Abstract
Background
Nerve compression syndromes in the posterior cranial fossa can severely impair patients’ quality of life. There is often uncertainty about the best treatment. In this article, we provide an overview of these conditions and the corresponding treatment strategies.
Methods
This review is based on pertinent publications retrieved by a selective search in PubMed and on a scientific analysis of the authors’ patient collective.
Results
These syndromes are caused by compression of a cranial nerve by an artery or vein at the zone of the nerve’s entry to or exit from the brainstem. The best-known neurovascular compression syndrome is trigeminal neuralgia, followed by hemifacial spasm. Less well known are glossopharyngeal neuralgia, nervus intermedius neuralgia, and vestibular paroxysmia. The initial treatment of trigeminal neuralgia is medical: the first line of treatment is with sodium-blocking anticonvulsants, such as carbamazepine. For patients with hemifacial spasm, botulinum toxin injection is the recommended initial treatment and often leads to a satisfactory regression of the spasms. If these treatments fail, a microvascular decompression operation is indicated. The aim of the procedure is to separate the irritating vessel from the nerve and to keep these structures apart permanently. There is hardly any available evidence on these treatment strategies from randomized controlled trials.
Conclusion
Nerve compression syndromes in the posterior cranial fossa can generally be treated nonsurgically at first. Over the course of the condition, however, treatment failure or intolerable side effects may arise. In such cases, a microvascular decompression operation is indicated. This is a causally directed form of treatment that generally yields very good results.
Neurovascular compression syndromes are clinically characterized by functional disturbances of individual cranial nerves. The most common compression syndrome affects the trigeminal nerve and leads to trigeminal neuralgia, followed by hemifacial spasm, which is caused by vascular compression of the facial nerve. Less well-known nerve compression syndromes affect the glossopharyngeal nerve, the nervus intermedius and the vestibulocochlear nerve. Very rarely, the oculomotor nerve or the abducens nerve is involved.
A single pathophysiological mechanism underlies all of these compression syndromes. In the area of the root entry zone or root exit zone (REZ) of the relevant cranial nerve at the brainstem, the nerve comes into contact with a blood vessel—usually an artery, less commonly a vein. This is a natural weak point of the nerve, the site of transition between central and peripheral myelin. The nerve is especially sensitive to mechanical irritation here, which provokes the clinical symptoms of nerve compression.
In this review, we discuss the individual neurovascular compression syndromes of the posterior fossa and various corresponding modes of treatment.
Methods
A literature search was carried out in PubMed with the following search terms: “neurovascular compression syndrome,” “cranial neuralgia,” “trigeminal neuralgia,” “hemifacial spasm,” “glossopharyngeal neuralgia,” “vestibular nerve compression,” “vestibular paroxysmia,” “intermedius neuralgia” and “microvascular decompression.” For this review, we mainly considered large-scale studies carried out in the past 20 years. There is hardly any evidence available from randomized controlled trials on treatment strategies for these compression syndromes. In particular, the evidence for the utility of interventional and invasive treatments is based mainly on longitudinal follow-up studies evaluating the long-term results in patient cohorts of variable sizes.
Diagnostic evaluation
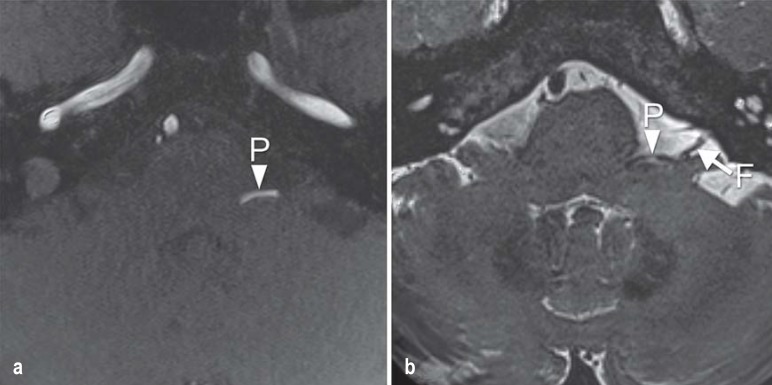
Whenever a nerve compression syndrome is suspected, magnetic resonance imaging (MRI) of the brain should be performed, with particular attention to the posterior fossa. High-resolution 3D-T2-weighted sequences, such as the CISS (constructive interference in steady-state) sequence, should be obtained, as well as 3D-TOF (time-of-flight) MR angiography, in order to distinguish reliably between arterial and venous compression (figure 1) (1, 2, e1). MRI also serves to rule out other processes that might cause the same clinical manifestations, such as a tumor or aneurysm.
Figure 1.
Magnetic resonance imaging in hemifacial spasm.
a) The TOF (time of flight) and b) CISS (constructive interference in steady-state) sequences in the axial plane clearly reveal vascular compression of the facial nerve (arrow, F) by the posterior inferior cerebellar artery (arrowhead, P)
Trigeminal neuralgia
Trigeminal neuralgia is the most common neurovascular compression syndrome in the posterior fossa, with an incidence of 4–5 cases per 100 000 persons per year (among persons over age 60: up to 20 per 100 000 persons per year) (3, 4). It can thus be estimated that some 4200 people newly develop symptoms of trigeminal neuralgia in Germany each year. The pain is generally strictly unilateral, lasting a few seconds to minutes. It is generally described as attacks of stabbing (lancinating) pain and is usually located in the distribution of the second or third division of the trigeminal nerve (the maxillary or mandibular nerve). The attacks arise spontaneously and can also be evoked by external stimuli, including touching the face or chewing. Spontaneous remission for months or years can occur. Over the course of time, the attacks may become more frequent and the pain longer-lasting (5). A distinction is drawn between classic (idiopathic) trigeminal neuralgia and the much less common symptomatic form of the condition. Classic trigeminal neuralgia is usually due to a neurovascular conflict in which the trigeminal nerve is compressed at its REZ by a blood vessel, often the superior cerebellar artery (e2). Symptomatic trigeminal neuralgia is defined as trigeminal neuralgia due to another disease; it is found, for example, among patients with multiple sclerosis, who often have typical demyelinating foci in the brainstem (e3, e4). It can also be caused by a tumor.
The primary treatment of both types of trigeminal neuralgia is with drugs, primarily sodium-channel blockers that are also used as antiepileptic drugs. The scientific evidence supports the use of carbamazepine as the drug of first choice (6, 7). In symptomatic trigeminal neuralgia, the underlying disease should be treated if possible (e.g., resection of a tumor). If pharmacotherapy brings inadequate relief or causes unacceptable side effects (e.g., fatigue, dizziness, cognitive disturbances), then a neurosurgical procedure can be recommended, such as microvascular decompression (MVD), percutaneous procedures (thermocoagulation, glycerol rhizolysis, balloon compression of the gasserian ganglion), or a radiosurgical procedure (e5, e6). In our opinion, percutaneous techniques should only be considered if there is no demonstrable neurovascular conflict, because, when such a conflict is present, MVD provides better and longer-lasting success and is associated with fewer complications (8– 10).
MVD (also called “the Jannetta procedure,” after the American neurosurgeon Peter Jannetta [1932–2016]) is considered a causally directed treatment for classic trigeminal neuralgia and has the highest long-term success rate (11). In large-scale case series, MVD yielded relief or improvement of pain in 68% to more than 90% of cases (12– 15, e7– e9). Moreover, meta-analyses have shown MVD to be clearly superior to radiosurgical treatment with respect to early as well as long-term pain relief (16, 17). Well over 80% of patients are still free of pain five years after MVD, and the success rate at 10 years has been reported at over 70% (14, 15). Our experience to date has shown comparably good to very good results (table 1) (e10). There is no age limit to MVD surgery; good results are achievable even in elderly patients (>75 years old) (e11). Complications of the operation are rare, including ipsilateral hearing loss or deafness and sensory deficits. Multiple studies with long-term follow-up have revealed permanent impairment of hearing in 0.1–3% of patients (18). The risk of cerebellar infarction is reported as less than 1% in most case series (12, 15, 19). The mortality, across multiple studies, is approximately 0.3% (12, 15, 18).
Table 1. Results of microvascular decompression for trigeminal neuralgia*.
| Trigeminal neuralgia | Overall: 175 patients | |
| Sex distribution | Men: 72 | Women: 103 |
| Mean age (range) | 62.3 years (20–89 years) | |
| Mean duration of pain (range) | 7.8 years (1–33 years) | |
| Outcome | Immediately after surgery (175 patients) | Late follow-up at >1 year (113 pts.); mean, 53 months |
| No pain | 155 (88.6%) | 91 (80.5%) |
| 50% relief of pain | 4 (2%) | 12 (10%) |
| Permanent morbidity | ||
| Hearing loss | 3 (1.7%) | |
| Hypesthesia | 5 (2.9%) | |
*Results from the authors’ own series of microvascular decompression surgery in 175 patients with trigeminal neuralgia. Pain relief immediately after surgery and on late follow-up is shown, as is the frequency of permanent morbidity (hearing loss and sensory loss)
Finally, partial sensory rhizotomy (PSR) should be mentioned as well. This is, in our opinion, a treatment of last resort for intractable trigeminal neuralgia. Patients who have already undergone multiple types of treatment without lasting relief of pain may benefit from this surgical procedure. We have had favorable results from it in patients with multiple sclerosis (among others). In PSR, up to two-thirds of the sensory fibers of the trigeminal nerve are cut (13, e12). The first division of the trigeminal nerve must not be cut, because of the danger of neuroparalytic keratitis; thus, PSR should only be used to treat trigeminal neuralgia of the second and/or third divisions. The patient must be informed preoperatively that PSR will cause numbness in the corresponding area of the face. Our patients have not found this distressing. There is a risk that PSR may be followed by burning dysesthesia in the numb cutaneous area (anesthesia dolorosa) or by difficulty eating or chewing (20), but our patients have not experienced these negative sequelae to date. Anesthesia dolorosa, a rare complication of neuroablative techniques, is difficult to treat (6).
In summary, it must be stated that the current evidence base is inadequate for a satisfactory direct comparison of the invasive and operative treatments for trigeminal neuralgia (21). An extensive Cochrane analysis published in 2011 additionally documents this fact (22).
Hemifacial spasm
The epidemiology of hemifacial spasm has not been extensively studied to date. In a study from Olmsted County, Minnesota, the mean annual incidence was found to be 0.81 and 0.74 cases per 100 000 persons per year among women and men, respectively, with a mean prevalence of 11 per 100 000 persons (23). It can thus be calculated that some 9000 persons in Germany now suffer from this condition. Hemifacial spasm is characterized by involuntary, strictly unilateral tonic and/or clonic contractions of the facial musculature, typically including the platysma (e13). The spasms cannot be voluntarily suppressed, and they persist during sleep. Examination reveals the Babinski-2 sign: during the contractions, the eyebrow rises while the eye is closed (figure 2). This pattern of movement cannot be reproduced in persons without the condition (e14). The diagnosis of hemifacial spasm can generally be made immediately on inspection, but it can also be confirmed electrophysiologically by the demonstration of pathological lateral spreading of activation (24). A source of additional distress to persons with this condition is that the facial spasms are often wrongly thought to be psychogenic (e15). The differential diagnosis of hemifacial spasm includes blepharospasm, facial dystonia, tardive dyskinesia, motor tics, and synkinesia after facial nerve injury, among other conditions (25, 26, e16, e17).
Figure 2.
Babinski-2 sign in hemifacial spasm. During the spasms, the eyebrow rises while the eye simultaneously closes
The current state of the evidence on the treatment of hemifacial spasm is poor. For drug therapy, anticonvulsants are usually recommended, but they often fail to provide adequate benefit. Botulinum neurotoxin (BTX) injections have become the standard symptomatic treatment. The efficacy of BTX is based on blockade of calcium-dependent acetylcholine release at the neuromuscular junction, leading to reversible paralysis of the affected muscles (e18). BTX is injected into the affected muscles directly; the clinically visible effect is first seen 3–6 days after injection and lasts approximately three months. Transient side effects arise in approximately 20% of patients, including ptosis, facial palsy, (rarely) diplopia, and headache (e19).
The only causally directed form of treatment is MVD. In patients with marked symptoms, we recommend early surgery, because some of our patients who already displayed structural damage of the facial nerve did not experience satisfactory regression of the spasms. Large-scale studies have yielded very good success rates, with postoperative freedom from spasms in 85–90% of cases (27). These figures are also consistent with our experience (table 2). The main risk of MVD for hemifacial spasm is ipsilateral hearing loss or deafness, with a reported frequency of 1.5–8% in large case series (28, 29, e20). The rate of deafness among our patients was 3%. We have not seen a permanent, high-grade facial palsy over the long term in any of our patients, but 7% of our patients have had a delayed postoperative facial palsy arising 10–14 days after surgery—a phenomenon also reported by other authors (30, 31, e20). It is conceivable that reactivation of herpes zoster plays a role in this phenomenon, but its cause has not yet been clearly identified (31). Fortunately, this delayed facial palsy generally regresses completely. In current reviews of MVD for hemifacial spasm including data from large numbers of patients, the risk of stroke is estimated at 0.1%, and the mortality is also estimated at 0.1% (27, 32, e21).
Table 2. Results of microvascular decompression for hemifacial spasm*.
| Hemifacial spasm | Overall: 320 patients | |
| Sex distribution | Men: 120 | Women: 200 |
| Mean age (range) | 54.89 years (22–81 years) | |
| Mean duration of symptoms | 7.84 years (1–35 years) | |
| Follow-up (FU) |
Immediately after surgery (320 patients) |
FU >12 months (201 patients) |
| – No spasms | 178 (55.63%) | 156 (77.61%) |
| – Spasms reduced by 90% | 53 (16.56%) | 22 (10.95%) |
| – Spasms reduced by 50% | 62 (19.38%) | 20 (9.95%) |
| – No improvement | 27 (8.44%) | 3 (1.49%) |
| Morbidity (in a total of 339 operations) | ||
| Transient | Permanent | |
| Vertigo | 11 (3.2%) | 2 (0.6%) |
| Partial hearing loss | 34 (10%) | 8 (2.4%) |
| Deafness | 9 (2.7%) | 9 (2.7%) |
| Facial palsy (immediate) due to surgical manipulation | 5 (1.5%) | 2 (0.6%, mild) |
| Facial palsy arising after a delay of >10 days | 22 (6.5%) | 1 (0.3%, mild) |
* Results from the authors’ own series of microvascular decompression surgery in 320 patients with hemifacial spasm (a total of 339 operations, including 19 second procedures because of inadequate initial relief or because of recurrence). The relief of spasms immediately after surgery and on late follow-up (>12 months) is shown, as is the frequency of transient and permanent morbidity on the affected side
The microvascular decompression operation
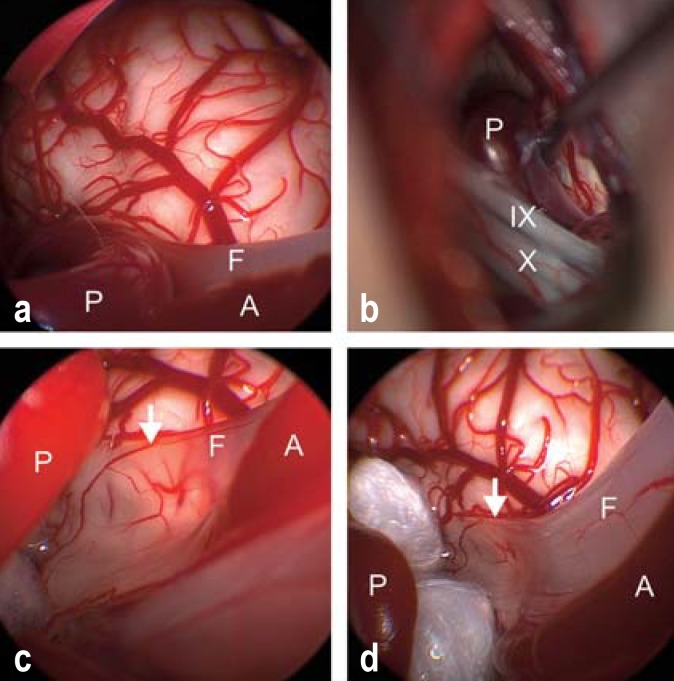
In our institution, microvascular decompression is performed as an endoscopically assisted microsurgical procedure (33). This means that the main operative moves are carried out under the operating microscope, while the endoscope is used at certain points during the operation as an aid to the inspection of the local anatomy at the REZ, which often (mainly in operations for hemifacial spasm) is less well seen under the microscope alone (figure 3). The goal of microvascular decompression surgery is to eliminate the neurovascular conflict by separating the nerve and the compressing vessel from each other and preventing recurrent contact over the long term.
Figure 3.
Endoscopically assisted microvascular decompression for hemifacial spasm.
a) Inspection through the endoscope reveals vascular compression of the facial nerve (F) by a loop of the posterior inferior cerebellar artery (P), while the anterior inferior cerebellar artery (A) courses parallel to the nerve.
b) Inspection through the operating microscope reveals the posterior inferior cerebellar artery (P) but does not directly reveal the site of compression, which is hidden behind the lower cranial nerve group (cranial nerve IX, the glossopharyngeal nerve, and cranial nerve X, the vagus nerve).
c) After mobilization of the artery (P), inspection through the endoscope reveals that the facial nerve (F) is paper-thin after years of compression (14 years of symptoms) (arrow). The brainstem vessels are visible through the flattened, translucent nerve.
d) Two Teflon pledgets have been interposed between the posterior inferior cerebellar artery (P) and the facial nerve (F) to hold these two structures apart without touching the nerve at the site of its previous compression. The hemifacial spasm finally regressed 13 months after surgery; the long delay may be due to the severe structural change of the facial nerve as a result of its longstanding compression. The anterior inferior cerebellar artery (A) is marked as well.
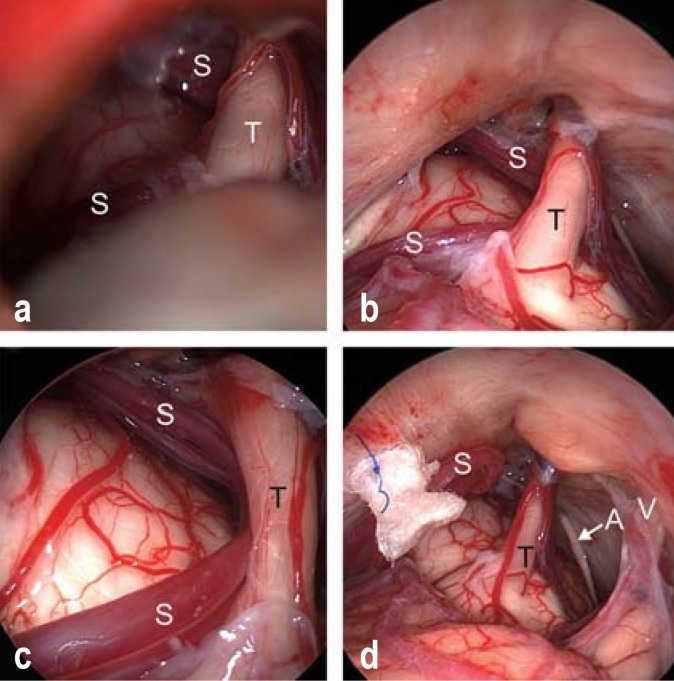
The procedure is carried out under general anesthesia through a retrosigmoid craniotomy. Continuous neuromonitoring with facial nerve EMG and auditory evoked potentials is mandatory (26). This enables the early recognition of, and response to, any changes in the function of the facial or auditory nerve. Under microscopic vision, the cerebellopontine angle is opened, and the target cranial nerve is exposed. Endoscopic inspection at this point in the operation reveals the vascular compression very well (figure 4). The vessel is mobilized away from the affected nerve and a Teflon pledget is inserted between the vessel and the nerve to keep these two structures apart (figure 4). In some cases, a Teflon sling containing the compressing vessel is sewn up to the dura mater to keep the vessel away from the nerve (Figure 4 d).
Figure 4.
Endoscopically assisted microvascular decompression for trigeminal neuralgia.
a) View of the trigeminal nerve (T) and the superior cerebellar artery (S) through the operating microscope.
b) Inspection through the endoscope (0° optics), revealing the entire cisternal course of the trigeminal nerve (T) and the superior cerebellar artery (S).
c) Inspection through the endoscope with 30° optics reveals severe vascular compression of the trigeminal nerve (T) by the elongated loop of the superior cerebellar artery (S).
d) The superior cerebellar artery (S) has been transposed and sewn upward toward the tentorium with a loop of Teflon. Ideally, the trigeminal nerve (T) should be entirely free after the decompression, without any contact either with the vessel or with the Teflon pledget. The abducens nerve (arrow A) and the vestibulocochlear nerve (V) can be seen in the vicinity.
Glossopharyngeal neuralgia (vago-glossopharyngeal neuralgia)
This is a rare entity, accounting for only 0.2–1.3% of all cases of “facial pain syndrome” (34, e22). Its incidence has been estimated at 0.7 cases per 100 000 persons per year (4). A neurovascular conflict at the REZ of the glossopharyngeal nerve can lead to attacks of sudden, lancinating pain in the sensory distribution of the auricular and pharyngeal branches of the ninth and tenth cranial nerves (the glossopharyngeal nerve and the vagus nerve). The pain is typically felt in the posterior portion of the tongue, the tonsils, the pharynx, the larynx, the middle ear, and the angle of the jaw. It can be precipitated by triggers such as swallowing or chewing. In rare cases, vagus involvement leads to attacks of bradycardia, asystole, cramps, or syncopal episodes (35). Like trigeminal neuralgia, this disorder can go into remission and possibly relapse at a later time (36).
The diagnosis of (vago-)glossopharyngeal neuralgia is made on clinical grounds; it can be additionally confirmed by immediate suppression of pain during an attack by local anesthesia of the throat. In differential diagnosis, this disorder may be difficult to distinguish from trigeminal neuralgia, because the affected areas are adjacent and the pain is of similar quality. Further differential diagnoses include superior laryngeal neuralgia and nervus intermedius neuralgia. The main form of conservative treatment is with anticonvulsant drugs, which can also be combined with antidepressants (e23). Surgical treatment by microvascular decompression should be considered the gold standard of treatment (37); further options include radiofrequency ablation and radiosurgery (38). The reported success rate of microvascular decompression is over 90% (34, 39, 40).
Neurovascular compression of the eighth cranial nerve
A series of microvascular decompression operations to relieve compression of the eighth cranial nerve was already reported on by Jannetta, who popularized the MVD procedure in the 1970s and -80s (11, e24). He found that some patients with vertigo profit from the operation, but that it was ineffective against tinnitus. It is still debated whether either of these symptoms is ever caused by neurovascular compression. Any potential case requires very precise neurophysiological, neuroradiological, and clinical evaluation (e25). Only a few studies have been dedicated to this topic (e26, e27). A clinically significant entity is vestibular paroxysmia, which is characterized by attacks of rotational or swaying vertigo lasting from a few seconds to one minute (e26). Drugs (mainly carbamazepine) are the first line of treatment, and MVD can be considered in intractable cases. No statement can be made about a potential association of chronic tinnitus with a neurovascular conflict. In a systematic review by Nash et al., the success rate for the treatment of tinnitus as an isolated symptom was 60%—a figure based on a total of only 43 cases culled from an extensive literature search (e27).
Nervus intermedius neuralgia
Nervus intermedius neuralgia, also called geniculate neuralgia, is a very rare condition. Only 174 cases were described from 1932 to 2018 (e28– e30). It is characterized by brief attacks of stabbing deep-seated ear pain (e31). The pain may be accompanied by lacrimation and taste sensations. The condition is usually due to vascular compression of the nervus intermedius, but can also be caused by herpes zoster. Aside from microvascular decompression, transection of the nervus intermedius is also recommended as a treatment for medically intractable cases. Unfortunately, because of the rarity of nervus intermedius neuralgia, the evidence base for its treatment is very small (e28– 30).
Overview
The most common neurovascular compression syndrome is trigeminal neuralgia, followed by hemifacial spasm. High-resolution 3D-MRI is now available and enables reliable confirmation of the diagnosis in patients with typical clinical manifestations. Microvascular decompression can then be carried out as a causally directed treatment. Patients for whom conservative therapy is insufficiently effective should be informed early about the option of surgical treatment. (Vago-)glossopharyngeal neuralgia, a rare condition, can also be treated surgically with MVD; the operation is indicated for patients with medically intractable symptoms.
Vestibular paroxysmia is due to compression of the eighth cranial nerve. Tinnitus, too, has been relieved by MVD in a few reported cases, but the evidence available from the literature does not permit any clear recommendation. A neurovascular conflict involving the eighth cranial nerve that has been revealed by an imaging study should be considered critically, as such findings are common in asymptomatic persons as well. The current state of knowledge suggests that nervus intermedius neuralgia and carbamazepine-responsive vestibular paroxysmia are potential indications for MVD.
Key Messages.
The most common nerve compression syndrome in the posterior cranial fossa is trigeminal neuralgia, followed by hemifacial spasm.
Neurovascular compression syndromes are generally due to compression of a cranial nerve by an artery or vein at the root entry or exit zone (REZ) near the brainstem.
In patients with typical clinical manifestations, the diagnosis can be confirmed by high-resolution 3D-MRI.
Most nerve compression syndromes are treated with drugs at first, e.g., trigeminal neuralgia with carbamazepine and hemifacial spasm with botulinum toxin injections.
Microvascular decompression is the only causally directed treatment of these conditions and should be considered early in patients for whom pharmacotherapy is insufficiently effective or causes unacceptable side effects.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Acknowledgement
The authors thank Marc Matthes for processing the images and for the statistical analysis of the authors’ own data, as presented in this paper.
Footnotes
Conflict of interest statement
Dr. Baldauf has received reimbursement of travel and accommodation expenses as well as lecture honoraria from the Karl Storz company.
Prof. Schroeder has served as a paid consultant for the Karl Storz company, from which he has also recieved reimbursement of travel and accommodation expenses as well as lecture honoraria.
Dr. Rosenstengel states that he has no conflict of interest.
References
- 1.Donahue JH, Ornan DA, Mukherjee S. Imaging of vascular compression syndromes. Radiol Clin North Am. 2017;55:123–138. doi: 10.1016/j.rcl.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 2.El Refaee E, Langner S, Baldauf J, Matthes M, Kirsch M, Schroeder HW. Value of 3-dimensional high-resolution magnetic resonance imaging in detecting the offending vessel in hemifacial spasm: comparison with intraoperative high definition endoscopic visualization. Neurosurgery. 2013;73:58–67. doi: 10.1227/01.neu.0000429838.38342.e2. [DOI] [PubMed] [Google Scholar]
- 3.Katusic S, Williams DB, Beard CM, Bergstralh EJ, Kurland LT. Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences Rochester, Minnesota, 1945-1984. Neuroepidemiology. 1991;10:276–281. doi: 10.1159/000110284. [DOI] [PubMed] [Google Scholar]
- 4.Manzoni GC, Torelli P. Epidemiology of typical and atypical craniofacial neuralgias. Neurol Sci. 2005;26(Suppl 2):s65–s67. doi: 10.1007/s10072-005-0410-0. [DOI] [PubMed] [Google Scholar]
- 5.Fromm GH. Trigeminal neuralgia and related disorders. Neurol Clin. 1989;7:305–319. [PubMed] [Google Scholar]
- 6.DGN. Leitlinie Trigeminusneuralgie. www.dgn.org/leitlinien/ 2287-ll-58-012-trigeminusneuralgie (last accessed on 30 October 2018) 2012 [Google Scholar]
- 7.Gronseth G, Cruccu G, Alksne J, et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology. 2008;71:1183–1190. doi: 10.1212/01.wnl.0000326598.83183.04. [DOI] [PubMed] [Google Scholar]
- 8.Spatz AL, Zakrzewska JM, Kay EJ. Decision analysis of medical and surgical treatments for trigeminal neuralgia: how patient evaluations of benefits and risks affect the utility of treatment decisions. Pain. 2007;131:302–310. doi: 10.1016/j.pain.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Degn J, Brennum J. Surgical treatment of trigeminal neuralgia Results from the use of glycerol injection, microvascular decompression, and rhizotomia. Acta Neurochirurgica. 2010;152:2125–2132. doi: 10.1007/s00701-010-0840-1. [DOI] [PubMed] [Google Scholar]
- 10.Tatli M, Satici O, Kanpolat Y, Sindou M. Various surgical modalities for trigeminal neuralgia: literature study of respective long-term outcomes. Acta Neurochirurgica. 2008;150:243–255. doi: 10.1007/s00701-007-1488-3. [DOI] [PubMed] [Google Scholar]
- 11.Jannetta PJ. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. 1967;26(Suppl):159–162. doi: 10.3171/jns.1967.26.1part2.0159. [DOI] [PubMed] [Google Scholar]
- 12.Barker FG, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 1996;334:1077–1083. doi: 10.1056/NEJM199604253341701. [DOI] [PubMed] [Google Scholar]
- 13.Zakrzewska JM, Lopez BC, Kim SE, Coakham HB. Patient reports of satisfaction after microvascular decompression and partial sensory rhizotomy for trigeminal neuralgia. Neurosurgery. 2005;56:1304–1311. doi: 10.1227/01.neu.0000159883.35957.e0. [DOI] [PubMed] [Google Scholar]
- 14.Sindou M, Leston J, Howeidy T, Decullier E, Chapuis F. Micro-vascular decompression for primary trigeminal neuralgia (typical or atypical) Long-term effectiveness on pain; prospective study with survival analysis in a consecutive series of 362 patients. Acta Neurochir (Wien) 2006;148:1235–1245. doi: 10.1007/s00701-006-0809-2. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Lei D, You C, Mao BY, Wu B, Fang Y. The long-term outcome predictors of pure microvascular decompression for primary trigeminal neuralgia. World Neurosurg. 2013;79:756–762. doi: 10.1016/j.wneu.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Mendelson ZS, Velagala JR, Kohli G, Heir GM, Mammis A, Liu JK. Pain-free outcomes and durability of surgical intervention for trigeminal neuralgia: a comparison of gamma knife and microvascular decompression. World Neurosurg. 2018;112:e732–e746. doi: 10.1016/j.wneu.2018.01.141. [DOI] [PubMed] [Google Scholar]
- 17.Gubian A, Rosahl SK. Meta-analysis on safety and efficacy of microsurgical and radiosurgical treatment of trigeminal neuralgia. World Neurosurg. 2017;103:757–767. doi: 10.1016/j.wneu.2017.04.085. [DOI] [PubMed] [Google Scholar]
- 18.Franzini A, Ferroli P, Messina G, Broggi G. Surgical treatment of cranial neuralgias. Handb Clin Neurol. 2010;97:679–692. doi: 10.1016/S0072-9752(10)97057-7. [DOI] [PubMed] [Google Scholar]
- 19.Broggi G, Ferroli P, Franzini A, Servello D, Dones I. Microvascular decompression for trigeminal neuralgia: comments on a series of 250 cases, including 10 patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;68:59–64. doi: 10.1136/jnnp.68.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jafree DJ, Williams AC, Zakrzewska JM. Impact of pain and postoperative complications on patient-reported outcome measures 5 years after microvascular decompression or partial sensory rhizotomy for trigeminal neuralgia. Acta Neurochir (Wien) 2018;160:125–134. doi: 10.1007/s00701-017-3350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruccu G, Gronseth G, Alksne J, et al. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008;15:1013–1028. doi: 10.1111/j.1468-1331.2008.02185.x. [DOI] [PubMed] [Google Scholar]
- 22.Zakrzewska JM, Akram H. Neurosurgical interventions for the treatment of classical trigeminal neuralgia. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD007312.pub2. CD007312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Auger RG, Whisnant JP. Hemifacial spasm in Rochester and Olmsted County, Minnesota, 1960 to 1984. Arch Neurol. 1990;47:1233–1234. doi: 10.1001/archneur.1990.00530110095023. [DOI] [PubMed] [Google Scholar]
- 24.El Damaty A, Rosenstengel C, Matthes M, Baldauf J, Schroeder HW. The value of lateral spread response monitoring in predicting the clinical outcome after microvascular decompression in hemifacial spasm: a prospective study on 100 patients. Neurosurg Rev. 2016;39:455–466. doi: 10.1007/s10143-016-0708-9. [DOI] [PubMed] [Google Scholar]
- 25.Deluca C, Tommasi G, Moretto G, Fiaschi A, Tinazzi M. Focal motor seizures mimicking hemifacial spasm. Parkinsonism Relat Disord. 2008;14:649–651. doi: 10.1016/j.parkreldis.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Yaltho TC, Jankovic J. The many faces of hemifacial spasm: differential diagnosis of unilateral facial spasms. Mov Disord. 2011;26:1582–1592. doi: 10.1002/mds.23692. [DOI] [PubMed] [Google Scholar]
- 27.Sindou M, Mercier P. Microvascular decompression for hemifacial spasm: outcome on spasm and complications A review. Neurochirurgie. 2018;64:106–116. doi: 10.1016/j.neuchi.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Barker FG, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD. Microvascular decompression for hemifacial spasm. J Neurosurg. 1995;82:201–210. doi: 10.3171/jns.1995.82.2.0201. [DOI] [PubMed] [Google Scholar]
- 29.Jo KW, Kim JW, Kong DS, Hong SH, Park K. The patterns and risk factors of hearing loss following microvascular decompression for hemifacial spasm. Acta Neurochir (Wien) 2011;153:1023–1030. doi: 10.1007/s00701-010-0935-8. [DOI] [PubMed] [Google Scholar]
- 30.El Damaty A, Rosenstengel C, Matthes M, et al. A new score to predict the risk of hearing impairment after microvascular decompression for hemifacial spasm. Neurosurgery. 2017;81:834–843. doi: 10.1093/neuros/nyx111. [DOI] [PubMed] [Google Scholar]
- 31.Lovely TJ, Getch CC, Jannetta PJ. Delayed facial weakness after microvascular decompression of cranial nerve VII. Surg Neurol. 1998;50:449–452. doi: 10.1016/s0090-3019(97)00314-5. [DOI] [PubMed] [Google Scholar]
- 32.Mizobuchi Y, Muramatsu K, Ohtani M, et al. The current status of microvascular decompression for the treatment of hemifacial spasm in Japan: an analysis of 2907 patients using the Japanese diagnosis procedure combination database. Neurologia medico-chirurgica. 2017;57:184–190. doi: 10.2176/nmc.oa.2016-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenstengel C, Matthes M, Baldauf J, Fleck S, Schroeder H. Hemifacial spasm: conservative and surgical treatment options. Dtsch Arztebl Int. 2012;109:667–673. doi: 10.3238/arztebl.2012.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Sindou M. Vago-glossopharyngeal neuralgia: a literature review of neurosurgical experience. Acta Neurochir (Wien) 2015;157:311–321. doi: 10.1007/s00701-014-2302-7. [DOI] [PubMed] [Google Scholar]
- 35.Blumenfeld A, Nikolskaya G. Glossopharyngeal neuralgia. Curr Pain Headache Rep. 2013;17 doi: 10.1007/s11916-013-0343-x. [DOI] [PubMed] [Google Scholar]
- 36.The International Classification of Headache Disorders 3rdedition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 37.Rey-Dios R, Cohen-Gadol AA. Current neurosurgical management of glossopharyngeal neuralgia and technical nuances for microvascular decompression surgery. Neurosurg Focus. 2013;34 doi: 10.3171/2012.12.FOCUS12391. [DOI] [PubMed] [Google Scholar]
- 38.Kano H, Urgosik D, Liscak R, et al. Stereotactic radiosurgery for idiopathic glossopharyngeal neuralgia: an international multicenter study. J Neurosurg. 2016;125:147–153. doi: 10.3171/2016.7.GKS161523. [DOI] [PubMed] [Google Scholar]
- 39.Ma Y, Li YF, Wang QC, Wang B, Huang HT. Neurosurgical treatment of glossopharyngeal neuralgia: analysis of 103 cases. J Neurosurg. 2016;124:1088–1092. doi: 10.3171/2015.3.JNS141806. [DOI] [PubMed] [Google Scholar]
- 40.Xia L, Li YS, Liu MX, et al. Microvascular decompression for glossopharyngeal neuralgia: a retrospective analysis of 228 cases. Acta Neurochir (Wien) 2018;160:117–123. doi: 10.1007/s00701-017-3347-1. [DOI] [PubMed] [Google Scholar]
- E1.Haller S, Etienne L, Kovari E, Varoquaux AD, Urbach H, Becker M. Imaging of neurovascular compression syndromes: trigeminal neuralgia, hemifacial spasm, vestibular paroxysmia, and glossopharyngeal neuralgia. Am J Neuroradiol. 2016;37:1384–1392. doi: 10.3174/ajnr.A4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001;124:2347–2360. doi: 10.1093/brain/124.12.2347. [DOI] [PubMed] [Google Scholar]
- E3.Meaney JF, Watt JW, Eldridge PR, Whitehouse GH, Wells JC, Miles JB. Association between trigeminal neuralgia and multiple sclerosis: role of magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 1995;59:253–259. doi: 10.1136/jnnp.59.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Gass A, Kitchen N, MacManus DG, Moseley IF, Hennerici MG, Miller DH. Trigeminal neuralgia in patients with multiple sclerosis: lesion localization with magnetic resonance imaging. Neurology. 1997;49:1142–1144. doi: 10.1212/wnl.49.4.1142. [DOI] [PubMed] [Google Scholar]
- E5.Udupi BP, Chouhan RS, Dash HH, Bithal PK, Prabhakar H. Comparative evaluation of percutaneous retrogasserian glycerol rhizolysis and radiofrequency thermocoagulation techniques in the management of trigeminal neuralgia. Neurosurgery. 2012;70:407–412. doi: 10.1227/NEU.0b013e318233a85f. [DOI] [PubMed] [Google Scholar]
- E6.Noorani I, Lodge A, Vajramani G, Sparrow O. Comparing percutaneous treatments of trigeminal neuralgia: 19 years of experience in a single centre. Stereotact Funct Neurosurg. 2016;94:75–85. doi: 10.1159/000445077. [DOI] [PubMed] [Google Scholar]
- E7.Kondo A. Follow-up results of microvascular decompression in trigeminal neuralgia and hemifacial spasm. Neurosurgery. 1997;40:46–51. doi: 10.1097/00006123-199701000-00009. [DOI] [PubMed] [Google Scholar]
- E8.Tronnier VM, Rasche D, Hamer J, Kienle AL, Kunze S. Treatment of idiopathic trigeminal neuralgia: comparison of long-term outcome after radiofrequency rhizotomy and microvascular decompression. Neurosurgery. 2001;48:1261–1267. [PubMed] [Google Scholar]
- E9.Sarsam Z, Garcia-Finana M, Nurmikko TJ, Varma TR, Eldridge P. The long-term outcome of microvascular decompression for trigeminal neuralgia. Br J Neurosurg. 2010;24:18–25. doi: 10.3109/02688690903370289. [DOI] [PubMed] [Google Scholar]
- E10.Baldauf J, Rosenstengel C, Matthes M, Fleck S, Marx S, Schroeder HWS. Microvascular decompression and partial sensory rhizotomy for trigeminal neuralgia with special reference to endoscopic-assisted microsurgery. J Neurol Surg B. 2016;77 LFP-12-03. [Google Scholar]
- E11.Gunther T, Gerganov VM, Stieglitz L, Ludemann W, Samii A, Samii M. Microvascular decompression for trigeminal neuralgia in the elderly: long-term treatment outcome and comparison with younger patients. Neurosurgery. 2009;65:477–482. doi: 10.1227/01.NEU.0000350859.27751.90. [DOI] [PubMed] [Google Scholar]
- E12.Abhinav K, Love S, Kalantzis G, Coakham HB, Patel NK. Clinicopathological review of patients with and without multiple sclerosis treated by partial sensory rhizotomy for medically refractory trigeminal neuralgia: a 12-year retrospective study. Clin Neurol Neurosurg. 2012;114:361–365. doi: 10.1016/j.clineuro.2011.11.018. [DOI] [PubMed] [Google Scholar]
- E13.Wang A, Jankovic J. Hemifacial spasm: clinical findings and treatment. Muscle Nerve. 1998;21:1740–1747. doi: 10.1002/(sici)1097-4598(199812)21:12<1740::aid-mus17>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- E14.Babinski J. “Hémispasme facial périphérique”. Nouvelle iconographie de la Salpétrière. 1905;18:418–423. [Google Scholar]
- E15.Tan EK, Jankovic J. Psychogenic hemifacial spasm. J Neuropsychiatry Clin Neurosci. 2001;13:380–384. doi: 10.1176/jnp.13.3.380. [DOI] [PubMed] [Google Scholar]
- E16.Tan EK, Fook-Chong S, Lum SY, Lim E. Botulinum toxin improves quality of life in hemifacial spasm: validation of a questionnaire (HFS-30) J Neurol Sci. 2004;219:151–155. doi: 10.1016/j.jns.2004.01.010. [DOI] [PubMed] [Google Scholar]
- E17.Jankovic J. Tourette syndrome Phenomenology and classification of tics. Neurol Clin. 1997;15:267–275. doi: 10.1016/s0733-8619(05)70311-x. [DOI] [PubMed] [Google Scholar]
- E18.Lim EC, Seet RC. Use of botulinum toxin in the neurology clinic. Nat Rev Neurol. 2010;6:624–636. doi: 10.1038/nrneurol.2010.149. [DOI] [PubMed] [Google Scholar]
- E19.Defazio G, Abbruzzese G, Girlanda P, et al. Botulinum toxin A treatment for primary hemifacial spasm: a 10-year multicenter study. Arch Neurol. 2002;59:418–420. doi: 10.1001/archneur.59.3.418. [DOI] [PubMed] [Google Scholar]
- E20.Dannenbaum M, Lega BC, Suki D, Harper RL, Yoshor D. Microvascular decompression for hemifacial spasm: long-term results from 114 operations performed without neurophysiological monitoring. J Neurosurg. 2008;109:410–415. doi: 10.3171/JNS/2008/109/9/0410. [DOI] [PubMed] [Google Scholar]
- E21.Lee MH, Jee TK, Lee JA, Park K. Postoperative complications of microvascular decompression for hemifacial spasm: lessons from experience of 2040 cases. Neurosurgical Review. 2016;39:151–158. doi: 10.1007/s10143-015-0666-7. [DOI] [PubMed] [Google Scholar]
- E22.Ceylan S, Karakus A, Duru S, Baykal S, Koca O. Glossopharyngeal neuralgia: a study of 6 cases. Neurosurg Rev. 1997;20:196–200. doi: 10.1007/BF01105564. [DOI] [PubMed] [Google Scholar]
- E23.Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders. Cephalalgia. (2) 2004;24(1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- E24.Jannetta PJ. Neurovascular compression in cranial nerve and systemic disease. Ann Surg. 1980;192:518–525. doi: 10.1097/00000658-198010000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E25.Best C, Gawehn J, Kramer HH, et al. MRI and neurophysiology in vestibular paroxysmia: contradiction and correlation. J Neurol Neurosurg Psychiatry. 2013;84:1349–1356. doi: 10.1136/jnnp-2013-305513. [DOI] [PubMed] [Google Scholar]
- E26.Brandt T, Strupp M, Dieterich M. Vestibular paroxysmia: a treatable neurovascular cross-compression syndrome. J Neurol. 2016;263(1):S90–S96. doi: 10.1007/s00415-015-7973-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E27.Nash B, Carlson ML, van Gompel JJ. Microvascular decompression for tinnitus: systematic review. J Neurosurg. 2017;126:1148–1157. doi: 10.3171/2016.2.JNS152913. [DOI] [PubMed] [Google Scholar]
- E28.Tang IP, Freeman SR, Kontorinis G, et al. Geniculate neuralgia: a systematic review. J Laryngol Otol. 2014;128:394–399. doi: 10.1017/S0022215114000802. [DOI] [PubMed] [Google Scholar]
- E29.Peris-Celda M, Oushy S, Perry A, et al. Nervus intermedius and the surgical management of geniculate neuralgia. J Neurosurg. 2018:1–9. doi: 10.3171/2018.3.JNS172920. [DOI] [PubMed] [Google Scholar]
- E30.Holste KG, Hardaway FA, Raslan AM, Burchiel KJ. Pain-free and pain-controlled survival after sectioning the nervus intermedius in nervus intermedius neuralgia: a single-institution review. J Neurosurg. 2018:1–8. doi: 10.3171/2018.3.JNS172495. [DOI] [PubMed] [Google Scholar]
- E31.O‘Neill F, Nurmikko T, Sommer C. Other facial neuralgias. Cephalalgia. 2017;37:658–669. doi: 10.1177/0333102417689995. [DOI] [PubMed] [Google Scholar]