Abstract
In humans, prior alcohol use is linked with impulsivity and impaired decision-making, but the nature of this relationship is unclear. In a previous study in rats, we found that prior alcohol access led to over-responding in go/no-go discrimination training, but had no effect on discrimination learning. It was unclear whether this over-responding effect would occur in a reversal learning task, or whether prior alcohol would impair reversal learning in our task. In the present experiments, we determined whether six weeks of chronic intermittent alcohol access would induce over-responding or impair reversal learning in our task. Our task allowed for multiple responses/trial with limited reinforcement, so over-responding could be assessed. In Exp. 1, we gave three days of discrimination training prior to access to 20% alcohol or water, then reversed task contingencies starting 4 days after the end of alcohol access. In Exp. 2, we gave either three or six days of discrimination training prior to the same alcohol access and reversal learning procedures to determine if the original training length would affect alcohol’s behavioral effects. We found no reversal learning deficits in either experiment. Across both experiments, we found that the Alcohol group exhibited over-responding to the active lever, but this effect was smaller than in our previous discrimination experiments. Our data suggest that there are behavioral changes after voluntary alcohol access that can be missed by some discrimination/reversal learning assessments, and our over-responding task can detect these transient changes. However, over-responding is more pronounced in discrimination than reversal learning.
Keywords: alcohol, response inhibition, reversal learning
1. Introduction
Prior extensive alcohol use is associated with impulsivity and poor decision-making, even months or years after the cessation of alcohol use [1-4]. However, the nature of this relationship is unclear. It is possible that previous extensive alcohol consumption causes impaired decision-making and impulsivity, impaired decision-making and impulsivity precede and possibly increase alcohol consumption, or the two co-occur due to some other pre-existing factor [5-8]. Further research is needed to determine the relationship between alcohol use, impulsivity, and decision making.
One task that is often used to assess impulsivity is go/no-go discrimination learning, in which one cue indicates that a response should be made while a second cue indicates that responses should be withheld. Detoxified alcoholics, heavy drinkers, and people with higher alcohol use disorder scores exhibit impaired performance or abnormal reaction times in these tasks [9, 10]. However, there is less known about the effects of alcohol consumption/exposure on the acquisition of go/no-go discrimination learning in rodent models [11, 12].
Prior alcohol consumption also has some relationship with abnormal reversal learning abilities, even when behavior is tested in an alcohol-free state. In reversal learning tasks, one response earns a reinforcer and a second response is not reinforced in a first phase. The identities of the reinforced and non-reinforced responses are then switched in a subsequent phase. Prior alcohol exposure leads to impairments in both initial discrimination and the subsequent reversal under some experimental conditions in rodent models [13, 14]. There are also several other examples of alcohol exposure-behavioral training combinations in which prior alcohol exposure leaves the original discrimination intact but impairs subsequent reversal learning [13, 15-18]. However, there are many examples of experiments that find that prior alcohol exposure does not impair reversal learning [19-23]. Further research is needed to disentangle the complex relationship between prior alcohol exposure and reversal learning.
Our lab recently determined the effects of prior alcohol consumption in a go/no-go discrimination task in which multiple responses/trial could be assessed [24]. In this task, the S+/active lever and S−/inactive levers were available for 40 seconds regardless of responding (although active lever-presses could only earn 2 pellets/trial), and lever-presses on the inactive lever earned no food reward. This allowed for a measure of both the ability to learn to discriminate between the two levers (assessed by the percent of active lever and inactive lever trials with at least one response) and the amount of over-responding to the active lever (defined as the number of responses beyond the 2/trial needed to earn all reinforcement) and the inactive lever (defined as the total number of responses to the S-). Prior alcohol consumption (4-6 weeks of chronic intermittent alcohol access [CIA: alcohol available 24-h/day 3 times/week [25, 26]] had no effect on the ability to discriminate between the active and inactive levers 4-5 days after the final access period. However, prior alcohol consumption led to an increase in over-responding on the active lever [24]. All rats responded more than the 2 responses/trial that were required to earn all available reinforcers, but the rats that had prior substantial alcohol consumption exhibited greater over-responding on the active lever than rats without prior alcohol access.
In the current report, we determined whether this over-responding effect would be replicated in a test of the go/no-go task that assessed reversal learning, rather than initial acquisition of the discrimination, and whether prior alcohol consumption would lead to a reversal learning deficit. In Exp. 1, we gave the rats 3 days of discrimination training (with 40-sec cues as in our previous experiments) before giving 6 weeks of alcohol access in order to isolate the effects of alcohol access on subsequent reversal learning. In the Alcohol group, we found over-responding to the active lever (but not the inactive lever) and no reversal learning impairments. In Exp. 2, we then determined whether a reversal learning deficit would be found if we varied the difficulty of the task. By the second reversal learning session in Exp. 1, all rats were responding on ~100% of the trials with the new active lever, but responding to the new inactive lever was well above zero (suggesting that it was more difficult to suppress lever-pressing to the inactive lever than to learn to press the new active lever). Thus, we extended the trial lengths to 50 seconds to make it more difficult to withhold responding to the inactive lever (which was expected to have no effect on the percent of trials with an active lever-press due to a ceiling effect). We also compared the effects of alcohol if we gave 3 or 6 days of discrimination training before alcohol access and reversal learning. Prior research has shown that over-training of an initial discrimination alters the difficulty of the subsequent reversal, although results differ on whether over-training makes the reversal more difficult, easier (termed the “over-training reversal effect), or has no effect (as reviewed in [27, 28]). Examining reversal after different lengths of discrimination training could increase the likelihood of discovering whether there are boundary conditions that affect whether prior alcohol access will affect reversal learning, regardless of whether over-training makes reversal learning more or less difficult. In Exp. 2, however, we found no evidence that the length of discrimination training affected the difficulty of reversal learning. We replicated the pattern from Exp. 1, with over-responding to the active lever, and no reversal learning deficits.
2. Materials and methods
2.1. Subjects
Male Long Evans rats (Charles River Laboratories, Portage, MI and Kingston, NY; n=52), weighing 150-200 g upon arrival in the facility, were used for the experiment. All animals were individually housed and maintained on a 12-hour reverse light-dark cycle with lights off at 07:30 am in a temperature and humidity controlled room. Once the rats had acclimated to the facility, they were food-restricted to 85% of their initial free-feeding weights by daily feedings with a minimum of 5 g of food chow per day. Once rats reached their initial target weight (85% of their initial free feeding weight), the target body weight was increased by 1 g/day from the 85% weight for the remainder of the experiment, such that the rats gained 7 g/week. Rats were fed to maintain them at their target weights. Research in our lab has found that this food restriction regimen leads to high levels of lever-pressing while still allowing the rats to gain weight over time [21, 24, 29]. Water was available ad libitum. All procedures and animal care were in accordance with the Kansas State University Institutional Animal Care and Use Committee guidelines, the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and United States federal law.
2.2. Behavioral apparatus
Experiments were conducted in standard self-administration chambers (Med Associates, St. Albans, VT). The chambers had two retractable levers on either side of the food cup at approximately one-third of the total height of the chamber, with a white stimulus light located above each lever. A red houselight was mounted on the top-center of the back wall. A speaker for delivering auditory stimuli was located on the left side of the back wall of the chambers, on the opposite wall from the food cup. A Dell Optiplex computer equipped with Med-PC for Windows controlled the equipment and recorded lever-presses.
2.3. General Behavioral Procedures
Once the rats had stabilized on the food-restriction conditions, they were trained in a go/no-go lever discrimination task. First, rats were given 1 session of magazine training. This session was 40 min long with delivery of a 45-mg food pellet (Catalogue # 1811155, TestDiet, Richmond, IN) every 125 sec. Next, rats had 3 or 6 sessions of a go/no-go lever discrimination task. In this task, the right and left lever were extended one at a time in alternating order for 40 sec (Exp. 1) or 50 sec (Exp. 2) each, with a cue-light illuminated above the extended lever. The left lever was extended with a cue-light steadily illuminated above it and the right lever was extended with the cue-light above it illuminated in a flashing pattern (2 Hz). For each rat, one of the two lever-light compounds was designated as the active lever and responses on this lever were rewarded on an intermittent reinforcement schedule. The reinforcement schedule within each trial was an alternating FI 1s: VI 25s, with one pellet available for the first lever-press after 1 second had passed, and another pellet available for the first press after a randomized time between 21-30 seconds into the cue presentation. This schedule was used to ensure that the first lever-press a rat made on the active lever would earn a food pellet, while also ensuring that the rats would have an incentive to press in the second half of the trial rather than potentially earning both food pellets in the first half of the trial. The other lever-light compound was designated as the inactive lever. Responses on the inactive lever were not reinforced, but there was no penalty for responding. Both lever-light compounds lasted the full 40 or 50 sec regardless of responding, and lever-presses did not terminate either lever-light. All sessions contained 20 presentations of the active lever and 20 presentations of the inactive lever presented in pseudo-random order, such that each block of 4 trials contained 2 active lever and 2 inactive lever trials. In Exp. 1, one rat failed to make a single lever-press on the active lever during the first session. In Exp. 2, one rat in the group given 3 days of discrimination training failed to make a single lever-press on the active lever during the first three sessions. These rats were given an additional 1 or 3 days of lever-press training, respectively, so that all rats would have at least 3 days of training in which they earned food pellets.
Next, the rats received 6 weeks of chronic intermittent alcohol (CIA) access. The rats were assigned to the two groups such that the groups did not differ in their pre-alcohol access discrimination learning. For each experiment, half of the rats comprised Water groups that received only water in both bottles, and the other half comprised Alcohol groups that received alcohol in one of the 2 bottles 3X/week. During the CIA access period, all rats had 2 bottles on their cages on all days, with at least one of the bottles containing tap water at all times (a permanent water bottle). The animals in the Water group received no alcohol during the experiment, and had tap water in both bottles for the duration of the access period. The Alcohol groups had 20% alcohol (v/v) in one of the bottles for a 24-h period every other day (3 days/week, separated by 24- or 48-h alcohol-free periods) and water in both bottles on other days. The location of the alcohol bottle was randomized to avoid side preference. The alcohol and water bottles were weighed and exchanged between 1:00 and 2:30 pm each afternoon.
After 6 weeks of CIA access, all rats were given a single water bottle for the remainder of the experiment. Three days after the end of the final alcohol exposure period, all rats received a single discrimination-retraining session with the original active and inactive lever contingencies. Starting the following day, all rats received 2 sessions (one session/day) in which the contingencies were reversed, such that the previously non-rewarded lever-light now earned food pellets and the previously rewarded lever-light no longer earned food pellets. All parameters of the reversal sessions were otherwise identical to the original lever-press training.
2.4. Individual experiments:
2.4.1. Experiment 1
In Exp. 1, all rats received 3 discrimination sessions (as described above). Next, the rats received 6 weeks of CIA access or water only access. There were 2 groups (n=8/group), with one group receiving Water only and the other group receiving CIA access. Then all rats received one session of discrimination-retraining 3 days after the final alcohol exposure, and then 2 sessions of reversal learning on subsequent days. All sessions lasted 40 min and both the active lever and inactive lever presentations lasted 40-sec in all sessions.
2.4.2. Experiment 2
In Exp. 2, two groups received 3 discrimination sessions and the other 2 groups received 6 discrimination sessions (as described above). Next, the rats received 6 weeks of CIA access or water only access. There were 4 groups (n=9/group): 3 day discrimination-Water only, 3 day discrimination-Alcohol, 6 day discrimination-Water only, 6 day discrimination-Alcohol. Then all rats received one session of discrimination-retraining 3 days after the final alcohol exposure, and then 2 once-daily sessions of reversal learning on the subsequent 2 days. All sessions lasted 44 min and both the active lever and inactive lever presentations lasted 50-sec in all sessions.
2.5. Statistical Analysis
For alcohol consumption, the alcohol consumed was calculated as the bottle weight difference between the start and end of the sessions, minus 2 grams for spillage, and then multiplied by 0.162 for the weight of alcohol in 1 g of a 20% v/v alcohol solution. The 2 g subtracted for spillage was determined on the basis of pilot research carried out in our laboratory, during which bottles were placed on empty cages and weighed daily to determine general spillage. In a few cases, experimenter error led to spillage of a bottle or incorrect data recording (defined as a recorded increase in the bottle weight after 24-h or a >100g decrease in the bottle weight). In these cases, the consumption on the day before and after the erroneous day was averaged to fill in the missing value. The data for the Alcohol rats were combined into weekly averages and then analyzed with a repeated-measures ANOVA with the within-subjects factor of Week (the 6 weeks of alcohol exposure that each contained 3 24-h alcohol access periods) for Exp. 1 and a mixed-factor ANOVA with the between-subjects factor of Training Length (3 or 6 discrimination days) and the within-subjects factor of Week for Exp. 2.
For all discrimination sessions and reversal sessions, data were analyzed for number of lever-presses/trial and for percent of trials with at least one lever-press. For Exp. 2, the discrimination data from the 3- and 6-day training session groups were analyzed separately. Data for the 3 or 6 discrimination sessions prior to alcohol access were analyzed with mixed-factor ANOVAs with the between-subjects factor of Exposure Group (Water and Alcohol), and the within-subjects factors of Lever (Active and Inactive lever) and Training Day (the 3 or 6 discrimination training days). One rat made zero active lever-presses on the first day of discrimination training in Exp. 1. This rat received an additional day of discrimination training and the data from days 2-4 were used in the analysis. One rat in Exp. 2 that was assigned to receive 3 discrimination sessions made zero active lever-presses in the first 3 days of discrimination training. This rat received three additional days of discrimination training, and the data from days 4-6 were used in the analysis.
For Exp. 1, data from the discrimination-retraining session after the alcohol access period were analyzed with a mixed-factor ANOVA with the between-subjects factor of Exposure Group (Water and Alcohol), and the within-subjects factors of Lever (Active and Inactive lever) and Trial Block (the five 4-trial blocks). For Exp. 2, data from the discrimination-retraining session after the alcohol access period were analyzed with a mixed-factor ANOVA with the between-subjects factors of Exposure Group (Water and Alcohol) and Training Length (Three and Six discrimination training sessions), and the within-subjects factors of Lever (Active and Inactive lever) and Trial Block (the five 4-trial blocks).
For Exp. 1, data from the reversal learning sessions were analyzed with a mixed-factor ANOVA with the between-subjects factor of Exposure Group (Water and Alcohol), and the within-subjects factors of Lever (Active and Inactive lever), Training Day (the 2 reversal training days), and Trial Block (the five 4-trial blocks for each lever within each day). For Exp. 2, data from the reversal learning sessions were analyzed with a mixed-factor ANOVA with the between-subjects factors of Exposure Group (Water and Alcohol) and Training Length (Three and Six discrimination training sessions), and the within-subjects factors of Lever (Active and Inactive lever), Training Day (the 2 reversal training days), and Trial Block (the five 4-trial blocks for each lever within each day).
All data were analyzed using Statistica 5.1 (StatSoft. Tulsa, OK). Post-hoc analyses using Tukey HSD tests were run when the main effects or interactions of Exposure Group were significant (p<0.05). Full ANOVA tables for all analyses can be found in the Supplementary Materials.
3. Results
3.1. Alcohol consumption
3.1.1. Alcohol consumption in Experiment 1
During the drinking phase, the rats given alcohol access maintained relatively steady alcohol consumption, except for a spike during week 3 (Fig 1A). A within-subjects ANOVA with the within-subjects factor of Week (the 6 weeks of alcohol access) found a significant effect of Week (F(5, 35)=2.8, p<0.05). Post-hoc analyses found that alcohol consumption in week 3 was significantly higher than consumption in week 1.
Figure 1. Alcohol consumption.
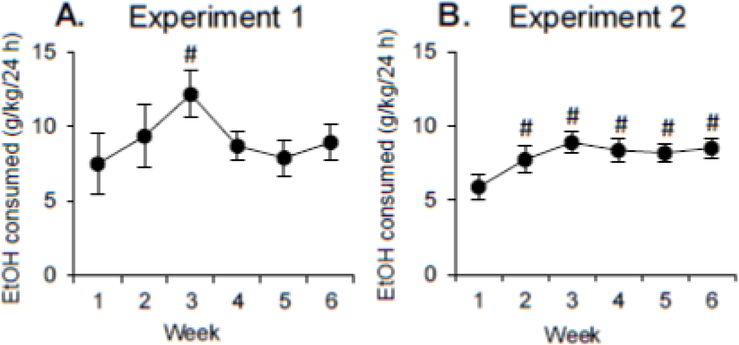
(A) Alcohol consumption (mean±SEM) in the Alcohol group for the six weeks of CIA access in Experiment 1. (B) Alcohol consumption (mean±SEM) in the Alcohol group for the six weeks of CIA access in Experiment 2. # = significant difference from week 1.
3.1.2. Alcohol consumption in Experiment 2
During the drinking phase, there was no difference in alcohol consumption between the two groups of rats that received the different lengths of discrimination training. Both groups of rats given alcohol access increased their consumption from week 1 to subsequent weeks, when alcohol consumption then leveled off (Fig 1B). A mixed-factor ANOVA with the between-subjects factor of Training Length and the within-subjects factor of Week (the 6 weeks of alcohol access) found a significant effect of Week (F)5, 80)=7.3, p<0.01), but no effect or interaction of Training Length (all F<1). Post-hoc analyses found that consumption in weeks 2-6 was significantly higher than consumption in week 1.
3.2. Operant training data
3.2.1. Experiment 1
3.2.1.1. Discrimination
In the original discrimination, there were no differences in percent of trials with a lever-press between the two groups (that would later receive alcohol or water). The percent of trials with a lever-press for discrimination training (Fig 2A) was analyzed with a mixed-factor ANOVA with the between-subjects factor of Exposure Group and the within-subjects factors of Lever and Training Day. This analysis found significant effects of Lever (F(1,14)=150.5, p<0.01), Training Day (F(2,28)=4.0, p<0.05), and Lever X Training Day (F(2,28)=63.4, p<0.01). There were no significant effects or interactions of Exposure Group (all F<1).
Figure 2. Experiment 1 behavioral data.
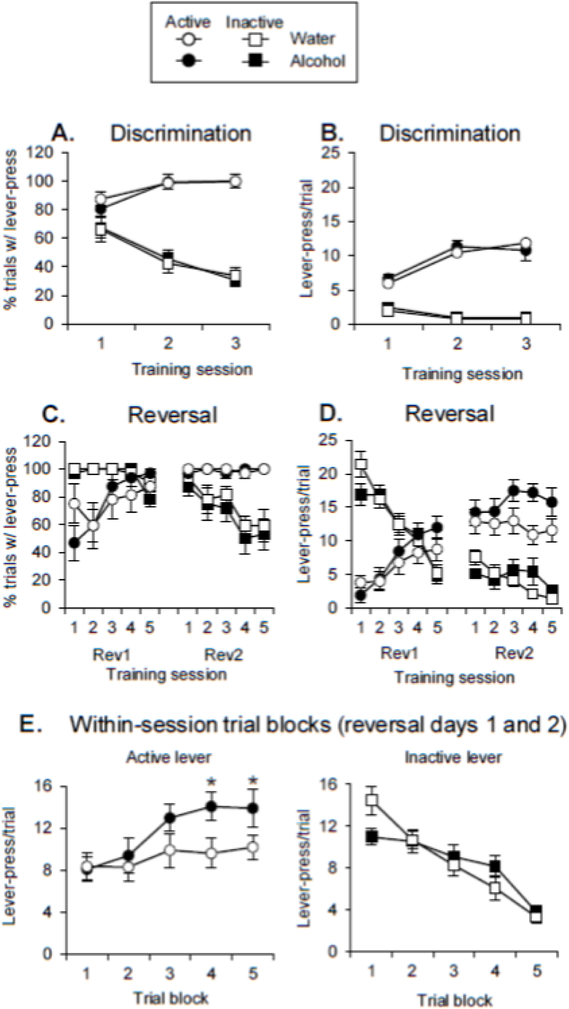
Mean±SEM for percent of trials with a lever-press (A) and lever-presses per trial (B) for the three days of initial discrimination training. Mean±SEM for percent trials with a lever-press (C) and lever-presses per trial (D) across the two reversal learning sessions. (Rev1 and Rev2 = reversal day 1 and reversal day 2, respectively). (E) Within-session lever-presses per trial (mean±SEM), averaged across reversal days one and two, for the active lever (left) and inactive lever (right). For figures 2D-2F, the #’s 1-5 on the x-axis represent the 5 4-trial blocks for each stimulus within each session. * = significant difference from the corresponding trial blocks for the Water group.
In the original discrimination, there were no differences in lever-presses/trial between the two groups (that would later receive alcohol or water). Lever-presses/trial for discrimination training (Fig 2B) were analyzed with a mixed-factor ANOVA with the between-subjects factor of Exposure Group and the within-subjects factors of Lever and Training Day. This analysis found significant effects of Lever (F(1,14)=195.3, p<0.01), Training Day (F(2,28)= 15.8, p<0.05), and Lever X Training Day (F(2,28)=41.5, p<0.01). There were no significant effects or interactions of Exposure Group (all p>0.05).
3.2.1.2. Discrimination Retraining
In the discrimination retraining session, there were no differences in percent of trials with a lever-press between the two groups that received alcohol or water. The percent of trials with a lever-press for discrimination retraining (data not shown) was analyzed with a mixed-factor ANOVA with the between-subjects factor of Exposure Group and the within-subjects factors of Lever and Trial Block. This analysis found significant effects of Lever (F(1,14)=293.5, p<0.01) and Trial Block (F(4,56)=11.8, p<0.01) and a significant interaction of Lever X Trial Block (F(4,56)= 11.8, p<0.01). There were no significant effects or interactions of Exposure Group (all p>0.05).
In the discrimination retraining session, there were no clear differences in lever-presses/trial between the two groups that received alcohol or water. The number of lever-presses/trial for discrimination retraining (data not shown) was analyzed with a mixed-factor ANOVA with the between-subjects factor of Exposure Group and the within-subjects factors of Lever and Trial Block. This analysis found significant effects of Lever (F(1,14)=171.4, p<0.01) and Trial Block (F(4,56)=5.9, p<0.01) and significant interactions of Lever X Trial Block (F(4,56)=4.5, p<0.01) and Exposure Group X Lever X Trial Block (F(4,56)=3.1, p<0.05). Despite the significant Exposure Group X Lever X Trial Block interaction, the Alcohol group did not differ from the Water group in responding on either lever on any trial block during the lever retraining session.
3.2.1.3. Reversal learning
In the reversal learning sessions, there were no differences in percent of trials with a lever-press between the two groups that received alcohol or water. The percent of trials with a lever-press for reversal learning (Fig 2C) was analyzed with a mixed-factor ANOVA with the between-subjects factor of Exposure Group and the within-subjects factors of Lever, Training Day, and Trial Block. This analysis found significant interactions of Lever X Training Day (F(1,4)=27.9, p<0.01), Lever X Trial Block (F(4,56)=17.2, p<0.01) and Training Day X Trial Block (F(4,56))=10.7, p<0.01). There were no significant effects or interactions of Exposure Group (all p>0.05).
In the reversal learning sessions, the group given prior alcohol access exhibited a pattern in which over-responding to the active lever emerged within the sessions. Lever-presses/trial for reversal learning (Fig 2D) was analyzed with a mixed-factor ANOVA with the between-subjects factor of Exposure Group and the within-subjects factors of Lever, Training Day, and Trial Block. This analysis found a significant main effect of Training Day (F(1,14)=27.9, p<0.01), and significant interactions of Exposure Group X Trial Block (F(4,56)=7.8, p<0.01), Lever X Training Day (F(1,14)=169.9, p<0.01), Lever X Trial Block (F(4,56)=55.0, p<0.01) and Lever X Training Day X Trial Block (F(4,56)=21.2, p<0.01). As the Exposure Group X Trial Block interaction was significant, we performed post-hoc tests to determine the source of this interaction and found that lever-pressing across both levers in the Alcohol group differed from lever-pressing in the Water group in trial block 4 (trials 13-16) across the 2 days (p<0.05), but overall responding on the two levers did not differ in the other blocks. One purpose of the current experiment was to determine whether the selective increase in responding on the active lever present in our previous experiment [24] would be present in a reversal learning task. For this reason, we performed post-hoc tests on the Exposure Group X Lever X Trial Block interaction and found that responding on the active lever differed between the Alcohol and Water groups in trial blocks 4 and 5 (trials 13-20, Fig 2E, left) (p<0.05), but lever-pressing on the inactive lever did not differ between the Alcohol and Water groups in any trial block (Fig 2E, right) (all p>0.05). This suggests that the overall Exposure Group X Trial Block effects was driven by an increase in active lever responding.
3.2.2. Experiment 2
3.2.2.1. Discrimination
For the groups given 3 discrimination sessions, there were no differences in the original discrimination in percent of trials with a lever-press between the two groups (that would later receive alcohol or water). The percent of trials with a lever-press for the groups given 3 days of discrimination training (Fig 3A, left) was analyzed with a mixed-factor ANOVA with the between-subjects factor of Exposure Group and the within-subjects factors of Lever and Training Day. This analysis found significant effects of Lever (F(1,16)=261.8, p<0.01) and Lever X Training Day (F(2,32)=38.7, p<0.01). There were no significant effects or interactions of Exposure Group (all F<1).
Figure 3. Experiment 2 behavioral data.
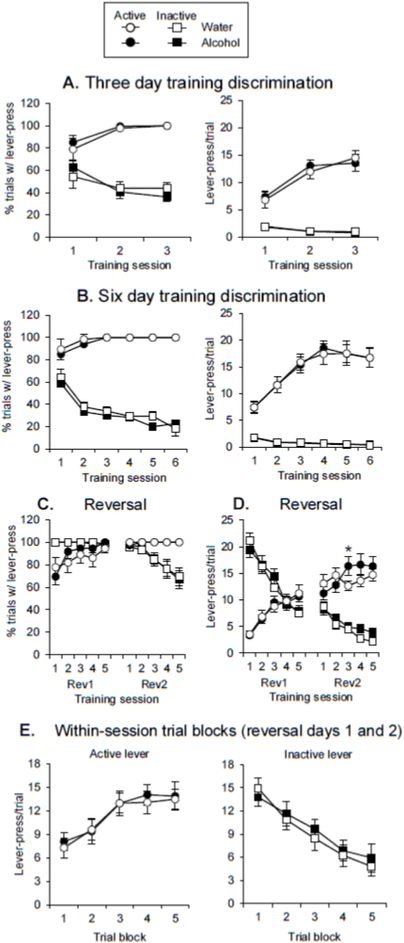
(A) For the three-day training group, percent of trials with a lever-press (mean±SEM; left) or lever-presses/trial (mean±SEM; right) during three discrimination sessions. (B) For the six-day training group, percent of trials with a lever-press (mean±SEM; left) or lever-presses/trial (mean±SEM; right) during six discrimination sessions. (C) Percent of trials with a lever-press (mean±SEM) for reversal learning. (D) Lever-presses per trial (mean±SEM) in reversal learning. (E) Within-session lever-presses per trial (mean±SEM), averaged across reversal days one and two, for the active lever (left) and inactive lever (right). For figures 3C-3E, the #’s 1-5 on the x-axis represent the 5 4-trial blocks for each stimulus within each session. * = significant difference from the corresponding trial blocks for the Water group.
For the groups given 6 discrimination sessions, there were no differences in the original discrimination in percent of trials with a lever-press between the two groups (that would later receive alcohol or water). The percent of trials with a lever-press for the groups given 6 days of discrimination training (Fig 3B, left) was analyzed with a mixed-factor ANOVA with the between-subjects factor of Exposure Group and the within-subjects factors of Lever and Training Day. This analysis found significant effects of Lever (F(1,16)=600.6, p<0.01), Training Day (F(5,80)=5.3, p<0.01), and Lever X Training Day (F(5,80)=36.3, p<0.01). There were no significant effects or interactions of Exposure Group (all F< 1).
For the groups given 3 discrimination sessions, there were no differences in the original discrimination in lever-presses/trial between the two groups (that would later receive alcohol or water). Lever-presses/trial for discrimination for the groups given 3 days of discrimination training (Fig 3A, right) were analyzed with a mixed-factor ANOVA with the between-subjects factor of Exposure Group and the within-subjects factors of Lever and Training Day. This analysis found significant effects of Lever (F(1,16)=153.5, p<0.01), Training Day (F(2,32))= 119.9, p<0.01), and Lever X Training Day (F(2,32)=84.6, p<0.01). There were no significant effects or interactions of Exposure Group (all F<1).
For the groups given 6 discrimination sessions, there were no differences in the original discrimination in lever-presses/trial between the two groups (that would later receive alcohol or water). Lever-presses/trial for discrimination for the groups given 6 days of discrimination training (Fig 3B, right) were analyzed with a mixed-factor ANOVA with the between-subjects factor of Exposure Group and the within-subjects factors of Lever and Training Day. This analysis found significant effects of Lever (F1,16)=284.8, p<0.01), Training Day (F(5,80)=20.5, p<0.01), and Lever X Training Day (F(5,80)=34.3, p<0.01). There were no significant effects or interactions of Exposure Group (all F<1).
3.2.2.2. Discrimination Retraining
In the discrimination retraining session, there were no differences in the percent of trials with a lever-press between the groups that received alcohol or water. The percent of trials with a lever-press for discrimination retraining (data not shown) was analyzed with a mixed-factor ANOVA with the between-subjects factors of Exposure Group and Training Length and the within-subjects factors of Lever and Trial Block. This analysis found significant effects of Training Length (F(1,32)=4.2, p<0.05), Lever (F(1,32)=1443.8, p<0.01), and Trial Block (F(4, 128) 22.6, p<0.01), and significant interactions of Training Length X Lever (F(1,32)=4.2, p<0.01), and Lever X Trial Block (F(4,128)=22.6, p<0.01). There were no significant effects or interactions of Exposure Group (all p>0.05).
In the discrimination retraining session, there were no differences in lever-presses/trial between the groups that received alcohol or water. Lever-presses/trial for discrimination retraining (data not shown) was analyzed with a mixed-factor ANOVA with the between-subjects factors of Exposure Group and Training Length and the within-subjects factors of Lever and Trial Block. This analysis found significant effects of Lever (F(1,32)=384.7, p<0.01), and Trial Block (F(4, 128)= 11.1, p<0.01), and a significant interaction of Lever X Trial Block (F(4,128)=5.1, p<0.01). There were no significant effects or interactions of Exposure Group or Training Length (all p>0.05).
3.2.2.3. Reversal learning
In the reversal learning sessions, there were no differences in percent of trials with a lever-press between the groups that received alcohol or water. The percent of trials with a lever-press for reversal learning (Fig. 3C) was analyzed with a mixed-factor ANOVA with the between-subjects factors of Exposure Group and Training Length, and the within-subjects factors of Lever, Training Day, and Trial Block. We found a significant effect of Trial Block (F(4,128)=2.5, p<0.05) and significant interactions of Lever X Training Day (F(1,32)=44.3, p<0.01), Lever X Trial Block (F(4,128)=24.8, p<0.01), and Training Day X Trial Block (F(4,128)=20.6, p<0.01). There were no significant effects or interactions of Exposure Group or Training Length (all p>0.05).
In the reversal learning sessions, the groups given prior alcohol access exhibited a pattern in which over-responding to the active lever emerged within the second reversal session. Lever-presses/trial for reversal learning (Fig. 3D) were analyzed with a mixed-factor ANOVA with the between-subjects factors of Exposure Group and Training Length, and the within-subjects factors of Lever, Training Day, and Trial Block. We found significant effects of Lever (F(1,32)=4.8, p<0.05), Training Day (F(1,32)=5.2, p<0.05), Trial Block (F(4,128)=9.6, p<0.01) and significant interactions of Lever X Training Day (F(1,32)=236.0, p<0.01), Exposure Group X Trial Block (F(4,128)=4.7, p<0.01), Lever X Trial Block (F(4,128)=149.6, p<0.01), Training Day X Trial Block (F(4,128)=3.5, p<0.01), Lever X Training Day X Trial Block (F(4,128)=24.3, p<0.01), and Exposure Group X Lever X Training Day X Trial Block (F(4,128)=2.7, p<0.05). There were no significant effects or interactions of Training Length (all p>0.05). Post-hoc Tukey’s tests determined that the Exposure Group X Lever X Training Day X Trial Block interaction was driven by a significant increase in active lever-pressing in the third trial block of reversal day 2 (trials 9-12) (p<0.05). While this is a more limited effect than that seen in Exp. 1 (as it was only seen on Day 2 and was not seen if the two reversal sessions were averaged [Fig. 3E]), it replicates the pattern that was observed in Exp. 1, with equivalent responding at the beginning of the session and active lever over-responding emerging within-session.
4. Discussion
We performed two experiments to determine the relationship between voluntary alcohol consumption and two variants of a go/no-go reversal learning task that allowed for multiple responses/trial. We found no effect of alcohol consumption on the acquisition of reversal learning, as there were no differences between the Alcohol and Water groups in the percent of trials with a response to the active and inactive lever in either experiment. In both experiments, the rate of responding on the active lever was equivalent between the groups at the beginning of each session and an increased over-responding effect emerged within-session in the Alcohol groups. Our results extend the over-responding pattern previously observed in discrimination learning [24] to a reversal learning procedure, although the over-responding effect was smaller than that observed in discrimination learning and emerged within-session rather than being present throughout the entire reversal learning sessions. Below, we discuss the lack of an effect in reversal learning, the replicated over-responding effect, and the implications of each.
4.1. No effect of prior alcohol access on reversal learning
We found no effect of prior alcohol consumption on reversal learning in our task under a variety of experimental conditions. These conditions included different cue-lengths or reversal learning following different lengths of prior discrimination training (although we failed to find any effect of training length on the difficulty of reversal learning, supporting the idea that this task dimension does not always affect reversal difficulty [27, 28]). Our lack of an effect in reversal learning adds to the mixed literature on the effects of a history of alcohol exposure on reversal learning tasks, with some experiments finding that alcohol exposure impairs reversal learning [13, 15-19, 30, 31] and other experiments finding no effect or improved reversal learning [19-23]. There are several possible explanations for the varied results of alcohol exposure on reversal learning, including the method and dose of alcohol exposure as well as differences in the specifics of the reversal task.
One possible explanation for our finding that alcohol access did not affect reversal learning is that the voluntarily consumed levels of alcohol failed to meet a threshold of sustained blood-ethanol concentration (BEC) levels required for reversal learning impairments. Using the same method of providing alcohol access in food-restricted rats (average consumption 9-11 g/kg/24-h), we previously found average BECs of 85-90 mg/dl [24]. In that experiment, BECs correlated with drinking levels and we were able to extrapolate a linear curve of 24-h consumption to BECs: (BEC = 15.4 X 24-h alcohol consumption in g/kg – 41.7). In the current experiments, the average alcohol consumption in the last 3 weeks of Exp. 1 and 2 ranged from 7-9 g/kg/24-h, and based on this curve we would estimate average BECs of 66-97 mg/dl in the two experiments. This range of BECs overlaps with those in several experiments in which pre-conditioning alcohol exposure altered conditioned fear after a withdrawal period (estimated BECs of ~80 mg/dl) [32, 33], suggesting that our BECs reached levels able to induce a pharmacological effect. However, our BECs are likely below the high BECs in previous experiments showing that alcohol exposure impairs reversal learning (~ 150-200 [13, 16, 18], ~257-310 [15, 17, 31] or ~500 mg/dl [19]). A recent study compared the effects of intraperitoneal (i.p.) injections (peak BEC = ~500 mg/dl) and i.g. injections (peak BEC = ~200 mg/dl) within the same reversal learning task and found that only the i.p. injections led to later reversal learning impairments [19], suggesting that the threshold to lead to reversal deficits is dose- and BEC-dependent. However, the pattern of effects associated with these alcohol exposure methods is complex. For example, some experiments found that 8-12 exposures to alcohol vapor (with sustained BECs of 150-225 mg/dl) for 16-h/exposure did not impair later reversal learning in touchscreen discrimination and T-maze tasks [20, 22], while 6 exposures to intragastric (i.g.) alcohol injections (which lead to peak BECs of 150 mg/dl that are not sustained) led to reversal learning impairments in a Barnes maze task [13]. It is unclear why fewer exposures with lower BECs/exposure would lead to a reversal learning deficit when more exposures with higher BECs would not, unless the particular reversal learning task used also affects the results. Likewise, 13 5-g/kg i.g. injections starting on post- natal day 28 impaired reversal learning in a bowl foraging task [16], while 16 5-g/kg i.g. injections starting on post-natal day 25 did not impair reversal learning in lever-press and Barnes maze tasks [23]. As the periods of alcohol exposure overlapped, it is unclear why the experiment with longer exposure did not lead to reversal learning impairments, unless the particular reversal learning task used also affects the results.
Differences in the tasks used to assess reversal learning could also be a source of variability in the results of alcohol access/exposure on reversal learning. Previous alcohol-reversal learning experiments utilized tasks involving touch-screen discrimination [20], rule-based T-maze [22], bowl-foraging [16, 18, 19], water-maze [15, 17], Barnes maze [13, 23, 30, 31], and lever-based discriminations (Exp. 1 and 2 in the current paper and [21, 23]). The wide variability in the types of behavioral tasks could explain some of the differing results in the literature. This cannot be the only source of variability, however, as different alcohol exposure procedures led to either impaired [13, 19, 30, 31] or unimpaired [19, 23] reversal learning in Barnes maze and bowl foraging tasks, with performance in each task being impaired by prior alcohol exposure in some experiments and not others. As the current experiments are the first investigation of the effects of alcohol exposure on reversal learning in our task, it is possible that the particular task parameters influenced the current findings.
As described above, the pattern of results on the effects of prior alcohol exposure is complex, and no single factor appears to be the sole determinant of whether prior alcohol exposure will lead to reversal learning impairments. As such, our results add to the mixed literature, but our results do not invalidate earlier studies finding that passive exposure methods (that cause higher BECs) or use of alternate behavioral testing procedures leads to patterns in which alcohol exposure leads to reversal learning impairments. Future research will need to be performed to determine the role of these factors.
4.2. Over-responding by alcohol groups during reversal sessions
In both Exp. 1 and Exp. 2, we found evidence that 6 weeks of CIA access in food-restricted rats (which have elevated alcohol consumption) led to over-responding on the active lever that provided limited food reinforcement for lever-pressing. The results of both experiments replicate our previous findings that CIA access increases responding on the active lever, but had no effect on lever-pressing on the inactive lever [24]. The over-responding effect observed here was relatively small and was limited to over-responding that emerged within-session rather than being present throughout the reversal learning sessions, as was observed in our previous discrimination learning experiments. However, the increased responding in trial block 3 (trials 9-12) of the second reversal session was replicable, as it was observed in two experiments and under different experimental parameters. This small within-session over-responding effect was observed regardless of the length of the original discrimination training (3 or 6 days of training) and across experiments in which the cues lasted 40- or 50-seconds.
Notably, while we collapsed across the first and second day of reversal learning to demonstrate our over-responding effect in Exp. 1, this was not necessary. Based on our finding in Exp. 2 that the over-responding effect was limited to reversal day 2, we ran an exploratory ANOVA of lever-presses/trial limited to day 2 in Exp. 1. We found a significant interaction of Exposure Group X Trial Block (F(4,56)=5.6, p<0.01), just as was found in the ANOVA with both reversal learning days included. Post-hoc tests found that lever-pressing across both levers in the Alcohol group differed from lever-pressing in the Water group in trial blocks 3 and 4 (trials 9-16; p<0.05), but overall responding on the two levers did not differ in the other blocks. As in the main ANOVA across both days, we performed post-hoc tests on the Exposure Group X Lever X Trial Block interaction and found that responding on the active lever differed between the Alcohol and Water groups in trial block 4 (trials 13-16; p<0.05), but lever-pressing on the inactive lever did not differ between the Alcohol and Water groups in any trial block (all p>0.05). Thus, the most consistent finding across the two experiments was an increase in over-responding on the active lever-pressing that emerged within-session in the second reversal learning session.
The over-responding pattern observed here and in our previous paper [24] resembles the over-responding seen in both humans and rodents after extensive alcohol exposure. Our findings suggest an inability to withhold responding, which is seen in humans with a history of excessive alcohol intake. In humans, a history of binge-drinking was associated with premature responding in a human version of the 5-choice serial reaction time task (5CSRTT), with significant correlations between the level of binge-drinking and the number of premature responses [34]. Alcohol-dependent humans also exhibit a failure to withhold responding during the two most common measure of response inhibition in humans, the stop-signal task and the no/no-go task. A meta-analysis by Smith and colleagues [35] found that alcohol dependence in humans was associated with impairments in withholding responding in both tasks, and heavy drinking was associated with impairments in withholding responding in the stop-signal task. In rodents, chronic ethanol treatment is associated with impairments in response inhibition in the 5CSRTT task [36-38]. Perhaps most relevantly, multiple cycles of alcohol consumption (with average consumption of ~13-14 g/kg/24-h) and withdrawal led to impairments in a fixed-interval task [11], Over-responding during this fixed-interval task occurred in a task where reinforcement was available at certain times (every 120 seconds), but increased responding occurred at times when reinforcement was predictably unavailable [11]. Responding to the active lever in our tasks was similar, in that the active lever was associated with a limited amount of reinforcement and over-responding occurred when reinforcement was predictably unavailable. Thus, our current results extend our previous findings of alcohol-induced over-responding to additional experimental conditions (different cue lengths, initial discrimination and reversal from this discrimination). The reliability of our over-responding effect in combination with concurring clinical evidence denotes the translational value of the current findings of over-responding following a history of alcohol consumption.
This analysis suggests that the over-responding effect represents an impairment in impulsivity and response inhibition. We believe that this is the case, although other explanations are possible, including increased hunger after the cessation of alcohol access, withdrawal symptoms, impairments in timing, or changes in executive control. First, increased hunger in the alcohol group (as the calories from alcohol are no longer available) leading to increased motivation for pellets is possible. However, this seems unlikely, as both the water- and alcohol access groups were food restricted to maintain a steady increase in their target weights. As such, the loss of calories in the alcohol group was reflected in their body weights in the days after the cessation of alcohol access and they received additional food chow to compensate for this loss. The rats had several days of this increased food chow before the reversal learning sessions began, which should prevent (or at least mitigate) any increase in hunger. Second, it is possible that this over-responding effect is associated with withdrawal symptoms. However, the lack of anhedonia, which would cause a decrease in responding for food rather than the increases we observe here, suggests that withdrawal symptoms are not likely generating our over-responding effect. Third, the over-responding effect could represent deficits in timing, as no additional pellets are available until after 20 seconds into the cue once the first pellet is earned, and any responses before this 20-second mark could occur because of errors in estimating 20-second intervals. It is difficult to exclude this possibility with our current data. We did not record the exact time of the lever-presses and food deliveries. In addition, any increased lever-pressing during the first 20-seconds of the cue could reflect either a failure in estimating the time of the next food availability (a timing deficit) or a failure to inhibit responding even when timing abilities were normal (an impulsivity/response inhibition deficit). Finally, it is possible that the over-responding reflects general changes in executive control (in choices of how to respond rather an inability to withhold a response that the animal is attempting to withhold). We admit that we cannot exclude this possibility, as there was no penalty for the excessive responding on the active lever (other than wasted effort). As such, while the alcohol-over-responding pattern we observe fits with human data on a relationship between alcohol use and impulsivity measures, it is possible that our over-responding effect reflects alterations in timing or executive functioning instead.
In the current experiments, the over-responding effect was not observed during the early trials of the reversal learning sessions. Instead, over-responding emerged within the lever-press sessions. This pattern contrasts with the results of our previous experiments, in which alcohol consumption preceded the original discrimination learning and over-responding was seen throughout the discrimination training sessions and the overall over-responding effect was larger [24]. The reason for this particular pattern of over-responding is unclear. One possibility is that the previous contingencies, in which the active lever for reversal learning was the inactive lever during the initial discrimination learning phase, led to low responding on the active lever that interfered during the early trials of the reversal learning sessions. The influence of the initial discrimination contingencies may have been increased by spontaneous recovery during the early trials of the second reversal learning session [39, 40] and interfered with the expression of the over-responding effect. Regardless of the cause of this within-session pattern of over-responding, this pattern was observed in both experiments and appears to be a reliable expression of the over-responding effect under reversal learning conditions. Additional research will need to be conducted to further characterize potential spontaneous recovery effects in reversal learning, and boundary conditions that might limit the generality of the over-responding effect.
It is noteworthy that the over-responding effect was also not present during the post-alcohol discrimination retraining session. This suggests several intriguing possibilities about the nature of the over-responding effect. One possibility is that the neurobiological changes induced by alcohol do not emerge until 4-5 days after the cessation of alcohol access and our retraining session 3 days after the cessation of alcohol access occurred too early to be affected by these changes. Some changes in neurobiological markers, such as altered gene expression in the prefrontal cortex and hippocampus [41], increased D1 receptors in the nucleus accumbens [42], and decreased SOX2-immunoreactive-labelled neural stem cells in the subventricular zone [43], appear or increase in magnitude between post-alcohol day 3 and post-alcohol day 7. Although this explanation remains speculative, this suggests that neurobiological changes that could alter behavior might emerge after the 3-4 day interval when the discrimination retraining session occurred. A second possibility is that alcohol access affects new learning that occurs after alcohol access rather than altering expression of learning that occurred prior to alcohol access. If the effects of alcohol access were on expression rather than performance (for example, by spontaneously increasing locomotor activity or increasing motivation for food), these alterations could be expected to immediately increase the amount of responding on the active lever during the discrimination retraining session. In essence, the prior alcohol access would instantly “turn up the gain” on how the prior learning was translated into behavior. Instead, the slower emergence of over-responding (absent during discrimination retraining, weak [Exp. 1] or absent [Exp. 2] in the first day of reversal learning, and strongest in the second day of reversal learning) suggests that prior alcohol access may have had its effects through altering what the animal was learning during their post-alcohol lever-pressing for food. Regardless of the nature of this potential alteration in learning (sensitization to the food, sensitization to the cues associated with the active lever, decreased impact of non-reinforced active lever-presses during over-responding, etc…), this could explain the slow emergence of the over-responding effect.
Notably, both of these explanations for alcohol having no effect on discrimination retraining (delayed effects until 4-5 days after cessation of alcohol or prior alcohol affecting learning rather than a mechanism that increased expression of prior learning) are consistent with our previous findings that pre-discrimination alcohol access caused over-responding during discrimination learning [24]. In that study, one experiment (Exp. 1) started lever-press training 4 days after the end of alcohol access and there was no significant over-responding until the third day of lever-press training, which was 6 days after the end of alcohol access. A second experiment (Exp. 2) started lever-press training 5 days after the end of alcohol access. In this experiment, consistent over-responding (2 days in a row) emerged in 2 out of 3 alcohol exposed groups on the 3rd day of lever-training, which was 7 days after the end of alcohol access. The delayed emergence of over-responding is consistent with either a delayed effect of prior alcohol access and withdrawal, or alcohol having effects on learning that require several sessions of response-reward pairings affect behavior. Future research studies, which give discrimination training prior to alcohol access and then systematically vary the delay from the end of alcohol access until discrimination training is resumed, will be required to differentiate between these possibilities.
5. Conclusions
Here we have demonstrated that prior alcohol access does not impair go/no-go reversal learning, but induce over-responding to the active lever in a task that allows for multiple responses/trial. Our results do not invalidate previous findings of alcohol-induced impairments in reversal learning utilizing different experimental procedures, but instead add valuable information to the mixed alcohol exposure-reversal learning literature. Our data suggest that there are behavioral changes after voluntary alcohol access that can be missed by some discrimination/reversal learning assessments, and our over-responding task can detect these transient changes. The over-responding task represents a sensitive measure that can be used to assess behavioral alterations associated with levels of alcohol that rats will voluntarily drink, rather than requiring forced exposure to cause behavioral alterations, although the magnitude of this effect is smaller during reversal learning than during discrimination learning. Therefore, assessment of over-responding during discrimination learning would be recommended as a task that is more sensitive to alcohol-induced behavioral alterations. Future research will also be needed to determine the neurobiological changes associated with over-responding after these moderate levels of alcohol exposure.
Supplementary Material
Acknowledgments
We would like to thank Samantha Aleman, Lexia Aurand, Nicholas Bright, Anna Cook, Brooke Gaeddert, Naomi Mwebaza, Rebecca Raastad, Brianna Rodgers, Karlyn Ruggle and Kennedy Schmitt for technical assistance. We would like to thank Dr. Sara Keefer, Eliza Greiner, and Danielle Lafferty for comments related to the revision of the manuscript. All procedures and animal care were in accordance with the Kansas State University Institutional Animal Care and Use Committee guidelines, the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and United States federal law.
Funding: This work was supported by the National Institutes of Health [grant number P20 GM113109-01A1] and by start-up funds from Kansas State University.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fein G, Klein L, Finn P, Impairment on a simulated gambling task in long-term abstinent alcoholics, Alcohol Clin Exp Res 28(10) (2004) 1487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tomassini A, Struglia F, Spaziani D, Pacifico R, Stratta P, Rossi A, Decision making, impulsivity, and personality traits in alcohol-dependent subjects, Am J Addict 21(3) (2012) 263–7. [DOI] [PubMed] [Google Scholar]
- [3].Petry NM, Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls, Psychopharmacology (Berl) 154(3) (2001) 243–50. [DOI] [PubMed] [Google Scholar]
- [4].Coskunpinar A, Dir AL, Cyders MA, Multidimensionality in impulsivity and alcohol use: a meta-analysis using the UPPS model of impulsivity, Alcohol Clin Exp Res 37(9) (2013) 1441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Verdejo-Garcia A, Lawrence AJ, Clark L, Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies, Neurosci Biobehav Rev 32(4) (2008) 777–810. [DOI] [PubMed] [Google Scholar]
- [6].Malone SM, Luciana M, Wilson S, Sparks JC, Hunt RH, Thomas KM, Iacono WG, Adolescent drinking and motivated decision-making: a cotwin-control investigation with monozygotic twins, Behav Genet 44(4) (2014) 407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Khemiri L, Kuja-Halkola R, Larsson H, Jayaram-Lindstrom N, Genetic overlap between impulsivity and alcohol dependence: a large-scale national twin study, Psychol Med 46(5) (2016) 1091–102. [DOI] [PubMed] [Google Scholar]
- [8].Heitzeg MM, Nigg JT, Yau WY, Zucker RA, Zubieta JK, Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics, Biol Psychiatry 68(3) (2010) 287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H, Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task, Biol Psychol 69(3) (2005) 353–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ames SL, Kisbu-Sakarya Y, Reynolds KD, Boyle S, Cappelli C, Cox MG, Dust M, Grenard JL, Mackinnon DP, Stacy AW, Inhibitory control effects in adolescent binge eating and consumption of sugar-sweetened beverages and snacks, Appetite 81 (2014) 180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Borlikova GG, Elbers NA, Stephens DN, Repeated withdrawal from ethanol spares contextual fear conditioning and spatial learning but impairs negative patterning and induces over-responding: evidence for effect on frontal cortical but not hippocampal function?, Eur J Neurosci 24(1) (2006) 205–16. [DOI] [PubMed] [Google Scholar]
- [12].Tombaugh TN, Ethanol-Metrecal diets: II. Failure to obtain impaired performance on a series of appetitively and aversively motivated tasks, Pharmacol Biochem Behav 15(3) (1981) 463–9. [DOI] [PubMed] [Google Scholar]
- [13].Kuzmin A, Liljequist S, Meis J, Chefer V, Shippenberg T, Bakalkin G, Repeated moderate-dose ethanol bouts impair cognitive function in Wistar rats, Addict Biol 17(1) (2012) 132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fernandez GM, Stewart WN, Savage LM, Chronic Drinking During Adolescence Predisposes the Adult Rat for Continued Heavy Drinking: Neurotrophin and Behavioral Adaptation after Long-Term, Continuous Ethanol Exposure, PLoS One 11(3) (2016) e0149987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Coleman LG Jr., He J, Lee J, Styner M, Crews FT, Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice, Alcohol Clin Exp Res 35(4) (2011) 671–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fernandez GM, Lew BJ, Vedder LC, Savage LM, Chronic intermittent ethanol exposure leads to alterations in brain-derived neurotrophic factor within the frontal cortex and impaired behavioral flexibility in both adolescent and adult rats, Neuroscience 348 (2017) 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Obernier JA, White AM, Swartzwelder HS, Crews FT, Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats, Pharmacol Biochem Behav 72(3) (2002) 521–32. [DOI] [PubMed] [Google Scholar]
- [18].Badanich KA, Becker HC, Woodward JJ, Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice, Behav Neurosci 125(6) (2011) 879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Badanich KA, Fakih ME, Gurina TS, Roy EK, Hoffman JL, Uruena-Agnes AR, Kirstein CL, Reversal learning and experimenter-administered chronic intermittent ethanol exposure in male rats, Psychopharmacology (Berl) 233(19–20) (2016) 3615–26. [DOI] [PubMed] [Google Scholar]
- [20].DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, Holmes A, Chronic alcohol produces neuroadaptations to prime dorsal striatal learning, Proc Natl Acad Sci U S A 110(36) (2013) 14783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fisher H, Bright N, Gallo M, Pajser A, Pickens CL, Relationship of low doses of alcohol voluntarily consumed during adolescence and early adulthood with subsequent behavioral flexibility, Behav Pharmacol 28(7) (2017) 531–544. [DOI] [PubMed] [Google Scholar]
- [22].Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ, Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex, PLoS One 7(5) (2012) e37541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fernandez GM, Savage LM, Adolescent binge ethanol exposure alters specific forebrain cholinergic cell populations and leads to selective functional deficits in the prefrontal cortex, Neuroscience 361 (2017) 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pickens CL, Fisher H, Bright N, Gallo M, Ray MH, Anji A, Kumari M, Prior alcohol consumption does not impair go/no-go discrimination learning, but causes over-responding on go trials, in rats, Behav Brain Res 312 (2016) 272–8. [DOI] [PubMed] [Google Scholar]
- [25].Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE, Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats, Alcohol Clin Exp Res 32(10) (2008) 1816–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wise RA, Voluntary ethanol intake in rats following exposure to ethanol on various schedules, Psychopharmacologia 29(3) (1973) 203–10. [DOI] [PubMed] [Google Scholar]
- [27].Lovejoy E, Analysis of the overlearning reversal effect, Psychol Rev 73(1) (1966) 87–103. [DOI] [PubMed] [Google Scholar]
- [28].Mackintosh NJ, Further analysis of the overtraining reversal effect, J Comp Physiol Psychol 67(2) (1969) Suppl:1–18. [DOI] [PubMed] [Google Scholar]
- [29].Pickens CL, Aurand L, Hunt J, Fisher H, Subchronic anesthetic ketamine injections in rats impair choice reversal learning, but have no effect on reinforcer devaluation, Behav Pharmacol (2017). [DOI] [PubMed] [Google Scholar]
- [30].Marszalek-Grabska M, Gibula-Bruzda E, Bodzon-Kulakowska A, Suder P, Gawel K, Talarek S, Listos J, Kedzierska E, Danysz W, Kotlinska JH, ADX-47273, a mGlu5 receptor positive allosteric modulator, attenuates deficits in cognitive flexibility induced by withdrawal from 'binge-like' ethanol exposure in rats, Behav Brain Res 338 (2018) 9–16. [DOI] [PubMed] [Google Scholar]
- [31].Coleman LG Jr., Liu W, Oguz I, Styner M, Crews FT, Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility, Pharmacol Biochem Behav 116 (2014) 142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ripley TL, O'Shea M, Stephens DN, Repeated withdrawal from ethanol impairs acquisition but not expression of conditioned fear, Eur J Neurosci 18(2) (2003) 441–8. [DOI] [PubMed] [Google Scholar]
- [33].Stephens DN, Brown G, Duka T, Ripley TL, Impaired fear conditioning but enhanced seizure sensitivity in rats given repeated experience of withdrawal from alcohol, Eur J Neurosci 14(12) (2001) 2023–31. [DOI] [PubMed] [Google Scholar]
- [34].Sanchez-Roige S, Baro V, Trick L, Pena-Oliver Y, Stephens DN, Duka T, Exaggerated waiting impulsivity associated with human binge drinking, and high alcohol consumption in mice, Neuropsychopharmacology 39(13) (2014) 2919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Smith JL, Mattick RP, Jamadar SD, Iredale JM, Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis, Drug Alcohol Depend 145 (2014) 1–33. [DOI] [PubMed] [Google Scholar]
- [36].Irimia C, Wiskerke J, Natividad LA, Polis IY, de Vries TJ, Pattij T, Parsons LH, Increased impulsivity in rats as a result of repeated cycles of alcohol intoxication and abstinence, Addict Biol 20(2) (2015) 263–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Walker SE, Pena-Oliver Y, Stephens DN, Learning not to be impulsive: disruption by experience of alcohol withdrawal, Psychopharmacology (Berl) 217(3) (2011) 433–42. [DOI] [PubMed] [Google Scholar]
- [38].Irimia C, Buczynski MW, Natividad LA, Laredo SA, Avalos N, Parsons LH, Dysregulated Glycine Signaling Contributes to Increased Impulsivity during Protracted Alcohol Abstinence, J Neurosci 37(7) (2017) 1853–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lopez-Romero LJ, Garcia-Barraza R, Vila J, Spontaneous recovery in human instrumental learning: Integration of information and recency to primacy shift, Behav Processes 84(2) (2010) 617–21. [DOI] [PubMed] [Google Scholar]
- [40].Devenport LD, Devenport JA, Time-dependent averaging of foraging information in least chipmunks and goladen-mantled ground squirrels, Animal Behaviour 47(787–802) (1994). [Google Scholar]
- [41].Smith ML, Lopez MF, Archer KJ, Wolen AR, Becker HC, Miles MF, Time-Course Analysis of Brain Regional Expression Network Responses to Chronic Intermittent Ethanol and Withdrawal: Implications for Mechanisms Underlying Excessive Ethanol Consumption, PLoS One 11(1) (2016) e0146257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hirth N, Meinhardt MW, Noori HR, Salgado H, Torres-Ramirez O, Uhrig S, Broccoli L, Vengeliene V, Rossmanith M, Perreau-Lenz S, Kohr G, Sommer WH, Spanagel R, Hansson AC, Convergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence, Proc Natl Acad Sci U S A 113(11) (2016) 3024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hansson AC, Nixon K, Rimondini R, Damadzic R, Sommer WH, Eskay R, Crews FT, Heilig M, Long-term suppression of forebrain neurogenesis and loss of neuronal progenitor cells following prolonged alcohol dependence in rats, Int J Neuropsychopharmacol 13(5) (2010) 583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


