Abstract
Objective
To determine the efficacy in improving pain and health-related quality of life (HRQOL) of an online self-management program for adolescents with juvenile idiopathic arthritis (JIA).
Methods
Youth ages 12–18 years with JIA were recruited from 10 rheumatology clinics across the United States and randomized to complete an online self-management program (n = 144) or an online disease education program (n = 145). Participants in the self-management group worked through multimedia-based modules comprising psychoeducation, training in cognitive–behavioral coping skills and stress management, and other self-management topics over a 12-week period. Participants in the control group viewed a series of preselected quality educational websites about JIA over the same interval. Online content for both groups was made available in English and Spanish to facilitate inclusion of Hispanic participants. Blinded assessment of main outcomes (pain intensity, pain interference, and HRQOL) and process outcomes (disease knowledge, self-efficacy, pain coping, and emotional adjustment) occurred at baseline, posttreatment, and at 6- and 12-month postrandomization follow-up visits.
Results
Participants on average demonstrated significant improvements over the study period in the main outcomes, with no significant group differences in the degree of improvement. Effect sizes for these improvements were small. The amount of improvement in self-efficacy, emotional avoidance coping, disease knowledge, and emotional functioning in part predicted improvement in pain and HRQOL outcomes.
Conclusions
Primarily self-directed online self-management training and online disease education comparably and modestly improve pain and HRQOL in youth with JIA.
Keywords: eHealth, juvenile idiopathic arthritis, pain, quality of life, randomized controlled trial
Juvenile idiopathic arthritis (JIA) is a chronic inflammatory disease affecting approximately .07% of children and is the most common rheumatologic condition of childhood (Thierry, Fautrel, Lemelle, & Guillemin, 2014). Although there has been significant recent advances in pharmacological options for reducing disease activity and minimizing risk of permanent joint damage in JIA, chronic musculoskeletal pain remains common with this condition particularly for adolescents (Bromberg, Connelly, Anthony, Gil, & Schanberg, 2014; Rashid et al., 2018). Results of daily diary studies suggest that youth treated for JIA report pain on 72% of days, with moderate to severe pain being reported about one-third of the time (Bromberg et al., 2014). In turn, pain has been found to be a reliable predictor of the reduced quality of life that has been reported for patients with JIA worldwide, including difficulties with routine physical activities, elevated symptoms of depression and anxiety, and problems with feeling distant from peers (Gutiérrez-Suárez et al., 2007; Haverman et al., 2012; Tong, Jones, Craig, & Singh-Grewal, 2012; Weitzman et al., 2017). Thus, there remains an important need to improve pain management and quality of life for this population.
Advances from pediatric psychology and other fields in understanding the cognitive and behavioral variables that modulate pain perception, including mood, types of coping strategies used, and self-efficacy, have provided an empirical foundation to develop “self-management interventions” that ostensibly target these variables to improve pain and functioning (Jensen, 2010; Palermo, Eccleston, Lewandowski, Williams, & Morley, 2010). Self-management interventions typically comprise components of psychoeducation and instruction in cognitive and behavioral coping strategies, with the intent of equipping patients with knowledge and behaviors that optimize health and reduce the potential impact of symptoms on development, physical functioning, mood, and relationships (Lorig & Holman, 2003). Youth with JIA remain infrequent consumers of such interventions, however, due in part to access challenges and limited knowledge about their availability (Slater et al., 2016; van Dijkhuizen et al., 2018).
Over the past several years, informational resources about chronic pediatric conditions increasingly have been made available online to be broadly accessible. However, for youth with JIA, online resources have been mostly targeted toward parents, highly variable in quality, and focused exclusively on disease education (Stinson et al., 2009). Online resources that are based on science for effecting changes in important patient outcomes such as pain and quality of life are desired by patients with JIA but historically unavailable (Stinson et al., 2008). In the broader field of pediatric pain, evidence mostly from small samples has been supportive of the benefit of online cognitive–behaviorally based interventions for pain outcomes in the short term, with inconclusive results regarding changes in functioning and mood and for long-term pain outcomes (Fisher, Law, Palermo, & Eccleston, 2015).
The “Teens Taking Charge” program is an online treatment that specifically was developed to meet the need for a high-quality, accessible, empirically grounded self-management skills training program for adolescents with JIA that could be readily adaptable for other languages and cultures. The program is rooted in cognitive–behavioral principles of improving pain and health-related quality of life (HRQOL) through psychoeducation (about pain and JIA), empowering patients with cognitive and behavioral coping skills for managing disease symptoms and stress, and enhancing perceived social connectedness and support through peer model videos and opportunities for interaction (e.g., discussion board). Results of an initial attention-controlled pilot study of this program were encouraging, with teens with JIA in the online intervention group reporting high satisfaction with the program and greater reductions in pain intensity and HRQOL over a 12-week time span relative to those in an attention control group (Stinson et al., 2010). Based on these promising pilot data, the current multisite trial was undertaken to definitively determine the extent to which the Teens Taking Charge program, when primarily self-directed, leads to sustained improvements in pain and HRQOL in a representative sample of youth with JIA; program content was made available both in English and Spanish for the trial to facilitate inclusion of Hispanic families. We hypothesized that adding this online program to the existing care of youth with JIA would produce improvements over the span of a 12-month study period in the main outcomes of pain and HRQOL, and that these improvements would exceed those attained by only viewing quality disease education information online. We also hypothesized that individual differences in changes on the main outcomes would be predicted by the extent to which patients changed on select “process” outcome variables often shown to be modifiable with psychoeducational treatment and related to pain and quality of life: self-efficacy, emotional adjustment, pain coping, and disease knowledge.
Methods
Participants and Recruitment
Enrollment of patients occurred between November 2012 and February 2015, with follow-up assessments continuing through June 2016. Patients were enrolled onsite at 10 American pediatric rheumatology practices affiliated with the Childhood Arthritis and Rheumatology Research Alliance (CARRA, Inc.); sites were clinics within mostly urban children’s hospitals and were from the four main regions of the United States (Northeast, Midwest, South, and West). Families of patients with upcoming clinic appointments for JIA and prescreened through medical record review by site research coordinators were called by the coordinators after receiving a study information letter and/or were approached at routine clinic visits to discuss the study. Eligibility was confirmed by the site coordinators at clinic visits based on information provided by the attending physician and family. Written parental permission/child assent or consent was attained by site coordinators at the clinic appointment. Bilingual study coordinators or interpreters were used when enrolling Spanish-speaking families. Study procedures were approved by the institutional review board (IRB) at the primary study site and by the IRBs of each of the other nine recruiting sites.
Patients were eligible to participate if they were between the ages of 12 and 18 years, diagnosed with JIA, reporting pain in one or more joints over the past 6 months, and able to speak and read English or Spanish. Patients were ineligible to participate if they had a chronic medical condition other than JIA (e.g., inflammatory bowel disease, genetic disorders, and diabetes), were judged by a physician to have significant cognitive impairment that would prevent understanding of the intervention or measures, or currently were involved in psychotherapy. Of 1,216 patients who received study letters and/or were attempted to be approached in clinics, 433 were determined to be ineligible (most commonly due to having conditions other than JIA or to a parent or patient unable to speak or read English or Spanish), 324 could not be fully screened (e.g., no clinic visit appearance during the recruiting period or no time to stay after a clinic visit to speak with study staff), and 154 reported not being interested in participating (most commonly because of concerns about time commitment). The final consented sample comprised 305 youth and their parents/caregivers. The enrolled sample did not significantly differ on known demographics from those that declined to participate. Four participants did not have home Internet access and were provided a laptop with a wireless card for the duration of the intervention period. No changes were made to routine care provided to study participants.
Study Groups
Teens Taking Charge Group
Participants in the Teens Taking Charge online self-management group accessed the Teens Taking Charge program via a password-protected Web portal. Content for the program was developed and subsequently refined for this trial by a team of interdisciplinary experts in pediatric rheumatology, pediatric psychology, pain, and adolescent development. Interactivity and multimedia (e.g., animations, audio files, monitored peer discussion board, goal-setting, forms, and video clips with peer models) were included to augment understanding and engagement with text content. All content was made available in Spanish to allow for enrollment of Spanish-speaking youth with JIA and their parents, with translated content being reviewed for cultural sensitivity by a consultant with expertise in Latino cultural adaptation of cognitive–behavioral treatments.
Participants were instructed to work through 1 of 12 program modules per week over a 12-week treatment phase, with each module comprising 20–30 content pages and expected to take approximately 30 min of time to complete. The first few modules were designed to increase patient confidence in understanding their condition and ability to manage its symptoms and impact by providing psychoeducation about arthritis and introducing the biopsychosocial model of pain. The next several modules were designed to teach and model cognitive and behavioral strategies that can help modulate the severity and impact of pain and other disease symptoms, including strategies for managing stress, relaxation training, distraction methods, and cognitive coping skills. The final few modules included additional content on optimizing health habits that are associated with pain and HRQOL (e.g., physical activity, healthy eating, and sleep habits), additional therapies and supports, and strategies for preventing and overcoming setbacks. No more than two modules could be completed per week. Parents/caregivers of the adolescent participants also were asked to complete two online modules about facilitating their child’s self-management skills.
Participants in the treatment group received brief (<30 min) monthly telephone support calls for 3 months by trained bilingual “health coaches” that were dedicated to this treatment condition only. Health coaches had completed undergraduate training in psychology or general science and received training and supervision in the protocol through initial training sessions with role-plays and recurring meetings with senior research staff. The health coach calls were scripted and comprised use of prompts to discuss the content of the four modules intended to be completed before each call and a review of answers to the knowledge quizzes contained in each of those modules. All calls were audio-recorded.
Online Education Control Group
The education control group was designed to control for the potential effects on outcomes of extra attention from a supportive individual and increased knowledge about JIA from viewing publicly available websites. Participants in this group accessed a password-protected study website containing links to 12 educational websites about JIA that had been vetted for quality during a systematic review (Stinson et al., 2009). Links to electronically translated Spanish versions of the educational sites were provided to Spanish-speaking participants. Information on the educational sites did not include training in specific cognitive–behavioral coping skills or opportunities for social exchange. Adolescents were instructed to view one educational website per week over 12 weeks, and no more than two sites could be viewed in any given week. Participants in this group also received 3 monthly calls by a bilingual health coach dedicated to this condition only. These calls comprised use of prompts to discuss current health and information obtained from the assigned websites.
Randomization
The trial was registered at ClinicalTrials.gov (NCT01541917) and used a two-arm parallel design. Site research coordinators entered patient information into a Web-based randomization service that was used to equally (1:1) allocate participants to the two trial groups following completion of baseline measures. Blocked randomization was used, with randomly varying block sizes of 4, 6, and 8. Randomization was stratified by study site, baseline physician-rated disease severity (mild, moderate, or severe), and primary language of the patient (English or Spanish) to help ensure equal representation on these variables between groups. Randomization results then were sent automatically by an e-mail to unblinded members of the central study team, who called families to explain procedures. Study physicians, investigators, and coordinators at each site were kept blind to patient group assignment; they did not participate in any aspect of the study intervention, and patients were instructed by coordinators at time of consent to not discuss content of the intervention with study staff. Study participants were not blinded to group assignment.
Assessment Procedures
Computer-administered self-report measures with built-in validity checks (e.g., for out of range or missing items) were completed during onsite study visits at baseline (prerandomization), posttreatment (3 months after randomization), and at 6-month and 12-month postrandomization. Coordinators also recorded clinical data at these time points and manually entered this information into the study database. All measures were available in translated/back-translated Spanish versions. Participants/caregivers received a $50 stipend for completing the assessment visits.
Measures
Primary Outcome Variables
Pain Intensity and Interference
Participants rated average pain intensity over the prior 2-week period on an 11-point (0–10) numeric rating scale with anchors “no pain” and “very much pain.” Participants also rated pain interference (the extent to which pain had gotten in the way of activities, mood, walking, sleeping, and enjoyment of life) using numeric rating scales with anchors “doesn’t get in the way at all” and “totally gets in the way.” Response values on the five pain interference items were averaged to form a single index of pain interference, ranging from 0 to 10, with higher scores indicating greater pain interference. The pain intensity and interference items have been previously validated in youth with JIA and shown to be responsive to treatment (Stinson et al., 2008). Cronbach’s alpha for the pain interference scale for the current sample was .93.
Health-Related Quality of Life
The PedsQL 3.0 Rheumatology Module, self-report version, is a 22-item questionnaire designed to measure the impact of having a rheumatologic condition on one’s quality of life, such as the perceived severity of problems with daily activity limitations, worry/anxiety, and communication (Varni et al., 2002). Responses are provided on a five-point scale (“never” to “almost always” a problem), with each response then converted to a 0–100 scale. An average across all items was used as a total score for analyses (ranging from 0 for poorest quality of life to 100 for excellent quality of life). Cronbach’s alpha for the average total score for the current sample was .91.
Process Outcome Variables
Self-Efficacy
The Children’s Arthritis Self-Efficacy (CASE) scale (Barlow, Shaw, & Wright, 2001) contains 11 items that inquire about patient’s confidence in their ability to manage the symptoms, emotional consequences, and activities related to their arthritis. A sample item is “I can find ways to control the hurt of arthritis.” Responses are provided on a five-point ordinal scale ranging from “not at all sure” to “very sure.” Item responses were averaged to form a total self-efficacy score that ranged from 1 to 5, with higher scores indicating higher self-efficacy. Cronbach’s alpha for the CASE total score for the current sample was .94.
Pain Coping
Pain coping strategies were measured using the Pain Coping Questionnaire (PCQ; Reid, Gilbert, & McGrath, 1998), a widely used measure of pain coping strategies in youth. Respondents indicate on a five-point scale how often they use various types of coping strategies when they are in pain “for a few hours or days.” Sample items include “talk to a friend about how I feel” and “do something fun.” Composite subscale scores can be derived based on prior factor analyses. The two composite subscales selected for the current study included approach coping (e.g., seeking social support), which typically relates to less pain and other positive health outcomes, and emotion-focused avoidance coping (e.g., catastrophizing), which has been shown to relate to greater pain in youth with arthritis (Thastum, Herlin, & Zachariae 2005). Subscale scores ranged from 1 to 5, with higher scores indicating greater frequency of use of the given type of coping strategies. Internal consistency (Cronbach’s alpha) for the approach coping and emotion-focused avoidance coping subscales was .88 and .78, respectively.
Emotional Adjustment
The presence and severity of anxiety and depression symptoms (as indicators of emotional adjustment) was measured using the PROMIS Pediatric Anxiety and Depression Short Forms. These measures each consist of eight items that have been shown through item response theory to provide maximal information about anxiety and depression in the pediatric age range (Irwin et al., 2010). Participants are asked to indicate on a five-point scale (“never” to “almost always”) how often they have experienced a given statement in the past 7 days. Scores on the measure were converted to T-scores based on published norms, with scores >50 indicating higher than typical symptoms of anxiety and depression. Cronbach’s alpha for the PROMIS Anxiety and Depression scales was .93 and .96, respectively.
Disease Knowledge
Knowledge related to the medical and management aspects of JIA was measured using the Medical Issues, Exercise, Pain, and Social Support Questionnaire (MEPS; André, Hedengren, Hagelberg, & Stenstrom, 1999), which has evidence of good construct validity, high test–retest reliability over 1 week, and responsiveness to education interventions. The questionnaire consists of eight items that are responded to using an 11-point numeric rating scale ranging from “none at all” to “enough.” A sample item is “How much knowledge do you have of how to manage pain?” Item scores are averaged to form a total score ranging from 0 to 10; higher scores indicate greater disease knowledge. Cronbach’s alpha for the MEPS total score for the current sample was .94.
Covariates and Other Measures
Patient Characteristics and Adverse Events
A questionnaire was used at baseline to collect information on sociodemographic data from parents/caregivers of patients. Information on JIA subtype (reduced to oligoarticular, polyarticular, and “other” for analyses) and medications being used at all time points (non-steroidal anti-inflammatory drugs, non-biological disease-modifying antirheumatic drugs, biological response modifiers, and/or corticosteroids) was obtained from attending providers and medical chart review. Adverse events were documented by study coordinators throughout the duration of the study using standardized electronic forms.
Adherence
Usage of the study group websites (logins and pages accessed) was tracked through analytics software, and completion of health coach calls was documented through a report completed by the health coaches. Participants were considered adherent to the group protocol if they had logged in to view at least 75% of the assigned modules/educational sites and completed at least two of the three health coach calls.
Quality Control
Study procedures for each site were standardized through investigator meetings, live and video-recorded site coordinator training, a comprehensive manual of operating procedures, monthly coordinator calls, and a monthly study e-newsletter that in part addressed frequently asked questions about study procedures. Site adherence in implementing the study protocol was monitored indirectly through review of monthly site data reports, and directly through three on-site monitoring visits. Fidelity for the monthly health coach calls was monitored by review of checklists completed by coordinators of information covered during each call and by intermittent review of recorded calls; fidelity was maintained at or above 90% for completing intended elements of the calls.
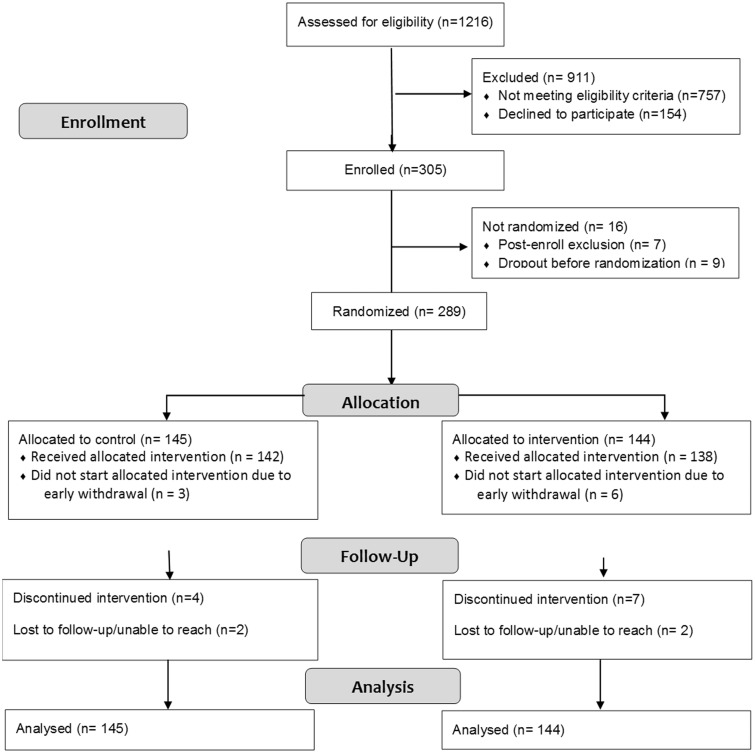
Participant Flow
Figure 1 shows the flow of participants from screening through follow-up. Of the 305 enrolled patients, 16 were not randomized either because of the study team determining after consent that the patient did not meet full eligibility criteria (n = 7) or because of the participant not fully completing baseline procedures (n = 9). Of the 289 randomized patients, 145 were allocated to the control group and 144 to the treatment group. Nine enrolled patients (three control/six treatment) never started the treatment phase because they could not be reached after baseline assessment (n = 1) or later decided they did not have enough time to do the study (n = 8). Eleven patients (four control/seven treatment) discontinued group procedures during the treatment phase because of time constraints, and four patients (two in each group) were lost to follow-up. Intention to treat procedures were used for data analyses.
Figure 1.
CONSORT flow diagram for participants in the trial.
Analyses
Sample Size Determination
For a priori sample size determination, model assumptions derived from pilot work (Stinson et al., 2010) were entered into Optimal Design Software (Liu, Spybrook, Martinez, & Raudenbush, 2009), with the resulting power curve showing that 288 participants (144 in each group) were the minimal sample size necessary to attain power of .80 for detecting a minimally important difference in outcomes of at least .35 standardized units.
Descriptive and Preliminary Analyses
Descriptive statistics were used to summarize the demographic characteristics of the sample and to estimate central tendency and variability of the main study variables. Chi-square analysis was used to evaluate the association of adverse events with study condition and the association of adherence with study condition. SPSS (version 23.0) software was used for conducting these analyses.
Analyses of Changes in Primary and Process Outcomes
Conditional multilevel growth models specified in HLM 7.0 (Raudenbush, Bryk, & Congdon, 2016) were used for the main analyses in this study and specified to estimate two effects of primary interest: (a) a time intercept coefficient, representing the average monthly rate of change since baseline through end of study in the outcome variables regardless of group assignment, and (b) a group × time slope coefficient, representing the relative increase or decrease to the average monthly rate of change associated with being in the treatment condition (Hesser, 2015; Singer & Willett, 2003). These regression coefficient estimates and their standard errors were compared against a t-sampling distribution for testing statistical significance (p < .05). Potential moderators of changes in outcomes entered as covariates in these models included age (at baseline), sex, disease subtype, baseline disease severity, medication type(s), and ethnicity (Hispanic/non-Hispanic). Effect size (ES) estimates (Cohen’s d equivalents) and their 95% confidence intervals were calculated using equations from Feingold (2009), with values of .2, .5, and .8 corresponding to small, medium, and large ESs, respectively.
Predictors of Change in Pain and HRQOL
To evaluate predictors of the amount of change since start of treatment in pain and HRQOL, Bayes estimates derived from growth models were computed to represent each individual’s estimated change (“growth”) over the study period in each of the process variables. These estimates then were specified in multilevel growth models as predictors (slope coefficients) of the extent to which pain intensity, pain interference, and HRQOL changed over the study period (time intercept coefficients).
Results
Descriptive and Preliminary Analyses
Table I shows a description of the analyzed study sample as a function of group. Groups did not significantly differ on measured demographic or clinical variables. Median combined annual family income for the sample was $80,000. Thirty-nine participants (13%) identified as Hispanic/Latino, of which 51% reported Spanish being their primary language.
Table I.
Demographic and Baseline Clinical Characteristics for Patients in the Study Sample
| Characteristic | Control group (n = 145) | Treatment group (n = 144) |
|---|---|---|
| Age (M ± SD) | 14.5 ± 1.7 | 14.6 ± 1.8 |
| Sex (n, %) | ||
| Male | 34 (23%) | 46 (32%) |
| Female | 111 (77%) | 98 (68%) |
| Race (n, %) | ||
| White | 126 (87%) | 122 (85%) |
| American Indian or Alaskan Native | 1 (1%) | 3 (2%) |
| Asian | 5 (3%) | 2 (1%) |
| Black or African American | 3 (2%) | 7 (5%) |
| Native Hawaiian or Other Pacific Islander | 0 (0%) | 0 (0%) |
| Mixed | 10 (7%) | 10 (7%) |
| Ethnicity (n, %) | ||
| Not Hispanic or Latino | 128 (88%) | 122 (85%) |
| Hispanic or Latino | 17 (12%) | 22 (15%) |
| Disease Subtype (n, %) | ||
| Oligoarticular (extended or persistent) | 29 (20%) | 31 (22%) |
| Polyarticular (RF−, RF+, or RF unknown) | 62 (43%) | 68 (47%) |
| Other (enthesitis-related JIA, psoriatic, systemic, and undifferentiated) | 54 (37%) | 45 (31%) |
| Medications (%) | ||
| NSAID | 55 (38%) | 49 (34%) |
| Non-biologic DMARD | 81 (56%) | 66 (46%) |
| Biologic response modifier | 67 (46%) | 66 (46%) |
| Corticosteroid | 10 (7%) | 6 (4%) |
| Physician-rated disease severity (n, %) | ||
| Mild | 118 (81%) | 118 (82%) |
| Moderate or severe | 27 (19%) | 26 (18%) |
Note. There were no significant group differences on any of the baseline demographic and clinical characteristics shown in this table. NSAID = non-steroidal anti-inflammatory drug; DMARD = disease-modifying anti-rheumatic drug.
Approximately three quarters (73%) of the sample met the minimum threshold for adherence (completed at least two of three health coach calls and logged in to view at least 75% of the Web modules). A higher proportion of patients in the control condition met the minimum criteria for being considered adherent (82% vs. 64%, χ2(1, N = 289) = 12.12, p < .01).
Seventy-two participants experienced an adverse event during the study period, and nine participants experienced a serious adverse event (hospitalization). The most common adverse events were infections (N = 18) and arthritis flares (N = 17). Four participants (three treatments and one control) reported suicidal thoughts at some point during the study. There was no association between study group and occurrence of adverse events, χ2 (1, N = 289) = .18, p = .67.
Changes in Primary Outcomes
Table II shows the Ms and SDs of the primary outcome variables as a function of group and assessment time point. Table III displays the unadjusted results of multilevel growth model analyses evaluating the significance of time and group by time effects for the primary outcomes. Participants on average demonstrated statistically significant linear improvements in pain intensity, pain interference, and HRQOL from baseline through the end of follow-up (ES = .21, .23, and .31, respectively). The group by time effects for the pain and HRQOL outcome variables was not significant, indicating that on average participants in both groups were comparable in their improvements in pain and HRQOL. Group improvements in the pain and HRQOL outcomes were not significantly different as a function of any of the covariates examined (age, sex, disease subtype, disease severity, medications being used for treating JIA, and ethnicity). Given the aforementioned group difference in participants’ adherence, a group by adherence interaction term also was explored as a predictor of changes in pain and HQROL outcomes; the interaction term was not significant, indicating that level of adherence was not associated with the amount of changes observed in the primary outcomes.
Table II.
Descriptive Statistics for Outcome and Process Variables as a Function of Group and Assessment Time Point
| Variable | Baseline |
Posttreatment |
6-month follow-up |
12-month follow-up |
||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | |
| Primary outcomes | ||||||||
| Pain intensity NRS | 3.6 ± 2.3 | 3.3 ± 2.4 | 3.1 ± 2.5 | 2.9 ± 2.5 | 2.9 ± 2.5 | 3.0 ± 2.3 | 3.1 ± 2.5 | 2.7 ± 2.4 |
| (0–9) | (0–9) | (0–9) | (0–10) | (0–8) | (0–8) | (0–10) | (0–9) | |
| Pain interference NRS | 2.6 ± 2.3 | 2.5 ± 2.3 | 2.2 ± 2.4 | 1.7 ± 2.2 | 2.20 ± 2.2 | 1.8 ± 2.0 | 2.0 ± 2.2 | 1.9 ± 2.2 |
| (0–9.8) | (0–9.4) | (0–9.6) | (0–9.4) | (0–8.6) | (0–10) | (0–8.6) | (0–7.4) | |
| PedsQL rheumatology | 72.6 ± 15.6 | 72.4 ± 15.8 | 75.7 ± 16.2 | 77.8 ± 16.2 | 77.3 ± 15.6 | 77.1 ± 14.4 | 78.3 ± 16.2 | 78.0 ± 14.3 |
| (34.1–100) | (17.0–98.9) | (34.1–100) | (34.1–100) | (43.2–100) | (37.5–100) | (42.0–100) | (43.2–100) | |
| Process variables | ||||||||
| CASE self-efficacy | 3.3 ± 1.0 | 3.3 ± 1.0 | 3.8 ± 1.0 | 3.7 ± .9 | 3.8 ± 1.0 | 3.7 ± .9 | 3.8 ± 1.0 | 3.8 ± 1.0 |
| (1.0–5.0) | (1.1–5.0) | (1.8–5.0) | (1.0–5.0) | (1.4–5.0) | (1.0–5.0) | (1.0–5.0) | (1.0–5.0) | |
| PCQ—approach coping | 2.6 ± .7 | 2.6 ± .7 | 2.8 ± .9 | 2.7 ± .8 | 2.8 ± .9 | 2.5 ± .9 | 3.8 ± 1.0 | 2.5 ± .9 |
| (1.0–5.0) | (1.0–4.7) | (1.0–4.6) | (1.0–5.0) | (1.0–5.0) | (1.0–4.9) | (1.0–5.0) | (1.0–5.0) | |
| PCQ—emotion-focused avoidance coping | 2.1 ± .8 | 2.0 ± .8 | 2.0 ± .8 | 1.9 ± .8 | 1.9 ± .8 | 1.9 ± .8 | 2.0 ± .9 | 1.9 ± .8 |
| (1.0–4.5) | (1.0–5.0) | (1.0–5.0) | (1.0–4.5) | (1.0–4.5) | (1.0–4.5) | (1.0–5.0) | (1.0–5.0) | |
| PROMIS pediatric anxiety | 48.6 ± 11.8 | 47.6 ± 10.7 | 46.8 ± 11.3 | 45.5 ± 11.0 | 45.1 ± 12.1 | 46.0 ± 10.8 | 45.3 ± 12.0 | 46.0 ± 11.4 |
| (33.5–71.8) | (33.5–81.1) | (33.5–70.6) | (33.5–70.6) | (33.5–76.0) | (33.5–83.3) | (33.5–70.6) | (33.5–79.3) | |
| PROMIS pediatric depression | 47.2 ± 11.6 | 46.5 ± 11.7 | 46.4 ± 11.2 | 45.2 ± 12.1 | 45.6 ± 11.2 | 45.1 ± 11.4 | 45.5 ± 11.0 | 45.0 ± 11.4 |
| (35.2–82.4) | (35.2–82.4) | (35.2–82.4) | (35.2–76.5) | (35.2–82.4) | (35.2–82.4) | (35.2–82.4) | (35.2–79.9) | |
| MEPS disease knowledge | 4.8 ± 2.0 | 4.6 ± 2.3 | 6.3 ± 2.0 | 6.5 ± 2.3 | 6.6 ± 2.4 | 6.4 ± 2.5 | 6.5 ± 2.3 | 6.6 ± 2.6 |
| (0.5–10) | (0.4–10) | (0–10) | (0–10) | (0–10) | (0–10) | (0–10) | (0–10) | |
Note. Values shown are M values ± SDs (score range). Treatment = Teens Taking Charge online self-management condition; control = online disease education condition. CASE = Children’s Arthritis Self-Efficacy Scale; HRQOL = health-related quality of life; MEPS = Medical Issues, Exercise, Pain and Social Support Questionnaire; NRS = Numeric Rating Scale; PCQ = Pain Coping Questionnaire; PedsQL = Pediatric Quality of Life Scale; PROMIS = Patient-Reported Outcomes Measurement Information System.
Table III.
Unadjusted Results of Multilevel Growth Modeling Predicting the Primary and Process Outcomes From Time Since Randomization and Group Assignment
| Time effect |
Group × time effect |
|||||||
|---|---|---|---|---|---|---|---|---|
| b ± SE | β | t | ES (95% CI) | b ± SE | β | t | ES (95% CI) | |
| Primary outcomes | ||||||||
| Pain intensity NRS | −.04 ± .01 | −.08 | −3.47* | −.19/−.24 | −.01 ± .02 | −.02 | −.67 | −.09/−.01 |
| Pain interference NRS | −.04 ± .01 | −.09 | −3.99* | −.21/−.25 | −.00 ± .02 | −.01 | −.19 | −.09/−.02 |
| PedsQL rheumatology | .37 ± .05 | .13 | 7.27* | .21/.41 | .06 ± .10 | .01 | .55 | −.16/.24 |
| Process variables | ||||||||
| CASE self-efficacy | .03 ± .01 | .17 | 7.67* | .38/.40 | −.01 ± .01 | −.01 | −.44 | −.15/−.12 |
| PCQ approach coping | .00 ± .01 | .00 | .05 | .14/.16 | .01 ± .01 | .01 | .69 | .14/.16 |
| PCQ emotion-focused avoidance coping | −.01 ± .01 | −.04 | −1.60 | −.15/−.17 | .01 ± .01 | .02 | .94 | .15/.17 |
| PROMIS anxiety | −.18 ± .04 | −.07 | −3.95* | −.12/−.29 | −.13 ± .09 | −.03 | −1.43 | −.33/.03 |
| PROMIS depression | −.12 ± .05 | −.05 | −2.56* | −.05/−.23 | −.02 ± .09 | −.01 | −.24 | −.20/.16 |
| MEPS disease knowledge | .12 ± .01 | .25 | 11.43* | .60/.66 | −.01 ± .02 | −.01 | −.45 | −.09/−.01 |
Notes. *p < .05. The b-coefficient for the time effect indicates the estimated average monthly raw unit change in the given outcome variable since before starting group procedures (baseline); a significant time effect indicates that on average (across all participants) there was a significant increase or decrease in the outcome variable over months since starting group procedures. The b-coefficient for the group × time effect indicates the extent to which being in the treatment condition, relative to the control condition, increased or decreased the average monthly raw unit change on the given variable. The β-values for the time effect and group × time effect are interpreted similarly as the b-coefficients but in SD units instead of raw units. ESs are shown as the lower and upper values corresponding to the 95% CIl for the ES estimate. CI = confidence interval; ES = effect size; NRS = Numeric Rating Scale; CASE = Children’s Arthritis Self-Efficacy Scale; HRQOL = health-related quality of life; MEPS = Medical Issues, Exercise, Pain and Social Support Questionnaire; NRS = Numeric Rating Scale; PCQ = Pain Coping Questionnaire; PedsQL = Pediatric Quality of Life; PROMIS = Patient-Reported Outcomes Measurement Information System.
Changes in Process Outcomes
Tables II and III also show descriptive statistics and results of multilevel growth model analyses, respectively, for the process variables. Over the study period, disease knowledge and self-efficacy on average significantly increased (ES = .63 and .39, respectively) and indicators of anxiety and depression significantly decreased (ES = −.21 and −.14, respectively). There was a nonsignificant time trend (p = .10, ES = −.16) for reduced emotion-focused avoidance coping; there was no reliable change for approach coping. There was no significant group by time interaction effects, indicating that improvements in the process outcomes were comparable regardless of group assignment. The magnitude of group changes in process outcomes did not significantly differ as a function of the examined covariates.
Process Variables as Predictors of Change in Primary Outcomes
Table IV shows the results of multilevel analyses evaluating the extent to which the process variables predicted changes in the pain outcomes and HRQOL. On average, participants who reported greater increases in self-efficacy and greater decreases in avoidance coping, anxiety, and depression reliably had more improvement in pain intensity and interference. Similarly, participants who reported greater increases in disease knowledge and self-efficacy, and greater decreases in avoidance coping and symptoms of anxiety and depression, demonstrated significantly greater improvements in HRQOL. These results did not change when analyzing the data for the treatment and control groups independently.
Table IV.
Results of Multilevel Growth Modeling Evaluating Predictors of Change in the Primary Outcome Variables of Pain and HRQOL
| Predictors | Outcomes |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pain intensity |
Pain interference |
HRQOL |
|||||||
| b ± SE | β | t | b ± SE | β | t | b ± SE | β | t | |
| CASE self-efficacy | −3.11 ± .82 | −.08 | −3.80* | −3.26 ± .71 | −.10 | −4.61* | 16.26 ± 3.70 | .10 | 4.40* |
| PCQ approach coping | 1.04 ± .56 | .04 | 1.83 | .57 ± .45 | .03 | 1.25 | −1.48 ± 2.36 | .00 | .53 |
| PCQ emotion-focused avoidance coping | 1.10 ± .33 | .06 | 3.33* | 1.00 ± .29 | .06 | 3.42* | −9.12 ± 2.15 | −.08 | −4.23* |
| PROMIS anxiety scale | 1.46 ± .42 | .09 | 3.47* | 1.46 ± .47 | .10 | 3.10* | −16.42 ± 2.94 | −.13 | −5.57* |
| PROMIS depression scale | .67 ± .38 | .08 | 1.76 | .91 ± .33 | .10 | 2.69* | −9.52 ± 1.90 | −.10 | −4.88* |
| MEPS disease knowledge | .46 ± .24 | .03 | 1.94 | .24 ± .21 | .02 | 1.14 | 3.44 ± 1.30 | .04 | 2.65* |
Notes. * p < .05. Positive b-values indicate the amount of increase expected in the given outcome variable for each raw unit increase (since preintervention) in the given predictor variable; positive β-values indicate the same but in SD units rather than raw units. Negative b-values indicate the expected amount of reduction in the given outcome variable for each raw unit increase (since preintervention) in the given predictor variable; negative β-values indicate the same but in standard deviation units rather than raw units. Pain intensity and pain interference were measured on numeric rating scales with a potential range from 0 to 10, with higher scores indicating greater pain intensity and pain interference. HRQOL (average item score from PedsQL Rheumatology Module) was measured on a scale ranging from 0 to 100, with higher scores indicating better HRQOL. CASE = Children’s Arthritis Self-Efficacy Scale; HRQOL = health-related quality of life; MEPS = Medical Issues, Exercise, Pain and Social Support Questionnaire; PCQ = Pain Coping Questionnaire; PROMIS = Patient-Reported Outcomes Measurement Information System.
Discussion
This study sought to determine through a definitive trial whether an online JIA self-management program would result in greater improvements in pain and HRQOL over a 12-month time span compared with accessing additional disease education online. Contrary to hypotheses, results indicated that participants in both study groups on average had comparable and statistically significant improvements in pain and HRQOL over the course of the study, with no significant between-group differences in these changes. Effect size estimates associated with improvements in these outcomes were small. Both groups on average also reported comparable improvements in variables that predicted the extent of improvement in pain and HRQOL, including self-efficacy, anxiety and depression symptoms, and disease knowledge. Average changes in pain and HRQOL observed over the study period were not modified by patient age, sex, disease severity, medications used to treat JIA, or ethnicity.
Prior preliminary studies have demonstrated benefit of in-person or Web-based cognitive–behavioral interventions for improving pain and (less reliably) functional ability in youth with painful conditions (Fisher et al., 2015) and specifically in youth with JIA (Lomholt, Thastum, Christensen, Leegaard, & Herlin, 2015; Stinson et al., 2010). The current study extends this work by evaluating outcomes of a primarily self-directed online self-management program for youth with JIA in a large representative sample, over a relatively long follow-up period, and against a more “active” control arm than the wait-list or standard care comparators often used in other studies. This latter feature of the current trial may be particularly relevant for explaining why between-group differences in outcomes were not found for the current trial but had been observed for some outcomes (including pain intensity) in a pilot wait-list-controlled study (Stinson et al., 2010). In a meta-analytic review of internet-delivered cognitive–behavioral interventions for pediatric conditions, Vigerland and colleagues (2016) found that between-group differences in outcomes only were observed if studies used a wait-list control and were not observed in the few studies that included an “active” comparator condition. Thus, the observed lack of between-group differences in the amount of improvement in primary outcomes in the current trial may have important implications for future eHealth work and consideration of resources. In particular, for some patient groups, it may be that adding a layer of support and guided disease education using quality online resources to regular patient care is sufficient on average to positively impact important patient outcomes; use of education control comparison groups thus might best be considered early on in the development and testing of eHealth interventions.
Alternatively, it may be that between-group differences in outcomes, or greater magnitude of changes in outcomes, would have been observed had we supplemented the online programs with more frequent human contact or therapist guidance. The current trial intentionally evaluated the online intervention with minimal provider contact to facilitate future reproducibility in routine practice settings and in fact used 75% less frequent human (phone) contact than was used in a pilot test of the intervention (Stinson et al., 2010). It may be that supplementing the online self-management intervention with regular contact with a pediatric psychologist or other provider would result in greater adherence, larger treatment effects, and/or a greater distinction in outcomes from only accessing general educational information about JIA online. This remains speculative given the wide variability in the amount and means of provider contact among Internet intervention studies (Vigerland et al., 2016), lack of consistent reporting and definitions of adherence for these types of studies, and no known studies of Internet interventions for pediatric conditions that systematically vary therapist contact. One study with JIA patients did demonstrate improved self-efficacy, a variable found in the current study to predict amount of change in pain and HRQOL, from weekly video calls with peer (young adult) mentors (Stinson et al., 2016); combining a peer video call program with the online resources evaluated in the current study may be a novel way to enhance treatment effects.
Another possibility for the similar improvements in outcomes across both study groups in the current trial, in the context of no other known systematic change to treatment or health status for participants over the study period, is that there are common variables that changed with the online approaches used in both groups to engender the observed outcomes. Identifying and exploiting such common factors therefore have the potential to optimize pain and HRQOL outcomes when providing care to youth with JIA and may also be informative when designing pediatric eHealth interventions. Extending from a behavior change model for Internet interventions (Ritterband, Thorndike, Cox, Kovatchev, & Gonder-Frederick, 2009), potential relevant nonspecific factors associated with outcomes in the current study could be the added layer of support received from calls with a health coach, the structure and guidance provided for accessing online content, the “contracted” (consented) commitment to gain additional knowledge about JIA online, and quality information provided to patients from reputable sources. These aspects of treatment common to both study conditions may have helped improve patients’ self-efficacy and helped optimize emotional functioning, which in turn were found in this study to partly predict the average magnitude of change in pain and HRQOL.
Although no between-group differences in outcomes were found in the current trial, and effect sizes for changes in primary outcomes were small, improvements observed over the study period for both groups may still have important implications for clinical care. Youth with JIA regard pain and HRQOL impairments to be among the highest priority clinical problems associated with the disease (Guzman et al., 2014). These outcomes also are difficult to improve with appropriate medical treatment alone (Bromberg et al., 2014; Wipff et al., 2016). Distance, scheduling, language, and other barriers also can make it difficult for youth with JIA to regularly access services with a pediatric psychologist as an adjunct to medical care. Thus, the finding of a reliable degree of even modest benefit for pain and HRQOL of mostly self-directed viewing of publicly available quality online disease education resources or custom-built online self-management modules has relevance for patient care. These resources can be easily accessed regardless of patient location and may have long-term dividends not directly measured in the current study, such as improved transition to adult care and reduced disease burden into adulthood.
There are several study limitations that should be considered when interpreting results. The sample for this study may not be representative of the general population of youth with JIA in that participants were agreeable to a study involving an online intervention. Positive expectancy effects for those agreeing to be in the current trial may have contributed to general improvement in many of the subjective outcomes examined. Indeed, recent studies have suggested that expectancy effects associated with trial participation can produce a large analgesic response that can match “active” treatments (Tuttle et al., 2015). The study sample, though on average comparable in demographics and characteristics to other samples of youth with JIA, also largely was comprised of youth having mild disease severity at baseline, and we did not restrict participation to only those meeting a specific threshold for pain or HRQOL impairment; this may have diluted treatment effects at the expense of increasing generalizability. Adherence also was moderate overall and inferred based on indirect and imperfect measures; for example, it is unclear the extent to which self-management skills learned were actively applied by treatment participants. However, it also is unclear how to define “optimal” adherence for online interventions; one potential benefit of this format is that patients can largely self-direct information they access based on perceived personal relevance. Although adherence in the context of internet interventions rarely is reported for pediatric samples and varies widely in definition (Vigerland et al., 2016), studies that include more frequent personal contact with a support person tend to report better adherence and treatment effects (Stinson et al., 2010). Future studies should consider directly exploring the optimal “dose” of therapist contact that maximizes benefits for eHealth interventions, and whether this varies as a function of patient demographic or clinical characteristics.
Overall, study findings support a modest benefit for pain and HRQOL of supplementing the medical care of patients with JIA with additional quality online disease education or a more formalized online self-management program. Additional research is needed to determine which patients may benefit most from one approach or the other (or neither), to determine effectiveness in the context of regular care, to determine criteria for adherence and adherence promotion strategies that best optimize outcomes, and to determine other treatment features that augment the effectiveness of online self-management interventions for youth with chronic conditions.
Funding
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant number R01AR061513). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no financial conflicts of interest.
Conflicts of interest: None declared.
References
- André M., Hedengren E., Hagelberg S., StenströM C. H. (1999). Perceived ability to manage juvenile chronic arthritis among adolescents and parents: Development of a questionnaire to assess medical issues, exercise, pain, and social support. Arthritis Care and Research, 12, 229–237. [PubMed] [Google Scholar]
- Barlow J., Shaw K., Wright C. (2001). Development and preliminary validation of a Children’s Arthritis Self‐Efficacy Scale. Arthritis and Rheumatism, 45, 159–166. [DOI] [PubMed] [Google Scholar]
- Bromberg M., Connelly M., Anthony K., Gil K., Schanberg L. (2014). Self-reported pain and disease symptoms persist in Juvenile Idiopathic Arthritis despite treatment advances: An electronic diary study. Arthritis and Rheumatology, 66, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold A. (2009). Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychological Methods, 14, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher E., Law E., Palermo T., Eccleston C. (2015). Psychological therapies (remotely delivered) for the management of chronic and recurrent pain in children and adolescents. The Cochrane Database of Systematic Reviews, 3, CD011118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Suárez R., Pistorio A., Cespedes Cruz A., Norambuena X., Flato B., Rumba I., Harjacek M., Nielsen S., Susic G., Mihaylova D., Huemer C., Melo-Gomes J., Andersson-Gare B., Balogh Z., De Cunto C., Vesely R., Pagava K., Romicka A. M., Burgos-Vargas R., Martini A., Ruperto N. (2007). Health-related quality of life of patients with juvenile idiopathic arthritis coming from 3 different geographic areas. The PRINTO multinational quality of life cohort study. Rheumatology, 46, 314–320. [DOI] [PubMed] [Google Scholar]
- Guzman J., Gómez-Ramírez O., Jurencak R., Shiff N. J., Berard R. A., Duffy C. M., Tucker L. B. (2014). What matters most for patients, parents, and clinicians in the course of juvenile idiopathic arthritis? A qualitative study. Journal of Rheumatology, 41(11), 2260–2269. [DOI] [PubMed] [Google Scholar]
- Haverman L., Grootenhuis M. A., van den Berg J. M., van Veenendaal M., Dolman K. M., Swart J. F., Kuijpers T. W., van Rossum M. A. J. (2012). Predictors of health-related quality of life in children and adolescents with juvenile idiopathic arthritis: Results from a web-based survey. Arthritis Care and Research, 64, 694–703. [DOI] [PubMed] [Google Scholar]
- Hesser H. (2015). Modeling individual differences in randomized experiments using growth models: Recommendations for design, statistical analysis and reporting of results of internet interventions. Internet Interventions, 2, 110–120. [Google Scholar]
- Irwin D. E., Stucky B., Langer M. M., Thissen D., DeWitt E. M., Lai J.-S., Varni J. W., Yeatts K., DeWalt D. A. (2010). An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Quality of Life Research, 19, 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M. (2010). A neuropsychological model of pain: Research and clinical implications. The Journal of Pain, 11, 2–12. [DOI] [PubMed] [Google Scholar]
- Liu X., Spybrook J., Congdon R., Martinez A., Raudenbush S. W. (2009). Optimal Design software for multi-level and longitudinal research (Version 2.0) [Computer software]. [Google Scholar]
- Lomholt J., Thastum M., Christensen A., Leegaard A., Herlin T. (2015). Cognitive behavioral group intervention for pain and well-being in children with juvenile idiopathic arthritis: A study of feasibility and preliminary efficacy. Pediatric Rheumatology, 13, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorig K. R., Holman H. R. (2003). Self-management education: History, definition, outcomes, and mechanisms. Annals of Behavioral Medicine, 26, 1–7. [DOI] [PubMed] [Google Scholar]
- Palermo T., Eccleston C., Lewandowski A., Williams A. C., Morley S. (2010). Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: An updated meta-analytic review. Pain, 148, 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G., Gilbert C., McGrath P. (1998). The pain coping questionnaire: Preliminary validation. Pain, 76, 83–96. [DOI] [PubMed] [Google Scholar]
- Rashid A., Cordingley L., Carrasco R., Foster H. E., Baildam E. M., Chieng A., Davidson J. E., Wedderburn L. R., Ioannou Y., McErlane F., Verstappen S. M. M., Hyrich K. L., Thomson W. (2018). Patterns of pain over time among children with juvenile idiopathic arthritis. Archives of Disease in Childhood, 103, 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S. W., Bryk A. S., Congdon R. (2016). HLM 7.02 for Windows [computer software]. Skokie, IL: Scientific Software International, Inc. [Google Scholar]
- Ritterband L. M., Thorndike F. P., Cox D. J., Kovatchev B. P., Gonder-Frederick L. A. (2009). A behavior change model for internet interventions. Annals of Behavioral Medicine, 38, 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J., Willett J. (2003). Applied longitudinal data analysis. Oxford: Oxford University Press. [Google Scholar]
- Slater H., Jordan J., Chua J., Schütze R., Wark J., Briggs A. (2016). Young people’s experiences of persistent musculoskeletal pain, needs, gaps and perceptions about the role of digital technologies to support their co-care: A qualitative study. BMJ Open, 6, e014007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson J., Ahola Kohut S., Forgeron P., Amaria K., Bell M., Kaufman M., Luca N., Luca S., Harris L., Victor C., Spiegel L. (2016). The iPeer2Peer Program: A pilot randomized controlled trial in adolescents with Juvenile Idiopathic Arthritis. Pediatric Rheumatology, 14, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson J. N., McGRATH P. J., Hodnett E. D., Feldman B. M., Duffy C. M., Huber A. M., Tucker L. B., Hetherington C. R., Tse S. M., Spiegel L. R., Campillo S., Gill N. K., White M. E. (2010). An internet-based self-management program with telephone support for adolescents with arthritis: A pilot randomized controlled trial. The Journal of Rheumatology, 37, 1944–1952. [DOI] [PubMed] [Google Scholar]
- Stinson J. N., Stevens B. J., Feldman B. M., Streiner D., McGrath P. J., Dupuis A., Gill N., Petroz G. C. (2008). Construct validity of a multidimensional electronic pain diary for adolescents with arthritis. Pain, 136, 281–292. [DOI] [PubMed] [Google Scholar]
- Stinson J. N., Toomey P. C., Stevens B. J., Kagan S., Duffy C. M., Huber A., Malleson P., McGrath P. J., Yeung R. S., Feldman B. M. (2008). Asking the experts: Exploring the self-management needs of adolescents with arthritis. Arthritis and Rheumatism, 59, 65–72. [DOI] [PubMed] [Google Scholar]
- Stinson J. N., Tucker L., Huber A., Harris H., Lin C., Cohen L., Gill N., Lukas-Bretzler J., Proulx L., Prowten D. (2009). Surfing for Juvenile Idiopathic Arthritis: Perspectives on quality and content of information on the internet. The Journal of Rheumatology, 36, 1755–1762. [DOI] [PubMed] [Google Scholar]
- Thastum M., Herlin T., Zachariae R. (2005). Relationship of pain-coping strategies and pain-specific beliefs to pain experience in children with juvenile idiopathic arthritis. Arthritis and Rheumatism, 53, 178–184. [DOI] [PubMed] [Google Scholar]
- Thierry S., Fautrel B., Lemelle I., Guillemin F. (2014). Prevalence and incidence of juvenile idiopathic arthritis: A systematic review. Joint Bone Spine, 81, 112–117. [DOI] [PubMed] [Google Scholar]
- Tong A., Jones J., Craig J. C., Singh-Grewal D. (2012). Children’s experiences of living with juvenile idiopathic arthritis: A thematic synthesis of qualitative studies. Arthritis Care and Research, 64, 1392–1404. [DOI] [PubMed] [Google Scholar]
- Tuttle A., Tohyama S., Ramsay T., Kimmelman J., Schweinhardt P., Bennett G., Mogil J. (2015). Increasing placebo responses over time in U.S. clinical trials of neuropathic pain. PAIN, 156, 2616–2626. [DOI] [PubMed] [Google Scholar]
- van Dijkhuizen E. H. P., Egert T., Egert Y., Costello W., Schoemaker C., Fernhout M., Kepic M., Martini A., Scala S., Rotstein-Grein I., Vastert S. J., Wulffraat N. M. (2018). Patient’s experiences with the care for juvenile idiopathic arthritis across Europe. Pediatric Rheumatology, 16, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varni J., Seid M., Smith Knight T., Burwinkle T., Brown J., Szer I. (2002). The PedsQL™ in pediatric rheumatology: Reliability, validity, and responsiveness of the Pediatric Quality of Life Inventory™ generic core scales and rheumatology module. Arthritis and Rheumatism, 46, 714–725. [DOI] [PubMed] [Google Scholar]
- Vigerland S., Lenhard F., Bonnert M., Lalouni M., Hedman E., Ahlen J., Olén O., Serlachius E., Ljótsson B. (2016). Internet-delivered cognitive behavior therapy for children and adolescents: A systematic review and meta-analysis. Clinical Psychology Review, 50, 1–10. [DOI] [PubMed] [Google Scholar]
- Weitzman E. R., Wisk L. E., Salimian P. K., Magane K. M., Dedeoglu F., Hersh A. O., Kimura Y., Mandl K. D., Ringold S., Natter M. (2017). Adding patient-reported outcomes to a multisite registry to quantify quality of life and experiences of disease and treatment for youth with juvenile idiopathic arthritis. Journal of Patient Reported Outcomes, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff J., Sparsa L., Lohse A., Quartier P., Kahan A., Deslandre C. (2016). Impact of juvenile idiopathic arthritis on quality of life during transition period at the era of biotherapies. Joint Bone Spine, 83, 69–74. [DOI] [PubMed] [Google Scholar]