Abstract
Background:
Different diagnostic interviews are used as reference standards for major depression classification in research. Semi-structured interviews involve clinical judgement, whereas fully structured interviews are completely scripted. The Mini International Neuropsychiatric Interview (MINI), a brief fully structured interview, is also sometimes used. It is not known whether interview method is associated with probability of major depression classification.
Aims:
To evaluate the association between interview method and odds of major depression classification, controlling for depressive symptom scores and participant characteristics.
Method:
Data collected for an individual participant data meta-analysis of Patient Health Questionnaire-9 (PHQ-9) diagnostic accuracy were analyzed. Binomial Generalized Linear Mixed Models were fit.
Results:
17,158 participants (2,287 major depression cases) from 57 primary studies were analyzed. Among fully structured interviews, odds of major depression were higher for the MINI compared to the Composite International Diagnostic Interview (CIDI) [OR (95% CI) = 2.10 (1.15–3.87)]. Compared to semi-structured interviews, fully structured interviews (MINI excluded) were non-significantly more likely to classify participants with low-level depressive symptoms (PHQ-9 scores ≤6) as having major depression [OR (95% CI) = 3.13 (0.98–10.00)], similarly likely for moderate-level symptoms (PHQ-9 scores 7–15) [OR (95% CI) = 0.96 (0.56–1.66)], and significantly less likely for high-level symptoms (PHQ-9 scores ≥16) [OR (95% CI) = 0.50 (0.26–0.97)].
Conclusions:
The MINI may identify more depressed cases than the CIDI, and semi- and fully structured interviews may not be interchangeable methods, but these results should be replicated.
INTRODUCTION
Historically, major depression classification in research was done by clinical judgement or unstructured interviews. Lack of agreement between interviewers led to the development of standardized diagnostic interviews, including semi-structured interviews, designed to be administered by clinicians, and fully structured interviews, which can be administered by lay interviewers.1,2 Semi-structured interviews are akin to a guided diagnostic conversation. Standardized questions are asked, but interviewers may insert additional queries and use clinical judgement to decide whether symptoms are present.2,3 Examples include the Structured Clinical Interview for DSM (SCID) and Schedules for Clinical Assessment in Neuropsychiatry (SCAN).4,5 In contrast, fully structured interviews typically involve fully scripted, standardized questions that are read verbatim, without additional probes.2,3 They are designed to be less subjective and provide greater standardization, but with less flexibility and without incorporating clinical judgment.2,3,6 Examples include the Composite International Diagnostic Interview (CIDI) and the Diagnostic Interview Schedule (DIS).7,8 The Mini International Neuropsychiatric Interview (MINI) is also a fully structured interview, but it differs from the CIDI and DIS in that it was described by its authors as designed to be able to be administered in a fraction of the time at the cost of being over-inclusive and generating a higher rate of false-positive diagnoses.9,10
Although fully structured interviews are sometimes referred to as imperfect reference standards compared to semi-structured interviews,11 both are considered appropriate reference standards for major depression classification in research.2 Consistent with this, existing meta-analyses on depression screening tool accuracy have treated both interview types as equivalent reference standards.12 For different interviews to be treated as equivalent diagnostic standards, the probability of being classified as meeting diagnostic criteria should not depend on the interview administered. Different interview formats, however, may lead to different diagnostic patterns. For instance, it is possible that the greater standardization and reliability across interviews gained in fully structured interviews, compared to clinician-administered semi-structured interviews, could increase misclassification.
Five studies have administered validated semi- and fully structured interviews to the same set of participants in non-psychiatric settings within a 2-week period to assess current major depression (SupplementaryTable1).11,13–16 Most included small numbers of participants and major depression cases. Nonetheless, in the three studies with ≥100 participants, prevalence of major depression was more than twice as high when assessed with fully structured interviews compared to semi-structured interviews. No studies have randomized participants to receive either a fully or semi-structured interview and compared major depression prevalence.
The high cost and burden of administering multiple diagnostic interviews to large numbers of participants or, alternatively, randomizing large numbers of participants to receive semi- or fully structured interviews, presents a substantial barrier to testing for differences between interview types. An alternative would be to compare the probability of being classified as having major depression using different interview types, controlling for depression symptom severity and other factors potentially related to classification. Individual participant data (IPD) meta-analysis, in which participant-level data from many studies are synthesized, offers a way to examine the association between diagnostic method and probability of major depression classification across a large number of participants, controlling for factors potentially associated with classification, including depressive symptom severity.
The objective of this study was to examine the association between diagnostic interview method and major depression classification. First, we compared the odds of major depression classification using different diagnostic interviews, first among semi-structured interviews and then separately among fully structured interviews, in each case controlling for depressive symptom severity and study- and participant-level characteristics. Second, we compared the odds of major depression classification between the semi- and fully structured interviews, including a focus on the interviews with the largest numbers of patients, the SCID and the CIDI, and controlling for depressive symptom severity and study and participant-level characteristics. Third, we tested whether differences in the odds of classification across interview types were associated with depressive symptom severity.
METHOD
This study used data accrued for an IPD meta-analysis on the diagnostic accuracy of the Patient Health Questionnaire-9 (PHQ-9) depression screening tool to detect major depression. Detailed methods were registered in PROSPERO (CRD42014010673), and a protocol was published.17 As an initial step, we assessed the comparability of diagnostic classifications generated by different diagnostic interviews.
Search Strategy
A medical librarian searched Medline, Medline In-Process & Other Non-Indexed Citations, PsycINFO, and Web of Science from January 2000 - December 2014 on February 7, 2015, using a search strategy (SupplementaryMethods1), which was peer-reviewed using PRESS.18 We limited our search to these databases based on research showing that adding other databases when the Medline search is highly sensitive does not identify additional eligible studies.19 The search was limited to the year 2000 forward because the PHQ-9 was published in 2001.20 We reviewed reference lists of relevant reviews and queried contributing authors about non-published studies. Search results were uploaded into RefWorks (RefWorks-COS, Bethesda, MD, USA). After de-duplication, unique citations were uploaded into DistillerSR (Evidence Partners, Ottawa, Canada), which was used to store and track search results and track the review process.
Identification of Eligible Studies
Datasets from articles in any language were eligible for inclusion if they included diagnostic classification for current Major Depressive Disorder (MDD) or Major Depressive Episode (MDE) based on a validated semi- or fully structured interview conducted within two weeks of PHQ-9 administration, since diagnostic criteria are for symptoms in the last two weeks. Datasets where not all participants were administered the PHQ-9 within two weeks of the diagnostic interview were included if the primary data allowed us to select participants administered the diagnostic interview and PHQ-9 within two weeks. Data from studies where the PHQ-9 was administered exclusively to patients known to have psychiatric diagnoses or symptoms were excluded, since screening is not done with patients already managed in psychiatric settings.21 For defining major depression, we considered MDD or MDE based on the Diagnostic and Statistical Manual of Mental Disorders (DSM), or MDE based on the International Classification of Diseases (ICD). If more than one was reported, we prioritized DSM over ICD, and DSM MDE over DSM MDD. We prioritized MDE over MDD because screening tests are intended to identify symptoms of depression and not rule out due to bipolar disorder. We prioritized DSM over ICD because DSM is more commonly used in existing studies. However, across all studies, there were only 23 discordant diagnoses that depended on classification prioritization (0.1% of participants).
Two investigators independently reviewed titles and abstracts for eligibility. If either reviewer deemed a study potentially eligible, a full-text article review was completed, also by two investigators independently. Seven members of the research team participated in the review process; however, each title and abstract and each full text was reviewed independently by only two of the seven investigators. Disagreement between reviewers after full-text review was resolved by consensus, including a third investigator (either BL or BDT) when necessary. Titles, abstracts and full-text articles in languages other than English were translated by members of the research team or by advanced research trainees who were native speakers of the language and familiar with the topic. They were not paid for their translation services.
Data Contribution and Synthesis
Authors of eligible datasets were invited to contribute de-identified primary data. Primary study country, clinical setting, language, and diagnostic interview administered were extracted from published reports by two investigators independently, with disagreements resolved by consensus. Countries were categorized as “very high”, “high”, or “low-medium” development level based on the United Nation’s human development index.22 Recruitment settings were categorized as “non-medical”, “primary care”, “inpatient specialty care”, or “outpatient specialty care”. Participant-level data included age, sex, major depression status, and PHQ-9 scores. In three primary studies, multiple settings were included, thus setting was coded at the participant-level.
Individual participant data were converted to a standard format and entered into a single dataset that also included study-level data. We compared published participant characteristics and diagnostic accuracy results with results obtained using the raw datasets. When primary data and original publications were discrepant, we identified and corrected errors when possible, and resolved outstanding discrepancies in consultation with the original investigators. Two investigators assessed risk of bias of included studies independently, using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool.23 See SupplementaryMethods2 for QUADAS-2 coding rules. Discrepancies in data extraction and risk of bias assessment were resolved by consensus.
Statistical Analyses
To isolate the association between diagnostic assessment method and major depression classification, we estimated binomial Generalized Linear Mixed Models (GLMMs) with a logit link function. In all analyses, the outcome was major depression classification. The predictor of interest was either the specific diagnostic interview or interview category, depending on the analysis. Covariates were depressive symptom severity (PHQ-9 score), age, sex, country human development index, and clinical setting. The PHQ-9 has been shown in many studies, across diverse populations in both medical and non-medical settings, to be a valid measure of depressive symptom severity with good convergent validity and a one-dimensional factor structure.20,24–27 Other covariates were chosen due to their potential influence on major depression classification and their availability across primary studies. To account for correlation between subjects within the same primary study, a random intercept was fit for each primary study. Fixed slopes were estimated for PHQ-9 score, assessment method, age, sex, human development index, and clinical setting.
First, we estimated a GLMM among studies that used semi-structured interviews (SCID, SCAN, Depression Interview and Structured Hamilton [DISH]). Then, we estimated a GLMM among studies that used fully structured interviews (CIDI, Clinical Interview Schedule-Revised [CIS-R], Diagnostic Interview Schedule [DIS], MINI). For each model, we used the interview with the greatest number of participants as the reference category.
Second, because the MINI was intentionally designed to be a brief, but overly inclusive, tool,9,10 and based on results from the first analyses, which were consistent with this, we compared fully structured diagnostic interviews, without the MINI, to semi-structured interviews. To do this, we estimated a GLMM to compare odds of major depression classification between the remaining semi- and fully structured interviews, (reference = semi-structured). As a sensitivity analysis, we further restricted our analysis to studies using either the CIDI or SCID (reference = SCID), as these interviews were used substantially more often than other included interviews.
Third, we investigated a possible interaction between interview assessment method and depressive symptom severity based on categorical PHQ-9 score classifications. To do this, we separated PHQ-9 scores into 3 categories: low (scores 0–6; reference group), medium (scores 7–15), and high (scores 16–27). Score ranges were chosen because recent meta-analyses of the PHQ-9 have evaluated cutoff scores from 7 to 15, suggesting a mid-level range.28 To compare models with and without the interaction term, a likelihood ratio test was used. We then replicated the model comparing semi- and fully structured interviews in each PHQ-9 category separately to obtain stratum-specific classification odds ratios for fully versus semi-structured interviews. Additionally, we conducted a separate interaction analysis between continuous PHQ-9 score and diagnostic interview method. As a sensitivity analysis, we further restricted our interaction analyses to studies using the CIDI or SCID.
In another set of sensitivity analyses, we reran all of our models adding domain scores for QUADAS-2. All analyses were run in R using the lme4 package.
Funding and ethics
The study sponsors had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. BDT had full access to all data in the study and had final responsibility for the decision to submit for publication. As this study involved secondary analysis of anonymized previously collected data, the Research Ethics Committee of the Jewish General Hospital declared that this project did not require research ethics approval. However, for each included dataset, we confirmed that the original study received ethics approval and that all patients provided informed consent.
RESULTS
Search Results and Inclusion of Primary Data
Of 5,248 unique titles and abstracts identified from the database search, 5,039 were excluded after title and abstract review and 113 after full-text review, leaving 96 eligible articles with data from 69 unique participant samples (SupplentaryFigure1). Of the 69 unique samples, 55 contributed data (80%). In addition, authors of included studies contributed data from three unpublished studies, for a total of 58 datasets. However, one primary dataset did not include data for key covariates included in analyses and was excluded, leaving 57 primary datasets. In total, 17,158 participants (2,287 major depression cases) were included. Included study characteristics are shown in SupplementaryTable2a. Characteristics of eligible studies that did not provide data for the present study are shown in SupplementaryTable2b. Of the 21,171 participants in 69 eligible published datasets, 16,757 were in the 54 published studies with data included in the present study (79%).
Of the 57 total included studies, 29 used semi-structured interviews, and 28 used fully structured interviews (Table 1). The SCID was the most commonly used semi-structured interview (26 studies, 4,732 participants), and the CIDI (11 studies, 6,271 participants) and MINI (14 studies, 2,756 participants) were the most commonly used fully structured interviews.
Table 1.
Participant data by diagnostic interview
| Diagnostic Interview | N Studies | N Participants | Major Depression |
|
|---|---|---|---|---|
| N | % | |||
| Semi-structured | ||||
| SCID | 26 | 4,732 | 785 | 17 |
| SCAN | 2 | 1,891 | 130 | 7 |
| DISH | 1 | 100 | 9 | 9 |
| Fully structured | ||||
| CIDI | 11 | 6,271 | 554 | 9 |
| DIS | 1 | 1,006 | 221 | 22 |
| MINI | 14 | 2,756 | 524 | 19 |
| CIS-R | 2 | 402 | 64 | 16 |
| Total | 57 | 17,158 | 2,287 | 13 |
Abbreviations: CIDI: Composite International Diagnostic Interview, CIS-R: Clinical Interview Schedule-Revised, DIS: Diagnostic Interview Schedule, DISH: Depression Interview and Structured Hamilton, MINI: Mini International Neuropsychiatric Interview, SCAN: Schedules for Clinical Assessment in Neuropsychiatry, SCID: Structured Clinical Interview for DSM Disorders
Association of Diagnostic Interview and Major Depression Classification
Semi-structured Interviews
Among semi-structured interviews, compared to the SCID, odds of major depression were not significantly different for the SCAN (adjusted odds ratio [aOR] = 0.56, 95% confidence interval [95% CI] = 0.18 to 1.78) or DISH (aOR = 1.13, 95% CI = 0.19 to 6.80). However, only two studies used the SCAN, and only one used the DISH.
Fully Structured Interviews
Among fully structured interviews, compared to the CIDI, odds of major depression were higher, but not significantly different for the DIS (aOR = 4.32, 95% CI = 0.95 to 19.62) or CIS-R (aOR = 1.53, 95% CI = 0.48 to 4.91), although these estimates were based on one and two studies, respectively. Participants interviewed with the MINI were substantially and statistically significantly more likely to be classified as having major depression (aOR = 2.10, 95% CI = 1.15 to 3.87).
Semi-structured versus Fully Structured Interviews
Excluding the MINI, odds of major depression were similar using fully versus semi-structured interviews (aOR = 0.90, 95% CI = 0.51 to 1.57). In a sensitivity analysis restricted to studies that used the SCID or CIDI, odds of major depression were lower for the CIDI compared to the SCID, but this was not statistically significantly different (aOR = 0.57, 95% CI = 0.32 to 1.02).
Interaction between PHQ-9 Scores and Diagnostic Interview Method
The proportion of participants classified as having major depression at each PHQ-9 score for semi-structured interviews, fully structured interviews (MINI excluded), and the MINI are shown in Figure 1a, with differences in proportions across interview types shown in Figure 1b. As shown in Figure 1 and SupplementaryTable3, compared to semi-structured interviews, fully structured interviews resulted in a somewhat higher probability of major depression classification for PHQ-9 scores from 0 to 10, but lower probability for PHQ-9 scores of 11 to 27. Consistent with this, there was a significant interaction between assessment method and PHQ-9 score category (Table 2), and the likelihood ratio test comparing models with and without the interaction term was statistically significant (p < 0.001). The interaction was also statistically significant when tested using the PHQ-9 as a continuous variable. The aOR for the interaction between PHQ-9 score and fully structured interview was 0.90 (95% CI = 0.88 to 0.92), which suggested a 10% dilution in the slope of the odds of a major depression diagnosis across PHQ-9 scores for fully structured interviews compared to semi-structured interviews.
Figure 1a.
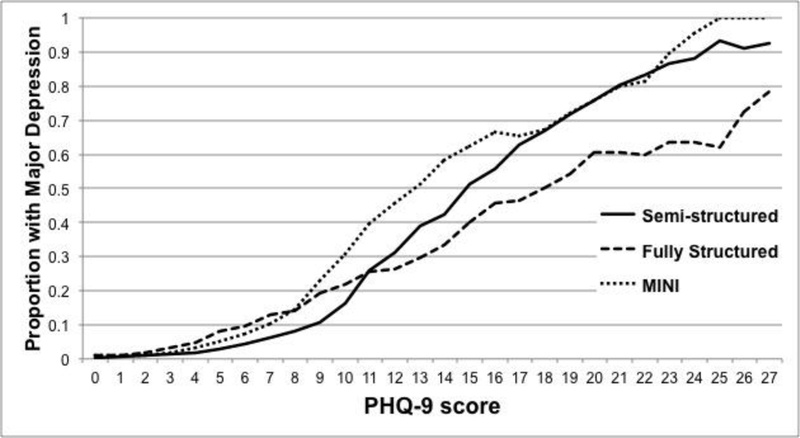
Probability of Major Depression Classification by PHQ-9 Score for Semi-structured Interviews, Fully structured Interviews (Excluding MINI), and MINI.
Proportions are plotted as 3-point moving averages (e.g., the proportions at the PHQ-9 score of 10 are averages of the proportions at PHQ-9 scores of 9, 10, and 11).
Figure 1b.
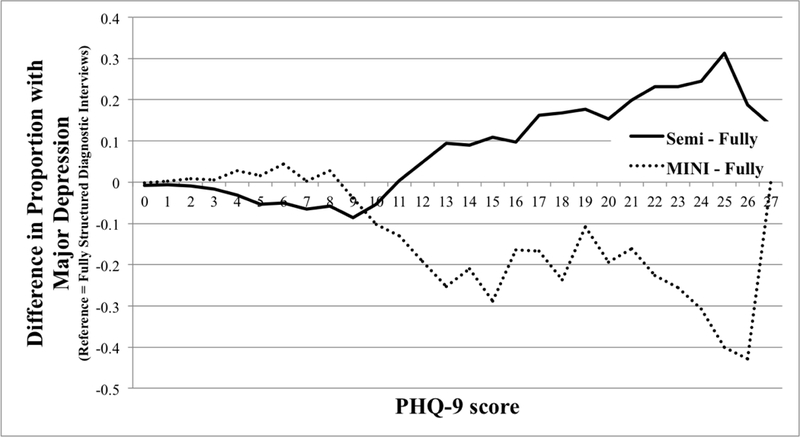
Difference in Probability of Major Depression Classification by PHQ-9 Score for Semi-structured Interviews and MINI compared to Fully structured Interviews (Excluding MINI).
Differences in proportions are plotted as 3-point moving averages (e.g., the differences in proportions at the PHQ-9 score of 10 are averages of the differences in proportions at PHQ-9 scores of 9, 10, and 11).
Table 2.
Model summary of fixed effects generalized linear mixed model considering a potential interaction between PHQ-9 score category and assessment methoda,b
| Variable | Odds ratio (OR) |
|
|---|---|---|
| OR | 95% CI | |
| Fully structured assessment method | 1.49 | 0.82–2.72 |
| PHQ-9 total score | 1.37 | 1.35–1.40 |
| Age (years) | 1.00 | 0.99–1.00 |
| Male sex | 0.89 | 0.77–1.03 |
| Clinical setting | -- | -- |
| Non-medical (reference) | -- | -- |
| Primary care | 0.67 | 0.27–1.64 |
| Specialty care: Inpatient | 0.33 | 0.13–0.85 |
| Specialty care: Outpatient | 0.64 | 0.26–1.54 |
| Human development index | -- | -- |
| Very high (reference) | -- | -- |
| High | 2.27 | 1.11–4.61 |
| Low to medium | 0.78 | 0.27–2.24 |
| PHQ-9 score category * fully structured assessment methodc | -- | -- |
| Low PHQ-9 (0–6) (reference) | -- | -- |
| Medium PHQ-9 (7–15) | 0.73 | 0.57–0.92 |
| High PHQ-9 (16–27) | 0.26 | 0.18–0.37 |
Excluding the MINI.
Estimate of random intercept variance = 0.58.
p < 0.001 in likelihood ratio test comparing models with and without interaction term.
When stratified based on PHQ-9 score category, participants with low PHQ-9 scores (0–6) were more likely to receive a major depression classification with a fully structured interview compared to a semi-structured interview (aOR = 3.13, 95% CI = 0.98 to 10.00), although this was not statistically significant. Semi- and fully structured interviews performed similarly among participants in the medium PHQ-9 group (scores 7–15: aOR = 0.96, 95% CI = 0.56 to 1.66). Among participants with high PHQ-9 scores (16–27), participants were significantly less likely to be classified with major depression using fully structured interviews (aOR = 0.50, 95% CI = 0.26 to 0.97, Table 3). These odds ratios corresponded to a crude prevalence of 3.2% among those administered a fully structured interview vs. 1.2% among those administered a semi-structured interview in the low range PHQ-9 group, 21.4% vs. 20.8% in the medium range group, and 54.2% vs. 72.5% in the high range group, not adjusting for PHQ-9 scores or participant characteristics.
Table 3.
Generalized linear mixed model summaries for each PHQ-9 score category
| PHQ-9 score category | Low PHQ scores (0–6) N = 9,339 |
Medium PHQ scores (7–15) N = 3,970 |
High PHQ scores (16–27) N = 1,093 |
|||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| ORa (95% CI) for fully structured assessment method | 3.13 | 0.98–10·00 | 0.96 | 0.56–1.66 | 0.50 | 0.26–0.97 |
| N receiving fully structured interview | 5,228 | 1,999 | 452 | |||
| N | % | N | % | N | % | |
| N (%) with major depression | 167 | 3.2 | 427 | 21.4 | 245 | 54.2 |
| N receiving semi-structured interview | 4,111 | 1,971 | 641 | |||
| N | % | N | % | N | % | |
| N (%) with major depression | 50 | 1.2 | 409 | 20.8 | 465 | 72.5 |
Excluding the MINI and adjusted for PHQ-9 score, age, sex, clinical setting and human development index.
In sensitivity analyses restricted to studies that used the SCID or CIDI, results for interaction models were similar.
Risk of Bias Sensitivity Analyses
See SupplentaryTable4 for QUADAS-2 ratings for each included primary study. In sensitivity analyses with models that included QUADAS-2 domains, no domains were significantly associated with major depression, and the inclusion of the QUADAS-2 domains did not substantially change coefficient estimates for any variables.
DISCUSSION
There were two main findings. First, among fully structured interviews, the adjusted odds of being classified as having major depression were approximately twice as high using the MINI compared to the CIDI. Second, excluding the MINI, there was a statistically significant interaction between fully structured versus semi-structured interview and depression symptom severity based on the PHQ-9. Compared to semi-structured interviews, the likelihood of diagnosis increased significantly less for fully structured interviews as symptom severity increased. Fully structured interviews tended to classify more participants with low-level symptoms as having major depression, although this was not statistically significant; they performed similar to semi-structured interviews for participants with moderate symptoms, and they classified fewer participants with high-level symptoms as having major depression compared to semi-structured interviews.
The finding that odds of major depression classification were twice as high for the MINI compared to the CIDI is consistent with the interviews’ designs. Whereas the CIDI and other fully structured interviews are in-depth interviews,7,8 the MINI was developed to be able to be administered in a fraction of the time as other interviews and was described by its developers as designed to be over-inclusive.9,10 Our findings suggest that, consistent with the developers’ intent, the MINI may identify substantially higher rates of major depression if used to determine case status than other fully structured interviews. The probability of being classified with major depression was also high based on the DIS and CIS-R, but evidence was too limited to draw conclusions. The formats of these interviews, however, are more similar to the CIDI than the MINI.
By standardizing all questions and probes and removing clinical judgment, fully structured interviews are designed to be as reliable as possible, but this may reduce advantages of semi-structured interviews related to the inclusion of a framework for incorporating clinical judgment. Consistent with this, our findings suggest that compared to semi-structured interviews, the association between symptom levels and probability of being classified as having major depression was lower for fully structured interviews (MINI excluded). Compared to semi-structured interviews, participants with low-level depressive symptoms assessed with fully structured interviews appeared more likely to be classified as having major depression, whereas participants with high-level symptoms appeared less likely. Participants with moderate symptoms were similarly likely to be classified as having major depression when semi- and fully structured interviews were used. This suggests that, in practice, the effect of the diagnostic interview that is selected on the prevalence that is generated likely depends on the underlying distribution of symptom levels in the population.
Existing data from other studies is roughly consistent with this. In general population samples, where depressive symptom levels are generally low, major depression prevalence has been found to be substantially higher when fully structured interviews are used versus semi-structured interviews (SupplementaryTable1).11,13 On the other hand, in a study of patients from an alcoholic treatment unit, where depressive symptoms would be expected to be much higher, major depression prevalence was similar based on semi- and fully structured interviews.15
In research settings, semi- and fully structured interviews are typically used interchangeably as appropriate reference standards in depression screening tool diagnostic accuracy studies, for inclusion and exclusion in treatment trials, and for determining major depression prevalence. Based on the findings of the present study, caution is warranted when deciding which interview to use. Prevalence estimates may be influenced, potentially substantially, by this choice. It is not clear to what degree estimates of screening tool accuracy may be influenced by using a fully versus semi-structured interview, and this should be determined by future studies, including a replication of this study using data from IPD meta-analyses of other depression screening tools.29,30
This is the first study to compare fully and semi-structured interviews for major depression using an IPD meta-analysis approach. Strengths of this study include the large overall sample size and the ability to consider both study and participant-level factors in analyses, including participant-specific depressive symptom severity. There are also limitations to consider. First, we were unable to include primary data from 15 of 69 eligible datasets (20% of eligible datasets, 21% of eligible participants), and we restricted our analyses to those with complete data for all variables in our models (98% of available data). Nonetheless, this was a very large sample, many times the size of existing studies that have attempted to compare fully and semi-structured interviews for major depression. None of those studies included more than 61 cases based on a fully structured interview or 22 cases based on a semi-structured interview. Second, despite the large overall sample size, there was substantial heterogeneity across studies. We were not able to conduct subgroup analyses based on medical comorbidity or cultural aspects such as country or language because comorbidity data were not available for over half of participants, and many countries and languages were represented in few primary studies. However, studies of differential item functioning with the PHQ-9 have shown that it performs equivalently across multiple languages and between people with and without medical disorders.31–35 Third, it is possible that residual confounding may exist, given that we were only able to consider variables collected in the original investigations, and the included study-level variables may not apply uniformly to all participants in a study. Fourth, although we coded for the qualifications of the interviewer for all semi-structured interviews as part of our QUADAS-2 rating, two studies used interviewers who did not meet typical standards, and approximately half of studies were rated unclear. This may have influenced the quality of the reference standard in some studies. Fifth, particularly for semi-structured interviews, lack of interviewer blinding may have influenced classifications. Although only two studies were coded as having non-blinded interviewers, 11 were coded as unclear. We did not query authors on interviewer characteristics and blinding if information was not published due to concern that recollection, in some cases, after over a decade had passed, may not have been accurate.
CONCLUSIONS
We found that the MINI diagnostic interview was associated with a substantially higher probability of major depression classification than the CIDI, controlling for depression symptom scores on the PHQ-9 and other patient characteristics. We also found that compared to semi-structured interviews, fully structured interviews tend to classify more people with low-level symptoms as depressed, but fewer people with high-level symptoms. This suggests that the choice of using a fully structured diagnostic interview or a semi-structured interviews may influence research findings. This is the first study that has used a large participant sample and IPD meta-analysis to compare diagnostic interview methods, and future research should replicate this study to verify results.
Supplementary Material
Acknowledgements:
This study was funded by the Canadian Institutes of Health Research (CIHR, KRS-134297). Ms. Levis was supported by a CIHR Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award. Dr. Benedetti was supported by a Fonds de recherche du Québec - Santé (FRQS) researcher salary award. Ms. Riehm and Ms. Saadat were supported by CIHR Frederick Banting and Charles Best Canada Graduate Scholarship Master’s Awards. Mr. Levis and Ms. Azar were supported by FRQS Masters Training Awards. Ms. Rice was supported by a Vanier Canada Graduate Scholarship. Collection of data for the study by Arroll et al. was supported by a project grant from the Health Research Council of New Zealand. Data collection for the study by Ayalon et al. was supported from a grant from Lundbeck International. The primary study by Khamseh et al. was supported by a grant (M-288) from Tehran University of Medical Sciences. The primary study by Bombardier et al. was supported by the Department of Education, National Institute on Disability and Rehabilitation Research, Spinal Cord Injury Model Systems: University of Washington (grant no. H133N060033), Baylor College of Medicine (grant no. H133N060003), and University of Michigan (grant no. H133N060032). Dr. Butterworth was supported by Australian Research Council Future Fellowship FT130101444. Dr. Cholera was supported by a United States National Institute of Mental Health (NIMH) grant (5F30MH096664), and the United States National Institutes of Health (NIH) Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Heart, Blood, and Lung Institute, and the NIH Office of Research for Women’s Health through the Fogarty Global Health Fellows Program Consortium (1R25TW00934001) and the American Recovery and Reinvestment Act. Dr. Conwell received support from NIMH (R24MH071604) and the Centers for Disease Control and Prevention (R49 CE002093). Collection of data for the primary study by Delgadillo et al. was supported by grant from St. Anne’s Community Services, Leeds, United Kingdom. Collection of data for the primary study by Fann et al. was supported by grant RO1 HD39415 from the US National Center for Medical Rehabilitation Research. The primary studies by Amoozegar and by Fiest et al. were funded by the Alberta Health Services, the University of Calgary Faculty of Medicine, and the Hotchkiss Brain Institute. The primary study by Fischer et al. was funded by the German Federal Ministry of Education and Research (01GY1150). Dr. Fischler was supported by a grant from the Belgian Ministry of Public Health and Social Affairs and a restricted grant from Pfizer Belgium. Data for the primary study by Gelaye et al. was supported by grant from the NIH (T37 MD001449). Collection of data for the primary study by Gjerdingen et al. was supported by grants from the NIMH (R34 MH072925, K02 MH65919, P30 DK50456). The primary study by Eack et al. was funded by the NIMH (R24 MH56858). Collection of data for the primary study by Hobfoll et al. was made possible in part from grants from NIMH (RO1 MH073687) and the Ohio Board of Regents. Dr. Hall received support from a grant awarded by the Research and Development Administration Office, University of Macau (MYRG2015–00109-FSS). The primary study by Hides et al. was funded by the Perpetual Trustees, Flora and Frank Leith Charitable Trust, Jack Brockhoff Foundation, Grosvenor Settlement, Sunshine Foundation and Danks Trust. The primary study by Henkel et al. was funded by the German Ministry of Research and Education. Data for the study by Razykov et al. was collected by the Canadian Scleroderma Research Group, which was funded by the CIHR (FRN 83518), the Scleroderma Society of Canada, the Scleroderma Society of Ontario, the Scleroderma Society of Saskatchewan, Sclérodermie Québec, the Cure Scleroderma Foundation, Inova Diagnostics Inc., Euroimmun, FRQS, the Canadian Arthritis Network, and the Lady Davis Institute of Medical Research of the Jewish General Hospital, Montreal, QC. Dr. Hudson was supported by a FRQS Senior Investigator Award. Collection of data for the primary study by Hyphantis et al. was supported by grant from the National Strategic Reference Framework, European Union, and the Greek Ministry of Education, Lifelong Learning and Religious Affairs (ARISTEIA-ABREVIATE, 1259). The primary study by Inagaki et al. was supported by the Ministry of Health, Labour and Welfare, Japan. Dr. Jetté was supported by a Canada Research Chair in Neurological Health Services Research. Collection of data for the primary study by Kiely et al. was supported by National Health and Medical Research Council (grant number 1002160) and Safe Work Australia. Dr. Kiely was supported by funding from a Australian National Health and Medical Research Council fellowship (grant number 1088313). The primary study by Lamers et al. was funded by the Netherlands Organisation for Health Research and development (grant number 945–03-047). Dr. Lamers received funding from the European Union Seventh Framework Programme (FP7/2007–2013, PCIG12-GA-2012–334065). The primary study by Liu et al. was funded by a grant from the National Health Research Institute, Republic of China (NHRI-EX97–9706PI). The primary study by Lotrakul et al. was supported by the Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand (grant number 49086). Dr. Bernd Löwe received research grants from Pfizer, Germany, and from the medical faculty of the University of Heidelberg, Germany (project 121/2000) for the study by Gräfe et al. The primary study by Mohd Sidik et al. was funded under the Research University Grant Scheme from Universiti Putra Malaysia, Malaysia and the Postgraduate Research Student Support Accounts of the University of Auckland, New Zealand. The primary study by Santos et al. was funded by the National Program for Centers of Excellence (PRONEX/FAPERGS/CNPq, Brazil). The primary study by Muramatsu et al. was supported by an educational grant from Pfizer US Pharmaceutical Inc. Collection of primary data for the study by Dr. Pence was provided by NIMH (R34MH084673). The primary studies by Osório et al. were funded by Reitoria de Pesquisa da Universidade de São Paulo (grant number 09.1.01689.17.7) and Banco Santander (grant number 10.1.01232.17.9). The primary study by Picardi et al. was supported by funds for current research from the Italian Ministry of Health. Dr. Persoons was supported by a grant from the Belgian Ministry of Public Health and Social Affairs and a restricted grant from Pfizer Belgium. Dr. Shaaban was supported by funding from Universiti Sains Malaysia. The primary study by Rooney et al. was funded by the United Kingdom National Health Service Lothian Neuro-Oncology Endowment Fund. The primary study by Sidebottom et al. was funded by a grant from the United States Department of Health and Human Services, Health Resources and Services Administration (grant number R40MC07840). Simning et al.’s research was supported in part by grants from the NIH (T32 GM07356), Agency for Healthcare Research and Quality (R36 HS018246), NIMH (R24 MH071604), and the National Center for Research Resources (TL1 RR024135). Dr. Stafford received PhD scholarship funding from the University of Melbourne. The study by van Steenbergen-Weijenburg et al. was funded by Innovatiefonds Zorgverzekeraars. Collection of data for the studies by Turner et al were funded by a bequest from Jennie Thomas through the Hunter Medical Research Institute. Dr Vöhringer was supported by the Fund for Innovation and Competitiveness of the Chilean Ministry of Economy, Development and Tourism, through the Millennium Scientific Initiative (grant number IS130005). Collection of data for the primary study by Williams et al. was supported by a NIMH grant to Dr. Marsh (RO1-MH069666). Collection of data for the primary study by Zhang et al. was supported by the European Foundation for Study of Diabetes, the Chinese Diabetes Society, Lilly Foundation, Asia Diabetes Foundation and Liao Wun Yuk Diabetes Memorial Fund. The primary study by Twist et al. was funded by the UK National Institute for Health Research under its Programme Grants for Applied Research Programme (grant reference number RP-PG-0606–1142). The primary study by Thombs et al. was done with data from the Heart and Soul Study (PI Mary Whooley). The Heart and Soul Study was funded by the Department of Veterans Epidemiology Merit Review Program, the Department of Veterans Affairs Health Services Research and Development service, the National Heart Lung and Blood Institute (R01 HL079235), the American Federation for Aging Research, the Robert Wood Johnson Foundation, and the Ischemia Research and Education Foundation. Dr. Thombs was supported by an Investigator Award from the Arthritis Society. No other authors reported funding for primary studies or for their work on the present study.
Declaration of Interest: This study was funded by the Canadian Institutes of Health Research (KRS-134297).
Conflict of Interest Disclosures:
Drs. Jetté and Patten declare that they received a grant, outside the submitted work, from the Hotchkiss Brain Institute, which was jointly funded by the Institute and Pfizer. Pfizer was the original sponsor of the development of the PHQ-9, which is now in the public domain. Dr. Chan is a steering committee member or consultant of Astra Zeneca, Bayer, Lilly, MSD and Pfizer. She has received sponsorships and honorarium for giving lectures and providing consultancy and her affiliated institution has received research grants from these companies. Dr. Hegerl declares that within the last three years, he was an advisory board member for Lundbeck, Servier and Otsuka Pharma; a consultant for Bayer Pharma; and a speaker for Medice Arzneimittel, Novartis, Roche Pharma, all outside the submitted work. Dr. Inagaki declares that he has received grants from Novartis Pharma, lecture fees from Pfizer, Mochida, Shionogi, Sumitomo Dainippon Pharma, Daiichi-Sankyo, Meiji Seika, and Takeda, and royalties from Nippon Hyoron Sha, Nanzando, Seiwa Shoten, Igaku-shoin, and Technomics, all outside of the submitted work. Dr. Yamada reports personal fees from Meiji Seika Pharma Co., Ltd., MSD K.K., Asahi Kasei Pharma Corporation, Seishin Shobo, Seiwa Shoten Co., Ltd, Igaku-shoin Ltd., Chugai Igakusha, and Sentan Igakusha, all outside the submitted work. All other authors declare no competing interests. No funder had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Contributor Information
Brooke Levis, Lady Davis Institute for Medical Research, Jewish General Hospital, 4333 Chemin de la Côte-Sainte-Catherine, Montréal, QC, H3T 1E4, Canada.
Andrea Benedetti, Centre for Outcomes Research & Evaluation, Research Institute of the McGill University Health Centre, 5252 boul de Maisonneuve, Office/Workstation # 3D.59, Montréal, QC, H4A 3S5, Canada.
Kira E. Riehm, Lady Davis Institute for Medical Research, Jewish General Hospital, 4333 Chemin de la Côte-Sainte-Catherine, Montréal, QC, H3T 1E4, Canada.
Nazanin Saadat, Lady Davis Institute for Medical Research, Jewish General Hospital, 4333 Chemin de la Côte-Sainte-Catherine, Montréal, QC, H3T 1E4, Canada.
Alexander W. Levis, Lady Davis Institute for Medical Research, Jewish General Hospital, 4333 Chemin de la Côte-Sainte-Catherine, Montréal, QC, H3T 1E4, Canada.
Marleine Azar, Lady Davis Institute for Medical Research, Jewish General Hospital, 4333 Chemin de la Côte-Sainte-Catherine, Montréal, QC, H3T 1E4, Canada.
Danielle B. Rice, Lady Davis Institute for Medical Research, Jewish General Hospital, 4333 Chemin de la Côte-Sainte-Catherine, Montréal, QC, H3T 1E4, Canada.
Matthew J. Chiovitti, Lady Davis Institute for Medical Research, Jewish General Hospital, 4333 Chemin de la Côte-Sainte-Catherine, Montréal, QC, H3T 1E4, Canada.
Tatiana A. Sanchez, Lady Davis Institute for Medical Research, Jewish General Hospital, 4333 Chemin de la Côte-Sainte-Catherine, Montréal, QC, H3T 1E4, Canada.
Pim Cuijpers, Department of Clinical, Neuro and Developmental Psychology, Faculty of Behavioural and Movement Sciences, Vrije Universiteit Amsterdam, Van der Boechorststraat 1, 1081 BT Amsterdam, The Netherlands.
Simon Gilbody, Mental Health and Addiction Research Group, Department of Health Sciences and Hull York Medical School, University of York, Heslington YO10 5DD, United Kingdom.
John P. A. Ioannidis, Stanford University, 1265 Welch Road, MSOB X306, Stanford, CA, 94305, USA.
Lorie A. Kloda, Concordia University, 1455, boul. de Maisonneuve Ouest, FB-802, Montréal, QC, H3G 1M8, Canada.
Dean McMillan, Mental Health and Addiction Research Group, Department of Health Sciences and Hull York Medical School, University of York, Heslington YO10 5DD, United Kingdom.
Scott B. Patten, Department of Community Health Sciences, 3rd Floor, TRW Building, University of Calgary, 3280 Hospital Drive NW, Calgary, AB, T2N 4Z6, Canada.
Ian Shrier, Centre for Clinical Epidemiology, Lady Davis Institute for Medical Research, Jewish General Hospital, 3755 Cote Ste-Catherine Rd, Montréal, QC, H3T 1E2, Canada.
Russell J. Steele, Department of Mathematics and Statistics, McGill University, 805 Rue Sherbrooke O., Montreal, QC, H3A 0B9, Canada.
Roy C. Ziegelstein, Johns Hopkins University School of Medicine, Miller Research Building, 733 N. Broadway, Suite 115, Baltimore, MD, 21205, USA.
Dickens H. Akena, Department of Psychiatry, Makerere University College of Health Sciences, P.O.Box 7062 Kampala, Uganda.
Bruce Arroll, Department of General Practice and Primary Health Care, University of Auckland, Private Bag 92019, Auckland 1142, New Zealand.
Liat Ayalon, Louis and Gabi Weisfeld School of Social Work, Ramat Gan, Bar Ilan University, 52900, Israel.
Hamid R. Baradaran, Endocrinology and Metabolism Research Institute, Shariati Hospital Complex, North Kargar Avenue, Tehran, 14114-13137, Iran.
Murray Baron, Jewish General Hospital, Suite A 725, 3755 Cote St Catherine Rd, Montréal, QC, H3T 1E2, Canada.
Anna Beraldi, Auenstraße 6, D-82467 Garmisch-Partenkirchen, Germany.
Charles H. Bombardier, Division of Clinical and Neuropsychology, Department of Rehabilitation Medicine, University of Washington, Box 359612, Harborview Medical Center, 325 9th Avenue, Seattle, WA, 98104, USA.
Peter Butterworth, Centre for Mental Health, Level 4, 207 Bouverie St, The University of Melbourne, Victoria 3010, Australia.
Gregory Carter, Locked Bag #7, Hunter Region Mail Centre, NSW 2310, Australia.
Marcos H. Chagas, University of São Paulo, Av. Bandeirantes, 3900, 14048-900-Ribeirão Preto, SP, Brazil.
Juliana C. N. Chan, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, 9/F Lui Che Woo Clinical Sciences Building, Prince of Wales Hospital, Shatin, Hong Kong.
Rushina Cholera, UNC School of Medicine, Department of Pediatrics, CB# 7593, Chapel Hill, NC, 27599-7593, USA.
Neerja Chowdhary, World Health Organization. Avenue Appia 20, 1211 Genève 27, Switzerland.
Kerrie Clover, Psycho-oncology #51, Calvary Mater Newcastle, Platt St, Waratah NSW 2301, Australia.
Yeates Conwell, University of Rochester Medical Center, 300 Crittenden Blvd., Rochester, NY, 14642, USA.
Janneke M. de Man-van Ginkel, University Medical Center Utrecht, Internal mail no Str. 6.131, P.O. Box 85500, 3508 GA, Utrecht, The Netherlands.
Jaime Delgadillo, Clinical Psychology Unit, University of Sheffield, Cathedral Court, Floor F, 1 Vicar Lane, Sheffield, S1 1HD, United Kingdom.
Jesse R. Fann, Department of Psychiatry & Behavioral Sciences, Univerisity of Washington, Box 356560, Seattle, WA 98195.
Felix H. Fischer, Medizinische Klinik mit Schwerpunkt Psychosomatik, Charité - Universitätsmedizin Berlin, Charitéplatz 1, 10098 Berlin, Germany.
Benjamin Fischler, Private practice Brussels rue du Pepin 4, Belgium.
Daniel Fung, Institute of Mental Health, 10 Buangkok View, 539747, Singapore.
Bizu Gelaye, Department of Epidemiology, 677 Huntington Ave, Room 505F, Boston, MA, 02115, USA.
Felicity Goodyear-Smith, Department of General Practice & Primary Health Care, University of Auckland, PB 92019, Auckland, 1142, New Zealand.
Catherine G. Greeno, 2204 Cathedral of Learning, University of Pittsburgh, 4200 Fifth Ave, Pittsburgh, PA, 15260, USA.
Brian J. Hall, Department of Psychology, Faculty of Social Sciences, Humanities and Social Sciences Building E21-3040, University of Macau, E21 Avenida da Universidade, Taipa, Macau, China.
John Hambridge, University of Newcastle, NSW 2310, Newcastle, Australia.
Patricia A. Harrison, City of Minneapolis Health Department, 250 S. Fourth St., Room 510, Minneapolis, MN 55415, USA.
Ulrich Hegerl, University of Leipzig, Department of Psychiatry and Psychotherapy, Semmelweisstrasse 10, 04103 Leipzig, Germany.
Leanne Hides, School of Psychology, University of Queensland, St Lucia, Brisbane, Queensland, 4072, Australia.
Stevan E. Hobfoll, 1645 W. Jackson Blvd, Suite 400, Dept of Behavioral Sciences, Rush University Medical Center, Chicago, IL, 60614, USA.
Marie Hudson, Jewish General Hospital and Lady Davis Research Institute, 3755 Côte Ste-Catherine Rd, Room A725, Montréal, QC, H3T 1E2, Canada.
Thomas Hyphantis, Department of Psychiatry, Faculty of Medicine, School of Health Sciences, University of Ioannina, Ioannina 451 10, Greece.
Masatoshi Inagaki, Department of Neuropsychiatry, Okayama University Hospital, 2-5-1, Shikata-cho, Kita-ku, Okayama, 700-8558, Japan.
Khalida Isamail, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, 10 Cutcombe Road, London, SE5 9AF, United Kingdom.
Nathalie Jetté, Department of Clinical Neurosciences, University of Calgary, 1403 29th Street NW, T2N 2T9, Canada.
Mohammad E. Khamseh, Endocrinology and Metabolism Research Institute, Shariati Hospital Complex, North Kargar Avenue, Tehran, 14114-13137, Iran.
Kim M. Kiely, Centre for Research on Ageing, Health and Wellbeing, The Australian National University, Building 54 Mills Road, ACT 2601, Australia.
Femke Lamers, VU University Medical Center, Department Psychiatry, A.J. Ernststraat 1187, room D2.14, 1081 HL Amsterdam, The Netherlands.
Shen-Ing Liu, Department of Psychiatry, Mackay Memorial Hospital, No. 92, Section 2, Chung-Shan North Rd, Taipei, Taiwan.
Manote Lotrakul, Department of Psychiatry, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand.
Sonia R. Loureiro, Rua Tenente Catão Roxo, 2650, CEP 14051-140, Ribeirão Preto, SP, Brazil.
Bernd Löwe, Universitätsklinikum Hamburg-Eppendorf, Institut und Poliklinik für Psychosomatische Medizin und Psychotherapie, Martinistr. 52, Gebäude O25, 20246 Hamburg, Germany.
Laura Marsh, Mental Health Care Line, Michael E DeBakey VA Medical Center, Departments of Psychiatry and Neurology, Baylor College of Medicine, 2002 Holcombe Blvd, Houston, TX, 77030, USA.
Anthony McGuire, Whites Bridge Rd., Standish, ME, 04084, USA.
Sherina Mohd Sidik, Cancer Resource & Education Centre / Department of Psychiatry, Faculty of Medicine & Health Sciences, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia.
Tiago N. Munhoz, Depto Medicina Social, Programa Pós-graduação Epidemiologia, Universidade Federal de Pelotas, Rua Marechal Deodoro 1160, 3º piso, 96020-220 - Pelotas, RS, Brasil.
Kumiko Muramatsu, Department of Clinical Psychology, Graduate School of Niigata Seiryo University, 1-5939, Suidocho, Chuo-ku, Niigata 951-8121, Japan..
Flávia L. Osório, Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto - USP. Avenida dos Bandeirantes 3900- 3 andar- alaC. Ribeirão Preto - São Paulo - Brasil - CEP 14049-900.
Vikram Patel, Department of Global Health and Social Medicine, Harvard Medical School, Boston, USA 02119, USA.
Brian W. Pence, Department of Epidemiology, UNC-Chapel Hill, McGavran-Greenberg 2103C, CB#7435, 135 Dauer Dr, Chapel Hill NC 27599-7435, USA.
Philippe Persoons, Katholieke Universiteit Leuven, Department of Neurosciences, Research Group Psychiatry, University Psychiatric Center KU Leuven, Herestraat 49, 3000 Leuven, Belgium.
Angelo Picardi, Italian National Institute of Health, Centre for Behavioural Sciences and Mental Health, Viale Regina Elena 299, 00161 Rome, Italy.
Alasdair G. Rooney, Division of Psychiatry, University of Edinburgh, Royal Edinburgh Hospital Edinburgh, EH10 5HF, Scotland.
Iná S. Santos, Depto Medicina Social, Programa Pós-graduação Epidemiologia, Universidade Federal de Pelotas, Rua Marechal Deodoro 1160, 3º piso 96020-220 - Pelotas, RS, Brasil.
Juwita Shaaban, School of Medical Science, Health Campus Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan, Malaysia.
Abbey Sidebottom, Allina Health, 800 E 28th Street, MR 15521, Minneapolis, MN 55407-3799, USA.
Adam Simning, Strong Behavioral Health, 300 Crittenden Blvd, Rochester, NY, 14642, USA.
Lesley Stafford, Centre for Women’s Mental Health, The Royal Women’s Hospital, Locked Bag 300, Parkville Victoria 3052, Australia.
Sharon Sung, Office of Clinical Sciences, Duke-NUS Medical School, 20 College Road, Level 6, 169856, Singapore.
Pei Lin Lynnette Tan, Tan Tock Seng Hospital, 11 Jalan Tan Tock Seng, 308433, Singapore.
Alyna Turner, School of Medicine and Public Health, Faculty of Health, University of Newcastle, University Drive, Callaghan, NSW, 2308, Australia.
Christina M. van der Feltz-Cornelis, Tranzo Academic Centre ‘Geestdrift’, Tilburg University, PO Box 90153, 5000 LE Tilburg, The Netherlands.
Henk C. van Weert, Dpt. General Practice, Academic Medical Centre Amsterdam, Meibergdree 9, 1105 AZ Amsterdam, The Netherlands.
Paul A. Vöhringer, Department of Psychiatry, 800 Washington St, Boston, MA 02111, USA.
Jennifer White, School of Primary and Allied Health Care, Faculty of Medicine, Nursing & Health Sciences, Monash University, Kingston Centre, 400 Warrigal Rd, Cheltenham Victoria 3192, Australia.
Mary A. Whooley, Department of Veterans Affairs Medical Center, 4150 Clement Street (111A1), San Francisco, CA 94121, USA.
Kirsty Winkley, King’s College London & Institute of Psychiatry, Psychology & Neuroscience, Weston Education Centre, London SE5 9RS, UK.
Mitsuhiko Yamada, National Center of Neurology and Psychiatry, 4-1-1 Ogawahigashi, Kodaira, Tokyo 187-8553, Japan.
Yuying Zhang, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, 9/F Lui Che Woo Clinical Sciences Building, Prince of Wales Hospital, Shatin, Hong Kong.
Brett D. Thombs, Room 302, Institute of Community and Family Psychiatry, Jewish General Hospital, 4333 Cote Ste Catherine Road, Montréal, QC, H3T 1E4, Canada.
REFERENCES
- 1.Jones KD. The Unstructured Clinical Interview. J Couns Dev. 2010;88:220–226. [Google Scholar]
- 2.Brugha TS, Bebbington PE, Jenkins R . A difference that matters: comparisons of structured and semi-structured psychiatric diagnostic interviews in the general population. Psychol Med. 1999;29:1013–1020. [DOI] [PubMed] [Google Scholar]
- 3.Nosen E, Woody SR. Chapter 8: Diagnostic Assessment in Research In, McKay D. Handbook of research methods in abnormal and clinical psychology. Sage; 2008. [Google Scholar]
- 4.First MB. Structured clinical interview for the DSM (SCID). John Wiley & Sons, Inc; 1995. [Google Scholar]
- 5.World Health Organization. Schedules for clinical assessment in neuropsychiatry: manual. Amer Psychiatric Pub Inc; 1994. [Google Scholar]
- 6.Kurdyak PA, Gnam WH. Small signal, big noise: performance of the CIDI depression module. Can J Psychiatry. 2005;50:851–856. [DOI] [PubMed] [Google Scholar]
- 7.Robins LN, Wing J, Wittchen HU, et al. The Composite International Diagnostic Interview: an epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988:45:1069–1077. [DOI] [PubMed] [Google Scholar]
- 8.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. [DOI] [PubMed] [Google Scholar]
- 9.Lecrubier Y, Sheehan DV, Weiller E et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry. 1997;12:224–231. [Google Scholar]
- 10.Sheehan DV, Lecrubier Y, Sheehan KH et al. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur. Psychiatry. 1997;12:232–241. [Google Scholar]
- 11.Brugha TS, Jenkins R, Taub N, Meltzer H, Bebbington PE. A general population comparison of the Composite International Diagnostic Interview (CIDI) and the Schedules for Clinical Assessment in Neuropsychiatry (SCAN). Psychol Medicine. 2001;31:1001–1013. [DOI] [PubMed] [Google Scholar]
- 12.Rice DB, Kloda LA, Shrier I, Thombs BD. Reporting completeness and transparency of meta-analyses of depression screening tool accuracy: A comparison of meta-analyses published before and after the PRISMA statement. J Psychosom Res. 2016;87:57–69. [DOI] [PubMed] [Google Scholar]
- 13.Anthony JC, Folstein M, Romanoski AJ, et al. Comparison of the lay Diagnostic Interview Schedule and a standardized psychiatric diagnosis: experience in eastern Baltimore. Arch Gen Psychiatry. 1985;42:667–675. [DOI] [PubMed] [Google Scholar]
- 14.Booth BM, Kirchner JA, Hamiltonc G, Harrell R, Smith GR. Diagnosing depression in the medically ill: validity of a lay-administered structured diagnostic interview. J Psychiatr Res. 1998;32:353–360. [DOI] [PubMed] [Google Scholar]
- 15.Hesselbrock V, Stabenau J, Hesselbrock M, Mirkin P, Meyer R. A comparison of two interview schedules: the Schedule for Affective Disorders and Schizophrenia-Lifetime and the National Institute for Mental Health Diagnostic Interview Schedule. Arch Gen Psychiatry. 1982;39:674–677. [DOI] [PubMed] [Google Scholar]
- 16.Jordanova V, Wickramesinghe C, Gerada C, Prince M. Validation of two survey diagnostic interviews among primary care attendees: a comparison of CIS-R and CIDI with SCAN ICD-10 diagnostic categories. Psychol Med. 2004;34:1013–1024. [DOI] [PubMed] [Google Scholar]
- 17.Thombs BD, Benedetti A, Kloda LA, et al. The diagnostic accuracy of the Patient Health Questionnaire-2 (PHQ-2), Patient Health Questionnaire-8 (PHQ-8), and Patient Health Questionnaire-9 (PHQ-9) for detecting major depression: protocol for a systematic review and individual patient data meta-analyses. Syst Rev. 2014;27:3:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.PRESS – Peer Review of Electronic Search Strategies: 2015 Guideline Explanation and Elaboration (PRESS E&E). Ottawa: CADTH; 2016. January. [Google Scholar]
- 19.Sampson M, Barrowman NJ, Moher D, et al. Should meta-analysts search Embase in addition to Medline? J Clin Epidemiol. 2003;56:943–955. [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB: The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thombs BD, Arthurs E, El-Baalbaki G, Meijer A, Ziegelstein RC, Steele RJ. Risk of bias from inclusion of patients who already have diagnosis of or are undergoing treatment for depression in diagnostic accuracy studies of screening tools for depression: systematic review. BMJ. 2011;343:d4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.United Nations. International Human Development Indicators. Available: http://hdr.undp.org/en/countries (accessed 2017 April 26).
- 23.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. [DOI] [PubMed] [Google Scholar]
- 24.Huang FY, Chung H, Kroenke K, Delucchi KL, Spitzer RL. Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. J Gen Intern Med. 2006;21:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin A, Rief W, Klaiberg A, Braehler E. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28:71–77. [DOI] [PubMed] [Google Scholar]
- 26.Adewuya AO, Ola BA, Afolabi OO. Validity of the patient health questionnaire (PHQ-9) as a screening tool for depression amongst Nigerian university students. J Affect Disord. 2006;96:89–93. [DOI] [PubMed] [Google Scholar]
- 27.Milette K, Hudson M, Baron M, Thombs BD. Comparison of the PHQ-9 and CES-D depression scales in systemic sclerosis: internal consistency reliability, convergent validity and clinical correlates. Rheumatology. 2010;49:789–796. [DOI] [PubMed] [Google Scholar]
- 28.Moriarty AS, Gilbody S, McMillan D, Manea L. Screening and case finding for major depressive disorder using the Patient Health Questionnaire (PHQ-9): a meta-analysis. Gen Hosp Psychiatry. 2015;37:567–576. [DOI] [PubMed] [Google Scholar]
- 29.Thombs BD, Benedetti A, Kloda LA, et al. Diagnostic accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for detecting major depression in pregnant and postnatal women: protocol for a systematic review and individual patient data meta-analyses. BMJ Open. 2015;5:e009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thombs BD, Benedetti A, Kloda LA, et al. Diagnostic accuracy of the Depression subscale of the Hospital Anxiety and Depression Scale (HADS-D) for detecting major depression: protocol for a systematic review and individual patient data meta-analyses. BMJ Open. 2016;6:e011913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arthurs E, Steele RJ, Hudson M, Baron M, Thombs BD; Canadian Scleroderma Research Group. Are scores on English and French versions of the PHQ-9 comparable? An assessment of differential item functioning. PLoS One. 2012;7:e52028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang FY, Chung H, Kroenke K, Delucchi KL, Spitzer RL. Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. J Gen Intern Med. 2006;21:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung H, Kim J, Askew RL, Jones SMW, Cook KF, Amtmann D. Assessing measurement invariance of three depression scales between neurologic samples and community samples. Qual Life Res. 2015;24;1829–1834. [DOI] [PubMed] [Google Scholar]
- 34.Cook KF, Kallen MA, Bombardier C, et al. Do measures of depressive symptoms function differently in people with spinal cord injury versus primary care patients: the CES-D, PHQ-9, and PROMIS-D. Qual Lif Res. 2017;26:139–148. [DOI] [PubMed] [Google Scholar]
- 35.Leavens A, Patten SB, Hudson M, Baron M, Thombs BD, Canadian Scleroderma Research Group. Influence of somatic symptoms on Patient Health Questionnaire-9 depression scores among patients with systemic sclerosis compared to a healthy general population sample. Arthritis Care Res. 2012;64:1195–1201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


