytes, keratin filaments, keratin associated fibrous proteins, water content, lipid bilayers, and the nail matrix.10,11 The integrity of keratin associated fibrous proteins is maintained by disulfide bonds between cysteine residues, resulting in the formation of the sulfur-containing amino acid cystine.11
Ibrutinib covalently binds to the cysteine residue at the active site of BTK.1 Since cystines are critical for nail hardness, ibrutinib-induced disruption of the disulfide bonds between cysteine residues could be responsible for increased nail brittleness. Fingernails require 3–6 months to complete a growth cycle. This time interval is consistent with the reported delay of 6.5 months between initiation of ibrutinib therapy and the appearance of nail changes. Toenail abnormalities were reported an average of 9 months after starting therapy, consistent with the slower growth rate of the toenail plate (12–18 months). We consider the nail abnormalities related to ibrutinib because these changes arose on ibrutinib and were not pre-existing, such changes are not part of disease-related complications or associated with standard chemotherapy, and because the timing of appearance of nail changes correlated well with the growth rate of the nail plates. Unfortunately, no pre-treatment photos were available for comparison at the time of the study. Keratinocyte associated proteins in the hair are also rich in sulfur-containing amino acids and form disulfide bonds that contribute to hair structure and tensile strength.12 Hair permanents that contain reducing agents straighten hair through disruption of disulfide bonds.12 Reduced disulfide bonds have free thiols that can migrate and form new disulfide bonds creating more curls as seen with permanent waving agents.12 Ibrutinib disruption of hair disulfide bonds may act in a similar manner to cause alterations in hair strength or texture.
Although ibrutinib demonstrates high selectivity for BTK (IC50= 0.5nM), it also inhibits other kinases, including human epidermal growth factor receptors 1 (EGFR, IC50=12nM) and 2 (HER2, IC50=22nM).6 Ibrutinib at 560 mg/day can reach a maximum concentration of > 100 ng/mL in the plasma13 which is equivalent to 227nM. At maximum concentration, ibrutinib may inhibit other target kinases, including the EGFR. EGFR inhibition by TKIs leads to well-established cutaneous adverse events, including acneiform rash, nail and hair changes8. However, acneiform rash, one of the most common manifestations of EGFR inhibitors, has not been described with ibrutinib.9
Concern over cosmetic appearance and nail discomfort may negatively impact quality of life in diseases affecting the nails.10 In our study, 55% of affected patients expressed concern related to nail appearance or pain. Treatment options for brittle nails include biotin supplementation and topical solutions such as hydrosoluble nail lacquer (Genadur) and polyureaurethane (Nuvail).
In one study of 32 patients with brittle nails, 25% improvement in nail thickness was noted following ingestion of 2.5 mg of biotin daily for 6–15 months.14 In another study of 44 patients with brittle nails, 63% reported improvement after 2 months of biotin, with re-emergence of brittle nails after discontinuing treatment.11 Biotin is a cofactor for several enzymes involved in fatty acid formation which may enhance the lipid content of the nail plate and improve keratinocyte binding.15 The benign nature of biotin and the absence of drug-drug interactions suggest that supplementation may be reasonable; however, further clinical study is needed to confirm efficacy in the setting of ibrutinib therapy.
Acknowledgment:
We are indebted to the patients who participated in this trial and their families; Pharmacyclics for providing ibrutinib, research support, and comments on the manuscript; and the Intramural Research Program of the NHLBI.
Funding/Support:
This study was supported in part by the Intramural Research Program of the NIH, the National, Heart, Lung and Blood Institute, and by Pharmacyclics that provided ibrutinib, research support, and comments on the manuscript.
Footnotes
Funding/Sponsor was involved?
Design and conduct of the study No
Collection, management, analysis and interpretation of data No
Preparation, review, or approval of the manuscript Yes
Decision to submit the manuscript for publication No
Financial Disclosure:
Dr. Wiestner received research funding from Pharmacyclics. Dr. Saba serves as consultant and a speaker for Pharmacyclics. All other authors declare no competing interests.
Role of Pharmacyclics: design and conduct of the study: No; collection, management, analysis, and interpretation of the data: No; preparation, review, or approval of the manuscript: Yes (review only); and decision to submit the manuscript for publication: No.
1. Employment: No
2. Consultancies: Yes (Saba)
3. Honoraria: Yes (Saba)
4. Speakers bureau: Yes (Saba)
5. Stock ownership or options: No
6. Expert testimony: No
7. Grants: Yes (Wiestner)
8. Patents filed, received, pending, or in preparation: No
9. Royalties: No
10. Donation of medical equipment: No
REFERENCES
- 1.Wiestner A. The role of B-cell receptor inhibitors in the treatment of patients with chronic lymphocytic leukemia. Haematologica 2015;100(12):1495–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013;369(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369(6):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N Engl J Med 2015;372(15):1430–1440. [DOI] [PubMed] [Google Scholar]
- 5.Herman SE, Mustafa RZ, Gyamfi JA, et al. Ibrutinib inhibits BCR and NF-kappaB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood 2014;123(21):3286–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger JA, Buggy JJ. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765). Leuk Lymphoma 2013;54(11):2385–2391. [DOI] [PubMed] [Google Scholar]
- 7.Mannis G, Wu D, Dea T, Mauro T, Hsu G. Ibrutinib rash in a patient with 17p del chronic lymphocytic leukemia. Am J Hematol 2015;90(2):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, Soria JC, Spatz A, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet. Oncol 2005;6(7):491–500. [DOI] [PubMed] [Google Scholar]
- 9.Farooqui MZ, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet. Oncol 2015;16(2):169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Kerkhof PC, Pasch MC, Scher RK, et al. Brittle nail syndrome: a pathogenesis-based approach with a proposed grading system. J Am Acad Dermatol 2005;53(4):644–651. [DOI] [PubMed] [Google Scholar]
- 11.Cashman MW, Sloan SB. Nutrition and nail disease. Clin Dermatol 2010;28(4):420–425. [DOI] [PubMed] [Google Scholar]
- 12.de Sa Dias TC, Baby AR, Kaneko TM, Robles Velasco MV. Relaxing/straightening of Afro-ethnic hair: historical overview. J Cosmet Dermatol 2007;6(1):2–5. [DOI] [PubMed] [Google Scholar]
- 13.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 2013;31(1):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombo VE, Gerber F, Bronhofer M, Floersheim GL. Treatment of brittle fingernails and onychoschizia with biotin: scanning electron microscopy. J Am Acad Dermatol 1990;23(6 Pt 1):1127–1132. [DOI] [PubMed] [Google Scholar]
- 15.Iorizzo M, Pazzaglia M, B MP, Tullo S, Tosti A. Brittle nails. J Cosmet Dermatol 2004;3(3):138–144. [DOI] [PubMed] [Google Scholar]


