Abstract
The high reactivity of bisphenol A (BPA) with disinfectant chlorine is evident in the instantaneous formation of chlorinated BPA derivatives (ClxBPA) in various environmental media that show increased estrogen-activity when compared with that of BPA. The documented health risks associated with BPA exposures have led to the gradual market entry of BPA structural analogs, such as bisphenol S (BPS), bisphenol F (BPF), bisphenol B (BPB), etc. A suite of exposure sources to ClxBPA and BPA analogs in the domestic environment is anticipated to drive the nature and range of halogenated BPA derivatives that can form when residual BPA comes in contact with disinfectant in tap water and/or consumer products. The primary objective of this review was to survey all available studies reporting biomonitoring protocols of ClxBPA and structural BPA analogs (BPS, BPF, BPB, etc.) in human matrices. Focus was paid on describing the analytical methodologies practiced for the analysis of ClxBPA and BPA analogs using hyphenated chromatography and mass spectrometry techniques, because current methodologies for human matrices are complex. During the last decade, an increasing number of ecotoxicological, cell-culture and animal-based and human studies dealing with ClxBPA exposure sources and routes of exposure, metabolism and toxicity have been published. Up to date findings indicated the association of ClxBPA with metabolic conditions, such as obesity, lipid accumulation, and type 2 diabetes mellitus, particularly in in-vitro and in-vivo studies. We critically discuss the limitations, research needs and future opportunities linked with the inclusion of ClxBPA and BPA analogs into exposure assessment protocols of relevant epidemiological studies.
Keywords: Biomonitoring, Bisphenol A, BPA analogs, BPA free, Analogs, Chlorinated derivatives, Disinfection, Emerging contaminants, Human exposure, Mass spectrometry, Metabolites
1. Introduction
Bisphenol A (BPA), 2,2-bis(4-hydroxyphenyl)propane, is a synthetic compound that is widely used as a monomer in polycarbonate plastics and epoxy resins, being one of the world’s highest production volume chemicals. Scientific reports linked BPA exposures to the development of obesity and type II diabetes mellitus (T2DM) in humans (Bodin et al., 2015; Chevalier and Fénichel, 2015; Lakind et al., 2014; Oppeneer and Robien, 2015; Rezg et al., 2014). Numerous studies reported the association between urine BPA levels and long-term metabolic disorders such as diabetes/impairment of glucose metabolism (Hong et al., 2009; Kim and Park, 2013; LaKind et al., 2012; Lang et al., 2008; Li et al., 2012; Melzer et al., 2010; Ning et al., 2011; Olsén et al., 2012; Shankar and Teppala, 2011; Silver et al., 2011; Takeuchi et al., 2004; Teppala et al., 2012; Wang et al., 2012a; Wang et al., 2012b) and obesity (Bloom et al., 2011; Carwile and Michels, 2011; Galloway et al., 2010; Kim et al., 2012; Ko et al., 2014; Lee et al., 2014; Melzer et al., 2012; Mok-Lin et al., 2010; Olsén et al., 2012; Shankar et al., 2012; Song et al., 2014a; Takeuchi and Tsutsumi, 2002; Takeuchi et al., 2004; Tarantino et al., 2013; Wang et al., 2012b; Yang et al., 2009; Zhao et al., 2012). The frequency of new incidences of the aforementioned metabolic diseases is expected to continue growing in the next couple of decades (Yach et al., 2006; Swinburn et al., 2011), suggesting the environment and lifestyle/behavior as major risk factors for metabolic diseases (Diamanti-Kandarakis et al., 2009; Jeon et al., 2015).
The BPA occurrence in the environment and consumer products is ubiquitous (Kang et al., 2006; Staples et al., 1998; Vandenberg et al., 2007; Vandenberg et al., 2010). Concerns over the aforementioned health outcomes associated with BPA exposures in human studies have resulted for the gradual market entry of BPA structural analogs in consumer products that are speculatively considered safer (BPA-free) than BPA, such as bisphenol S (BPS), bisphenol F (BPF), bisphenol B (BPB), bisphenol AF (BPAF), and observed entering environment and human systems (Liao et al., 2012a; Liao et al., 2012b; Liao et al., 2012c; Liao et al., 2012d). The high reactivity of BPA with disinfectant chlorine (in the forms of either hypochlorite or free chlorine radicals) is evident in the instantaneous formation of chlorinated BPA derivatives (ClxBPA) (Gallard et al., 2004; Liu et al., 2009; Yamamoto and Yasuhara, 2002). Similar reactivity to disinfectant chlorine is anticipated for structural BPA analogs but this remains to be experimentally documented. The formation kinetics and reactions of ClxBPA derivatives has been documented in laboratory experiments using chlorinated tap water and BPA (Gallard et al., 2004). Hypochlorite ions are often added to finished tap water as disinfectant and they are held responsible for the electrophilic attack of phenolic groups in BPA, acting as a precursor of ClxBPA formation (Gallard et al., 2004; Liu et al., 2009; Yamamoto and Yasuhara, 2002). The main ClxBPA derivatives reported so far in the literature are: mono-(ClBPA), di-(Cl2BPA), tri-(Cl3BPA) and tetrachlorobisphenol (Cl4BPA) (Lee et al., 2004; Rebenne et al., 1996). Available carbon atom positions for chlorination on the BPA molecule and resulting in the formation of respective ClxBPA, and the structural analogs of BPA are presented in Table 1.
Table 1.
Representative structure, common and systematic names, formulae, and molecular masses of bisphenol A and its chlorinated derivatives, along with structural BPA analogs, such as, bisphenol B, bisphenol F, bisphenol S, bisphenol AF, and bisphenol A diglycidyl ether.
| Common name | Systematic name | Abbreviation | Ri | R2 | R3 | R4 | Formula | Molecular mass | Representative structure |
|---|---|---|---|---|---|---|---|---|---|
| Bisphenol A | 2,2-Bis(4-hydroxyphenyl)propane | BPA | C15H16O2 | 228.29 | 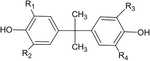 |
||||
| 3-Chlorobisphenol A | 2-Chloro-4-[l-(4-hydroxyphenyl)-l-methylethyl]phenol | ClBPA | Cl | C15H15ClO2 | 262.73 | ||||
| 3,5-Dichlorobisphenol A | 2,6-Dichloro-4-[l-(4-hydroxyphenyl)-l-methylethyl]phenol | Cl2BPA or 2,6-Cl2BPA | Cl | Cl | C15H14Cl2O2 | 297.18 | |||
| 3,3′-Dichlorobisphenol A | 2-Chloro-4-[l-(3-chloro-4-hydroxyphenyl)-l-methylethyl]phenol | 2,2-Cl2BPA | Cl | Cl | C15H14Cl2O2 | 297.18 | |||
| 3,3’,5-Trichlorobisphenol A | 2,6-Dichloro-4-[l-(3-chloro-4-hydroxyphenyl)-l-methylethyl]phenol | Cl3BPA | Cl | Cl | Cl | C15H13Cl3O2 | 331.62 | ||
| 3,5,3’,5’-Tetrachlorobisphenol A | 2,6-Dichloro-4-[l-(3,5-dichloro-4-hydroxyphenyl)-l-methylethyl]phenol | Cl4BPA | Cl | Cl | Cl | Cl | C15H12Cl4O2 | 366.07 | |
| Bisphenol B | 2,2-Bis(4-hydroxyphenyl)butane | BPB | C16H18O2 | 242.31 | 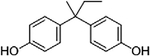 |
||||
| Bisphenol F | l,l-Bis(4-hydroxyphenyl)methane | BPF | C13H12O2 | 200.23 | |||||
| Bisphenol S | 4,4’-Sulfonyldiphenol | BPS | C12H10O4S | 250.27 | 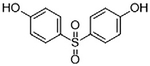 |
||||
| Bisphenol AF | 4-[l,l,l,3,3,3-Hexafluoro-2-(4-hydroxyphenyl)propan-2-yl]phenol | BPAF | C15H10F6O2 | 336.23 | 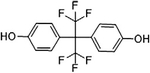 |
||||
| Bisphenol A diglycidyl ether | 2,2-Bis(4-glycidyloxyphenyl)propane | BADGE | C21H24O4 | 340.42 |
Occurrence of ClxBPA derivatives has been widely reported in a suite of water bodies bodies (Ballesteros et al., 2006; Bastos et al., 2008; Bourgin et al., 2013a; Bourgin et al., 2013b; Bulloch et al., 2015; Casatta et al., 2015; Chang et al., 2014; Chang et al., 2012; Dorival-Garcia et al., 2012a; Dorival-Garcia et al., 2012b; Dupuis et al., 2012; Fan et al., 2013; Fukazawa et al., 2001; Fukazawa et al., 2002; Gallard et al., 2004; Gallart-Ayala et al., 2007; Gallart-Ayala et al., 2010; Kosaka et al., 2012; Lane et al., 2015; Li et al., 2015; Ruan et al., 2015; Song et al., 2014b; Voordeckers et al., 2002; Yamamoto and Yasuhara, 2002; Yang et al., 2014a; Yang et al., 2014b; Yuan et al., 2011; Yuan et al., 2010; Zafra-Gómez et al., 2008; Zafra et al., 2003). In addition, BPA is frequently detected in thermal receipts (Fan et al., 2015; Hormann et al., 2014) and certain personal care- and household-cleaning products, such as, bar soaps, facial/body lotions, shampoo, dishwashing and laundry detergent, and toilet bowl cleaner (Dodson et al., 2012). Reported BPA levels in these consumer products ranged between <10 μg g−1 and up to ~100 μg g−1 (Dodson et al., 2012), while it was as high as 20 mg g−1 on thermal receipt paper (Hormann et al., 2014). Residual BPA in these products when come in contact with chlorine-containing water or household cleaning products may react to yield ClxBPA (unpublished experimental observations in our laboratory). Recycled plastic and paper raw materials often contain residual BPA that can react yield ClxBPA in a suite of personal care, and household cleaning products and food contact papers (Zhou et al., 2015). A suite of exposure sources to ClxBPA in the domestic environment is anticipated to drive the nature and range of halogenated derivatives that can form when residual BPA comes in contact with chlorine and other chemical constituents in household tap water and consumer products. This may lead to subsequent exposure to humans with unknown intensities, duration of exposures and possible health effects.
During the past decade, structural BPA analogs have been replacing BPA in numerous industrial, commercial and consumer products, such as, container linings (Oldring et al., 2006), infant food formulae (Cunha et al., 2011), polycarbonate food container linings (Fromme et al., 2002), thermal receipts (Becerra and Odermatt, 2012; Liao et al., 2012c), and canned and packaged food and beverages (Cacho et al., 2012; Grumetto et al., 2008; Liao and Kannan, 2013; Viñas et al., 2010). As a result, BPA structural analogs have been also detected in various environmental media, such as, indoor dust (Liao et al., 2012b; Wang et al., 2012c), food (Petersen et al., 2003), food contact recycled paper items (Perez-Palacios et al., 2012), water and sediment (Liao et al., 2012d), etc.
An increasing frequency of scientific reports are found in the literature dealing with the sources and routes of human exposure, biomonitoring, metabolism, and toxicity of ClxBPA and BPA structural analogs in ecotoxicological and animal studies, albeit less in humans. The occur-rence of BPA structural analogs in human matrices has been recently reported (Vela-Soria et al., 2014a; Vela-Soria et al., 2014b; Xue et al., 2015; Yang et al., 2014a; Zhou et al., 2014). Hence, it is a timely topic to summarize the current research status and discuss future opportunities in this review. The primary objective of this review was to survey all available studies reporting biomonitoring of ClxBPA and BPA structural analogs in human matrices. Focus was paid on describing the analytical methodologies practiced for the analysis of ClxBPA and BPA structural analogs using hyphenated chromatography and mass spectrometry techniques, because current methodologies for extraction and analysis in human matrices are often complex and time-consuming. A brief discussion was also provided on the human exposure sources and routes to ClxBPA, their metabolism and toxicity observed from in vitro and in vivo studies and human health effects, including current limitations and future research needs. In the following sub-sections, we review each one of these topics by gathering relevant reported studies in the literature.
2. Chlorinated derivatives and structural analogs of bisphenol A
2.1. Literature search
A comprehensive literature search in Scopus (1960 onwards) was performed in order to identify studies reporting biomonitoring of ClxBPA and BPA structural analogs in human matrices. Using multiple combinations of keywords (bisphenol* AND (chlorin* OR chlorinated OR chloro*) AND (derivative* OR analog* OR substitute*)) we performed the search on 25–26 May 2015 that resulted in 442 articles. PubMed and Web of Science search using the same keywords resulted in 58 and 272 articles, respectively; henceforth we used the results of the Scopus database. Further screening for studies of human relevance from the aforementioned search was achieved by using keywords “(urine OR blood OR plasma OR serum OR placenta OR hair OR cord OR milk OR adipose OR colostrum OR nail* OR tissue* OR fluid* OR human*)”. Resulting efforts narrowed the hits to 156 articles which were assessed for inclusion by reading either the abstract or full text or both. Eligible studies were screened to obtain relevant back referenced citations and concurrent citing articles for possible inclusion. Altogether, 14 and 9 relevant articles reporting ClxBPA and structural BPA analogs in human matrices, respectively, were selected for further reviewing. Studies reporting ClxBPA in human matrices ranged from analysis of (i) adipose tissue (Fernandez et al., 2007), (ii) placenta (Jimenez-Diaz et al., 2010; Vela-Soria et al., 2011; Vela-Soria et al., 2015), (iii) breast milk (Cariot et al., 2012; Rodriguez-Gomez et al., 2014a,b), (iv) urine (Kalyvas et al., 2014; Liao and Kannan, 2012; Vela-Soria et al., 2014b; Venisse et al., 2014; Yang et al., 2014a), (v) colostrum (Migeot et al., 2013), (vi) plasma (del Olmo et al., 2005) and (vii) serum (Liao and Kannan, 2012). Studies reporting structural BPA analogs in human biospecimen ranged from: (i) urine (Yang et al., 2014a,b; Zhou et al., 2014; Asimakopoulos et al., 2014; Xue et al., 2015; Vela-Soria et al., 2014a,b; Cunha and Fernandez, 2014; Liao et al., 2012a) and (ii) breast milk, (Deceuninck et al., 2015).
2.2. Sources and routes of exposure
The widespread occurrence of ClxBPA derivatives in a suite of environmental media has been already documented (Table 2), such as in, (i) wastewater (Ballesteros et al., 2006; Bulloch et al., 2015; Fukazawa et al., 2001; Fukazawa et al., 2002; Gallart-Ayala et al., 2007; Gallart-Ayala et al., 2010; Zafra-Gómez et al., 2008; Zafra et al., 2003), (ii) wastewater treatment plants (Bulloch et al., 2015; Dupuis et al., 2012; Gallart-Ayala et al., 2007; Gallart-Ayala et al., 2010), (iii) drinking water distribution pipes (Kosaka et al., 2012), (iv) finished and household tap water (Dupuis et al., 2012; Fan et al., 2013; Lane et al., 2015; Yang et al., 2014b), (v) sediment (Casatta et al., 2015; Chang et al., 2012; Chang et al., 2014; Voordeckers et al., 2002; Yuan et al., 2010; Yuan et al., 2011), (vi) sewage (Dorival-Garcia et al., 2012a,b; Ruan et al., 2015; Song et al., 2014b), (vii) bench-scale and simulated water treatment experiments in a laboratory set-up (Bastos et al., 2008; Bourgin et al., 2013a; Gallard et al., 2004; Gallart-Ayala et al., 2010; Kosaka et al., 2012; Li et al., 2015; Liu et al., 2009; Yamamoto and Yasuhara, 2002) and (viii) food contact paper (Zhou et al., 2015).
Table 2.
Highlights of analytical methods for the quantification of chlorinated derivatives of bisphenol A in environmental samples and non-human biological matrices.
| Table 2. Item # | BPA and its chlorinated derivatives | Matrix | Sample source and number | Sample extraction/ clean-up/preparation | Instrumental analysis |
Analytical column/mobile phase | LOD or MDL or LOQ | Recovery | Concentration | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BPA, ClBPA, 3,5-Cl2BPA, 3,3’-Cl2BPA, Cl3BPA, and Cl4BPA | Final effluent | Paper manufacturing plants; (n=8) |
LLE/dichloromethane/sylation with N,O-bis(trimethylsilyl) trifluoroacetamide | GC-MSD | HP-5 Trace Analysis capillary column with 5% diphenyl and 95% dimethyl arylene siloxane (30 m × 0.25 mm × 0.1 μm)/Helium (carrier gas) | Trace limits, tr (μg L−1): <0.2 (for all analytes) | n.a. | Range (μg L−1): BPA (8–370), ClBPA (<0.2–2.0), 3,5-Cl2BPA(< 0.2–1.0), 3,3’-Cl2BPA (<0.2–0.5), Cl3BPA (0.9–1.2), and Cl4BPA (1.3–1.4) | Fukazawa et al. (2001) |
| 2 | BPA, ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | Synthetic raw water | Beaker setup | SPE (polystyrene/divinylbenzene sorbent cartridge, 500 mg) | HPLC-MS (APCI, −ve mode) | Capacell Pak C18 UG120S3 silica packed LC column (150 mm × 4.6 mm × 3.0 μm)/mobile phase: acetonitrile/water (20:80, v/v) with 0.1% acetic acid. | n.a. | n.a. | n.a. | Hu et al. (2002) |
| 3 | BPA, ClBPA, 3,5-Cl2BPA, 3,3’-Cl2BPA, Cl3BPA, and Cl4BPA | Wastewater effluent | Paper recycling plants; (n = 20) |
LLE/dichloromethane/sylation with N,O-bis(trimethylsilyl) trifluoroacetamide | GC-MSD | HP-5 Trace Analysis capillary column (30 m × 0.25 mm × 0.1 μm)/Helium (carrier gas) | Trace limits, tr (μg L−1): <0.2 (for all analytes) | Range (%): BPA-d16 (88–94) | Range (μg L−1): BPA (0.2–370), ClBPA (<0.2–2.0), 3,5-Cl2BPA (<0.2–1.0), 3,3’-Cl2BPA (<0.2–0.5), Cl3BPA (0.9–1.2), and Cl4BPA (1.3–1.4) | Fukazawa et al. (2002) |
| 4 | BPA, ClBPA, Cl2BPA, and Cl4BPA | Sediment | Tidal strait (estuarine) | BPA and Cl4BPA: LLE/methanol ClBPA and Cl2BPA: acylation of the LLE extract/acetic anhydride | BPA and Cl4BPA: HPLC with UV detector/280 nm ClBPA and Cl2BPA: GC-MSD | BPA and Cl4BPA: Sphereclone 5
μm ODS (250 mm × 4.60 mm × 5 μm)/Mobile
phase: methanol: water: glacial acetic acid ClBPA and Cl2BPA: HP 5MS column cross-linked with 5% PH ME siloxane (30 m × 0.25 mm × 0.25 μm)/Helium (carrier gas) |
n.a. | n.a. | n.a. | Voordeckers et al. (2002) |
| 5 | BPA, ClBPA, 2,6-Cl2BPA, 2,2’-Cl2BPA, Cl3BPA, and Cl4BPA | Water | Beaker setup | LLE/dichloromethane | GC-MSD (electron impact ionization) | PTE-5 capillary column (30 m × 0.25 mm × 0.25 μm)/Helium (carrier gas) | Detection limits, DLs (μmol L−1): BPA (0.002), and Cl4BPA (0.005) | n.a. | n.a. | Yamamoto and Yasuhara (2002) |
| 6 | BPA, ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | Wastewater (urban) | Different places in WWTPs | LLE/dichloromethane: carbon tetrachloride (75:25, v/v)/sylation with N,O-bis(trimethylsilyl) trifluoroacetamide | GC-MSD | HP1-MS fused silica capillary column (30 m × 0.25 mm × 0.25 μm) (coated with methyl silicone gum phase) | Detection limits, DLs (ng L−1): BPA (0.3), ClBPA (0.6), Cl2BPA (2.1), Cl3BPA (4.7), and Cl4BPA (12.9) | n.a. | Range (ng mL−1): BPA (104.1–106.7), ClBPA (91.1–107.2), Cl2BPA (96.0–106.5), Cl3BPA (94.5–104.7), and Cl4BPA (93.4–101.7) | Zafra et al. (2003) |
| 7 | BPA, ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | Water | Beaker setup | LLE/fractionation on a HPLC (Hichrom Spherisorb S5ODS2, 250 mm × 4.6 mm)/methanol: water (60:40, v/v)/225 nm detection wavelength | GC-MSD | AT-SMS column (30 m × 0.25 mm × 0.25 μm) | Detection limits, DLs (μmol): BPA (2 × 10−5 μmol) | n.a. | n.a. | Gallard et al. (2004) |
| 8 | BPA, ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | Wastewater (urban) | WWTPs; (n = 6) |
SPE/diethyl ether: methanol (9:1, v/v) | GC-MSD (electron impact ionization) | ZB-5 MS Zebron (30 m × 0.25 mm × 0.25 μm)/Helium (carrier gas) | Detection capabilities, DCs (ng L−1): BPA (50), ClBPA (40), Cl2BPA (90), Cl3BPA (100), and Cl4BPA (80) | Range (%): BPA (95.2–105.0) | Range (ng mL−1): BPA (<DC), ClBPA (<DC), Cl2BPA (<DC), Cl3BPA (< DC), and Cl4BPA (<DC) | Ballesteros et al. (2006) |
| 9 | BPA, ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | Wastewater | Paper recycling plant; (n = 1) | SPE (Bond Elut C18, 500 mg)/methanol: water (20:80, v/v) | HPLC–MS/MS (ESI, − ve mode) | SunFire C18 column (150 mm × 2.1 mm × 3.5 μm)/Mobile phase: methanol and water | MLODs (ng mL−1): BPA (0.38), ClBPA (0.23), Cl2BPA (0.062), Cl3BPA (0.067), and Cl4BPA (0.016) | Mean (%): >85% (for all analytes) | Range (ng mL−1): 464–810 (for all chlorinated derivatives of BPA) | Gallart-Ayala et al. (2007) |
| 10 | BPA and Cl4BPA | Water | Beaker setup | HPLC-UV detector/Ace 5 C4 reversed phase column (250 mm × 4.6 mm × 5.0 μm)/235 nm detection wavelength/mixture of methanol and 0.1% trifluoroacetic acid in water and methanol (4:1, v/v) | GC-MSD | DB-5 fused silica capillary column (30 m × 0.25 mm × 0.25 μm)/Helium (carrier gas) | n.a. | n.a. | n.a. | Bastos et al. (2008) |
| 11 | BPA, ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | Wastewater (urban) | Different points; (n = 6) | SPE (LiChrolut RP-18 cartridge)/diethyl ether: methanol (9:1, v/v) | HPLC—MS/MS (APCI, − ve mode) | Gemini C18 column (150 mm × 4.6 mm × 5.0 μm)/mobile phase [A]: aqueous acetic acid (1%, v/v) and [B]: acetonitrile | Detection capabilities, DCs (ng L−1): BPA (20), ClBPA (9), Cl2BPA(12), Cl3BPA (12), and Cl4BPA (17) | Range(%): BPA (98.0–103.2), ClBPA (96.4–97.8), Cl2BPA (98.4–103.1), Cl3BPA (96.8–102.8), and Cl4BPA (95.6–102.0) | Range (ng mL−1): BPA (<DC), ClBPA (<DC), Cl2BPA (<DC), Cl3BPA (<DC), and Cl4BPA (<DC) | Zafra-Gómez et al. (2008) |
| 12 | BPA and chlorinated derivatives | Water | Beaker setup | SPE (cleanert PEP-SPE)/-dichloromethane: methanol (6:4, v/v) | BPA: HPLC-photodiode array detector Chlorinated derivatives of BPA: GC-MSD |
BPA: SunFire ODS reverse-phase column (150 mm
× 4.6 mm × 5.0 μm)/mobile phase: methanol and water
(70:30, v/v) Chlorinated derivatives of BPA: HP–5MS column (30 m × 0.22 mm × 0.25 μm)/Helium (carrier gas) |
Quantitative detection limits,
QDLs (ng
L−1): Cl2BPA (0.4) |
Mean (%):
ClBPA (107), and Cl2BPA (108) |
n.a. | Liu et al. (2009) |
| 13 | BPA, ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | Water samples | Multiple sources: (i) effluent from paper recycling plant, (ii) WWTPs, (iii) river, and (iv) DWTPs (influent and samples at different points) |
Online SPE (Ascentis Express C18 column with a fused core)/acetonitrile: ethanol: water | HPLC–MS/MS (ESI, −ve mode) | Hypersil Gold C18 column (20 mm × 2.1 mm × 12 μm, 175 Å)/acetonitrile: methanol: water | Method LOQs Range (ng L−1): BPA (57–115), ClBPA (57–176), Cl2BPA (60–183), Cl3BPA (60–180), and Cl4BPA (57–140) | Range (%): 85–100 (for all the analytes) |
Paper recycling plant
effluent, Mean (ng L−1): BPA (679), ClBPA (739), Cl2BPA (836), Cl3BPA (460), and Cl4BPA (530) |
Gallart-Ayala et al. (2010) |
| 14 | Cl4BPA | Sediment | River; (n = 3) | LLE (hexane: acetone, 9:1) | GC-electron capture detector | HP-5 capillary column/nitrogen (carrier gas) |
LODs (mg
L−1): Cl4BPA (1.0) |
Mean (%): Cl4BPA (96.5) | Range (ng g−1): Cl4BPA (<LOD-542.6) | Yuan et al. (2010) |
| 15 | BPA, Cl2BPA, and Cl4BPA | Sediment | River; (n = 3) | LLE (hexane: acetone, 9:1) | Cl4BPA: GC-electron capture detector Cl4BPA degradation products: GC-ion-trap MS | Cl4BPA: HP-5 capillary
column/nitrogen (carrier gas) Cl4BPA degradation products:
DB-5 MS capillary column (30 mm × 0.25 mm × 0.25 μm)/electron impact ionization/Helium (carrier gas) |
LODs (mg
L−1): Cl4BPA (1.0) |
Mean (%): Cl4BPA (96.5) | n.a. | Yuan et al. (2011) |
| 16 | BPA, ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | Sewage sludge |
WWTPs; (n = 2) |
Ultrasound-assisted extraction or Microwave-assisted extraction or pressurized liquid extraction (ethyl acetate) | HPLC–MS/MS (APCI, −ve mode) | Gemini-C18 (100 mm × 2.0 mm × 3.0 μm) (with C18 guard column)/mobile phase [A]: ammonical aqueous solution (0.025%, v/v) and [B]: ammonia in methanol (0.025%, v/v) |
Microwave-aasisted extraction: LODs (ng g−1): BPA (6), ClBPA (9), Cl2BPA (7), Cl3BPA (6), and Cl4BPA (7) |
Microwave-assisted
extraction: Range (%): BPA
(98.7–100.6), ClBPA (97.0–98.9), Cl2BPA
(99.0–101.0), Cl3BPA (99.8–101.4), and Cl4BPA (97.2–99.3) |
n.a. | Dorival-Garcia et al. (2012a) |
| 17 | BPA, ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | Sewage sludge |
WWTPs; (n = 17) | Pressurized liquid extraction (ethyl acetate) | HPLC–MS/MS (APCI, −ve mode) | Gemini-C18 (100 mm × 2.0 mm × 3.0 μm) (with C18 guard column)/mobile phase [A]: ammonical aqueous solution (0.025%, v/v) and [B]: ammonia in methanol (0.025%, v/v) |
LODs (ng
g−1: BPA (5), ClBPA (4), Cl2BPA (7), Cl3BPA (8), and Cl4BPA (8) |
Range(%):
BPA (99.4–99.5), ClBPA (99.3–100.6), Cl2BPA (98.9–99.4), Cl3BPA (97.7–99.7), and Cl4BPA (97.7–99.3) |
Range (ng g−1):BPA (<LOD-680), ClBPA (<LOD), Cl2BPA (<LOD), Cl3BPA (<LOD), and Cl4BPA (<LOD) | Dorival-Garcia et al. (2012b) |
| 18 | BPA, ClBPA, 2,6-Cl2BPA, 2,2’-Cl2BPA, and Cl3BPA | (A) Surface water | DWTPs; (n = 8) | SPE (glass C18 upti-clean endcapped
cartridge, 200 mg) |
HPLC–MS–MS (APCI, −ve mode) | Supercosil ABZ (150 mm × 4.6 mm × 3.0 μm)/mobile phase [A]: methanol/water (50:50, v/v) and [B]: methanol |
Method LOD, mLODs
(ng
L−1): BPA (0.5), ClBPA (0.7), 2,6-Cl2BPA (0.4), 2,2’-Cl2BPA (0.3), and Cl3BPA (2.3) |
Mean (%):
BPA (108), ClBPA (99), 2,6-Cl2BPA (101), 2,2’-Cl2BPA (100), and Cl3BPA (88) |
Range (ng L−1): BPA (6.7–29.7), ClBPA (<mLOD), 2,6-Cl2BPA (<mLOD), 2,2’-Cl2BPA (<mLOD), and Cl3BPA (<mLOD) | Dupuis et al. (2012) |
| (B) Treated water | Range (ng L−1): BPA (2.0–16.9), ClBPA (<mLOD), 2,6-Cl2BPA (<mLOD), 2,2’-Cl2BPA (<mLOD), and Cl3BPA (<mLOD) | |||||||||
| 19 | BPA, ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | Tap water | Simulated water pipe system (laboratory setup) | LLE | HPLC–MS/MS | n.a. |
LOQs (ng
L−1): BPA (1.0), ClBPA (0.9), Cl2BPA (1.5), Cl3BPA (0.7), and Cl4BPA (0.6) |
n.a. | n.a. | Kosaka et al. (2012) |
| 20 | BPA
and halogenated derivatives (primarily chlorinated and brominated) |
Water from DWTP | Beaker setup | BPA: LLE (dichloromethane); BPA chlorination products: SPE (Macherey Nagel HR-X, 6 mL, 500 mg) |
BPA: GC-MSD;
BPA chlorination products: HPLC-LTQ-Orbitrap HRMS |
BPA: DB-5HT column (15 m × 0.25 mm
× 0.1 μm)/Helium (carrier gas) BPA chlorination products: Gemini C18 column (50 mm × 2 mm × 3 μm)/Mobile phase [A]: acetonitrile and [B]: water |
LOQ (ng
L−1):
BPA (10) |
n.a. | n.a. | Bourgin et al. (2013a) |
| 21 | BPA, ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | (A) source water | DWTPs; (n = 62) | SPE (Oasis HLB cartridge)/dansylation (with aqueous sodium bicarbonate | UPLC–MS/MS (ESI, −ve mode) | Acquity UPLC BEH C18 (100 mm × 2.1 mm × 1.7 μm)/Mobile phase [A]: acetonitrile with 0.1% formic |
IDLs (ng
mL−1): BPA (0.001), ClBPA (0.001), Cl2BPA (0.002), Cl3BPA |
Range(%):
BPA (101–109), ClBPA (102–110), Cl2BPA (94–102), Cl3BPA |
Range (ng
L−1):
BPA (51.3–512), ClBPA (<IDL-18.5), Cl2BPA (<IDL-3.6), Cl3BPA |
Fan et al. (2013) |
| (100 mmol L−1, pH 10.5) and dansyl chloride) | acid and [B]: water with 0.1% formic acid | (0.001), and Cl4BPA (0.001) | (101–109), and Cl4BPA (97–105) | (< IDL-2.2), and Cl4BPA (<IDL-0.2) | ||||||
| (B) Drinking water | Range (ng L−1): BPA (10.8–128), ClBPA (2.8–26.7), Cl2BPA (0.7–6.3), Cl3BPA (1.5–7.7), and Cl4BPA (0.3–4.9) | |||||||||
| 22 | BPA and Cl4BPA | Sediment | River | BPA: ultrasonic extraction Cl4BPA: LLE (hexane/acetone, 9:1, v/v) | BPA: HPLC-fluorescence detector; Cl4BPA: GC-electron capture detector |
BPA: Polymetric bound silica column; Cl4BPA: HP-5 capillary column | LODs (mg L−1): BPA (0.1) and Cl4BPA (1.0) |
Mean (%):
BPA (96.3) and Cl4BPA (96.5) |
n.a. | Chang et al. (2014) |
| 23 | BPA and Cl4BPA | Sewage sludge |
DWTPs; (n = 52) | SPE (ENVI-Carb cartridge and Sep-Pak C18 cartridge) | HPLC–MS/MS (ESI, −ve mode) | Symmetry Shield C18 analytical column (150 mm × 2.1 mm × 5.0 μm)/Mobile phase [A]: methanol with water (1:9, v/v) and [B]: methanol | MQLs (ng g−1): BPA (0.61) and Cl4BPA (1.33) |
Range(%):
BPA (87–100) and Cl4BPA (75–90) |
Range (ng
g−1):
BPA (<MQL-152) and Cl4BPA (< MQL-143) |
Song et al. (2014b) |
| 24 | BPA and Cl4BPA | Source water, river water, effluent water and tap water | Multiple locations (n = 7) | Online-SPE (Direct Connect HP XBridge C18 column (30 mm × 2.1 mm × 10 μm) | UPLC–MS/MS | Acquity Shield RP 18 column (100 mm ×
2.1 mm, 1.7 μm)/Mobile phase: methanol/water (20:80, v/v) |
Method LODs, MLODs
(ng
L−1): BPA (3.0–18.0) and Cl4BPA (0.5–2.0) |
Range(%):
BPA (83.8–103.3) and Cl4BPA (84.0–107.4) |
Range (ng
L−1):
BPA (<MLOQ-77) and Cl4BPA (<MLOD) |
Yang et al. (2014b) |
| 25 | BPA, ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | Waste water (point of secondary and tertiary effluent) | WWTPs (n = 9) |
SPE (Oasis HLB cartridges, mL/200 mg) | HPLC–MS/MS (ESI, −ve mode) | Aquasil column (5.0 mm × 2.1 mm, 3.0 μm)/mobile phase [A]: aqueous ammonium acetate and [B]: methanol |
Reporting
Limits, RLs (ng L−1): BPA (10), ClBPA (4), Cl2BPA (4), Cl3BPA (4), and Cl4BPA (4) |
Range(%):
BPA (102–105), ClBPA (94–102), Cl2BPA (97–101), Cl3BPA (97–109), and Cl4BPA (96–97) |
Range (ng
L−1):
BPA (<RL-648), ClBPA (<RL), Cl2BPA (<RL), Cl3BPA (<RL), and Cl4BPA (<RL) |
Bulloch et al. (2015) |
| 26 | BPA and Cl4BPA | (A) Sediment (B) Clams |
Sites; (n = 3) |
Soxhlet extraction (n-hexane/acetone; 3:1, v/v)/pressurized liquid extraction (acetone/n-hexane, 1:1, v/v) | UPLC–MS/MS | n.a. | n.a. |
Range (%):
All analytes (65–112) |
Range (ng
g−1):
BPA (<LOD-9.5) and Cl4BPA (<LOD) Range (ng g−1): BPA (<LOD-4.2) and Cl4BPA (<LOD-1.4) |
Casatta et al. (2015) |
| 27 | BPA, ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | Reagent grade water | Beaker setup | n.a. | HPLC–MS/MS (ESI, −ve mode) | Gemini-NX C18 (150 mm × 3.0 mm × 3.0 μm) (with TMS end capping column)/mobile phase [A]: water and [B]: methanol |
MDLs (ng
mL−1): BPA (0.057), ClBPA (13.6), Cl2BPA (1.8), Cl3BPA (3.2), and Cl4BPA (5.9) |
n.a. | n.a. | Lane et al. (2015) |
| 28 | BPA, ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | Municipal drinking water |
Water distribution system (pilot scale model) (laboratory setup) | SPE (C18 cartridge) | GC-MS (electron impact ionization) | HP-5 MS capillary column (30 m × 0.25 mm x 0.25 μm)/Helium (carrier gas) |
n.a. | n.a. | n.a. | Li et al. (2015) |
| 29 | BPA, ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | Food contacting papers |
Market basket survey; (n = 74) |
LLE (methanol)/silica cartridges (hexane :
ethyl acetate, 38:62, v/v)/dansylation (dansyl chloride,
4-(dimethylamino)- pyridine, dichloromethane) |
UPLC–MS/MS (ESI, +ve mode) | Acquity UPLC BEH C18 (100 mm × 2.1 mm × 1.7 μm)/Mobile phase [A]: acetonitrile and [B]: water with 0.1% formic acid |
LOQs (ng
g−1): BPA (0.3), ClBPA (0.003), Cl2BPA (0.002), Cl3BPA (0.005), and Cl4BPA (0.006) |
Range(%):
BPA (93–108), ClBPA (88–103), Cl2BPA (87–101), Cl3BPA (88–102), and Cl4BPA (87–101) |
GM (ng g−1): BPA (0.80), ClBPA (0.004), Cl2BPA (0.001), Cl3BPA (0.001), and Cl4BPA (0.002) | Zhou et al. (2015) |
Bisphenol analogs are used in a range of industrial, commercial and consumer products, and occur widely in environmental media, such as, (i) bisphenol A diglycidyl ethers (BADGEs) in container linings (Oldring et al., 2006), (ii) bisphenol B (BPB) in infant food formulae (Cunha et al., 2011), (iii) bisphenol F (BPF) in polycarbonate food container linings (Fromme et al., 2002), (iv) bisphenol S (BPS) in thermal receipts (Becerra and Odermatt, 2012; Liao et al., 2012c), (v) BADGE and derivatives in indoor dust (Wang et al., 2012c) and food (Petersen et al., 2003), (vi) BPF, BADGE and BFDGE in food contact recycled paper items (Perez-Palacios et al., 2012), (vii) BPB, BPF and BPS in canned and packaged food and beverages (Cacho et al. 2012; Grumetto et al. 2008; Liao and Kannan 2013; Viñas et al. 2010), (viii) BPAF, BPB, BPF and BPS in indoor dust (Liao et al., 2012b) and water and sediment (Liao et al., 2012d), etc.
BPA and ClxBPA derivatives are ubiquitous in environmental matrices, including water resources. For example, BPA has been reported in surface waters (Fromme et al., 2002; Stachel et al., 2003), and in finished drinking water (Fan et al., 2013). Application of chlorine-based disinfectants to water is necessary for the removal of harmful microorganisms from tap water prior to reaching consumer taps. Thus, BPA may react with chlorine compounds in water (Fukazawa et al., 2001; Gallard et al., 2004; Hu et al., 2002; Lee et al., 2004; Yamamoto and Yasuhara, 2002), resulting in the addition of chlorine atoms to the phenolic aromatic moieties on BPA by electrophilic substitution at ortho-position. A higher frequency of detection and magnitude of ClxBPA concentrations in finished tap water than in source waters has been observed (Fan et al., 2013), underlying the prerequisite of disinfectant presence for the formation of ClxBPA. The percent detection and levels of ClxBPA in drinking water samples were (i) 97% and 3–27 ng L−1 for ClBPA, (ii) 98% and 1–6 ng L−1 for Cl2BPA, (iii) 60% and 2–8 ng L−1 for Cl3BPA, and (iv) 50% and 0.3–5 ng L−1 for Cl4BPA (Table 2) (Fan et al., 2013).
Recent developments in studying transformation products of BPA in water resources took into consideration the presence of dissolved natural organic matter and inorganic bromine, which potentially compete with chlorine leading to the formation of a new set of by-products and derivatives (Von Gunten, 2003; Von Gunten and Salhi, 2003). Moreover, presence of bromide ions favors the formation of hypobromite ions that react vigorously with phenol groups and resulting in formation of a suite of halogenated derivatives of BPA. A metabolomics-type approach was undertaken for untargeted profiling of BPA transformation products using high resolution mass spectrometry (LC-HRMS), which resulted in the identification of a novel set of 21 chlorination products and 17 brominated compounds of BPA (Bourgin et al., 2013a). However, mechanisms and environmental conditions behind the formation of these BPA transformation products have not been proposed. A targeted profiling approach for the identification and quantification of halogenated BPA transformation products in drinking water reaching household units to estimate human exposure is yet to be undertaken.
Residual BPA often found in chlorine-containing household cleaning (e.g., dishwashing and laundry detergent, and toilet cleaning solution) and personal hygiene products (e.g., bar soap, body lotion, shampoo/ conditioner, shaving cream) (Dodson et al., 2012) could act as a source for ClxBPA formation, when in contact with chlorinated tap water.Chlorine-containing household products often take the form of (i) cleaning products that contain sodium hypochlorite (kitchen countertop/floor/toilet cleaners, bleaching and scouring powders, stain removing sprays/gels, etc.) (Odabasi, 2008), (ii) bleach-containing laundry detergents (Nazaroff and Weschler, 2004), (iii) hypochlorite containing dishwasher detergents (Olson and Corsi, 2004), and (iv) bleached clothes and fabrics (Leri and Anthony, 2013). Other than oral ingestion of ClxBPA from drinking water and food sources; dermal uptake and inhalation may be also considered relevant routes of exposure because the addition of chlorine atoms to BPA may increase the lipophilicity of ClxBPA derivatives, and related dermal uptake rates. This is putatively supported by the evidence that higher ClxBPA to BPA concentration ratios were measured in fatty tissues when compared to the corresponding urine-based ratios (Cariot et al., 2012; Fernandez et al., 2007; Jimenez-Diaz et al., 2010; Liao and Kannan, 2012; Migeot et al., 2013). It was also speculated that the presence of gaseous free chlorine atoms or chloroform in the air, could react with BPA resulting in chlorinated BPA formation and subsequent exposures via the inhalation route, but this remains to be experimentally investigated. Use of chlorine-based products in routine activities (mopping, dish/clothes washing, etc.) was associated with increased urinary ClxBPA concentrations in an adult study population (Kalyvas et al., 2014); however, further research in this field is needed.
Food contact papers (FCP) (coffee filter papers, etc.) have been recently reported to contain ClxBPA derivatives, because of the widespread occurrence of residual BPA in recycled paper and the possibility of chlorine-containing bleached paper due to the pulp bleaching procedure (Zhou et al., 2015). Bleached coffee filter paper when in contact with liquid coffee extract facilitated high migration rates of ClxBPA into filtered coffee (Zhou et al., 2015). Mean concentrations of ClxBPA derivatives in bleached FCP were 3 pg g−1 (Cl2BPA) and 19 pg g−1 (ClBPA) compared to 0.7 pg g−1 (Cl2BPA) and 2 pg g−1 (ClBPA) in un-bleached FCP (Zhou et al., 2015). The authors speculated that BPA in paper reacted with sodium hypochlorite during pulp bleaching procedures of paper production, and there by generating and accumulating ClxBPA in FCP.
2.3. Toxicity and Health Outcomes: from in-vitro, in-vivo, to human studies
Based on in-vitro and in-vivo studies, the health risks of structural BPA analogs, such as for BPS and BPF have been extensively reviewed in recently published works (Eladak et al., 2015; Rochester and Bolden, 2015; Rosenmai et al., 2014); no human health studies involving structural BPA analogs’ exposures have been published so far. Although toxicity studies are important to establish the purpose of the analytical method development, metabolism and pharmacokinetic aspects are also crucial as they determine what metabolites/biomarkers as well as which biological matrices are important for human biomonitoring studies. However, no pharmacokinetics data were available for ClxBPA either in animals or humans. Hence, the metabolism and/or detoxification pathways, tissue distribution and percent elimination from the body remains unclear.
The biological plausibility of ClxBPA health effects was based on low-dose in vitro and in vivo experiments suggesting a higher (about 10 to 40 times) estrogenic activity of chlorinated BPA compared to BPA (Hu et al., 2002) that resulted in proliferation of breast cancer cells (Rivas et al., 2002) and uterine endometrium cells (Takemura et al., 2005). The estrogenic activity of chlorinated derivatives of BPA is considered to be higher than BPA (Nishikawa et al., 1999). For example, a yeast bioassay with equal concentrations of ClBPA, 2,6-Cl2BPA, 2, 2′-Cl2BPA, Cl3BPA, and Cl4BPA showed 8, 8, 38, 20 and 3-fold higher estrogenic activity than that of BPA (Fukazawa et al., 2002). The estrogenic activity of ClxBPA is being studied and these compounds exhibit similar activity compared to BPA, which depending on the receptors can be slightly lower (Kuruto-Niwa et al., 2002; Molina-Molina et al., 2013), or higher (Fukazawa et al., 2002; Liu et al., 2005; Takemura et al., 2005; Terasaki et al., 2011; Yamauchi et al., 2003). However, certain studies indicated that the offset of estrogenic activity of ClxBPA occurs at lower concentrations than those of BPA (Babu et al., 2012; Kuruto-Niwa et al., 2002; Viñas et al., 2013) and that biologically-relevant ClxBPA concentrations triggered non-monotonic responses (Viñas et al., 2013). Animal and cell culture toxicological studies reported adverse effects of ClxBPA, such as endocrine disruption (Viñas et al., 2013), estrogenicity (Kuruto-Niwa et al., 2002; Kuruto-Niwa et al., 2005), genotoxicity (Ozaki et al., 2004; Riu et al., 2011a; Riu et al., 2011b; Riu et al., 2014), energy disruption metabolism (le Maire et al., 2009; Riu et al., 2011a; Riu et al., 2014), and other minor and localized effects.
Few toxicological studies reported the link between the formation of BPA derivatives, altered BPA metabolism (Jaeg et al., 2004; Nakamura et al., 2011) and induction of inflammatory outcomes (oxidative stress and oxidative cellular damage) that related to insulin resistance pathophysiology in rat hepatocytes (Bindhumol et al., 2003). Possible reactions between BPA and cellular oxidants (e.g., peroxynitrite, hypochlorite or hypochlorous acid) may yield ClxBPA due to oxidative biotransformation reactions (Babu et al., 2012). The authors’ demonstrated the formation of chlorinated and nitrated derivatives when BPA reacted with hypochlorite/hypochlorous acid and peroxynitrite at neutral pH in a beaker setup. Further, they performed a molecular docking study showing that the putatively formed derivatives had stronger binding affinity for the human estrogen-related receptor-gamma (ERRγ) compared to estradiol. Under oxidative stress conditions, the neutrophil and macrophage derived oxidants, such as peroxynitrite, hypochlorite or hypochlorous acid prevailed in biological systems. Hence, the likelihood of BPA reactions with cellular oxidants to form ClxBPA via phase I biotransformation (Babu et al., 2012). Such alternative metabolic pathways may account for 20–25% of BPA that do not follow the conventional glucuronidation pathway (Yoshihara et al., 2004). These findings merit further investigation on alternate metabolites of BPA with varied estrogenic potencies (Ye et al., 2011), and presumably varying half-lives of elimination. The presence of such alternative metabolic pathways in the formation of ClxBPA in humans has not yet been reported. Halogenated BPA compounds showed 10- to 100-times higher binding affinity to peroxisome proliferator-activated receptors than BPA (Riu et al., 2011a,b) whose dysfunction was associated with the onset of obesity and T2DM in vivo (Somm et al., 2009; Swedenborg et al., 2009). In addition, photodegradation of ClxBPA altered their estrogenic activity (Gallart-Ayala et al., 2007; Ibuki et al., 2008; Mutou et al., 2006, 2008), while sulfonation of ClxBPA (viz.,Cl4BPA) did not eliminate their estrogenic activity, contrary to the effect of sulfonation on BPA (Riu et al., 2011a,b). It is expected that ClxBPA derivatives are detoxified to non-toxic forms in humans similar to BPA molecule (e.g. Cl4BPA bio-transformed to sulfonated metabolites in Zebra fish, (Riu et al., 2014)). However, recent in-vitro findings suggested that the glucuronide form of BPA was able to induce adipocyte differentiation in human and 3T3L1 murine preadipocytes (Boucher et al., 2015). The pharmacokinetics and toxicodynamics of ClxBPA derivatives in humans is currently unclear. Similar to BPA, a wide inter-, and intra-individual exposure variability and clearance patterns are also anticipated for ClxBPA derivatives in the human physiological system, but this remains to be investigated.
Limited evidence is currently available on the health effects associated with ClxBPA exposures. It was shown that Cl3BPA and Cl4BPA increased thyroid hormone activities but inhibited triiodothyronine activity compared to Cl2BPA, ClBPA, and BPA using a yeast two-hybrid assay on rat liver S9 preparation (Terasaki et al., 2011). Tetrachloro (C14BPA) and tetrabromobisphenol (Br4BPA) induced lipid accumulation in a cell-culture study (Riu et al., 2011a,b). In a zebra fish model, these ClxBPA derivatives acted as obesogens (Riu et al., 2014; Tingaud-Sequeira et al., 2011). It was suggested that ClxBPA exposure disrupted energy balance mechanisms due to agonism of peroxisome proliferator-activated receptor y (PPARy) and activation of retinoid × receptors (RXRs), leading to lipid accumulation (le Maire et al., 2009; Riu et al., 2011a, 2014). Grow-out studies on zebrafish exposed to halogenated BPA during the early developmental phase showed an induction of obese condition at a later life stage (Riu et al., 2014), supporting the theory of later onset of obesity due to exposure to endocrine disrupting chemicals at early-life stages (Janesick and Blumberg, 2011a,b, 2012). In contrast to conjugated metabolites of BPA, monosulfonated forms of tetrachloro- and tetrabromo-BPA remained biologically active, acted as PPARy agonists and promoted lipid deposits in a Zebrafish animal model (Riu et al., 2014). If an association between sulfated forms of halogenated BPA derivatives and lipid accumulation and obesity is con-firmed, then the default concept of benign conjugated BPA forms (Boucher et al., 2015) should be revisited in related toxicological studies.
In humans, BPA prenatal exposure effects on later life obesity have been already demonstrated, albeit with mixed results (Braun et al., 2014; Harley et al., 2013; Valvi et al., 2013). A few epidemiological studies reported a positive association between BPA in biological matrices and obesity (Li et al., 2012; Ning et al., 2011; Shankar et al., 2012; Wang et al., 2012b; Zhao et al., 2012), whereas other human studies did not confirm the positive association (Carwile and Michels, 2011; Duan et al., 2013; Galloway et al., 2010; Kim and Park, 2013; Ko et al., 2014; Lee et al., 2014; Melzer et al., 2012; Mok-Lin et al., 2010; Song et al., 2014a; Yang et al., 2009). Similar human studies on BPA derivatives or analogs are lacking; the exception is a human study (n = 223) reporting on the association between exposures to ClxBPA (monochlorinated BPA, mono-ClBPA) and obesity. Relatively weak positive association was observed between creatinine (Cr)-adjusted urinary mono-ClBPA and BMI, such as (i) 76 ng g−1 Cr in participants with above normal BMI (≥25 kg m−2) versus 55 ng g−1 Cr in those with normal BMI (<25 kg m−2) (p for mean difference = 0.053) and (ii) higher percentage of participants with above normal BMI in the high urinary mono-ClBPA tertile (63% in tertile 3 and 57% in tertile 2 versus 50% in tertile 1, p for trend = 0.056) (Andra and Makris, 2015). Similar tests of association between urinary BPA and BMI showed null outcome (Andra and Makris, 2015). A dichotomously-classified group analysis showed an increased odds ratio (OR) for higher BMI in the group with high creatinine-adjusted urinary levels of BPA and mono-ClBPA when compared with the participants group with low levels for both compounds [logistic model adjusted for gender and health status as potential confounders; adjusted OR (95% CI): 2.34 (1.06, 4.36), p = 0.027] (Andra and Makris, 2015). Also, higher odds for developing T2DM per unit increase in creatinine-adjusted urinary mono-ClBPA levels [ln (ng g−1)] were observed in a pilot human study [adjusted OR (95% CI): 3.29 (1.10, 11.4), p < 0.05] (Andra et al., 2015). These findings underscored the importance of monitoring both BPA and its ClxBPA derivatives in human matrices being part of a comprehensive exposure assessment towards improving our understanding of their obesogenic and metabolic-disruptive effects. Whether it is useful to bio-monitor trace-level ClxBPA derivatives when the main effect of the exposure to parent compound (BPA) is non-significant (either due to small sample size or due to differential species toxicities) remains an unanswered research question.
2.4. Analytical methods for human matrices
Analyses of chlorinated derivatives of BPA have been performed in a wide range of human matrices, such as urine, blood, placenta, breast milk and adipose tissue, while biomonitoring studies on BPA structural analogs have been conducted only in urine and breast milk. Each of these matrices is complex, requiring specific analytical steps that include pre-treatment, analyte(s) extraction and pre-concentration, separation using chromatographic techniques, and detection using mass spectrometry. We summarized and discussed the bioanalytical protocols of ClxBPA and BPA analogs in the following sub-sections (Tables 3 and 5).
Table 3.
Analytical method parameters and instrumental variables for measuring chlorinated derivatives of bisphenol A in human tissue and matrices.
| Table 3. Item # | Biomarker of exposure to BPA and its chlorinated derivatives | (i) Bio-matrix (ii) Sample volume or mass (iii) Enzymatic deconjugation (Yes/No) |
(i) Analytical method (ii) Sample injection volume (iii) Reference to the original method(s) |
Sample pretreatment | Extraction and clean-up method | LC or GC separation Column | LC or GC conditions | MS system | MS condition | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ClBPA(t—total), Cl2BPA, Cl3BPA, and Cl4BPA |
(i) Plasma (ii) 5 mL (iii) Yes |
(i) GC—MS (ii) 2 μL (iii) n.a. |
(i) (a) Protein precipitation with
ZnSO4 and NaOH, and (b) membrane filtration to remove
particulate matter. (ii) Internal standard addition (BPA-dl6) (iii)Solid phase microextraction (polyacrylate-coated fiber) (iv) Incubation and LLE (acetonitrile) (iv) Aqueous phase collection and evaporation (nitrogen) |
SPME: (a) Polyacrylate-coated fiber, (b) SPME fiber immersed in NaCl solution for 40 min and 40 °C, and (c) thermal desorption at 300 °C Derivatization: (a) BSTFA (N,O-bis(trimethylsilyl) trifluoroacetamide) diluted in dichloromethane, and (b) desorption and subsequent derivatization allowed during 8 min in splitless mode with split closed. |
HP1 fused silica capillary column (30 m × 0.25 mm i.d.; 0.25 μm film thickness) |
Mode: Splitless
Injector temp: 300 °C Oven temp
program: 5 min at 50 °C, 30 °C/min to 300 °C, and 7 min at 300 °C. Run time: ~20.3 min. |
Mass selective detector, Selected ion monitoring | Ion source: Electron impact | del Olmo et al. (2005) |
| 2 | BPA(f—free), ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | (i) Adipose tissue (ii) 200 mg (iii) No |
GC–MS (ii) 2 μL (iii) del Olmo et al. (2005) |
(i) Homogenization (n-hexane) (ii) Internal standard addition (Bisphenol F) (iii) Incubation and LLE (acetonitrile) (iv) Aqueous phase collection and evaporation (nitrogen) |
SPE: AccuBONDII ODS-C18
(silica-based) Conditioners: diethylether, methanol,
and deionized water Eluent: Mixture of diethylether and
methanol (9:1 v/v) Derivatization: Evaporation and
esterification with ethyl acetate, and BSTFA (N,O-bis(trimethylsilyl)
trifluoroacetamide) and TMCS (trimethylchlorosilane). |
ZB-5 MS Zerbon capillary column (30 m × 0.25 mm i.d.; 0.25 μm film thickness) |
Mode: splitless
Carrier gas: helium, 1.0
mL/min Injector temp: 250 °C Oven temp program: 2 min at 120 °C, 30 °C/min to 230 °C, 2 min at 230 °C, 40 °C/min to 270 °C, and held for 6 min. Run time: ~14.7 min. |
Mass selective detector, Selected ion monitoring |
Ion source: Electron impact
Ion source temp: 250
°C Interface temp: 270 °C |
Fernandez et al. (2007) |
| 3 | BPA(f—free), ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | (i) Placenta issue (ii) 1.5 g (iii) No |
(i) LC–MS/MS (ii) 40 μL (iii) n.a. |
(i) Homogenization in water (ii) Ethyl acetate addition and centrifugation (iii)Organic layer collection and evaporation (nitrogen) (iv) Reconstitution in 0.1% v/v ammonia in methanol containing internal standard (BPA-d16) |
LLE: (i)Addition of 0.1% v/v ammoniacal aqueous solution (ii) Vigorous shaking (iii) Centrifugation and extract filtration |
Gemini C18 column (100 mm × 2 mm i.d.; 3 μm particle size) |
Mobile phase: Solvent A: 0.1%
v/v ammoniacal aqueous solution Solvent B: 0.1% v/v ammonia in methanol
Gradient: 0.0–3.5 min, 60% B; 3.5–4.0
min, 60–100% B; 4.0–6.5 min, 100% B; 7.0–10.5 min,
60% B. Flow rate: 0.25 mL min−1 Column temperature: 40 °C Run time: 7.0 min. |
Triple quad, APCI, negative ion mode |
Ion source temp: 350
°C Ion spray voltage: −3kV Curtain gas: Nitrogen, 30 psi Ion source gas 1: Nitrogen, 50 psi Ion source gas 2: Nitrogen, 30 psi collision gas: helium, 10 psi Dwell time: 200 ms |
Jimenez-Diaz et al. (2010) |
| 4 | BPA(f—free), ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | (i) Placenta issue (ii) 1.5 g (iii) No |
(i) LC–MS/MS (ii) 40 μL Jimenez-Diaz et al. (2010) |
(i) Homogenization in wáter* and with
ultrasonication (ii) Ethyl acetate** addition, shaking and centrifugation (iii) Organic layer separation and evaporation (nitrogen) * Most suitable extraction media was water in comparison to various pH adjusted media with formic acid or ammonia, and salt-saturated aqueous solution **Most effective extractant was ethyl acetate compared to methanol, ethanol, and acetonitrile |
LLE: (i) Reconstitution in 0.1% v/v ammonia in methanol and ammoniacal aqueous solution (ii) Extract filtration (iii) BPA-d16 used as a surrogate indicator |
*Gemini C18 column (100 mm × 2 mm i.d.;
3 μm particle size) *Best results obtained with Gemini C18 column
compared to (i) an Acquity UPLC (100 mm × 2.1 mm i.d.; 1.7
μm particle size), (ii) Chromolith SpeedROD RP-18e (50 mm × 4.6 mm i.d.; 2 μm particle size), and (iii) Zorbax Eclipse XDB-C8 (100 mm × 2.0 mm i.d.; 1.8 μm particle size) |
*Mobile phase: Solvent A:
0.1% v/v ammoniacal aqueous solution Solvent B: 0.1% v/v ammonia in methanol Gradient: 0.0–3.5 min, 60% B; 3.5–4.0 min, 60–100% B; 4.0–6.5 min, 100% B; 7.0–10.5 min, 60% B. Flow rate: 0.25-mL min−1 *Column temperature: 40 °C *from an earlier report: Jimenez-Diaz et al. (2010) Run time: 7.0 min. |
Triple quad, *APCI, negative ion mode * Best results obtained with the APCI negative mode compared to ESI in both modes |
Ion source temp: 350
°C Ion spray voltage: −3 kV Curtain gas: Nitrogen, 30 psi Ion source gas 1 : Nitrogen, 50 psi Ion source gas 2: Nitrogen, 30 psi Collision gas: Helium, 10 psi Dwell time: 200 ms |
Vela-Soria et al. (2011) |
| 5 | BPA(f—free), ClBPA, 2,6-Cl2BPA, 2,2-Cl2BPA, and Cl3BPA | (i) Breast milk (ii) 0.5 mL (iii) No |
(i) LC–MS/MS (ii) 50 μL (iii) n.a. |
(i) Addition of internal standards (ii) Homogenization by shaking (iii) BPA-d16 used as an internal standard |
LLE plus
SPE 1. LLE: (i) Addition of methanol (ii) Vortex, sonication, and centrifugation (iii) Supernatant collection, evaporation, and reconstitution in water/methanol mixture (70%/30%v/v) 2. SPE: Online SPE setup, Xbridge C8 column (30 mm × 2.1 mm i.d.) Eluent: Methanol and water (80%/20% v/v) |
Acquity CSH C18 column (100 mm × 2.1 mm i.d.; 1.7 μm particle size) |
Mobile phase: Solvent A:
Methanol and water (50%/50% v/v) Solvent B: Methanol
Gradient: 2.0–2.5 min, 0% B; 2.5–4.0
min, 90% B; 4.0–4.5 min, 99% B; 4.5–10.0 min, 99% B;
10.0–13.0 min, 0%B Flow rate: 0.40 mL min−1 Column temperature: 40 °C Run time: 13.0 min. |
Triple quad, ESI, negative ion mode |
Ion source temp: 150
°C Desolvation temp: 550 °C Cone gas: 50 Lh−1 Desolvation gas: Nitrogen, 1000 Lh−1 Collision gas : Argon, 0.28 mL min−1 Capillary potential: 3.5 V Cone potential: −66 V Extractor potential: −29 V |
Cariot et al. (2012) |
| 6 | BPA(f—free,
t—total), ClBPA, Cl2BPA, and Cl3BPA |
1. (i) Urine (ii) 0.5 mL (iii) Both, yes and no* 2. (i) Serum (ii) 0.5 mL (iii) Both, yes and no* * Two different steps: (a) Yes for analyzing total form (free + conjugated); and (b) No for the analysis of free form (unconjugated). |
(i) LC–MS/MS (ii) 10 μL (iii) n.a. |
(i) Spike Internal Standard
(13C12-BPA) (ii) Incubation with in a mix of 1 M ammonium acetate buffer (pH 5.0), 1 M formic acid (pH 1.0), and water. (iii)Enzymatic hydrolysis with β-glucuronidase |
1. Urine SPE: (i) Oasis HLB (60 mg/3 cc) (ii) Eluate was evaporated to 0.5 mL under nitrogen stream Conditioners: methanol and water in series Wash: mixture of 0.1 N HCl and 10% methanol in water Eluent: methanol 2. Serum SPE: (i)Strata NH2 in continuation with a second cartridge of Oasis MCX (60 mg/3 cc) (ii) Eluate was evaporated to 0.5 mL under nitrogen |
Betasil C18 column (100 mm × 2.1 mm
i.d.; 5 μm particle size) and Betasil C18 guard column (20 mm × 2.1 mm i.d.; 5 μm particle size) |
Mobile phase: Solvent A:
methanol and 10 mM ammonium acetate Solvent B: Methanol Gradient: 0.0–2.0 min, 15% B; 2.0–2.5 min, 75% B; 2.5–5.0 min, 75% B; 5.0–10.0 min, 99% B; 10.0–13.5 min, 99%B; 13.5–20.0 min, 15%B Flow rate: 0.30 mL min−1 Run time: 20.0 min. |
Triple quad, ESI, negative ion mode |
Ion source temp: 700
°C Ion spray voltage: − 4.5 kV Curtain gas: Nitrogen, 10–11 psi Ion source gas 1 : 70 psi Ion source gas 2: 65 psi Collision gas: Nitrogen, 10–11 psi |
Liao and Kannan (2012) |
|
Conditioners: Methanol and
water in series Wash: (i) Each cartridge washed with a
mixture of 0.1 N HCl and 25% methanol in water (ii) Strata NH2 is further washed with methanol Eluent: (i) Oasis MCX cartridge was eluted with methanol to collect fraction with BPA and BPA chlorides |
||||||||||
| 7 | BPA(f—free), ClBPA, 2,6-Cl2BPA, 2,2-Cl2BPA, and Cl3BPA | (i) Colostrum (ii) 0.5 mL (iii) No |
(i) LC–MS/MS (ii) 50 μL (iii)n.a. |
(i) Spike of internal standard
(BPA-d16) (ii) Incubation with methanol (iii) Vortex, sonicate, and centrifugation (iv) Supernatant collection and evaporation (nitrogen) (iv) Reconstitution in methanol and water mixture (50%/50% v/v) |
SPE: Online SPE setup, Xbridge C8 column (30 mm × 2.1 mm i.d., 10 μm particle size) Conditioners and wash: methanol and water (80%/20% v/v) | Acquity CSH C18 column (30 mm × 2.1 mm i.d.; 10 μm particle size) |
Mobile phase: Solvent A:
methanol and water (50%/50% v/v); Solvent B: methanol
Gradient: Initial: Methanol and water(50%/50%v/v);
Linear increase: 90% methanol; Final: 99% methanol Flow
rate: 0.40 mL min−1 Column temperature: 40 °C Run time: n.a. |
Triple quad, ESI, negative ion mode | NA | Migeot et al. (2013) |
| 8 | BPA(t—total) and ClBPA | (i) Urine (ii) 4 mL (iii) Yes |
(i) GC–MS/MS (ii) 20 μL Fukazawa et al. (2001) |
(i) Spike of surrogate standard
(BPA-d16) (ii) Incubation with 0.5 M sodium acetate buffer (pH 4.75) (iii) Enzymatic hydrolysis with β-glucuronidase |
LLE: (i) Extraction with ethyl acetate and hexane mixture (1:4) Derivatization: Evaporation and esterification with trifluoroacetic anhydride Reconstitution: Evaporation and reconstitution with dichloro methane Spike of internal standard (Decafluoro-biphenyl) |
Restek Rxi-5 ms [5% diphenyl/95% dimethylpolysiloxane] (30 m × 0.25 mm i.d.; 0.25 μm film thickness) |
Mode: PTV Carrier
gas: helium 1 mL/min Injector temp: 35 °C Oven temp program: 30 °C for 1.5 min, 30 °C to 220 °C for 5 min with 300 °C/min, 220 °C to 300 °C for 1.75 min with 80 °C/min Injector temp ramp: 35 °C for 0.35 min, 35 °C to 300 °C with 300 °C/min Run time: 14.0 min. |
Triple
quad, multi reaction monitoring (MRM) |
Ion source: Electron
impact Carrier gas and quenching gas: Helium 99.999% Collision gas: nitrogen 99.999% Transfer line temp: 250 °C Ion source temp: 250 °C Quadrupole 1 and 2 temp: 150 °C |
Kalyvas et al. (2014) |
| 9 | BPA(f—free), ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | (i) Breast milk (ii) 9.9 mL (iii) No |
(i)LC—MS/MS (ii) 10 μL (iii) n.a. |
(i) Spike of acetonitrile solution with
surrogate standard
(BPA-d16) Vortex |
LLE: (i) Addition of acetonitrile and a fat/proteins precipitation solution (1:1). This solution consisted of mixture of zinc acetate, phosphor-tungstic acid and glacial acetic acid. (ii) Vortex and centrifugation (iii) Supernatant collection and evaporation (vacuum) (iv) Residue reconstitution in 40% mobile phase B (ammonia in methanol, 0.1% (v/v)) |
Acquity UPLC BEH C18 column (100 mm × 2.1 mm i.d.; 1.7 μm particle size) |
Mobile phase: Solvent A:
Aqueous ammonium formate (0.1% v/v) (pH 9.0) Solvent B: ammonia in methanol (0.1% v/v) Gradient: 0.0–4.0 min, 40% B; 4.0–6.0 min, 40–90%B; 6.0–6.1 min, 90–100%B; 6.1–7.5 min, 100% B; 7.5–8.0 min, 40% B; 8.0–13.0 min, 40% B Flow rate: 0.30 mL min−1 Column temperature: 40 °C Run time: 13.0 min. |
Triple quad, ESI,negative ion mode |
Ion source temp: 150
°C Capillary voltage: 0.6 kV Desolvation temp: 500 °C Cone gas: Nitrogen (99.999%), 150 Lh−1 Desolvation gas: Nitrogen (99.999%), 500 Lh−1 Collision gas: Argon (99.999%), 0.15 mL min−1 Nebulizer gas: 7.0 bar |
Rodriguez-Gomez et al. (2014a) |
| 10 | BPA(f—free), ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | (i) Breast milk (ii) 9.9 mL (iii) No |
[A] LC method: (i) LC–MS/MS (ii) 10 μL (iii) n.a. [B] GC method: (i) GC–MS/MS (ii) 1 μL (iii) n.a. |
Spike of acetonitrile solution with surrogate
standard
(BPA-d16) (ii) Vortex for a minute |
LLE and Stir-bar sorptive extraction
(SBSE): (i) Addition of acetonitrile and a fat/proteins precipitation solution (1:1) (ii) Vortex and centrifugation (iii) Underlying lipid layer collection and evaporation (vacuum) (iv) Residue reconstitution in water and vortex (v) Transfer extract to sodium chloride solution (vi) Add polydimethyl-siloxane (PDMS) twister bar (20 mm (l), 0.5 mm (dia)) (vii)Stir for 24 h at 600 rpm (viii)Collect PDMS twister, wash to remove remaining salts (ix) Desorption of analytes in acetonitrile, solvent evaporation (nitrogen) (x) Reconstitution with ethyl acetate and N,O-bis(trimethyl silyl) trifluoro-acetamide with trimethyl chlorosilane (BSTFA/1%TMCS) (60:40%, v/v) (xi) Heat at 60 °C for 20 min. (xii) Dissolve the extract in 30% of mobile phase (ammonia in methanol, 0.1% (v/v)) |
[A] LC method: Acquity UPLC BEH C18 column (100 mm × 2.1 mm i.d.; 1.7 μm particle size) GC method: HP-5MS capillary column (30 m × 0.25 mm i.d.; 0.25 μm film thickness) |
[A] LC method: Mobile phase: Solvent A: Aqueous ammonium formate (0.1% v/v) Solvent B: Ammonia in methanol (0.1% v/v) Gradient: 0.0–2.0 min, 30% B; 2.0–5.0 min, 30–90%B; 5.0–5.1 min, 90–100%B; 5.1–7.0 min, 100% B; 7.0–7.1 min, 30% B; 7.1–10.0 min, 30% B Flow rate: 0.25 mL min−1 Column temperature: 40 °C Run time: 10.0 min. GC method: Mode: Splitless Carrier gas: Helium, 20 psi Injector temp: 250 °C Oven temp program: 2 min at 70 °C, 25 °C/min to 120 °C, 10 °C/min to 260 °C, 2 min at 260 °C, 20 °C/min to 280 °C, and held for 5 min. Injector temp ramp: 12 °C/second to 325 °C to transfer the analytes to GC column Run time: 26.0 min. |
[A] LC method: Triple quad, ESI, negative ion
mode [B] GC method: Triple quad, selected reaction monitoring (SRM) |
[A] LC method: Ion source temp: 150 °C Capillary voltage: 0.6 kV Desolvation temp: 500 °C Cone gas: Nitrogen (99.999%), 150 Lh−1 Desolvation gas: Nitrogen (99.999%), 500 Lh−1 Collision gas: Argon (99.999%), 0.15 mL min− 1 Nebulizer gas: 7.0 bar Dwell time: 20 ms [B] GC method: Ion source: Electron impact Carrier gas and quenching gas: Helium (99.999%) Collision gas: Nitrogen (99.999%) Transfer line temp: 290 °C Ion source temp: 290 °C Quadrupole 1 and 2 temp: 180 °C |
Rodriguez-Gomez et al. (2014b) |
| 11 | BPA(f—free, t—total), ClBPA, Cl2BPA, Cl3BPA, Cl4BPA | (i) Urine (ii) 5.0 mL (iii) Both, yes and no* * Two different steps: (a) Yes for analyzing total form (free + conjugated); and (b) No for the analysis of free form (unconjugated). |
(i) LC–MS/MS (ii) 10 μL (iii)n.a. |
(i) Spike of surrogate standard
(BPA-d16) (ii) Two sets of incubation: (iiA) with no enzyme hydrolysis (to determine free forms) (iiB) with enzyme hydrolysis (for total forms): Addition of (a) β-glucuronidase/sulfatase, and (b) a mixture with 4-methylumbelliferyl glucuronide, 4-methylumbelliferyl sulfate, and 13C4-4-methylumbelliferone to assess success rate of deconjugation step (iiC) Incubation at 37 °C for 24 h |
Dispersive
liquid-liquid micro-extraction (DLLME): (i)Addition of 10% (w/v) sodium chloride solution (ii) pH adjustment to 2.0 with 0.1 M HCl (iii) A mix of acetone (dispenser solvent) and trichloromethane (extraction solvent) is rapidly injected into the aqueous sample with a syringe (iii) Vortex gently and centrifugation (iv) Collection of sedimented phase and evaporation (v) Reconstitution with a mixture of methanol and water (0.1% ammonia) (60%/40% v/v) and vortex |
Acquity UPLC BEH C18 column (50 mm × 2.1 mm i.d.; 1.7 μm particle size) |
Mobile phase: Solvent A:
Aqueous ammonium formate (0.1% v/v) Solvent B: Ammonia in methanol (0.1% v/v) Gradient: 0.0–3.5 min, 60% B; 3.5–4.0 min, 60–100% B; 4.0–6.5 min, 100% B; 6.5–6.6 min, 60% B; 6.6–10.0 min, 60% B Flow rate: 0.25 mL min −1 Column temperature: 40 °C Run time: 10.0 min. |
Triple quad, ESI,negative ion mode |
Ion source temp: 150
°C Capillary voltage: 0.6 kV Desolvation temp: 500 °C Cone gas: Nitrogen (99.995%), 150 Lh−1 Desolvation gas: Nitrogen (99.995%), 500 Lh−1 Collision gas: Argon (99.999%), 0.15 mL min−1 Nebulizer gas: 7.0 bar Dwell time:25 ms |
Vela-Soria et al. (2014) |
| 12 | BPA(f—free),
ClBPA, 2,6-Cl2BPA, 2,2’-Cl2BPA, Cl3BPA, and Cl4BPA |
(i) Urine (ii) 0.3 mL (iii) No |
(i) LC–MS/MS (ii) 30 μL (iii) n.a. |
(i) Addition of internal standard
(BPA-d16 and
2,2’-Cl2BPA-d12)
and homogenization |
LLE: (i) Addition of acetonitrile and vortex (iii) Addition of 10 M ammonium formate (salting-out reagent) and vortex (ii) Centrifugation, collection of upper organic layer, evaporation (nitrogen) and reconstitution with water |
Acquity UPLC CSH C18 column (100 mm × 2.1 mm i.d.; 1.7 μm particle size) |
Mobile phase: Solvent A:
Deionized water Solvent B: Methanol Gradient:
0.0–0.5 min, 30% B; 0.5–7.0 min, 90% B; 7.0–7.5
min, 99% B; 7.5–12.5 min, 99% B; 12.5–13.0 min, 30% B;
13.0–15.5 min, 30% B Flow rate: 0.35 mL min−1 Column temperature: 40 °C Run time: 15.5 min. |
Triple quad, ESI, negative ion mode |
Ion source temp: 150
°C Capillary potential: 1.5 kV Desolvation temp: 550 °C Cone gas: Nitrogen, 150 Lh−1 Desolvation gas: Nitrogen, 800 Lh−1 Collision gas: Argon (99.999%), 0.15 mL min−1 |
Venisse et al. (2014) |
| 13 | BPA(t—total)
and Cl4BPA |
(i)Urine (ii) 2 mL (iii) Yes |
(i) LC–MS/MS (ii) 5 μL (iii) n.a. |
(i) Addition of internal standard
(BPA-d4) (ii) Enzymatic hydrolysis (β-glucuronidase/sulfatase) (iii) Addition of 0.2 M sodium acetate buffer (pH 5.4) and vortex (iv) Incubation at 37 °C for 12 h in dark |
LLE: (i) Addition of acetonitrile for protein precipitation (iii) Addition of ethyl acetate Sonication (iv) Centrifugation, supernatant collection, evaporation and reconstitution with methanol and water (50%/50%, v/v) |
Acquity BEH C18 column (100 mm × 2.1 mm i.d.; 1.7 μm particle size) |
Mobile phase: Solvent A:
Methanol (LC/MS grade) Solvent B: Water (LC/MS grade) Gradient: 0.0–1.0 min, 40% A; 1.0–6.0 min, 40–80% A; 6.1–8.0 min, 100% A Flow rate: 0.40 mL min−1 Run time: 8.0 min. |
Triple quad, ESI, negative ion mode |
Ion source temp: 150
°C Capillary potential: 2.9 kV Desolvation temp: 400 °C Cone gas: Nitrogen (99%), 150 Lh−1 Desolvation gas: Nitrogen (99%), 1000Lh−1 |
Yang et al. (2014a,b) |
| 14 | BPA(f—free), ClBPA, Cl2BPA, Cl3BPA, and Cl4BPA | (i) Placenta (ii) 0.25 g (iii) No |
(i) LC–MS/MS (ii) 10 μL (iii)n.a. |
(i) Sample homogenization with silica in mortar |
Manually packed
SPE: (i) Load the sample mixture onto primary secondary amine (PSA) sorbent filled polypropylene cartridge (ii) Extraction with methanol (iii)Extract evaporation (nitrogen) and residue reconstitution with a mixture containing 60:40 (v/v) of methanol and water containing ammonia (0.1% (v/v)) (iv) Addition of surrogate standard (BPA-d16) (iv) Vortex and centrifugation |
Acquity BEH C18 column (50 mm × 2.1 mm i.d.; 1.7 μm particle size) |
Mobile phase: Solvent A:
Aqueous ammonium formate (0.1% v/v) Solvent B: ammonia in methanol (0.1% v/v) Gradient: 0.0–3.5 min, 60% B; 3.5–4.0 min, 60–100% B; 4.0–6.5 min, 100% B; 6.5–6.6 min, 60% B; 6.6–10.0 min, 60% B Flow rate: 0.25 mL min−1 Column temperature: 40 °C Run time: 10.0 min. |
Triple quad, ESI,negative ion mode |
Ion source temp: 150
°C Capillary voltage: 0.6 kV Desolvation temp: 500 °C Cone gas: Nitrogen (99.995%), 150 Lh−1 Desolvation gas: Nitrogen (99.995%), 500 Lh−1 Collision gas: Argon (99.999%), 0.15 mL min−1 Nebulizer gas: 7.0 bar Dwell time: 25 ms |
Vela-Soria et al. (2015) |
Table 5.
Analytical methods and highlights for biomonitoring of structural analogs of bisphenol A in human tissue and matrices.
| Table 5 Item# | Study (i) Size (ii) Location (iii) Year |
(i) Matrix (ii) Sample volume (iii) Injection volume |
Analytical method (i) Enzymatic deconjugation (yes/no) (ii) Sample extraction/clean-up (iii) Internal standards (iv) Instrumentation (v) Column (vi) Mobile phase (vii) Run time |
Analytical performance (i) LOD (ng mL−1) (ii) LOQ(ng mL−1) (iii) Recovery (%) (iv) RSD (%) |
Bisphenol A and its structural
analogs (i) detection frequency (ii) concentration (ng mL−1) |
Reference |
|---|---|---|---|---|---|---|
| 1 | (i) n = 20 (ii) Portugal (iii) n.a. |
(i) Urine (ii) 5.0 mL (iii) 2 μL (splitless) |
(i) Two different steps: (a) Yes for analyzing
total form (free + conjugated); and (b) No for the analysis of free form
(unconjugated). (ii) Dispersive-LLE (tetrachloroethylene) (iii) BPA-d16 (iv) Multi-dimensional GC-MS(electron impact, SRM transitions) (v) Heart-cutting GC separation of analytes with two columns, (a) Primary column: DB-5HT (5 m × 0.32 mm × 0.10 μm); and (b) secondary column: DB-5MS (20 m × 0.18 mm × 0.18 (μm) with a restrictor (2 m × 0.10 mm). (vi) Helium (carrier gas) (vii) 10.0 min. |
(i) BPA (0.03); BPB (0.05) (ii) BPA (0.1); BPB (0.1) (iii) Dispersive-LLE yield: BPA (68–77); BPB (56–63) (iv) BPA (7–15); BPB (11–20) |
(i) BPA (85%); BPB (10%) (ii) BPA (<0.03b−4.99d); BPB (<0.05b−1.15d) |
Cunha and Fernandes (2010) |
| 2 | (i) n = 315 (ii) Multiple countries (iii) 2010–2011 |
(i) Urine (ii) 0.5 mL (iii) 10 μL |
(i) Yes for the total form (unconjugated +
conjugated) (ii) SPE (Oasis MCX cartridge; 60 mg, 3 mL) (iii) BPA-13C12 (iv) HPLC-MS/MS (ESI, −ve mode) (v) Betasil C18 (100 mm × 2.1 mm × 5 μm) (vi) [A] Methanol; [B] Water (vii) 20.0 min. |
(i) n.a. (ii) BPS (0.02) (iii) BPS (92–94) (iv) n.a. |
(i) BPS (81%) (ii) BPS (<0.02e−21.0d) |
Liao et al. (2012a) |
| 3 | (i) n = 30 (ii) Greece (iii) n.a. |
(i) Urine (ii) 500 μL (iii) 10 μL |
(i) Yes for the total form (unconjugated +
conjugated) (ii) LLE (ethyl acetate) (iii) BADGE-2D6 (iv) HPLC-MS/MS (ESI, +ve mode)f (v) Betasil C18 (100 mm × 2.1 mm × 5 μm) (vi) [A] Methanol; [B] Water/methanol (90:10, v/v) with ammonium acetate (1.5%, w/v). (vii) 30.0 min. |
(i) n.a. BADGE (26–45); BADGE· H2O (63–83); BADGE· HCl (25–40); BADGE· 2H2O (78–135); BADGE#x00B7;H2O·HCl (82–122) (ii) BADGE (6.8–11.4); BADGE#x00B7;H2O (7.3–12.2); (iii) BADGE· HCl (9.5–12.8); BADGE· 2H2O (9.5–16.4); BADGE.H2O#x00B7;HCl (9.0–13.7) |
(i) BADGE (3%); BADGE #x00B7;2H2O
(93%) (ii) BADGE (<0.50e); BADGE· 2H2O (<0.50e−13.8d,f) |
Asimakopoulos et al. (2014) |
| 4 | (i) n = 20 (ii) Spain (iii) n.a. |
(i) Urine (ii) 5.0 mL (iii) 1 μL (splitless) |
(i) Two different steps: (a) Yes for analyzing
total form (free + conjugated); and (b) No for the analysis of free form
(unconjugated). (ii) Dispersive-LLE (trichloromethane) (iii) BPA-d16 (iv) GC–MS/MS (electron impact, SRM transitions) (v) HP-5MS (30-m × 0.25 mm × 0.25 μm) (vi) Helium (carrier gas) (vii) 26.0 min. |
(i) BPA (0.2); BPS (0.1) (ii) BPA (0.5); BPS (0.4) (iii) BPA (98–105); BPS (96–104) (iv) BPA (4.3–7.3); BPS (6.3–9.7) |
(i) BPA (65%); BPS (0%) (ii) BPA (<0.20b−46.0d); BPS (<0.10b) |
Vela-Soria et al. (2014a) |
| 5 | (i) n = 20 (ii) Spain (iii) n.a. |
(i) Urine (ii) 5.0 mL (iii) 2 μL |
(i) Two different steps: (a) Yes for analyzing
total form (free + conjugated); and (b) No for the analysis of free form
(unconjugated). (ii) Dispersive-LLE (trichloromethane) (iii) BPA-d16 (iv) UHPLC-MS/MS (ESI, +ve and − ve mode) (v) Acquity UPLC BEH C18 column (50 mm × 2.1 mm × 1.7 μm) (vi) [A] ammoniac aqueous solution (0.1%, v/v); and [B] ammonia in methanol (0.1%, v/v). |
(i) BPA (0.2); BPS (0.1) (ii) BPA (0.6); BPS (0.3) (iii) BPA (98–102); BPS (98–105) (iv) BPA (6.7–13.8); BPS (3.8–9.3) |
(i) BPA (30%); BPS (0%) (ii) BPA (<0.20b−40.0d); BPS (<0.10b) |
Vela-Soria et al. (2014b) |
| (vii) 10.0 min. | ||||||
| 6 | (i) n = 94 (ii) China (iii) 2013 |
(i) Urine (ii) 2 mL (iii) 5 μL |
(i) Two different steps: (a) Yes for analyzing
total form (free + conjugated); and (b) No for the analysis of free form
(unconjugated). (ii) LLE (ethyl acetate) (iii) BPS-13C12; BPF-d10; BPA-d4; TCBPA-13C12; TBBPA-13C12 UPLC-MS/MS (ESI, −ve mode) (v) Acquity BEH Cl8 column (100 mm × 2.1 mm × 1.7 μm) (vi) [A] Methanol; [B] Water (vii) 8.0 min. |
(i) BPA (0.09); BPS (0.010); BPF (0.10); BPAF
(0.008). (ii) BPA (0.27); BPS (0.032); BPF (0.31); BPAF (0.024). (iii) BPA (93.7–106.7); BPS (82.5–104.4); BPF (83.2–103.6); BPAF (93.4–116.8); BPB (86.2–98.6); TBBPA (90.2–104.8); TCBPA (81.6–97.8). (iv) <16.4% (for all the analytes) |
(i) BPA (97%a); BPS (40%a); BPF
(20%a);
BPAF (20%a); BPB
(0%a);
TBBPA (0%a). (ii) BPA (<0.09b−8.073c,d); BPS (<0.01b−7.046c,d); BPF (<0.10b−1.207c,d); BPAF (<0.008b−0.217c,d); BPB (<0.04b); TBBPA (<0.04b). |
Yang et al. (2014a) |
| 7 | (i) n = 100 (ii) USA (iii) 2009–2012 |
(i) Urine (ii) 100 μL (iii) 350 μL |
(i) Two different steps: (a) Yes for analyzing
total form (free + conjugated); and (b) No for the analysis of free form
(unconjugated). (ii) On-line SPE (LiChrospher RP-18ADS (25 mm × 4 mm × 25 μm; 60°A) (iii) BPA-13C12; BPS-13C12 (iv) HPLC–MS/MS (APCI, −ve mode) (v) Chromolith High Resolution RP-18e (100 mm × 4.6 mm) (vi) [A] Water; [B] Methanol (vii) 19.0 min. |
(i) BPA (0.1); BPS (0.03); BPF
(0.06) (ii) n.a. (iii) BPA (99–104); BPS (104–107); BPF (91–103) (iv) BPA (5.4–5.9); BPS (6.1–6.4); BPF (6.7–12.1) |
(i) BPA (95%); BPS (78%); BPF
(55%) (ii) BPA (<0.10b−37.7d); BPS (<0.03b−12.3d); BPF (<0.06b−212.0c,d) |
Zhou et al. (2014) |
| 8 | (i) n = 30 (ii) France (iii) n.a. (iv) Multiple BPA analogs |
(i) Breast milk (ii) 3 g (iii) 2 μL |
(i) Yes for the total form (unconjugated +
conjugated) (ii) Two successive SPE (first: polystyrene-divinylbenzene stationary phase (HR-X); and second: molecularly imprinted polymers stationary phase (MIP)). (iii) BPA-13C12 (internal standard); Biphenyl-2,2′-diol (external standard) (iv) GC-MS/MS (electron impact, MRM) (v) Optima 17 MS column (30 m × 0.25 mm × 0.25 μm) (vi) Helium (carrier gas) (vii) 19.0 min. |
(i) BPA (<0.003c); BPS (0.001c); BPF
(0.006c);
BPAF (0.001c);
BPB (0.006c); BPM
(0.002c);
BPP (0.004c);
BPAP (0.002c);
BPBP (0.002c);
BPC (0.003c);
BPC2 (0.001c);
BPE (0.006c);
BPPH (0.003c);
BPFL (0.004c);
BPZ (0.002c) (ii) BPA (<0.01c); BPS (0.003c); BPF (0.018c); BPAF (0.003c); BPB (0.020c); BPM (0.010c); BPP (0.012c); BPAP (0.007c); BPBP (0.006c); BPC (0.009c); BPC2 (0.003c); BPE (0.018c); BPPH (0.009c); BPFL (0.012c); BPZ (0.006c) (iii) BPA (94–105); BPS (93–100); BPF (103–109); BPAF (90–100); BPB (96–102); BPM (0%); BPP (0%); BPAP (90–100); BPBP (99–109); BPC (92–97); BPC2 (93–102); BPE (94–102); BPPH (93–102); BPFL (96–103); BPZ (97–103) (iv) BPA (13–20) |
(i) BPA (90%); BPS (3%); BPF (0%); BPAF (0%);
BPB (0%); BPM (0%); BPP (0%); BPAP (0%); BPBP (0%); BPC (0%); BPC2 (0%);
BPE (0%); BPPH (0%); BPFL (0%); BPZ (0%) (i) BPA (<0.01c,e−1.16d); BPS (<0.003c,e−0.23d); BPF (<0.018c,e); BPAF (<0.003c,e); BPB (<0.020c,e); BPM (<0.010c,e); BPP (<0.012c,e); BPAP (<0.007c,e); BPBP (<0.006c,e); BPC (<0.009c,e); BPC2 (<0.003c,e); BPE (<0.018c,e); BPPH (<0.009c,e); BPFL (<0.012c,e); BPZ (<0.006c,e) |
Deceuninck et al. (2015) |
| 9 | (i) n = 76 (ii) India (iii) 2012–2013 |
(i) Urine (ii) 500 μL (iii) 10 μL |
(i) Yes for the total form (unconjugated +
conjugated) (ii) LLE (ethyl acetate) (iii) BPA-13C12; BADGE-D6 (iv) HPLC-MS/MS (ESI, +ve mode)f (v) Javelin guard column (Betasil Cl8,20 mm × 2.1mm × 5 μm) + Betasil C18 (100 mm × 2.1 mm × 5 μm) (vi) (a) for BPA: [A] Methanol; [B] Water; and (b) for BADGEs: [A] Methanol; [B] Water/methanol (90:10, v/v) with ammonium acetate (1.5%, w/v). (vii) 20.0 min. |
(i) n.a. (ii) BPA (0.10); BPS (0.02); BPAF (0.01); BPAP (0.01); BPB (0.01); BPP (0.01); BPZ (0.01); BADGE (0.10); BADGE·H2O (0.20); BADGE·HCl(0.02); BADGE·2H2O (0.10); BADGE· 2HCl (0.05); BADGE·H2O·HCl (0.50); BFDGE (1.00); BFDGE·2H2O (2.00); BFDGE·2HCl (0.50). (iii) n.a. (iv) n.a. |
(i) BPA (99%); BPS (70%); BADGE (99%);
BADGE·2H2O (78%) (ii) BPA (<0.10e−41.4d); BPS (<0.10e−12.2d); BADGE (<0.10e−295d,f); BADGE·2H2O (<0.10e−1450d,f) |
Xue et al. (2015) |
Bisphenol A [2,2-bis(4-hydroxyphenyl)propane, (BPA)]; bisphenol B [2,2-bis(4-hydroxyphenyl)butane, (BPB)]; bisphenol AP [1,1-bis(4-hydroxyphenyl)-1-phenyl-ethane, (BPAP)]; bisphenol AF [2,2-bis(4-hydroxyphenyl)hexafluoropropane, (BPAF)]; bisphenol BP [bis-(4-hydroxyphenyl)diphenylmethane, (BPBP)]; bisphenol C [2,2-bis(3-methyl-4-hydroxyphenyl)propane, (BPC)]; bisphenol Cl2 [bis(4-hydroxyphenyl)-2,2-dichlorethylene, (BPC2)]; bisphenol E [1,1-bis(4-hydroxyphenyl)ethane, (BPE)]; bisphenol PH [5,5′-(1-methylethyliden)-bis[1,1′-(bisphenyl)-2-ol]propane, (BPPH)]; bisphenol S [bis(4-hydroxyphenyl)sulfone, (BPS)]; bisphenol F [bis(4-hydroxydiphenyl)methane, (BPF)]; bisphenol FL [9,9′-bis(4-hydroxyphenyl)fluorene, (BPFL)]; bisphenol Z [1,1-bis(4-hydroxyphenyl)-cyclohexane, (BPZ)]; bisphenol M [1,3-bis(2-(4-hydroxyphenyl)-2-propyl)benzene, (BPM)]; bisphenol P [1,4-bis(2-(4-hydroxyphenyl)-2-propyl)benzene, (BPP)]; Bisphenol A diglycidyl ether (BADGE); bisphenol A (2, 3-di-hydroxypropyl) glycidyl ether (BADGE·H2O); bisphenol A (3-chloro-2-hydroxypropyl) glycidyl ether (BADGE·HCl); bisphenol A bis(2,3-dihydroxypropyl) glycidyl ether (BADGE·2H2O); bisphenol A bis (3-chloro-2-hydro-xypropyl) glycidyl ether (BADGE·2HCl); bisphenol A (3-chloro-2-hydroxypropyl)(2,3-dihydroxypropyl) glycidyl ether (BADGE·H2O·HCl); bisphenol F diglycidyl ether (BFDGE); Bisphenol F bis(3-chloro-2-hydroxypropyl) glycidyl ether (BFDGE·2HCl); bisphenol F bis(2,3-dihydroxypropyl) glycidyl ether (BFDGE·2H2O); tetrachlorobisphenol A (TCBPA); tetrabromobisphenol A (TBBPA).
Data interpreted from a figure and hence a visual approximation.
Limit of detection (LOD).
μg kg−1.
Range.
Limit of quantification (LOQ).
LC–MS/MS (ESI, +ve mode) for BADGE analysis.
2.4.1. Sample pretreatment and extraction
Considering the diverse composition of each human biospecimen matrices, a pretreatment step either to remove interfering matrix components or to facilitate the enzymatic deconjugation of BPA and/or its derivatives or analogs is warranted. Phase II metabolism in humans facilitates the biotransformation of BPA and its derivatives to yield glucuronide and sulfate conjugates that are eventually excreted in urine; such evidence for chlorinated derivatives or structural analogs are lacking so far. In the case of urine samples, sample pre-treatment refers to hydro-lysis of conjugated forms (e.g., glucuronidated and sulfonated) to respective unconjugated/free forms of BPA and ClxBPA using a β-glucuronidase/sulfatase enzyme (Kalyvas et al., 2014; Liao and Kannan, 2012; Vela-Soria et al., 2014b; Yang et al., 2014a). This procedure provides a total bisphenol concentration comprised of both conjugated and unconjugated forms. Because conjugated forms have been traditionally considered having minimal estrogenic activity, few research groups measured only the unconjugated (free) forms of BPA and ClxBPA in urine, occurring at much lower concentrations than the corresponding conjugated forms (Liao and Kannan, 2012; Venisse et al., 2014). Also, the lipophilic nature of ClxBPA compounds is responsible for their accumulation in lipid-rich tissues, hence, deconjugation step was not performed for the analysis of adipose tissue (Fernandez et al., 2007), placenta (Jimenez-Diaz et al., 2010; Vela-Soria et al., 2015), and breast milk (Cariot et al., 2012; Migeot et al., 2013; Rodriguez-Gomez et al., 2014a,b).
During the sample pretreatment step, first interfering endogenous compounds such as salts, lipids and proteins were removed, while BPA, ClxBPA and structural BPA analytes were concentrated using sample clean-up procedures such as protein precipitation, liquid–liquid extraction (LLE), and solid-phase extraction (SPE) (Tables 3 and 5). Protein precipitation with an organic modifier and acid mixture was usually performed on breast milk samples (Rodriguez-Gomez et al., 2014a,b), while salting out with ammonium formate was used for urine analysis (Venisse et al., 2014), followed by centrifugation. LLE is a popular procedure for cleaner extracts and greater extraction sensitivity, and it is performed either alone or in combination with SPE. LLE is typically preceded by an alkalization step and/or enzyme hydrolysis step. LLE for ClxBPA extraction from human matrices was performed with a wide range of solvents such as (i) acetonitrile for adipose (Fernandez et al., 2007), (ii) ammoniacal solution (Jimenez-Diaz et al., 2010), and ammonia in methanol and ammoniacal solution mixture (Vela-Soria et al., 2011) for placenta, (iii) methanol (Cariot et al., 2012) and acetonitrile (Rodriguez-Gomez et al., 2014a,b) for breast milk, (iv) ethyl acetate for serum (Liao and Kannan, 2012), (v) ethyl acetate (Liao and Kannan, 2012), ethyl acetate and hexane mixture, acetone and trichloromethane mixture (Vela-Soria et al., 2014b), acetonitrile and ammonium formate mixture (Venisse et al., 2014), and acetonitrile and ethyl acetate mixture (Yang et al., 2014a) for urine. Typical sample volumes used for LLE were in the range of 0.5–9.9 mL of breast milk (Cariot et al., 2012; Rodriguez-Gomez et al., 2014a; Rodriguez-Gomez et al., 2014b). LLE is succeeded by evaporation of the organic extractant and reconstitution in a LC mobile phase or GC solvent. Recoveries were affected by the initial sample volume used in LLE. For example, ClxBPA recoveries in breast milk were in the range of 81–119% from a 0.5 mL sample volume (Cariot et al., 2012) compared to 92–110% with 9.9 mL sample (Rodriguez-Gomez et al., 2014a,b). Similar was the case in urine with recoveries in the range of 37–45% from 0.3 mL (Venisse et al., 2014) versus 98–104% from 5.0 mL urine sample volume (Vela-Soria et al., 2014b).
Stir-bar sorptive extraction (SBSE) was applied for the first time to extract ClxBPA along with BPA, parabens and benzophenones from breast milk (Rodriguez-Gomez et al., 2014b). BSE is based on the principles of solid-phase micro-extraction (SPME), relying on the equilibrium process between the sorbent and sample (Baltussen et al., 1999). Unlike conventional SPME, SBSE showed higher analytes extraction capacity due to sorbent’s larger surface area (David and Sandra, 2007; De Coensel et al., 2009; Kawaguchi et al., 2006; Rodriguez-Gomez et al., 2014b). Polydimethylsiloxane coated stir bar (20 mm length × 0.5 mm thickness) was used as sorptive extraction phase to preconcentrate ClxBPA in breast milk. SBSE parameters were optimized in regards to matrix modifiers, sample volume, ionic strength, extraction time, stirring speed, and desorption time and solvent for obtaining an enhanced sensitivity and performance (precision and trueness). Achieved recoveries were greater than 90% for the four ClxBPA analytes. Moreover, this method yielded successful extraction and recovery of 14 analytes from three different chemical classes (Rodriguez-Gomez et al., 2014b). Further research from the same group obtained similar or better recoveries (~100%) of multi-class analytes from breast milk by using a simple extraction protocol to precipitate proteins and fats with a mixture of zinc acetate, phosphotungstic acid and glacial acetic acid (Rodriguez-Gomez et al., 2014a).
Dispersive liquid–liquid micro-extraction (DLLME) is gaining attention as a useful alternative to LLE because of its simplicity, cost and time- efficiency, while enhancing analytes recovery and enrichment factor (Rezaee et al., 2006). DLLME applies the working principle of mixing an extract with high-density solvent and disperser with water miscible polar solvent, which speeds up analytes mass transfer process when rapidly comes in contact with the sample. DLLME has been applied for the extraction of BPA and other environmental phenols in human matrices (Cunha and Fernandes, 2010; Tarazona et al., 2013; Vela-Soria et al., 2013), and also for chlorinated derivatives and structural analogs of BPA in human urine (Vela-Soria et al., 2014b). DLLME procedure appears to require a small sample volume of 5 mL human urine (Vela-Soria et al., 2014b) compared to SBSE that utilized 9.9 mL breast milk (Rodriguez-Gomez et al., 2014b), but provided comparable recoveries for both BPA and ClxBPA (>90%) (Rodriguez-Gomez et al., 2014b; Vela-Soria et al., 2014b).
Solid phase extraction (SPE) of BPA and its chlorinated derivatives in human matrices was performed using conventional sorbents, such as, (i) reversed-phase Octadecylsilane (ODS)-C18 for adipose tissue (Fernandez et al., 2007) (ii) C8 sorbent for breast milk (Cariot et al., 2012; Migeot et al., 2013), and (iii) a new approach of combining sorbents, such as NH2 (a weak anion-exchange sorbent) and a mixed-mode MCX (a reversed-phase and strong cation-exchange sorbent) for serum and urine analysis (Liao and Kannan, 2012). On-line SPE (Cariot et al., 2012; Migeot et al., 2013) and manually-packed SPE (Vela-Soria et al., 2015) were also used. Vela-Soria et al. (2015) tested several clean-up sorbents made of C18, silica, florisil, alumina and a poly secondary amine (PSA) by packing each of these manually into polypropylene cartridges. PSA sorbent was selected, because it demonstrated best extraction efficiency and minimal attenuation of relative signal for ClxBPA in human placenta. In general, SPE required significantly smaller volume of solvents compared to LLE, while providing higher analyte selectivity and recovery. For example, extraction and clean-up of placental tissue with SPE required 0.25 g (Vela-Soria et al., 2015) compared to 1.5 g by using LLE (Vela-Soria et al., 2011). Similarly, 0.5 mL urine sample volume was required for SPE (Liao and Kannan, 2012) compared to LLE that required urine in the range of 0.3–5.0 mL (Vela-Soria et al., 2014b; Venisse et al., 2014). Comparable recoveries of ClxBPA were obtained from placenta using SPE (0.25 g, 98–105%) (Vela-Soria et al., 2015) and LLE protocols (1.5 g, 96–102%) (Vela-Soria et al., 2011), respectively; however, this was not the case with urine. For example, SPE yielded recoveries in the range of 78–129% from 0.5 mL urine (Liao and Kannan, 2012) compared to 37–45% from using a 0.3 mL urine with LLE (Venisse et al., 2014). Further information on the re-agents, solvents and solutions, and conditions used during the sample pretreatment of human matrices, and extraction and clean-up for ClxBPA analysis were detailed in Table 3. Precision of the human sample extraction and clean-up protocols, represented as percent relative standard deviation, were comparable and acceptable for LLE and SPE (relative standard deviation < 20%) (Table 4).
Table 4.
Biomonitoring of chlorinated derivatives of bisphenol A in human tissue and matrices.
| Table 4. Item # | Biomarker of exposure to BPA and its chlorinated derivatives | Study objective(s) | Study location Sampling year Population Age BMI Sample size |
Human bio-matrix |
Analytical method |
Analytical method performance | Detection rate [n (%)] |
Concentration | Study Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD / LOQ (ng mL-−1) |
Recovery (%) [L: low level spike; H: high level spike] | RSD (%) | |||||||||
| 1 | Chlorinated-BPA • ClBPA • Cl2BPA • Cl3BPA • Cl4BPA |
SPME-based analytical method development to quantify ClXBPA in human plasma. | (i) Spain (ii) n.a. (iii) Healthy volunteers (iv) n.a. (v) n.a. (vi) N = 9 |
Plasma | GC-MS | • 0.5 A/
0.8 B • 0.5 A/ 0.8 B • 2.7 A / 4.5 B • 3.0A/ 5.0 B A Decision limit B Detection capability |
94–109% (for all the analytes) |
n.a. | n.a. | n.a. | del Olmo et al. (2005) |
| 2 | BPA(f-free)
Chlorinated-BPA • ClBPA • Cl2BPA • Cl3BPA • Cl4BPA |
Quantify BPA and ClXBPA in adipose tissue from women. | (i) Spain (ii) n.a. (iii) Adult females Mean (SD): (iv) 59.7 (14.1) years (v) 31.9 (11.5) kgm−2 (vi)N = 20 |
Adipose tissue |
GC-MS | • 0.5 / n.a. • 0.5 / n.a. • 0.5 / n.a. • 2.7 / n.a. • 3.0 / n.a. |
95–105% (for all the analytes) |
n.a. | • 11 (55%) • 3 (15%) • 16 (80%) • 2 (20%) • 0 (0%) |
• 5.83
(3.48)Y • 3.05 (0.28)Y • 9.21 (9.26)Y • 0.74 (0.15)Y • <LODx Mean (SD)X ng mL−1 Y ng g−1tissue |
Fernandez et al. (2007) |
| 3 | • BPA(f-free)
Chlorinated-BPA • ClBPA • C12BPA • Cl3BPA • Cl4BPA |
Method development for “free” BPA and ClXBPA in placenta |
(i) Spain (ii) n.a. (iii) Females (iv) n.a. (v) n.a. (vi) N = 49 |
Placenta tissue |
LC-MS/MS | • 0.20Y /
0.50Y • 0.30Y / 1.00Y • 0.30Y / 1.00Y • 0.40Y / 1.40Y • 0.60Y / 2.00Y Y ng g−1 tissue |
• L(0.50): 99,
H(30.0): 99 • L(0.50): 97, H(30.0): 100 • L(0.50): 98, H(30.0): 99 • L(0.50): 101, H(30.0): 101 • L(0.50): 97, H(30.0)): 101 L(0.50): 0.5 ng g−1 H (30.0):,30ng g−1 |
• 4.9,2.5 • 8.1,2.2 • 4.5,1.8 • 5.1,1.9 • 5.1,2.4 |
• 10 (20%) • 25 (51%) • 25 (51%) • 24 (49%) • 0 (0%) |
•
<LOD-34.9Y • <LOD-21.4Y • <LOD-58.8Y • <LOD-31.2y • <LODy Range Y ng g−1 tissue |
Jimenez-Diaz etal. (2010) |
| 4 | • BPA(f-free)
Chlorinated-BPA • ClBPA • Cl2BPA Cl3BPA Cl4BPA |
Multi-class method for environmental phenols in human placenta. | (i) Spain (ii) n.a. (iii) Volunteers (iv) n.a. (v) n.a. (vi) N = 50 |
Placenta tissue |
LC-MS/MS | •
0.2Y/0.5Y • 0.3Y/1.0Y • 0.3Y/1.0Y • 0.4Y/1.4Y • 0.6Y/2.0Y Y ng g−1 tissue |
• L(5): 99,
H(30): 99 •L(5): 97,H(30): 100 • L(5): 98, H(30): 99 • L(5):102, H(30): 101 L(5): 96, H(30): 101 L(5) :5ng g−1 H(30): 30 ng g−1 |
•5, 2 • 8, 2 • 5, 2 • 5,2 • 5,2 |
• 20 (40%) • 0 (0%) • 0 (0%) • 0 (0%) • 0 (0%) |
•
<LOD-12.7Y • <LODY • <LODY • <LODY • <LODY Range Y ng g−1 tissue |
Vela-Soria et al. (2011) |
| 5 | • BPA(f-free)
Chlorinated-BPA • ClBPA • 2,6-Cl2BPA • 2,2-Cl2BPA • Cl3BPA |
Develop a method for unconjugated BPA and ClXBPA in human breast milk. | (i) France (ii) n.a. (iii) Females (iv) n.a. (v) n.a. (vi) N = 3 |
Breast milk | LC-MS/MS | • 0.09/0.40 • 0.01/0.40 • 0.05/0.40 • 0.05/0.40 • 0.04/0.40 |
• L(0.4): 101,
H(32): 93 • L(0.4): 90, H(3.2): 81 • L(0.4): 81,H(3.2) 107 • L(0.4): 91, H(32): 119 • L(0.4): 103, H(3.2): 91 L(0.4): 0.4 ng mL−1 L(3.2): 3.2 ng mL−1 |
• 15, 1 • 6, 15 • 18, 20 • 6, 2 • 18, 7 |
• 3 (100%) • 0 (0%) • 3 (100%) • 3 (100%) • 1 (33%) |
•
0.80–3.49X • <LODX • <LOQ-1.40X • <LOQ-4.13X • <LOD-0.68X Range X ng mL. −1 |
Cariot et al. (2012) |
| 6 | •
BPA(f-free,t-total)
Chlorinated-BPA • ClBPA • Cl2BPA • Cl3BPA |
Determination of free and conjugated BPA forms, and C1XBPA in human urine and serum matrices. | (i) USA (ii) 2011 (iii) Healthy volunteers Range: (iv) 11–66 years (v) n.a. (vi)N = 31 (urine) and 14 (serum) |
Urine(U) Serum(s) |
LC-MS/MS | • 0.003/0.01 • 0.02/0.05 • 0.02/0.05 • 0.02/0.05 |
L(10)u: 78–123%
for all analytes in urine; • H(100)U 78–129% for all analytes in urine. • L(10)s: 72–118% for all analytes in serum; H(100)s 76–123% for all analytes in serum. L(10): 10 ng H(100): 100 ng |
• 5–16% • 2–19% • 5–11% • 8–18% |
• 30(96.8%)Uf,
8(57.1%)Sf,
31(100%)Ut,
14(100%)St • 5(16.1%)U, 0(0%)S • 6(19.4%)U, 0(0%)S • 6(19.4%)U, 0(0%)S |
•
<LOQ-18.7UfX,
<LOQ-12.8UfV,
<LOQ-0.588SfX,
0.222–66.2Utx,
0.093–40.4UtV,
<LOQ-13.8St,X • <LOQ-1.68Utx, • <LOQ-2.31UtV, • <LODStX • <LOQ-1.06UtX, |
Liao and Kannan (2012) |
| <LOD-0.617UtV
<LODStX •
<LOQ.-0.675UtX,<LOQ-0.394UtV <LODStX RangeXng mL−1 V μg g−1Cr |
|||||||||||
| 7 | •BPA(f-free)
Chlorinated-BPA • ClBPA • 2,6-Cl2BPA • 2,2-Cl2BPA • Cl3BPA |
Develop a method for unconjugated BPA and ClXBPA in human breast milk. | (i) France (ii) n.a. (iii) Females Mean (SD): (iv)33 (4) years (v) 22.1 (2.8) kg m−2 (vi) N = 21 |
Colostrum (Breast milk) |
LC-MS/MS | • 0.09/0.40 • 0.01 / 0.40 • 0.05/0.40 • 0.05/0.40 • 0.04/0.40 |
Range: 80–120% for all the analytes |
≤20% | • 19(90%) • 0 (0%) • 21 (100%) • 11(52%) • 4 (19%) |
•
<LOD-6.12X • <LODX • <0.4(LOQ)-2.89- X • <LOM.13X • <LOD-O.68X |
Migeot et al. (2013) |
| 8 | • BPA(t-total)
Chlorinated-BPA • ClBPA |
Find associations between domestic activities
that involve chlorine-based cleaning products and mono-chlorinated BPA levels in urine. |
(i) Cyprus (ii) 2012 (iii) Adults Mean (SD):(iv) 51 (17) years (v) 26 (5) kg m −2 (vi) N = 224 |
Urine | GC-MS/MS | • 0.095/0.319 • 0.032/0.108 |
• L(0.1)
>80%, H(1.5):>80% •L(0.1) >80%, H(1.5): >80% L(0.1): 0.1 ng mL−1 L(1.5): 1.5ng mL−1 |
5% (inter- and intra-day) | •224(100%) • 202(90%) |
Range X ng
mL.−1 • 3.75 (7.63)X 2.85 (4.38)U • 0.08 (0.05)X 0.12 (0.14)U Mean (SD)Xng mL−1 Ung g−1Cr |
Kalyvas et al. (2014) |
| 9 | • BPA(f-free)
Chlorinated-BPA • ClBPA • Cl2BPA • Cl3BPA • Cl4BPA |
Development of a method for simultaneous quantification of environmental phenols based on sample preparation of fat and proteins precipitation. | (i) Spain (ii) n.a. (iii) Females (iv) n.a. (v) n.a. (vi) N = 10 |
Breast milk | LC-MS/MS | • 0.05/0.15 • 0.04/0.12 • 0.04/0.12 • 0.04/0.14 • 0.04/0.13 |
• L(0.50):
109.8, H(25.0): 100.2 • L(0.50): 102.6, H(25.0): 100.4 • L(0.50) 108.0, H(25.0): 100.5 • L(0.50): 93.1, H(25.0): 100.8 • L(0.50): 110.0, H(25.0): 100.3 |
• 4.6, 3.5 • 5.4, 1.5 • 6.9, 2.9 • 4.2, 5.2 • 3.2, 2.0 |
• 6(60%) • 0 (0%) • 2(20%) • 0 (0%)• 0 (0%) | •
<LOD-13.8X • <LODX • <LOD-O.4X • <LODX • <LODX Range X ng ml.−1 |
Rodriguez-Gomez et al. (2014a) |
| L(0.50):0.5 ng
mL−1 H(25.0):25 ng mL−1 |
|||||||||||
| 10 | • BPA(f-free)
Chlorinated-BPA • ClBPA • C12BPA • Cl3BPA • Cl4BPA |
GC and LC based methods development for selected endocrine disrupting chemicals in human breast milk. | (i) Spain (ii) n.a. (iii) Healthy women (iv) n.a. (v) n.a. (vi) N = 10 |
Breast milk | [A] LC-MS/MS [B] GC-MS/MS |
• LC:0.1/0.3,
GC:0.2/0.5 • LC:0.1/0.2, GC:0.1/0.5 • LC:0.1/0.3,GC: 0.3/1.0 • LC:0.2/0.5,GC:l.0/3.0 • LC: 0.3/1.0,GC:l.5/5.0 • LC: 0.3/1.0, GC:1.5/5.0 |
• L(1.0)LC: 106, H(100)LC: 99, L(1.0)GC:100, ,H(100)GC 100 go L(1.0)LC: 92, H(100)LC:100, L(1.0)GC:95, H(100)GC: 100 • L(1.0)LC: 109, H(100)LC:100, L(1.0)GC: 92, H(100)GC: 99 • L(1.0)LC: 108, H(100)LC: 98, L(1.0)GC: 106, H(100)GC: 105 L(1.0)LC: 106, H(100)LC: 98, L(1.0)GC: 94, H(100)GC: 96 L(1.0)GC: 1.0 ng mL−1 H(100): 100 ng mL−1 |
• 8.0, 2.8, 8.4, 3.9 • 7.3, 3.4, 7.3, 3.4 • 8.1, 5.3, 5.8, 5.5 • 5.5, 3.1, 5.7, 3.5 • 9.6, 4.0, 8.6, 3.3 |
• 8 (80%)LC, 8 (80%) GC • 0 (0%)LC, 0 (0%) GC • 0 (0%)LC, 0 (0%) GC • 0 (0%)LC, 0 (0%)GC • 0 (0%)LC, 0 (0%)GC |
•
<LOD-11.5XLC,
<LOD-10.8XGC • <LODX • <LODX • <LODX • <LODX Range X ng mL.−1 |
Rodriguez-Gomez et al. (2014b) |
| 11 | • BPA(f-free,
t-total)
Chlorinated-BPA C1BPA • Cl2BPA • Cl3BPA • Cl4BPA |
Single method for multi-classes of environmental phenols, and their concentration in free and total (free + conjugated) forms in human urine. | (i) Spain (ii) n.a. (iii) Male and female volunteers (iv) n.a. (v) n.a. (vi) N = 20 |
Urine | LC-MS/MS | • 0.2/0.6 • 0.03/0.1 • 0.03/0.1 • 0.03/0.1 • 0.03/0.1 |
• L(2): 102, H(40): 98 • L(2): 103, H(40): 99 • L(2): 98, H(40): 98 • L(2):102, H(40): 104 L(2): 98, H(40): 103 L(2):2 ng mL−1 H(40): 40 ng mL−1 |
• 13.8, 6.7 • 7.8, 3.1 • 4.1, 4.4 • 11.7, 4.5 • 11.3, 5.6 |
• 6 (30%)f, 6
(30%)t • 0 (0%) • 0 (0%) • 0 (0%) • 0 (0%) |
•
<LOD-4.3fX,
<LOD-40.0tX • <LODX • <LODX • <LODX • <LODX RangeXng mL−1 |
Vela-Soria et al. (2014) |
| 12 | • BPA(f-free)
Chlorinated-BPA • ClBPA • 2,6-Cl2BPA • 2,2-Cl2BPA • Cl3BPA • Cl4BPA |
Develop a method for unconjugated BPA and ClXBPA in human urine. | (i) France (ii) n.a. (iii) Donors (iv) n.a. (v) n.a. (vi) N = 10 |
Urine | LC-MS/MS | • 0.048/0.5 • 0.014/0.05 • 0.009/0.05 • 0.023/0.05 • 0.018/0.05 • 0.014/0.05 |
• L(1): 34.5 (20.0),
H(S): 33.0 (16.6) • L(0.1):41.2 (8.2), H(0.8): 36.5 (6.7) • L(0.1): 41.3 (8.0), H(0.8): 39.9 (8.2) • L(0.1)45.1 (17.3), H(0.8): 39.9 (8.0) • L(0.1): 38.7 (9.2), H(0.8): 39.8 (11.6) • L(0.1): 38.1 (13.6), H(0.8): 36.6 (9.2) L(1): 1 ng mL−1 L(8):0.8 ng mL−1L(0.1):0.1 ng mL−1 L(0.8):0.8 ng mL −1 |
n.a. | • 5 (50%) • 6 (60%) • 4 (40%) • 2 (20%) • 4 (40%) • 4 (40%) |
•
<LOQ-l.378X • <LOQ-0.202X • <LOQ-0.109X • <LOQX • <LOQ-0.368X • <LOQ-l.501X RangeXng mL−1 |
Venisse et al. (2014) |
| 13 | • BPA(t- total)
Chlorinated-BPA • Cl4BPA |
To monitor urinary BPA analogues in residents near a bisphenol F manufacturing unit. | (i) China (ii) 2013 (iii) Adults Range: (iv) 26–84 years (v) n.a. (vi) N = 94 |
Urine | LC-MS/MS | • 0.09/0.27 • 0.01 / 0.03 |
• Range(X):
93.7–106.7% • Range(X): 81.6–97.8% Range (X) spiked concentrations at 2, 5, and 10 times the LOQ for the respective analyte. |
<16.4% for all analytes | • n.a. (~70%) • 0 (0%) |
• <LOD-4.480X,
<LOD-8.073V • <LODX,<LODV RangeX ng mL−1 Vμg g−1Cr |
Yang et al. (2014a) |
| 14 | • BPA(f-free)
Chlorinated-BPA • ClBPA • Cl2BPA • Cl3BPA • Cl4BPA |
Simultaneous measurement of multi-residue environmental phenols in human placenta. | (i) Spain (ii) n.a. (iii) Volunteers (iv) n.a. (v) n.a. (vi) N = 10 |
Placenta tissue | LC-MS/MS | •
0.1Y/0.3Y • 0.1Y/0.4Y • 0.1Y/0.3 Y • 0.1Y/0.3 Y • 0.1Y/0.3 Y Yng g−1tissue |
• L(0.50): 104,
H(20.0): 101 • L(0.50): 103, H(20.0): 102 • L(0.50): 105, H(20.0): 102 • L(0.50): 99, H(20.0):100 • L(0.50): 100, H(20.0): 98 L(0.50): 0.5 ng g−1 H(20.0):,20 ng g−1 |
• 14.8, 10.4 • 8.9, 7.1 • 10.7, 7.9 • 12.4, 8.7 • 13.6, 7.1 |
• 5(50%) • 0 (0%) • 0 (0%) • 0 (0%) • 0 (0%) |
•
<LOD-14.5Y • <LODY • <LODY • <LODY • <LODY Range Yng g−1tissue |
Vela-Soria et al. (2015) |
2.4.2. Analyte separation, detection, and quantification
Separation of ClxBPA and BPA structural analogs in extracts of human matrices has been primarily achieved by either liquid (LC) or gas chromatography (GC) techniques (Tables 3 and 5). Analysis of ClxBPA in human matrices using LC-based methods require larger injection volume (range: 5–50 μL) (Migeot et al., 2013; Yang et al., 2014a) and shorter analysis time per sample (range: 7–20 min.) (Jiménez-Díaz et al., 2010; Liao and Kannan, 2012; Vela-Soria et al., 2011), compared to the reported GC protocols (range: 1–20 μL, 14– 26 min.) (Kalyvas et al., 2014; Rodriguez-Gomez et al., 2014b). Use of a C18-reversed phase column was reported in all studies that employed LC: (i) Gemini C18 (Jimenez-Diaz et al., 2010; Vela-Soria et al., 2011) and Acquity BEH C18 (Vela-Soria et al., 2015) for placenta, (ii) Acquity CSH C18 (Cariot et al., 2012; Migeot et al., 2013) and Acquity BEH C18 (Rodriguez-Gomez et al., 2014a,b) for breast milk, (iii) Betasil C18 for serum (Liao and Kannan, 2012), and (iv) Betasil C18 (Liao and Kannan, 2012) and Acquity BEH C18 (Vela-Soria et al., 2014b; Yang et al., 2014a) for urine. A commonly used mobile phase in these studies was methanol with solvent modifiers such as ammonia (Jimenez-Diaz et al., 2010; Rodriguez-Gomez et al., 2014a,b; Vela-Soria et al., 2011), ammonium acetate (Liao and Kannan, 2012) and ammonium formate (Vela-Soria et al., 2015; Vela-Soria et al., 2014b) as proton acceptors. Individual study details on the LC conditions including (i) LC column characteristics, (ii) binary solvent composition and pH, (iii) mobile phase gradient, flow duration and rate, and (iv) column temperature are presented in Table 3 (BPA derivatives) and Table 5 (BPA structural analogs).
GC-based separation of ClxBPA, structural BPA analogs and BPA was achieved following derivatization step of native non-volatile analytes to GC-amenable volatile derivatives. This procedure was followed for the analysis of adipose (Fernandez et al., 2007), urine (Kalyvas et al., 2014), and breast milk (Rodriguez-Gomez et al., 2014b). The GC conditions including (i) derivatization reagents and steps, (ii) GC column characteristics, (iii) injector mode and temperature, (iv) carrier gas and flow rate, (v) injector temperature ramp program, and (vi) oven temperature, gradient and duration are available in Tables 3 and 5.
Mass spectrometry has been the preferred detection technique for ClxBPA extracted from human matrices (Tables 3 and 4). Quantification with highly sensitive tandem mass spectrometry methods (MS/MS) are widely preferred (12 out of 14 studies), except for two studies that utilized a less sensitive single quadruple mass spectrometer (mass selective detector/MSD) (del Olmo et al., 2005; Fernandez et al., 2007). The applied methods were (i) LC–MS/MS for placenta (Jimenez-Diaz et al., 2010; Vela-Soria et al., 2011; Vela-Soria et al., 2015), breast milk (Cariot et al., 2012; Migeot et al., 2013; Rodriguez-Gomez et al., 2014a, b), serum (Liao and Kannan, 2012), urine (Liao and Kannan, 2012; Vela-Soria et al., 2014b; Venisse et al., 2014; Yang et al., 2014a) (ii) GC–MS/MS for urine (Kalyvas et al., 2014) and breast milk (Rodriguez-Gomez et al., 2014b), and (iii) GC-MSD for adipose tissue (Fernandez et al., 2007). The preferred ionization mode for the LC–MS/MS analysis was electrospray ionization (Cariot et al., 2012; Liao and Kannan, 2012; Migeot et al., 2013; Rodriguez-Gomez et al., 2014b; Vela-Soria et al., 2014b; Vela-Soria et al., 2015; Venisse et al., 2014; Yang et al., 2014a) followed by atmospheric pressure chemical ionization (APCI) (Jimenez-Diaz et al., 2010; Vela-Soria et al., 2011), and the mass spectrometer polarity in either ionization was a negative ion mode. APCI mode was reported to give better sensitivity and lower detection limits compared to the ESI mode for ClxBPA in placental tissue (Vela-Soria et al., 2011). This is probably because APCI mode is less prone to matrix effects compared to the ESI mode. Electron impact was the most commonly used ionization method for GC-based mass spec-trometry methods (Fernandez et al., 2007a; Kalyvas et al., 2014; Rodriguez-Gomez et al., 2014b). Individual ClxBPA study details on the LC-based mass spectrometry conditions such as (i) ion source, (ii) desolvation temperature, (iii) cone, desolvation, collision, nebulizer, and ion source gas, (iv) capillary, cone, and extractor potential, and (v) dwell time were presented in Table 3. Similar details on the GC-based mass spectrometry conditions from relevant studies such as (i) ion source, (ii) carrier, quenching, and collision gas, and (iii) ion source, transfer line, interface, and first and second quadruple temperatures were also presented in Tables 3 and 5. Information on the precursor and product ion transitions (m/z), cone voltage (V), and collision energy (eV) from each of the relevant ClxBPA studies was presented in Table S1–1–1 (Supplementary information).
The extraction and clean up protocols for structural analogs of BPA were similar to those of chlorinated BPA derivatives (Table 5). LLE was the widely practiced extraction method (Asimakopoulos et al., 2014; Cunha and Fernandes, 2010; Vela-Soria et al., 2014a,b; Xue et al., 2015; Yang et al., 2014a), followed by SPE (Deceuninck et al., 2015; Liao et al., 2012a; Zhou et al., 2014). Advantages of LLE were acceptable recoveries (>80%) and cost-effective, while the main disadvantage was a need for larger sample volume ranging between 0.5 mL (Asimakopoulos et al., 2014; Xue et al., 2015) and 5.0 mL (Cunha and Fernandes, 2010; Vela-Soria et al., 2014a,b). An online SPE protocol requires low sample volume such as 0.1 mL for urine (Zhou et al., 2014). In general dispersive LLE showed a distinct advantage in the percent recoveries of structural BPA analogs (>95%) (Vela-Soria et al., 2014a,b) that are on par with SPE (Deceuninck et al., 2015; Liao et al., 2012a; Zhou et al., 2014) in biological matrices, with an exception of <65% recovery for BPB in urine (Cunha and Fernandes, 2010). Nevertheless, the percent relative standard deviations of the accuracy and precision measurements were acceptable in all the reported studies (Table 5). Most suitable separation techniques for BPA analogs were based on LC compared to GC instrumentation. LC-based methods used larger injection volume (2–350 μL) and shorter analysis time per sample (8–30 min.), compared to the reported GC protocols (1–2 μL, 10–26 min.) (Table 5). Electron impact ionization in association with selected ion monitoring was the commonly used GC–MS method (Cunha and Fernandes, 2010; Vela-Soria et al., 2014a), while electrospray ionization in negative ion mode and in association with multiple reaction monitoring was the most widely used LC–MS based method for analyzing BPA analogs in biological matrices (Liao et al., 2012a; Yang et al., 2014a). While mass spectrometry is a widely preferred detector for the quanti-fication of BPA analogs (Table 5), a diode array detector coupled with HPLC was recently used for eight bisphenols’ extract from milk and urine by a dummy molecularly imprinted solid phase extraction (DMISPE) method using 1,1,1-tris(4-hydroxyphenyl)ethane as the sor-bent (Sun et al., 2014).
Limits of detection (LODs) obtained for ClxBPA in human matrices using GC–MS based methods were in the range from 0.032 ng mL−1 for ClBPA in urine (Kalyvas et al., 2014) to 3.0 ng mL−1 (decision limit) for Cl4BPA in plasma (del Olmo et al., 2005). LC–MS based methods for ClxBPA had LODs in the range from 0.009 ng mL−1 for 2,6-Cl2BPA in urine (Venisse et al., 2014) to 0.3 ng mL−1 for Cl4BPA in breast milk (Rodriguez-Gomez et al., 2014b). Similarly, the limits of quantification (LOQs) for ClxBPA in human samples obtained with GC–MS based methods ranged from 0.108 ng mL−1 for ClBPA in urine (Kalyvas et al., 2014) to 5.0 ng mL−1 (decision limit) for Cl4BPA in plasma (del Olmo et al., 2005) and breast milk (Rodriguez-Gomez et al., 2014b). LC–MS based methods for ClxBPA had LOQs in the range from 0.05 ng mL−1 for all ClxBPA in urine and serum (Liao and Kannan, 2012) to 4.0 ng mL−1 for all ClxBPA in colostrum (Migeot et al., 2013) and breast milk (Cariot et al., 2012). Most sensitive LOD and LOQ for ClBPA was achieved because of a 20 μL large-volume injection of extract in a solvent-vent mode using programmed temperature vaporization inlet on the GC–MS/MS (Kalyvas et al., 2014). This required special injection inlet and cleaner sample extracts to avoid contamination. Tandem mass spectrometry (MS/MS) offered better analytical sensitivity because of multiple reactions monitoring capability, compared to the single quadrupole’s (MS) selected reaction monitoring. Overall, it was unclear how the LODs and LOQs were determined in certain studies. Similarly, the linear range of the analytical method was not mentioned by all studies.
2.4.3. Comparison with analysis of environmental samples
LLE and SPE are of equal choice for the extraction and clean-up of environmental samples for ClxBPA analysis (Table 2). Dichloromethane was the popular choice of extractant for LLE (Bourgin et al., 2013a; Fukazawa et al., 2001; Fukazawa et al., 2002; Yamamoto and Yasuhara, 2002; Zafra et al., 2003). A suite of SPE material was used for the clean-up of environmental samples with the most popular material being made of C18 (Dupuis et al., 2012; Gallart-Ayala et al., 2007; Gallart-Ayala et al., 2010; Li et al., 2015; Song et al., 2014b; Zafra-Gómez et al., 2008). SPE-based sample preparation yielded higher pre-concentration of ClxBPA in water samples and lower LODs in the range of 1–2 ng L−1 (Fan et al., 2013) compared to 0.6–12.9 ng L−1 obtained with LLE (Zafra et al., 2003). Online SPE, an effective sample preparation method, was used only in couple of studies (Gallart-Ayala et al., 2010; Yang et al., 2014b). Analyte recoveries in all the reported studies were above 80% and satisfactory (Table 2). LC-based methods were widely used in comparison to the GC for analyzing ClxBPA in environmental media. Individual study details on the (i) LC conditions including LC column characteristics and mobile phases, and (ii) GC conditions such as column details are available in Table 2. As it was apparent from Tables 2 and 3; the analytical methods used for ClxBPA in environmental media and human matrices shared several similarities. SPE coupled with LC–MS/MS with ESI negative mode appeared to be the popular choice of analytical methodology, obtaining better sensitivities and lower LODs and LOQs. Moving forward, there is a quintessential need for developing multi-analyte methodology for simultaneous detection of chlorinated and other halogenated derivatives of BPA and as well as structural analogs of BPA using a single method for environmental samples.
2.5. Human biomonitoring
The first human biomonitoring report of ClxBPA concentrations in adipose tissue was published in 2005 (del Olmo et al., 2005) while the first study for structural BPA analogs measured them in urine and it was published in 2010 (Cunha and Fernandes, 2010). Since then, 13 and 8 peer-reviewed studies have been published reporting internal exposure measurements of ClxBPA and BPA analogs in various biospecimen, such as, in adipose, serum, placenta, breast milk, and urine. BPA analogs were reported in worldwide populations, for example, China (Yang et al., 2014a), India (Xue et al., 2015), Spain (Vela-Soria et al., 2014b), United States of America (Zhou et al., 2014), and a multinational study (Liao et al., 2012a). In Tables 4 and 5, we summarized these studies, providing key details of study population groups, analyzed bio-matrix, analytical method and features, detection rates and concentrations in human matrices.
Limits of detection (LOD) varied widely, which was primarily determined by the nature of human matrix, choice of sample preparation, and the chromatographic and mass spectrometry conditions used in the respective ClxBPA biomonitoring studies (Table 4). Most sensitive LODs reported for each matrix were in the range of (i) 0.5 ng mL−1 (ClBPA and Cl2BPA)–3.0 ng mL−1 (Cl4BPA) in adipose tissue (Fernandez et al., 2007), (ii) 0.5 ng g−1 (ClBPA and Cl2BPA)–0.6 ng g−1 (Cl4BPA) in placenta (Jimenez-Diaz et al., 2010; Vela-Soria et al., 2011), (iii) 0.01 ng mL−1 (ClBPA)–0.05 ng mL−1 (Cl2BPA) in breast milk (Cariot et al., 2012; Migeot et al., 2013), and (iv) 0.009 ng mL−1 (2,6-Cl2BPA)–0.023 ng mL−1 (2,2-Cl2BPA) in urine (Venisse et al., 2014). Cl2BPA was frequently detected when compared with the rest of chlorinated derivatives, while detection rates in the study samples were: 80% in adipose tissue (Fernandez et al., 2007), 51% in placenta (Jimenez-Diaz et al., 2010), 100% in breast milk (Cariot et al., 2012; Migeot et al., 2013), 0% in serum (Liao and Kannan, 2012), and 40% in urine (Venisse et al., 2014). In comparison, detection of BPA in the study samples was 55% in adipose (Fernandez et al., 2007), 50% in placenta (Vela-Soria et al., 2015), 100% in breast milk (Cariot et al., 2012), 100% in serum (Liao and Kannan, 2012), and 100% in urine (Kalyvas et al., 2014; Liao and Kannan, 2012; Venisse et al., 2014). These findings in conjunction with the greater lipophilic nature of a ClxBPA compared to BPA indicated their accumulation and higher detection rates in lipid-rich tissues (Migeot et al., 2013). Limits of quantification for BPA and ClxBPA, detection frequency, percent matrix spike recovery and relative standard deviation of the analyses, where available, were presented in Table 4. Limits of detection (LODs) obtained for structural analogs of BPA in human matrices using GC–MS based methods were in the range from 0.05 ng mL−1 for BPB in urine (Cunha and Fernandes, 2010) to 0.1 ng mL−1 for BPS in urine (Vela-Soria et al., 2014a) (Table 5). LC–MS based methods for BPA structural analogs had LODs in the range from 0.008 ng mL−1 for BPAF in urine (Yang et al., 2014a) to 0.1 ng mL−1 for BPS in urine (Vela-Soria et al., 2014b) (Table 5).
In the studied populations, Cl2BPA was measured in almost all human matrices. For example, reported concentrations of Cl2BPA above limits of detection were (i) 2.6–21.5 ng g−1 (5th–95th percentiles) in adipose (Fernandez et al., 2007), (ii) 12.7–58.8 ng g−1 (range) in placenta (Jimenez-Diaz et al., 2010), (iii) 1.87 [1.23] ng mL−1 (arithmetic mean [sd]) in breast milk (Migeot et al., 2013), and (iv) 0.048 ng mL−1 (geometric mean) in urine (Liao and Kannan, 2012). Reported BPA levels in the same studies were (i) 2.07–11.8 ng g−1 (5th– 95th percentiles) in adipose (Fernandez et al., 2007), (ii) 5.7– 22.2 ng g−1 (range) in placenta (Jimenez-Diaz et al., 2010), (iii) 1.87 [1.38] ng mL−1 (arithmetic mean [sd]) in breast milk (Migeot et al., 2013), and (iv) 5.4 ng mL−1 (geometric mean) in urine (Liao and Kannan, 2012) (Table 4). Because ClxBPA are more lipophilic in nature compared to BPA (Migeot et al., 2013), it could be possible that ClxBPA compounds were present at higher concentrations in lipid-containing matrices, such as adipose and breast milk rather in urine or blood. For example, (i) Cl2BPA was detected in 20–100% of the studied breast milk samples (Cariot et al., 2012; Migeot et al., 2013; Rodriguez-Gomez et al., 2014a) compared to 0–40% in urine (Liao and Kannan, 2012; Vela-Soria et al., 2014b; Venisse et al., 2014), and (ii) maximum Cl2BPA concentrations were in the range of 0.40– 4.13 ng mL−1 in breast milk (Cariot et al., 2012; Migeot et al., 2013; Rodriguez-Gomez et al., 2014a) compared to 0.11–1.06 ng mL−1 in human urine (Liao and Kannan, 2012; Vela-Soria et al., 2014b; Venisse et al., 2014). But, given our limited understanding of the pharmacokinetics and half-lives of ClxBPA derivatives and bisphenol analogs in humans, it is premature to suggest an appropriate biological matrix or a biomarker for ClxBPA exposure assessment in humans based on the available studies, thus far. Among the structural analogs of BPA, bisphenol S (BPS) was the most studied structural analog of BPA in human matrices, with detection rates of 81% (Liao and Kannan, 2012), 65% and 30% (Vela-Soria et al., 2014a,b), 70% (Xue et al., 2015), 40% (Yang et al., 2014a), and 78% (Zhou et al., 2014) in urine and 3% in breast milk (Deceuninck et al., 2015). Reported BPS levels were in the range of (i) <0.02–21.0 ng mL−1 (Liao et al., 2012a), (ii) <0.02 ng mL−1 (Vela-Soria et al., 2014a,b), (iii) <0.10–12.2 ng mL−1 (Xue et al., 2015), (iv) <0.01–7.046 μg kg−1 (Yang et al., 2014a), and (v) <0.03– 12.3 ng mL−1 (Zhou et al., 2014) in urine and (vi) <0.003– 0.23 μg kg−1 in breast milk (Deceuninck et al., 2015) (Table 5).
3. Current challenges and future perspectives
3.1. Methodological advances in biomonitoring protocols
Biomonitoring-based protocols to assess internal exposures to ClxBPA and structural BPA analogs relied upon GC–MS/MS and LC–MS/MS techniques both satisfactorily performing in regards to analytical method accuracy and sensitivity for ClxBPA quantitation (as in the example of breast milk, Rodriguez-Gomez et al., 2014b). However, LC– MS technology is most commonly used in human biomonitoring protocols of ClxBPA derivatives (10 out of 14 studies, Table 4). A single methodology for ClxBPA extraction and assay from multiple matrices does not exist due to differences in sample preparation procedures and differences in optimal analyte recoveries from different matrices. Although not used for human matrices, a novel derivatization of ClxBPA in water samples using dansyl chloride resulted in at least a 10 fold increase in sensitivity with UPLC–ESI-MS/MS analysis (Fan et al., 2013). The achieved detection limits were 0.001 ng mL−1 (ClBPA), 0.002 ng mL−1 (Cl2BPA), 0.001 ng mL−1 (Cl3BPA), and 0.001 ng mL−1 (Cl4BPA) in water samples (Fan et al., 2013) compared to the best achieved limits of detection in human matrices such as 0.01 ng mL−1 for ClBPA in adipose tissue (Fernandez et al., 2007), and 0.009 ng mL−1, 0.018 ng mL−1, and 0.014 ng mL−1 for Cl2BPA, Cl3BPA, and Cl4BPA in urine, respectively (Venisse et al., 2014). Dansyl chloride as a derivatization agent exhibited faster reaction rates with phenolic hydroxyl groups and thereby greater sensitivity using LC– MS/MS in electrospray ionization positive mode (Chang et al., 2010; Naassner et al., 2002). Adapting the dansylation procedure to human biospecimen could perhaps increase the sensitivity of existing ClxBPA methodologies for human matrices. GC methods still probably offers few advantages over LC because of (i) greater analytes separation on the GC column, (ii) cost effective sample preparation GC protocols, and (iii) lower matrix effects in electron impact ionization mode in GC compared to the ESI mode in LC. However, the disadvantage is that GC methods required an additional step for derivatizing polar analytes.
Sample volume or mass is a major consideration in human biomonitoring studies part of large cohort studies. The typical volume of urine samples required for analysis was in the range of 0.5 mL (Liao and Kannan, 2012) to 5.0 mL (Vela-Soria et al., 2014a,b), and breast milk from 0.5 mL (Cariot et al., 2012; Migeot et al., 2013) to 9.9 mL (Rodriguez-Gomez et al., 2014a,b). In contrast to LLE that required extensive solvent extraction volumes and SPE that required expensive sample preparation material, stir-bar sorptive extraction (SBSE) is gaining attention as a cost-effective, low-volume solvent use, and environment-safe sample preparation procedure. Moreover, SBSE showed a high pre-concentration capacity for ClxBPA in human biospecimen (e.g. breast milk) (Rodriguez-Gomez et al., 2014b). Another emerging sample clean-up protocol is the use of online SPE that could help to (i) minimize manual handling of samples and thereby human errors, and solvent(s) exposures for the primary analyst, (ii) avoid additional steps, such as solvent evaporation and extract reconstitution, and thereby preventing loss of analytes, and (iii) high throughput extractions and time-conservative clean-up steps that are ideal to process a large number of samples. Need for a simplified analytical method is felt to minimize variance in the recoveries of spiked standards and internal standards that vary significantly within and between sample batches. A simplified method may also help to perform blank corrections at ease. Hence, the development of time-, and cost-effective sample preparation procedures, faster chromatography run times, and greater sensitive mass spectrometry detection conditions are needed to facilitate adoption of such protocols by large epidemiological cohort studies. Additional research is needed to identify the conjugated forms of BPA derivatives and analogs, if any, towards the development of generic analytical workflows for the simultaneous detection of parent and conjugated forms in a single method.
3.2. Matrix effects and role in biomonitoring
Matrix effects vary by the nature of biological sample, yielding either ion suppression or enhancement that eventually interferes with trace level quantification of BPA derivatives and analogs. These affect significantly the method performance variables, such as LOD, LOQ, linearity range, and inter- and intra-batch variability. This necessitates the use of an internal standard (stable isotope-labeled compound) that could overcome matrix effects present during extraction, clean-up, chromatography and ionization in the mass spectrometer source. LC–MS methods were more susceptible to matrix effects during the electrospray ionization process and hence required internal standardization compared to GC–MS protocols. However, GC methods offered higher LOD compared to the LC protocols. For example, a side by side comparison of the LODs from using LC–MS/MS and GC–MS/MS were 0.1 and 0.3 ng mL−1 for Cl2BPA, 0.2 and 1.0 ng mL−1 for Cl3BPA, and 0.3 and 1.5 ng mL−1 for Cl4BPA, respectively (Rodriguez-Gomez et al., 2014b). A preventive measure for minimizing matrix effect could be to follow the best sample clean-up protocol, though excessive pre-concentration of the study analytes would also concentrate in parallel the components that contribute to matrix effects. Hence, the pre-concentration factor needs to be carefully evaluated on a case by case basis. Matrix effects affect the analytical method LOQ but not necessarily the instrument LOQ, which are generally determined with spiked matrix and pure standards, respectively. Hence, it is of absolute importance to report method LOQ compared to the instrumentation LOQ, which is commonly reported in the literature.
Matrix effects on ClxBPA analysis in human samples were reported, except for a few studies (del Olmo et al., 2005; Fernandez et al., 2007; Kalyvas et al., 2014; Liao and Kannan, 2012; Migeot et al., 2013; Rodriguez-Gomez et al., 2014a). The widely practiced measure to minimize matrix effects was to use internal standards. The most commonly used internal standard was BPA-d16 in the so far available ClxBPA studies, with few exceptions such as use of (i) 13C12-BPA (Liao and Kannan, 2012), (ii) BPA-d4 (Yang et al., 2014a), and (iii) a surrogate, bisphenol F (Fernandez et al., 2007). A notable effort is the use of 2,2′-Cl2BPA-d12, a custom made internal standard, specifically to eliminated matrix effects on ClxBPA measurements in human urine (Venisse et al., 2014). Though expensive, it is suggested to having 13C labeled-compounds because they share similar physico-chemical properties that of 12C in comparison to the 1H versus 2H (deuterium) labeled internal standards (Briscoe et al., 2007; Van Eeckhaut et al., 2009; Wang et al., 2007). Few of the studies assessed matrix effects by comparing calibration curves build in the (i) initial mobile phase (solvent) and the human matrix under consideration (Jiménez-Díaz et al., 2010; Vela-Soria et al., 2011), (ii) washed sand and placenta (Vela-Soria et al., 2015), and (iii) distilled water and respective human sample (Rodriguez-Gomez et al., 2014b; Vela-Soria et al., 2014b). Few other studies assessed matrix effects by (i) analyte signal suppression (Cariot et al., 2012), (ii) post-column infusion and matrix factor calculation (Venisse et al., 2014), and (iii) a variance between samples (Yang et al., 2014a). Suggested calculation and presentation of matrix effects as percent relative signal suppression or enhancement (% ME) is missing in the available ClxBPA studies, while few studies compared the slopes of calibration curves built in different media towards assessing this effect (Jimenez-Diaz et al., 2010; Rodriguez-Gomez et al., 2014b; Vela-Soria et al., 2011; Vela-Soria et al., 2014b; Vela-Soria et al., 2015). Despite the use of precautionary measures and additional experimentation, matrix effects still prevailed during the analysis of ClxBPA in human matrices, because (i) assessment was made on a subset of samples or aliquots, while rest of real samples varied widely in composition, and (ii) recovery of internal standards varied significantly within and between batches of samples. Moreover, availability of commercial internal standards for ClxBPA is currently lacking for their wider use to correct for matrix effects.
3.3. Emerging BPA sub-classes: other halogenated derivatives
Recently, it was shown that BPA in a simulated water system reacted with chlorine giving rise to ClxBPA, which may further undergo benzene ring opening, followed by halogenation resulting in the formation of trihalomethanes (a major class of disinfection by-products) and minor haloacetic acids (Li et al., 2015). If transformation of BPA to halogenated BPA congeners that further transform to disinfection by-products is confirmed in drinking water distribution systems, then it should be emphasized to monitor the association between exposures to BPA and dis-infection by-products in relation to human health effects from exposure to water contaminants (Li et al., 2015; Zhai and Zhang, 2011). In addition, the range and types of possible halogenated derivatives formed when BPA comes in contact with chlorine and other chemical constituents present in domestic household consumer products is currently unknown. Calculated BPA-equivalent estrogenic activity (EQBPA) was higher for finished drinking water (user end) compared to the source water (prior to water treatment), indicating a plethora of estrogenic compounds formed after water treatment and within the drinking water pipe network (Fan et al., 2013). Taking these aspects into consideration, the scope of environmental monitoring of BPA derivatives and analogs should not only include the parent compounds but also a suite of transformation products that they can potentially form in the presence of reactive chlorine readily available in various ecosystems.
Research is needed to assess the magnitude and variability of exposures to emerging derivatives of BPA not only in finished tap water (Bourgin et al., 2013a,b), but also in relevant human matrices (for example, urine and blood). Apart from the chlorinated derivatives and structural analogs of BPA, occurrence of brominated forms such as tetrabromobisphenol A and its derivatives viz., tri-, di-, and monobromobisphenol A in human matrices is gaining attention. Reported mean concentrations of tetra- and tri-bromo BPAs in human breast milk samples were 1.9 and 5.5 ng g−1 lipid wt., respectively, while mono- and di-bromo BPA were below LOQ of 0.01 ng g−1 lipid (Nakao et al., 2015). It should be noted that tribromo BPA is reported to having interfering sugar and fatty acid metabolic pathways by acting as a ligand for peroxisome proliferator-activated receptor (Fini et al., 2012). Hence, in addition to ClxBPA there is a need to biomonitor other halogenated forms of BPA that have shown adverse health outcomes in cell culture and animal studies.
3.4. Biomarkers of exposure and epidemiological studies
The main focus of most of the so far published studies reporting ClxBPA and BPA analogs in human matrices was on the bioanalytical method development followed by validation in a small human sample size (Cariot et al., 2012; Jimenez-Diaz et al., 2010; Liao and Kannan, 2012; Liao et al., 2012a; Rodriguez-Gomez et al., 2014a; Rodriguez-Gomez et al., 2014b; Vela-Soria et al., 2015; Vela-Soria et al., 2014a; Vela-Soria et al., 2014b; Vela-Soria et al., 2011; Venisse et al., 2014; Xue et al., 2015; Yang et al., 2014a; Zhou et al., 2014). The rest of the published studies focused on biomonitoring and assessment of human exposures to ClxBPA (Fernandez et al., 2007; Kalyvas et al., 2014; Liao and Kannan, 2012; Migeot et al., 2013; Yang et al., 2014a). Different sample sizes were used in the existing ClxBPA biomonitoring studies, ranging from 224 participants (Kalyvas et al., 2014) to 94 participants (Yang et al., 2014a), 10 participants (Rodriguez-Gomez et al., 2014a; Rodriguez-Gomez et al., 2014b; Vela-Soria et al., 2015; Venisse et al., 2014), and 3 participants (Cariot et al., 2012). Sample sizes for human biomonitoring studies on BPA structural analogs ranged from 315 participants (from multiple countries, Liao et al., 2012a) to 20 participants (Vela-Soria et al., 2014a,b). Moreover, detection rates and concentrations in most of the reported studies thus far, mainly served (i) as a preliminary assessment of the range of concentrations expected to be found in the general population and (ii) as an indication on which bio-matrix would be appropriate to quantify their magnitude of exposure accounting for matrix effects (for example, lipid-rich tissue versus urine).
In addition to oral ingestion, non-ingestion routes of exposure to ClxBPA could be important and yet to be fully elucidated. In addition to water intake and dermal contact, inhalation route was speculated to be one of their primary routes of exposure. It was speculated that free chlorine atoms or chloroform in the air, could react with BPA resulting in ClxBPA formation and subsequent exposures via the inhalation route, but this remains to be experimentally investigated (Kalyvas et al., 2014). The variability in urinary concentrations of monochlorinated BPA was recently studied as a function of specific indoor chlorine-based water-use activities (household cleaning, swimming, etc.); results indicated non-ingestion routes as the primary contributor to human exposures to chlorinated derivatives of BPA (unpublished data from our laboratory). BPA derivatives (such as chlorinated BPA) have not been yet considered in the studies affiliated with the National Health and Nutrition Examination Survey (NHANES). If domestic cleaning and personal care and hygiene activities were indeed considered as relevant BPA and ClxBPA exposure sources, then the issue of non-food BPA exposures could be further investigated (Geens et al., 2011; Stahlhut et al., 2009).
For better assessment of biomarkers of exposures and effects, the premise is to overcome the most common limitations in the reviewed studies, such as (i) small sample size, (ii) cross-sectional studies that cannot rule out plausible biological causality, (iii) likely misclassification error due to mismatch between stage of critical window of susceptibility and exposure assessment, (iv) spot urine or a single sample of a biological matrix that may not shed information on short-lived, nonbioaccumulating chemicals in humans, (v) not accounting for residual confounding effects, and (vi) reverse causality effects. Most importantly, as recently demonstrated in the case of BPA (Vandenberg et al., 2014), a round robin approach to validate sample collection and analysis protocols is quintessential before deriving at associations between human exposures to these chemicals and their possible health effects.
4. Conclusion
Collective evidence reviewed in this report suggested a widespread occurrence of ClxBPA and structural analogs of BPA in human biospecimen matrices and various environmental media as fueled by recent years’ growing scientific interest. Exposure sources and pathways of ClxBPA and structural BPA analogs in a suite of environmental media and consumer products were evident, yet to be fully elucidated. It was suggested that the increased halogen content of ClxBPA could modify the physicochemical properties of BPA derivatives allowing them to partition between the gas/liquid phases, giving rise to all three human routes of exposure. Similarly, structural analogs of BPA were detected in human biospecimen during the last couple of years (2014–2015) indicative of their gradually increasing detection in consumer products as safer alternatives to BPA. In the absence of human data on structural analogs of BPA and chlorinated derivatives of BPA, in-vitro and in-vivo studies hint towards their obesogenic and diabetogenic potential. Hence, it is warranted that the inclusion of BPA analogs and derivatives in prospective cohort studies would shed light to their health effects in a systematic fashion.
Human studies are needed to answer the research questions: (i) whether ClxBPA exposures occur internally from metabolic conversion of BPA to respective derivatives in human systems or externally as reported to occur in environment or both, (ii) what the biochemical pathways are that yield ClxBPA metabolites for internal exposure, (iii) what are the exposure sources and frequency of occurrence in environmental media and consumer products, and (iv) how chlorinated BPA metabolites and BPA analogs could act as endocrine-disrupting compounds in human studies. The exposure sources of ClxBPA in the indoor environment and the contribution of non-ingestion and ingestion routes to the total ClxBPA body burden remains to be determined, including the contribution of various consumer products mediating ClxBPA formation in environmental compartments. Low-dose BPA health effects may need to be revisited by incorporating knowledge on its chlorinated analogs (ClxBPA). In addition, the investigation of alternative physiological pathways of BPA resulting in higher estrogenic-active metabolites that could aggravate adverse biological responses is needed. It may be prudent to study whether halogenated derivatives of bisphenol and other environmental phenols induce obesogenic effects, and if so, whether they induce lipid accumulation in adipose or non-adipose tissue or both.
Supplementary Material
Acknowledgments
KCM would like to thank the European Structural Funds and the Cyprus Research Promotion Foundation project #0713/18 for partially funding this study. J. V. van Vliet-Ostaptchouk is supported by a Diabetes Funds Junior Fellowship from the Dutch Diabetes Research Foundation (project no. 2013.81.1673).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.envint.2015.09.011.
Competing financial interests
The authors declare no competing financial interests.
References
- Andra SS, et al. , 2015. Preliminary evidence of the association between monochlorinated bisphenol A exposure and type II diabetes mellitus: A pilot study. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng 50, 243–259. [DOI] [PubMed] [Google Scholar]
- Andra SS, Makris KC, 2015. Association between urinary levels of bisphenol A and its monochlorinated derivative and obesity. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng 50, 1169–1179. [DOI] [PubMed] [Google Scholar]
- Asimakopoulos AG, et al. , 2014. A multi-class bioanalytical methodology for the determination of bisphenol A diglycidyl ethers, p-hydroxybenzoic acid esters, benzophe-none-type ultraviolet filters, triclosan, and triclocarban in human urine by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1324, 141–148. [DOI] [PubMed] [Google Scholar]
- Babu S, et al. , 2012. Molecular docking of bisphenol A and its nitrated and chlorinated metabolites onto human estrogen-related receptor-gamma. Biochem. Biophys. Res. Commun 426, 215–220. [DOI] [PubMed] [Google Scholar]
- Ballesteros O, et al. , 2006. Sensitive gas chromatographic-mass spectrometric method for the determination of phthalate esters, alkylphenols, bisphenol A and their chlorinated derivatives in wastewater samples. J. Chromatogr. A 1121, 154–162. [DOI] [PubMed] [Google Scholar]
- Baltussen E, et al. , 1999. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep 11, 737–747. [Google Scholar]
- Bastos PM, et al. , 2008. A standardized method for assessment of oxidative transformations of brominated phenols in water. Chemosphere 70, 1196–1202. [DOI] [PubMed] [Google Scholar]
- Becerra V, Odermatt J, 2012. Detection and quantification of traces of bisphenol A and bisphenol S in paper samples using analytical pyrolysis-GC/MS. Analyst 137, 2250–2259. [DOI] [PubMed] [Google Scholar]
- Bindhumol V, et al. , 2003. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology 188, 117–124. [DOI] [PubMed] [Google Scholar]
- Bloom MS, et al. , 2011. Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertil. Steril 96, 672–677. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin J, et al. , 2015. Can exposure to environmental chemicals increase the risk of diabetes type 1 development? BioMed. Res. Int [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher JG, et al. , 2015. Effects of Bisphenol A beta-D-Glucuronide (BPA-G) on Adipo-genesis in Human and Murine Preadipocytes. Environ. Health Perspect [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgin M, et al. , 2013a. Chlorination of bisphenol A: non-targeted screening for the identification of transformation products and assessment of estrogenicity in generated water. Chemosphere 93, 2814–2822. [DOI] [PubMed] [Google Scholar]
- Bourgin M, et al. , 2013b. Differential chemical profiling to identify ozonation by-products of estrone-sulfate and first characterization of estrogenicity in generated drinking water. Water Res. 47, 3791–3802. [DOI] [PubMed] [Google Scholar]
- Braun JM, et al. , 2014. Early-life bisphenol a exposure and child body mass index: a prospective cohort study. Environ. Health Perspect 122, 1239–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe CJ, et al. , 2007. System suitability in bioanalytical LC/MS/MS. J. Pharm. Biomed. Anal 44, 484–491. [DOI] [PubMed] [Google Scholar]
- Bulloch DN, et al. , 2015. Occurrence of halogenated transformation products of selected pharmaceuticals and personal care products in secondary and tertiary treated waste-waters from southern California. Environ. Sci. Technol 49, 2044–2051. [DOI] [PubMed] [Google Scholar]
- Cacho JI, et al. , 2012. Stir bar sorptive extraction coupled to gas chromatography–mass spectrometry for the determination of bisphenols in canned beverages and filling liquids of canned vegetables. J. Chromatogr. A 1247, 146–153. [DOI] [PubMed] [Google Scholar]
- Cariot A, et al. , 2012. Reliable quantification of bisphenol A and its chlorinated derivatives in human breast milk using UPLC–MS/MS method. Talanta 100, 175–182. [DOI] [PubMed] [Google Scholar]
- Carwile JL, Michels KB, 2011. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ. Res 111, 825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casatta N, et al. , 2015. Tracing endocrine disrupting chemicals in a coastal lagoon (Sacca di Goro, Italy): sediment contamination and bioaccumulation in Manila clams. Sci. Total Environ 511, 214–222. [DOI] [PubMed] [Google Scholar]
- Chang BV, et al. , 2014. Aerobic degradation of bisphenol-A and its derivatives in river sediment. Environ. Technol 35, 416–424. [DOI] [PubMed] [Google Scholar]
- Chang BV, et al. , 2012. Aerobic degradation of tetrabromobisphenol-A by microbes in river sediment. Chemosphere 87, 535–541. [DOI] [PubMed] [Google Scholar]
- Chang H, et al. , 2010. Simultaneous quantification of multiple classes of phenolic compounds in blood plasma by liquid chromatography-electrospray tandem mass spec-trometry. J. Chromatogr. A 1217, 506–513. [DOI] [PubMed] [Google Scholar]
- Chevalier N, Fénichel P, 2015. Endocrine disruptors: New players in the pathophysiology of type 2 diabetes? Diabete Metab. 41, 107–115. [DOI] [PubMed] [Google Scholar]
- Cunha SC, et al. , 2011. Simultaneous determination of bisphenol A and bisphenol B in beverages and powdered infant formula by dispersive liquid-liquid micro-extraction and heart-cutting multidimensional gas chromatography–mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 28, 513–526. [DOI] [PubMed] [Google Scholar]
- Cunha SC, Fernandes JO, 2010. Quantification of free and total bisphenol A and bisphenol B in human urine by dispersive liquid-liquid microextraction (DLLME) and heart-cutting multidimensional gas chromatography–mass spectrometry (MDGC/MS). Talanta 83, 117–125. [DOI] [PubMed] [Google Scholar]
- David F, Sandra P, 2007. Stir bar sorptive extraction for trace analysis. J. Chromatogr. A 1152, 54–69. [DOI] [PubMed] [Google Scholar]
- De Coensel N, et al. , 2009. Study on the migration of bisphenol-A from baby bottles by stir bar sorptive extractionthermal desorption-capillary GC-MS. J. Sep. Sci 32, 3829–3836. [DOI] [PubMed] [Google Scholar]
- Deceuninck Y, et al. , 2015. Determination of bisphenol A and related substitutes/analogues in human breast milk using gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem 407, 2485–2497. [DOI] [PubMed] [Google Scholar]
- del Olmo M, et al. , 2005. Use of solid-phase microextraction followed by on-column silylation for determining chlorinated bisphenol A in human plasma by gas chromatography–mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 817, 167–172. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, et al. , 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev 30, 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, et al. , 2012. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ. Health Perspect 120, 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorival-Garcia N, et al. , 2012a. Analysis of bisphenol A and its chlorinated derivatives in sewage sludge samples Comparison of the efficiency of three extraction techniques. J. Chromatogr. A 1253, 1–10. [DOI] [PubMed] [Google Scholar]
- Dorival-Garcia N, et al. , 2012b. Improved sample treatment for the determination of bisphenol A and its chlorinated derivatives in sewage sludge samples by pressurized liquid extraction and liquid chromatography-tandem mass spectrometry. Talanta 101, 1–10. [DOI] [PubMed] [Google Scholar]
- Duan B, et al. , 2013. The relationship between urinary bisphenol A levels and meningioma in Chinese adults. Int. J. Clin. Oncol 18, 492–497. [DOI] [PubMed] [Google Scholar]
- Dupuis A, et al. , 2012. Quantification of bisphenol A, 353-nonylphenol and their chlorinated derivatives in drinking water treatment plants. Environ. Sci. Pollut. Res. Int 19, 4193–4205. [DOI] [PubMed] [Google Scholar]
- Eladak S, et al. , 2015. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril 103, 11–21. [DOI] [PubMed] [Google Scholar]
- Fan R, et al. , 2015. Levels of bisphenol-A in different paper products in Guangzhou, China, and assessment of human exposure via dermal contact. Environ. Sci.: Processes Impacts 17, 667–673. [DOI] [PubMed] [Google Scholar]
- Fan Z, et al. , 2013. Detection and occurrence of chlorinated byproducts of bisphenol a, nonylphenol, and estrogens in drinking water of china: comparison to the parent compounds. Environ. Sci. Technol 47, 10841–10850. [DOI] [PubMed] [Google Scholar]
- Fernandez MF, et al. , 2007. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod. Toxicol 24, 259–264. [DOI] [PubMed] [Google Scholar]
- Fini J, et al. , 2012. Parallel biotransformation of tetrabromobisphenol A in Xenopus laevis and mammals: Xenopus as a model for endocrine perturbation studies. Toxicol. Sci 125, 359–367. [DOI] [PubMed] [Google Scholar]
- Fromme H, et al. , 2002. Occurrence of phthalates and bisphenol A and F in the environment. Water Res. 36, 1429–1438. [DOI] [PubMed] [Google Scholar]
- Fukazawa H, et al. , 2001. Identification and quantification of chlorinated bisphenol A in wastewater from wastepaper recycling plants. Chemosphere 44, 973–979. [DOI] [PubMed] [Google Scholar]
- Fukazawa H, et al. , 2002. Formation of chlorinated derivatives of bisphenol A in waste paper recycling plants and their estrogenic activities. J. Health Sci 48, 242–249. [Google Scholar]
- Gallard H, et al. , 2004. Chlorination of bisphenol A: kinetics and by-products formation. Chemosphere 56, 465–473. [DOI] [PubMed] [Google Scholar]
- Gallart-Ayala H, et al. , 2007. Liquid chromatography/multi-stage mass spectrometry of bisphenol A and its halogenated derivatives. Rapid Commun. Mass Spectrom 21, 4039–4048. [DOI] [PubMed] [Google Scholar]
- Gallart-Ayala H, et al. , 2010. On-line solid phase extraction fast liquid chromatography-tandem mass spectrometry for the analysis of bisphenol A and its chlorinated derivatives in water samples. J. Chromatogr. A 1217, 3511–3518. [DOI] [PubMed] [Google Scholar]
- Galloway T, et al. , 2010. Daily bisphenol A excretion and associations with sex hormone concentrations: results from the InCHIANTI adult population study. Environ. Health Perspect 118, 1603–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geens T, et al. , 2011. Are potential sources for human exposure to bisphenol-A overlooked? Int. J. Hyg. Environ. Health 214, 339–347. [DOI] [PubMed] [Google Scholar]
- Grumetto L, et al. , 2008. Determination of bisphenol A and bisphenol B residues in canned peeled tomatoes by reversed-phase liquid chromatography. J. Agric. Food Chem 56, 10633–10637. [DOI] [PubMed] [Google Scholar]
- Harley KG, et al. , 2013. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ. Health Perspect 121, 514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YC, et al. , 2009. Community level exposure to chemicals and oxidative stress in adult population. Toxicol. Lett 184, 139–144. [DOI] [PubMed] [Google Scholar]
- Hormann AM, et al. , 2014. Holding thermal receipt paper and eating food after using hand sanitizer results in high serum bioactive and urine total levels of bisphenol A (BPA). PLoS One 9, e110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JY, et al. , 2002. Products of aqueous chlorination of bisphenol A and their estrogenic activity. Environ. Sci. Technol 36, 1980–1987. [DOI] [PubMed] [Google Scholar]
- Ibuki Y, et al. , 2008. UVB-exposed chlorinated bisphenol a generates phosphorylated his-tone H2AX in human skin cells. Chem. Res. Toxicol 21, 1770–1776. [DOI] [PubMed] [Google Scholar]
- Jaeg JP, et al. , 2004. Characterization of new bisphenol a metabolites produced by CD1 mice liver microsomes and S9 fractions. J. Agric. Food Chem 52, 4935–4942. [DOI] [PubMed] [Google Scholar]
- Janesick A, Blumberg B, 2011a. Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity. Birth Defects Res. C Embryo Today 93, 34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janesick A, Blumberg B, 2011b. Minireview: PPARγ as the target of obesogens. J. Steroid Biochem. Mol. Biol 127, 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janesick A, Blumberg B, 2012. Obesogens, stem cells and the developmental programming of obesity. Int. J. Androl 35, 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon JY, et al. , 2015. New risk factors for obesity and diabetes: Environmental chemicals. J. Diabetes Investig 6, 109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Diaz I, et al. , 2010. Determination of Bisphenol A and its chlorinated derivatives in placental tissue samples by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 878, 3363–3369. [DOI] [PubMed] [Google Scholar]
- Kalyvas H, et al. , 2014. Influence of household cleaning practices on the magnitude and variability of urinary monochlorinated bisphenol A. Sci. Total Environ 490, 254–261. [DOI] [PubMed] [Google Scholar]
- Kang JH, et al. , 2006. Human exposure to bisphenol A. Toxicology 226, 79–89. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, et al. , 2006. Novel stir bar sorptive extraction methods for environmental and biomedical analysis. J. Pharm. Biomed. Anal 40, 500–508. [DOI] [PubMed] [Google Scholar]
- Kim DH, et al. , 2012. Serum bisphenol A concentration in postmenopausal women with osteoporosis. J. Bone Metab 19, 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Park H, 2013. Association between urinary concentrations of bisphenol A and type 2 diabetes in Korean adults: a population-based cross-sectional study. Int. J. Hyg. Environ. Health 216, 467–471. [DOI] [PubMed] [Google Scholar]
- Ko A, et al. , 2014. Association between Urinary Bisphenol A and Waist Circumference in Korean Adults. Toxicol. Res 30, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K, et al. , 2012. Elution of bisphenol A and its chlorination by-products from lined pipes in water supply process. Water Sci. Technol. Water Supply 12, 791–798. [Google Scholar]
- Kuruto-Niwa R, et al. , 2005. Estrogenic activity of alkylphenols, bisphenol S, and their chlorinated derivatives using a GFP expression system. Environ. Toxicol. Pharmacol 19, 121–130. [DOI] [PubMed] [Google Scholar]
- Kuruto-Niwa R, et al. , 2002. Identification of estrogenic activity of chlorinated bisphenol A using a GFP expression system. Environ. Toxicol. Pharmacol 12, 27–35. [DOI] [PubMed] [Google Scholar]
- Lakind JS, et al. , 2014. Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: a systematic review of epidemiologic research. Crit. Rev. Toxicol 44, 121–150. [DOI] [PubMed] [Google Scholar]
- LaKind JS, et al. , 2012. Use of NHANES Data to Link Chemical Exposures to Chronic Diseases: A Cautionary Tale. PLoS One 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RF, et al. , 2015. Chlorination and chloramination of bisphenol A, bisphenol F, and bisphenol A diglycidyl ether in drinking water. Water Res. 79, 68–78. [DOI] [PubMed] [Google Scholar]
- Lang IA, et al. , 2008. Association of Urinary Bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 300, 1303–1310. [DOI] [PubMed] [Google Scholar]
- le Maire A, et al. , 2009. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep. 10, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BC, et al. , 2004. Effects of chlorine on the decrease of estrogenic chemicals. Water Res. 38, 733–739. [DOI] [PubMed] [Google Scholar]
- Lee MR, et al. , 2014. Urinary bisphenol A concentrations are associated with abnormal liver function in the elderly: a repeated panel study. J. Epidemiol. Community Health 68, 312–317. [DOI] [PubMed] [Google Scholar]
- Leri AC, Anthony LN, 2013. Formation of organochlorine by-products in bleached laundry. Chemosphere 90, 2041–2049. [DOI] [PubMed] [Google Scholar]
- Li C, et al. , 2015. Transformation of bisphenol A in water distribution systems: a pilot-scale study. Chemosphere 125, 86–93. [DOI] [PubMed] [Google Scholar]
- Li M, et al. , 2012. Exposure to bisphenol A is associated with low-grade albuminuria in Chinese adults. Kidney Int. 81, 1131–1139. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K, 2012. Determination of free and conjugated forms of bisphenol A in human urine and serum by liquid chromatography-tandem mass spectrometry. Environ. Sci. Technol 46, 5003–5009. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K, 2013. Concentrations and profiles of bisphenol a and other bisphenol analogues in foodstuffs from the united states and their implications for human exposure. J. Agric. Food Chem 61, 4655–4662. [DOI] [PubMed] [Google Scholar]
- Liao C, et al. , 2012a. Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ. Sci. Technol 46, 6860–6866. [DOI] [PubMed] [Google Scholar]
- Liao C, et al. , 2012b. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ. Sci. Technol 46, 9138–9145. [DOI] [PubMed] [Google Scholar]
- Liao C, et al. , 2012c. Bisphenol S, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ. Sci. Technol 46, 6515–6522. [DOI] [PubMed] [Google Scholar]
- Liao C, et al. , 2012d. Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: Spatial and temporal distributions. Environ. Sci. Technol 46, 11558–11565. [DOI] [PubMed] [Google Scholar]
- Liu, et al. , 2009. Formation of chlorinated intermediate from bisphenol A in surface saline water under simulated solar light irradiation. Environ. Sci. Technol 43, 7712–7717. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. , 2005. Screening estrogenic oxidized by-products by combining ER binding and ultrafiltration. Environ. Toxicol. Pharmacol 20, 269–278. [DOI] [PubMed] [Google Scholar]
- Melzer D, et al. , 2012. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 125, 1482–1490. [DOI] [PubMed] [Google Scholar]
- Melzer D, et al. , 2010. Association of urinary bisphenol A concentration with heart disease: Evidence from NHANES 2003/06. PLoS One 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeot V, et al. , 2013. Bisphenol a and its chlorinated derivatives in human colostrum. Environ. Sci. Technol. 47, 13791–13797. [DOI] [PubMed] [Google Scholar]
- Mok-Lin E, et al. , 2010. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int. J. Androl 33, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Molina JM, et al. , 2013. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol. Appl. Pharmacol 272, 127–136. [DOI] [PubMed] [Google Scholar]
- Mutou Y, et al. , 2006. Change of estrogenic activity and release of chloride ion in chlorinated bisphenol A after exposure to ultraviolet B. Biol. Pharm. Bull 29, 2116–2119. [DOI] [PubMed] [Google Scholar]
- Mutou Y, et al. , 2008. Induction of apoptosis by UV-irradiated chlorinated bisphenol A in Jurkat cells. Toxicol. in Vitro 22, 864–872. [DOI] [PubMed] [Google Scholar]
- Naassner M, et al. , 2002. Determination of the xenoestrogens 4-nonylphenol and bisphenol A by high-performance liquid chromatography and fluorescence detection after derivatisation with dansyl chloride. J. Chromatogr. A 945, 133–138. [DOI] [PubMed] [Google Scholar]
- Nakamura S, et al. , 2011. Ipso substitution of bisphenol A catalyzed by microsomal cyto-chrome P450 and enhancement of estrogenic activity. Toxicol. Lett 203, 92–95. [DOI] [PubMed] [Google Scholar]
- Nakao T, et al. , 2015. Levels of tetrabromobisphenol A, tribromobisphenol A, dibromobisphenol A, monobromobisphenol A, and bisphenol a in Japanese breast milk. Chem. Res. Toxicol 28, 722–728. [DOI] [PubMed] [Google Scholar]
- Nazaroff WW, Weschler CJ, 2004. Cleaning products and air fresheners: Exposure to primary and secondary air pollutants. Atmos. Environ 38, 2841–2865. [Google Scholar]
- Ning G, et al. , 2011. Relationship of urinary bisphenol A concentration to risk for prevalent type 2 diabetes in Chinese adults: a cross-sectional analysis. Ann. Intern. Med 155, 368–374. [DOI] [PubMed] [Google Scholar]
- Nishikawa JI, et al. , 1999. New screening methods for chemicals with hormonal activities using interaction of nuclear hormone receptor with coactivator. Toxicol. Appl. Pharmacol 154, 76–83. [DOI] [PubMed] [Google Scholar]
- Odabasi M, 2008. Halogenated volatile organic compounds from the use of chlorine-bleach- containing household products. Environ. Sci. Technol 42, 1445–1451. [DOI] [PubMed] [Google Scholar]
- Oldring PKT, et al. , 2006. Migrants from food cans revisited – application of a stochastic model for a more realistic assessment of exposure to bisphenol A diglycidyl ether (BADGE). Packag. Technol. Sci 19, 121–137. [Google Scholar]
- Olsén L, et al. , 2012. Associations between circulating levels of bisphenol A and phthalate metabolites and coronary risk in the elderly. Ecotoxicol. Environ. Saf 80, 179–183. [DOI] [PubMed] [Google Scholar]
- Olson DA, Corsi RL, 2004. In-home formation and emissions of trihalomethanes: The role of residential dishwashers. J. Expo. Anal. Environ. Epidemiol 14, 109–119. [DOI] [PubMed] [Google Scholar]
- Oppeneer SJ, Robien K, 2015. Bisphenol A exposure and associations with obesity among adults: a critical review. Public Health Nutr. 18, 1847–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki A, et al. , 2004. Chemical analysis and genotoxicological safety assessment of paper and paperboard used for food packaging. Food Chem. Toxicol 42, 1323–1337. [DOI] [PubMed] [Google Scholar]
- Perez-Palacios D, et al. , 2012. Determination of bisphenol-type endocrine disrupting compounds in food-contact recycled-paper materials by focused ultrasonic solid–liquid extraction and ultra performance liquid chromatography-high resolution mass spectrometry. Talanta 99, 167–174. [DOI] [PubMed] [Google Scholar]
- Petersen H, et al. , 2003. Determination of bisphenol A diglycidyl ether (BADGE) and its derivatives in food: Identification and quantification by internal standard. Eur. Food Res. Technol 216, 355–364. [Google Scholar]
- Rebenne LM, et al. , 1996. Aqueous chlorination kinetics and mechanism of substituted dihydroxybenzenes. Environ. Sci. Technol 30, 2235–2242. [Google Scholar]
- Rezaee M, et al. , 2006. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J. Chromatogr. A 1116, 1–9. [DOI] [PubMed] [Google Scholar]
- Rezg R, et al. , 2014. Bisphenol A and human chronic diseases: current evidences, possible mechanisms, and future perspectives. Environ. Int 64, 83–90. [DOI] [PubMed] [Google Scholar]
- Riu A, et al. , 2011a. Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ. Health Perspect 119, 1227–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riu A, et al. , 2011b. Characterization of novel ligands of ERα, Erβ, and PPARγ: The case of halogenated bisphenol A and their conjugated metabolites. Toxicol. Sci 122, 372–382. [DOI] [PubMed] [Google Scholar]
- Riu A, et al. , 2014. Halogenated bisphenol-A analogs act as obesogens in zebrafish larvae (Danio rerio). Toxicol. Sci 139, 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas A, et al. , 2002. Estrogenic effect of a series of bisphenol analogues on gene and protein expression in MCF-7 breast cancer cells. J. Steroid Biochem. Mol. Biol 82, 45–53. [DOI] [PubMed] [Google Scholar]
- Rochester JR, Bolden AL, 2015. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Gomez R, et al. , 2014a. A multiresidue method for the determination of selected endocrine disrupting chemicals in human breast milk based on a simple extraction procedure. Talanta 130, 561–570. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gomez R, et al. , 2014b. Gas chromatography and ultra high performance liquid chromatography tandem mass spectrometry methods for the determination of selected endocrine disrupting chemicals in human breast milk after stir-bar sorptive extraction. J. Chromatogr. A 1349, 69–79. [DOI] [PubMed] [Google Scholar]
- Rosenmai AK, et al. , 2014. Are structural analogues to bisphenol a safe alternatives? Toxicol. Sci 139, 35–47. [DOI] [PubMed] [Google Scholar]
- Ruan T, et al. , 2015. Evaluation of the in vitro estrogenicity of emerging bisphenol analogs and their respective estrogenic contributions in municipal sewage sludge in China. Chemosphere 124, 150–155. [DOI] [PubMed] [Google Scholar]
- Shankar A, Teppala S, 2011. Relationship between urinary bisphenol A levels and diabetes mellitus. J. Clin. Endocrinol. Metab 96, 3822–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, et al. , 2012. Urinary bisphenol a levels and measures of obesity: results from the national health and nutrition examination survey 2003–2008. ISRN Endocrinol. 2012, 965243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MK, et al. , 2011. Urinary Bisphenol a and type-2 diabetes in U.S. Adults: Data from NHANES 2003–2008. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somm E, et al. , 2009. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ. Health Perspect 117, 1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, et al. , 2014a. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int. J. Obes. (Lond) 38, 1532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, et al. , 2014b. Occurrence and profiles of bisphenol analogues in municipal sewage sludge in China. Environ. Pollut 186, 14–19. [DOI] [PubMed] [Google Scholar]
- Stachel B, et al. , 2003. Xenoestrogens in the River Elbe and its tributaries. Environ. Pollut 124, 497–507. [DOI] [PubMed] [Google Scholar]
- Stahlhut RW, et al. , 2009. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ. Health Perspect 117, 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples CA, et al. , 1998. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 36, 2149–2173. [DOI] [PubMed] [Google Scholar]
- Swedenborg E, et al. , 2009. Endocrine disruptive chemicals: mechanisms of action and involvement in metabolic disorders. J. Mol. Endocrinol 43, 1–10. [DOI] [PubMed] [Google Scholar]
- Swinburn BA, et al. , 2011. The global obesity pandemic: shaped by global drivers and local environments. Lancet 378, 804–814. [DOI] [PubMed] [Google Scholar]
- Takemura H, et al. , 2005. In vitro and in vivo estrogenic activity of chlorinated derivatives of bisphenol A. Toxicology 207, 215–221. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, et al. , 2004. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr. J 51, 165–169. [DOI] [PubMed] [Google Scholar]
- Tarantino G, et al. , 2013. Bisphenol A in polycystic ovary syndrome and its association with liver-spleen axis. Clin. Endocrinol 78, 447–453. [DOI] [PubMed] [Google Scholar]
- Tarazona I, et al. , 2013. Determination of benzophenone-3 and its main metabolites in human serum by dispersive liquid-liquid microextraction followed by liquid chromatography tandem mass spectrometry. Talanta 116, 388–395. [DOI] [PubMed] [Google Scholar]
- Teppala S, et al. , 2012. Bisphenol A and metabolic syndrome: Results from NHANES. Int. J. Endocrinol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, et al. , 2011. Assessment of thyroid hormone activity of halogenated bisphenol A using a yeast two-hybrid assay. Chemosphere 84, 1527–1530. [DOI] [PubMed] [Google Scholar]
- Tingaud-Sequeira A, et al. , 2011. Zebrafish obesogenic test: A tool for screening molecules that target adiposity. J. Lipid Res 52, 1765–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvi D, et al. , 2013. Prenatal bisphenol a urine concentrations and early rapid growth and overweight risk in the offspring. Epidemiology 24, 791–799. [DOI] [PubMed] [Google Scholar]
- Van Eeckhaut A, et al. , 2009. Validation of bioanalytical LC-MS/MS assays: evaluation of matrix effects. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 877, 2198–2207. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, et al. , 2010. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect 118, 1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, et al. , 2014. A round robin approach to the analysis of bisphenol A (BPA) in human blood samples. Environ. Health 13, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, et al. , 2007. Human exposure to bisphenol A (BPA). Reprod. Toxicol 24, 139–177. [DOI] [PubMed] [Google Scholar]
- Vela-Soria F, et al. , 2015. Matrix solid phase dispersion for the extraction of selected endocrine disrupting chemicals from human placental tissue prior to UHPLC-MS/MS analysis. Microchem. J 118, 32–39. [Google Scholar]
- Vela-Soria F, et al. , 2013. A new treatment by dispersive liquid-liquid microextraction for the determination of parabens in human serum samples. Anal. Bioanal. Chem 405, 7259–7267. [DOI] [PubMed] [Google Scholar]
- Vela-Soria F, et al. , 2014a. A multiclass method for the analysis of endocrine disrupting chemicals in human urine samples Sample treatment by dispersive liquid-liquid microextraction. Talanta 129, 209–218. [DOI] [PubMed] [Google Scholar]
- Vela-Soria F, et al. , 2014b. UHPLC-MS/MS method for the determination of bisphenol A and its chlorinated derivatives, bisphenol S, parabens, and benzophenones in human urine samples. Anal. Bioanal. Chem 406, 3773–3785. [DOI] [PubMed] [Google Scholar]
- Vela-Soria F, et al. , 2011. A multiclass method for endocrine disrupting chemical residue analysis in human placental tissue samples by UHPLC-MS/MS. Anal. Methods 3, 2073–2081. [Google Scholar]
- Venisse N, et al. , 2014. Reliable quantification of bisphenol A and its chlorinated derivatives in human urine using UPLC-MS/MS method. Talanta 125, 284–292. [DOI] [PubMed] [Google Scholar]
- Viñas P, et al. , 2010. Comparison of two derivatization-based methods for solid-phase microextraction–gas chromatography–mass spectrometric determination of bisphenol A, bisphenol S and biphenol migrated from food cans. Anal. Bioanal. Chem 397, 115–125. [DOI] [PubMed] [Google Scholar]
- Viñas R, et al. , 2013. Rapid estrogenic signaling activities of the modified (chlorinated, sulfonated, and glucuronidated) endocrine disruptor bisphenol A. Endocrine Disruptors. 1 e25411 [Google Scholar]
- Von Gunten U, 2003. Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 37, 1469–1487. [DOI] [PubMed] [Google Scholar]
- Von Gunten U, Salhi E, 2003. Bromate in drinking water: A problem in Switzerland? Ozone Sci. Eng 25, 159–166. [Google Scholar]
- Voordeckers JW, et al. , 2002. Anaerobic biotransformation of tetrabromobisphenol A, tetrachlorobisphenol A, and bisphenol A in estuarine sediments. Environ. Sci. Technol 36, 696–701. [DOI] [PubMed] [Google Scholar]
- Wang, et al. , 2012a. High urinary bisphenol A concentrations in workers and possible laboratory abnormalities. Occup. Environ. Med [DOI] [PubMed] [Google Scholar]
- Wang, et al. , 2012b. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J. Clin. Endocrinol. Metab 97, E223–E227. [DOI] [PubMed] [Google Scholar]
- Wang, et al. , 2012c. Occurrence and human exposure of p-hydroxybenzoic acid esters (parabens), bisphenol A diglycidyl ether (BADGE), and their hydrolysis products in indoor dust from the United States and three East Asian countries. Environ. Sci. Technol 46, 11584–11593. [DOI] [PubMed] [Google Scholar]
- Wang S, et al. , 2007. Does a stable isotopically labeled internal standard always correct analyte response? A matrix effect study on a LC/MS/MS method for the determination of carvedilol enantiomers in human plasma. J. Pharm. Biomed. Anal 43, 701–707. [DOI] [PubMed] [Google Scholar]
- Xue J, et al. , 2015. Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ. Res 137, 120–128. [DOI] [PubMed] [Google Scholar]
- Yach D, et al. , 2006. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat. Med 12, 62–66. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yasuhara A, 2002. Chlorination of bisphenol A in aqueous media: formation of chlorinated bisphenol A congeners and degradation to chlorinated phenolic compounds. Chemosphere 46, 1215–1223. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, et al. , 2003. Competitive interactions of chlorinated phenol compounds with 3,3′,5-triiodothyronine binding to transthyretin: Detection of possible thyroid-disrupting chemicals in environmental waste water. Toxicol. Appl. Pharmacol 187, 110–117. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. , 2014a. Urinary levels of bisphenol analogues in residents living near a manufacturing plant in south China. Chemosphere 112, 481–486. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. , 2014b. Simultaneous determination of bisphenol A, bisphenol AF, tetrachlorobisphenol A, and tetrabromobisphenol A concentrations in water using on-line solid-phase extraction with ultrahigh-pressure liquid chromatography tandem mass spectrometry. Int. J. Environ. Anal. Chem 94, 16–27. [Google Scholar]
- Yang YJ, et al. , 2009. Bisphenol A exposure is associated with oxidative stress and in-flammation in postmenopausal women. Environ. Res. 109, 797–801. [DOI] [PubMed] [Google Scholar]
- Ye X, et al. , 2011. in-vitro oxidation of bisphenol A: Is bisphenol A catechol a suitable bio-marker for human exposure to bisphenol A? Anal. Bioanal. Chem 399, 1071–1079. [DOI] [PubMed] [Google Scholar]
- Yoshihara S, et al. , 2004. Potent estrogenic metabolites of bisphenol A and bisphenol B formed by rat liver S9 fraction: their structures and estrogenic potency. Toxicol. Sci 78, 50–59. [DOI] [PubMed] [Google Scholar]
- Yuan SY, et al. , 2011. Anaerobic degradation of tetrachlorobisphenol-A in river sediment. Int. Biodeterior. Biodegrad 65, 185–190. [Google Scholar]
- Yuan SY, et al. , 2010. Biodegradation of tetrachlorobisphenol-A in river sediment and the microbial community changes. J. Environ. Sci. Health B 45, 360–365. [DOI] [PubMed] [Google Scholar]
- Zafra-Gómez A, et al. , 2008. Determination of some endocrine disrupter chemicals in urban wastewater samples using liquid chromatography-mass spectrometry. Microchem. J 88, 87–94. [Google Scholar]
- Zafra A, et al. , 2003. Gas chromatographic-mass spectrometric method for the determination of bisphenol A and its chlorinated derivatives in urban wastewater. Water Res. 37, 735–742. [DOI] [PubMed] [Google Scholar]
- Zhai H, Zhang X, 2011. Formation and decomposition of new and unknown polar brominated disinfection byproducts during chlorination. Environ. Sci. Technol 45, 2194–2201. [DOI] [PubMed] [Google Scholar]
- Zhao HY, et al. , 2012. The effects of bisphenol A (BPA) exposure on fat mass and serum leptin concentrations have no impact on bone mineral densities in non-obese premenopausal women. Clin. Biochem 45, 1602–1606. [DOI] [PubMed] [Google Scholar]
- Zhou X, et al. , 2014. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantifica tion of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 944, 152–156. [DOI] [PubMed] [Google Scholar]
- Zhou Y, et al. , 2015. Ubiquitous Occurrence of Chlorinated Byproducts of Bisphenol A and Nonylphenol in Bleached Food Contacting Papers and their Implications for Human Exposure. Environ. Sci. Technol [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


