Abstract
Background
Chronic lymphocytic leukemia (CLL) mainly affects older persons and is the commonest form of leukemia, with an incidence of 6 cases per 100 000 persons per year. In Germany, approximately 1000 men and 850 women die of CLL each year.
Methods
This review is based on pertinent publications retrieved by a selective literature search in PubMed and on the authors’ scientific and clinical experience.
Results
The diagnosis of CLL requires the detection of at least 5000 B-lymphocytes per microliter in the peripheral blood. Courses of CLL may be indolent and require no treatment, but may also be aggressive and progress rapidly. Treatment should be initiated when there is marked evidence of bone-marrow suppression or disease-related symptoms such as B symptoms or fatigue. In the past ten years, a number of targeted drugs have been introduced that can achieve a very good, long-lasting response, particularly when used in combination. The combination of chemotherapy with anti-CD20 antibodies (chemoimmunotherapy) is the standard first-line treatment. In younger patients without any relevant accompanying illnesses, the combination of fludarabine, cyclophosphamide, and rituximab prolongs survival. Patients with comorbidities should be treated with a combination of chlorambucil and obinutuzumab. In the last few years, ibrutinib, idelalsib, and venetoclax have been approved for clinical use. These substances inhibit cellular signal transduction pathways and are being increasingly used.
Conclusion
Recent progress in the development of novel treatment options gives hope that CLL may soon be a controllable disease. Even at present, chemoimmunotherapy can achieve a progression-free survival of more than eight years in certain genetically defined subgroups of CLL patients.
Chronic lymphocytic leukemia (CLL) is the most common type of leukemia, typically affecting older adults. The disease can take an indolent course without need for treatment, but may also present as aggressive disease with rapid progression. By the combined use of chemotherapy and monoclonal antibodies (chemoimmunotherapy), today progression-free survival of more than 8 years has already become a reality in subgroups of CLL patients with specific genetic features. Over the past 10 years, several targeted drugs capable of achieving excellent and sustained responses, especially as combination therapies, have been introduced into clinical practice. Altogether, the advances have given rise to hopes that treatments to control CLL could become available in the near future.
Epidemiology
With an incidence of approximately 6 per 100 000 population, CLL is the most common type of leukemia in Germany. Men are more frequently affected than women (ratio of 1.9 : 1.4). With a median age of 73 years at the time of first diagnosis, CLL is also referred to as leukemia of the elderly (1).
Pathogenesis
CLL is characterized by the clonal proliferation of mature, CD5-positive B cells, accumulating in the blood, the bone marrow, in lymph nodes and in the spleen (2). Only a few risk factors for the development of the disease are known (3), for example living on a farm or exposure to herbicides and pesticides (3). Approximately 10% of all CLL patients have a positive family history for the disease (4). Furthermore, inverse correlations between the risk of developing CLL und recreational sun exposure as well as the presence of any atopic condition were reported (3). There is also weak evidence indicating that hepatitis C and other infectious diseases can increase the risk of developing the disease (3).
The pathogenesis of CLL is explained by acquired genetic aberrations, developing in multiple steps. Typically, CLL is associated with the destruction of large parts of chromosomal material; for example, the deletion of the long arm of chromosome 13 [del(13q)]) is the most common chromosomal abnormality, present in half of the CLL patients. During the course of the disease, further mutations or chromosomal alterations occur, resulting in increased aggressiveness and resistance (5). Here, the deletion of the short arm of chromosome 17 [del(17p)], in approximately 5% to 8% of cases), is of particular note as it results in the loss of the TP53 gene. This loss, but also mutations of the TP53 gene, lead to resistance to chemotherapeutic agents. Another characteristic feature of CLL cells is their dependency on a microenvironment in the bone marrow or lymphatic organs, i.e. they survive outside the body only for a short time (6).
Clinical presentation, differential diagnosis, diagnostic evaluation, and prognosis
In many cases, CLL is diagnosed only because of an incidental finding of lymphocytosis on a routine complete blood cell count obtained for other reasons. Besides that, lymphadenopathy is a common first manifestation of the disease. Less common initial signs and symptoms include
B symptoms (fever, night sweats, weight loss)
Fatigue
Cytopenias (anemia, thrombocytopenia, neutropenia) and associated clinical signs (infection, fatigue, hemorrhage)
Autoimmune phenomena, such as autoimmune hemolytic anemia (AIHA).
The diagnosis of CLL requires the presence of ≥ 5000 B lymphocytes/µL in the peripheral blood. The disease is typically diagnosed by immunophenotyping (7), which helps to distinguish CLL from reactive, benign B lymphocytosis or other types of low-grade non-Hodgkin lymphoma (8). Clonality of CLL cells is demonstrated by the detection of kappa or lambda light chain restriction. If immunophenotyping does not allow to definitely confirm the diagnosis of CLL (8), a lymph node biopsy should be performed (9).
If, despite detection of clonality, the number of B lymphocytes is below 5000/µL in the peripheral blood in the absence of cytopenia, lymphadenopathy, hepatomegaly or splenomegaly, a monoclonal B lymphocytosis (MBL) is diagnosed (8). MBL typically has a good prognosis and does not require treatment. MBL progresses to overt CLL at a rate of 1% to 2% of cases per year (10) and is associated with an increased risk of infection. The risk of secondary malignancy is about twice as high in MBL patients (11).
Once the diagnosis of CLL has been established, investigations are carried out which help to estimate the stage of the disease and the prognosis. Initial staging is performed using the clinical staging systems suggested by Rai et al. (12) or Binet et al. (13) which are based on physical examination findings and complete blood counts. Over the last 20 years, many biological and genetic markers have been described which add further prognostic information (5). Especially the detection of del(17p) or genetic changes of the TP53 tumor suppressor gene located on chromosome 17 are predictive of an aggressive course of the disease and resistance to chemotherapy (14). The confusing abundance of new prognostic factors and the decreasing prognostic discriminatory power of the clinical staging systems with the advent of improved treatment methods led to the development of the CLL-IPI (CLL-International Prognostic Index) (15), combining 5 independent prognostic factors and distinguishing 4 risk groups. This index can be calculated using an online tool (www.qxmd.com/) (table 1).
Table 1. International Prognostic Index for Chronic Lymphocytic Leukemia (CLL, CLL-IPI*).
| Variable | Risk factor | Points | Score | Risk group | 5-year survival rates (%) | Subgroup frequency (%) | |
| TP53 (17p) | Deletion and/or mutation | 4 | 0–1 | low | 93.2 | 28 | |
| IGHV | unmutated | 2 | 2–3 | intermediate | 79.3 | 39 | |
| B2M (mg/L) | >3.5 | 2 | 4–6 | high | 63.3 | 28 | |
| Age | >65 years | 1 | 7–10 | very high | 23.3 | 5 | |
| Stage | Binet B/C, Rai I–IV | 1 | |||||
| Total risk score | 0–10 | ||||||
* For the variables listed in the Table, differently weighted points are awarded, resulting in total risk scores between 0 and 10.
From the total risk score, 4 risk groups can be identified (low, intermediate, high, and very high) which differ in their overall survival rates.
The classification of the risk groups originates from the time before the introduction of chemoimmunotherapy. Nevertheless, it has proven to be useful until today.
The introduction of new substances has significantly improved prognosis.
Binet or Rai staging system: disease progression is defined based on laboratory tests and physical examination;
IPI, International Prognostic Index, B2M, ß2 -microglobulin.
Indication for treatment
The majority of patients does initially not require treatment because the disease is asymptomatic in the early stages. Only when patients develop marked signs of bone marrow suppression (anemia or thrombocytopenia) and disease-related symptoms (e.g. fatigue, B symptoms or discomfort caused by large nodal masses), treatment of CLL is indicated. Until now, no evidence of benefits from using one of the treatments detailed below in early stages of the disease or in asymptomatic patients has been reported (16).
First-line therapy
For many decades, chlorambucil was the gold standard treatment for all patients with CLL (17, 18). Building on this standard of care, the German CLL Study Group (GCLLSG, Deutsche CLL-Studiengruppe) has systematically improved the initial treatment of CLL. The CLL5 trial showed that older CLL patients did not benefit from first-line therapy with fludarabine (F) (median overall survival of 46 months versus 64 months with chlorambucil), despite the fact that the substance fludarabine is in principle more effective than chlorambucil (18). The CLL4 trial found that the combination of fludarabine, a purine analog, with cyclophosphamide (FC) improved the quality and duration of response in younger patients below age 65 years compared with fludarabine alone (19). By combining FC therapy with rituximab, an anti-CD20 monoclonal antibody, a very effective treatment regimen (FCR) was obtained. The CLL8 trial compared the chemoimmunotherapy FCR with the FC regimen alone. For the first time it was shown in a prospective, randomized trial that the use of a specific first-line therapy for CLL, the FCR regimen, improved overall survival and changed the natural course of the disease (20). Since then, FCR has been the standard of care for young CLL patients without relevant concomitant diseases (20, 21). The long-term follow-up of the CLL8 trial revealed that a genetically defined subgroup of 40% of the physical fit CLL patients significantly benefited from this therapy. In more than half of these patients (patients with mutated IGHV status), no recurrence has been detected even after a period of 12 years (22). In comparison with FCR therapy, bendamustine in combination with rituximab (BR) is clearly less effective (CLL10 study) (23). However, in CLL patients older than age 65 years, FCR is associated with significantly increased bone marrow toxicity and higher rates of severe infection compared to BR. Thus, in physically fit patients older than age 65, the use of BR can be advantageous (23).
In patients with significant comorbidity who are labeled as “unfit” (or “slow go“) based on an assessment with the Comorbidity Illness Rating Scale (CIRS; score >6), FCR chemoimmunotherapy is also associated with excessive toxicity and thus not usable. In this patient group, chlorambucil was the standard of care. In this patient group, too, the addition of anti-CD20 antibodies has resulted in significantly improved outcomes. Especially the use of the anti-CD20 antibody obinutuzumab in combination with chlorambucil (CLL11 trial) was associated with prolonged overall survival compared to treatment with chlorambucil and to treatment with chlorambucil plus rituximab (24). Median overall survival in patients treated with chlorambucil alone was 58.5 month; chlorambucil plus obinutuzumab did not reach the median overall survival (25). Based on these results, chemoimmunotherapy using anti-CD20 antibodies is now the established standard of care in the first-line treatment of CLL patients.
In recent years, new oral kinase inhibitors (ibrutinib, idelalisib) and B-cell lymphoma-2 (Bcl-2) inhibitors (venetoclax) have been introduced for the treatment of CLL. Ibrutinib received approval for first-line treatment of CLL based on the results of the RESONATE-2 trial which compared continuous ibrutinib therapy with chlorambucil alone given over a limited period of time in patients aged 65 years and older. Ibrutinib treatment resulted in significantly prolonged overall survival, improved response rates and longer progression-free survival (18 months with chlorambucil, while for ibrutinib the median was not reached) (26). Thus, ibrutinib is an alternative first-line therapy for CCL in unfit patients. Only by the end of 2018, a head-to-head comparison of single-agent ibrutinib with chemoimmunotherapy became available. It now appears that ibrutinib will become a potential first-line option for subgroups of CLL (IGHV-unmutated patients).
Treatment of patients with cytogenetic risk factors
Patients with defined genetic risk factors, especially those with a genetic change affecting the TP53 gene [mutations and/or del(17p)] have a poor prognosis. For example, CLL patients with del(17p) have a median overall survival of only 33.1 months after FCR treatment (27). These patients are often refractory to cytostatic agents and should not receive chemotherapy according to current evidence.
Development of targeted therapies
The already mentioned, recently approved new inhibitors of signaling pathways critical to CLL (ibrutinib, idelalisib and venetoclax) have changed the management of CLL. Therefore, a brief summary of their features is provided below.
Ibrutinib, an inhibitor of Bruton‘s tyrosine kinase (BTK), has been available for the treatment of patients with CLL since 2017. Its adverse reactions differ from those of chemotherapeutic agents and are less severe which is reflected in the lower rate of treatment discontinuations compared to the chlorambucil group (28). Patients treated with ibrutinib rarely develop severe bone marrow toxicity and infections. Important adverse reactions include increased susceptibility to bleeding and cardiac arrhythmias, especially atrial fibrillation in 8% to 10% of patients which is partly reversible after temporary interruption of the treatment or dose reduction. With an estimated 30-month overall survival of 65%, ibrutinib also showed good efficacy in patients with del(17p) who received ibrutinib as first-line treatment (29). Consequently, ibrutinib is recommended as a first-line treatment for patients with genetic TP53 changes, provided that its use is not contraindicated (history of atrial fibrillation, cardiac problems or hemorrhagic events).
Idelalisib is a phosphatidylinositol 3-kinase-delta (PI3Kd) inhibitor. It was directly evaluated in combination with rituximab and approved in 2014, because the combination of idelalisib plus rituximab significantly prolonged survival compared to rituximab alone in older, comorbid patients with previously treated CLL (30). Since some studies reported fatal, mainly opportunistic infections (pneumocystis, cytomegalovirus [CMV]), idelalisib is recommended for patients with genetic TP53 changes who are not eligible for alternative first-line treatment options (31).
Venetoclax is a BH3-only mimetic and inhibits the anti-apoptotic protein BCL-2. Venetoclax has shown excellent short-term efficacy in all patient groups, in particular also in patients with genetic TP53 changes (32). However, due to the rapid response to the substance, patients may develop tumor lysis syndrome (TLS). Therefore, the dose has to be increased gradually and regular laboratory testing is required. Venetoclax was approved in 2016 for the treatment of CLL patients with del(17p) or genetic TP53 changes in patients who are not eligible for or failed to respond to ibrutinib and idelalisib, as well as for patients who failed to respond to chemoimmunotherapy or kinase inhibitor therapy (32).
The new inhibitors are increasingly used to treat patients with relapsed CLL (33, 34), in particular if after an initial chemotherapy patients develop an early relapse within three years.
Today, allogeneic stem cell transplantation (HSCTx) is only rarely indicated due to these numerous treatment options that have become available.
Current trials
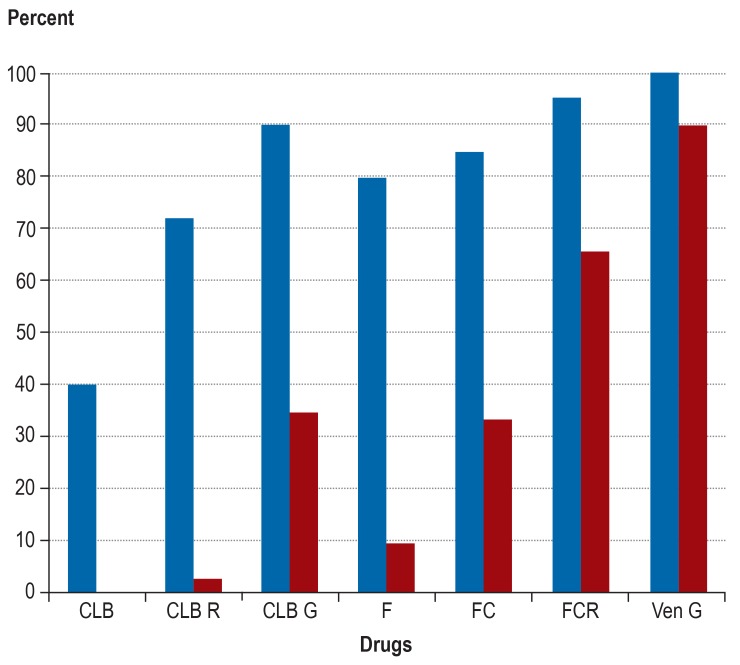
Besides the substances mentioned above, numerous other substances are available, but their detailed description would go beyond the scope of this paper (35– 37). At this time, however, it is more important to develop optimal combinations, making use of all available agents. Therefore, the German CLL Study Group (GCLLSG) is currently evaluating these combinations in several trials, especially as first-line treatments. The CLL13 trial in physically fit patients without cytogenetic risk factors is designed to compare several chemotherapy-free regimes with chemoimmunotherapy in the first-line setting. This study is evaluating combinations of venetoclax and rituximab (RVe), venetoclax and obinutuzumab (GVe) as well as venetoclax, obinutuzumab and ibrutinib (GIVe). The CLL2-GIVe trial is assessing the efficacy and safety of first-line GIVe therapy in patients with genetic TP53 changes. The CLL14 trial is comparing chemoimmunotherapy with chlorambucil plus obinutuzumab to GVe in the first-line treatment of older, comorbid patients. First results from the safety run-in of this study have shown that the GVe combination is safe and very effective (38) (figure). For further information about currently recruiting GCLLSG studies visit http://www.dcllsg.de/.
Figure.
Development of response rates and remissions with no longer detectable minimal residual disease (MRD) with the various first-line treatments for chronic lymphocytic leukemia. The results show the efficacy improvements achieved by combining various agents and the excellent efficacy of the new combination of venetoclax (Ven) plus obinutuzumab (GA101, G).
CLB, chlorambucil; R, rituximab; F, fludarabine; C, cyclophosphamide;
Blue bar: overall response (%); red bar: rate of patients in whom no MRD was detected in the peripheral blood using immunophenotyping (<104).
New minimal residual disease-guided treatment concepts
Based on our concept of a targeted and tailored (personalized) therapy aiming at total eradication of minimal residual disease (MRD)—the so called ‘sequential triple T concept’—, the GCLLSG has initiated several phase II studies. CLL patients receive—depending on the tumor mass— a debulking regimen consisting of up to 2 cycles of bendamustine, followed by 6 cycles of induction therapy with 1 to a maximum of 3 of the new oral substances (ibrutinib, acalabrutinib, idelalisib, or venetoclax) in combination with an anti-CD20 antibody (obinutuzumab or ofatumumab), followed by MRD-guided maintenance therapy. When testing for MRD in the peripheral blood has repeatedly yielded negative results, treatment is terminated. Here, again, the first results are promising with MRD-negative remissions in more than 90% of patients in the first-line setting (figure).
Transfer to current clinical practice and conclusion
CLL is the most common type of leukemia in Germany, with an incidence of approximately 6 per 100 000 population. Approximately 1000 men and 850 women die of the disease every year. CLL patients with sufficient bone marrow reserves and without B symptoms (fever, night sweats, weight loss) are initially followed with close observation only and do not require treatment.
Patients with symptomatic or advanced CLL receive treatment according to their levels of fitness. FCR is the standard treatment for fit patients without genetic TP53 changes. Combinations of chlorambucil and an anti-CD20 antibody, mainly obinutuzumab, are used for initial treatment in comorbid patients. Patients with genetic TP53 changes should be treated with targeted substances because chemotherapies are usually less effective. In the relapse setting, the initial treatment can be repeated if the first remission lasted for at least 3 years. In all other cases, treatment is switched to one of the new targeted substances.
Table 2 shows the current GCLLSG-recommended treatment algorithm which is based on the available evidence. Further information about the disease is available from the clinical practice (S3) guidelines of the AWMF (39) and the German Society of Hematology and Medical Oncology (40) which have been published online.
Table 2. Current treatment recommendations for chronic lymphocytic leukemia (CLL)*1.
| Binet stage | Fitness*2 |
Genetic TP53 changes |
First-line treatment |
| A/B without symptoms | irrelevant | Irrelevant | None |
| C, A/B with symptoms | “go go“ | No | FCR (BR ≥ 65 years) |
| Yes | Ibrutinib, if contraindications for ibrutinib: venetoclax, idelalisib + R, (allo-HSCTx) |
||
| “slow go“ | Yes | ||
| No | Chlorambucil + obinutuzumab or ibrutinib | ||
| Binet stage | Fitness*2 |
Genetic TP53 changes |
Relapse treatment |
| Early (<3 years) | “go go“ | Irrelevant | Ibrutinib, idelalisib + R, venetoclax*3, alemtuzumab, where appropriate, discuss allo-HSCTx as consolidation |
| “slow go“ | Ibrutinib, idelalisib + R, venetoclax*3, alemtuzumab, BR/BO, Ofatumumab mono, HD rituximab, lenalidomide (+ R) |
||
| Late (>3 years) | Irrelevant | Yes | Ibrutinib, idelalisib + R, venetoclax*3, alemtuzumab, where appropriate, discuss allo-HSCTx as consolidation |
| “go go“ | No | Repeat first-line treatment | |
| “slow go“ |
*1 Modified according to the AWMF Clinical Practice (S3) Guideline for CLL and according to the Onkopedia guideline for CLL
*2 Physical fitness was determined using the Comorbidity Illness Rating Scale (CIRS).
Patients with a score of or below 6 were labelled as “fit“ (or “go go“), patients with a score above 6 as “ unfit“ (or “ slow go“).
*3 In patients without del(17p) / TP53 mutations only after failure of chemotherapy and BCR inhibitor
FCR, fludarabine, cyclophosphamide plus Rituximab; BR, bendamustine plus rituximab; R, rituximab; allo-HSCTx, allogeneic hematopoietic stem cell transplantation; BO, bendamustine plus ofatumumab; HD, high dose.
The significant global progress in the treatment of CLL was made possible by the consistent implementation of new insights from basic research and by systematically planned clinical trials, including the GCLLSG studies. The advances achieved so far are reasons for hope that before long it could be possible to therapeutically control CLL.
Key Messages.
The treatment of chronic lymphocytic leukemia (CLL) has been improved significantly in recent years.
Depending on physical fitness, various regimes are used in the first-line treatment of CLL.
New substances have been approved which are progressively changing the management of CLL.
Due to the new, effective treatment options, allogeneic hematopoietic stem cell transplantation is now rarely performed.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest
Prof. Hallek received consulting fees from Abbvie, Janssen, Roche, and Gilead. He received reimbursement of travel expenses and lecture fees from Roche, Gilead and Abbvie as well as reimbursement of meeting participation fees from Roche. He received financial support from Gilead and Roche for a research project that he initiated.
Dr. von Tresckow received consulting fees and reimbursement of meeting participation fees, travel and accommodation expenses as well as lecture fees from Abbvie, Janssen and Roche. She received financial support from Janssen and Roche for a research project that she initiated.
PD Eichhorst received consulting fees from Janssen, Roche, Abbvie, Gilead, Novartis, and Celegne. She received reimbursement of meeting participation fees as well as travel and accommodation expenses from Roche, Janssen, Gilead, and Abbvie. She received fees for preparing continuing medical education events from Roche, Janssen, Gilead, Novartis, and Abbvie.
For the conduct of clinical contract studies, she received funds from Roche, Abbvie, Janssen, and Gilead.
Dr. Bahlo received reimbursement of meeting participation fees, travel and accommodation expenses as well as fees for preparing scientific meetings from Roche.
References
- 1.Barnes B, Kraywinkel K, Nowossadeck E, et al. Bericht zum Krebsgeschehen in Deutschland 2016. Robert Koch-Institut. 2016 [Google Scholar]
- 2.Rozman C, Montserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052–1057. doi: 10.1056/NEJM199510193331606. [DOI] [PubMed] [Google Scholar]
- 3.Slager SL, Benavente Y, Blair A, et al. Medical history, lifestyle, family history, and occupational risk factors for chronic lymphocytic leukemia/small lymphocytic lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr. 2014:41–51. doi: 10.1093/jncimonographs/lgu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerhan JR, Slager SL. Familial predisposition and genetic risk factors for lymphoma. Blood. 2015;126:2265–2273. doi: 10.1182/blood-2015-04-537498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landau DA, Tausch E, Taylor-Weiner AN, et al. Mutations driving CLL and their evolution in progression and relapse. Nature. 2015;526:525–530. doi: 10.1038/nature15395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger JA, Gribben JG. The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: insight into disease biology and new targeted therapies. Semin Cancer Biol. 2014;24:71–81. doi: 10.1016/j.semcancer.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Moreau EJ, Matutes E, A‘Hern RP, et al. Improvement of the chronic lymphocytic leukemia scoring system with the monoclonal antibody SN8 (CD79b) Am J Clin Pathol. 1997;108:378–382. doi: 10.1093/ajcp/108.4.378. [DOI] [PubMed] [Google Scholar]
- 8.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morice WG, Kurtin PJ, Hodnefield JM, et al. Predictive value of blood and bone marrow flow cytometry in B-cell lymphoma classification: comparative analysis of flow cytometry and tissue biopsy in 252 patients. Mayo Clin Proc. 2008;83:776–785. doi: 10.4065/83.7.776. [DOI] [PubMed] [Google Scholar]
- 10.Vardi A, Dagklis A, Scarfò L, et al. Immunogenetics shows that not all MBL are equal: the larger the clone, the more similar to CLL. Blood. 2013;121:4521–4528. doi: 10.1182/blood-2012-12-471698. [DOI] [PubMed] [Google Scholar]
- 11.Solomon BM, Chaffee KG, Moreira J, et al. Risk of non-hematologic cancer in individuals with high-count monoclonal B-cell lymphocytosis. Leukemia. 2016;30:331–336. doi: 10.1038/leu.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 13.Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–206. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Zenz T, Eichhorst B, Busch R, et al. TP53 Mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28:4473–4479. doi: 10.1200/JCO.2009.27.8762. [DOI] [PubMed] [Google Scholar]
- 15.International CLLIPIwg. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol. 2016;17:779–790. doi: 10.1016/S1470-2045(16)30029-8. [DOI] [PubMed] [Google Scholar]
- 16.Schweighofer C. Early versus deferred treatment with combined fludarabine, cyclophosphamide and rituximab (FCR) improves event-free survival in patients with high-risk binet stage a chronic lymphocytic leukemia - first results of a randomized German-French cooperative phase III trial. ASH conference. 2013 [Google Scholar]
- 17.Han T, Ezdinli EZ, Shimaoka K, Desai D. Chlorambucil vs combined chlorambucil-corticosteroid therapy in chronic lymphocytic leukemia. Cancer. 1973;31:502–508. doi: 10.1002/1097-0142(197303)31:3<502::aid-cncr2820310303>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114:3382–3391. doi: 10.1182/blood-2009-02-206185. [DOI] [PubMed] [Google Scholar]
- 19.Eichhorst BF, Busch R, Hopfinger G, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–891. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- 20.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 21.Keating MJ, O‘Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 22.Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127:208–215. doi: 10.1182/blood-2015-06-651125. [DOI] [PubMed] [Google Scholar]
- 23.Eichhorst B, Fink A-M, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–942. doi: 10.1016/S1470-2045(16)30051-1. [DOI] [PubMed] [Google Scholar]
- 24.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 25.Goede V, Fischer K, Dyer MJS, et al. Overall survival benefit of obinutuzumab over rituximab when combined with chlorambucil in patients with chronic lymphocytic leukemia and comorbidities: final survival analysis of the CLL11 study EHA 2018. EHA Learning Center. Goede. 2018 215923. [Google Scholar]
- 26.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stilgenbauer S, Schnaiter A, Paschka P, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood. 2014;123:3247–3254. doi: 10.1182/blood-2014-01-546150. [DOI] [PubMed] [Google Scholar]
- 28.Paul Barr, Tadeusz Robak, Carolyn J Owen, et al. Updated efficacy and safety from the phase 3 resonate-2 study: Ibrutinib as first-line treatment option in patients 65 years and older with chronic lymphocytic leukemia/small lymphocytic leukemia. Blood. 2016;128 [Google Scholar]
- 29.Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–2506. doi: 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrientos JC. Idelalisib for the treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma. Future Oncol. 2016;12:2077–2094. doi: 10.2217/fon-2016-0003. [DOI] [PubMed] [Google Scholar]
- 32.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17:768–778. doi: 10.1016/S1470-2045(16)30019-5. [DOI] [PubMed] [Google Scholar]
- 33.O‘Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17:1409–1418. doi: 10.1016/S1470-2045(16)30212-1. [DOI] [PubMed] [Google Scholar]
- 34.Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in chronic lymphocytic leukemia. N Engl J Med. 2018;378:2141–2144. [Google Scholar]
- 35.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:323–332. doi: 10.1056/NEJMoa1509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter HS, Jayne S, Rule SA, et al. Long-term follow-up of patients with CLL treated with the selective Bruton’s tyrosine kinase inhibitor ONO/GS-4059. Blood. 2017;129:2808–2810. doi: 10.1182/blood-2017-02-765115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharman J, Hawkins M, Kolibaba K, et al. An open-label phase 2 trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2015;125:2336–2343. doi: 10.1182/blood-2014-08-595934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer K, Al-Sawaf O, Fink AM, et al. Venetoclax and obinutuzumab in chronic lymphocytic leukemia. Blood. 2017;129:2702–2705. doi: 10.1182/blood-2017-01-761973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.AWMF. Interdisziplinäre S3-Leitlinie zur Diagnostik, Therapie und Nachsorge für Patienten mit einer chronischen lymphatischen Leukämie. www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/CLL/LL_Chronisch_Lymphatische_Leukaemie_ Langversion_Konsultationsfassung.pdf (last accessed on 26 November 2018) [Google Scholar]
- 40.Wendtner, CM, Dreger P, Gregor M. Chronische Lymphatische Leukämie Onkopedia. www.onkopedia.com/de/onkopedia/guidelines/chronische-lymphatische-leukaemie-cll/@@view/html/index.html (last accessed on 26 November. 2018) [Google Scholar]