Abstract
Background:
The debate continues regarding the best way to reconstruct posterior cruciate ligament (PCL). The objective of this study was to compare the knee stability and clinical outcomes after single and double bundle (SB and DB) PCL reconstruction.
Materials and Methods:
A total of 98 patients with PCL injury were enrolled for PCL reconstruction with four-strand semitendinosus and gracilis tendon autograft in the SB technique (n = 65) or two-strand Achilles allograft in the DB technique (n = 33). Each bundle fixation was achieved by the means of femoral Endo Button CL and tibial bioabsorbable interference screw. Demographic data, knee stability, and clinical outcomes were collected for analysis.
Results:
The SB and DB groups showed comparable demographic data. After a minimum followup interval of 24 months, the data of 59 patients in the SB group and 30 patients in the DB group were analyzed. There was no statistical difference between the SB and DB group in terms of both knee stability and clinical outcomes (P > 0.05).
Conclusions:
Compared with the SB technique, the DB technique did not exhibit any superiority in knee stability or clinical outcomes.
Keywords: Clinical outcome, double bundle, posterior cruciate ligament, single bundle, stability
Introduction
Posterior cruciate ligament (PCL) is the primary restraint for posterior translation in uninjured knees.1 The injury of the PCL has recently become an intriguing topic of discussion.2,3 The complex anatomy of the PCL has initially been identified as consisting of two bundles based on the ligament function in flexion and extension.4 The anterolateral (AL) bundle is the main part of the PCL, which accounts for at least 2/3 of the entire cross-sectional area.4,5,6 It is the primary restraint for maintaining the posterior stability of tibia at 0°–120° of flexion, while the posteromedial (PM) bundle maintains the posterior stability of tibia at hyperextension and flexion over 120°.4,6,7
Hence, an intense interest has been provoked with respect to the methods of reconstruction. Initial PCL reconstructions aimed at reconstructing the AL bundle of the PCL, with the objective to restore most of the PCL functions. However, early reports of these reconstructions concerned about persistent posterior laxity, particularly in extension and over-flexion.8,9 To simulate the intact knee posterior tibial translation across the full range of motion, double bundle (DB) reconstruction was presented.10 Subsequent studies made comparisons between the single bundle (SB) and DB reconstruction. However, due to the heterogeneity of the studies, contradictory conclusions had been drawn. For instance, different grafts have been employed in previous randomized controlled trials (RCTs). Nine RCTs has been found in PubMed, in which, one used tibialis anterior allografts;11 three used hamstring tendon autografts;12,13,14 four used Achilles tendon allografts;15,16,17,18 and one used Achilles tendon–bone allografts for SB and posterior tibialis tendon allograft for DB.19 Some scholars preferred the DB reconstruction as it is conducive to rotational and posterior stability.11,15,20 While others questioned its superiority arguing that even though it does exist, it would not be clinically significant.17
Therefore, the purpose of this study was to compare the knee stability and short-term clinical outcomes after the SB and DB PCL reconstruction. It is hypothesized that the DB PCL reconstruction is superior in restoring the knee stability and able to provide better short-term clinical outcomes than the SB reconstruction.
Materials and Methods
This prospective comparative study was designed to identify the differences in functions, stability, and the knee muscle strength recovery of PCL reconstruction between the SB and DB technique. The study was carried out at Qingdao University. It has been approved by the institutional review board and the ethics committee of Qingdao University. Meanwhile, all patients had signed the letter of consent regarding which graft to be used for preoperation.
From January 2004 to September 2012, a total of 130 patients with PCL injury were enrolled for PCL reconstruction with four-strand semitendinosus and gracilis tendon autograft in the SB technique or two-strand Achilles allograft in the DB technique. The diagnosis of PCL injury was based on the latest diagnosis standard of PCL injury.21 The inclusion criteria were as follows: (1) no history of surgeries on bilateral knees; (2) closed physes on MRI; and (3) no or minimal degenerative osteochondral changes on radiographic examination (Stage 0 or 1 in the Kellgren and Lawrence staging system22). Patients with concomitant injuries, including multiple-ligament injuries, fracture, posterolateral corner injuries or massive cartilage injuries requiring operative treatments (such as microfracture and autologous chondrocyte implantation in the ipsilateral knee), were excluded from the present study. Patients with ligament injuries in the contralateral knee were also excluded from the study. Patients who met the standard of diagnosis, inclusion criteria and exclusion criteria would be allocated to SB or DB group. Assignment to the SB or DB group was not randomized, but up to the patients’ choice of grafts in view of the following concerns: cost variance of the two techniques, religious factors, rejecting allograft, etc.
Operative procedure
All arthroscopic procedures were performed by the same surgeon (XT). Under spinal anesthesia, routine diagnostic arthroscopy was performed before PCL reconstruction, and associated injuries, including small chondral lesion and meniscus tear, if any, were treated first. Small chondral lesion was treated with debridement, and meniscus tear was treated with partial meniscectomy or meniscal suture.
Single bundle group
The semitendinosus and gracilis tendons were harvested through an anteromedial longitudinal incision [Figure 1a]. The grafts were doubled, and the graft loop ends were connected to the EndoButton CL® with 20-mm loops, and the free ends were prepared with whip stitches.
Figure 1.
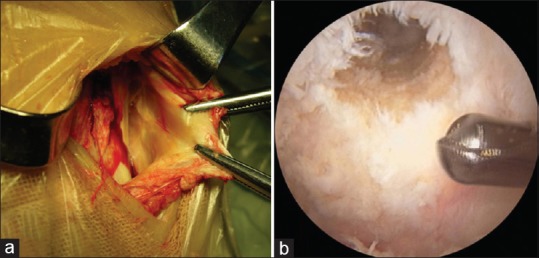
Single bundle posterior cruciate ligament reconstruction using semitendinosus and gracilis tendon autograft. (a) Semitendinosus and gracilis tendon harvest. (b) View of the femoral tunnel
The tibial tunnel was prepared under arthroscopic visualization through the posterior transseptal portal. For tibial tunnel, the tibia guide pin exited posteriorly at approximately 1.5 cm below the articular surface of the medial tibial plateau slightly lateral to the midline at the PCL insertion site. The tip of the guide pin was carefully protected with a curved curette to prevent injury of the neurovascular structures while being exited posteriorly. The tibial tunnel was then created with a graft-size matched core-reamer.
The femoral guide was introduced through the AL portal. To create the femoral tunnel, a 2.5 mm K-wire was passed through the additional AL approach with the knee flexed to 100° and then placed at the orientation of 1:30 (right knee) or 10:30 (left knee) position, which corresponds to 8 mm posterior to the anteromedial articular margins of the medial femoral condyle. This location was close to the insertion site of the AL bundle of the normal PCL [Figure 1b]. The guide wire was driven through the femur and exited on the medial epicondyle of the femur. Then, it was over-drilled carefully with a 4.5 mm diameter cannulate reamer in order not to damage the remnant PCL stump. Subsequently, a 25–30 mm deep femoral tunnel was created with a graft-size matched reamer. The graft was delivered through the tibial tunnel into the knee joint and the femoral tunnel. The distal end of the graft was pulled tightly inside the tibial tunnel. The knee was then cycled for approximately 20 times through a full range of motion, and a manual tension was applied with an anterior Drawer Test position during the tibial fixation. The graft was secured with a 30 mm long bioabsorbable intrafix screw (Smith and Nephew®; Andover, MA) of the graft diameter.
Double bundle group
Deep-frozen human Achilles allograft was used in the DB group. The bony portion was made in the cylindrical, 10 mm in diameter and 25 mm in length. The tendinous portion was split into two bundles and fashioned to be 6 mm in diameter each [Figure 2a]. The hook of the PCL tibial drill guide was introduced through the anteromedial portal and advanced to the only distal, lateral portion of the PCL tibial attachment.
Figure 2.
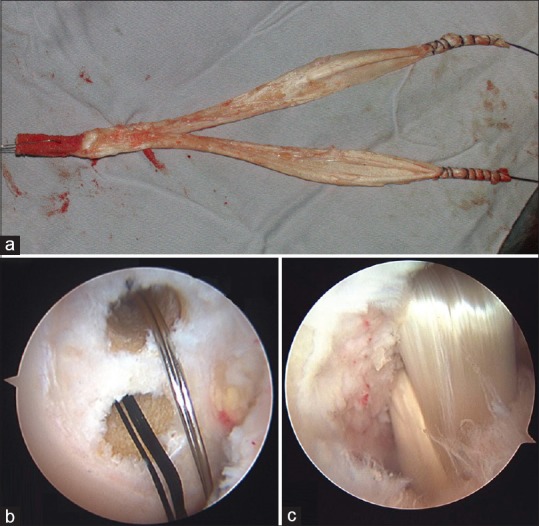
Double bundle posterior cruciate ligament reconstruction using Achilles allograft. (a) Achilles allograft preparation. (b) The femoral tunnels were drilled. And guide wire were placed in the position of the anterolateral and posteromedial bundle of the posterior cruciate ligament. (c) Arthroscopic image after reconstruction of the posterior cruciate ligament using the anterolateral and posteromedial band
The tibial tunnel was prepared in a similar way as in the SB group, with a diameter of 10 mm.
The anatomic insertions of the AL and PM bundles were located on the medial wall of the intercondylar notch. This location was identified and remnant preserved for the two femoral tunnels. The AL femoral tunnel was prepared at the 10:30–11:00 (left knee) or 1:00–1:30 (right knee) clock-face position, which was located at 13 mm posterior to the anteromedial articular margins of the medial femoral condyle and 13 mm distal to the top of the intercondylar notch. The PM femoral tunnel was determined as follows: 8 mm posterior to the anteromedial articular margins of the medial femoral condyle and 20 mm distal to the top of the intercondylar notch with 100° knee flexion and at the 8:30–9:00 (left knee) or 3:00–3:30 (right knee) clock-face position.23,24 The two femoral tunnels were created with a 6 mm reamer [Figure 2b] To establish a femoral fixation, the grafts for the PM and AL were then passed through each bone tunnel and the EndoButton loop was flipped over the anteromedial femoral cortical surface. The knee was then cycled for approximately 20 times through a full range of motion. The distance between the two tunnels should be at least 4 mm to avoid tunnel bridge collapse. A bioabsorbable intrafix screw (Smith and Nephew) was used for tibial fixation. The graft was tightened and fixed at 70° of knee flexion with an anteriorly directed load to restore normal tibial step-off [Figure 2c].
Postoperative rehabilitation
The two groups were provided the same rehabilitation protocol supervised by a physical therapist. The protocol was fine-tuned according to the purposes of regaining strength, endurance, agility, balance training, and muscular strength exercises.
Phase I (0–6 weeks postoperative): in the first 2 weeks postoperatively (postoperative), patients were installed with a long leg brace with toe-touch weight bearing, after which weight bearing was gradually increased to 75% of the body weight at the 4th week postoperative. Full weight bearing was permitted at the 6th week postoperative. With the assistance of the pad behind tibial plateau, the range of motion exercise during therapy was limited to 90° for the first 4 weeks, and then gradually increased to 120° for the next 2 weeks. During this phase, patients were required to walk with the brace locked at the 0° extension position.
Phase II (7–10 weeks postoperative): full knee flexion exercise was to be achieved at the end of this phase. The long leg brace was locked at the 65° flexion at the 7th week and increased to 120° for the next 3 weeks.
Phase III (11 weeks-): The brace was completely dismounted at 3rd month postoperative, and at the same time, jogging was allowed. High impact strengthening activities were permitted 1 year after operation.
Followup and outcome evaluation
The patients were required to record pain intensity on a visual analog scale (VAS)25 at a consistent time in the evening for the first 2 weeks postoperative. Differences in the mid-patellar knee circumference between the affected and unaffected extremities were measured to evaluate the swollen degree of the knee; difference >1 cm was considered clinically significant. Thereafter, the pain and swelling statuses were no longer documented because most of the patients were discharged from the hospital.
Followup evaluation index: patients were reexamined at the clinic 6 weeks, 10 weeks, 6 months, 1 year, and 2 years postoperative, respectively, and once a year thereafter. The primary outcome measures concentrated on knee stability, including subjective posterior stability (reverse-Lachman test, posterior drawer test), objective posterior stability: tibial posteriorization at 30° and 90° flexion (evaluated by KNEEELAX3® arthrometer), posterolateral rotatory instability (dial test with the knee at 30° of flexion), and single-legged hop test. The secondary outcome was the indices related to clinical outcomes (range of motion, thigh girth difference between the operative knee and healthy knee, Larson score,26 Lysholm score27). Adverse events were also monitored and documented [Table 1].
Table 1.
Demographic data and clinical scores of patients of the single bundle posterior cruciate ligament reconstruction group and double bundle posterior cruciate ligament reconstruction group
| Parameters | SB | DB | P |
|---|---|---|---|
| Number of patients | 65 | 33 | |
| Male: female | 42:18 | 22:8 | 0.742 |
| Average age (year) | 33.6±9.5 | 31.5±7.6 | 0.273 |
| Height (cm) | 177.0±13.6 | 182.2±16.1 | 0.096 |
| Weight (kg) | 81.3±6.4 | 83.6±7.6 | 0.118 |
| Causes of injuries | |||
| Car accident | 33 | 19 | 0.532 |
| Sports injury | 27 | 11 | 0.431 |
| Associated injuries | |||
| Meniscal tear | 10 | 6 | 0.723 |
| Partial meniscectomy | 6 | 4 | |
| Meniscal suture* | 4 | 2 | 0.066 |
| Cartilage injury† | 11 | 5 | 0.823 |
| Interval between injury and operation (month) | 4.5±1.9 | 5.0±2.1 | 0.238 |
Preoperative data show no statistically-significant difference between the two groups. *Fisher’s exact test was employed because cells have expected count <5, †Cartilage injury of Grade 1 or 2 by International Cartilage Repair Society Grading System. SB=Single bundle, DB=Double bundle
Demographic data, including gender, age, body mass index, the cause of injury, associated injury, the interval between injury and operation, were extracted from the medical records. The in-patient evaluation index and followup evaluation index were determined using two observers separately (one orthopedic surgeon and one physical therapist).
Statistical analysis
Statistical analysis was performed using the SPSS statistical software package, version 20.0 (IBM Corp., Armonk, New York, USA). Chi-square was employed to compare the demographic data between the two groups, and the incidence of complication and revision in the SB and DB groups. The independent t-test was applied to compare the two groups in terms of stability indices and clinical outcomes. P < 0.05 was considered statistically significant.
Results
With a minimum interval of 24 months (mean 28 months), 59 patients in the SB group and 30 patients in the DB patients continued to be followed up for analysis [Figure 3]. There was no difference between the two groups in terms of demographic data [Table 1]. The complications were caused by two superficial infections, one posterior cortical disruption of femoral tunnel in SB group (3.4%), one tunnel communication of femoral tunnel in the DB group (3.3%), and 31 injuries of the infrapatellar branch of the saphenous nerve in the SB group. According to the Pearson Chi-square test, there was no statistically significant difference between the SB and DB PCL reconstruction (P = 0.706).
Figure 3.
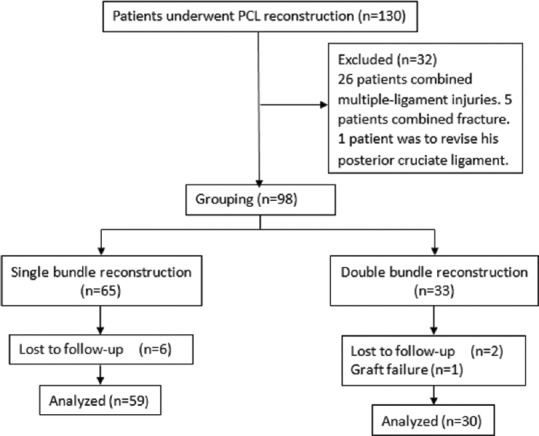
The flow diagram of the study. PCL = Posterior cruciate ligament
Two superficial infections in SB group were treated with antibiotics and routine dressing change. Only one PCL revision was performed before the last followup. The posterior cortical disruption of the femoral tunnel in the SB group contributed to the PCL graft healing failure, which in turn led to laxity of PCL grafts. However, the present study has not been able to detect any statistical difference in graft failure rate between the two techniques (P = 0.473).
In-patient evaluation
Four of 65 patients in the SB group and five of 33 in the DB group still suffered from pain (VAS score >3) by the time of discharge. However, no statistically significant difference was observed between the two groups (P = 0.225). The VAS scores of these patients were collected and analyzed, but no statistical difference was detected between the two groups (P = 1.000).
In the 2nd week postoperative, five of 65 patients in the SB group still had joint swelling. There was no statistical difference between them and patients in the DB group (5 of 33) (P = 0.238) Table 2.
Table 2.
Symptoms assessments in the 2nd week postoperation
| Parameters | SB (n=65) | DB (n=33) |
|---|---|---|
| Pain (%) | 4 (6.15) | 5 (15.15) |
| VAS score* | 3 (1-5) | 3 (1-5) |
| Swelling (%) | 5 (7.69) | 5 (15.15) |
*VAS was used to measure the intensity of knee pain ranging from 0 to 10, with 0 for no pain and 10 for most severe pain, only the patients who suffered from pain (4 in 65 of the SB and 5 in 33 of the DB) were enrolled in the VAS score assessment. VAS=Visual analog scale, SB=Single bundle, DB=Double bundle
Followup evaluation
At the last followup, two of 60 in the SB group and one of 30 in the DB group received closed-to-normal results in the reverse-Lachman test (P = 1.000). Meanwhile, one patient in the SB group received closed-to-normal results in the posterior drawer test and Dial test, while the results of all patients in the DB group were negative (P = 0.477). Such results neither favored the SB nor the DB technique. Similarly, other evaluation indices, including a range of motion, thigh girth difference, single-legged hop test, tibial posteriorization at 30° and 90° flexion, Larson score and Lysholm score, did not suggest statistical differences between the SB and DB group as well [Table 3].
Table 3.
Postoperation outcomes of the single bundle and double bundle posterior cruciate ligament reconstructions at the last followup
| Parameters | Preoperation | 2 years postoperation | P value of SB versus DB | |||
|---|---|---|---|---|---|---|
| SB (n=65) | DB (n=33) | SB (n=60) | DB (n=30) | Preoperation | 2 years postoperation | |
| Range of motion* (°) | 0-(133±9.2) | 0-(130±8.9) | 0-(136±9.1) | 0-(134±8.3) | 0.126 | 0.315 |
| Thigh girth difference (mm) | 6.3±3.8 | 7.9±4.7 | 0.093 | |||
| Single-legged hop test (cm) | 90.2±16.9 | 87.6±18.3 | 0.505 | |||
| Tibial posteriorization at 30° flexion† (mm) | 13.22±2.76 | 13.16±2.88 | 2.25±0.26 | 2.24±0.25 | 0.920 | 0.862 |
| Tibial posteriorization at 90° flexion† (mm) | 13.98±2.99 | 13.89±3.02 | 2.28±0.29 | 2.26±0.25 | 0.889 | 0.748 |
| Larson score | 49.54±4.38 | 48.76±3.98 | 92.76±5.48 | 91.62±4.86 | 0.393 | 0.337 |
| Lysholm score | 41.63±3.45 | 43.78±4.66 | 90.63±5.78 | 89.76±4.87 | 0.011 | 0.481 |
*Range of motion was evaluated by IsoMed 2000® isokinetic dynamometer, †Posterior translation at different flexion angles was evaluated by KNEEE LAX3® arthrometer.SB=Single bundle, DB=Double bundle
Discussion
In this study, knee stability and clinical outcomes were compared after SB and DB reconstruction for PCL deficient knees. The results and clinical outcomes showed that DB PCL reconstruction with an allograft was not a superior technique in restoring the posterior tibial translation in comparison with the SB reconstruction with an autograft. The data obtained from this study was comparable to previous studies in terms of clinical outcomes.13,14,16,17,18,19 Nevertheless, the conclusion of early reports on issues of knee stability were inconsistent. Kim,16 Xu,14 Li11 reported that the DB PCL reconstruction provided more satisfying stability than the SB technique. However, there are concerns about the clinical significance of its superiority.18
The following factors might contribute to the results of this study:
First, the anatomy foundation of PCL splitting was not robust. PCL is more accurately defined as a continuum of fibers that rotate during the knee flexion and extension. With certain posterior loading, the particular bundle of PCL showed a different pattern of tension and relaxation cycle. In addition to quartering28 and trichotomy,29 a variety of patterns of splitting have been developed. Based on the function in flexion and extension, most scholars regarded PCL as a two-division ligament (AL bundle and PM bundle). However, previous studies showed that PCL was divided at different flexion angles, leading to a nonuniform division standard.
Second, the AL bundle has a significantly greater linear stiffness and ultimate load than the PM bundle.30 Race claimed that the AL bundle was six times as strong as the PM bundle.5 The AL bundle is primarily responsible for the stabilizing effect of the PCL. Thus, at the theoretical level, reconstruction of the AL bundle is sufficient to restore the PCL biomechanics.
Third, the present study is concerned about the reconstruction of PCL in its anatomic insertion, rather than isometric reconstruction. Although substitutes for PCL and PCL itself were morphologically different, the function of PCL fibers is determined primarily by the femoral attachment site.31 By drilling the tunnel at the center of its anatomical insertion, the original tension– relaxation pattern had been simulated to the best extent in this study.
Fourth, the indices used to evaluate stability were practised at the flexed position, while the AL bundle is responsible for the knee stability at flexion. As respect to clinical outcomes, since the conservative treatment has provided tolerate clinical and biomechanical results, PCL reconstruction theoretically will generate better outcomes.
The present study is subject to several limitations. First, the relatively small sample size of this hypothesis-generating study was underpowered to indicate statistical differences between the two groups. The power analysis (results not shown) showed that the smallest study population in each group should be 158 to achieve >90% of statistical power. However, due to the low incidence of PCL injury, this problem is universal in PCL studies.11,12,13,14,15,17,19 Second, different grafts were used to reconstruct PCL (semitendinosus and gracilis tendons for SB group and deep-frozen human Achilles allograft for DB group). The DB reconstruction technique requires grafts to be >12 mm in diameter; yet unilateral autologous hamstring tendon does not meet such requirement. The impact imposed by different tendon grafts is unclear. Third, patient assignment to the SB or DB group was not randomized, but subject to other factors such as the financial cost of the two techniques. This may be a significant bias factor. Fourth, in view of the uniqueness of the incision of hamstring tendon harvesting, neither the patients nor observers were blinded at the followup evaluation.
Conclusions
SB and DB PCL reconstruction showed comparable results in stability and clinical outcomes. The differences between these two techniques, if any, can be clarified only by larger sample size. Further multicentric randomized controlled studies are required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This study was funded by medicine and health technology development plan project of Shandong province (2015WS0328), Qingdao outstanding health professional development fund, and Qingdao key health discipline development fund.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am. 1980;62:259–70. [PubMed] [Google Scholar]
- 2.Kennedy JC, Hawkins RJ, Willis RB, Danylchuck KD. Tension studies of human knee ligaments. Yield point, ultimate failure, and disruption of the cruciate and tibial collateral ligaments. J Bone Joint Surg Am. 1976;58:350–5. [PubMed] [Google Scholar]
- 3.Trent PS, Walker PS, Wolf B. Ligament length patterns, strength, and rotational axes of the knee joint. Clin Orthop Relat Res. 1976;117:263–70. [PubMed] [Google Scholar]
- 4.Girgis FG, Marshall JL, Monajem A. The cruciate ligaments of the knee joint.Anatomical, functional and experimental analysis. Clin Orthop Relat Res. 1975 Jan-Feb;106:216–31. doi: 10.1097/00003086-197501000-00033. [DOI] [PubMed] [Google Scholar]
- 5.Race A, Amis AA. The mechanical properties of the two bundles of the human posterior cruciate ligament. J Biomech. 1994;27:13–24. doi: 10.1016/0021-9290(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 6.Harner CD, Janaushek MA, Kanamori A, Yagi M, Vogrin TM, Woo SL, et al. Biomechanical analysis of a double-bundle posterior cruciate ligament reconstruction. Am J Sports Med. 2000;28:144–51. doi: 10.1177/03635465000280020201. [DOI] [PubMed] [Google Scholar]
- 7.Liu P, Ao YF. The clinical anatomical research of the tibial attachment of the posterior cruciate ligament and the tibial tunnel position in double-bundle posterior cruciate ligament reconstruction. Zhonghua Wai Ke Za Zhi. 2008;46:1080–4. [PubMed] [Google Scholar]
- 8.Markolf KL, Slauterbeck JR, Armstrong KL, Shapiro MS, Finerman GA. A biomechanical study of replacement of the posterior cruciate ligament with a graft. Part 1: Isometry, pre-tension of the graft, and anterior-posterior laxity. J Bone Joint Surg Am. 1997;79:375–80. doi: 10.2106/00004623-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Wang CJ, Chen HS, Huang TW. Outcome of arthroscopic single bundle reconstruction for complete posterior cruciate ligament tear. Injury. 2003;34:747–51. doi: 10.1016/s0020-1383(02)00197-3. [DOI] [PubMed] [Google Scholar]
- 10.Race A, Amis AA. PCL reconstruction. In vitro biomechanical comparison of ‘isometric’ versus single and double-bundled ‘anatomic’ grafts. J Bone Joint Surg Br. 1998;80:173–9. doi: 10.1302/0301-620x.80b1.7453. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Li J, Wang J, Gao S, Zhang Y. Comparison of single-bundle and double-bundle isolated posterior cruciate ligament reconstruction with allograft: A prospective, randomized study. Arthroscopy. 2014;30:695–700. doi: 10.1016/j.arthro.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 12.Hatayama K, Higuchi H, Kimura M, Kobayashi Y, Asagumo H, Takagishi K, et al. A comparison of arthroscopic single- and double-bundle posterior cruciate ligament reconstruction: Review of 20 cases. Am J Orthop (Belle Mead NJ) 2006;35:568–71. [PubMed] [Google Scholar]
- 13.Wang CJ, Weng LH, Hsu CC, Chan YS. Arthroscopic single- versus double-bundle posterior cruciate ligament reconstructions using hamstring autograft. Injury. 2004;35:1293–9. doi: 10.1016/j.injury.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Yin Y, Wang JQ, Ao YF. Comparison of single and double bundle isolate posterior cruciate ligament reconstruction with hamstring autograft. Zhonghua Wai Ke Za Zhi. 2013;51:247–51. [PubMed] [Google Scholar]
- 15.Kim SJ, Kim SH, Kim SG, Kung YP. Comparison of the clinical results of three posterior cruciate ligament reconstruction techniques: Surgical technique. J Bone Joint Surg Am. 2010;92(Suppl 1)(Pt 2):145–57. doi: 10.2106/JBJS.J.00185. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Kim TE, Jo SB, Kung YP. Comparison of the clinical results of three posterior cruciate ligament reconstruction techniques. J Bone Joint Surg Am. 2009;91:2543–9. doi: 10.2106/JBJS.H.01819. [DOI] [PubMed] [Google Scholar]
- 17.Yoon KH, Bae DK, Song SJ, Cho HJ, Lee JH. A prospective randomized study comparing arthroscopic single-bundle and double-bundle posterior cruciate ligament reconstructions preserving remnant fibers. Am J Sports Med. 2011;39:474–80. doi: 10.1177/0363546510382206. [DOI] [PubMed] [Google Scholar]
- 18.Valdevit A, Kambic H, Lilly D, Graham S, Parker R, Bergfeld J, et al. Non-linear fitting of mechanical data for efficacy determination of single versus double bundle achilles tendon grafts for PCL reconstructions. Biomed Mater Eng. 2002;12:309–17. [PubMed] [Google Scholar]
- 19.Kim SJ, Jung M, Moon HK, Kim SG, Chun YM. Anterolateral transtibial posterior cruciate ligament reconstruction combined with anatomical reconstruction of posterolateral corner insufficiency: Comparison of single-bundle versus double-bundle posterior cruciate ligament reconstruction over a 2- to 6-year followup. Am J Sports Med. 2011;39:481–9. doi: 10.1177/0363546510385398. [DOI] [PubMed] [Google Scholar]
- 20.Takeda Y, Sato R, Ogawa T, Fujii K, Naruse A. In vivo magnetic resonance imaging measurement of tibiofemoral relation with different knee flexion angles after single- and double-bundle anterior cruciate ligament reconstructions. Arthroscopy. 2009;25:733–41. doi: 10.1016/j.arthro.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Margheritini F, Mariani PF, Mariani PP. Current concepts in diagnosis and treatment of posterior cruciate ligament injury. Acta Orthop Belg. 2000;66:217–28. [PubMed] [Google Scholar]
- 22.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stähelin AC, Südkamp NP, Weiler A. Anatomic double-bundle posterior cruciate ligament reconstruction using hamstring tendons. Arthroscopy. 2001;17:88–97. doi: 10.1053/jars.2001.20661. [DOI] [PubMed] [Google Scholar]
- 24.Ao YF. Beijing: Peking University Medical Press; 2009. Repair or reconstruction of posterior cruciate ligament injury. Cruciate Ligament Surgery; p. 326. [Google Scholar]
- 25.Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13:227–36. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]
- 26.Larson DE, Premer RF, Gustilo RB. Acute ligamentous injuries of the knee joint. Minn Med. 1973;56:374–6. [PubMed] [Google Scholar]
- 27.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43–9. [PubMed] [Google Scholar]
- 28.Makris CA, Georgoulis AD, Papageorgiou CD, Moebius UG, Soucacos PN. Posterior cruciate ligament architecture: Evaluation under microsurgical dissection. Arthroscopy. 2000;16:627–32. doi: 10.1053/jars.2000.9238. [DOI] [PubMed] [Google Scholar]
- 29.Inderster A, Benedetto KP, Klestil T, Künzel KH, Gaber O. Fiber orientation of posterior cruciate ligament: An experimental morphological and functional study, part 2. Clin Anat. 1995;8:315–22. doi: 10.1002/ca.980080502. [DOI] [PubMed] [Google Scholar]
- 30.Harner CD, Xerogeanes JW, Livesay GA, Carlin GJ, Smith BA, Kusayama T, et al. The human posterior cruciate ligament complex: An interdisciplinary study. Ligament morphology and biomechanical evaluation. Am J Sports Med. 1995;23:736–45. doi: 10.1177/036354659502300617. [DOI] [PubMed] [Google Scholar]
- 31.Oakes DA, Markolf KL, McWilliams J, Young CR, McAllister DR. The effect of femoral tunnel position on graft forces during inlay posterior cruciate ligament reconstruction. Am J Sports Med. 2003;31:667–72. doi: 10.1177/03635465030310050601. [DOI] [PubMed] [Google Scholar]


