Abstract
Background
Hypertrophic cardiomyopathy (HCM) is caused by mutations in a number of genes. Its prevalence is 0.2% to 0.6%.
Methods
This review is based on publications retrieved by a selective literature search and on the authors’ clinical experience.
Results
70% of patients with HCM suffer from the obstructive type of the condition, clinically characterized by highly dynamic and variable manifestations in the form of dyspnea, angina pectoris, and stress-dependent presyncope and syncope. Younger patients are at particular risk of sudden cardiac death; thus, all patients need not only symptomatic treatment, but also risk assessment, which can be difficult in individual cases. Left ventricular obstruction, which usually causes symptoms, is treated medically at first, with either a beta-blocker or verapamil. If medical treatment fails, two invasive treatments are available, surgical myectomy and percutaneous septum ablation. Both of these require a high level of expertise. If performed successfully, they lead to sustained gradient reduction and clinical improvement. Septum ablation is associated with low perioperative and peri-interventional mortality but necessitates permanent pacemaker implantation in 10–20% of patients.
Conclusion
In the absence of evidence from randomized comparison trials, a suitable method of reducing the gradient should be determined by an HCM team in conjunction with each individual patient. Important criteria for decision-making include the anatomical findings and any accompanying illnesses.
Hypertrophic cardiomyopathy (HCM) is a genetic disorder of the myocardium. It is characterized by marked myocardial hypertrophy (>15 mm) that cannot be explained by pressure load or the presence of myocyte disarray (1, 2, e1). The prevalence of HCM has been stated as 0.2%, but with modern imaging modalities and including healthy carriers of the genes concerned, prevalence of 0.6% can be assumed (3, 4).
HCM is caused by mutations in genes that, for the most part, code for sarcomere proteins and have autosomal dominant inheritance. HCM is thus a disease of the myofilaments, changes in the structure and function of which lead to the characteristic, variably pronounced pathological and pathophysiological phenomena. No connection has yet been described between the mutations identified to date and the phenotype.
The cardinal symptoms of HCM are dyspnea, angina pectoris, and stress-induced (pre)syncopes, which also vary greatly in degree (box). The patients often remain oligosymptomatic or even asymptomatic for many years (5, 6). However, persons with HCM are at increased risk of sudden cardiac death, particularly the younger among them. HCM is the most frequently documented cause of cardiac death in athletes (7, 8).
Box. Clinical and drug-related determinants of gradient increase.
-
Clinical conditions that may cause gradient increase
-
Acute volume depletion
Bleeding and diarrhea
-
Cardiac arrhythmias
Emergency VVI pacemaker in induced/spontaneous AV block grade III
After meals and alcohol consumption
-
-
Provocation
Valsalva maneuver
-
Physiological stress test (echocardiography)
Yields more information than Valsalva in <20% of patients
Postextrasystolic (Brockenbrough phenomenon)
-
Drugs with side effects that are associated with gradient increase
-
Reduction of preload and afterload
ACE inhibitors/AT1 blockers
Nitrates
Calcium antagonist of nifedipine type, occasionally verapamil type
-
Positively inotropic drugs
Catecholamines
Digitalis
-
ACE, angiotensin-converting enzyme; VVI, ventricular pacing on demand
The typical pathophysiological changes comprise diastolic dysfunction, variable intracavitary obstruction (hypertrophic obstructive cardiomyopathy, HOCM), and ischemia. The most important result of HOCM is obstruction of the left ventricular outflow tract (2, 9, e1– e3).
This obstruction is dynamic and largely influenced by changes in left ventricular loading and contractility (9, e2, e3). It leads to increased left ventricular systolic pressure, which in turn gives rise to a complex interplay of elevated wall stress, prolongation of ventricular relaxation, hindrance of left ventricular filling, increased filling pressure, secondary mitral insufficiency, myocardial ischemia, and reduction in cardiac output.
Around 70% of patients with HCM display relevant obstruction with a peak pressure difference of more than 30 mm Hg (10). In half of these patients the obstruction is present even at rest, while in the remainder it is latent, i.e., a gradient can be demonstrated only under stress. It should be noted that an obstruction can progress with time, so regular monitoring is indicated (5, 9).
The dynamic obstruction and its secondary pathophysiological consequences play a decisive role in development of the clinical symptoms. Their detection is of importance because septal reduction treatment can lead to considerable relief of the symptoms even in patients whose obstruction is latent (5, 11, e4). In this article we describe the current execution and results of myectomy and percutaneous septal ablation on the basis of a literature survey (PubMed) and our own experience. The PubMed search terms were “hypertrophic obstructive cardiomyopathy,” “myectomy,” and “septal ablation,” and the survey spanned the period 1995 to 2018.
The pathophysiological basis of obstruction
The original assumption was that the systolic obstruction arose purely from narrowing of the outflow tract caused by the hypertrophy of the basal septum (e2, e5). Later, echocardiographic studies showed that systolic anterior motion (SAM) of the mitral valve is a crucial component of the obstruction (e6). Rarer causes of gradient formation such as abnormal insertion of the papillary muscles or central ventricular thickening of the septum with subsequent obstruction must also be taken into consideration, because they determine the choice of the best treatment to reduce the gradient (12).
The systolic distortion of the mitral valve associated with SAM often leads to secondary mitral insufficiency (13, e7), the severity of which mostly depends on the degree of obstruction (e8).
In patients with mid-ventricular obstruction, apical aneurysms may form. These are often symptomatic and may give rise to ventricular arrhythmia or embolic complications (14).
Diagnosis of HOCM
Patients with HOCM/HCM should be informed about their disease and its genetic aspects. Genetic investigation demonstrates a mutation in less than 60% of the patients with a corresponding phenotype, so no direct prognostic or therapeutic conclusions can currently be drawn. The onset and severity of clinical HOCM/HCM in relatives in whom mutations are detected also cannot confidently be predicted, but those affected should receive genetic counseling and be fully informed of the potential consequences (15). Therefore, screening of close blood relatives should always be recommended on an individual basis.
Regular risk stratification with regard to sudden cardiac death—independent of symptom severity—is an integral component of the care given to HOCM/HCM patients (1, 15– 17). The follow-up examinations are currently carried out according to the guidelines of the European Society of Cardiology (ESC), taking the patient’s medical history and the findings of noninvasive diagnostic tests (table 1) into account to determine the 5-year risk of sudden cardiac death and the associated individual indication for primary prophylactic placement of an implantable cardioverter–defibrillator (ICD) (1).
Table 1. Parameters for estimation of the 5-year risk of sudden cardiac death according to the guidelines of the European Society of Cardiology (1).
| Parameter | Definition | Unit |
| Age | At time of evaluation | Years |
| Gradient in left ventricle | Maximal gradient at rest or during exercise as measured on transthoracic Doppler echocardiogram | mm Hg |
| Left ventricular diameter | As measured in M mode or on 2D echocardiogram | mm |
| Maximal wall thicness of left ventricle | As measured on 2D echocardiogram | mm |
| Sudden cardiac death (SCD) in the family | SCD at <40 years in the family or scd in an hcm family irrespective of age | Y/N |
| Nonsustained ventricular tachycardia (NSVT) | ≥ 3 consecutive ventricular beats at a rate of ≥ 120/min on 24- to 48-h ECG | Y/N |
| Unexplained syncope | At time of evaluation or previously | Y/N |
Y/N, Yes/no; HCM, hypertrophic cardiomyopathy
All HCM patients with stress-induced symptoms, particularly when the latter are variable, should undergo determination of the form and degree of obstruction at rest or under provocation (2, e4).
Together with intensive questioning regarding the history of the symptoms (dyspnea, angina pectoris, dizziness, stress-dependent [pre]syncopes) and physical examination with auscultation at rest and during exercise, echocardiography is essential.
Provocation of a systolic murmur of increasing intensity to the left of the sternum in the fourth intercostal space by the Valsalva maneuver points to the presence of a dynamic obstruction. The same is true for a systolic sound occurring during or directly after exercise. In the case of mid-ventricular obstruction, there is not uncommonly a diastolic sound as a result of the obstruction-related impedance of inflow (18).
In experienced hands, echocardiography (eFigure) is an important and reliable method for diagnosing HCM and deciding among the options for treatment. Echocardiography enables determination of the extent of the left ventricular hypertrophy and of the site, severity, and mechanism of any obstruction. For quantification of the obstruction, the peak gradient should be determined. Mid-ventricular obstruction can be identified by turbulent flow in the middle of the left ventricle. If neither SAM nor an obstruction is found, echocardiography should be repeated with provocation, most simply by means of a Valsalva maneuver. It would also be clinically relevant to perform the examination under physiological stress or after a meal. The postextrasystolic left ventricular outflow tract (LVOT) gradient is comparable to the results during exercise. Gradient measurements after drug-induced provocation, especially dobutamine infusion, should not be used to decide on the appropriate treatment, because the readings are often false positive under such circumstances.
eFigure:
Echocardiography in a patient with HOCM: apical four-chamber view in systole.
a) With SAM (arrow) and in parasternal longitudinal plane in diastole;
b) hypertrophic interventricular septum (IVS) with narrow left ventricular cavity (LV)
HOCM, hypertrophic obstructive cardiomyopathy; LA, left atrium; LVPW, left ventricular posterior wall; RA, right atrium; RV, right ventricle;
SAM, systolic anterior motion
Treatment of HOCM
It must be emphasized at the outset that no randomized controlled trials have yet been conducted to compare either drug treatment with gradient-reducing procedures or the results of myectomy with those of percutaneous septal ablation. The cornerstone of the management of patients with symptomatic HOCM is reduction of the obstruction by means of lifestyle modification and conservative treatment (1, 2, 15, 16, e1). The patients should be strongly advised to drink enough to ensure maintenance of adequate left ventricular filling. In their daily lives, they should avoid activities involving sudden changes of preload and afterload, e.g., saunas and long hot baths.
Medicinal treatment
Treatment with ß-blockers is effective for most patients; in this way contractility and heart rate can be reduced, particularly under stress. The dose of ß-blocker is generally set to achieve either amelioration of the symptoms or a resting pulse of 60/min. Beta-blocker treatment is also useful after successful gradient reduction with myectomy and percutaneous septal ablation. Alternatively, the calcium channel blocker verapamil can be given. It should be noted that even in early series with low numbers of patients, significant gradient increases were described after the initial administration of verapamil, going as far as the triggering of pulmonary edema. Owing to this potential complication, the first doses of verapamil in patients with HOCM must be administered under the supervision of a physician. Other medications, such as disopyramide, are not available in Germany. Preload- and afterload-lowering drugs (nitroglycerin, ACE inhibitors, AT1 blockers, calcium antagonists) should be avoided because they increase the gradient and thus aggravate the symptoms (5).
Nonmedicinal treatment
A large proportion of patients with dynamic obstruction remain symptomatic despite optimal drug treatment or complain of major adverse effects. In 90–95% of these patients, septal reduction treatment will lead to relief of the symptoms. This can take the form of either septal myectomy or percutaneous alcohol ablation of the septum (2, 19). The use of a two-chamber pacemaker with short conduction, with the aim of achieving dyssynchrony of the septal contraction, was tried three decades ago but is now of merely historical significance (e9).
Surgical septal myectomy
The first attempts to treat HCM by surgical septal myectomy took place over 50 years ago (20). Initially the myectomy was limited in scope, but nowadays the resection is much more aggressive in terms of both width and length (19, 21). Distally, the resection must take in the point of contact between the mitral valve and the septum, which is often rendered recognizable by augmented fibrosis; this widens the left ventricular outflow tract and results in reduction or elimination of the obstruction. In patients with simultaneous mitral insufficiency due to SAM, the insufficiency is almost always reduced or eliminated by myectomy alone. Occasionally there may be accompanying lesions of the aortic valve and/or fibrous (usually discreet) subaortic stenosis; these also require surgical correction, depending on their severity. Intraoperative echocardiography helps the surgeon to determine the individual extent of the disease and decide on the necessary scope of resection (e10). Direct pressure measurement can provide supplementary information. Other interventions are required only in the presence of additional structural disease.
Over the course of time, improvements in surgical technique have increased the effectiveness of treatment. The refinements include distal continuation of the resection to the level of the papillary muscles in order to avoid residual mid-ventricular obstruction if at all possible (figure 1). In the case of abnormality of the papillary muscles, partial detachment from the adjacent myocardium has been suggested (e11).
Figure 1.
Schematic representation of septal myectomy, with access via the mitral valve in almost all cases (a), in hypertrophy of the ventricular septum (IVS) with subaortic obstruction (blue arrow in b), leading to mitral insufficiency (green arrow) of variable degree. Sparing the cardiac conduction system, septal myocardium is resected, depending on the area of hypertrophy and extending if necessary as far as the level of the papillary muscles, to an extent (dotted lines in a and b) sufficient to eliminate the obstruction, retaining septal thickness of 1 cm. LA = Left atrium, LV = left ventricle
More difficult to treat are patients with an apical variant of HCM; they have obliteration of the left ventricular lumen with severe diastolic dysfunction (e12). As in patients with mid-ventricular obstruction and an apical aneurysm, hemodynamic and clinical improvement can be achieved by means of an apical approach.
The results of myectomy depend essentially on the experience and competence of the surgeon and his/her team (e13). Only in specialized centers can myectomy be carried out with a risk of perioperative complications under 1%. The hemodynamic results are usually excellent: in most cases the postoperative gradient is = 10 mm Hg (22). The likelihood of an undesired outcome (ventricular septal defect, atrioventricular block, residual obstruction) is lower for experienced surgeons. In expert hands the risk should be below 3% (23).
The long-term results of operative treatment are good. More than 90% of patients who undergo surgery are subsequently asymptomatic and can lead a normal life (24, e14). The results in persons with a latent, provocable obstruction are equally good. Renewed occurrence of obstruction and symptoms is rare. A recurrence is usually related to inadequate septal resection or takes the form of a new mid-ventricular obstruction. In both cases the HCM team should decide on an individual basis whether further gradient reduction by repeat myectomy or percutaneous septal ablation would be beneficial.
Although prevention of sudden cardiac death by septal myectomy has not been documented in randomized controlled trials, recent results suggest that survival can be prolonged in young patients with severe obstruction (25, e15). Lower frequency of appropriate shock was found in surgically treated patients with an ICD (26).
Percutaneous septal ablation
Following the introduction of percutaneous balloon dilatation by Andreas Grüntzig in the 1970s, percutaneous treatment procedures were developed for a large number of cardiac diseases. After numerous preliminary studies (e16, e17, 27), the first written documentation of successful percutaneous septal ablation by occlusion of a septal branch in HOCM came from the Berlin cardiologist G. Berghöfer in 1989. The inducement of infarction in the segments of septal myocardium forming the obstruction achieves a result resembling that of myectomy. This leads to widening of the left ventricular outflow tract, subsequent reduction of the gradients and the SAM-related mitral insufficiency, and amelioration of the diastolic dysfunction.
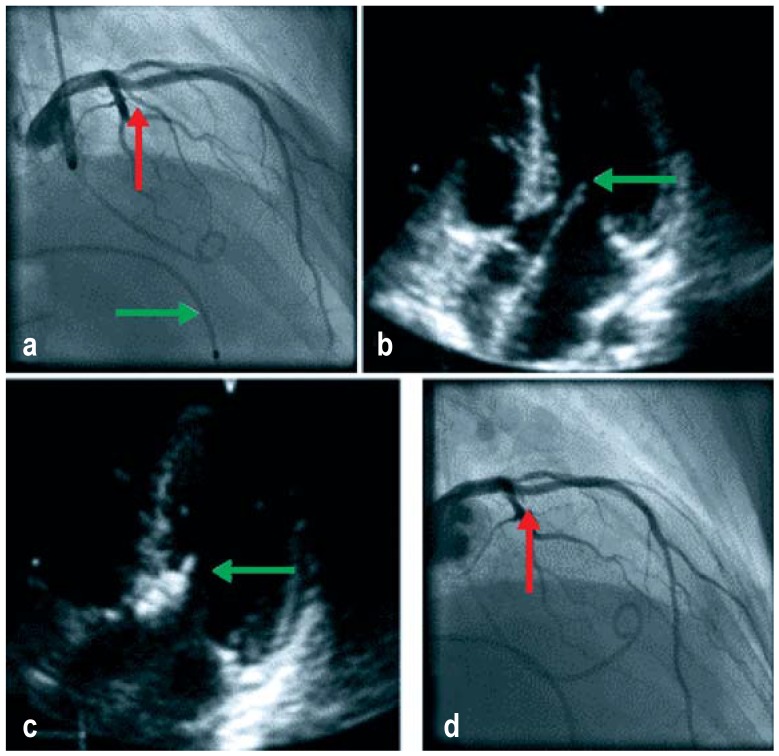
From the performance of the first alcohol ablation by Sigwart in 1994 (28) right up to the recent past, concerns were expressed regarding the danger of an increased incidence of sudden cardiac death (29, e18– e20). Nevertheless, due mainly to the work of German groups this procedure has become an established, widely practiced form of treatment (28, 30– 32). A crucial part was played by the introduction of intraprocedural contrast echocardiographic guidance (figure 2) in 1996 (33).
Figure 2.
Steps in echocardiographically guided septal ablation (from [6])
a) Initial angiogram of the left coronary artery showing the targeted septal branch (red arrow) and the temporary pacemaker probe (green arrow)
b) Modified initial four-chamber view echocardiogram with systolic contact between the mitral valve and the septum (green arrow)
c) The echocardiographic contrast medium depot in the subaortic segment of the ventricular septum at the level of the contact point between the mitral valve and the septum (green arrow)
d) Occluded septal branch (red arrow) following balloon removal 10 min after the final injection of alcohol without damage to the anterior interventricular branch
In experienced centers, gradient reduction to <50% of the initial value and simultaneous amelioration of the symptoms are achieved in >90 to 95% of the patients treated (19). The in-hospital mortality should be <1%, and in our own patients it is <0.2% (34, e21). Since most deaths occur following the intervention, it has proved beneficial to keep the patients in the critical care unit for at least 48 h and in the hospital for a week. For both acute and long-term results the success and complication rates have been shown to be associated with the treatment volumes (35). The quantitatively most significant complication is the need for implantation of a permanent pacemaker in the presence of persisting postinterventional AV block. The incidence of this complication is given as 10 to 20%.
In contrast to myectomy, the hemodynamic success of septal ablation often kicks in only after 3 to 12 months. This is because of the remodeling after induced septal infarction, with delayed scar formation in the ablated area. Particularly in young patients, one should wait for this remodeling before deciding to repeat ablation. It is generally necessary to ablate more than one septal branch in up to 20% of patients treated, in some cases due to anatomic variants in the arterial supply to the segments of the septum forming the obstruction.
Numerous observational studies over periods of up to 17 years show continuing relief of symptoms and no increase in mortality, particularly sudden cardiac death, or morbidity (34, 36, 37, e22). In our own long-term observation of 952 patients, overall survival was 79.7% and survival without cardiac events was 96.5% at 15 years after percutaneous septal ablation (38). While clearly no conclusions can be drawn in the absence of randomized controlled trials, the demonstrated reduction of modifiable risk factors for the occurrence of sudden cardiac death certainly suggests that the prognosis is improved.
Nonrandomized studies have shown comparable survival of HOCM patients after septal ablation and an age-adjusted group of patients with nonobstructive HCM, with 10-year survival rates of 90% in patients under 55 and 82% over 55 years of age (39). Comparable survival rates and hemodynamic results after alcohol septal ablation and myectomy have also been documented in nonrandomized observational studies (40, e23).
Conclusion
Two effective procedures, myectomy and percutaneous septal ablation, are available to the experienced operator for the management of HOCM that has not responded to conservative treatment. Until randomized trials of the two methods produce conclusive results, the best treatment for each individual HOCM patient should be determined by an HCM team with expertise in both procedures and the disease as such.
The criteria for the decision between the two treatment modalities are the anatomical situation and the presence or otherwise of cardiac and noncardiac comorbidities (table 2). The two procedures should be viewed as complementary rather than competing treatments. The success rate and complication rate depend mainly on the experience of the surgeon or interventional cardiologist involved. Finally, the patient’s own wishes, after detailed discussion of the options, are crucial in deciding how to proceed.
Table 2. Factors relevant to the HCM team’s decision on gradient-reducing treatment in patients for whom medication is inadequate.
| Criteria | Myectomy | Percutaneous alcohol septal ablation |
| Site of obstruction | Subvalvular Mid-ventricular Apical |
Subvalvular Mid-ventricular |
| Extent of hypertrophy | Massive hypertrophy >30 mm | Hypertrophy ≤ 30 mm |
| Mitral valve insufficiency | Non-SAM-associated/SAM-associated | SAM-associated |
| Further cardiac disease necessitating surgery | Subvalvular membrane Aortic valve stenosis Multiple-vessel coronary disease Rare diseases inaccessible to interventional procedures |
Single-vessel coronary disease |
| Patients’ age | Adolescents and adults | Adults |
| Hemodynamic success | Immediate | Delayed (up to 3–12 months) |
| Complexity of procedure | Operation with HLM | Less invasive |
| Postprocedural risk for pacemaker dependency | 2–10% Up to 50% with pre-existing right bundle branch block |
10–20% Up to 50% with pre-existing left bundle branch block |
| Experience of method | Over 50 years | Over 20 years |
| Structural availability | Low, small number of centers with experience | High, but few centers with extensive experience |
HCM, hypertrophic cardiomyopathy; HLM, heart–lung machine; SAM, systolic anterior motion
Key Messages.
The essential pathophysiological criterion of hypertrophic obstructive cardiomyopathy is dynamic left ventricular obstruction.
Myectomy has been an established method for treating left ventricular obstruction in drug-refractory patients for over 50 years.
Percutaneous alcohol septal ablation (PTSMA) has been achieving comparable results for over 20 years.
PTSMA and myectomy have not yet been compared in randomized controlled trials.
Ventricular septal defect, atrioventricular block, and residual obstruction are the most frequently occurring complications of myectomy. Postinterventional AV block with subsequent implantation of a pacemaker takes place in 10 to 20% of patients after PTSMA.
Acknowledgments
Translated from the original German by David Roseveare
Footnotes
Conflict of interest statement The authors declare that no conflict of interests exists.
References
- 1.Elliott PM, Anastasakis A, et al. Authors/Task Force members. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2733–2779. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura RA, Holmes DR. Clinical practice Hypertrophic obstructive cardiomyopathy. N Engl J Med. 2004;350:1320–1327. doi: 10.1056/NEJMcp030779. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults Echocardiographic analysis of 4111 subjects in the CARDIA study. Coronary artery risk development in (young) adults. Circulation. 1995;92:785–789. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- 4.Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65:1249–1254. doi: 10.1016/j.jacc.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Koljaja-Batzner A, Pfeiffer B, Seggewiss H. Die hypertrophe Kardiomyopathie - häufig und nicht erkannt. Internistische Praxis. 2018;59:187–201. [Google Scholar]
- 6.Seggewiss H, Koljaja-Batzner A, Seggewiss K, Meesmann M. [Syncope in hypertrophic (obstructive) cardiomyopathy] Herzschrittmacherther Elektrophysiol. 2018;29:141–143. doi: 10.1007/s00399-018-0567-x. [DOI] [PubMed] [Google Scholar]
- 7.Maron BJ. Sudden death in hypertrophic cardiomyopathy. J Cardiovasc Transl Res. 2009;2:368–380. doi: 10.1007/s12265-009-9147-0. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ, Epstein SE, Roberts WC. Hypertrophic cardiomyopathy: a common cause of sudden death in the young competitive athlete. Eur Heart J. 1983;4(F):135–144. doi: 10.1093/eurheartj/4.suppl_f.135. [DOI] [PubMed] [Google Scholar]
- 9.Maron MS, Olivotto I, Zenovich AG, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239. doi: 10.1161/CIRCULATIONAHA.106.644682. [DOI] [PubMed] [Google Scholar]
- 10.Prinz C, Farr M, Hering D, Horstkotte D, Faber L. The diagnosis and treatment of hypertrophic cardiomyopathy. Dtsch Arztebl Int. 2011;108:209–215. doi: 10.3238/arztebl.2011.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaff HV, Dearani JA, Ommen SR, Sorajja P, Nishimura RA. Expanding the indications for septal myectomy in patients with hypertrophic cardiomyopathy: results of operation in patients with latent obstruction. J Thorac Cardiovasc Surg. 2012;143:303–309. doi: 10.1016/j.jtcvs.2011.10.059. [DOI] [PubMed] [Google Scholar]
- 12.Sherrid MV. Pathophysiology and treatment of hypertrophic cardiomyopathy. Prog Cardiovasc Dis. 2006;49:123–151. doi: 10.1016/j.pcad.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28:1–83. doi: 10.1016/0033-0620(85)90024-6. [DOI] [PubMed] [Google Scholar]
- 14.Goel K, Schaff HV, Nishimura RA. Natural history of apical hypertrophic cardiomyopathy and novel surgical treatment. J Thorac Cardiovasc Surg. 2016;152:626–627. doi: 10.1016/j.jtcvs.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121:749–770. doi: 10.1161/CIRCRESAHA.117.311059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.et al. American College of Cardiology Foundation/American Heart Association. Task Force on Parctice, American Association for Thoracic Surgery, American Society of Echocardiography, 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2011;142:e153–e203. doi: 10.1016/j.jtcvs.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 17.O‘Mahony C, Jichi F, Pavlou M, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD) Eur Heart J. 2014;35:2010–2020. doi: 10.1093/eurheartj/eht439. [DOI] [PubMed] [Google Scholar]
- 18.Seggewiss H, Faber L. Percutaneous septal ablation for hypertrophic cardiomyopathy and mid-ventricular obstruction. Eur J Echocardiogr. 2000;1:277–280. doi: 10.1053/euje.2000.0032. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic obstructive cardiomyopathy: surgical myectomy and septal ablation. Circ Res. 2017;121:771–783. doi: 10.1161/CIRCRESAHA.116.309348. [DOI] [PubMed] [Google Scholar]
- 20.Morrow AG, Brockenbrough EC. Surgical treatment of idiopathic hypertrophic subaortic stenosis: technic and hemodynamic results of subaortic ventriculomyotomy. Ann Surg. 1961;154:181–189. doi: 10.1097/00000658-196108000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messmer BJ. Extended myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg. 1994;58:575–577. doi: 10.1016/0003-4975(94)92268-3. [DOI] [PubMed] [Google Scholar]
- 22.Maron BJ, Dearani JA, Ommen SR, et al. Low operative mortality achieved with surgical septal myectomy: role of dedicated hypertrophic cardiomyopathy centers in the management of dynamic subaortic obstruction. J Am Coll Cardiol. 2015;66:1307–1308. doi: 10.1016/j.jacc.2015.06.1333. [DOI] [PubMed] [Google Scholar]
- 23.Kim LK, Swaminathan RV, Looser P, et al. Hospital volume outcomes after septal myectomy and alcohol septal ablation for treatment of obstructive hypertrophic cardiomyopathy: US nationwide inpatient database, 2003-2011. JAMA Cardiol. 2016;1:324–332. doi: 10.1001/jamacardio.2016.0252. [DOI] [PubMed] [Google Scholar]
- 24.Mohr R, Schaff HV, Puga FJ, Danielson GK. Results of operation for hypertrophic obstructive cardiomyopathy in children and adults less than 40 years of age. Circulation. 1989;80:I191–I196. [PubMed] [Google Scholar]
- 25.Ball W, Ivanov J, Rakowski H, et al. Long-term survival in patients with resting obstructive hypertrophic cardiomyopathy comparison of conservative versus invasive treatment. J Am Coll Cardiol. 2011;58:2313–2321. doi: 10.1016/j.jacc.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 26.McLeod CJ, Ommen SR, Ackerman MJ, et al. Surgical septal myectomy decreases the risk for appropriate implantable cardioverter defibrillator discharge in obstructive hypertrophic cardiomyopathy. Eur Heart J. 2007;28:2583–2588. doi: 10.1093/eurheartj/ehm117. [DOI] [PubMed] [Google Scholar]
- 27.Brugada P, de Swart H, Smeets JL, Wellens HJ. Transcoronary chemical ablation of ventricular tachycardia. Circulation. 1989;79:475–482. doi: 10.1161/01.cir.79.3.475. [DOI] [PubMed] [Google Scholar]
- 28.Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet. 1995;346:211–214. doi: 10.1016/s0140-6736(95)91267-3. [DOI] [PubMed] [Google Scholar]
- 29.Geske JB, Gersh BJ. Myectomy versus alcohol septal ablation: experience remains key. JACC Cardiovasc Interv. 2014;7:1235–1236. doi: 10.1016/j.jcin.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Seggewiss H, Gleichmann U, Faber L, Fassbender D, Schmidt HK, Strick S. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: acute results and 3-month follow-up in 25 patients. J Am Coll Cardiol. 1998;31:252–258. doi: 10.1016/s0735-1097(97)00508-1. [DOI] [PubMed] [Google Scholar]
- 31.Gietzen FH, Leuner CJ, Raute-Kreinsen U, et al. Acute and long-term results after transcoronary ablation of septal hypertrophy (TASH) Catheter interventional treatment for hypertrophic obstructive cardiomyopathy. Eur Heart J. 1999;20:1342–1354. doi: 10.1053/euhj.1999.1520. [DOI] [PubMed] [Google Scholar]
- 32.Gleichmann U, Seggewiss H, Faber L, Fassbender D, Schmidt HK, Strick S. [Catheter treatment of hypertrophic obstructive cardiomyopathy] Dtsch Med Wochenschr. 1996;121:679–685. doi: 10.1055/s-2008-1043055. [DOI] [PubMed] [Google Scholar]
- 33.Faber L, Seggewiss H, Gleichmann U. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: results with respect to intraprocedural myocardial contrast echocardiography. Circulation. 1998;98:2415–2421. doi: 10.1161/01.cir.98.22.2415. [DOI] [PubMed] [Google Scholar]
- 34.Veselka J, Jensen MK, Liebregts M, et al. Long-term clinical outcome after alcohol septal ablation for obstructive hypertrophic cardiomyopathy: results from the Euro-ASA registry. Eur Heart J. 2016;37:1517–1523. doi: 10.1093/eurheartj/ehv693. [DOI] [PubMed] [Google Scholar]
- 35.Sorajja P, Binder J, Nishimura RA, et al. Predictors of an optimal clinical outcome with alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Catheter Cardiovasc Interv. 2013;81:E58–E67. doi: 10.1002/ccd.24328. [DOI] [PubMed] [Google Scholar]
- 36.Nagueh SF, Groves BM, Schwartz L, et al. Alcohol septal ablation for the treatment of hypertrophic obstructive cardiomyopathy A multicenter North American registry. J Am Coll Cardiol. 2011;58:2322–2328. doi: 10.1016/j.jacc.2011.06.073. [DOI] [PubMed] [Google Scholar]
- 37.Jensen MK, Prinz C, Horstkotte D, et al. Alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy: low incidence of sudden cardiac death and reduced risk profile. Heart. 2013;99:1012–1017. doi: 10.1136/heartjnl-2012-303339. [DOI] [PubMed] [Google Scholar]
- 38.Batzner A, Pfeiffer B, Neugebauer A, Aicha D, Blank C, Seggewiss H. Long-term survival after alcohol septal ablation in hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2018;72:3087–3094. doi: 10.1016/j.jacc.2018.09.064. [DOI] [PubMed] [Google Scholar]
- 39.Liebregts M, Steggerda RC, Vriesendorp PA, et al. Long-term outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy in the young and the elderly. JACC Cardiovasc Interv. 2016;9:463–469. doi: 10.1016/j.jcin.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 40.Singh K, Qutub M, Carson K, Hibbert B, Glover C. A meta analysis of current status of alcohol septal ablation and surgical myectomy for obstructive hypertrophic cardiomyopathy. Catheter Cardiovasc Interv. 2016;88:107–115. doi: 10.1002/ccd.26293. [DOI] [PubMed] [Google Scholar]
- E1.Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242–255. doi: 10.1016/S0140-6736(12)60397-3. [DOI] [PubMed] [Google Scholar]
- E2.Braunwald E, Lambrew CT, Rockoff SD, Ross J Jr., Morrow AG. Idiopathic hypertrophic subaortic stenosis. I. A description of the disease based upon an analysis of 64 patients. Circulation. 1964;30(4):3–119. doi: 10.1161/01.cir.29.5s4.iv-3. [DOI] [PubMed] [Google Scholar]
- E3.Wigle ED, David PR, Labroose CJ, McMeekan J. Muscular subaortic stenosis; the interrelation of wall tension, outflow tract “distending pressure” and orifice radius. Am J Cardiol. 1965;15:761–772. doi: 10.1016/0002-9149(65)90378-4. [DOI] [PubMed] [Google Scholar]
- E4.Nishimura RA, Ommen SR. Hypertrophic cardiomyopathy: the search for obstruction. Circulation. 2006;114:2200–2202. doi: 10.1161/CIRCULATIONAHA.106.660928. [DOI] [PubMed] [Google Scholar]
- E5.Brock R. Functional obstruction of the left ventricle; acquired aortic subvalvar stenosis. Guys Hosp Rep. 1957;106:221–238. [PubMed] [Google Scholar]
- E6.Shah PM, Gramiak R, Kramer DH. Ultrasound localization of left ventricular outflow obstruction in hypertrophic obstructive cardiomyopathy. Circulation. 1969;40:3–11. doi: 10.1161/01.cir.40.1.3. [DOI] [PubMed] [Google Scholar]
- E7.Nishimura RA, Tajik AJ, Reeder GS, Seward JB. Evaluation of hypertrophic cardiomyopathy by Doppler color flow imaging: initial observations. Mayo Clin Proc. 1986;61:631–639. doi: 10.1016/s0025-6196(12)62027-8. [DOI] [PubMed] [Google Scholar]
- E8.Wigle ED. Hypertrophic cardiomyopathy: a 1987 viewpoint. Circulation. 1987;75:311–322. doi: 10.1161/01.cir.75.2.311. [DOI] [PubMed] [Google Scholar]
- E9.Nishimura RA, Trusty JM, Hayes DL, et al. Dual-chamber pacing for hypertrophic cardiomyopathy: a randomized, double-blind, crossover trial. J Am Coll Cardiol. 1997;29:435–441. doi: 10.1016/s0735-1097(96)00473-1. [DOI] [PubMed] [Google Scholar]
- E10.Ashikhmina EA, Schaff HV, Ommen SR, Dearani JA, Nishimura RA, Abel MD. Intraoperative direct measurement of left ventricular outflow tract gradients to guide surgical myectomy for hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg. 2011;142:53–59. doi: 10.1016/j.jtcvs.2010.08.011. [DOI] [PubMed] [Google Scholar]
- E11.Rowin EJ, Maron BJ, Lesser JR, Rastegar H, Maron MS. Papillary muscle insertion directly into the anterior mitral leaflet in hypertrophic cardiomyopathy, its identification and cause of outflow obstruction by cardiac magnetic resonance imaging, and its surgical management. Am J Cardiol. 2013;111:1677–1679. doi: 10.1016/j.amjcard.2013.01.340. [DOI] [PubMed] [Google Scholar]
- E12.Klarich KW, Attenhofer Jost CH, Binder J, et al. Risk of death in long-term follow-up of patients with apical hypertrophic cardiomyopathy. Am J Cardiol. 2013;111:1784–1791. doi: 10.1016/j.amjcard.2013.02.040. [DOI] [PubMed] [Google Scholar]
- E13.Morrow AG. Hypertrophic subaortic stenosis Operative methods utilized to relieve left ventricular outflow obstruction. J Thorac Cardiovasc Surg. 1978;76:423–430. [PubMed] [Google Scholar]
- E14.Maron BJ, Nishimura RA. Surgical septal myectomy versus alcohol septal ablation: assessing the status of the controversy in 2014. Circulation. 2014;130:1617–1624. doi: 10.1161/CIRCULATIONAHA.114.011580. [DOI] [PubMed] [Google Scholar]
- E15.Desai MY, Smedira NG, Bhonsale A, Thamilarasan M, Lytle BW, Lever HM. Symptom assessment and exercise impairment in surgical decision making in hypertrophic obstructive cardiomyopathy: relationship to outcomes. J Thorac Cardiovasc Surg. 2015;150:928–935 e1. doi: 10.1016/j.jtcvs.2015.07.063. [DOI] [PubMed] [Google Scholar]
- E16.Sigwart U, Grbic M, Essinger A, Bischof-Delaloye A, Sadeghi H, Rivier JL. Improvement of left ventricular function after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1982;49:651–657. doi: 10.1016/0002-9149(82)91942-7. [DOI] [PubMed] [Google Scholar]
- E17.Come PC, Riley MF. Hypertrophic cardiomyopathy. Disappearance of auscultatory, carotid pulse, and echocardiographic manifestations of obstruction following myocardial infarction. Chest. 1982;82:451–454. doi: 10.1378/chest.82.4.451. [DOI] [PubMed] [Google Scholar]
- E18.Goodwin JF, Oakley CM. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet. 1995;346 doi: 10.1016/s0140-6736(95)91954-6. [DOI] [PubMed] [Google Scholar]
- E19.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- E20.Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64:83–99. doi: 10.1016/j.jacc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- E21.Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J Interv Cardiol. 2006;19:319–327. doi: 10.1111/j.1540-8183.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- E22.Seggewiss H, Rigopoulos A, Welge D, Ziemssen P, Faber L. Long-term follow-up after percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Clin Res Cardiol. 2007;96:856–863. doi: 10.1007/s00392-007-0579-8. [DOI] [PubMed] [Google Scholar]
- E23.Steggerda RC, Damman K, Balt JC, Liebregts M, ten Berg JM, van den Berg MP. Periprocedural complications and long-term outcome after alcohol septal ablation versus surgical myectomy in hypertrophic obstructive cardiomyopathy: a single-center experience. JACC Cardiovasc Interv. 2014;7:1227–1234. doi: 10.1016/j.jcin.2014.05.023. [DOI] [PubMed] [Google Scholar]