Abstract
Background: Repeated nonextreme sun exposures induce skin pigmentation by increasing melanin production and by oxidizing preexisting melanin and melanin precursors. This leads to skin disorders and skin color heterogeneity such as hyperpigmented spots. Objective: We assessed 31 randomized, controlled clinical trials to determine the potential of vitamin C to limit ultraviolet (UV) daylight-induced pigmentation, considering dose response and different skin type populations (Caucasian and Chinese). Materials and Methods: Thirty-one intraindividual, randomized, controlled clinical trials involving Caucasian and Chinese subjects (15–35 healthy male or female volunteers per study, 741 total volunteers) 18 to 50 years of age with Phototype III and individual typology angle (ITA) value between 28 and 49 degrees were analyzed. The 31 studies assessed the potential of vitamin C (formulated with the copolymer Styrène-Anhydride Maléique [SMA]) to decrease pigmentation induced by UV daylight exposure. Results were combined using a Bayesian meta-analysis to provide probabilistic evidence of the effects of vitamin C by dose and population. Results: Vitamin C was effective in reducing pigmentation induced by UV daylight-simulated expositions (4 days at 0.75 Individual Minimal Erythemal Dose [MEDi]) in a dose-dependent manner. During the depigmentation phase, no additive value was provided by the vitamin C, suggesting that the lightening properties described in the literature for vitamin C correspond to an antipigmenting quality rather than a depigmenting effect. Conclusion: Vitamin C is a valuable and safe dermocosmetic antipigmenting compound with a strong effect at 10% possibly useful in preventing signs of photoaging.
Keywords: Antipigmentation, topical, vitamin C, Bayesian
Repeated nonextreme sun exposure induces skin hyperpigmentation by increasing melanin production by melanocytes and its transfer to the keratinocytes over the epidermis. It induces the oxidation of preformed melanin and melanin precursors. Hyperpigmentation is the visible result of those phenomena and is frequently linked to a melanocyte homeostasis disturbance in the basal layer of the epidermis.1,2 Over time, this can lead to skin disorder and heterogeneity such as hyperpigmented spots.3-5
Hyperpigmentation is frequently managed by targeting the process of melanin production within melanocytes through topical application of antityrosinase compounds, while topical antioxidants provide protection against free radicals produced during ultraviolet (UV) exposures.1 Vitamin C (ascorbic acid) is a naturally occurring, potent, water-soluble molecule with antioxidant properties. It neutralizes the oxidative stress by a process of electron transfer and/or donation.7 Vitamin C is an effective skin lightener that has been described as a melanogenesis inhibitor due to its inhibition of tyrosinase and reduction of melanin and melanin intermediates, such as dopaquinone.8 Accordingly, several in-vitro data and clinical studies support the use of topically applied vitamin C for antiaging, anti-inflammatory, antipigmentation, skin-lightening, and photoprotection uses, as well as for managing skin pigmentation disorders, such as melasma or hyperpigmented spots.1,6–9
Vitamin C is usually added to cosmetic products. Most plants and animals are able to synthesize vitamin C, but humans are unable to do this due to the absence of the enzyme L-glucono-gamma lactone oxidase.6 Humans can ingest vitamin C from dietary sources, such as fruits and vegetables. However, oral supplementation with vitamin C produces only a limited increase in skin concentration, so topically applied vitamin C has become an alternate approach. The formulation of a cosmeceutical product containing vitamin C is important to the product efficacy, as vitamin C is a hydrophilic and unstable molecule with a low level of penetration into the skin.7 Reducing its acidity by decreasing the pH or using an instrumentation method, such as ultrasound, iontophoresis, or laser microdermabrasion, can enhance its penetration.9
Several options with different mechanisms prevent UV-induced pigmentation, such as hydroquinone, phenolic compounds, melano-toxic agents, retinoids, epidermal renewal agents, ellagic acid, antioxidants, kojic acid, and antityrosinase.10–18
Vitamin C is considered a safe and effective method of preventing UV-induced skin pigmentation. However, clinical studies on the efficacy of topical formulations of vitamin C using simulated daily UV conditions remain limited in number. Most of the studies are performed in vitro and most of the in-vivo studies are nonhuman studies, done under UVB alone or performed in association with other agents or with a derivative of vitamin C.6,7,9 Furthermore, all of these studies contained only a small number of subjects.
This article describes a meta-analysis of 31 randomized, controlled clinical trials assessing vitamin C on healthy skin in volunteers under UV daylight (UVDL)-simulated pigmentation in a standard protocol. A Bayesian meta-analysis of the skin-lightening potential of vitamin C was performed by dose and skin type population.19,20 Due to this standard protocol, our meta-analysis was able to include data from more than 700 volunteers, providing a unique observation of vitamin C efficacy with different doses and skin types.
MATERIALS AND METHODS
Clinical settings and study design. The skin lightening effect of vitamin C was compared with its vehicle in 31 randomized, double-blind, intraindividual clinical trials. The studies were conducted at seven investigational sites in France, Romania, the United States, and China between the years 2003 and 2016. The studies conformed to the local legal requirements and were performed according to the principles of the Declaration of Helsinki; each subject provided written informed consent prior to undergoing any procedure. Research studies were performed to assess skin lightening agents on a standard protocol for which vitamin C was used as a reference.
Population. For each trial, 15 to 35 healthy women and men, aged 18 to 50 years, with Phototype III and individual typology angles (ITAs) ranging from 28 to 49 degrees were recruited. The ITA21 is derived from the L*a*b* color space (Commission of Illumination, 1976) using the following formula: ITA°=arctan (L*–50)/b*)180/π.
Subjects without any history of abnormal response to sun and with no UV tanning marks, freckles, nevi, or hair on the back were selected. Subjects were requested to not expose themselves to solar or artificial sources of light during the entire study duration (Table 1).
TABLE 1.
Number of studies per concentration of vitamin C with corresponding number of volunteers, population, and ITA mean at baseline
| VITAMIN C CONCENTRATION, % | NUMBEROF STUDIES | NUMBER OF VOLUNTEERS | POPULATION | ITA°MEAN |
|---|---|---|---|---|
| 10 | 1 | 15 | Caucasian | 38° |
| 7 | 27 | 229 | Chinese:8 studies | 38° |
| 467 | Caucasian: 19 studies | 35° | ||
| 5 | 6 | 105 | Caucasian | 38° |
| 3 | 1 | 15 | Caucasian | 40° |
| 2 | 1 | 15 | Caucasian | 38° |
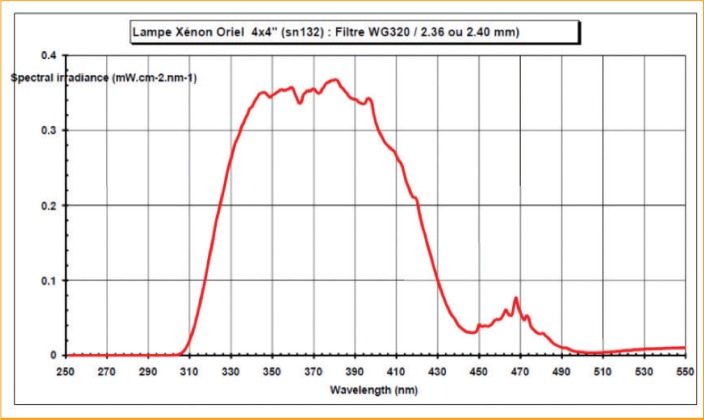
Treatment and ultraviolet daylioht exposures. UVDL was produced by a solar simulator (ORIEL 1600 Watt lamp; Oriel Instruments, Stratford, Connecticut) equipped with a filter WG 320 for UVDL exposures.22 The spectrum delivered by this exposure contains UVB starting at 310nm, the entire UVA portion, and visible light up to 460nm, with a U VA/UVB ratio of close to 25 (Figure 1).
FIGURE 1.
Ultraviolet (UV) daylight spectrum (with filter WG320) used for exposures representing the spectral irradiance (in mW/cm2) for each nanometer (nm)—The obtained spectrum started after 310nm and finished around 460nm. It contains a portion of UVB, the entire UVA, plus a portion of visible light with a ratio UVA/UVB close to 25.
UVDL-simulated exposure induces the basic pigmentation phenomena like those observed with sunlight exposure throughout the span of a day. Extreme exposures only occur at limited conditions linked to latitude (from 33.5° north to 33.5° south), season, cloud coverage, and degree of pollution. Most people never receive such exposure, and so the UVDL spectrum represents a more realistic irradiance.22 The immediate pigment darkening (IPD) occurs within minutes of exposure and can last up to two hours. IPD is followed by persistent pigment darkening (PPD), which can last from 24 hours to several months. Delayed tanning (DT) occurs 3 to 5 days after exposure and can persist for several days to weeks or sometimes even months.23
To induce these phenomenon, five areas (5cm × 6cm or 30cm2) were delineated on both sides of the spinal column on the backs of the subjects. According to a pre-established randomization plan, each subject received vitamin C and the vehicle on the application areas. Products were applied five days a week during seven weeks at a dose of 4mg/cm2 to ensure there was enough product administered to the skin. As vitamin C is a hydrophilic-active ingredient particularly sensitive to oxidation, it must be introduced within an anhydrous formulation or in a formulation containing a high ratio of glycols (low water activity). To incorporate it into cosmetically acceptable formulations that preserve its biological properties, it should be stabilized. In these studies, the vitamin C was formulated with a copolymer, Styrène-Anhydride Maléique (SMA), neutralized by soda (SMA 1000HNA) in an aqueous medium at an ambient temperature with a pH equal to 6. In these conditions, the SMA polymer and vitamin C combine in the form of hydrophobic entanglements, which preferentially position themselves at the interface. The formed complex is more stable, and the efficacy of the vitamin C is not reduced in comparison to the pure molecule.
From Days 8 to 11, investigational areas were exposed for Caucasian skin to 0.75 Individual Minimal Erythemal Dose (MEDi) previously determined during the first week. For Chinese skin, during other studies not described here, we observed irritative reaction under 0.75 MEDi.24,25,26 A specific study was performed to compare 0.5 MEDi and 0.75 MEDi on Chinese skin, during which we observed that 0.5 MEDi induced the requested pigmentation without any irritative reaction (data not shown).27 Therefore, in the Chinese study described in this article, skin areas were exposed to 0.5 MEDi.
Colorimetricand visual assessments. Skin color measurements and visual evaluations were assessed at baseline before the first product application and before first UVDL exposure, 24 hours after each UVDL exposure, four and eight days after the last UVDL exposure, and once per week during the follow-up period. Assessments were performed on each UVDL exposed and treated, unexposed and treated, and on untreated and unexposed sites (Table 2).
TABLE 2.
Treatment and assessments design*
| WEEK/DAY NUMBER | TREATMENT FREQUENCY | UV EXPOSURE | ASSESSMENTS |
|---|---|---|---|
| Week 1 | |||
| Day 1 | 2x/day | No | Yes |
| Day 2 | No | ||
| Day 3 | No | ||
| Day 4 | No | ||
| Day 5 | No | ||
| Week 2 | |||
| Day 8 | 3x/day | Yes | Yes |
| Day 9 | Yes | Yes | |
| Day 10 | Yes | Yes | |
| Day 11 | Yes | Yes | |
| Day 12 | No | Yes | |
| Week 3 | |||
| Day 15 | 2x/day | No | Yes |
| Day 16 | No | ||
| Day 17 | No | ||
| Day 18 | No | ||
| Day 19 | Yes | ||
| Weeks 4–6 | |||
| Days 22–26 | 1x/day | No | Yes** |
| Week 7 | |||
| Day 43 | 1x/day | No | No |
| Day 44 | Once daily | No | |
| Day 45 | No | ||
| Day 46 | No | ||
| Day 47 | No treatment | yes | |
| *Treatment was applied at site for 5 days a week during 7 weeks (except on Day 47). Number of applications per day varied from 1 to 3 times daily, depending on the week. UV exposures were done once a day on Days 8, 9, 10, and 12. Assessments (colorimetry and visual scoring of pigmentation) were completed before product application. | |||
| **Assessments on Fridays (Days 26, 33, and 40) UV: ultraviolet | |||
Colorimetric measurements were done using a Chromameter® Minolta CR400 (Konika Minolta, Tokyo, Japan) with L*a*b* color system (Commission of Illumination, 1976). Visual scoring of pigmentation was performed by a trained technician using a 14-unit grading scale (from “no pigmentation” to “pronounced brown +”) under a daylight ceiling D65.
Safety was assessed through adverse event reporting, including local tolerance.
The efficacy of the vitamin C on UV-induced skin pigmentation was assessed based on the difference in Delta E between vitamin C and its vehicle, with Delta E defined in 1976 by the international Commission of Illumination as follows, where π represents the difference between exposed and unexposed treated areas:

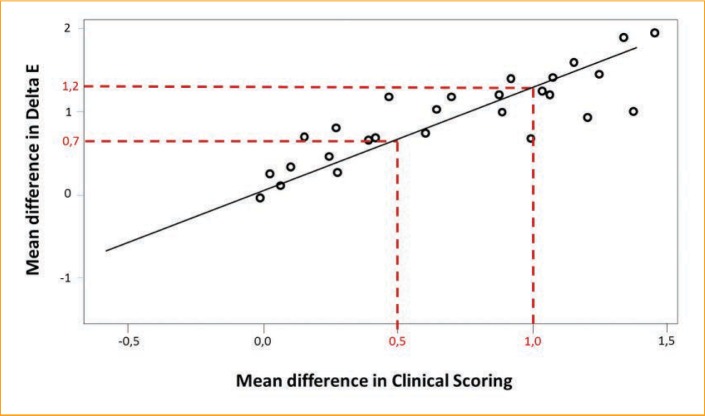
The aim of the Bayesian approach for the meta-analysis was to estimate the probabilities of belonging to performance classes for the vitamin C expressed as the Delta E difference between the vehicle and vitamin C. To determine the class thresholds, the colorimetric measurements (difference in Delta E) were matched with the Visual Score of Pigmentation (VSP) value. Our expertise indicates that a VSP value of 0.5 points is perceptible by an expert, and a VSP value of 1 or greater can be perceived by a consumer. The observed correlation allowed us to determine three performance classes: 1) the nonperceptible effect (corresponding to a Delta E difference from 0.0–0.7 points); 2) the perceptible effect for a trained assesser or expert (Delta E difference from 0.7–1.2 points); and 3) the perceptible effect for a consumer (Delta E difference >1.2 points) (Figure 2). The nonperceptible effect was referred to as the “weak class,” the perceptible effect by the expert was labeled the “moderate class,” and the perceptible effect for a consumer was called the “strong class.”
FIGURE 2.
Crossing graphic of Delta E and Visual Scoring of Pigmentation for vitamin C 7% vs. vehicle to determine performance classes—Each dot represents a study (i.e., the 27 studies with vitamin C 7%). For regression, looking at the values 0.5 and 1 for the visual scoring, the corresponding Delta E are respectively 0.7 and 1.2, providing the threshold for the performance classes.
Statistical analysis. The primary endpoint of this clinical study was the skin pigmentation modulation induced by the treatments, which was determined by calculating the colorimetric distance Delta E (ΔE) between the exposed and unexposed treated areas. A Delta a* calculation was also performed with the combination of visual assessment of erythema to control the product efficacy on erythema. The a* value represents the red color of the skin that is driven by the redness of both pigmentation and erythema.
Prior to any analysis, the homogeneities of the treated and untreated unexposed areas were tested to ensure that the products would have no effect on the unexposed area.
Using individual subject measurements at Day 12 from the 31 trials, a Bayesian metaanalysis was performed by skin type population (Chinese and Caucasian) and concentration on the difference in Delta E, between vehicle and vitamin C, to allow for establishment of a global ranking of vitamin C efficacy as a function of skin type population (Chinese and Caucasian) and dose concentration. The interest of using a Bayesian meta-analysis compared to a frequentist one is to explicitly use probability distributions as a way to quantify uncertainties on the parameters.
Let ΔΔEj(i) represent the difference in Delta E, between vehicle and vitamin C for subject j in study i. The model can therefore be written as: ΔΔEj(i)=θi+εj(i), where ![]() .The parameter θi is given with the prior distribution θi
~N(θ,τ2), with vague prior distributions for the variance ε2i, and the hyperparameters θ and
.The parameter θi is given with the prior distribution θi
~N(θ,τ2), with vague prior distributions for the variance ε2i, and the hyperparameters θ and ![]() ~ IG(0.01, scale=0.01), θ~N(0,1000), τ2~IG (0.01, scale=0.01).
~ IG(0.01, scale=0.01), θ~N(0,1000), τ2~IG (0.01, scale=0.01).
To derive posterior distributions for the parameters, conjugate sampling, when available, or random-walk Metropolis algorithm (Markov chain Monte Carlo method) were used to generate a sequence of draws from the joint posterior distribution.
Credibility intervals for θi were graphically displayed using Forest plots as well as distribution profiles with associate performance classes.
The Bayesian meta-analysis was performed using the Proc MCMC function in the SAS® 9.4 statistical software program (SAS Institute Inc., Cary, North Carolina).
RESULTS
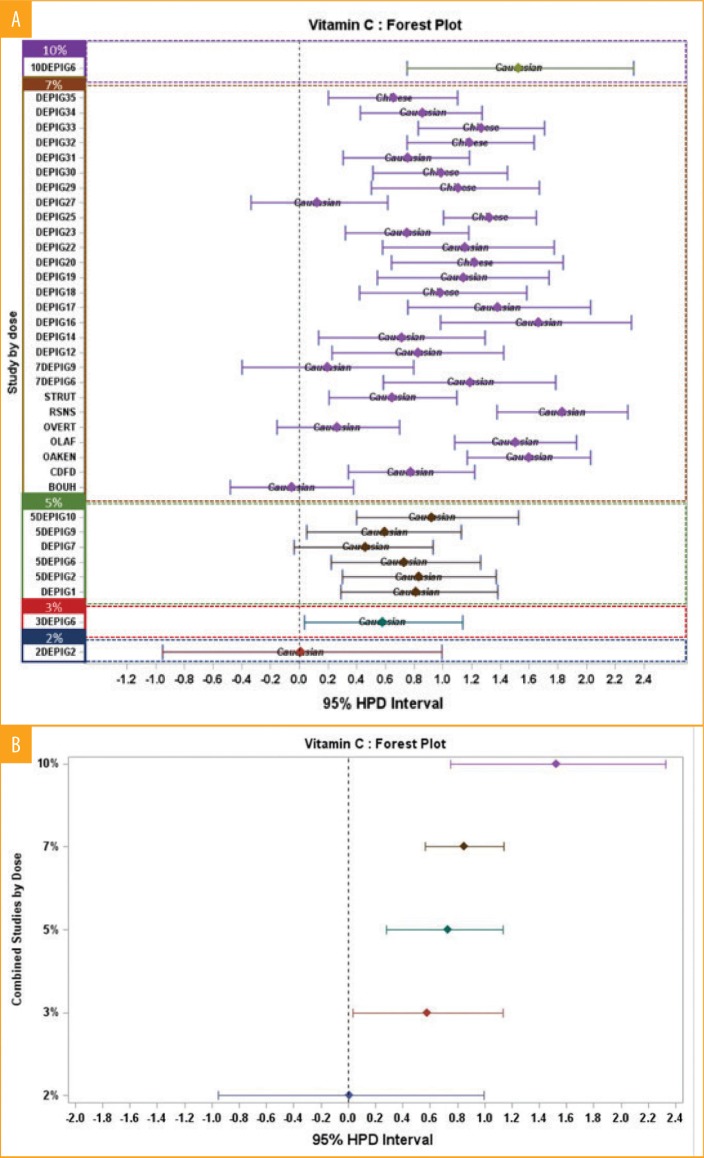
The Forest plot (Figure 3a) shows estimates of treatment effect by study along with 95-percent credible intervals. Overall effect estimates by concentration and associated 95-percent credible intervals are also displayed in Figure 3b. This representation allowed us to observe that vitamin C had a very low effect at 2%. At 3%, 5%, and 7%, vitamin C had a moderate effect. Finally, a higher effect is observed at the 10% concentration, estimated as being a strong effect. Vitamin C 10% was assessed only once (Depig6) and compared to 7%, 5%, and 3%. In this study, we observed a dose effect. However, in other studies (Depig16, RNSN, OLAF, OAKEN), vitamin C 7% had a similar efficacy as that seen at 10% in the Depig6 study, indicating that the results of 10% must be taken with caution and ideally should be reinforced with additional research. The response at 7% is globally a moderate effect. However, this concentration has been observed in some studies to have a weak or nil effect or, contrarily, a strong effect. The distribution of the effect of vitamin C at 7% is quite large, from a negative to a strong effect, with a global moderate effect. As for vitamin C 5%, we observed a moderate effect (6 studies involving Caucasian populations). With regard to skin type, at 7%, effects on Caucasian and Chinese are in the moderate class even though the effect upon the Chinese cohorts was higher compared with that in the Caucasian group (8 Chinese and 19 Caucasian cohorts were studied).
FIGURE 3.
A) Forest plot graphic per studies and concentration with both Caucasian and Chinese populations—As an example, the concentration 7% varies from 0 to 1.6 of Delta E difference; B) Forest plot graphic with mean per concentration with both Caucasian and Chinese populations—As an example, the concentration 7% is globally superior to 0.8%, corresponding to the moderate effect class.
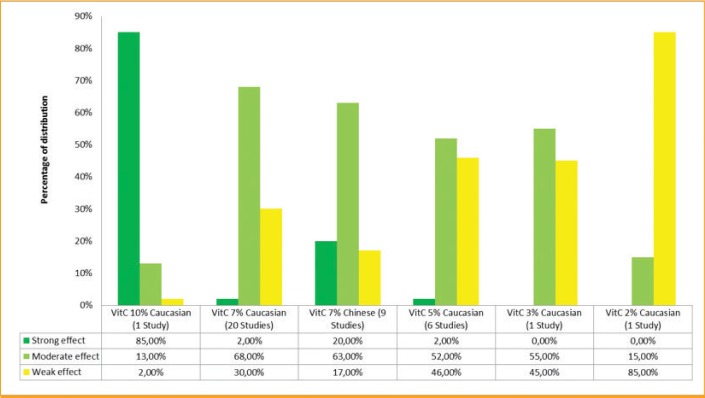
To better determine the effect of each concentration depending on the population, the combination of data for a concentration and a population can be done in order to have a global result. It allows the observation that vitamin C 10% (Caucasian population in 1 study, Figure 4) presents an 85-percent strong effect class membership and 13 percent in the moderate effect class. Vitamin C at 7% in Chinese populations has a 20-percent strong effect and a 63-percent moderate effect (9 studies; graphic not shown). The vitamin C 7% in Caucasian subjects has a 68-percent moderate effect without any strong effect (20 studies; graphic not shown). The 5% and 3% concentrations in Caucasian subjects have around a 55-percent moderate effect, while vitamin C 2% has only a 15-percent moderate effect (6 studies and 1 study, respectively; graphic not shown).
FIGURE 4.
Vitamin C 10% distribution profile in Caucasian population—85% of its effect is strong, 13% moderate, and 2% weak.
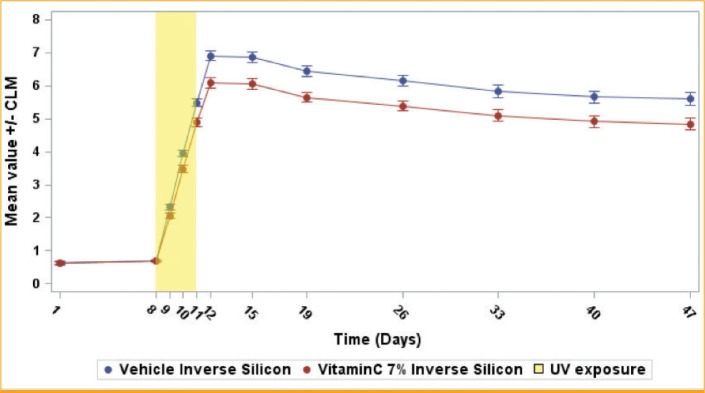
Descriptive results and observation. This meta-analysis is based on the difference in Delta E between the vehicle and vitamin C at Day 12. At this time point, the vehicle presents a higher pigmentation level. This corresponds to the maximum effect observed for vitamin C. The other time points confirmed such observations (data not shown). However, it is also important to look at the global kinetic of the effect. Looking at the mean curve of the 31 studies (Figure 5), the observed effect occurred after the first exposure and increased after each new exposure, but became particularly significant at 24 hours after the third exposure. The maximum was achieved at Day 12 and the effect versus vehicle remained stable or slightly decreased.
FIGURE 5.
Vitamin C 7% vs. vehicle inverse silicon mean Delta E evolution in time for Caucasian population—The vehicle, known to have no effect against ultraviolet (UV), illustrates the skin reaction under the exposures. Pigmentation increases in an incremental manner after each exposure until 7 points of Delta E at 24 hours after the last exposure. Then, pigmentation slightly decreases until the end of the study, without recovering to the initial level. Vitamin C 7% demonstrates efficacy during the increase of pigmentation after each exposure. Then, vitamin C does not have any effect during the natural decreasing of pigmentation. The same comportment is observed in Chinese skin and for the other effective concentrations (10%, 5%, and 3%).
Tolerance and safety observation. Regarding safety, no intolerance was recorded in any of the studies. Vitamin C has a very good safety profile on nonexposed and UV-exposed skin at every concentration.
DISCUSSION
The present meta-analysis describes the results obtained by several clinical evaluations with more than 700 healthy volunteers assessing the skin lightening effect of vitamin C at different concentrations and on different skin type populations. To allow an objective ranking of vitamin C, a Bayesian meta-analysis was conducted, confirming the higher skin lightening benefit of vitamin C 10% when compared with the lower concentrations of 7%, 5%, 3%, and 2% (Figure 6). This meta-analysis allowed us to observe a globally similar moderate effect was observed for vitamin C at 5% and 7% and a strong effect was noted at 10%. A higher effect of vitamin C 7% was also observed in Chinese subjects, compared with in Caucasian subjects, as 20 percent of its effect was in the strong class. However, is it important to note the difference in the number of studies per concentration—the more studies there are, the more robust the results are.
FIGURE 6.
Distribution of the performance per classes for vitamin C 2%, 3%, 5%, 7%, and 10%, depending on the population, using the Bayesian analysis. For vitamin C at 10%, it was observed that 85% of its effect belongs to the strong class. For the concentrations 7%, 5%, and 3%, the majority of the effects belong to the moderate class. However, vitamin C at 7% in the Chinese population has 20% of its effect belonging to the strong class, indicating that this concentration has a higher effect in this population.
The observed induction of pigmentation during the exposure phase and at 24 to 72 hours after the last exposure is mainly due to the IPD/ PPD (related to the creation of reactive oxygen species and oxidation of melanin precursors). A significant preventive effect of UV-induced skin pigmentation was observed for vitamin C during the exposure days and just after. This is coherent with the antioxidant properties of vitamin C.6–9 According to the literature, DT, which is related to neosynthesized melanin, occurs three days after UV exposure.23 In these clinical trials, it is assumed that melanin neosynthesis starts between Days 12 and 15, followed by a natural depigmenting phase. In these last two phases, the vehicle and vitamin C have similar behaviors, indicating that vitamin C does not have any effect on depigmentation. The results also suggest that vitamin C is an antidarkening or antipigmenting agent rather than a depigmenting or whitening/ lightening agent, as described in the literature.
The Bayesian meta-analysis allowed us to observe a higher effect of vitamin C 7% on Chinese skin, compared with Caucasian skin. This could be explained by the difference in melanosome distribution into the skin depending on the population. In 2003, Thong et al28 showed that Chinese skin has an intermediate pattern of melanosomes, in between Caucasian and African skin, that corresponds to moderately pigmented skin also described in 2017 by Hurbain et al.29 However, melanosomes in individuals with moderately pigmented skin also have a different distribution in the epidermis. In particular, the melanosome density is more than six times higher in the stratum corneum of moderately pigmented skin than in lightly pigmented skin, and almost 10 times higher if we take into account the stratum granulosum.29 This can induce a higher oxidation of superficial melanin, leading to a more visible response to exposure in Chinese skin, compared with Caucasian skin, and therefore a higher effect of vitamin C’s antioxidant properties.
Results from those studies confirm that vitamin C at 10%, 7%, 5%, and 3% offers a fast onset of action, a good safety profile, and suitability for the long-term management of hyperpigmentation and skin heterogeneity.
Fighting against signs of photoaging, especially against visible signs such as skin hyperpigmentation and skin heterogeneity, has become an important and growing aesthetic issue in the last few decades. Many pharmaceutical and cosmeceutical preparations have been developed to support this need. Many of those products, especially those containing hydroquinone, have been negatively considered with high caution due to their carcinogenic potential or possibility of causing ochronosis.30,31 Other naturally occurring products, such as kojic acid, are safe but are of a modest efficacy.32,33 Due to its proven efficacy and good safety, however, vitamin C has become the compound of choice to achieve a temporary antipigmenting effect. However, vitamin C must be incorporated into products at high dosages to produce a visible temporary effect against stress-induced pigmentation.
Vitamin C constitutes a valuable and well-tolerated alternative to currently available dermocosmetic antipigmenting compounds with a dose-related efficacy in Caucasian and Chinese skin.
REFERENCES
- 1.Vashi NA, Kundu RV. Facial hyperpigmentation: causes and treatment. Br J Dermatol. 2013;169(Suppl 3):41–56. doi: 10.1111/bjd.12536. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Madhunapantula SV, Gowda R et al. Identification of aurora kinase B and Wee1-like protein kinase as downstream targets of (V600E)B-RAF in melanoma. Am J Pathol. 2013;182(4):1151–1162. doi: 10.1016/j.ajpath.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastiaens M, Hoefnagel J, Westendorp R et al. Solar lentigines are strongly related to sun exposure in contrast to ephelides. Pigment Cell Res. 2004;17(3):225–229. doi: 10.1111/j.1600-0749.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 4.Monestier S, Gaudy C, Gouvernet J et al. Multiple senile lentigos of the face, a skin ageing pattern resulting from a life excess of intermittent sun exposure in dark-skinned caucasians: a case–control study. Br J Dermatol. 2006;154(3):438–444. doi: 10.1111/j.1365-2133.2005.06996.x. [DOI] [PubMed] [Google Scholar]
- 5.Plensdorf S, Martinez J. Common pigmentation disorders. Am Fam Physician. 2009;79(2):109–116. [PubMed] [Google Scholar]
- 6.Farris PK. Cosmetical vitamins: vitamin C. In: Draelos ZD, Dover JS, Alam M, editors. Cosmoceuticals. Procedures in Cosmetic Dermatology. 2nd ed. New York, NY: Saunders Elsevier; 2009. pp. 51–56. [Google Scholar]
- 7.Al-Niaimi F, Chiang NYZ. Topical vitamin c and the skin: mechanisms of action and clinical applications. J Clin Aesthet Dermatol. 2017;10(7):14–17. [PMC free article] [PubMed] [Google Scholar]
- 8.Farris PF. Topical vitamin C: a useful agent for treating photoaging and other dermatologic conditions. Dermatol Surg. 2005;31:814–817. doi: 10.1111/j.1524-4725.2005.31725. 7 Pt 2. [DOI] [PubMed] [Google Scholar]
- 9.Ochiai Y, Kaburagi S, Obayashi K et al. A new lipophilic pro-vitamin C, tetra-isopalmitoyl ascorbic acid (VC-IP), prevents UV-induced skin pigmentation through its anti-oxidative properties. J Dermatol Sci. 2006;44:37–44. doi: 10.1016/j.jdermsci.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Draelos ZD. Skin lightening preparations and the hydroquinone controversy. Dermatol Ther. 2007;20(5):308–313. doi: 10.1111/j.1529-8019.2007.00144.x. [DOI] [PubMed] [Google Scholar]
- 11.Jimbow K, Obata H, Pathak MA, Fitzpatrick TB. Mechanism of depigmentation by hydroquinone. J Invest Dermatol. 1974;62(4):436–449. doi: 10.1111/1523-1747.ep12701679. [DOI] [PubMed] [Google Scholar]
- 12.Briganti S, Camera E, Picardo M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003;16(2):101–110. doi: 10.1034/j.1600-0749.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 13.Ortonne JP. Retinoid therapy of pigmentary disorders. Dermatol Ther. 2006;19(5):280–288. doi: 10.1111/j.1529-8019.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- 14.Ortonne JP, Pandya AG, Lui H, Hexsel D. Treatment of solar lentigines. J Am Acad Dermatol. 2006;54(5) Suppl 2:S262–S271. doi: 10.1016/j.jaad.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 15.Cozzi R, Ricordy R, Bartolini F et al. Taurine and ellagic acid: Two difierently-acting natural antioxidants. Environ Mol Mutagen. 1995;26(3):248–254. doi: 10.1002/em.2850260310. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura M, Watanabe Y, Kasai K et al. Inhibitory efiect of an ellagic acid-rich pomegranate extract on tyrosinase activity and ultraviolet-induced pigmentation. Biosci Biotechnol Biochem. 2005;69(12):2368–2373. doi: 10.1271/bbb.69.2368. [DOI] [PubMed] [Google Scholar]
- 17.Kim YJ, Uyama H. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci. 2005;62(15):1707–1723. doi: 10.1007/s00018-005-5054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim JT. Treatment of melasma using kojic acid in a gel containing hydroquinone and glycolic acid. Dermatol Surg. 1999;25(4):282–284. doi: 10.1046/j.1524-4725.1999.08236.x. [DOI] [PubMed] [Google Scholar]
- 19.Spiegelhalter DJ, Abrams KR, Myles JP. West Sussex, England: John Wiley and Sons; 2004. Bayesian Approaches to Clinical Trials and Health-Care Evaluation. [Google Scholar]
- 20.Whitehead A. West Sussex, England: John Wiley & Sons; 2002. Meta-Analysis of Controlled Clinical Trials. [Google Scholar]
- 21.Chardon A, Cretois I, Hourseau C. Skin color typology and suntanning pathways. Int J Cosmet Sci. 1991;13(4):191–208. doi: 10.1111/j.1467-2494.1991.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 22.Christiaens F, Chardon A, Fourtanier A, Frederick JE. Standard ultraviolet daylight for nonextreme exposure conditions. Photochem Photobiol. 2005;81(14):874–878. doi: 10.1562/2004-05-14-RA-167. [DOI] [PubMed] [Google Scholar]
- 23.Sklar LR, Almutawa F, Lim HW, Hamzavi I. Efiects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: a review. Photochem Photobiol Sci. 2013;12(1):54–64. doi: 10.1039/c2pp25152c. [DOI] [PubMed] [Google Scholar]
- 24.DEPIG 11—STUDY OF THE ANTI / DE-PIGMENTING EFFICACY of vitamin C/ SMA (7%) ON U.V. INDUCED PIGMENTATION Shanghai, 06 / MAY / 2009, N0 report: RCL-COS-09–0117. Unpublished data on file at L’Oréal
- 25.DEPIG 13—STUDY OF THE ANTI / DE-PIGMENTING EFFICACY of vitamin C/ SMA (7%) ON U.V. INDUCED PIGMENTATION Shanghai, 12 / NOV / 2009, N0 report: RCL-COS-09–0194. Unpublished data on file at L’Oréal
- 26.DEPIG 15—STUDY OF THE ANTI / DE-PIGMENTING EFFICACY of vitamin C/ SMA (7%) ON U.V. INDUCED PIGMENTATION Shanghai, 23 / AUG / 2010, N0 report: RCL-COS-10–0024. Unpublished data on file at L’Oréal
- 27.DEPIG 18 —STUDY OF THE ANTI / DE-PIGMENTING EFFICACY of vitamin C/ SMA (7%) ON U.V. INDUCED PIGMENTATION Shanghai, 16 / FEB / 2011, N0 report: CH-2011–003(I). Unpublished data on file at L’Oréal
- 28.Thong HY, Jee SH, Sun CC, Boissy RE. The patterns of melanosome distribution in keratinocytes of human skin as one determining factor of skin colour. Br J Dermatol. 2003;149(3):498–505. doi: 10.1046/j.1365-2133.2003.05473.x. [DOI] [PubMed] [Google Scholar]
- 29.Hurbain I, Romao M, Sextius P et al. Melanosome distribution in keratinocytes in difierent skin types: melanosome clustersare not degradative organelles. J Invest Dermatol. 2018;138(3):647–656. doi: 10.1016/j.jid.2017.09.039. [DOI] [PubMed] [Google Scholar]
- 30.Charlin R, Barcaui CB, Kac BK et al. Hydroquinone-induced exogenous ochronosis: a report of four cases and usefulness of dermoscopy. Int J Dermatol. 2008;47(1):19–23. doi: 10.1111/j.1365-4632.2007.03351.x. [DOI] [PubMed] [Google Scholar]
- 31.Jow T, Hantash BM. Hydroquinone-induced depigmentation: case report and review of the literature. Dermatitis. 2014;25(1):e1–e5. doi: 10.1097/01.DER.0000438425.56740.8a. [DOI] [PubMed] [Google Scholar]
- 32.Monteiro RC, Kishore BN, Bhat RM et al. A comparative study of the efficacy of 4% hydroquinone vs 0.75% kojic acid cream in the treatment of facial melasma. Indian J Dermatol. 2013;58(2):157. doi: 10.4103/0019-5154.108070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dayal S, Sahu P, Jain VK, Khetri S. Clinical efficacy and safety of 20% glycolic peel, 15% lactic peel, and topical 20% vitamin C in constitutional type of periorbital melanosis: a comparative study. J Cosmet Dermatol. 2016;15(4):367–373. doi: 10.1111/jocd.12255. [DOI] [PubMed] [Google Scholar]