Abstract
Purpose
Quantitative susceptibility mapping (QSM) is influenced by iron as well as myelin, which makes interpretation of pathologic changes challenging. Concurrent acquisition of MR sequences that are sensitive to axonal/myelin integrity, such as diffusion tensor imaging (DTI), may provide context for interpreting quantitative susceptibility (QS) signal. The purpose of our study was to investigate alterations in normal-appearing white matter (NAWM) in multiple sclerosis (MS) using QSM in conjunction with DTI.
Methods
Twenty relapsing–remitting MS patients and 20 age-matched healthy controls (HC) were recruited for this prospective study. QS, radial diffusivity (RD), fractional anisotropy (FA), and R2* maps within the whole brain as well as individual tracts were generated for comparison between NAWM and HC white matter (HCWM).
Results
MS lesions demonstrated significant differences in QS, FA, RD, and R2* compared to HCWM (p < 0.03). These metrics did not show a significant difference between whole-brain NAWM and HCWM. Among NAWM tracts, the cingulate gyri demonstrated significantly decreased QS compared to HCWM (p = 0.004). The forceps major showed significant differences in FA and RD without corresponding changes in QS (p < 0.01).
Conclusion
We found discordant changes in QSM and DTI metrics within the cingulate gyri and forceps major. This may potentially reflect the influence of paramagnetic substrates such as iron, which could be decreased along these NAWM tracts. Our results point to the potential role of QSM as a unique biomarker, although additional validation studies are needed.
Keywords: Quantitative susceptibility mapping, Multiple sclerosis, Diffusion tensor imaging, Normal-appearing white matter
Introduction
Conventional MR imaging has a high sensitivity for detecting focal demyelinating lesions in multiple sclerosis (MS) and plays an important role in diagnosis as per the 2010 revised McDonald Criteria [1]. Early studies in MS focused on associating disease severity with the spatiotemporal dissemination of these lesions but yielded limited correlations. The absence of an imaging biomarker specific for disability progression in MS has led to an interest in looking beyond focal white matter lesions, including the normal-appearing white matter (NAWM). However, unlike MS lesions, pathologic alterations in NAWM, including diffuse axonal damage and changes in myelination, are largely occult on conventional MR sequences [2, 3].
To this end, MRI techniques for characterizing myelin and axonal damage have been developed to interrogate these alterations in vivo [4–6]. Quantitative susceptibility mapping (QSM) is a novel MRI method that has drawn interest as a potential imaging biomarker in MS [7–10]. In contrast to diffusion-weighted imaging, quantitative magnetization transfer, and T2-relaxometry, which are influenced by changes in axonal and myelin integrity, quantitative susceptibility (QS) as characterized by QSM is thought to be predominantly influenced by iron and myelin content within human white matter [11–13].
Previous studies have shown that white matter QS increases with decreasing diamagnetic myelin content resulting from “unmasking” of the underlying tissue QS [14]. Tissue iron also affects susceptibility, although in contrast to myelin, decreasing iron content will lead to decreases in QS [7, 14, 15]. As both myelin and iron contents are perturbed in MS, interpreting the biological basis for QS signal presents a challenge. Histologic correlation has been proposed for resolving this dilemma but is hindered by the logistical barriers associated with tissue sampling, particularly over multiple time points [16]. Alternatively, concurrent acquisition of MR sequences that differ in their sensitivities to myelin and tissue susceptibility could provide a surrogate to interpreting QS in vivo. For example, diffusion tensor imaging (DTI) metrics, such as fractional anisotropy (FA) and radial diffusivity (RD), are sensitive to axonal/myelin integrity but comparatively insensitive to tissue susceptibility. In the setting of increased QS, relative preservation of DTI metrics (such as RD which is thought to be more specific for myelin) would suggest an increase in iron content [17, 18]. The transverse relaxation rate of GRE sequences, R2*, is also affected by variations in myelin and iron concentrations in the brain [19]. However, in contrast to QS, decreases in myelin and iron would both decrease R2*.
There have been no published studies to date that have performed a multiparametric evaluation using QSM, DTI, and R2* in MS patients focusing on NAWM. The purpose of this study was (1) to compare the QS of focal lesions and NAWM in MS to the white matter of healthy subjects and (2) to correlate the QS of NAWM with FA, RD, and R2* over the whole brain as well as along major fiber tracts.
Methods
Study participants
This prospective study was approved by the institutional review board at the University of Texas Health Science Center at San Antonio. Twenty patients with relapsing–remitting MS (mean age 36.2 ± 9.7 years, 17 females) and twenty healthy age-matched controls (HC) (mean age 36.8 ± 10.4 years, 7 females) were recruited consecutively from August 2014 through October 2016. Inclusion criteria for MS patients were age from 18 to 60 years and fulfillment of 2010 revised McDonald criteria for MS [1]. Exclusion criteria were evidence of other structural brain diseases, severe claustrophobia, or other contraindication to MRI. Demographics and clinical characteristics of MS patients and HC volunteers are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of study participants
| Healthy controls | MS | |
|---|---|---|
| Subjects | 20 | 20 |
| Sex ratio (M/F) | 13:7 | 3:17 |
| Age (years) mean ± SD [range] | 36.8 ± 10.4 [25–53] | 36.2 ± 9.7 [21–53] |
| Median EDSS [range] | N/A | 1.5 [1–7.5] |
| Disease duration (years) [range] | N/A | 6.9 ± 8.1 [1–25] |
Data are presented as mean ± standard deviation unless otherwise indicated
EDSS Expanded Disability Status Scale, MS multiple sclerosis
Imaging protocol
Brain imaging was performed using a 3-T MRI scanner (Achieva; Philips, Amsterdam, Netherlands) with an eight-channel head coil. A three-dimensional T2*-weighted multi-echo (5 echoes) gradient echo sequence was acquired with the following parameters: flip angle, 20°; first echo time, 4.2 ms; echo spacing, 4.8 ms; repetition time, 36.56 ms; field of view, 230 mm × 230 mm; matrix size, 256 × 256; slice thickness, 2 mm; SENSE factor, 2; and acquisition time, 4.25 min. Anatomic images were obtained using a 3D T1-weighted turbo field-echo sequence at 1-mm isotropic resolution. Diffusion-weighted images were acquired using a single-shot echo-planar imaging sequence with the following parameters: 16, directions; two b-values (0 and 800 s/mm2); echo time, 60.1 ms; repetition time, 9593 ms; field of view, 224 mm × 224 m; matrix size, 112 × 112; and slice thickness, 2 mm. High-resolution 3D turbo spin-echo FLAIR images were also acquired with 1-mm3 isotropic resolution. Additionally, 1-mm isotropic gadolinium-enhanced (intravenous gadobutrol [Bayer AG, Leverkusen, Germany]) 3D T1-weighted turbo field-echo sequences were acquired as part of the clinical protocol (acquisition time for research protocol 19.2 min; total acquisition time 29.5 min).
Image analysis
The phase data from the five echoes was unwrapped using Laplacian-based unwrapping [15]. The normalized phase was subsequently calculated as previously described [12]. Background phase removal was then performed using the variable kernel sophisticated harmonic artifact reduction for phase data method (V-SHARP), with a spherical mean radius increasing from 0.6 mm at the boundary of the brain to 25 mm towards the center of the brain [20, 21]. QSM images were generated from the processed phase images using the improved sparse linear equation and least-squares (iLSQR) algorithm [12]. R2* maps were generated using a least square mono-exponential fit of the GRE magnitude data from the five echoes.
The T1-weighted images were processed and segmented using FreeSurfer version 5.3.0 (http://surfer.nmr.mgh.harvard.edu) [22, 23]. Diffusion data was reviewed for significant geometric distortions, and preprocessing was performed with motion and eddy current correction using the eddy tool in FSL (www.fmrib.ox.ac.uk/fsl). DTI metrics including FA and RD maps were derived along white matter tracts in all MS patients and HCs using the Tracts Constrained by Underlying Anatomy (TRACULA) tool within FreeSurfer as previously described [24]. These tracts included the forceps major, forceps minor, bilateral anterior thalamic radiations (ATR), cingulum angular bundles (CAB), cingulate gyri endings (CCG), corticospinal tracts (CST), inferior longitudinal fasciculi (ILF), superior longitudinal fasciculi parietal and temporal endings (SLFP and SLFT), and uncinate fasciculi (UNC). The TRACULA-derived white matter tract masks were then binarized and thresholded to include only voxels above 15th percentile using the fslmaths tool (www.fmrib.ox.ac.uk/fsl). White matter tract orientation with respect to the main magnetic field (B0) was determined based on the direction of the main eigenvector from the DTI data.
The diffusion-weighted, multi-echo GRE and FLAIR images were registered to the T1w anatomic image using the boundary based registration tool in FreeSurfer. White matter lesion segmentation was performed using the lesion prediction algorithm as implemented in the Lesion Segmentation Toolbox (v. 2.0.13; www.statistical-modelling.de/lst.html) for SPM12 [25]. The generated lesion masks were then manually edited by an experienced neuroradiologist (F.Y., 7 years of experience) using the FLAIR images for reference. Masks of whole-brain cerebral white matter were created by combining the FreeSurfer segmentations of right and left hemispheric white matter and the corpus callosum. NAWM masks were generated by subtracting the lesion masks from the supratentorial cerebral hemispheric white matter masks. In order to compare tissue QS between individuals, the CSF QS within the lateral ventricular atrium was selected as an internal reference [26].
Comparisons of metrics derived from QSM and DTI in NAWM and lesions in the MS patient group was performed using the two-sample t test. Comparisons of NAWM and lesions to HC white matter (HCWM) metrics were performed using analysis of variance (ANOVA), adjusting for sex (due to the difference in sex ratios between MS and HC groups). The Mann–Whitney U test was used to compare subsets of MS patients with only enhancing lesions and non-enhancing lesions to healthy subjects, while the Wilcoxon signed rank test was used to compare these MS subsets to each other. Correlation between QS and different MRI parameters was performed using a two-sided Pearson correlation. A statistical threshold of P < 0.05, false discovery rate (FDR) correction, was used to address the issue of multiple comparisons. Statistical analyses were performed using the Statistical Package for the Social Sciences (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.).
Results
MRI metrics in whole-brain white matter
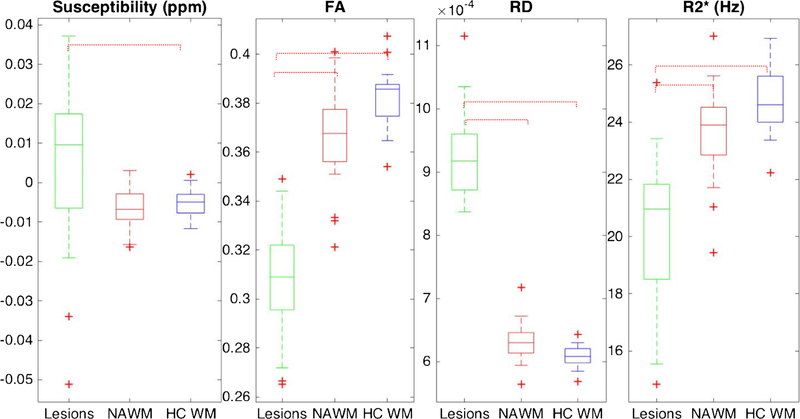
Figure 1 shows the QS and DTI parameters (FA, MD, RD, AD) within NAWM, MS lesions, and the white matter of healthy controls (HCWM) (Supplemental Materials - Table 1). There were a total of 367 lesions with an average of 18.35 ± 12 lesions per patient. Lesions showed significantly higher QS (3.9 × 10−3 ± 2.2 × 10−2 ppm) compared to HCWM (− 5.0 × 10−3 ± 3.9 × 10−3 ppm; p = 0.03), and a trend towards increased QS compared to NAWM (− 6.6 × 10−3 ± 5.3 × 10−3 ppm; p = 0.06). NAWM in MS patients did not demonstrate a significant difference in QS compared to HCWM (p = 0.6). Of the 20 MS patients, 4 had one or more gadolinium-enhancing lesions (13 enhancing lesions total) with an average disease duration of 3.25 ± 1.7 years (compared to 7.81 ± 8.8 years for those without enhancing lesions) (Fig. 2). The QS values of NAWM in MS patients with gadolinium-enhancing lesions (− 5.4 × 10−3 ± 8.1 × 10−3 ppm) was not significantly different compared to those without enhancing lesions (− 6.9 × 10−3 ± 4.8 × 10−3 ppm; p = 0.539) or to HCWM (p = 0.96). Likewise, the QS values of NAWM in patients with only non-enhancing lesions did not differ significantly from HCWM (p = 0.33). There was no significant QS difference between the combined lesions of patients with gadolinium-enhancing lesions (1.4 × 10−2 ± 5.6 × 10−3 ppm) and those with only non-enhancing lesions (1.3 × 10−3 ± 2.3 × 10−2 ppm; p = 0.32).
Fig. 1.
Whole-brain magnetic susceptibility, diffusion metrics (FA and RD) and R2* for lesions (green), NAWM (red), and HC WM (blue). Adjusted statistically significant differences are delineated by red dashed brackets
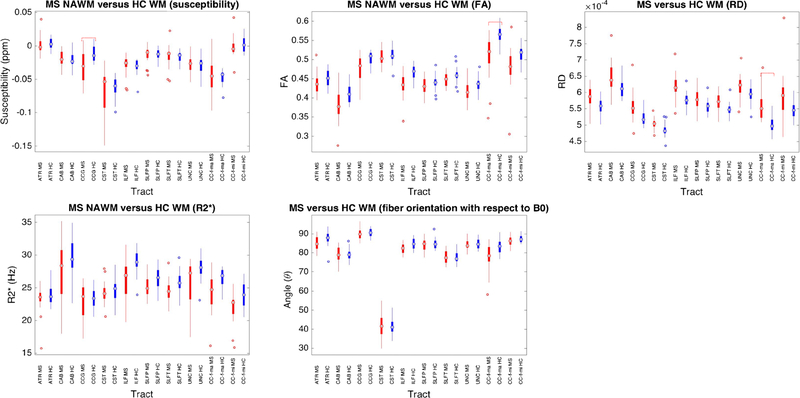
Fig. 2.
Tract-based susceptibility and DTI metrics in NAWM (red) compared to HCWM (blue). Magnetic susceptibility (QS), fractional anisotropy (FA), radial diffusivity (RD), R2*, and tract orientation are shown. Adjusted statistically significant differences are delineated by red dashed brackets
Both RD and FA demonstrated statistically significant differences between lesions and NAWM, as well as between lesions and HCWM. FA was markedly reduced in MS lesions (0.31 ± 0.025) compared to NAWM (0.36 ± 0.021, p < 0.0001). RD was significantly higher in MS lesions (9.3 × 10−4 ± 7 × 10−5) compared to NAWM (6.3 × 10−4 ± 3.0 × 10−5 in NAWM, p < 0.0001). However, there was no significant difference between NAWM and HCWM for either DTI metric after adjusting for sex and multiple comparisons (p > 0.05).
R2* was significantly reduced in MS lesions (20.1 ± 2.6 Hz) compared to both NAWM (23.7 ± 1.6 Hz, p < 0.0001) as well as HCWM (24.7 ± 1.1 Hz, p < 0.0001). However, no statistically significant difference in R2* was noted between NAWM and HCWM. There was no significant correlation between QS and the diffusion metrics or EDSS within lesions or NAWM (Supplemental Materials - Table 2). However, there was a mild positive correlation between R2* and QS within lesions (R = 0.463, p = 0.04).
MRI metrics in individual white matter tracts
QS varied between individual white matter tracts for both NAWM and HCWM. (Fig. 3) (Supplemental Materials - Table 3). The ATR demonstrated the highest QS (approximately 0.001 ppm) in both NAWM and HCWM, while the CST demonstrated the lowest QS (− 0.06 to − 0.08 ppm). Regarding the DTI metrics, the highest FA values were found within the CST and forceps major (approximately 0.5), while the lowest FA values were found within the CAB (approximately 0.3). RD was highest within the CAB (6.7 × 10−4) and lowest within the CST (5 × 10−4, respectively).
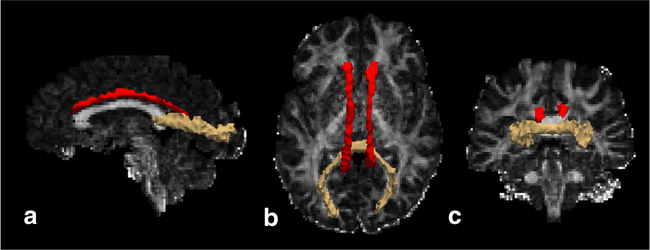
Fig. 3.
Sagittal (a), axial (b), and coronal (c) schematic renderings of statistically significant tract-based differences in susceptibility and RD between NAWM and HCWM superimposed on an FA map from a healthy subject. The cingulate gyri (CCG; red tracts) demonstrated significantly decreased susceptibility in NAWM, while the forceps major demonstrated significantly increased RD (beige tract). *Schematic renderings generated using TRACULA and displayed in Freeview (Freesurfer v5.3.0; http://surfer.nmr.mgh.harvard.edu
Among the white matter tracts, the CCG demonstrated a significant difference in QS between NAWM and HCWM (Figs. 3 and 4), which was significantly lower in the NAWM (− 0.031 ± 0.023 ppm versus − 0.012 ± 0.012 ppm, p = 0.004). On the other hand, the forceps major demonstrated significantly lower FA in NAWM compared to HCWM (0.51 ± 0.06 versus 0.57 ± 0.03, p = 0.004). RD was also significantly increased along the forceps major in NAWM (5.8 × 10−4 ± 6.1 × 10−6 versus 5.2 × 10−4 ± 3.2 × 10−5, p = 0.008).
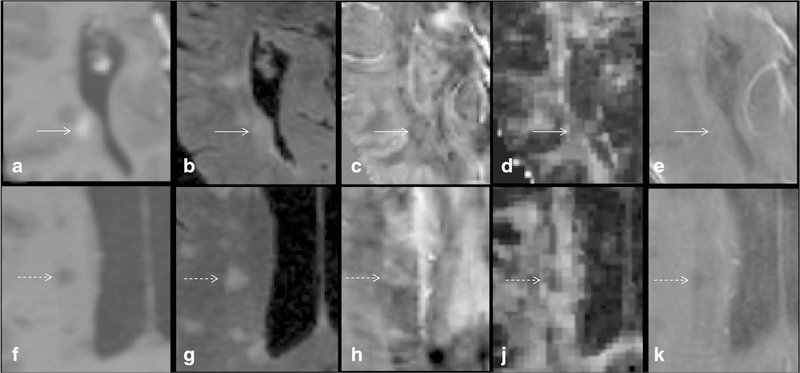
Fig. 4.
Contrast-enhanced T1W (a), T2-FLAIR (b), QSM (c), FA (d), and R2* (e) images of an acute-enhancing MS lesion (solid arrow) are shown. For comparison, a more chronic non-enhancing lesion shows (dashed arrow; f, g, h, i, j, k) increased susceptibility (0.0492 ppm versus − 0.0153 ppm) predominantly within the periphery of the lesion with decreased FA (0.272 versus 0.325) and R2* (8.74 Hz versus 19.8 Hz)
Among white matter tracts, the CST was aligned most parallel to the applied B0 at approximately 40–60°, while the other tracts were aligned between 80−90° relative to B0. However, there was no significant difference in orientation between the NAWM and HCWM tracts. There were non-significant trends (p = 0.08–0.09) towards decreased NAWM R2* along the forceps minor, ILF, and UNC.
No significant correlations were found between QS and EDSS along the NAWM tracts. Prior to adjusting for multiple comparisons, the forceps major, ILF, and SLFT showed negative correlations between QS and FA (Supplemental Materials - Table 4). Positive correlations between QS and RD were noted along the forceps major, CAB, CCG, and SLFT. There were also negative correlations between QS and tract orientation with respect to B0 along the ATR, CAB, ILF, and SLFT. R2* showed positive correlations with QS along the CCG and UNC. Of note, after adjusting for multiple comparisons, these correlations did not demonstrate significance.
Discussion
In this study, we probed QS in conjunction with DTI metrics (RD and FA) and R2* in the white matter of MS and HC subjects. We found that there was a trend towards decreased QS in whole-brain NAWM compared to lesions, but this did not differ significantly from HCWM. Our tract-based analysis showed NAWM QS was significantly lower compared to HCWM along the CCG. Additionally, NAWM DTI metrics differed most significantly from HCWM along the forceps major without a corresponding change in QS. Non-significant trends towards decreased R2* was noted along several NAWM tracts, which could reflect iron or myelin loss. These results suggest that there may be decreased paramagnetic substrate along the NAWM tracts such as the CCG and forceps major.
Evaluation of QS and DTI parameters in MS lesions
Consistent with previous reports, MS lesions showed higher average QS than NAWM as well as HCWM [8, 10, 18]. Additionally, the DTI metrics showed evidence of perturbed tissue microstructure, including myelin loss as suggested by increased RD [17]. The decreased R2* also provides evidence of myelin loss within lesions. Similar to iron deposition, demyelination increases QS by removing the diamagnetic “masking” effect of myelin [8, 9, 15].
The lack of a significant correlation between QS and DTI results suggests that these metrics are primarily influenced by separate substrates within lesions. A number of mechanisms have been suggested to account for QS alterations in lesions, including increased iron content from infiltration of microglia/macrophages and release of extracellular iron from degenerating oligodendrocytes [27, 28]. Demyelination would increase the QS within a lesion but only to that of CSF; positive QS would necessarily reflect the presence of paramagnetic substrates (iron). On the other hand, DTI metrics are likely influenced by alterations in white matter integrity conferred by myelin as well as axons, without a significant contribution from iron (at the concentrations found in MS) [6, 23, 29].
Of note, the relationship between DTI metrics and QS may be variable as one could potentially observe a positive correlation in acutely demyelinating lesions that do not have significant iron accumulation. However, with increasing iron deposition over time having a significant contribution to tissue QS, that correlation may be lost (the majority [~ 97%] of the lesions in our study appear to have been subacute or chronic given the lack of gadolinium enhancement) [9, 10].
We identified a mild positive correlation between QS and R2*, which likely corresponds to the observation that iron accumulation increases both parameters [30, 31]. In contrast to QS, R2* was overall significantly decreased in lesions compared to NAWM and HCWM. This may be accounted for by the fact that myelin loss has an opposing effect on R2*; within lesions, it appears to outweigh the effect of iron accumulation. However, this likely depends on lesion age and the corresponding changes in myelin and iron content.
Evaluation of QS and DTI parameters in NAWM
From our tract-based analysis, we found differences in QS between white matter tracts within both MS and HC groups. These findings could be partly accounted for by susceptibility anisotropy, wherein the anisotropic contribution should become increasingly diamagnetic for more perpendicularly oriented tracts relative to B0 [14, 32]. This is in agreement with the negative correlations we found between QS and orientation along several NAWM tracts. However, susceptibility anisotropy is only one factor that can influence bulk tissue QS [14]. For example, although the CST was the most parallel tract to B0, it was the most diamagnetic fiber tract rather than the most paramagnetic. This indicates that other factors, such as myelin integrity and iron content, can also contribute to bulk tissue QS to an equal or greater magnitude than the anisotropic effects [8, 16, 33]. Of note, we did not observe substantial differences in tract orientation between NAWM and HCWM, suggesting that significant variations in QS between the two groups were unlikely the result of susceptibility anisotropy.
Consistent with prior studies utilizing DTI, we found trends of decreased FA and increased RD along several NAWM tracts suggesting pathological changes such as demyelination and axonal loss [29, 33–37]. Although decreased myelin content should lead to an increase in QS, the majority of NAWM tracts instead demonstrated no significant difference (or decreased QS along the CCG) compared to HCWM [8, 16]. These findings suggest that like lesions, alterations in DTI metrics and QS in the NAWM may be predominantly influenced by different substrates.
Along these lines, changes in paramagnetic substrates such as iron could play an important role in the observed QS in NAWM [14]. Specifically, the lack of a significant increase in QS in the NAWM despite evidence of myelin/axonal loss suggests that there may be concurrent iron loss. The mild trend we observed towards decreased R2* along several of the NAWM tracts, which can result from iron loss and demyelination, is congruent with this. Taken together, our QSM results are consistent with prior imaging and pathology studies that have found evidence of decreased iron content in NAWM [18, 30, 38, 39]. Such depletion could have important implications as iron plays an important role in maintaining cellular functions such as metabolism and lipid synthesis [40, 41]. Interestingly, other QSM studies have reported increased iron accumulation in the basal ganglia of MS patients [18, 27, 30]. From this perspective, iron dysregulation in MS may be characterized as a redistribution between different brain regions rather than as strictly iron accumulation or depletion [28].
As noted above, we found that the CCG demonstrated the most significant decrease in QS compared to HCWM. However, no significant correlation with clinical impairment (EDSS scores) was appreciated. It has been proposed that the CCG is involved in cognitive tasks such as emotion and memory, while also forming part of the default mode network [42, 43]. Therefore, clinical tests that focus on cognition such as the multiple sclerosis functional composite may be more appropriate for determining this relationship.
A recent study reported increased NAWM QS in patients who had only non-enhancing lesions compared to HC subjects, and no significant difference in patients with enhancing lesions [44]. However, among patients in our study with only non-enhancing lesions, we found no significant QS difference between whole-brain NAWM compared to HCWM. This difference could potentially be attributed to different analysis methods, as we interrogated the whole-brain NAWM as opposed to selected ROIs. Specifically, QS may vary depending on the relative white matter location (i.e., periventricular, deep, subcortical, and perilesional) as well as within different brain regions (i.e., centrum semiovale versus corpus callosum). Along these lines, a separate study focusing on thalamic WM found decreased QS in MS compared to HC [39].
Furthermore, iron content in NAWM may vary between different patients (i.e., relapsing–remitting versus primary progressive subtypes) as well as within the same patient over time [44]. With regard to the latter, Hametner et al. found iron deposition in the NAWM of patients with acute MS similar to healthy populations, while patients with chronic disease exhibited iron depletion [38]. Unfortunately, we were not able to further interrogate these issues in the current study due to the lack of serial imaging, although future longitudinal studies could help to elucidate this relationship.
Limitations
Although the DTI metrics we analyzed are thought to correlate with white matter structural integrity, they have limited specificity for distinguishing between myelin and axonal changes [45]. Dispersion of fiber orientations further limits the interpretation as does the relatively low diffusion resolution (16 directions in our protocol) [33, 46]. Future research could address this limitation through the addition of more specific myelin (i.e., quantitative magnetization transfer ratio and macromolecular tissue volume) and axonal imaging markers (AxCaliber, neurite orientation dispersion and density imaging [NODDI]) [46–48].
The small size of our cohort as well as unequal sex distribution between the MS and HC study groups also limits generalizability of the findings. We accounted for the latter in our statistical analysis as described in the “Methods” section, which yielded decreased statistical significance of the results (see Supplemental Tables). Future work utilizing a larger matched study populations as well as with longitudinal data to assess changes over time would be helpful in this regard. The interpretation of QS alterations in NAWM would also benefit from the direct correlation with histology, in conjunction with other advanced imaging techniques, as has been previously suggested [16].
Conclusions
We found increased QS in lesions compared to HCWM, but no significant difference between NAWM and HCWM for the whole brain. Among white matter tracts, the CCG showed significantly decreased QS in NAWM compared to HCWM. DTI metrics showed evidence of NAWM injury, including probable myelin loss, that was most pronounced within the forceps major without a significant corresponding change in QS. Non-significant trends of decreased R2* was also noted along several NAWM tracts, which can be seen with myelin as well as iron loss. Considering that diamagnetic myelin loss should increase QS, the unchanged and/or slightly decreased QS suggests that there may be concurrent paramagnetic substrate loss from these NAWM tracts. Taken together, our results point to a potential complementary role for QSM as a novel imaging contrast for assessing changes in paramagnetic substrates in MS. Validating these findings and their clinical implications, particularly concerning determining prognosis and monitoring therapy, are potential areas for future research.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Dr. Wei Li for his invaluable help with the MRI protocol and data processing. The authors would also like to acknowledge Mr. Gilbert Gortez for his help with data collection.
Funding This work was funded, in part, by grants from the Conrad N. Hilton Foundation 17330 and the Radiological Society of North America Research Resident Grants RR1427 and RR1577.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00234-018-2137-7) contains supplementary material, which is available to authorized users.
References
- 1.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kutzelnigg A, Lucchinetti CF, Stadelmann C, Brück W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain J Neurol 128:2705–2712 [DOI] [PubMed] [Google Scholar]
- 3.Evangelou N, Esiri MM, Smith S, Palace J, Matthews PM (2000) Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol 47:391–395 [PubMed] [Google Scholar]
- 4.Guo AC, Jewells VL, Provenzale JM (2001) Analysis of normalappearing white matter in multiple sclerosis: comparison of diffusion tensor MR imaging and magnetization transfer imaging. Am J Neuroradiol 22:1893–1900 [PMC free article] [PubMed] [Google Scholar]
- 5.Traboulsee A, Dehmeshki J, Peters KR, Griffin CM, Brex PA, Silver N, Ciccarrelli O, Chard DT, Barker GJ, Thompson AJ, Miller DH (2003) Disability in multiple sclerosis is related to normal appearing brain tissue MTR histogram abnormalities. Mult Scler J 9:566–573 [DOI] [PubMed] [Google Scholar]
- 6.Kolasinski J, Stagg CJ, Chance SA, DeLuca GC, Esiri MM, Chang EH, Palace JA, McNab JA, Jenkinson M, Miller KL, Johansen-Berg H (2012) A combined post-mortem magnetic resonance imaging and quantitative histological study of multiple sclerosis pathology. Brain J Neurol 135:2938–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, Li W, Johnson GA, Wu B (2011) High-field (9.4 T) MRI of brain dysmyelination by quantitative mapping of magnetic susceptibility. NeuroImage 56:930–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wisnieff C, Ramanan S, Olesik J, Gauthier S, Wang Y, Pitt D (2015) Quantitative susceptibility mapping (QSM) of white matter multiple sclerosis lesions: interpreting positive susceptibility and the presence of iron. Magn Reson Med 74:564–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Gauthier SA, Gupta A, Comunale J, Liu T, Wang S, Pei M, Pitt D, Wang Y (2014) Quantitative susceptibility mapping of multiple sclerosis lesions at various ages. Radiology 271:183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Gauthier SA, Gupta A, Comunale J, Chia-Yi Chiang G, Zhou D, Chen W, Giambrone AE, Zhu W, Wang Y (2016) Longitudinal change in magnetic susceptibility of new enhanced multiple sclerosis (MS) lesions measured on serial quantitative susceptibility mapping (QSM). J Magn Reson Imaging 44:426–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schweser F, Sommer K, Deistung A, Reichenbach JR (2012) Quantitative susceptibility mapping for investigating subtle susceptibility variations in the human brain. NeuroImage 62:2083–2100 [DOI] [PubMed] [Google Scholar]
- 12.Li W, Wang N, Yu F, Han H, Cao W, Romero R, Tantiwongkosi B, Duong TQ, Liu C (2015) A method for estimating and removing streaking artifacts in quantitative susceptibility mapping. NeuroImage 108:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu T, Spincemaille P, de Rochefort L, Kressler B, Wang Y (2009) Calculation of susceptibility through multiple orientation sampling (COSMOS): a method for conditioning the inverse problem from measured magnetic field map to susceptibility source image in MRI. Magn Reson Med 61:196–204 [DOI] [PubMed] [Google Scholar]
- 14.Duyn JH, Schenck J (2016) Contributions to magnetic susceptibility of brain tissue. NMR Biomed 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Wu B, Liu C (2011) Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. NeuroImage 55:1645–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stüber C, Morawski M, Schäfer A et al. (2014) Myelin and iron concentration in the human brain: a quantitative study of MRI contrast. Neuroimage 93(Part 1):95–106 [DOI] [PubMed] [Google Scholar]
- 17.Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002) Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage 17: 1429–1436 [DOI] [PubMed] [Google Scholar]
- 18.Rudko DA, Solovey I, Gati JS, Kremenchutzky M, Menon RS (2014) Multiple sclerosis: improved identification of disease-relevant changes in gray and white matter by using susceptibility-based MR imaging. Radiology 272:851–864 [DOI] [PubMed] [Google Scholar]
- 19.Deistung A, Schäfer A, Schweser F, Biedermann U, Turner R, Reichenbach JR (2013) Toward in vivo histology: a comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase-, and R2*-imaging at ultra-high magnetic field strength. NeuroImage 65:299–314 [DOI] [PubMed] [Google Scholar]
- 20.Wu B, Li W, Guidon A, Liu C (2012) Whole brain susceptibility mapping using compressed sensing. Magn Reson Med 67:137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Avram AV, Wu B, Xiao X, Liu C (2014) Integrated Laplacian-based phase unwrapping and background phase removal for quantitative susceptibility mapping. NMR Biomed 27:219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage 9: 179–194 [DOI] [PubMed] [Google Scholar]
- 23.Fischl B, van der Kouwe A, Destrieux C et al. (2004) Automatically parcellating the human cerebral cortex. Cereb Cortex 1991 14:11–22 [DOI] [PubMed] [Google Scholar]
- 24.Yendiki A, Panneck P, Srinivasan P et al. (2011) Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinformatics 5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt P (2017) Bayesian inference for structured additive regression models for large-scale problems with applications to medical imaging
- 26.Straub S, Schneider TM, Emmerich J, Freitag MT, Ziener CH, Schlemmer HP, Ladd ME, Laun FB (2017) Suitable reference tissues for quantitative susceptibility mapping of the brain. Magn Reson Med 78:204–214 [DOI] [PubMed] [Google Scholar]
- 27.Langkammer C, Schweser F, Krebs N et al. (2012) Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. NeuroImage 62:1593–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephenson E, Nathoo N, Mahjoub Y, Dunn JF, Yong VW (2014) Iron in multiple sclerosis: roles in neurodegeneration and repair. Nat Rev Neurol 10:459–468 [DOI] [PubMed] [Google Scholar]
- 29.Sbardella E, Tona F, Petsas N et al. (2013) DTI measurements in multiple sclerosis: evaluation of brain damage and clinical implications. Mult Scler Int 2013:e671730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paling D, Tozer D, Wheeler-Kingshott C, Kapoor R, Miller DH, Golay X (2012) Reduced R2’ in multiple sclerosis normal appearing white matter and lesions may reflect decreased myelin and iron content. J Neurol Neurosurg Psychiatry 83:785–792 [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Gauthier SA, Gupta A, Chen W, Comunale J, Chiang GCY, Zhou D, Askin G, Zhu W, Pitt D, Wang Y (2016) Quantitative susceptibility mapping and R2* measured changes during white matter lesion development in multiple sclerosis: myelin breakdown, myelin debris degradation and removal, and Iron accumulation. Am J Neuroradiol 37:1629–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu C, Li W, Wu B, Jiang Y, Johnson GA (2012) 3D fiber tractography with susceptibility tensor imaging. NeuroImage 59: 1290–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groeschel S, Hagberg GE, Schultz T, Balla DZ, Klose U, Hauser TK, Nägele T, Bieri O, Prasloski T, MacKay AL, Krägeloh-Mann I, Scheffler K (2016) Assessing white matter microstructure in brain regions with different myelin architecture using MRI. PLoS One 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moll NM, Rietsch AM, Thomas S, Ransohoff AJ, Lee JC, Fox R, Chang A, Ransohoff RM, Fisher E (2011) Multiple sclerosis normal-appearing white matter: pathology-imaging correlations. Ann Neurol 70:764–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tillema JM, Leach J, Pirko I (2012) Non-lesional white matter changes in pediatric multiple sclerosis and monophasic demyelinating disorders. Mult Scler Houndmills Basingstoke Engl 18:1754–1759 [DOI] [PubMed] [Google Scholar]
- 36.Filippi M, Cercignani M, Inglese M, Horsfield MA, Comi G (2001) Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology 56:304–311 [DOI] [PubMed] [Google Scholar]
- 37.Roosendaal SD, Geurts JJG, Vrenken H et al. (2009) Regional DTI differences in multiple sclerosis patients. NeuroImage 44:1397–1403 [DOI] [PubMed] [Google Scholar]
- 38.Hametner S, Wimmer I, Haider L, Pfeifenbring S, Brück W, Lassmann H (2013) Iron and neurodegeneration in the multiple sclerosis brain. Ann Neurol 74:848–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergsland N, Schweser F, Dwyer MG, Weinstock-Guttman B, Benedict RHB, Zivadinov R (2018) Thalamic white matter in multiple sclerosis: a combined diffusion-tensor imaging and quantitative susceptibility mapping study. Hum Brain Mapp 39:4007–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saher G, Brügger B, Lappe-Siefke C, Möbius W, Tozawa RI, Wehr MC, Wieland F, Ishibashi S, Nave KA (2005) High cholesterol level is essential for myelin membrane growth. Nat Neurosci 8:468–475 [DOI] [PubMed] [Google Scholar]
- 41.Schonberg DL, McTigue DM (2009) Iron is essential for oligodendrocyte genesis following intraspinal macrophage activation. Exp Neurol 218:64–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geisseler O, Pflugshaupt T, Bezzola L et al. (2015) Cortical thinning in the anterior cingulate cortex predicts multiple sclerosis patients’ fluency performance in a lateralised manner. Neuroimage Clin 10:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maddock RJ, Garrett AS, Buonocore MH (2001) Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104:667–676 [DOI] [PubMed] [Google Scholar]
- 44.Chen W, Zhang Y, Mu K, Pan C, Gauthier SA, Zhu W, Wang Y (2017) Quantifying the susceptibility variation of normal-appearing white matter in multiple sclerosis by quantitative susceptibility mapping. Am J Roentgenol 209:889–894 [DOI] [PubMed] [Google Scholar]
- 45.Wheeler-Kingshott CAM, Cercignani M (2009) About “axial” and “radial” diffusivities. Magn Reson Med 61:1255–1260 [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC (2012) NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 61:1000–1016 [DOI] [PubMed] [Google Scholar]
- 47.Mezer A, Yeatman JD, Stikov N, Kay KN, Cho NJ, Dougherty RF, Perry ML, Parvizi J, Hua LH, Butts-Pauly K, Wandell BA (2013) Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nat Med 19:1667–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang SY, Tobyne SM, Nummenmaa A, Witzel T, Wald LL, McNab JA, Klawiter EC (2016) Characterization of axonal disease in patients with multiple sclerosis using high-gradient-diffusion MR imaging. Radiology 280:244–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.